Introduction
Renal ischemia reperfusion (IR) often results in
acute kidney injury (AKI), a clinical condition with no effective
treatment, which increases the risk of morbidity and mortality
perioperatively (1,2). Because of excessive workload and
greater metabolic demand, as well as limited anaerobic energy
production, proximal S3 segment tubular epithelial cells (TECs) of
the outer medulla are most commonly affected by acute ischemic
injury (3). The unique
microvasculature of this structure makes it vulnerable to renal
hypoxia, hypoperfusion and mitochondrial damage (4).
Inflammation is associated with the pathophysiology
of renal IR injury (IRI) (5).
Following ischemic injury, endothelial cells and leukocytes serve a
role in initiating inflammation and damaged TECs contribute to the
inflammatory process. Injured tubular epithelium produces numerous
cytokines including IL-6, IL-1β, TNF-α and TGF-β, thereby affecting
the behavior of macrophages and inducing a pro-inflammatory
phenotype (3,6,7). To
the best of our knowledge, it has not been determined whether renal
epithelial cells serve as antigen-presenting cells (APCs) and exert
an immunomodulatory function during renal IR.
During inflammation, inducible nitric oxide synthase
(iNOS) is upregulated and converts arginine into citrulline and NO
(8). This enzyme is found in the
renal tubules, interlobar and arcuate arteries and glomerulus of
normal rat kidney (9). Studies have
documented the involvement of iNOS and NO in renal IRI development
and suggested that iNOS inhibitors may prove beneficial as a
therapeutic strategy in clinical scenarios where renal IRI is
prevalent (10,11). Arginase-II (Arg-II), which is highly
expressed within the S3 proximal TECs (12), catalyzes the conversion of
L-arginine to L-ornithine and urea, which is needed for the
synthesis of polyamines (13).
Since iNOS and Arg-II use the same substrate, stimulating Arg-II
expression exerts anti-inflammatory effects via shifting of
arginine metabolism to produce polyamine at the expense of NO
production (14).
Signal transducer and activator of transcription 3
(STAT3) was identified by studies on acute response factor
signaling (15,16). During the binding of cytokines, JAK
protein stimulates canonical STAT3 signaling. The most common
activators of STAT3 are IL-6-type cytokines via IL-6-induced
tyrosine phosphorylation of STAT3(17). Dysregulation in the activation of
STAT3 is typically associated with multiple pathologies, including
autoimmune and malignant disorders (18). The role of STAT3 in the progression
of diabetic nephropathy, development of HIV-associated nephropathy,
activation of renal interstitial fibroblasts and progression of
renal fibrosis has been investigated (19-21).
Numerous studies have also noted an association between IRI
progression and the activation of STAT3 (22,23),
some of which found that activation of STAT3 in renal proximal TECs
may be protective during IRI (24,25).
Although there is limited data regarding the therapeutic potential
of STAT3 inhibitors in pathological renal models, evidence suggests
that STAT3 inhibitors may be beneficial (26,27).
Peroxisome proliferator-activated receptor (PPAR)γ,
a nuclear receptor superfamily member, is a transcription factor
involved in regulating glucose and lipid metabolism as well as
cancer progression and inflammation (28). PPARγ agonists [such as pioglitazone
(Pio)] inhibit inflammation by stopping the phosphorylation of
proteins involved in JAK-STAT signaling pathway (29,30).
PPARγ binds to miR-124 promoter, causing the upregulation of
miR-124(31), thereby regulating
gene expression. Sun et al (32) reported that miR-124 targets STAT3 to
decrease the production IL-6 and TNF-α converting enzyme to
decrease TNF-α release.
More studies are required to understand the
inflammatory response mechanisms during ischemic kidney injury to
identify the molecular targets for therapeutic intervention. The
present study aimed to determine the role of renal TECs as drivers
of inflammation in renal IRI and their potential function as
antigen-presenting cells by analyzing inflammatory markers involved
in pathogenesis of renal IRI, as well as the renal epithelial cell
expression of CD86, STAT3 expression in renal IRI and the molecular
basis underlying the anti-inflammatory action of the PPARγ agonist
Pio by investigating its effect on the expression of miRNA-124,
STAT3, pro-inflammatory cytokines, iNOS, Arg-II and CD86.
Materials and methods
Chemicals and reagents
Pio was purchased from Arab Pharmaceutical
Manufacturing Co., Ltd. Dimethyl sulfoxide (DMSO) was purchased
from Loba Chemie Pvt. Ltd.
Animals
A total of 50 adult Wistar male albino rats (age,
6-8 weeks; weight, 160-180 g) were obtained from the Faculty of
Agriculture, Benha University, Moshtohor, Egypt. Animals were
randomly divided into five groups (all n=10) and each group was
placed in a separate cage. The cages were maintained at 25˚C with
12/12-h light and dark cycles, relative humidity (45±5%) and all
animals had access to food and water ad libitum. All rats
were acclimatized to the laboratory setting for one week prior to
experiments. The study followed the criteria of care and use of
laboratory animals (33) and was
approved by the Medical Research Ethics Committee of Benha
University, Egypt (approval no. RC.11.6. 2022).
Rat model of renal IRI
The animals were divided into the following groups:
i) Sham operation + DMSO; ii) sham operation + Pio; iii) renal IRI
+ DMSO; iv) IRI + prophylactic preoperative (pre) Pio and v) IRI +
postoperative (post) Pio. All rats were anesthetized using
Thiopental Na [40 mg/kg, administered intraperitoneally (i.p.)] and
injected intramuscularly with antibiotic (Penicillin G procaine;
40,000 U/kg). Renal IR was performed by clamping the renal arteries
bilaterally for 45 min, followed by reperfusion for 24 h, as
described by Hu et al (34).
Rats in sham operation groups underwent similar surgical
interventions and were anesthetized but did not undergo bilateral
renal pedicle clamping. Pio was dissolved in DMSO and injected i.p
(10 mg/kg) as previously described (35). The drug was administered 2 h before
sham operation or induction of ischemia in groups II and IV
respectively, and 2 h after surgery in the IRI + postoperative
(post) Pio group. Respiratory rate and pattern of rats was
monitored every 10-15 min and rats were turned from side to side
during the recovery period to promote a quicker recovery. Food and
water intake was also monitored after recovery. At 24 h
post-reperfusion, rats were euthanized via decapitation following
anesthetization with 1.5 g/kg urethane (i.p). Death was verified by
cessation of heartbeat and respiration, then bilateral nephrectomy
was performed and each kidney was cut into two.
Renal function assessment
Blood samples (2 ml) taken from the abdominal aorta,
24 h after reperfusion, were left to clot for 15-30 min at room
temperature, centrifuged at 3,000 x g at 4˚C for 10 min and
supernatant was obtained to monitor renal function. Serum
creatinine and blood urea nitrogen (BUN) levels were estimated
using Rat Creatinine (cat. no. #MBS749827) and BUN ELISA kits (cat.
no. #MBS2611086; both MyBioSource, Inc.), according to the
manufacturer's instructions.
Biochemical analysis
The kidney specimens were rinsed in ice cold saline
and homogenized using a Mixer Mill MM400 (Retsch GmbH) in phosphate
buffer (pH 6-7). Tissue homogenate was centrifuged at 10,000 x g,
4˚C for 15 min. Supernatant was used for quantitative detection
using ELISA kits, according to the manufacturer's instructions, as
follows: Rat IL-1β (cat. no. E-EL-R0012; Elabscience Biotechnology,
Inc.), IL-6 (cat. no. ab100772; Abcam), TNF-α (cat. no. E-CL-R0019)
and TGF-β1 (cat. no. E-EL-0162; both Elabscience Biotechnology,
Inc.), Arg-II (cat. no. MBS7216305) and iNOS ELISA kit (cat. no.
MBS023874; both MyBioSource, Inc.).
Histopathological examination
The kidney samples were fixed in 10% buffered
formalin (pH 7.8) for 72 h at room temperature, then sliced into
very thin sections (4 µm), stained with hematoxylin and eosin and
visualized using the high-power option of the light microscope
(magnification, x400). Histopathological samples were scored using
the system described by El-Nabarawy et al (36) as follows: -, no abnormal
cellularity; +, minor focal lesions in 1-3 samples/group; ++, mild
focal lesions in 4-6 samples/group; +++, moderate diffuse lesions
in 4-6 samples/group and ++++, severe diffuse lesions in all
samples.
Immunohistochemistry staining
Deparaffinized, rehydrated 4-µm tissue sections in
descending alcohol series at room temperature were subjected to
antigen-retrieval at 95˚C, then blocked by 0.3%
H2O2 for 20 min at room temperature. Sections
were incubated with anti-CD86 primary antibody (cat. no. bs-1035R;
BIOSS USA; 1:150) overnight at 4˚C, washed with PBS, then incubated
with secondary antibody HRP Envision kit (Dako; Agilent
Technologies, Inc.) for 20 min and DAB for 15 min. Sections were
washed with PBS, counterstained with hematoxylin, dehydrated and
cleared in xylene and finally cover slipped for microscopic
examination. A total of six non-overlapping fields were randomly
selected and scanned from each sample for the determination of mean
area percentage of immunohistochemical expression levels of CD86
positive cells. All light microscopic examination and morphometric
data were obtained using Leica Application module for histological
analysis attached to Full HD microscopic imaging system (Leica
Microsystems GmbH).
Western blot analysis
Western blotting was performed to detect STAT3
expression levels. Total protein was extracted using RIPA lysis
buffer (Sigma-Aldrich; Merck KGaA) and protein concentration was
determined colorimetrically in kidney tissue samples using the
Bradford method (37). A total of
25 µg protein/lane was mixed and boiled with SDS Loading buffer for
5 min. The solution was left to cool on ice for 7 min before
loading into a 10% SDS-polyacrylamide gel and separated using the
Cleaver electrophoresis unit (Cleaver Scientific Ltd., UK) and
placed on PVDF membranes for 30 min via Semi-dry Electroblotter
(Bio-Rad Laboratories, Inc.). Blocking was performed with 5%
non-fat dry milk in Tris-buffered saline-0.05% Tween-20 (TBS-T),
for 2 h at 37˚C. Incubation of the membrane was performed overnight
at 4˚C with primary antibodies against STAT-3 (1:500; cat. no.
ab119352; Abcam) and β-actin (1:500; cat. no. A5060; Sigma-Aldrich;
Merck KGaA). Blots were washed three times (10 min each) using
TBS-T, incubated at room temperature for 1 h using horseradish
peroxidase-linked secondary antibodies (Dako; Agilent Technologies,
Inc.), then washed three times (10 min each) with TBS-T.
Chemiluminescent Western ECL substrate (PerkinElmer, Inc.) was
applied according to the manufacturer's guidelines. Signals were
captured using the Chemi Doc imager (Bio-Rad Laboratories, Inc.).
Band intensity was normalized to β-actin.
Reverse transcription-quantitative
(RT-q)PCR analysis of miRNA-124 gene expression
Total RNA was extracted from frozen kidney tissue
samples using TRIzol™ Plus RNA Purification kit (cat.
no. 12183555; Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's guidelines. The concentration and purity of
the RNA were determined by measuring the absorbance at 260 and 280
nm using a NanoDrop One spectrophotometer (Thermo Fisher
Scientific, Inc.). Pure RNA has A260/A280 ratio of 1.8-2.1(38). Rat rno-mir-124 Real-time RT-PCR
Detection and U6 Calibration kit (cat. no. MBS826191; MyBioSource,
Inc.) was used for the detection and quantification of mir-124. RT
was performed according to the Standard RT Reaction Program (30 min
at 25˚C, 30 min at 42˚C, 5 min at 85˚C) followed by PCR reaction
(95˚C for 3 min hold, 40 cycles of 95˚C, 12 sec; 62˚C, 40 sec)
using Step One Plus Real-Time PCR System (Thermo Fisher Scientific,
Inc.). The relative expression was calculated using the
2-∆∆Cq method described by Livak and Schmittgen
(39). The results are expressed as
the fold-change relative to the Sham operation + DMSO group.
Statistical analysis
Data are presented as the mean ± SD. Differences
between groups were evaluated using one-way ANOVA followed by post
hoc Tukey's test using Statistical Package for Social Science
program, Version 16 (SPSS, Inc.). P≤0.05 was considered to indicate
a statistically significant difference.
Results
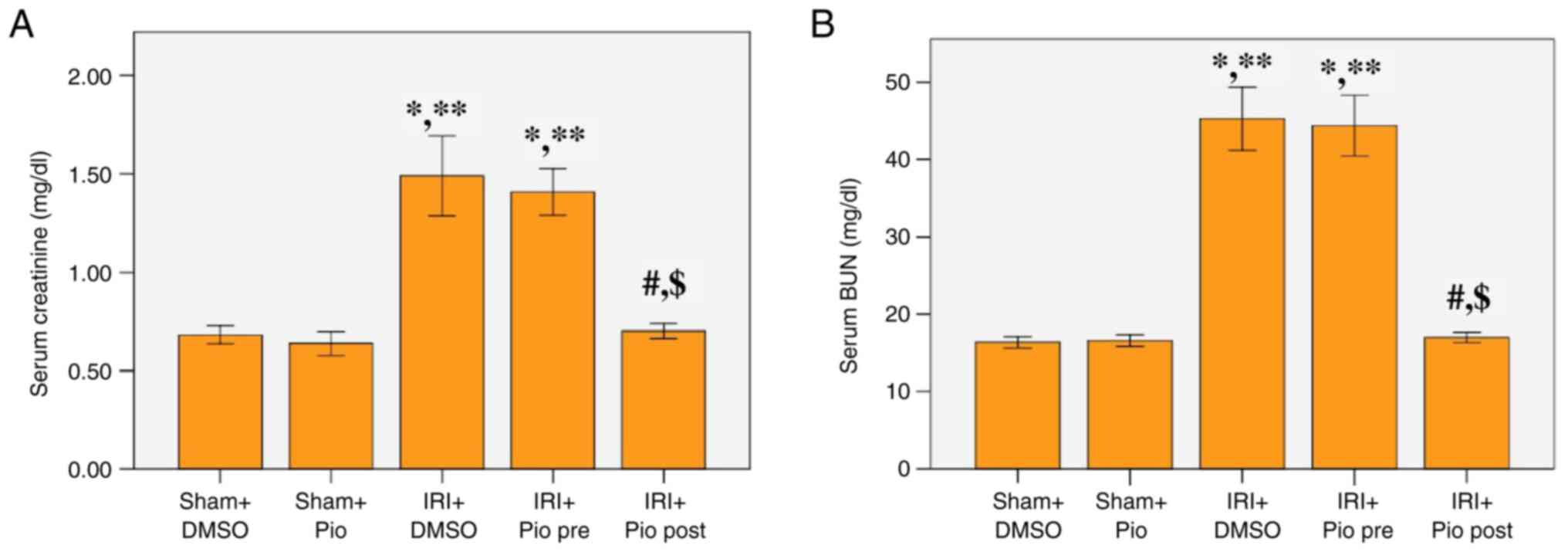
Effect of Pio on serum levels of
creatinine and BUN
Serum creatinine and BUN levels at 24 h after
reperfusion were significantly increased in the renal IRI + DMSO
group compared with the sham groups (Sham operation + DMSO, Sham
operation + Pio). Administration of Pio prior to ischemia induction
did not cause a significant decrease in serum creatinine and BUN
levels compared with the renal IRI + DMSO, while its administration
in the post-IR phase caused a significant decrease in the serum
creatinine and BUN levels compared with the renal IRI + DMSO and
the group administered Pio prior to ischemia induction (Fig. 1A and B).
Effect of Pio on iNOS, Arg-II and
proinflammatory cytokines levels
In the renal IRI + DMSO group, levels of
pro-inflammatory cytokines (IL-6, IL-1 β and TNF-α), TGF-β and iNOS
were significantly increased compared with the Sham groups (Sham
operation + DMSO, Sham operation + Pio) (P<0.05; Table I). Administration of Pio prior to
ischemia or post-IR caused a significant decrease in all assessed
pro-inflammatory cytokines as well as iNOS levels compared with the
IRI + DMSO group. A significant decrease in IL-1 β and iNOS levels
was detected when Pio was administered in post-IR compared with
administration before induction of ischemia. A significant decrease
in Arg-II was demonstrated in the renal IRI + DMSO group compared
with the sham groups (Sham operation + DMSO, Sham operation + Pio),
while Pio administration prior to ischemia induction or in the
post-IR phase significantly increased Arg-II.
 | Table IiNOS, Arginase II and proinflammatory
cytokines levels in renal tissue. |
Table I
iNOS, Arginase II and proinflammatory
cytokines levels in renal tissue.
| Parameter | Sham + DMSO
(n=10) | Sham + Pio
(n=10) | IRI + DMSO
(n=10) | IRI + Pio pre
(n=10) | IRI + Pio post
(n=10) |
|---|
| iNOS, pg/mg
protein | 3.38±1.03 | 2.75±0.68 |
15.71±2.36a,b |
6.38±1.54a,b,c |
4.33±1.20c,d |
| Arginase-II, pg/mg
protein | 3.12±0.76 | 3.40±0.70 |
2.32±0.74a,b |
3.04±0.67c |
3.66±0.65c |
| IL-6, pg/mg
protein | 5.31±1.28 | 4.65±0.64 |
8.05±1.69a,b |
6.69±0.72b,c |
5.96±0.92c |
| IL-1 β, pg/mg
protein | 5.79±0.90 | 3.90±0.81 |
18.59±3.55a,b |
13.76±1.80a,b,c |
6.75±1.23b,c,d |
| TNF-α, pg/mg
protein | 10.77±1.22 | 9.12±1.84 |
55.69±15.09a,b |
16.43±1.95c |
11.82±1.52c |
| TGF-β, pg/mg
protein | 16.48±1.17 | 16.20±1.45 |
42.54±7.87a,b |
21.84±2.02a,b,c |
17.50±1.82c |
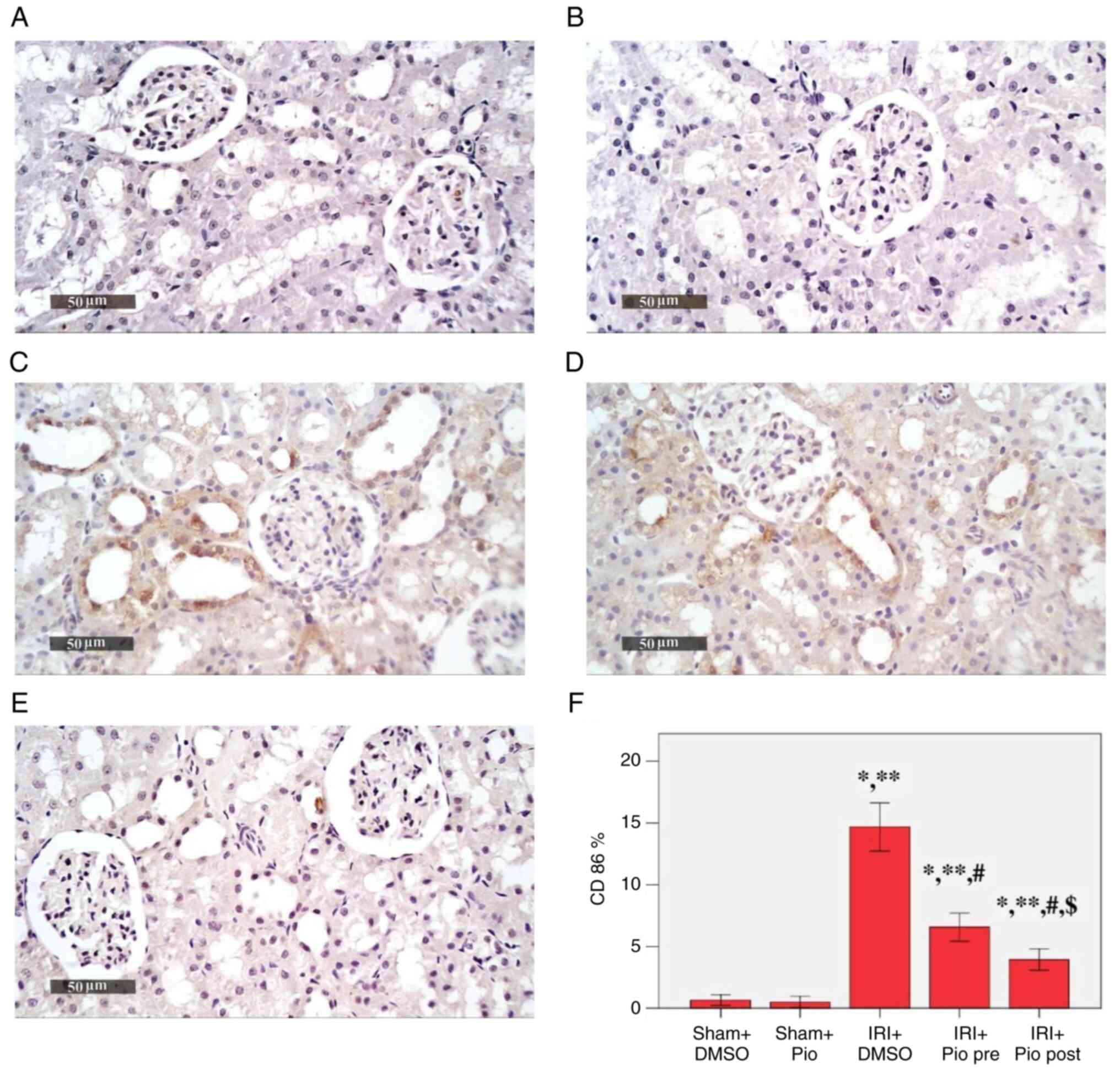
Effect of Pio on histopathological
changes in renal tissue samples
Sham groups (Sham operation + DMSO, Sham operation +
Pio) showed normal histological features of renal parenchyma (both
medullary and cortical components) with intact renal corpuscles and
tubular segments with almost intact tubular epithelium as well as
intact vasculature (Fig. 2A and
B; Table II). Renal IRI + DMSO group showed
notable degenerative alterations within the epithelium of tubules
with moderate dilatation in different segments, occasional focal
records of tubular necrosis with intraluminal casts, congested
glomerular tufts and interstitial blood vessels (BVs) and mild
inflammatory cell infiltrate (Fig.
2C). Pio administration prior to ischemia induction caused only
a mild focal improvement of renal tissue architecture without
notable protective efficacy (Fig.
2D), while Pio was effective at improving renal tissue
architecture post-IR, as shown by the organized morphological
features of renal parenchyma, notable protective efficacy on renal
tubular epithelium, mild focal records of degenerated TECs,
occasional nuclear pyknosis, mild congested interstitial BVs and
glomerular tufts (Fig. 2E).
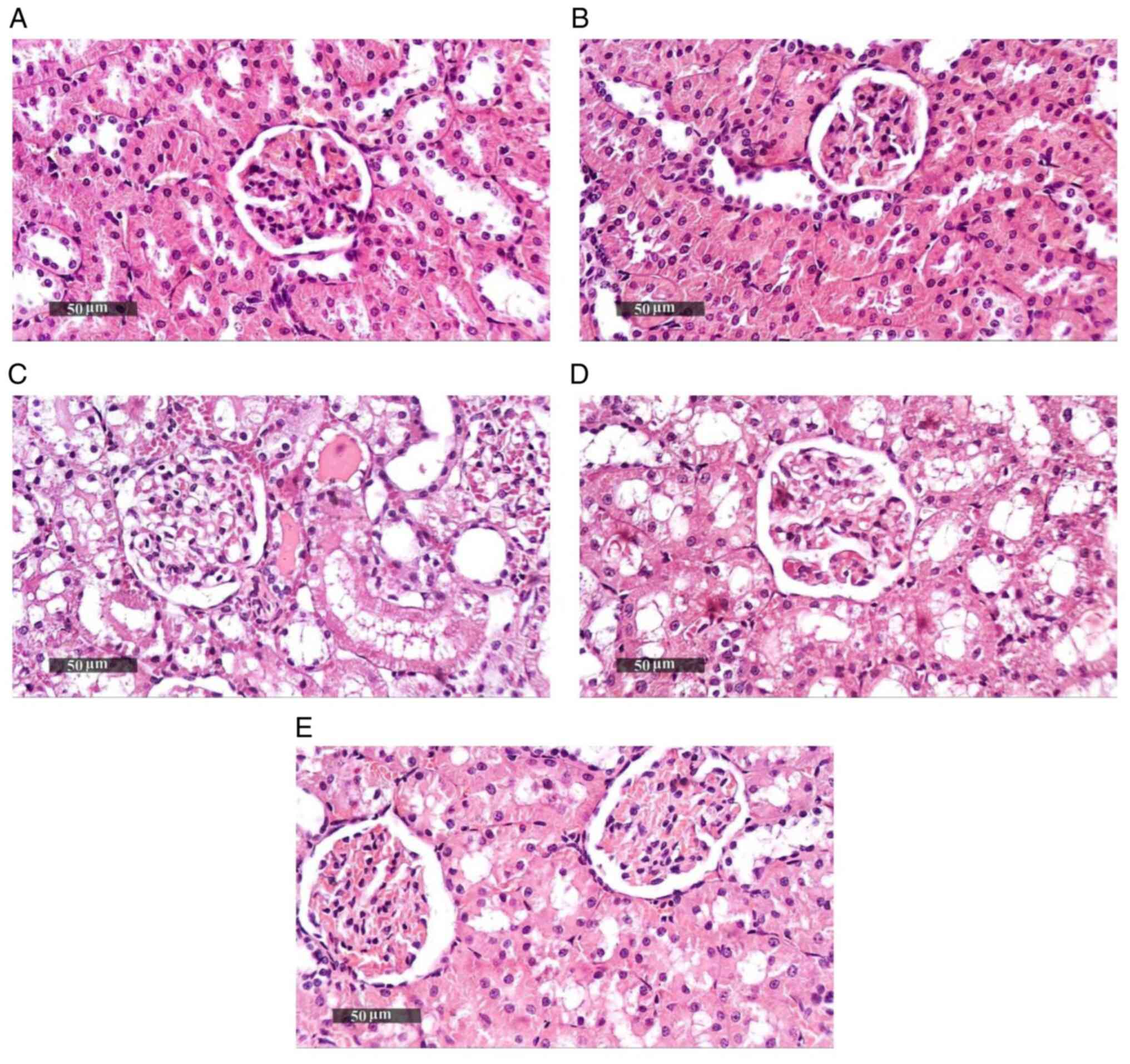 | Figure 2Photomicrographs of hematoxylin and
eosin-stained renal tissue sections. (A) Sham + DMSO shows normal
histological features of cortical and medullary components of renal
parenchyma with apparently intact renal corpuscles and tubular
segments with almost intact tubular epithelium as well as intact
vasculature. (B) Sham + Pio shows almost the same records as Sham +
DMSO without abnormal histological changes. (C) IRI + DMSO shows
severe degenerative changes of tubular epithelium with moderate
dilatation in different segments, occasional focal records of
tubular necrosis with intraluminal casts, severe congested
glomerular tufts, congested interstitial BVs and mild inflammatory
cell infiltrate. (D) IRI + Pio pre shows almost the same records as
IRI group without notable protective efficacy and mild focal
improvement of renal tissue architecture. (E) IRI + Pio post shows
more organized renal parenchyma with notable protective efficacy on
renal tubular epithelium, mild focal records of degenerated tubular
epithelial cells, occasional nuclear pyknosis and mild congested
interstitial BVs and glomerular tufts. Magnification, x400. IRI,
ischemia reperfusion injury; Pio, pioglitazone; pre, preoperative;
post, postoperative; BVs, blood vessels. |
 | Table IIHistopathological scoring of renal
tissue samples. |
Table II
Histopathological scoring of renal
tissue samples.
| Histopathological
changes | Sham + DMSO | Sham + Pio | IRI + DMSO | IRI + Pio pre | IRI + Pio post |
|---|
| Tubular
degenerative changes | - | - | ++++ | ++++ | ++ |
| Tubular
necrosis | - | - | ++ | + | - |
| Congested BVs | - | - | +++ | ++ | ++ |
| Inflammatory cell
infiltrates | - | - | + | - | - |
Effect of Pio on the expression of
CD86 in renal tissue
IRI was a potent inducer for CD86 immunoexpression
(Fig. 3). A significant increase in
the mean area % of CD86 immunoexpression was detected in the IRI +
DMSO, IRI + prophylactic preoperative (pre) Pio, IRI + post Pio
groups (14.68, 6.58 and 3.95% respectively) compared with the Sham
groups (Sham + DMSO, 0.67%; Sham + Pio, 0.52%). Pio, whether
administered prior to ischemia induction or in the post-IR phase,
caused a significant decrease in CD86 immunoexpression compared
with the IRI + DMSO group. Moreover, the decrease in
immunoexpression was more significant when Pio was administered in
the post-IR phase.
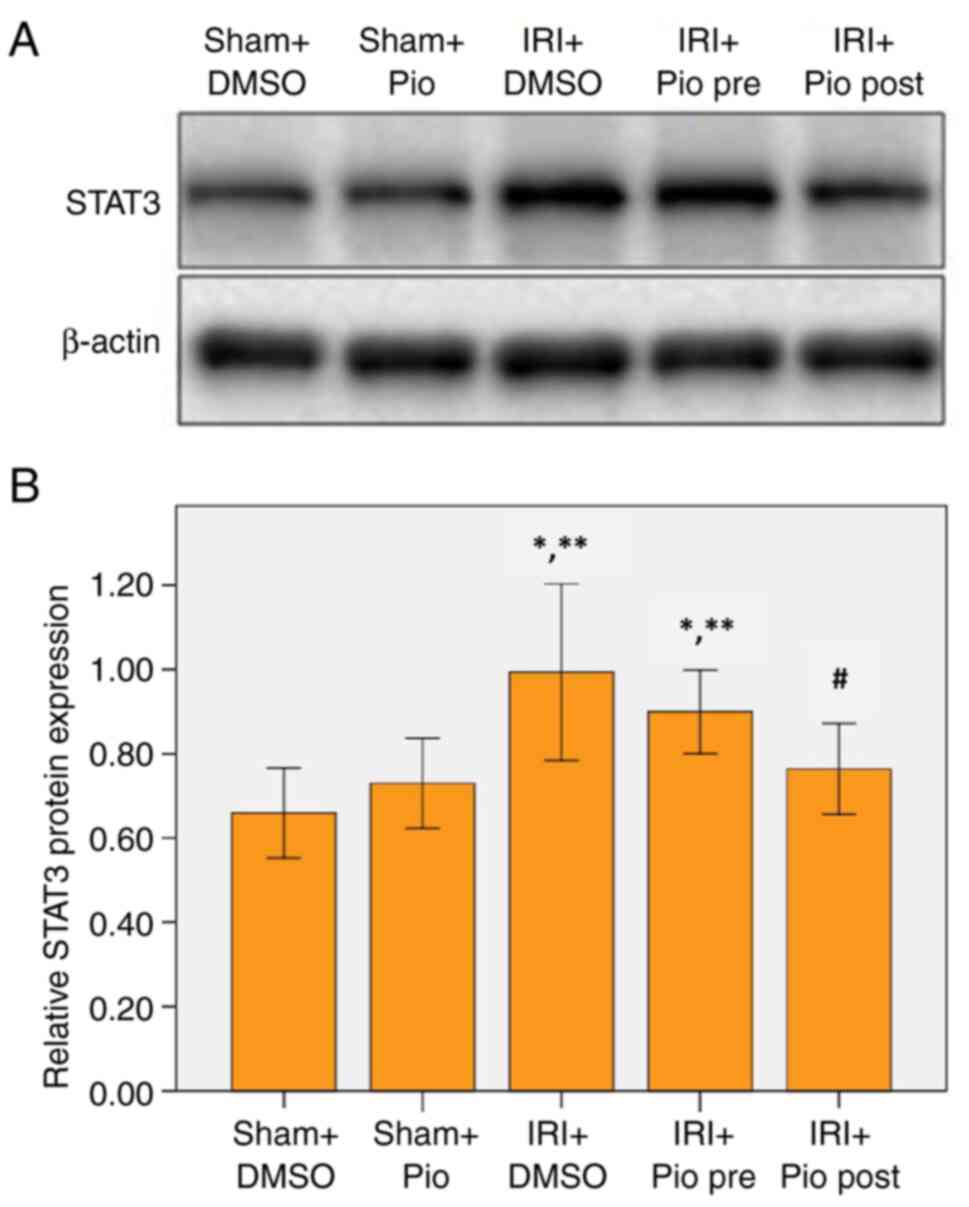
Effect of Pio on expression of
STAT3
Western blotting was performed to detect STAT3
expression levels. The renal IRI + DMSO group showed a significant
increase in STAT3 expression compared with Sham groups (Sham
operation + DMSO, Sham operation + Pio). Pio administration prior
to ischemia did not cause a significant decrease in STAT3 compared
with renal IRI + DMSO group, while its administration in the
post-IR phase caused a significant decrease in STAT3 compared with
the IRI + DMSO group (Fig. 4A and
B).
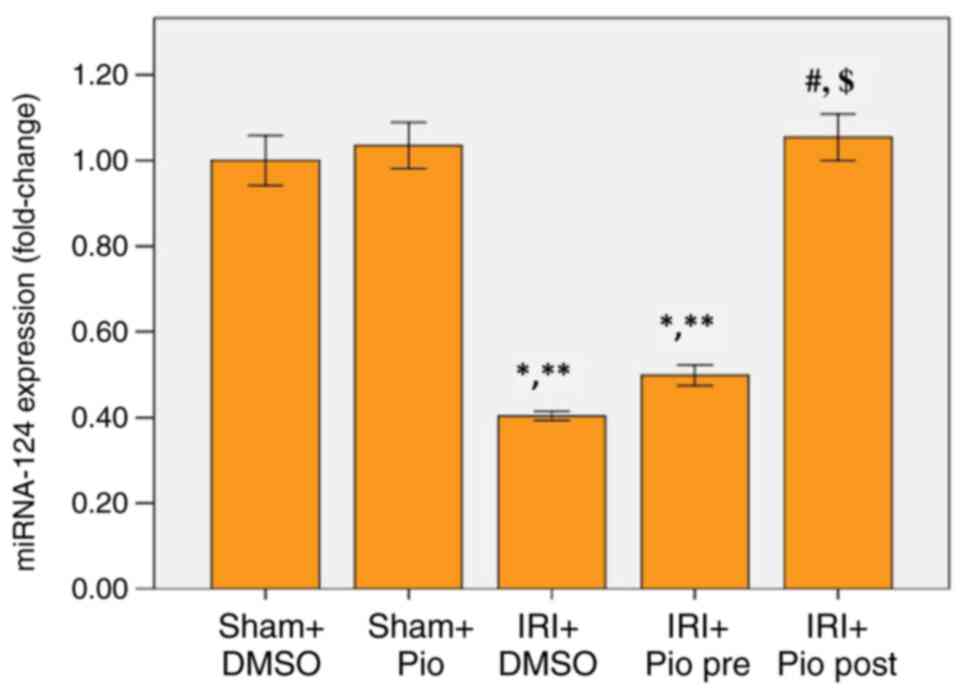
Effect of Pio on expression of
miRNA-124 in renal tissue
miR-124 was significantly downregulated in renal
tissue of IRI + DMSO group and the group administered Pio prior to
ischemia compared with Sham groups (Sham operation + DMSO, Sham
operation + Pio). Pio administration prior to ischemia caused a
mild increase in miR-124 levels but was not significantly different
compared with the IRI + DMSO. A marked increase in miR-124
expression was detected when Pio was administered in post-IR phase
compared with IRI + DMSO and IRI + prophylactic preoperative (pre)
Pio (Fig. 5).
Discussion
Inflammation serves a key role in the
pathophysiology and development of renal ischemia-induced AKI
(5,40). The tubulointerstitium and renal
tubules, which are key sites that respond to injury, comprise a
notable part of the kidney. Injured TECs directly (via autocrine
function) or indirectly (infiltrating leukocytes via a paracrine
process) increase production of inflammatory cytokines (41). TECs are considered key fibrogenic
and inflammatory cells (42).
A medication which has been found to have protective
functions against renal IRI mouse models is Pio, a synthetic ligand
of PPAR-γ. The majority of studies has investigated the
renoprotective effect of Pio in renal IR rat models with Pio
administered prior to renal ischemia induction (35,43,44).
The present study assessed the ability of Pio to provide protection
prior to renal ischemia induction as well as in the post-IR to
demonstrate the potential for acute use in AKI.
Here, Pio administration prior to ischemia or in the
post-IR phase significantly decreased levels of TNF-α, IL-1β, IL-6,
TGF- β and iNOS in renal tissue. Studies have found that PPARγ
agonists inhibit inflammation by stopping inflammatory factor
synthesis and signaling pathways (45,46).
Notably, two markers of inflammation, iNOS and
IL-1β, were significantly decreased in the group administered Pio
in the post-IR phase demonstrating the specific differential action
of Pio and further supports the findings of previous studies
demonstrated the specific effect of PPARγ on iNOS expression and
IL-1β levels (47-49).
According to Crosby et al (50), in mesangial cells, PPARγ agonists
directly inhibit iNOS transcription as well as NO production. PPARγ
is a negative regulator of NLRP3 inflammasome activation. PPARγ
binding sites are located in the promoter regions of a member of
the NLRP3 family, which decrease downstream molecules (such as
IL-1β). Activating the NLRP3 inflammasome is associated with renal
injury and inflammation in cases of I/R-induced AKI (51-54).
One of the most important reno-protective mechanisms
of PPARγ agonists is mediated by inhibitory action on iNOS, as NO
generated by iNOS contributes notably to renal IRI. NO reacts with
superoxide anion to form peroxynitrite. Peroxynirtrite induces
injury by direct oxidant injury and protein tyrosine nitration
(55). Furthermore, several studies
have reported that inactivation of iNOS expression and activity
ameliorates NO-mediated renal injury (11,56,57).
The results of the present study showed
significantly decreased levels of Arg-II in the IRI + DMSO group,
while Pio increased Arg-II levels. Inhibitory effects of PPARγ
agonists on iNOS expression increase the concentration of arginine,
a substance used by both Arg and NOS enzymes, resulting in the
stimulation of Arg expression (58). Erbas et al (59) reported that the inhibitory effects
of N-Acetylcysteine on iNOS activity increased arginine
availability, which caused an increase in Arg activity.
In general, the observed decrease in
pro-inflammatory cytokines as well as iNOS in the group
administered Pio in the post-IR phase compared with dosing prior to
ischemia may be associated with Pio pharmacokinetics including time
at maximum plasma concentration and elimination half-life.
The present histopathological changes demonstrated
the reno-protective effects of Pio administration and confirmed
that increased expression of iNOS contributed to increased IR-
mediated renal tissue injury. The group given Pio in the post-IR
phase showed significantly lower iNOS levels with notably decreased
histological evidence of IR-mediated renal tissue injury compared
with the group given Pio prior to renal ischemia induction.
To assess the role of TECs as drivers of
inflammation, kidney tissue was stained for CD86 to investigate
whether they served as APCs. There is conflicting data in terms of
expression of CD80 and CD86, which are needed for the activation of
CD4+ T cells, in renal epithelium (60,61).
The results of the study showed that IRI was a potent inducer for
CD86 expression in TECs. Breda et al (62) observed high expression of CD86 in
proximal tubular epithelial cells and suggested an
inflammation-dependent regulation of epithelium-expressed CD80 and
CD86. Niemann-Masanek et al (63) reported that, in addition to
generating pro-inflammatory cytokines and chemokines, tubular cells
also express complement and their receptors, toll-like receptors,
and co-stimulatory molecules (such as CD80 and CD86) which interact
with CD28 on T lymphocytes to facilitate production of
cytokines.
The present results revealed that Pio administration
significantly suppressed the expression of CD86. This raises the
question of which mechanism underlies the inhibitory effect of
PPARγ agonists on CD86 expression in tubular epithelial cells.
To understand the molecular mechanisms in IRI,
expression of STAT3 was assessed in renal tissue as its
dysregulated activation is implicated in various types of kidney
disease. Here, STAT3 expression was significantly increased in IRI
+ DMSO group and Pio administration in the post-IR phase
significantly decreased STAT3 expression. Evidence suggests
therapeutic potential for STAT3 inhibition in numerous pathological
renal models, but results of STAT3 inhibition role in AKI is
contradictory (24,26). To clarify the mechanism by which
PPARγ agonists suppresses expression of STAT3, the present study
assessed the levels of miRNA-124 expression in kidney tissue as it
negatively regulates inflammation by targeting several pathways.
Previous studies have reported that miRNA-124 targets STAT3 3'
untranslated region and inhibits protein translation (32,64-67).
The present study showed a significant downregulation of miRNA-124
in the IRI + DMSO group. Pio administered in the post-IR phase
significantly upregulated miRNA-124 expression, which explains the
significant decrease in STAT3 expression observed in this group.
These findings support those of Wang et al (31) who demonstrated that activation of
PPARγ upregulates miRNA-124 and inhibits miRNA-124 target
genes.
To conclude, the present study demonstrated that
tubular epithelium serves an important role in the inflammatory
response in kidney IRI, not only generating proinflammatory
cytokines which activate inflammatory cells, but also expressing
CD86, which is required for T lymphocyte activity regulation.
Targeting STAT3 by enhancing expression of miRNA-124 may exert
beneficial anti-inflammatory effects in kidney IRI. The molecular
mechanism by which Pio exerted its anti-inflammatory effect
includes upregulation of miRNA-124 with subsequent inhibition of
STAT3 expression. Better understanding of the molecular aspects
underlying the inflammatory component in kidney IRI may provide
novel therapeutic strategies to attenuate inflammation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WBEG conceived the study, designed and performed the
experiments and wrote and edited the manuscript. MMA conceived the
study, designed and performed the experiments and edited the
manuscript. SAS wrote the manuscript and contributed to analysis
and interpretation of the data. LAM and HEN designed and performed
the experiments and wrote the manuscript. AMS performed the
histological examination of the kidney and wrote the manuscript.
WBEG and MMA confirm the authenticity of all raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee, Benha Faculty of Medicine, Benha University,
Egypt (approval no. RC.11.6. 2022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han SJ and Lee HT: Mechanisms and
therapeutic targets of ischemic acute kidney injury. Kidney Res
Clin Pract. 38:427–440. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jia P, Xu S, Ren T, Pan T, Wang X, Zhang
Y, Zou Z, Guo M, Zeng Q, Shen B and Ding X: LncRNA IRAR regulates
chemokines production in tubular epithelial cells thus promoting
kidney ischemia-reperfusion injury. Cell Death Dis.
13(562)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sharfuddin AA and Molitoris BA:
Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol.
7:189–200. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Funk JA and Schnellmann RG: Persistent
disruption of mitochondrial homeostasis after acute kidney injury.
Am J Physiol Renal Physiol. 302:F853–F864. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bonventre JV and Zuk A: Ischemic acute
renal failure: An inflammatory disease? Kidney Int. 66:480–485.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Y, Chang J, Yao B, Niu A, Kelly E,
Breeggemann MC, Abboud Werner SL, Harris RC and Zhang MZ: Proximal
tubule-derived colony stimulating factor-1 mediates polarization of
renal macrophages and dendritic cells, and recovery in acute kidney
injury. Kidney Int. 88:1274–1282. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huen SC, Huynh L, Marlier A, Lee Y,
Moeckel GW and Cantley LG: GM-CSF promotes macrophage alternative
activation after renal ischemia/reperfusion injury. J Am Soc
Nephrol. 26:1334–1345. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cinelli MA, Do HT, Miley GP and Silverman
RB: Inducible nitric oxide synthase: Regulation, structure, and
inhibition. Med Res Rev. 40:158–189. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Joles JA, Vos IH, Gröne HJ and Rabelink
TJ: Inducible nitric oxide synthase in renal transplantation.
Kidney Int. 61:872–875. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mark LA, Robinson AV and Schulak JA:
Inhibition of nitric oxide synthase reduces renal
ischemia/reperfusion injury. J Surg Res. 129:236–241.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chatterjee PK, Patel NS, Kvale EO,
Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H and Thiemermann C:
Inhibition of inducible nitric oxide synthase reduces renal
ischemia/reperfusion injury. Kidney Int. 61:862–871.
2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Levillain O, Balvay S and Peyrol S:
Localization and differential expression of arginase II in the
kidney of male and female mice. Pflugers Arch. 449:491–503.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marselli L, Bosi E, De Luca C, Del Guerra
S, Tesi M, Suleiman M and Marchetti P: Arginase 2 and polyamines in
human pancreatic beta cells: Possible role in the pathogenesis of
type 2 diabetes. Int J Mol Sci. 22(12099)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Marathe C, Bradley MN, Hong C, Lopez F,
Ruiz de Galarreta CM, Tontonoz P and Castrillo A: The arginase II
gene is an anti-inflammatory target of liver X receptor in
macrophages. J Biol Chem. 281:32197–32206. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: A
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: How intimate is the relationship? Ann N Y Acad Sci.
1171:59–76. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Billing U, Jetka T, Nortmann L, Wundrack
N, Komorowski M, Waldherr S, Schaper F and Dittrich A: Robustness
and information transfer within IL-6-induced JAK/STAT signalling.
Commun Biol. 2(27)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Zheng C, Huang L, Luo W, Yu W, Hu X, Guan
X, Cai Y, Zou C, Yin H, Xu Z, et al: Inhibition of STAT3 in tubular
epithelial cells prevents kidney fibrosis and nephropathy in
STZ-induced diabetic mice. Cell Death Dis. 10(848)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Feng X, Lu TC, Chuang PY, Fang W, Ratnam
K, Xiong H, Ouyang X, Shen Y, Levy DE, Hyink D, et al: Reduction of
Stat3 activity attenuates HIV-induced kidney injury. J Am Soc
Nephrol. 20:2138–2146. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pang M, Ma L, Gong R, Tolbert E, Mao H,
Ponnusamy M, Chin YE, Yan H, Dworkin LD and Zhuang S: A novel STAT3
inhibitor, S3I-201, attenuates renal interstitial fibroblast
activation and interstitial fibrosis in obstructive nephropathy.
Kidney Int. 78:257–268. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L,
Liu C, Wang J, Yang X, Vohra A and Ma D: Dexmedetomidine protects
against renal ischemia and reperfusion injury by inhibiting the
JAK/STAT signaling activation. J Transl Med. 11(141)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao X, Zhang E, Ren X, Bai X, Wang D, Bai
L, Luo D, Guo Z, Wang Q and Yang J: Edaravone alleviates cell
apoptosis and mitochondrial injury in ischemia-reperfusion-induced
kidney injury via the JAK/STAT pathway. Biol Res.
53(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu MJ, Feng D, Wang H, Guan Y, Yan X and
Gao B: IL-22 ameliorates renal ischemia-reperfusion injury by
targeting proximal tubule epithelium. J Am Soc Nephrol. 25:967–977.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dube S, Matam T, Yen J, Mang HE, Dagher
PC, Hato T and Sutton TA: Endothelial STAT3 modulates protective
mechanisms in a mouse ischemia-reperfusion model of acute kidney
injury. J Immunol Res. 2017(4609502)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pace J, Paladugu P, Das B, He JC and
Mallipattu SK: Targeting STAT3 signaling in kidney disease. Am J
Physiol Renal Physiol. 316:F1151–F1161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park JY, Yoo KD, Bae E, Kim KH, Lee JW,
Shin SJ, Lee JS, Kim YS and Yang SH: Blockade of STAT3 signaling
alleviates the progression of acute kidney injury to chronic kidney
disease through antiapoptosis. Am J Physiol Renal Physiol.
322:F553–F572. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kersten S, Desvergne B and Wahli W: Roles
of PPARs in health and disease. Nature. 405:421–424.
2000.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Park EJ, Park SY, Joe EH and Jou I:
15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory
signaling through induction of suppressor of cytokine signaling 1
(SOCS1) and SOCS3 in glia. J Biol Chem. 278:14747–14752.
2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kapadia R, Yi JH and Vemuganti R:
Mechanisms of anti-inflammatory and neuroprotective actions of
PPAR-gamma agonists. Front Biosci. 13:1813–1826. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Wang D, Shi L, Xin W, Xu J, Xu J, Li Q, Xu
Z, Wang J, Wang G, Yao W, et al: Activation of PPARγ inhibits
pro-inflammatory cytokines production by upregulation of miR-124 in
vitro and in vivo. Biochem Biophys Res Commun. 486:726–731.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF
and Liu X: MicroRNA-124 mediates the cholinergic anti-inflammatory
action through inhibiting the production of pro-inflammatory
cytokines. Cell Res. 23:1270–1283. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals, Institute for Laboratory
Animal Research, Division on Earth and Life Studies, National
Research Council. Guide for the care and use of laboratory animals.
8th edition. National Academies Press, 2010.
|
|
34
|
Hu H, Zou C, Xi X, Shi Z, Wang G and Huang
X: Protective effects of pioglitazone on renal ischemia-reperfusion
injury in mice. J Surg Res. 178:460–465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zou C, Hu H, Xi X, Shi Z, Wang G and Huang
X: Pioglitazone protects against renal ischemia-reperfusion injury
by enhancing antioxidant capacity. J Surg Res. 184:1092–1095.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
El-Nabarawy NA, Gouda AS, Khattab MA and
Rashed LA: Effects of nitrite graded doses on hepatotoxicity and
nephrotoxicity, histopathological alterations, and activation of
apoptosis in adult rats. Environ Sci Pollut Res Int.
27:14019–14032. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lucena-Aguilar G, Sánchez-López AM,
Barberán-Aceituno C, Carrillo-Ávila JA, López-Guerrero JA and
Aguilar-Quesada R: DNA source selection for downstream applications
based on DNA quality indicators analysis. Biopreserv Biobank.
14:264–270. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zuk A and Bonventre JV: Recent advances in
acute kidney injury and its consequences and impact on chronic
kidney disease. Curr Opin Nephrol Hypertens. 28:397–405.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ding C, Zheng J, Wang B, Li Y, Xiang H,
Dou M, Qiao Y, Tian P, Ding X and Xue W: Exosomal MicroRNA-374b-5p
from tubular epithelial cells promoted M1 macrophages activation
and worsened renal ischemia/reperfusion injury. Front Cell Dev
Biol. 8(587693)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu BC, Tang TT, Lv LL and Lan HY: Renal
tubule injury: A driving force toward chronic kidney disease.
Kidney Int. 93:568–579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen W, Xi X, Zhang S, Zou C, Kuang R, Ye
Z, Huang Y and Hu H: Pioglitazone protects against renal
ischemia-reperfusion injury via the AMP-activated protein
kinase-regulated autophagy pathway. Front Pharmacol.
9(851)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zou G, Zhou Z, Xi X, Huang R and Hu H:
Pioglitazone ameliorates renal ischemia-reperfusion injury via
inhibition of NF-κB activation and inflammation in rats. Front
Physiol. 12(707344)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li Q, Tian Z, Wang M, Kou J, Wang C, Rong
X, Li J, Xie X and Pang X: Luteoloside attenuates neuroinflammation
in focal cerebral ischemia in rats via regulation of the
PPARγ/Nrf2/NF-κB signaling pathway. Int Immunopharmacol.
66:309–316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ding Y, Kang J, Liu S, Xu Y and Shao B:
The protective effects of peroxisome proliferator-activated
receptor gamma in cerebral ischemia-reperfusion injury. Front
Neurol. 11(588516)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hiben MG, de Haan L, Spenkelink B,
Wesseling S, Vervoort J and Rietjens IMCM: Induction of peroxisome
proliferator activated receptor γ (PPARγ) mediated gene expression
and inhibition of induced nitric oxide production by Maerua
subcordata (Gilg) DeWolf. BMC Complement Med Ther.
20(80)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hong W, Hu S, Zou J, Xiao J, Zhang X, Fu
C, Feng X and Ye Z: Peroxisome proliferator-activated receptor γ
prevents the production of NOD-like receptor family, pyrin domain
containing 3 inflammasome and interleukin 1β in HK-2 renal tubular
epithelial cells stimulated by monosodium urate crystals. Mol Med
Rep. 12:6221–6226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ramirez-Moral I, Ferreira BL, de Vos AF
and van der Poll T: Post-treatment with the PPAR-γ agonist
pioglitazone inhibits inflammation and bacterial growth during
Klebsiella pneumonia. Respir Res. 22(230)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Crosby MB, Svenson J, Gilkeson GS and
Nowling TK: A novel PPAR response element in the murine iNOS
promoter. Mol Immunol. 42:1303–1310. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yin F, Zheng PQ, Zhao LQ, Wang YZ, Miao
NJ, Zhou ZL, Cheng Q, Chen PP, Xie HY, Li JY, et al: Caspase-11
promotes NLRP3 inflammasome activation via the cleavage of
pannexin1 in acute kidney disease. Acta Pharmacol Sin. 43:86–95.
2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang Y, Yu B, Wang L, Yang M, Xia Z, Wei
W, Zhang F and Yuan X: Pioglitazone ameliorates glomerular NLRP3
inflammasome activation in apolipoprotein E knockout mice with
diabetes mellitus. PLoS One. 12(e0181248)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang X, Li R, Wang X, Fu Q and Ma S:
Umbelliferone ameliorates cerebral ischemia-reperfusion injury via
upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3
inflammasome. Neurosci Lett. 600:182–187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Meng QQ, Feng ZC, Zhang XL, Hu LQ, Wang M,
Zhang HF and Li SM: PPAR-γ activation exerts an anti-inflammatory
effect by suppressing the NLRP3 inflammasome in spinal cord-derived
neurons. Mediators Inflamm. 2019(6386729)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bartesaghi S and Radi R: Fundamentals on
the biochemistry of peroxynitrite and protein tyrosine nitration.
Redox Biol. 14:618–625. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang M, Deng J, Lai H, Lai Y, Meng G, Wang
Z, Zhou Z, Chen H, Yu Z, Li S and Jiang H: Vagus nerve stimulation
ameliorates renal ischemia-reperfusion injury through inhibiting
NF-κB activation and iNOS protein expression. Oxid Med Cell Longev.
2020(7106525)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Korkmaz A and Kolankaya D: Inhibiting
inducible nitric oxide synthase with rutin reduces renal
ischemia/reperfusion injury. Can J Surg. 56:6–14. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Aydogdu N, Erbas H, Atmaca G, Erten O and
Kaymak K: Melatonin reduces nitric oxide via increasing arginase in
rhabdomyolysis-induced acute renal failure in rats. Ren Fail.
28:435–440. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Erbas H, Aydogdu N and Kaymak K: Effects
of N-acetylcysteine on arginase, ornithine and nitric oxide in
renal ischemia-reperfusion injury. Pharmacol Res. 50:523–527.
2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Waeckerle-Men Y, Starke A, Wahl PR and
Wüthrich RP: Limited costimulatory molecule expression on renal
tubular epithelial cells impairs T cell activation. Kidney Blood
Press Res. 30:421–429. 2007.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hagerty DT, Evavold BD and Allen PM:
Regulation of the costimulator B7, not class II major
histocompatibility complex, restricts the ability of murine kidney
tubule cells to stimulate CD4+ T cells. J Clin Invest.
93:1208–1215. 1994.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Breda PC, Wiech T, Meyer-Schwesinger C,
Grahammer F, Huber T, Panzer U, Tiegs G and Neumann K: Renal
proximal tubular epithelial cells exert immunomodulatory function
by driving inflammatory CD4+ T cell responses. Am J
Physiol Renal Physiol. 317:F77–F89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Niemann-Masanek U, Mueller A, Yard BA,
Waldherr R and van der Woude FJ: B7-1 (CD80) and B7-2 (CD 86)
expression in human tubular epithelial cells in vivo and in vitro.
Nephron. 92:542–556. 2002.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Xiao YT, Wang J, Lu W, Cao Y and Cai W:
Downregulated expression of microRNA-124 in pediatric intestinal
failure patients modulates macrophages activation by inhibiting
STAT3 and AChE. Cell Death Dis. 7(e2521)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Koukos G, Polytarchou C, Kaplan JL,
Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M,
Pothoulakis C, Winter HS and Iliopoulos D: MicroRNA-124 regulates
STAT3 expression and is down-regulated in colon tissues of
pediatric patients with ulcerative colitis. Gastroenterology.
145:842–852.e2. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wang S, Wu G, Han Y, Song P, Chen J, Wu Y,
Yang J and Liang P: miR-124 regulates STAT3-mediated cell
proliferation, migration and apoptosis in bladder cancer. Oncol
Lett. 16:5875–5881. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Lin S, Liu Q, Wen J, Bai K, Guo Y and Wang
J: Mir-124 attenuates STAT3-mediated TH17 differentiation in
colitis-driven colon cancer. Front Oncol. 10(570128)2020.PubMed/NCBI View Article : Google Scholar
|