Introduction
Chronic prostatitis/chronic pelvic pain syndrome
(CP/CPPS) is a clinical syndrome characterized by pain in the
perineum, pelvis, suprapubic area, or external genitalia, and
variable degrees of voiding and ejaculatory disturbance, without
evidence of a bacterial infection. Symptoms are usually prolonged
and the treatment results are unsatisfactory. CP/CPPS is classified
as type 3 prostatitis following the National Institutes of Health
(NIH) classification of prostatitis (1). There are several theories which are
regarded as possible causes of CP/CPPS, such as pelvic floor
disfunction, stress, hormone levels and nerve disfunctions;
however, none of these theories have yet been proven (2,3).
CP/CPPS remains one of the most challenging pathological condition
for urologists. The diagnosis is one of exclusion and, based on
significant subjective criteria, the prediction of progression is
not possible, prognosis is unpredictable, and treatment is very
challenging and the optimal management of category III prostatitis
is not known. The impact on the quality of life of patients is
thereby high (4). The current
management of CPPS is based on several pharmacological and
non-pharmacological approaches (5-7).
Among all non-drug-based therapies, physical therapies [such as
extracorporeal shock wave therapy (ESWT) and intrarectal digital
massage of the pelvic floor)] (8-10),
psychological therapies (11) and
acupuncture (12) have exhibited
notable results in terms of clinical efficacy and improving the
quality of life of patients. Moreover, several other approaches
have been used and evaluated for the management of patients with
CP/CPPS, such as thermobalancing, transurethral needle ablation,
transcutaneous electrical nerve stimulation or sono-electromagnetic
therapy, and patients treated using these approaches have exhibited
a significant clinical improvement (5-7).
On the other hand, phytotherapy has been used over the past years
with satisfactory results as regards the relief of symptoms and
quality of life (7,13,14).
Several studies have been published on the roles of quercetin, bee
pollen, pumpkin seed oil, eviprostat or terpene mixture, with
promising results (7,13,14).
In particular, Cai et al (7)
reported that flower pollen extract was able to relieve symptoms in
patients affected by CP/CPPS through the reduction of interleukin
(IL)-8 levels. Previous studies have demonstrated the use of escin
and bromelin in the management of CP/CPPS due to its
anti-inflammatory properties (15,16).
The aim of the present study was to investigate the therapeutic
effects of ESWT in combination with bromelain and escin in patients
affected by CP/CPPS. The present study focused on changes in
urinary symptoms, pain and quality of life of patients with CP/CPPS
who had not undergone any other related treatments.
Patients and methods
Study design
A prospective, randomized study was conducted from
February, 2019 to November, 2020 on 100 male patients affected by
CPPS, attending the ‘Mater Domini’ Hospital. The study was approved
by the local ethics committee (Ethical Committee of Calabria
Region, Central Section). The enrolled subjects were randomly
assigned to receive either low-intensity ESWT (Li-ESWT) or Li-ESWT
plus bromelain and escin. Urinary symptoms, pain and satisfaction
were assessed at baseline evaluation.
Questionnaires
Urinary symptoms were evaluated using the
international prostatic symptoms score (IPSS) (17), pain with the visual analog scale
(VAS) score ranging from 0-10(18).
The NIH-Chronic Prostatitis Symptom Index (NIH-CPSI) with three
domains was used to assess the urinary symptoms, pain and the
quality of life of patients (19).
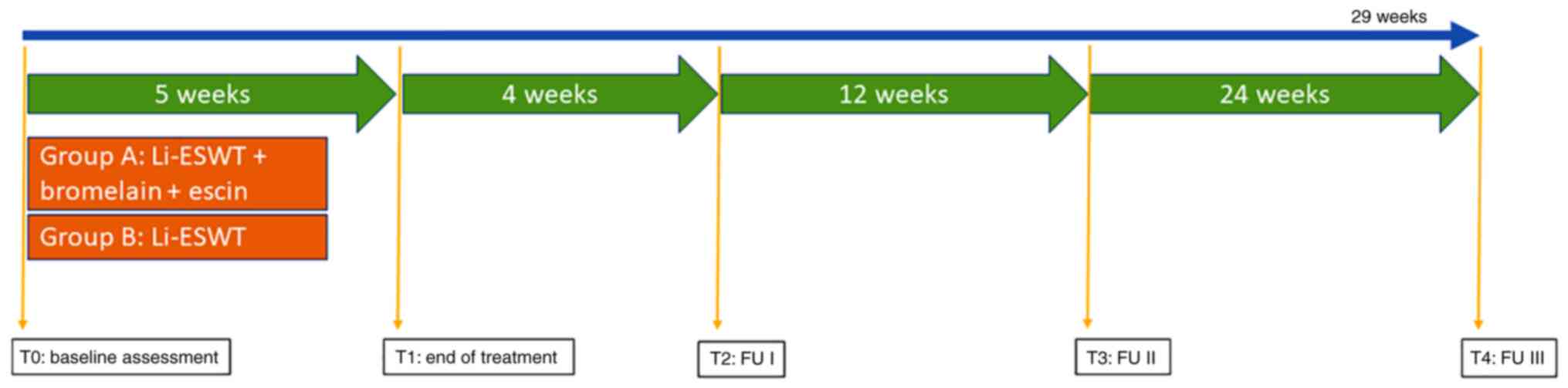
Study schedule
A total of 95 patients with a clinical diagnosis of
CP/CPSS were enrolled in the study. The patients were randomly
allocated to either the ESWT plus bromelain and escin group (group
A; n=48) or the ESWT only group (group B; n=47). Participants in
group A received identical Li-ESWT therapy plus bromelain (a dose
of 160 mg/day) and escin (a dose of 500 mg/day) for 5 weeks.
Treatments were performed without anesthesia. Treatment
complications were recorded. Follow-up evaluations were performed
at 4 and 12 weeks after the final intervention session (Fig. 1). The Storz Duolith Li-ESWT system
(Storz Medical AG) was used for the treatment sessions, which were
performed once weekly for 5 consecutive weeks in both groups by the
same operator. A total of 3,000 impulses were applied at each
Li-ESWT session with an energy flux density of 0.25
mJ/mm2 and an emission frequency of 4 Hz on the perineum
area. Urinary symptoms, pain and quality of life were evaluated
using the IPSS, VAS and NIH-CPSI. Each outcome was reassessed by
the same operator, as reported in previous studies by the authors
(20,21).
Inclusion and exclusion criteria
The inclusion criteria were the presence of pelvic
pain symptoms for at least 3 months over the past 6 months prior to
study entry in accordance with the European Association of Urology
(EAU) guidelines: A score in the pain domain of the NIH-CPSI of
>4; and a microbiologically negative result in the Meares-Stamey
four-glass test (19,22,23).
Patients with the following characteristics were excluded: Subjects
<18 and >50 years of age; patients affected by major
concomitant diseases with known anatomical abnormalities of the
urinary tract or with evidence of other urological diseases;
patients with residual urine volume >50 ml resulting from
bladder outlet obstruction; subjects with a reported allergy to
pollen extract; patients who had recently (<4 weeks) undergone
oral or parenteral treatment, or who were currently using
prophylactic antibiotic drugs; all patients positive to tests for
Chlamydia trachomatis, Ureaplasma urealyticum,
Neisseria gonorrhoeae, herpes viruses (HSV 1/2) and human
papillomavirus (HPV) (22).
Ethical considerations
The present study was approved by the University of
Catanzaro Institutional Review Board (no. 48 of 22th February
2019). The study was conducted in line with Good Clinical Practice
guidelines, in compliance with the ethical principles published in
the latest version of the Declaration of Helsinki (24). Written informed consents were
obtained from all patients prior to treatment.
Statistical analysis
The homogeneity of the groups at baseline was
assessed using Mann-Whitney U test for continuous variables.
Multiple comparisons between the two groups at the baseline and
each follow-up evaluation time point was performed using the
Kruskal-Wallis test. Post hoc analysis was performed using the
Dunn's multiple comparison test. General characteristics of the
study participants were expressed with descriptive statistics
(means, standard deviations or ranges). The calculation of the
sample size needed for enrollment was based on the expected
questionnaire results (improvement of quality of life) in line with
published results from other studies (14,20,21).
The required sample size was calculated under the following
conditions: Difference between the groups, 35% of patients who
reach a reduction in 15% of the NIH-CPSI total score; α error
level, 0.05 two-sided; statistical power, 80%; and anticipated
effect size, Cohen's d=0.5. The calculations yielded at least 44
individuals per group. A value of P<0.05 was considered to
indicate a statistically significant difference. All reported
P-values were two-sided. Statistical analyses were performed using
SPSS software, version 11.0 (SPSS, Inc.) for Apple-Macintosh.
Results
At the end of the follow-up period, 95 patients were
available for follow-up examinations and analyzed: 48 patients in
group A (Li-ESWT in association with bromelain and escin) and 47 in
group B (Li-ESWT only). Differences in pre-treatment
characteristics between the Li-ESWT and control groups were not
statistically significant. No major complications were observed in
patients receiving both treatments, and all patients tolerated the
treatments well. None of the patients required the administration
of analgesics during treatment. The patient clinical
characteristics at baseline are presented in Table I.
 | Table IBaseline assessment. |
Table I
Baseline assessment.
| Parameter | Group A (n=48 | Group B (n=47) | P-value |
|---|
| Age (years) | 32(5) | 31(6) | 0.89 |
| IPSS | 15(4) | 15(4) | 0.99 |
| VAS | 5(2) | 6(3) | 0.78 |
| NIH-CP/CPSI | | | |
|
Pain
domain | 13(2) | 13(2) | 0.99 |
|
Urinary
symptoms | 6(2) | 6(2) | 0.99 |
|
Quality of
life | 9(3) | 10(4) | 0.84 |
Follow-up assessment at 4 weeks
At 4 weeks follow-up, out of the patients assigned
to the group treated with Li-ESWT alone, 3 (6%) patients reported
pain disappearance, 25 (53%) reported pain reduction, 17 (36%)
reported pain stability and 5 (10%) reported pain worsening. In the
Li-ESWT plus bromelain and escin group, 4 (8%) reported pain
disappearance and 29 (60%) reported pain reduction; pain remained
stable in 14 patients (29%) and worsened in 3 patients (6%). The
median IPSS scores were significantly lower in both groups when
compared with the baseline values [15; (IQR 4) and 15 (IQR 4) vs.
10 (IQR 5) and 10 (IQR 6) for group A and B, respectively;
P<0.001; P<0.001] (Table
II). No significant differences emerged in the scores between
the two different groups. The same trend was observed for the
median VAS score (5; IQR 2 and 6; IQR 3 vs. 4; IQR 2 and 4; IQR 2
for groups A and B, respectively; P<0.001; P<0.001). The
median scores of the three domains of NIH-CPSI exhibited
significant differences compared to baseline values [13 (IQR 2) and
13 (IQR 2) vs. 8 (IQR 4) and 7 (IQR 4) for pain domain (P<0.001;
P<0.001); 6 (IQR 2) and 6 (IQR 2) vs. 4 (IQR 4) and 4 (IQR 3)
for urinary symptoms domain (P<0.001; P<0.001); 9 (IQR 3) and
10 (IQR 4) vs. 7 (IQR 4) and 7 (IQR 5) for quality-of-life domain
(P<0.001; P<0.001) in group A and B, respectively], although
no differences were found between the two groups at 4 weeks of
follow-up (Table II. All follow-up
findings at 4 weeks are presented in Table III.
 | Table IIComparison between baseline and
follow-up data. |
Table II
Comparison between baseline and
follow-up data.
| | Group A (n=48) | |
|---|
| Parameter | Baseline | 4 weeks | 12 weeks | 24 weeks | P-value (vs.
baseline) |
|---|
| IPSS | 15(4) | 10(5) | 10(5) | 11(5) | <0.001
(all) |
|
P-value | | <0.001 | 0.99 | 0.33 | |
| VAS | 5(2) | 4(2) | 1(2) | 1(1) | <0.001
(all) |
|
P-value | | <0.001 | <0.001 | 0.99 | |
| NIH-CP/CPSI | | | | | |
|
Pain
domain | 13(2) | 8(4) | 8(4) | 8(2) | <0.001
(all) |
|
P-value | | <0.001 | 0.99 | 0.87 | |
|
Urinary
symptoms | 6(2) | 4(4) | 4(3) | 4(2) | <0.001
(all) |
|
P-value | | <0.001 | 0.88 | 0.76 | |
|
Quality of
life | 9(3) | 7(4) | 4(2) | 5(2) | <0.001
(all) |
|
P-value | | <0.001 | <0.001 | 0.67 | |
| | Group B (n=47) | |
| Parameter | Baseline | 4 weeks | 12 weeks | 24 weeks | P-value (vs.
baseline) |
| IPSS | 15(4) | 10(6) | 9(5) | 11(4) | <0.001
(all) |
|
P-value | | <0.001 | 0.08 | 0.33 | |
| VAS | 6(3) | 4(2) | 4(3) | 4(2) | <0.001
(all) |
|
P-value | | <0.001 | 0.98 | 0.98 | |
| NIH-CP/CPSI | | | | | |
|
Pain
domain | 13(2) | 7(4) | 4(2) | 5(2) | <0.001
(all) |
|
P-value | | <0.001 | 0.98 | 0.65 | |
|
Urinary
symptoms | 6(2) | 4(3) | 4(2) | 4(2) | <0.001
(all) |
|
P-value | | <0.001 | 0.98 | 0.99 | |
|
Quality of
life | 10(4) | 7(5) | 7(3) | 7(2) | <0.001
(all) |
|
P-value | | <0.001 | 0.98 | 0.98 | |
 | Table IIIAssessment at 4 weeks of
follow-up. |
Table III
Assessment at 4 weeks of
follow-up.
| Parameter | Group A (n=48) | Group B (n=47) | P-value |
|---|
| IPSS | 10(5) | 10(6) | 0.99 |
| VAS | 4(2) | 4(2) | 0.99 |
| NIH-CP/CPSI | | | |
|
Pain
domain | 8(4) | 7(4) | 0.86 |
|
Urinary
symptoms | 4(4) | 4(3) | 0.99 |
|
Quality of
life | 7(4) | 7(5) | 0.99 |
Follow-up assessment at 12 weeks
At 12 weeks of follow-up in both groups, the median
IPSS scores were lower, although not significantly, when compared
with the follow-up values at 4 weeks [10 (IQR 5) and 10 (IQR 6) vs.
10 (IQR 5) and 9 (IQR 5) for groups A and B, respectively; P=0.99;
P=0.08]. No significant differences emerged in the scores between
the two different groups. The median VAS score was not
significantly lower compared to the 4 weeks of follow-up for group
B [4 (IQR 2) vs. 4 (IQR 3); P=0.98]. Conversely the median score
was significantly lower in group A when compared to the first
follow-up [4 (IQR 2) vs. 1 (IQR 2) P<0.001] and to group B [4
(IQR 3) vs. 1 (IQR 2); P<0.001] (Table II). As regards the median scores of
the three domains of NIH-CPSI, the pain and quality of life domains
exhibited significant differences between groups A and B [8 (IQR 4)
vs. 4 (IQR 2) 7 (IQR 3) vs. 4 (IQR 2) P<0.001; P<0.001]. At
12 weeks follow-up, out of the patients assigned to the ESWT plus
bromelain and escin group, 2 (4.16%) reported pain disappearance
and 25 (52.0%) reported pain reduction; pain remained stable in 14
patients (29.7%) and worsened in 6 patients (12.5%). In the
ESWT-alone group, 2 (4.25%) patients reported pain disappearance,
22 (46.8%) reported pain reduction, 20 (42.5%) reported pain
stability, and 4 (8.5%) reported pain worsening. All follow-up
findings at 12 weeks are displayed in Table IV.
 | Table IVAssessment at 12 weeks of
follow-up. |
Table IV
Assessment at 12 weeks of
follow-up.
| Parameter | Group A (n=48) | Group B (n=47) | P-value |
|---|
| IPSS | 10(5) | 9(5) | 0.78 |
| VAS | 4(3) | 1(2) | 0.001 |
| NIH-CP/CPSI | | | |
|
Pain
domain | 8(4) | 4(2) | 0.001 |
|
Urinary
symptoms | 4(3) | 4(2) | 0.99 |
|
Quality of
life | 7(3) | 4(2) | 0.001 |
Follow-up assessment at 24 weeks
At 24 weeks follow-up in both groups, the median
IPSS scores were similar compared with the 4- and 12-week follow-up
values, without any significant difference between the two groups
[11 (IQR 5) vs. 11 (IQR 5) for A and B group respectively; P=0.99].
The median VAS score was significantly lower for group A than group
B [1 (IQR 2) vs. 4 (IQR 2) P<0.001]. As regards the median
scores of the three domains of NIH-CPSI, the pain and quality of
life domains exhibited significant differences between groups A and
B [8 (IQR 2) vs. 5 (IQR 2); P<0.001; 7 (IQR 2) vs. 4 (IQR 2)
P<0.001]. At 24 weeks of follow-up, out of the patients assigned
to the Li-ESWT plus bromelain and escin group, 2 (4.16%) reported
pain disappearance and 19 (39.58%) reported stable pain reduction;
pain remained stable in 18 patients (37.5%) and worsened in 8
patients (16.6%). In the Li-ESWT -alone group, 2 (4.25%) patients
reported pain disappearance, 15 (31.91%) reported stable pain
reduction, 23 (48.93%) reported pain stability, and 8 (17.02%)
reported pain worsening. The mean IPSS score was lower when
compared with baseline values in the Li-ESWT plus bromelain and
escin group, but not significantly, while no statistically
significant differences were found in the Li-ESWT group (Table II). All follow-up findings at 24
weeks are presented in Table V.
 | Table VAssessment at 24 weeks of
follow-up. |
Table V
Assessment at 24 weeks of
follow-up.
| Parameter | Group A (n=48) | Group B (n=47) | P-value |
|---|
| IPSS | 11(5) | 11(4) | 0.99 |
| VAS | 4(2) | 1(1) | 0.001 |
| NIH-CP/CPSI | | | |
|
Pain
domain | 8(2) | 5(2) | 0.001 |
|
Urinary
symptoms | 4(2) | 4(2) | 0.99 |
|
Quality of
life | 7(2) | 4(2) | 0.001 |
Adverse effects
No clinically significant adverse effects were
reported. Two patients reported mild pain (VAS 1) during the
procedure in the ESWT application area. All results of safety
profile are presented in Table
VI.
 | Table VISafety profile. |
Table VI
Safety profile.
| Parameter | Group A (n=48) | Group B (n=47) | P-value |
|---|
| Discomfort | | | |
|
VAS
(0-2) | 1(2) | 1(2) | 0.99 |
| Gastrointestinal
symptoms | | | |
|
Mild | 1 | 0 | 0.87 |
Discussion
The present study demonstrated that the use of
bromelain plus escin improved the clinical efficacy of Li-ESWT in
patients affected by CP/CPPS, by ameliorating urinary symptoms,
pain and the quality of life.
At the present time, to the best of our knowledge,
there are no studies available that used both Li-ESWT and
phytotherapy and that have compared the two treatments. It was
hypothesized that the higher clinically significant improvement
observed in the quality of life of patients treated with Li-ESWT
and phytotherapy was due to the anti-inflammatory effects of
bromelain and escin which were enhanced by Li-ESWT. Several
researchers, in this sense, have reported a significant
anti-inflammatory effects of bromelain and escin in several aspects
of clinical practice (15,16,25).
The efficacy of Li-ESWT treatment in patients affected by CP/CPPS
has been demonstrated in several clinical studies, reporting a
significant relief in pelvic pain and voiding symptoms in patients
with CPPS (26-28).
Recently, Kim et al (29) in
a randomized control trial, demonstrated that Li-ESWT improved the
NIH-CPSI score, pain and the quality of life of patients with CPPS
IIIb. Moreover, they concluded that Li-ESWT could be an effective
alternative treatment modality for CPPS IIIb (29). The clinical efficacy of Li-ESWT in
patients with CP/CPPS is probably due some hypothesized mechanism,
such as nociceptor hyperstimulation, nitric oxide synthesis
induction, passive muscle tone decreasing, the interruption of
nerve impulses and an increase in local microvascularization.
Moreover, Jeon et al (30)
highlighted, by using an animal model, that Li-ESWT reduced COX-2
levels by inhibiting the TLR3-NF-κB pathway. Furthermore, in their
study, the TRAF2 regulator in ERK1/2 inhibition significantly
decreased inflammation (30). The
authors also demonstrated that these signaling pathways facilitated
inflammation with different levels of the expression of IL-1β, IL-6
and other inflammatory molecular markers via different stimulation
models (30). From a clinical
perspective, recently, Li and Man (31) reported the results of a systematic
review and meta-analysis, including six studies involving 317 male
patients exhibiting significant clinical improvements in terms of
total NIH-CPSI scores, quality of life, pain scores and urinary
symptom scores in the Li-ESWT group compared to the control group
at 12 weeks following treatment. The present study considered the
efficacy of the no-drug approach with a phytotherapy compound. The
hypothesis to combine two approaches, a no-drug and a drug approach
was based on the necessity to act in several pathophysiological
pathways in patients with CP/CPPS, as suggested by Magistro et
al (32). The association
between Li-ESWT and bromelain and escin was able to improve the
clinical efficacy due to the anti-inflammatory effects of bromelain
and escin. It has been demonstrated that bromelain is able to
reduce the levels of certain inflammatory mediators, such as NF-кB,
IL-1β, IL-6, TNF-α, PGE2 and nitrate concentrations (33). In the same manner, escin, a natural
mixture of triterpenoid saponins has been demonstrated to exert
anti-edematous and anti-inflammatory effects (16,34).
In this sense, the efficacy of the association between Li-ESWT and
phytotherapy is increased by the effects of the association to
inhibit several inflammatory pathways involved in the complex
pathogenesis of the CP/CPPS. Due to these notable results in males,
this approach may be also evaluated for managing pelvic pain
syndrome in female patients in the future.
The present study has certain limitations. Firstly,
the lack of a placebo should be considered. This aspect was
considered in the analysis and interpretation of the results. The
placebo effect in phytotherapy research ranges from 20 to 30%, as
highlighted by Capasso et al (35). On the basis of this consideration, a
clinically significant difference between the two groups was
considered when >30%. Finally, the short follow-up period should
be considered among the study limitations.
In conclusion, the present study demonstrates that
in patients with CP/CPPS, Li-ESWT plus bromelain and escin leads to
pain resolution and both treatments ameliorate IPSS, VAS and
NIH-CP/CPSI. However, further studies are warranted to confirm
these results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to Italian law on
privacy but are available from the corresponding author on
reasonable request.
Authors' contributions
LDL, ADG, LC and GLC collected and analyzed the
data. AP, CDA, TC, LG and MC were involved in the study conception,
design, analysis of data and in the writing of the manuscript. LDL
and AP confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the University of
Catanzaro Institutional Review Board (no. 48 of 22th February
2019). The study was conducted in line with Good Clinical Practice
guidelines, in compliance with the ethical principles published in
the latest version of the Declaration of Helsinki. Written informed
consents were obtained from all patients prior to treatment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clemens JQ, Meenan RT, O'Keeffe Rosetti
MC, Gao SY and Calhoun EA: A. Incidence and clinical
characteristics of national institutes of health type III
prostatitis in the community. J Urol. 174:2319–2322.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shoskes DA, Berger R, Elmi A, Landis JR,
Propert KJ and Zeitlin S: Chronic Prostatitis Collaborative
Research Network Study Group. Muscle tenderness in men with chronic
prostatitis/chronic pelvic pain syndrome: The chronic prostatitis
cohort study. J Urol. 179:556–560. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pontari MA and Ruggieri MR: Mechanisms in
prostatitis/chronic pelvic pain syndrome. J Urol. 179 (Suppl
5):S61–S67. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai T, Verze P and Bjerklund Johansen TE:
The quality of life definition: Where are we going? Uro. 1:14–22.
2021.
|
|
5
|
Franco JV, Turk T, Jung JH, Xiao YT,
Iakhno S, Tirapegui FI, Garrote V and Vietto V: Pharmacological
interventions for treating chronic prostatitis/chronic pelvic pain
syndrome. Cochrane Database Syst Rev. 10(CD012552)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Franco JV, Turk T, Jung JH, Xiao YT,
Iakhno S, Garrote V and Vietto V: Non-pharmacological interventions
for treating chronic prostatitis/chronic pelvic pain syndrome.
Cochrane Database Syst Rev. 1(CD012551)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cai T, Verze P, La Rocca R, Anceschi U, De
Nunzio C and Mirone V: . The role of flower pollen extract in
managing patients affected by chronic prostatitis/chronic pelvic
pain syndrome: A comprehensive analysis of all published clinical
trials. BMC Urol. 17(32)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salama AB and Abouelnaga WA: Effect of
radial shock wave on chronic pelvic pain syndrome/chronic
prostatitis. J Phys Ther Sci. 30:1145–1149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li G and Man L: Low-intensity
extracorporeal shock wave therapy for III B chronic pelvic pain
syndrome. Transl Androl Urol. 9:1323–1328. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Strauss AC and Dimitrakov JD: New
treatments for chronic prostatitis/chronic pelvic pain syndrome.
Nat Rev Urol. 7:127–135. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang J, Liang CZ, Shang X and Li H:
Chronic prostatitis/chronic pelvic pain syndrome: A disease or
symptom? current perspectives on diagnosis, treatment, and
prognosis. Am J Mens Health. 14(1557988320903200)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu X, Cheng K, Xu C, Liu S, Sun Q, Yang Z,
Dai X and Li N: Mechanism of acupuncture and moxibustion on chronic
prostatitis/chronic pelvic pain syndrome: A narrative review of
animal studies. Pain Res Manag. 2021(2678242)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu M, Wazir J, Ullah R, Wang W, Cui X,
Tang M and Zhou X: Phytotherapy and physical therapy in the
management of chronic prostatitis-chronic pelvic pain syndrome. Int
Urol Nephrol. 51:1081–1088. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cai T, Anceschi U, Tamanini I, Verze P and
Palmieri A: Soybean extracts (glycine max) with curcuma, boswellia,
pinus and urtica are able to improve quality of life in patients
affected by CP/CPPS: Is the pro-inflammatory cytokine IL-8 level
decreasing the physiopathological link? Uro. 2:40–48. 2022.
|
|
15
|
Karlsen M, Hovden AO, Vogelsang P, Tysnes
BB and Appel S: Bromelain treatment leads to maturation of
monocyte-derived dendritic cells but cannot replace PGE2 in a
cocktail of IL-1β, IL-6, TNF-α and PGE2. Scand. J Immunol.
74:135–143. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gallelli L: Escin: A review of its
anti-edematous, antiinflammatory, and venotonic properties. Drug
Des Devel Ther. 13:3425–3437. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Badía X, García-Losa M and Dal-Ré R:
Ten-language translation and harmonization of the international
prostate symptom score: Developing a methodology for multinational
clinical trials. Eur Urol. 31:129–140. 1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Couper M, Tourangeau R, Conrad F and
Singer E: Evaluating the effectiveness of visual analog scales: A
web experiment. Soc Sci Comput Rev. 24:227–245. 2006.
|
|
19
|
Giubilei G, Mondaini N, Crisci A, Raugei
A, Lombardi G, Travaglini F, Del Popolo G and Bartoletti R: The
Italian version of the national institutes of health chronic
prostatitis symptom index. Eur Urol. 47:805–811. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cai T, Gallelli L, Cione E, Verze P,
Palmieri A, Mirone V, Bonkat G, Wagenlehner FM and Bjerklund
Johansen TE: The efficacy and tollerability of pollen extract in
combination with hyaluronic acid and vitamins in the management of
patients affected by chronic prostatitis/chronic pelvic pain
syndrome: A 26 weeks, randomized, controlled, single-blinded, phase
III study. Minerva Urol Nephrol: Mar 29, 2021 (Epub ahead of
print).
|
|
21
|
Cai T, Wagenlehner FM, Luciani LG,
Tiscione D, Malossini G, Verze P, Mirone V and Bartoletti R: Pollen
extract in association with vitamins provides early pain relief in
patients affected by chronic prostatitis/chronic pelvic pain
syndrome. Exp Ther Med. 8:1032–1038. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
European Association of Urology (EAU):
Guidelines on Chronic Pelvic Pain. EAU, Arnhem, 2020. https://uroweb.org/guideline/chronic-pelvic-pain/.
Accessed January 27, 2020.
|
|
23
|
Meares EM Jr and Stamey TA: The diagnosis
and management of bacterial prostatitis. Br J Urol. 44:175–179.
1972.PubMed/NCBI View Article : Google Scholar
|
|
24
|
World Medical Association (WMA): WMA
Declaration of Helsinki-Ethical Principles for Medical Research
Involving Human Subjects. WMA, Ferney-Voltaire, 2020. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
Accessed January 27, 2020.
|
|
25
|
Hale LP, Greer PK and Sempowski GD:
Bromelain treatment alters leukocyte expression of cell surface
molecules involved in cellular adhesion and activation. Clin
Immunol. 104:183–190. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zimmermann R, Cumpanas A, Miclea F and
Janetschek G: Extracorporeal shock wave therapy for the treatment
of chronic pelvic pain syndrome in males: A randomised,
double-blind, placebo-controlled study. Eur Urol. 56:418–424.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pajovic B, Radojevic N, Dimitrovski A and
Vukovic M: Comparison of the efficiency of combined extracorporeal
shockwave therapy and triple therapy versus triple therapy itself
in Category III B chronic pelvic pain syndrome (CPPS). Aging Male.
19:202–207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mykoniatis I, Kalyvianakis D, Zilotis F,
Kapoteli P, Fournaraki A, Poulios E and Hatzichristou D: Evaluation
of a low-intensity shockwave therapy for chronic prostatitis type
IIIb/chronic pelvic pain syndrome: A double-blind randomized
sham-controlled clinical trial. Prostate Cancer Prostatic Dis.
24:370–379. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim KS, Choi YS, Bae WJ, Cho HJ, Ha US,
Hong SH, Lee JY, Ahn ST, Moon DG and Kim SW: Efficacy of
low-intensity extracorporeal shock wave therapy for the treatment
of chronic pelvic pain syndrome IIIb: A prospective-randomized,
double-blind, placebo-controlled study. World J Mens Health.
40:473–480. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jeon SH, Zhu GQ, Kwon EB, Lee KW, Cho HJ,
Ha US, Hong SH, Lee JY, Bae WJ and Kim SW: Extracorporeal shock
wave therapy decreases COX-2 by inhibiting TLR4-NFκB pathway in a
prostatitis rat model. Prostate. 79:1498–1504. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li G and Man L: Low-intensity
extracorporeal shock wave therapy for male chronic pelvic pain
syndrome: A systematic review and meta-analysis. Transl Androl
Urol. 10:1202–1211. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Magistro G, Wagenlehner FM, Grabe M,
Weidner W, Stief CG and Nickel JC: Contemporary management of
chronic prostatitis/chronic pelvic pain syndrome. Eur Urol.
69:286–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bakare AO and Owoyele BV: Bromelain
reduced pro-inflammatory mediators as a common pathway that mediate
antinociceptive and anti-anxiety effects in sciatic nerve ligated
Wistar rats. Sci Rep. 11(289)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xin W, Zhang L, Sun F, Jiang N, Fan H,
Wang T, Li Z, He J and Fu F: Escin exerts synergistic
anti-inflammatory effects with low doses of glucocorticoids in vivo
and in vitro. Phytomedicine. 18:272–277. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Capasso F, Grandolini G and Izzo A:
Placebo effect. In: Phytotherapy. Springer, Milan, 2006.
|