Introduction
Meningitis is a life-threatening disease associated
with increased mortality rates amongst newborns, children,
adolescents, and adults. Furthermore, survivors may be at risk of
developing a permanent disability (1). Infectious and non-infectious processes
can cause meningitis, and among the infectious agents involved are
bacteria, viruses, and fungi (2).
These agents can cause inflammation of the membranes (meninges)
and/or cerebrospinal fluid (CSF) surrounding the brain and spinal
cord (3). Bacteria are an important
cause of meningitis and it is estimated that >1.2 million cases
of bacterial meningitis occur annually worldwide, with incidence
varying by region, country, age, and pathogen (4). A global and regional analysis of
meningitis from 1990-2016 showed that incident meningitis cases
increased from 2.5 million in 1990 to 2.82 million in 2016 and the
overall incidence rate in 2016 varied from 0.5 cases per 100,000
individuals in Australia to 4.2 cases per 100,000 individuals in
South Sudan (5). In Iraq, the
annual incidence of laboratory-confirmed bacterial meningitis was
1.47 cases per 100,000 individuals (6).
Several risk factors associated with meningitis have
been described including age, sex, otitis or sinusitis,
neurosurgery, diabetes, splenectomy, pneumonitis, endocarditis,
chronic hepatitis with cirrhosis, head trauma, and impaired
consciousness (7). In addition, it
has been indicated that inflammatory reactions in the CSF play an
essential role in the pathogenesis of brain injury associated with
various meningitis pathogens, and bacteria are among the most
important pathogenic components that have been shown to stimulate
the release of pro-inflammatory substances (8). Thus, the permeability of the
blood-brain barrier increases, and leukocytes are attracted to the
central nervous system (CNS), which is observed as pleocytosis in
the CSF. Therefore, the CSF profile of inflammatory mediators, such
as the cytokines tumor necrosis factor-α, interleukin (IL)-1β, and
IL-6, may predict the severity and consequences of meningitis
(9). The secretion of cytokines can
also be induced by components of the innate immune response, such
as antimicrobial peptides (AMPs), which have been shown to play
several potential roles in inflammatory responses, and their role
in the pathogenesis of meningitis has also been proposed (10,11).
AMPs are essential components of innate immunity and
play an important role in fending off invasive microbial pathogens.
Most AMPs can directly kill microbial pathogens, whilst others act
indirectly by modulating the immune defense mechanisms of the host
(12). Based on their structure,
AMPs are classified into four classes, which include linear
α-helical peptides, β-sheet peptides, or both, as well as a linear
extension structure (13). The most
common class is β-sheet peptides, and this class includes the
largest group of AMPs, defensins, which in humans consist of two
major types, α- and β-defensins. Both types play a significant role
in mediating antibacterial, antiviral, antifungal, immune, and
anti-inflammatory responses (14).
Six human β-defensins (HBD1, HBD2, HBD3, HBD4, HBD5,
and HBD6) have been described, although gene-based analysis
indicates an additional 28 HBDs (15). The first three HBDs are primarily
expressed by epithelial cells, but peripheral blood mononuclear
cells, macrophages, and plasmacytoid dendritic cells also express
them (16). HBD4 is expressed by
neutrophils, the thyroid glands, testes, gastric antrum, uterus,
lungs, and kidneys, whilst expression of HBD5 and HBD6 is
restricted to the epididymis (17).
The most widely studied HBDs are HBD1, HBD2, HBD3, and HBD4
(15,16). Recent evidence indicates that in
addition to being components of innate antimicrobial immunity, HBDs
also play a key role as pro-inflammatory mediators and
immunostimulators that increase the response to infection (18-20).
In meningitis, HBDs have not been well investigated, but their
roles in neuroimmune function and neurodegeneration have been
proposed (21). Additionally,
insights have been provided to describe their function as part of
the innate immune defense against pathogens in bacterial CNS
infections (22). Recently, the
expression of HBD2 was shown to be modulated by Neisseria
meningitides, a Gram-negative bacteria that causes meningitis
(11,23).
The present study analyzed the levels of HBD1, HBD2,
HBD3, and HBD4 in the CSF of suspected meningitis cases. These
cases were divided into two groups based on the PCR assessment of
bacterial CSF infection (PCR-positive and PCR-negative). Next,
PCR-negative CSF samples were classified as abnormal (ABN) or
normal (NOR) based on the leukocyte counts, and glucose and protein
concentrations.
Patients and methods
Suspected meningitis cases
A cross-sectional study was performed on 176 cases
of suspected meningitis (mean age, 26.5±20.8 years; minimum age,
<1 year; maximum age, 81 years; 78 males, 98 females) following
a diagnosis of hydrocephalus in infants and children and increased
intracranial pressure in adults. Cases were admitted to two major
neurological hospitals in Baghdad (Iraq), Neurosurgery Teaching
Hospital and Alwitri Neuroscience Teaching Hospital, during a
period of 11 months (January to November 2020). Patients with
hydrocephalus or intracranial pressure who agreed to participate
were included in the present study. Excluded patients were those
who did not provide written consent by themselves or through their
guardian. Patients were also excluded if they had neurosurgical
disease or traumatic lumbar puncture. From each participant, one
CSF sample was obtained by lumbar puncture and transferred to a
sterile tube. Information regarding age, sex, and antibiotic use
was recorded. The Institutional Ethics Committee of the College of
Science, University of Baghdad (Baghdad, Iraq) approved the study
(approval no. CSEC/1022/0136) and written informed consent was
obtained from patients or their legal guardian prior to sample
collection.
Leukocyte count
The direct microscopic method was used to count the
number of leukocytes in the CSF using a Neubauer chamber. Leukocyte
counts are expressed as cells/mm3. CSF samples were
classified as having either normal leukocyte counts (0-15
cells/mm3 for infants ≤28 days; 0-9 cells/mm3
for infants 29-60 days of age; and 0-5 cells/mm3 for
children and adults) or pleocytosis as previously described
(24).
Measurement of glucose and protein
concentrations
Quantitative determination of glucose and protein
concentrations in the CSF was performed using commercially
available kits (cat. nos. MDBSIS46-P and MDBSIS29-I, respectively;
Spinreact) according to the manufacturer's protocol. The normal
ranges for glucose and protein concentrations in the CSF are 40-70
and 15-45 mg/dl, respectively (25,26).
Immunoassay for HBDs
ELISA kits were used to measure the CSF levels of
HBD1, HBD2, and HBD3 (cat. nos. CSB-E14186h, CSB-E13201h, and
CSB-E14187h, respectively; Cusabio Technology LLC), and HBD4 (cat.
no. GWB-SKR005; GenWay Biotech) according to the manufacturer's
protocol.
PCR analysis of CSF
DNA was isolated from CSF samples using a HiPurA
Mycobacterium tuberculosis DNA purification kit (cat. no.
MB545-250PR; Himedia Laboratories) according to the manufacturer's
protocol. PCR analysis was performed following a previously
developed universal PCR protocol to amplify a 996-bp DNA fragment
of the eubacteria 16S rRNA gene in CSF samples (27). PCR amplification was performed using
two primers: U1, 5'-CCAGCAGCCGCGGTAATACG-3'; and U2,
5'-ATCGG[C/T]TACCTTGTTACGACTTC-3'. PCR products were separated by
1.5% agarose gel electrophoresis, and those that showed an
amplified band (PCR-positive) were subjected to Sanger sequencing
(performed by Macrogen, Inc.) using a Genetic Analyzer System
(model ABI-310; Macrogen, Inc.). Two broad categories of bacteria,
Gram-negative (G-ve) and Gram-positive (G+ve), were considered
based on DNA sequence alignment with NCBI sequence databases using
the BLAST function (https://blast.ncbi.nlm.nih.gov).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 8.0.0 (GraphPad Software, Inc.) and IBM SPSS
Statistics 25.0 (IBM Corp.). Categorical variables are displayed by
number and percentage, and significant differences were assessed
using a Pearson's χ2-test. Continuous variables were
subjected to two normality tests, Kolmogorov-Smirnov and
Shapiro-Wilk tests. Normally distributed (parametric) variables are
presented as the mean ± SD and significant differences and were
compared using a one-way ANOVA (with Fisher LSD test post hoc).
Variables that did not exhibit normal distribution (non-parametric)
are expressed as the median and interquartile range (IQR), and
significant differences were assessed using a Mann-Whitney U test
(to compare two groups) or a Kruskal-Wallis test (to compare more
than two groups). Receiver operating characteristic (ROC) curve
analysis (Wilson/Brown method) was applied to calculate the area
under the curve (AUC), the 95% confidence interval (CI), the
cut-off value, and the sensitivity and specificity. The cut-off
value was optimized using the Youden index (YI). Spearman's rank
correlation analysis was used to estimate the correlation
coefficient (rs) between variables. P<0.05 was
considered to indicate a statistically significant difference. In
the case of multiple comparisons, the P-value was adjusted using a
Dunn's test.
Results
Characteristics of participants
Molecular analysis of 176 CSF samples demonstrated
that 66 samples (37.5%) were PCR-positive (presence of a 996-bp
band after agarose gel electrophoresis), whilst 110 samples (62.5%)
showed no band (PCR-negative). DNA sequence analysis of
PCR-positive products identified two broad categories of bacteria,
G-ve (45/66; 68.2%) and G+ve (21/66; 31.8%). When PCR-negative CSF
samples were explored for leukocyte counts, and glucose and protein
concentrations, 88 samples exhibited pleocytosis, glucose
concentrations lower or higher than normal, and/or protein
concentrations lower or higher than normal. These CSF samples were
considered ABN. The remaining 22 CSF samples showed normal
leukocyte counts, as well as glucose and protein concentrations,
and were included in the NOR group. Accordingly, the 176 CSF
samples were classified into three groups: PCR-positive (37.5%),
ABN (50.0%), or NOR (12.5%) (Table
I).
 | Table IBaseline characteristics of suspected
meningitis cases and cerebrospinal fluid laboratory data. |
Table I
Baseline characteristics of suspected
meningitis cases and cerebrospinal fluid laboratory data.
| | PCR-negative CSF,
n=110 | |
|---|
|
Characteristics/laboratory
dataa | | PCR-positive,
n=66 | ABNb, n=88 | NOR, n=22 | P-value |
|---|
| Age, years | | 28.8±20.7 | 25.1±22.2 | 25.6±14.7 | 0.549 |
| Age group,
years | <2 | 14 (21.2) | 32 (36.4) | 3 (13.6) | 0.085 |
| | 3-12 | 4 (6.1) | 3 (3.4) | 1 (4.5) | |
| | 13-18 | 5 (7.6) | 4 (4.5) | 3 (13.6) | |
| | 19-39 | 21 (31.8) | 17 (19.3) | 10 (45.5) | |
| | 40-59 | 17 (25.8) | 29 (33.0) | 5 (22.7) | |
| | ≥60 | 5 (7.6) | 3 (3.4) | 0 (0.0) | |
| Sex | Male | 27 (40.9) | 44 (50.0) | 7 (31.8) |
<0.001 |
| | Female | 39 (59.1) | 44 (50.0) | 15 (68.2) | |
| Leukocyte count,
cells/mm3 | | 2.0 (0-62.5) | 11.5 (1-74.3) | 0.5 (0-2) |
<0.001 |
| Leukocyte
count | Normal | 43 (65.2) | 42 (47.7) | 22 (100.0) |
<0.001 |
| | Pleocytosis | 23 (34.8) | 46 (52.3) | 0 (0.0) | |
| Glucose, mg/dl | | 60±30 | 58±34 | 57±9 | 0.868 |
| Glucose
percentile | ≤25 | 18 (27.3) | 28 (31.8) | 0 (0.0) |
<0.001 |
| | 26-50 | 14 (21.2) | 19 (21.6) | 10 (45.5) | |
| | 51-75 | 13 (19.7) | 19 (21.6) | 12 (54.5) | |
| | >75 | 21 (31.8) | 22 (25.0) | 0 (0.0) | |
| Protein, mg/dl | | 106±137 | 128±151 | 27±7 | 0.009 |
| Protein
percentile | ≤25 | 17 (25.8) | 18 (20.7) | 10 (45.5) |
<0.001 |
| | 25-50 | 18 (27.3) | 13 (14.9) | 12 (54.5) | |
| | 51-75 | 15 (22.7) | 29 (33.3) | 0 (0.0) | |
| | >75 | 16 (24.2) | 27 (31.0) | 0 (0.0) | |
| Broad categories of
bacteria | Gram-ve | 45 (68.2) | NA | NA | |
| | Gram+ve | 21 (31.8) | NA | NA | |
| Antibiotic
medication | Yes | 23 (34.8) | 40 (45.5) | 6 (27.3) | 0.194 |
| | No | 43 (65.2) | 48 (54.5) | 16 (72.7) | |
Mean age did not show a significant difference
between participants in the three groups [28.8±20.7 (PCR-positive),
25.1±22.2 (ABN), and 25.6±14.7 years (NOR), P=0.549]. In addition,
when participants were categorized into age groups (≤2, 3-12,
13-18, 19-39, 40-59, and ≥60 years), no significant differences
were found with regard to the frequency of participants in each
group (P=0.085). The median of the leukocyte counts was
significantly higher in the PCR-positive and ABN CSFs compared to
the NOR CSF [2.0 (IQR: 0-62.5) and 11.5 (IQR: 1-74.3) vs. 0.5 (IQR:
0-2) cell/mm3, respectively; P<0.001]. According to
the leukocyte count, CSF samples were classified into two groups
(normal and pleocytosis). It was found that 34.8 and 52.3% of
PCR-positive and ABN CSFs were classed as pleocytotic,
respectively, whilst none of the NOR CSFs were considered
pleocytotic; these differences were significantly different
(P<0.001). Glucose concentrations exhibited no significant
differences between PCR-positive, ABN, and NOR CSFs (60±30, 58±34,
and 57±9 mg/dl, respectively; P=0.868). However, when these
concentrations were ordered by percentiles (≤25, 26-50, 51-75, and
>75%), significant differences were revealed (P<0.001). Low
glucose concentrations (≤25 mg/dl) were observed in 27.3 and 31.8%
of PCR-positive and ABN CSFs, respectively, compared with 0% in the
NOR CSFs. Protein concentrations were significantly higher in the
PCR-positive and ABN CSFs than in the NOR CSFs (106±137 and 128±151
vs. 27±7 mg/dl, respectively; P=0.009). A similar observation was
made when protein concentrations were classified into percentiles,
and >56% of PCR-positive and ABN CSFs were classified in the
percentiles 51-75, and >75, whilst none of the NOR CSFs were
placed in these percentiles; these differences were significant
(P<0.001). Some participants were on antibiotic medication at
the time of CSF collection, but their distribution in the three
groups of participants did not show significant differences (34.8,
45.5, and 27.3% of PCR-positive, ABN, and NOR CSF groups,
respectively; P=0.194; Table
I).
CSF levels of HBDs
Median HBD1, HBD2, and HBD4 levels did not exhibit
significant differences between PCR-positive, ABN, and NOR CSFs.
Conversely, HBD3 levels were significantly higher in ABN CSFs than
in NOR CSFs [1,470 (IQR: 671-2,001) vs. 572 (IQR: 427-1,679) ng/l;
P=0.005]. HBD3 levels were also elevated in PCR-positive CSFs
compared with the NOR CSFs, but the difference was not significant
[1,086 (IQR: 659-1,584) vs. 572 (IQR: 427-1,679) ng/l; P=0.151].
Additionally, HBD3 levels showed no significant differences between
PCR-positive and ABN CSFs (P=0.303, Fig. 1).
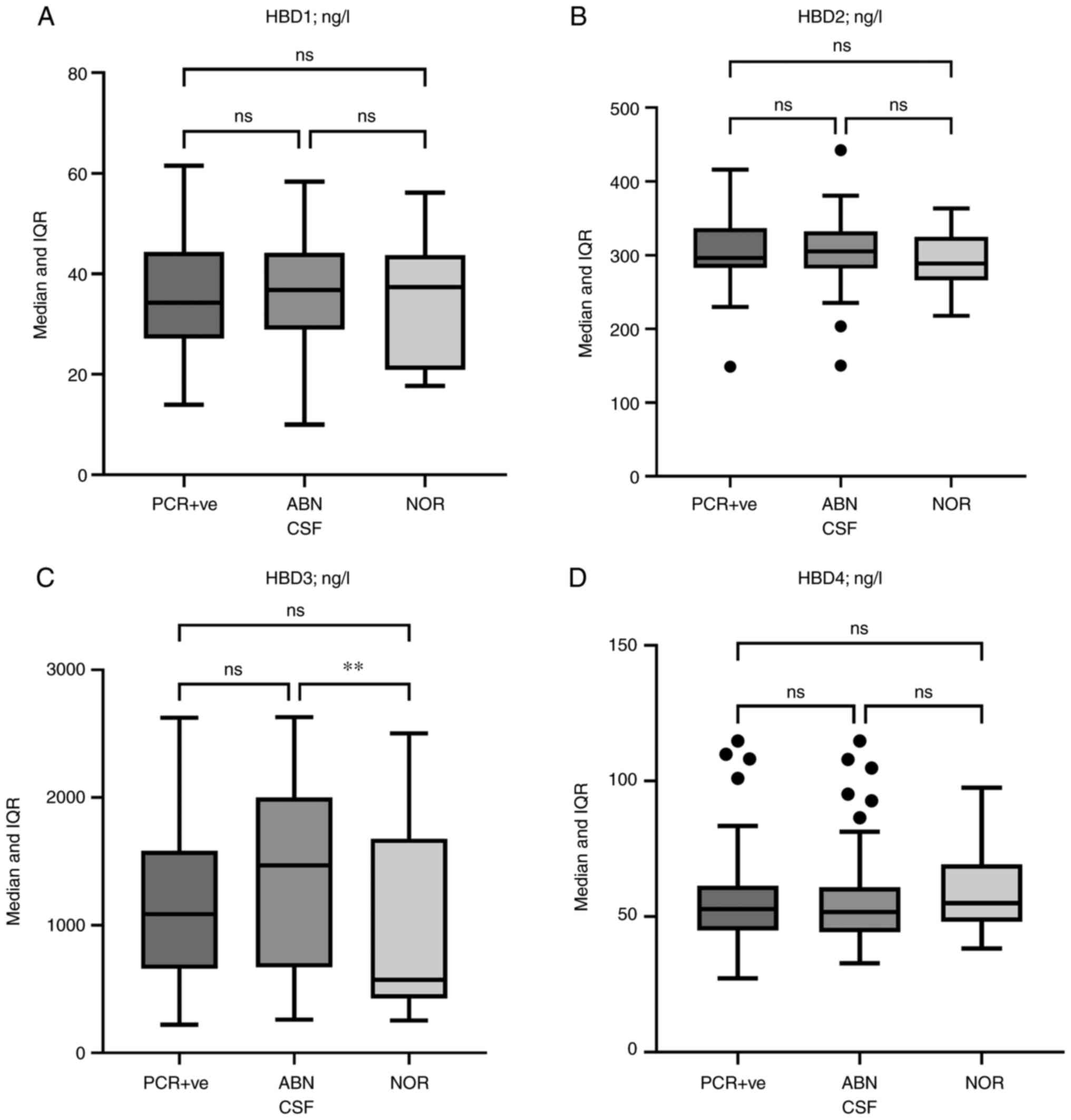 | Figure 1Box and whisker plots of HBD levels.
(A) HBD1, (B) HBD2, (C) HBD3, and (D) HBD4 levels in the CSF of
PCR-positive and PCR-negative cases, and in the ABN or NOR CSF
cases. Horizontal lines inside the boxes indicate the median value,
whilst the whiskers indicate the IQR. Outliers are presented as
black circles. Only HBD3 levels showed differences between the
three groups; the difference was significant between the ABN and
NOR groups [1,470 (IQR: 671-2,001) vs. 572 (IQR: 427-1,679) ng/l;
P=0.005]. **P<0.01. HBD, human β-defensin; CSF,
cerebrospinal fluid; ABN, abnormal; NOR, normal; IQR, interquartile
range; ns, not significant. |
ROC curve analysis of HBD3
ROC curve analysis of HBD3 in the PCR-positive group
vs. the NOR group revealed an AUC value of 0.652 (95%
CI=0.495-0.809; P=0.033). The YI-adjusted cut-off value of HBD3 was
652 ng/l with sensitivity and specificity percentages of 78.8 and
63.3%, respectively. When the analysis was conducted on the ABN
group vs. the NOR group, a higher discriminatory power was found
for HBD3 (AUC=0.707; 95% CI=0.573-0.841; P=0.003; cut-off value=650
ng/l, YI=0.44, sensitivity=63.6%, specificity=80.0%; Fig. 2).
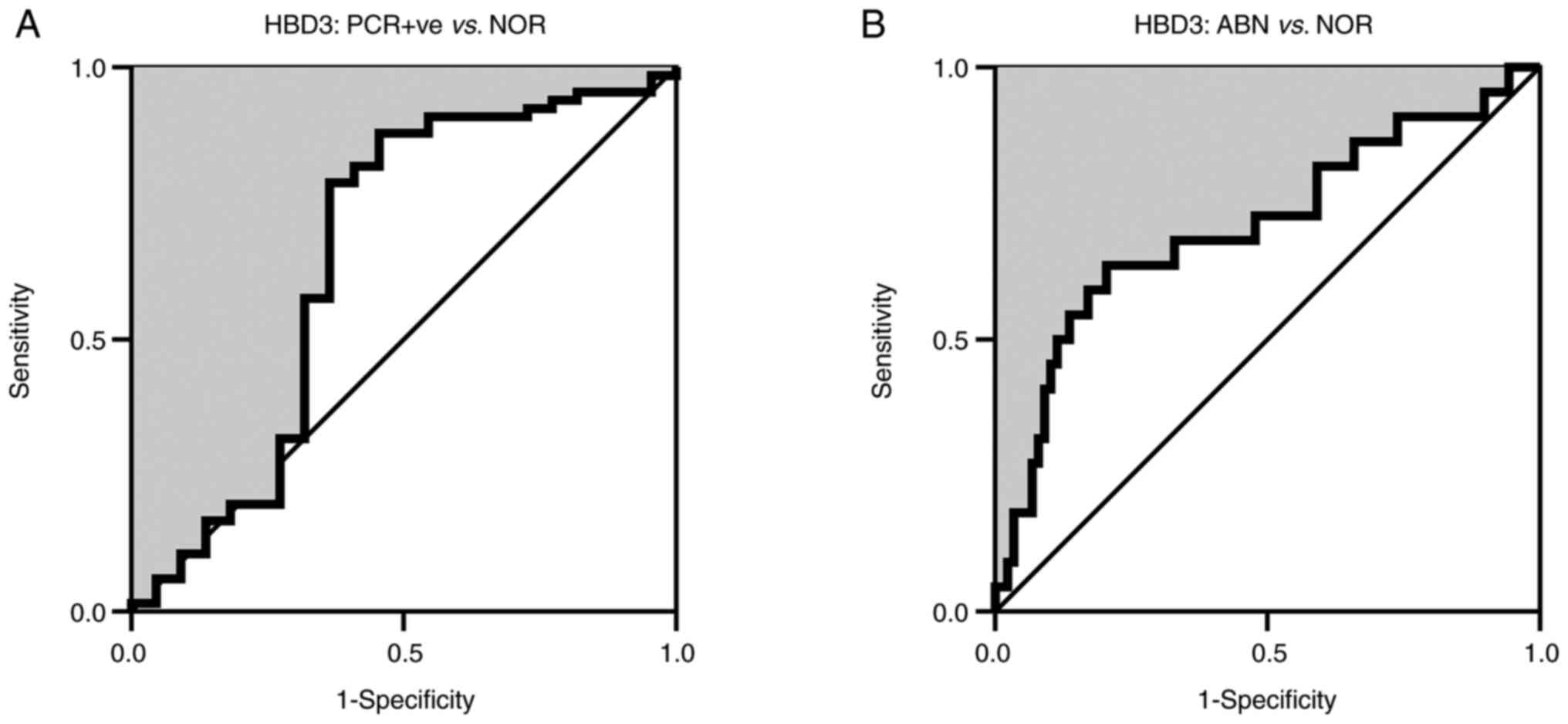 | Figure 2ROC curve analysis (Wilson/Brown
method) of HBD3 in the CSF of (A) PCR-positive cases and (B)
PCR-negative cases in the ABN vs. NOR CSF. In PCR-positive vs. NOR,
the estimated AUC was 0.652 (95%=0.495-0.809; P=0.033; cut-off
value=652 ng/l; YI=0.42; sensitivity=78.8%; specificity=63.3%). In
ABN vs. NOR, the AUC was higher than in the PCR-positive vs. NOR
(AUC=0.707; 95% CI=0.573-0.841; P=0.003; cut-off value=650 ng/l;
YI=0.44; sensitivity=63.6%; specificity=80.0%). ROC, receiver
operating characteristic; HBD, human β-defensin; CSF, cerebrospinal
fluid; ABN, abnormal; NOR, normal; AUC, area under the curve; CI,
confidence interval; YI, Youden index. |
Stratification of HBD levels by
characteristics
The median levels of HBD1, HBD2, HBD3, and HBD4 were
stratified by age group, sex, leukocyte counts (pleocytosis and
normal), glucose and protein percentiles, and antibiotic medication
in the PCR-positive and ABN groups. In the PCR-positive group, six
significant differences were observed. Upregulated HBD2 levels were
associated with the fourth percentile (>75) of protein
concentration (P=0.012). HBD3 levels were increased in age groups
≤2, 3-12, and 40-59 years compared to age groups 13-18, 19-39, and
≥60 years (P<0.001). Upregulated HBD3 levels were associated
with the first percentile (≤25) of glucose concentration (P=0.013),
whilst downregulated levels were associated with the first
percentile of protein concentration (P<0.001). Antibiotic
medication was also associated with higher CSF levels of HBD3
(P=0.005). HBD4 levels were higher in males than in females
(P=0.021, Table II).
 | Table IICerebrospinal fluid levels of HBD1,
HBD2, HBD3 and HBD4 classified by characteristics of PCR-positive
cases. |
Table II
Cerebrospinal fluid levels of HBD1,
HBD2, HBD3 and HBD4 classified by characteristics of PCR-positive
cases.
| | Median (IQR),
ng/l |
|---|
|
Characteristics | | HBD1 | HBD2 | HBD3 | HBD4 |
|---|
| Age group,
years | ≤2 | 36 (29-49) | 300 (282-342) | 1660
(1310-2147) | 54 (46-75) |
| | 3-12 | 36 (30-44) | 349 (319-380) | 1364
(751-2254) | 54 (44-86) |
| | 13-18 | 31 (27-53) | 287 (284-322) | 609 (271-711) | 61 (59-62) |
| | 19-39 | 33 (24-40) | 291 (283-336) | 770 (606-1206) | 48 (44-58) |
| | 40-59 | 30 (26-40) | 292 (274-306) | 1198
(803-1483) | 52 (45-74) |
| | ≥60 | 44 (42-46) | 304 (299-327) | 629 (432-1139) | 48 (47-53) |
| | P-value | 0.435 | 0.315 |
<0.001 | 0.543 |
| Sex | Male | 30 (25-42) | 297 (282-348) | 1194
(770-1575) | 58 (48-74) |
| | Female | 38 (28-44) | 296 (283-330) | 1033
(609-1626) | 48 (45-57) |
| | P-value | 0.282 | 0.51 | 0.527 | 0.021 |
| Leukocyte
count | Normal | 34 (27-44) | 292 (283-331) | 845 (629-1575) | 53 (45-61) |
| | Pleocytosis | 35 (28-46) | 311 (281-343) | 1194
(773-1628) | 51 (45-62) |
| | P-value | 0.691 | 0.278 | 0.261 | 0.732 |
| Glucose
percentile | ≤25 | 33 (27-49) | 300 (281-347) | 1488
(1194-1814) | 57 (46-61) |
| | 26-50 | 39 (30-44) | 291 (280-324) | 869 (770-1256) | 47 (40-62) |
| | 51-75 | 36 (27-44) | 303 (288-354) | 684 (549-824) | 52 (45-71) |
| | >75 | 33 (27-42) | 296 (284-311) | 846 (629-1611) | 52 (47-58) |
| | P-value | 0.835 | 0.608 | 0.013 | 0.704 |
| Protein
percentile | ≤25 | 33 (27-42) | 295 (285-343) | 630 (561-782) | 51 (47-69) |
| | 26-50 | 40 (24-44) | 296 (283-331) | 1156
(660-1483) | 55 (45-58) |
| | 51-75 | 30 (23-40) | 282 (272-304) | 1229
(790-1814) | 53 (40-62) |
| | >75 | 36 (29-46) | 328 (294-351) | 1437
(1004-1620) | 53 (46-74) |
| | P-value | 0.504 | 0.012 |
<0.001 | 0.808 |
| Antibiotic
medication | Yes | 36 (29-45) | 311 (290-346) | 1310
(845-1882) | 58 (47-75) |
| | No | 31 (25-44) | 292 (280-329) | 794 (606-1494) | 50 (45-59) |
| | P-value | 0.350 | 0.172 | 0.005 | 0.086 |
Regarding the ABN group, four significant
differences were found. HBD2 levels showed variations between the
protein percentiles, and the lowest levels were associated with the
first percentile (P=0.006). Upregulated HBD3 levels were associated
with the first and second percentiles (≤25 and 26-50) of glucose
concentration (P=0.002), whilst the opposite was observed in
protein concentrations, and these percentiles were associated with
downregulated levels (P<0.001). Finally, as in the PCR-positive
group, upregulated HBD3 levels were associated with antibiotic
medication (P=0.004, Table
III).
 | Table IIICerebrospinal fluid levels of HBD1,
HBD2, HBD3 and HBD4 classified by characteristics of PCR-negative
cases with abnormal cerebrospinal fluid. |
Table III
Cerebrospinal fluid levels of HBD1,
HBD2, HBD3 and HBD4 classified by characteristics of PCR-negative
cases with abnormal cerebrospinal fluid.
| | Median (IQR),
ng/l |
|---|
|
Characteristics | | HBD1 | HBD2 | HBD3 | HBD4 |
|---|
| Age group,
years | ≤2 | 40 (32-44) | 316 (280-334) | 1763
(1342-2008) | 51 (44-60) |
| | 3-12 | 34 (24-44) | 320 (298-381) | 1996
(343-2263) | 47 (35-56) |
| | 13-18 | 38 (31-45) | 294 (265-332) | 785 (478-1695) | 77 (67-86) |
| | 19-39 | 40 (32-44) | 320 (286-353) | 827 (598-2003) | 50 (46-74) |
| | 40-59 | 34 (29-43) | 298 (274-311) | 969 (704-1773) | 51 (44-58) |
| | ≥60 | 52 (10-56) | 348 (284-357) | 668 (614-2152) | 55 (47-57) |
| | P-value | 0.784 | 0.243 | 0.282 | 0.143 |
| Sex | Male | 36 (30-44) | 315 (278-335) | 1549
(654-2028) | 50 (44-61) |
| | Female | 41 (31-45) | 303 (282-327) | 975 (687-1965) | 53 (45-65) |
| | P-value | 0.341 | 0.576 | 0.494 | 0.537 |
| Leukocyte
count | Normal | 35 (27-44) | 311 (284-341) | 858 (592-2003) | 51 (44-62) |
| | Pleocytosis | 40 (32-44) | 301 (276-332) | 1493
(887-1996) | 53 (45-59) |
| | P-value | 0.168 | 0.488 | 0.078 | 0.796 |
| Glucose
percentile | ≤25 | 42 (35-45) | 316 (275-333) | 1813
(1360-2182) | 54 (44-61) |
| | 26-50 | 37 (32-44) | 321 (297-357) | 1540
(745-2003) | 57 (43-75) |
| | 51-75 | 36 (28-44) | 310 (282-342) | 781 (574-2060) | 48 (46-54) |
| | >75 | 33 (26-45) | 292 (271-311) | 730 (598-1510) | 49 (43-62) |
| | P-value | 0.173 | 0.252 | 0.002 | 0.577 |
| Protein
percentile | ≤25 | 37 (26-42) | 278 (263-310) | 674 (574-950) | 52 (44-61) |
| | 26-50 | 34 (30-47) | 304 (293-338) | 781 (527-1510) | 46 (44-49) |
| | 51-75 | 37 (32-44) | 324 (303-348) | 1522
(766-1956) | 51 (42-64) |
| | >75 | 42 (30-46) | 300 (284-333) | 1850
(1244-2134) | 55 (47-59) |
| | P-value | 0.584 | 0.006 |
<0.001 | 0.306 |
| Antibiotic
medication | Yes | 41 (32-44) | 317 (291-332) | 1653
(984-2061) | 51 (44-63) |
| | No | 36 (28-44) | 299 (272-340) | 803 (595-1954) | 52 (45-58) |
| | P-value | 0.185 | 0.283 | 0.004 | 0.821 |
HBD levels stratified by G-ve and G+ve
bacteria
HBD1, HBD2, HBD3, and HBD4 levels were examined in
the CSF of PCR-positive cases after being classified into two broad
categories of bacteria, G-ve and G+ve. Although there were no
significant differences, HBD3 [1,200 (IQR: 662-1,620) vs. 803 (IQR:
631-1,466) ng/l; P=0.28] and HBD4 [56 (IQR: 46-68) vs. 47 (IQR:
43-57) ng/l; P=0.07] tended to have higher levels in the G-ve group
than in the G+ve group (Fig.
3).
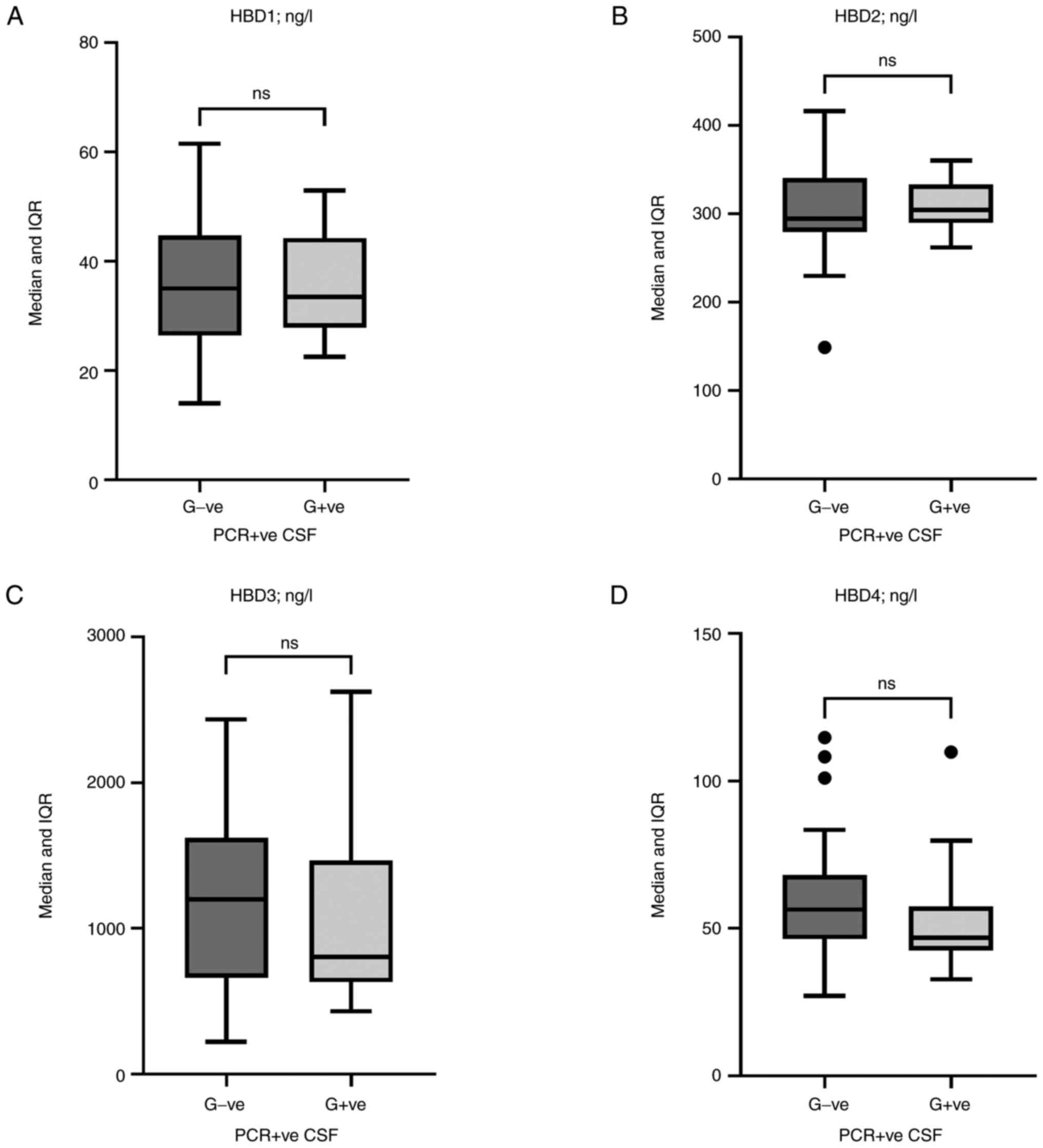 | Figure 3Box and whisker plots of human
β-defensin levels. (A) HBD1, (B) HBD2, (C) HBD3, and (D) HBD4
levels in the CSF of PCR-positive cases (G-ve and G+ve bacteria).
Horizontal lines inside the boxes indicate the median value, whilst
the whiskers indicate the IQR. Outliers are presented as black
circles. Although there were no significant differences, HBD3
[1,200 (IQR: 662-1,620) vs. 803 (IQR: 631-1,466) ng/l; P=0.28)] and
HBD4 [56 (IQR: 46-68) vs. 47 (IQR: 43-57) ng/l; P=0.07] tended to
have higher levels in the G-ve than in the G+ve group. HBD, human
β-defensin; CSF, cerebrospinal fluid; G-ve, Gram-negative; G+ve,
Gram-positive; IQR, interquartile range. |
Correlation analysis
Spearman's rank correlation analysis was performed
between age, leukocytes, glucose concentrations, protein
concentrations, and HBD1, HBD2, HBD3, and HBD4 levels in the CSF of
all participants. Age was positively correlated with glucose
(rs=0.534; P<0.001) and negatively correlated with
protein concentrations (rs=-0.216; P=0.004) and HBD3
levels (rs=-0.226; P=0.003). Leukocyte counts were
negatively correlated with glucose concentrations
(rs=-0.344; P<0.001), and positively correlated with
protein concentrations (rs=0.506; P<0.001), HBD1
(rs=0.164; P=0.031) and HBD3 levels
(rs=0.258; P<0.001). Glucose concentrations were
negatively correlated with protein concentrations
(rs=-0.452; P<0.001) and HBD3 levels
(rs=-0.329; P<0.001). Protein concentrations were
positively correlated with HBD1 (rs=0.162; P=0.033),
HBD2 (rs=0.186; P=0.014) and HBD3 levels
(rs=0.457; P<0.001). HBD1 was positively correlated
with HBD3 levels (rs=0.283; P<0.001) Finally, HBD2
levels were positively correlated with HBD4 levels
(rs=0.202; P=0.007) (Table
IV).
 | Table IVSpearman's rank correlation analysis
of cerebrospinal fluid variables in all participating subjects. |
Table IV
Spearman's rank correlation analysis
of cerebrospinal fluid variables in all participating subjects.
| Variables | Statistics | Age | Leukocytes | Glucose | Protein | HBD1 | HBD2 | HBD3 | HBD4 |
|---|
| Age | rs | 1.000 | -0.102 | 0.534 | -0.216 | -0.120 | -0.083 | -0.226 | -0.014 |
| | P-value | | 0.179 |
<0.001 | 0.004 | 0.113 | 0.275 | 0.003 | 0.853 |
| Leukocytes | rs | | 1.000 | -0.344 | 0.506 | 0.164 | 0.112 | 0.258 | 0.007 |
| | P-value | | |
<0.001 |
<0.001 | 0.031 | 0.140 |
<0.001 | 0.931 |
| Glucose | rs | | | 1.000 | -0.452 | -0.117 | -0.102 | -0.329 | -0.100 |
| | P-value | | | |
<0.001 | 0.123 | 0.179 |
<0.001 | 0.187 |
| Protein | rs | | | | 1.000 | 0.162 | 0.186 | 0.457 | 0.011 |
| | P-value | | | | | 0.033 | 0.014 |
<0.001 | 0.883 |
| HBD1 | rs | | | | | 1.000 | 0.099 | 0.283 | -0.124 |
| | P-value | | | | | | 0.190 |
<0.001 | 0.102 |
| HBD2 | rs | | | | | | 1.000 | 0.048 | 0.202 |
| | P-value | | | | | | | 0.529 | 0.007 |
| HBD3 | rs | | | | | | | 1.000 | -0.133 |
| | P-value | | | | | | | | 0.079 |
| HBD4 | rs | | | | | | | | 1.000 |
Discussion
The present study focused on four HBDs (HBD1, HBD2,
HBD3, and HBD4), which represent an important class of innate
immune modulators that act non-specifically against microbial
challenges (12). The systemic
profile of HBDs has been extensively studied in infectious,
autoimmune, and inflammatory diseases, and dysregulated production
has been associated with disease progression (18-20).
In the case of meningitis, the CSF profile of HBDs is the least
studied and there have been limited data assessing their role in
the development of meningitis. Meningitis is generally described as
an inflammation-based disease associated with high mortality and
significant morbidity rates (1).
HBDs are also functionally linked to inflammation, and induction of
HBD1, HBD2, HBD3, and HBD4 can occur due to exposure to microbial
infection and inflammatory stimuli, as well as endogenous danger
signals (15). Additionally, it has
been hypothesized that abnormal expression and regulatory function
of some antimicrobial peptides, such as HBDs, are associated with
neuropathological changes due to chronic CNS diseases (21). Therefore, it is necessary to examine
HBD levels in the CSF of patients with meningitis as information in
this regard is scarce.
The present study included suspected cases of
meningitis, where the cause of meningitis was not known. The
initial interest was bacterial meningitis, thus a PCR-based method
capable of detecting all types of bacteria using the eubacteria 16S
rRNA gene as a target was adopted (27). The method successfully identified 66
meningitis cases (PCR-positive) and DNA sequence analysis grouped
the cases into two broad categories, G-ve and G+ve. The remaining
110 CSF samples were PCR-negative, but analysis of leukocyte count
and glucose and protein concentrations revealed that 88 CSF samples
had abnormal results and were assigned to a group called ABN. The
three parameters of analysis used in the present study are the most
relevant for diagnosis of meningitis, and elevated leukocyte counts
(pleocytosis) or protein concentrations, and lower glucose
concentrations may be associated with viral, bacterial, and fungal
infections (28,29). Since a bacterial cause was excluded
in the ABN group, as indicated by the PCR analysis, a viral or
fungal infection could not be ruled out. CSF samples with normal
leukocyte counts, as well as normal glucose and protein
concentrations, were also encountered and were used as the control
(NOR group). The rationale for adopting this classification for CSF
samples (PCR-positive, ABN, and NOR CSFs) was to reduce causative
differences between samples in each group. This may aid in better
understanding the role of HBDs in the development of meningitis of
various etiologies.
Among the four HBDs studied, HBD3 levels were
significantly higher in the ABN CSF than in the NOR CSF,
particularly in patients ≤12 years old. In addition, higher levels
of HBD3 were associated with lower glucose concentrations and
higher protein concentrations in the CSF. PCR-positive CSFs showed
a nearly similar profile to ABN CSF, in addition, higher levels of
HBD3 were associated with G-ve bacteria over G+ve bacteria. These
findings suggest a role for HBD3 in meningitis regardless of the
cause, bacterial or otherwise. Another interesting issue is the
association of upregulated HBD3 levels with abnormal CSF
concentrations of glucose and proteins, which are important
diagnostic tests in meningitis (28,29).
This may also highlight the diagnostic value of HBD3 in meningitis,
and ROC analysis showed acceptable discriminatory power between ABN
CSF and NOR CSF (AUC=0.707). HBD3 is an important AMP involved in
protection against bacterial and viral infections, and is also
known to have immunomodulatory functions (20). Regarding its antibacterial effects,
studies have shown significant bactericidal activity of HBD3
against different G+ve and G-ve bacteria and this may be related to
the cationic charges of HBD3 molecules (30,31).
Additionally, high expression of HBD3 significantly enhanced wound
closure in diabetic animal models infected with Staphylococcus
aureus (32). The antiviral
effects of HBD3 have also been demonstrated and experimental
evidence has shown the effectiveness of HBD3 against various
viruses, for example, West Nile virus and human immunodeficiency
virus (33,34). Both effects (antibacterial and
antiviral) are also potentiated by the immunomodulatory functions
of HBD3. In this context, the role of HBD3 in innate immunity is
well-recognized due to its antimicrobial activity. However, it has
also been indicated that it contributes to the adaptive immune
response, and immune-modulating properties of HBD3 such as
chemotaxis to T lymphocytes, macrophages, neutrophils, and immature
dendritic cells have been described (35). Therefore, HBD3 has been revealed to
be associated with inflammatory diseases; for instance,
dysregulated expression of HBD3 has been reported in inflammatory
bowel disease, and periodontitis patients (36,37).
Taken together, these findings suggest a role for HBD3 in
inflammatory reactions associated with bacterial and viral
infection, and this role may extend to the CNS and dysregulated
expression of HBD3 in CSF could be expected.
The levels of HBD1, HBD2, and HBD4 in the CSF of the
current suspected meningitis cases did not show significant
differences between PCR-positive, ABN, and NOR CSF. However, CSF
levels of HBD2 tended to parallel CSF protein concentrations.
Significantly elevated levels of HBD2 were associated with protein
concentrations >25% in the PCR-positive and ABN CSFs. Elevated
levels of CSF proteins are a reliable marker in diagnosing
bacterial and viral meningitis (28). HBD2 is recognized as an AMP that
integrates innate immune defenses against bacterial and viral
infections and may also be considered a marker of inflammation.
Therapeutic administration of HBD2 has been suggested to maintain
systemic homeostasis on the basis of an appropriate microbial
composition (38). Thus, HBD2 may
have a similar functional role in the CSF of meningitis patients.
In addition to HBD2, HBD4 levels were significantly elevated in
male patients with PCR-positive CSF compared to female patients.
There is no supporting evidence for this observation, but in
patients with allergic rhinitis, the opposite observation was made
and serum HBD4 levels were significantly elevated in female
patients compared to male patients (39). In COVID-19 patients, there were no
significant differences between males and females regarding serum
HBD4 levels (19). With these
conflicting results, the association between HBD4 and sex remains
uncertain, and further studies are warranted.
Correlation analysis showed that CSF levels of HBD1
and HBD3, as well as HBD2 and HBD4, were positively correlated.
Similar positive correlations have been found in the serum of
COVID-19 patients (19).
Furthermore, a positive correlation between HBD2 and HBD4 has been
reported in the serum of allergic rhinitis patients and healthy
controls (39). This may indicate
functional associations between these HBDs. In fact, it has been
recognized that in addition to their common antimicrobial activity,
HBDs in general enhance certain immune functions such as chemotaxis
(20). Additional correlation
findings included positive correlations between HBD1 with leukocyte
counts and protein concentration, as well as between HBD2 and
glucose concentration. In the case of HBD3, more correlations were
found. It was negatively correlated with age and glucose
concentration, and positively correlated with leukocyte counts and
protein concentration. The most important of these correlations was
with leukocyte counts, glucose concentration, and protein
concentration, which are diagnostic parameters in the CSF for
meningitis (28,29).
The present study has some limitations. First, a
detailed clinical history of suspected meningitis cases was not
obtained. Second, only one CSF sample was collected from each
participant, and a second sample is necessary to confirm the
results of the first. Third, molecular analysis of CSF included
evaluation of only bacterial DNA, whilst viral and fungal presence
was not assessed. Fourth, the plasma concentrations of glucose were
not determined.
In conclusion, among the four HBDs studied, HBD3
levels were significantly elevated in the CSF of suspected
meningitis cases regardless of the cause of meningitis. CSF levels
of some HBDs were affected by specific diagnostic laboratory
parameters for meningitis, including leukocyte counts, glucose
concentrations, and protein concentrations.
Acknowledgements
The authors appreciate the cooperation of
consultants Dr Samir H. Al-delfi (Neurosurgery Teaching Hospital)
and Dr Basim H. Jabbar (Alwitri Neuroscience Teaching
Hospital).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (LKJ, MAU, KBJK, and AHA) conceptualized
the study and contributed to data curation, data analysis, the
methodology, and validation of the results, as well as wrote the
original draft, and revised and edited the manuscript. LKJ and AHA
confirm the authenticity of the raw data. MAU and AHA supervised
the study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Ethics Committee of the College of
Science, University of Baghdad (Baghdad, Iraq) approved the study
(approval no. CSEC/1022/0136) and written informed consent was
obtained from patients or their legal guardian prior to sample
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Young N and Thomas M: Meningitis in
adults: Diagnosis and management. Intern Med J. 48:1294–1307.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hersi K, Gonzalez FJ, Kondamudi NP and
Sapkota R: Meningitis (Nursing). StatPearls [Internet]. Treasure
Island (FL): StatPearls Publishing, 2022.
|
|
3
|
Griffiths MJ, McGill F and Solomon T:
Management of acute meningitis. Clin Med (Lond). 18:164–169.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Centers for Disease Control and
Prevention: Chapter 2: Epidemiology of Meningitis Caused by
Neisseria meningitidis, Streptococcus pneumoniae, and
Haemophilus influenza. https://www.cdc.gov/meningitis/lab-manual/chpt02-epi.html.
Accessed February 19, 2022.
|
|
5
|
GBD 2016 Meningitis Collaborators. Global,
regional, and national burden of meningitis, 1990-2016: A
systematic analysis for the Global Burden of Disease Study 2016.
Lancet Neurol. 17:1061–1082. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Al-Sanouri T, Mahdi S, Khader IA, Mahdi A,
Dogu A, Amiche A, Iweir S, Qader M, Belbaisi A and AlHilfi R: The
epidemiology of meningococcal meningitis: multicenter,
hospital-based surveillance of meningococcal meningitis in Iraq.
IJID Reg. 1:100–106. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bagheri-Nesami M, Babamahmoodi F and
Nikkhah A: Types, risk factors, clinical symptoms and diagnostic
tests of acute adult meningitis in northern Iran during 2006-2012.
J Clin Diagn Res. 9:IC01–IC05. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grandgirard D, Gäumann R, Coulibaly B,
Dangy JP, Sie A, Junghanss T, Schudel H, Pluschke G and Leib SL:
The causative pathogen determines the inflammatory profile in
cerebrospinal fluid and outcome in patients with bacterial
meningitis. Mediators Inflamm. 2013(312476)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Coutinho LG, Grandgirard D, Leib SL and
Agnez-Lima LF: Cerebrospinal-fluid cytokine and chemokine profile
in patients with pneumococcal and meningococcal meningitis. BMC
Infect Dis. 13(326)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tzeng YL and Stephens DS: Antimicrobial
peptide resistance in Neisseria meningitidis. Biochim Biophys Acta.
1848 (11 Pt B):3026–3031. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wassing GM, Ilehag N, Frey J and Jonssona
AB: Modulation of human beta-defensin 2 expression by pathogenic
neisseria meningitidis and commensal lactobacilli. Antimicrob
Agents Chemother. 65:e02002–e02020. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mahlapuu M, Håkansson J, Ringstad L and
Björn C: Antimicrobial peptides: An emerging category of
therapeutic agents. Front Cell Infect Microbiol.
6(194)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huan Y, Kong Q, Mou H and Yi H:
Antimicrobial peptides: Classification, design, application and
research progress in multiple fields. Front Microbiol.
11(582779)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kumar P, Kizhakkedathu JN and Straus SK:
Antimicrobial peptides: Diversity, mechanism of action and
strategies to improve the activity and biocompatibility in vivo.
Biomolecules. 8(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fruitwala S, El-Naccache DW and Chang TL:
Multifaceted immune functions of human defensins and underlying
mechanisms. Semin Cell Dev Biol. 88:163–172. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Park MS, Kim JI, Lee I, Park S, Bae JY and
Park MS: Towards the application of human defensins as antivirals.
Biomol Ther (Seoul). 26:242–254. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang L, Ching CB, Jiang R and Leong SS:
Production of bioactive human beta-defensin 5 and 6 in Escherichia
coli by soluble fusion expression. Protein Expr Purif. 61:168–174.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ali ZA, Mankhi AA and Ad'hiah AH:
Significance of the chemokine CXCL10 and human beta-defensin-3 as
biomarkers of pulmonary tuberculosis. Tuberculosis (Edinb).
128(102078)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Al-Bayatee NT and Ad'hiah AH: Human
beta-defensins 2 and 4 are dysregulated in patients with
coronavirus disease 19. Microb Pathog. 160(105205)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shelley JR, Davidson DJ and Dorin JR: The
dichotomous responses driven by β-Defensins. Front Immunol.
11(1176)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Williams WM, Castellani RJ, Weinberg A,
Perry G and Smith MA: Do β-defensins and other antimicrobial
peptides play a role in neuroimmune function and neurodegeneration?
ScientificWorldJournal. 2012(905785)2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Merres J, Höss J, Albrecht LJ, Kress E,
Soehnlein O, Jansen S, Pufe T, Tauber SC and Brandenburg LO: Role
of the cathelicidin-related antimicrobial peptide in inflammation
and mortality in a mouse model of bacterial meningitis. J Innate
Immun. 6:205–218. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wassing GM, Lidberg K, Sigurlásdóttir S,
Frey J, Schroeder K, Ilehag N, Lindås AC, Jonas K and Jonsson AB:
DNA Blocks the lethal effect of human beta-defensin 2 against
Neisseria meningitidis. Front Microbiol. 12(697232)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fleischer E, Neuman MI, Wang ME, Nigrovic
LE, Desai S, DePorre AG, Leazer RC, Marble RD, Sartori LF and
Aronson PL: FEBRILE YOUNG INFANT RESEARCH COLLABORATIVE.
Cerebrospinal fluid profiles of infants ≤60 days of age with
bacterial meningitis. Hosp Pediatr. 9:979–982. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Martín-Ancel A, García-Alix A, Salas S,
Del Castillo F, Cabañas F and Quero J: Cerebrospinal fluid
leucocyte counts in healthy neonates. Arch Dis Child Fetal Neonatal
Ed. 91:F357–F358. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kononen TR, Mooney KM and Hoekstra KA: A
slight shade of green. Clin Chem. 65:939–940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lu JJ, Perng CL, Lee SY and Wan CC: Use of
PCR with universal primers and restriction endonuclease digestions
for detection and identification of common bacterial pathogens in
cerebrospinal fluid. J Clin Microbiol. 38:2076–2080.
2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hrishi AP and Sethuraman M: Cerebrospinal
fluid (CSF) analysis and interpretation in neurocritical care for
acute neurological conditions. Indian J Crit Care Med. 23 (Suppl
2):S115–S119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carbonnelle E: Laboratory diagnosis of
bacterial meningitis: Usefulness of various tests for the
determination of the etiological agent. Med Mal Infect. 39:581–605.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu C, Bao NR, Chen S and Zhao JN: The
mechanism of human β-defensin 3 in MRSA-induced infection of
implant drug-resistant bacteria biofilm in the mouse tibial bone
marrow. Exp Ther Med. 13:1347–1352. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dhingra H, Kaur K and Singh B: Engineering
and characterization of human β-defensin-3 and its analogues and
microcin J25 peptides against Mannheimia haemolytica and bovine
neutrophils. Vet Res. 52(83)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hirsch T, Spielmann M, Zuhaili B, Fossum
M, Metzig M, Koehler T, Steinau HU, Yao F, Onderdonk AB,
Steinstraesser L and Eriksson E: Human beta-defensin-3 promotes
wound healing in infected diabetic wounds. J Gene Med. 11:220–228.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Chessa C, Bodet C, Jousselin C, Larivière
A, Damour A, Garnier J, Lévêque N and Garcia M: Antiviral effect of
hBD-3 and LL-37 during Human primary keratinocyte infection with
west nile virus. Viruses. 14(1552)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Quiñones-Mateu ME, Lederman MM, Feng Z,
Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B,
Kiser P, et al: Human epithelial β-defensins 2 and 3 inhibit HIV-1
replication. AIDS. 17:F39–F48. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Al Mansour N, Al-Kafaji G, Al Mahmeed A
and Bindayna KM: Dysregulation of human beta-defensin-3 expression
in the peripheral blood of patients with sepsis. SAGE Open Med.
9(20503121211041515)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Meisch JP, Nishimura M, Vogel RM, Sung HC,
Bednarchik BA, Ghosh SK, Fu P, McCormick T, Weinberg A and Levine
AD: Human β-Defensin 3 peptide is increased and redistributed in
Crohn's Ileitis. Inflamm Bowel Dis. 19:942–953. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cui D, Lyu J, Li H, Lei L, Bian T, Li L
and Yan F: Human β-defensin 3 inhibits periodontitis development by
suppressing inflammatory responses in macrophages. Mol Immunol.
91:65–74. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cieślik M, Bagińska N, Górski A and
Jończyk-Matysiak E: Human β-defensin 2 and its postulated role in
modulation of the immune response. Cells. 10(2991)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ahmed MB and Ad'hiah AH: Allergic Rhinitis
and asthma: A profile of beta-defensins in serum of Iraqi patients.
Iraqi J Sci. 63:1941–1954. 2022.
|

















