Introduction
Depression and anxiety are complex neurological and
psychological diseases that are rated as two of the most severe
health problems by the World Health Organization (1). These diseases have a high incidence
and low diagnosis and treatment rates; however, anxiety and
depression seriously affect the health of individuals (2). The clinical symptoms of anxiety and
depression disorders include depressed mood, irritability, impaired
concentration, poor appetite and insomnia (3). Moreover, in addition to these
aforementioned symptoms, anxiety and depression can induce nervous
dread, hypervigilance, an increased heart rate and blood pressure
and can result in suicide (4-6).
At present, the pathological mechanisms of anxiety
and depression are unclear and complex. An increasing number of
studies have reported that their pathogenesis is related to the
autonomic nervous system, hypothalamic-pituitary-adrenal (HPA)
axis, neural circuits and the immune system (7). Several neurotransmitters, including
dopamine (DA), 5-hydroxytryptamine (5-HT), glutamate (Glu), and
γ-aminobutyric acid (GABA), serve an important role in nervous
system regulation (8). 5-HT
reuptake inhibitors can reduce the treatment of depression by
reducing central 5-HT production, which damages nerve cells and
leads to depression (9). The
excitatory Glu-inhibitory GABA neurotransmitter secretion balance
is critical for transmission of neural signals. Anxiety-like
responses are accompanied by abnormal synaptic transmission, which
results in an imbalance of excitatory/inhibitory (E/I)
neurotransmission (10). GABA and
its receptors serve an important role in regulating anxiety and
depression (11). Moreover, the
various receptors of GABA and Glu are potential therapeutic targets
for anxiety and depression (12,13).
Agarwood is an important spice that has been widely
used in incense, religion and aromatherapy for centuries (14-16).
As a traditional Chinese medicine, agarwood is considered to serve
a major role in pain relief, arresting vomiting, relieving asthma
and it also has been reported to exert sedative effects (17). Agarwood has also previously been
used to treat digestive, neurodegenerative and sedative diseases
(18). Modern pharmacological
studies have reported that agarwood essential oil (AEO) (19,20),
extracts (21) and the
sesquiterpene and chromone components (22,23)
exert favorable sedative and hypnotic, anti-anxiety/depression,
neuroinflammatory and neuroprotective effects. In traditional
Chinese medicine, agarwood incense is used for its availability and
the incense is also the main product of the agarwood market.
Generally, AEO is the main active constituent of agarwood and
serves numerous pharmacological functions, especially in neural
regulation. Moreover, AEO vapor is able to sedate mice (24). Agarofuran, a constituent of AEO, has
been revealed to exert anxiolytic and antidepressant activity
(25). Furan compounds have been
isolated from agarwood and synthesized via structural modification
of bugufuran. These have further been developed and declared as
class 1.1 anti-anxiety novel therapeutics, for which a phase Ⅲ
clinical trial has been launched (26). Diterpenoids of agarwood have also
been demonstrated to exert antidepressant activity via the
inhibition of the synaptic reuptake of serotonin and norepinephrine
(NE) (27). The antianxiety,
antidepressant and other neuroregulatory effects of AEO are
currently the focus of research and AEO is expected to be developed
into innovative novel neuroregulatory drugs. Furthermore, in
previous studies by the authors it was demonstrated that an AEO
injection could promote sleep and provide antianxiety and
antidepressant effects (19,20).
However, its pharmacological components and mechanism of action
remain unclear.
Therefore, in the present study AEO incense
inhalation was used to study the antianxiety and antidepressant
effects of AEO and investigate its possible underlying mechanism in
regulating neurotransmitters. The effects of AEO on
meta-chlorophenylpiperazine (mCPP)-induced anxiety and
chronic unpredictable mild stress (CUMS)-induced depression-like
behaviors were assessed using animal behavioral tests.
Materials and methods
Instruments and reagents
A video analysis system for spontaneous activity
(model: JLBehv-LAM-4) was purchased from Shanghai Jiliang Software
Technology Co., Ltd. The incense box (50x50x40 cm) was formed of
plexiglass with a 20x20x20 cm3 hollow cylinder inside
with incense inserts in the middle. The centrifuge and microplate
reader were purchased from Thermo Fisher Scientific, Inc. (model:
Multiskan Go, serial no. 1510-04123). The neurotransmitter and its
receptor kits glutamate (Glu; cat. no. SRA-EMS-20177),
5-hydroxytryptamine (5-HT; cat. no. SRA-ESM-00901) and
γ-aminobutyric acid receptor A (GABAA; cat. no. SRA-ES-31001; all
from DXY.cn; Hangzhou Lianke Meixun Biomedical Technology Co.,
Ltd.) were purchased from Beijing Bosheng Jingwei Biotechnology
Co., Ltd. The primary antibodies GABA transaminase (GABAT; cat. no.
AG1008), glutamate metabotropic receptor 5 (GRM5; cat. no. AF1744),
glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B) (cat.
no. AF7029), and glutamate ionotropic receptor AMPA type subunit 1
(GluR1; cat. no. AF2473) were purchased from Beyotime Institute of
Biotechnology. Vesicular glutamate transporter 1 (VGluT1; cat. no.
bs-11167R) was purchased from BIOSS. The secondary antibodies HRP
goat anti-rabbit (cat. no. A0208) and HRP goat anti-mouse (cat. no.
A0216) were also obtained from Beyotime Institute of Biotechnology.
Diazepam (cat. no. DZ-225336; www.biaowu.com) was purchased from Shangcheng Beina
Chuanglian Biological Technology Co., Ltd. mCPP (cat. no. R031956)
and paroxetine (a positive drug on antidepressant experiment) (cat.
no. 200317) were purchased from the Beijing Lianshi Yunshang
Network Technology Co., Ltd.
Materials
The agarwood raw material was artificially
propagated agarwood (28) and
tested according to the standards of the Chinese Pharmacopoeia by
the Agarwood Identification Center of Hainan Branch, Institute of
Medicinal Plant, Chinese Academy of Medical Sciences (Haikou,
China). Voucher specimens (no. JC2016112) were deposited at the
Agarwood Identification Center of our institute. AEO was prepared
via steam distillation.
Animals
A total of 96 adult male KM mice (weight, 18-20 g)
were purchased from the Hainan Provincial Institute of Medicine
[cat. no. SCXK (Qiong) 2020-0007]. All animals were kept in a
specific pathogen-free (SPF) animal facility at a temperature of
23±2˚C, a humidity of 60±5% and a 12-h light/dark
cycle. Animals had free access to food and water. The animal
experiments were performed in the SPF animal room of Hainan
Institute of Materia Medica Co., Ltd. (Haikou, China) and followed
the guidelines for the care and use of laboratory animals of this
institute under the approval and supervision of the Animal Ethics
Committee of the Hainan Institute of Materia Medica Co., Ltd.
(approval no. 2022HL014).
Anxiolytic effects of AEO
inhalation
The anxiolytic effects and the underlying mechanisms
of AEO inhalation in an anxious mouse induced by mCPP were
explored. The animals were divided into the following six groups (8
mice/group): i) Control group; ii) mCPP (8 mg/kg) model group; iii)
diazepam (2.5 mg/kg) group; and iv) to vi) the AEO (2, 4 or 8 µl)
inhalation groups. With the exception of the control group, the
mice were administered with mCPP intraperitoneal injections for 2
days to create the anxiety animal model. The control group was
given an equal volume of saline. The diazepam group was injected
intraperitoneally for 7 days with 2.5 mg/kg (10 ml/kg) diazepam.
The AEO groups were administered with AEO via inhalation for 7
days. After 7 days, animal behavior was assessed via the open field
test (OFT) and the light-dark exploration test (LDET). Following
the behavioral tests, the mice were sacrificed via cervical
dislocation and blood samples and brain tissues were rapidly
collected and preserved in liquid nitrogen. The blood samples were
centrifugated at 314 x g for 15 min at 4˚C and stored at -20˚C.
Antidepressant effects of AEO
inhalation
The antidepressant effects of AEO were assessed in
depressed mice induced via CUMS. The animals were divided
into the following six groups (8 mice/group): i) Control group; ii)
model group; iii) paroxetine (10 mg/kg) group, and iv) to vi) the
AEO inhalation (2, 4 and 8 µl) groups. With the exception of the
control group, mice were stimulated using CUMS molding boxes, which
included plantar stimulation for 1 min, light/dark stimulation for
2 min, fasting for 24 h, water deprivation for 24 h, sleep
deprivation for 24 h and restraint stress for 3 h/day. For random
unpredictability, half of the mice were selected per day and were
treated with continuous stimulation for 28 days. The paroxetine and
AEO were administered from day 22 to day 28 for 7 days. The
inactive time of tail suspension test (TST) and the forced swimming
test (FST) were used to assess depression in the mice. After the
behavior was assessed, the mice were sacrificed via cervical
dislocation and the blood samples and the brain tissues of the mice
were rapidly collected and preserved in liquid nitrogen. The blood
samples were centrifugated at 314 x g for 15 min at 4˚C and the
supernatant was stored at -20˚C.
LDET
The LDET was used to observe the exploratory
behavior of mice when they were added to a new environment. As the
rodents were innately averse to brightly lit areas, they developed
anxiety and the animal activity was disordered. The apparatus
contained two parts, a light box and a dark box, which were used to
detect the movement of the animals. The apparatus was connected to
a computer to record the time spent, distance moved and number of
transitions in each part during a 6-min session. For this
experiment, the mice were administered diazepam as aforementioned,
to detect the anxiolytic effects of AEO. In the present study, the
data from the last 4 min were selected for analysis.
OFT
The OFT evaluated the general exploratory behaviors
of mice. For this experiment, the mice were administered diazepam
as aforementioned, to detect the anxiolytic effects of AEO.
Briefly, 1 h after the last treatment was administered, OFT
real-time detection analysis was performed. The mice were placed
into the open field along the barrel wall for 10 min of observation
and the system automatically recorded the spontaneous activities of
the animals, such as movement distance and movement time. The time
spent and distance moved in the central area were collected to
reveal the anxiolytic effect of AEO.
TST. The TST was performed as previously
stated with certain modifications. In brief, 1 h after the last
treatment was administered, the mice were fixed in place with
adhesive tape and hung upside down. The tension change signal of
the mice struggling was transmitted to the computer via a sensor,
as well as to the signal conditioning unit and transmission
circuit. The computer online detection system for the suspended
tail automatically recorded the accumulated immobility time and
movement time of mice within 6 min. It also detected the immobility
time of the suspended tail in the last 4 min.
FST
The FST was performed according the Porsolt swim
test method. In brief, 1 h following the last treatment
administration, forced swimming real-time detection and analysis
were performed. After setting the experimental parameters, geometry
and illumination calibration, the mice were placed in a
thermostatic swimming apparatus (height, 20 cm; diameter, 18 cm;
water depth, 12 cm; water temperature, 23-25˚C). The system
automatically recorded the activity status of the mice over 6 min
and assessed the accumulated immobility time of the mice within the
last 4 min.
ELISA
In this experiment, the mice were administered with
diazepam and paroxetine as aforementioned, to detect the anxiolytic
and antidepressant effects of AEO, respectively. Next, 1 h after
the last administration, the mice were sacrificed via cervical
dislocation and blood was collected. The brain tissue was also
collected. The hippocampus was removed and frozen in liquid
nitrogen. Subsequently, the tissue was weighed and added to nine
volumes of normal saline before being fully homogenized in an ice
bath and centrifuged at 314 x g for 15 min at 4˚C. The supernatant
was collected and stored at -20˚C. The precipitate was used for
protein extraction. The levels of 5-HT, Glu and GABAA in
the supernatant were determined using the aforementioned ELISA
kits, according to the manufacturer's protocols.
Western blotting
The precipitate of hippocampal tissues was added to
RIPA (cat. no. BL504A; Biosharp; Beijing Lianshi Yunshang Network
Technology Co., Ltd.) buffer supplemented with protease and
phosphatase inhibitors. The homogenate was centrifuged at 1,364 x
g, for 10 min at 4˚C. The protein concentration was determined
using a BCA Protein Assay Kit. According to the molecular weight of
the target protein, the protein samples (10 µg) were separated
using 10% gel electrophoresis. Subsequently, the separated proteins
were transferred to a PVDF membrane and blocked with 5% skimmed
milk powder for 1 h at room temperature. The membranes were then
incubated with the following primary antibodies; GABAT, GRIN2B,
GRM5, GluR, VGluT1 (all at 1:1,000) and β-actin (1:2,000; cat. no.
AF0003; Beyotime Institute of Biotechnology) at 4˚C overnight.
Following the primary incubation, the membranes were incubated with
secondary antibodies (1:2,000) for 2 h at room temperature. The
membrane was treated with an ECL kit (cat. no. BL520A; Biosharp;
Beijing Lianshi Yunshang Network Technology Co., Ltd.) and was
observed using a gel imager. The grayscale of the protein bands
were scanned and quantitative analysis was performed using the
Gel-Pro Analyzer 4.0 (Media Cybernetics, Inc.). Subsequently,
histograms were produced.
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for data
analysis. Experimental data are presented as the mean ± SD and
experiments were perfromed in triplicate. All the data were first
analyzed for normal distribution and variance homogeneity. The
statistical comparisons between more than three groups were
performed using one-way ANOVA followed by Tukey's post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
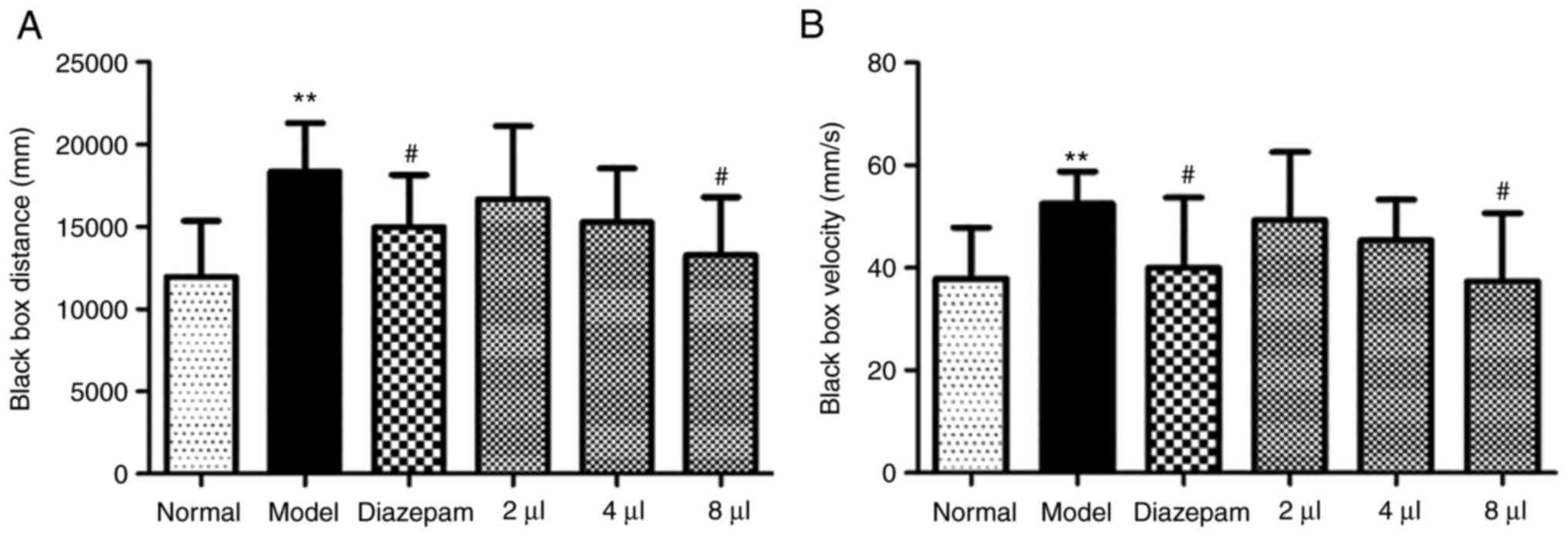
Effects of AEO on the LDET in
mCPP-induced anxious mice
The distance and velocity of the animals were
markedly enhanced in the model group compared with the control
group in the dark box (P<0.01), which suggested that the anxiety
model was successful. However, AEO significantly shortened the
distance travelled in a dose-dependent manner (P<0.05 in the
8-µl group) (Fig. 1A) and decreased
the velocity (P<0.05 in the 8-µl group) (Fig. 1B). This indicated that AEO
inhalation potentially exerted an antianxiety effect. These results
indicated that the effect of the high dose of AEO inhalation was
the same as that of the diazepam.
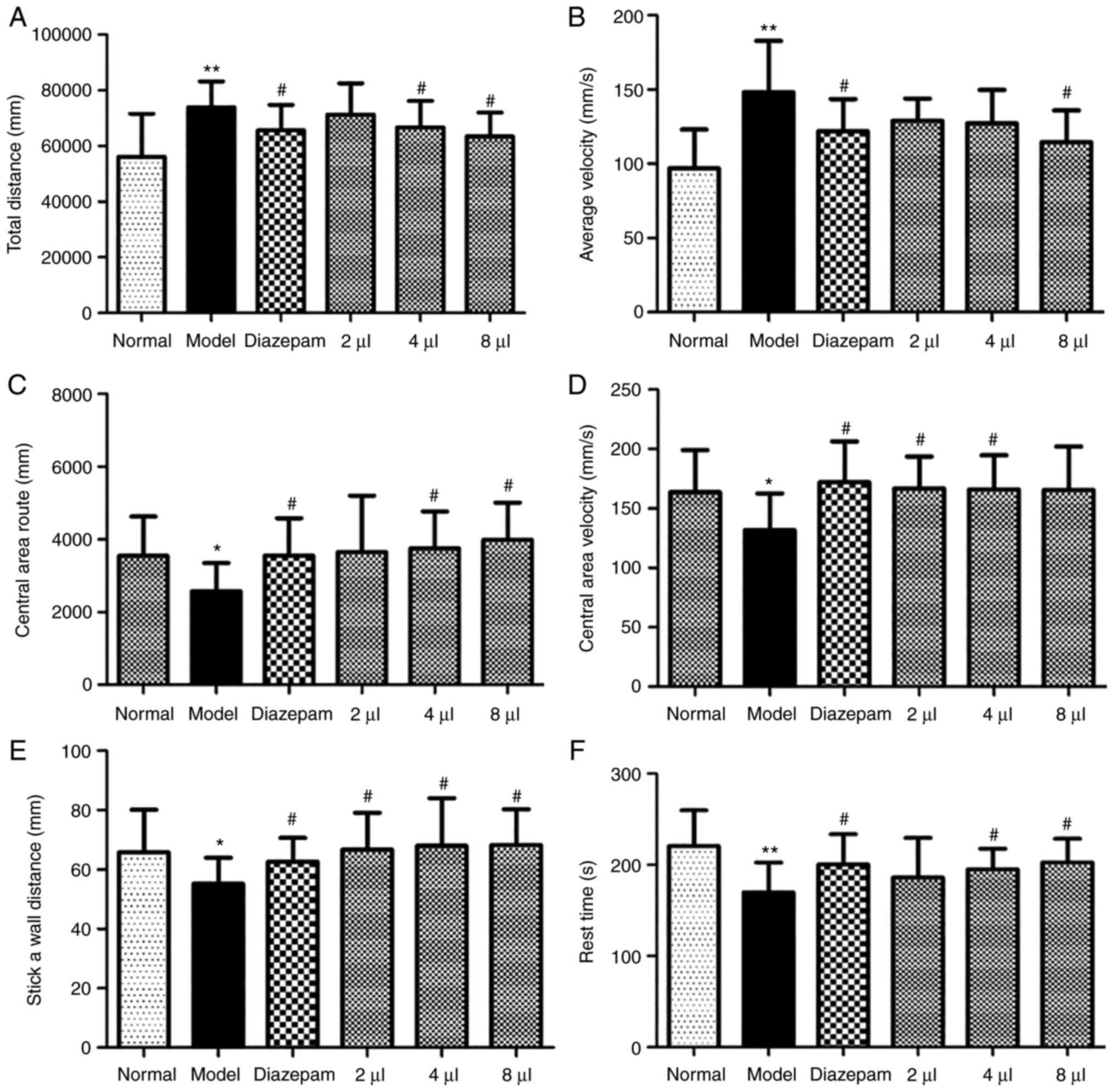
Effects of AEO on the OFT in
mCPP-induced anxious mice
The distance and velocity were significantly
enhanced in the model mice (P<0.01) compared with the control
mice, which demonstrated that the mice exhibited symptoms of
anxiety. The results further demonstrated that AEO markedly
shortened these distance increases in a dose-dependent manner
(Fig. 2A). Furthermore, following
treatment with AEO the velocity of the animals was decreased
(Fig. 2B) and increases were
observed in the central area route (Fig. 2C), central area velocity (Fig. 2D), stick-a-wall distance (Fig. 2E) and rest time (Fig. 2F) (P<0.05). These data indicated
that AEO inhalation potentially resulted in improved antianxiety
effects. Moreover, the effect of the high dose of AEO inhalation
was the same as that of the positive drug.
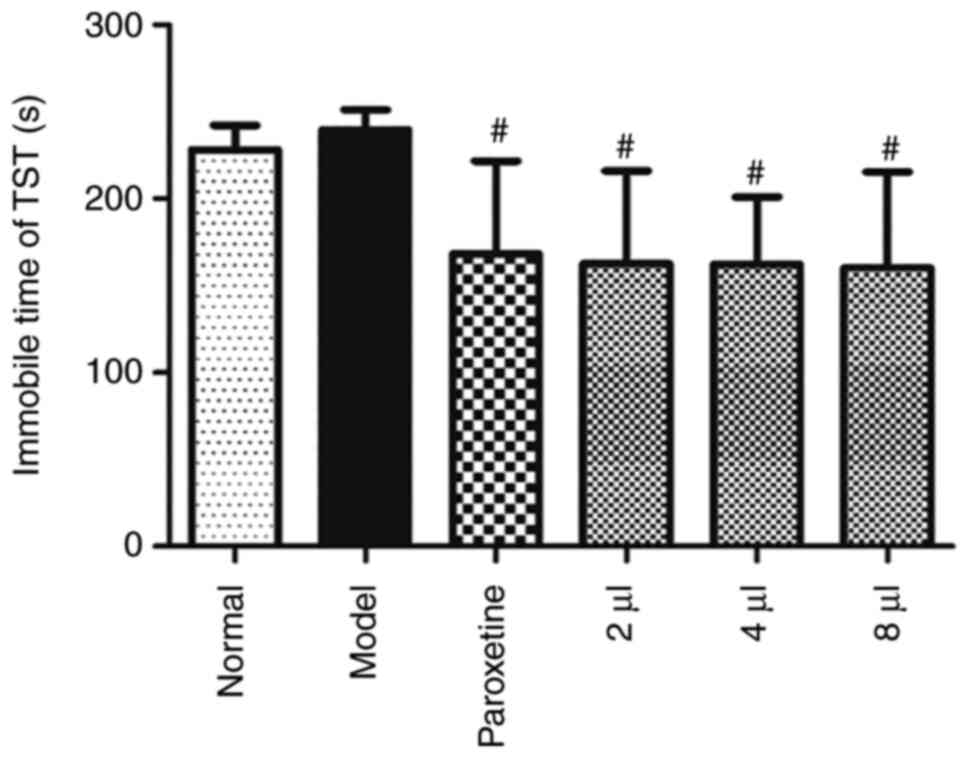
Effects of AEO on the TST in
CUMS-induced depressed mice
The immobility time of TST was prolonged in the
model mice, which indicated that the mice exhibited depression-like
symptoms. AEO treatment markedly shortened the immobility time
(Fig. 3; P<0.05), which
indicated that AEO treatment potentially exerted an improved
antidepressant effect.
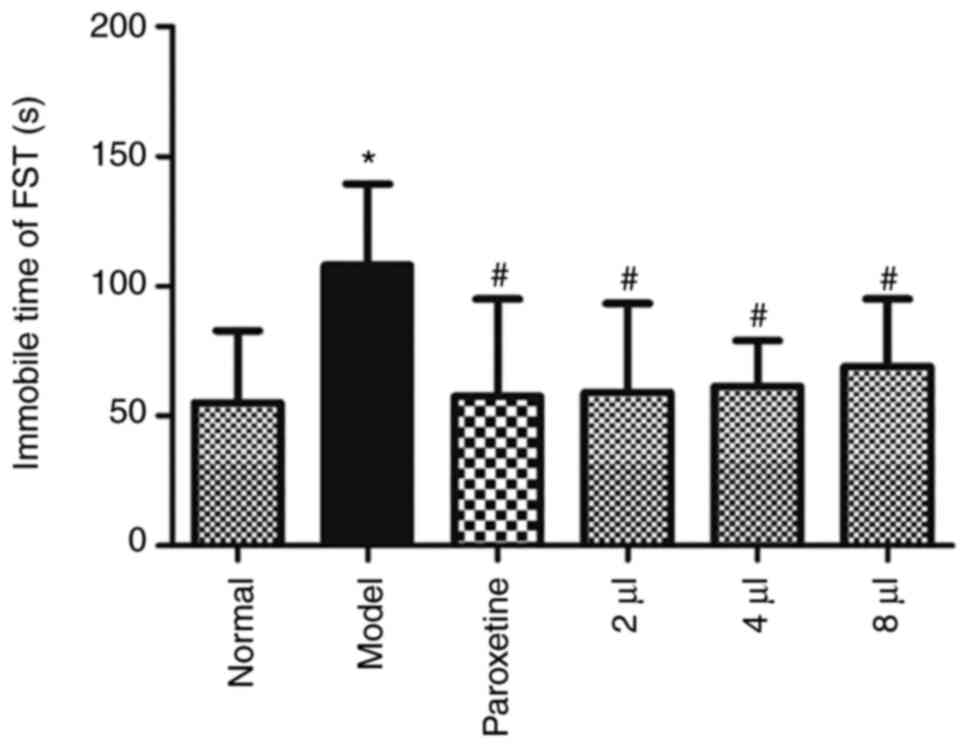
Effects of AEO on the FST in
CUMS-induced depressant mice
The immobility time in the FST was significantly
prolonged in the model mice (P<0.05), which indicated that the
animals were exhibiting depression-like symptoms. AEO significantly
reduced the immobility time (Fig.
4) (P<0.05), which indicated that AEO administration
potentially exerted an antidepressant effect.
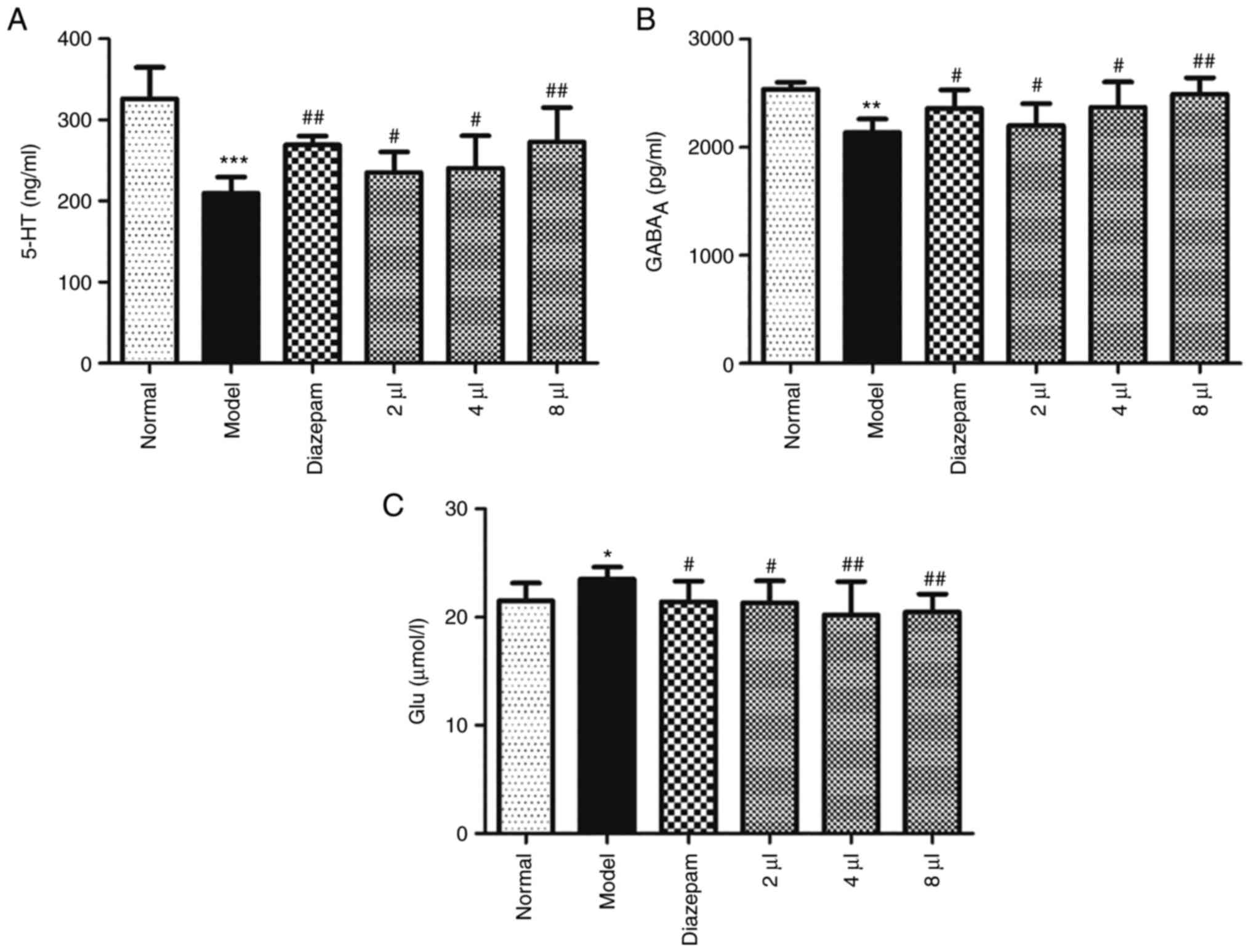
Effects of AEO on the 5-HT,
GABAA and Glu levels in mCPP-induced anxious mice
The levels of 5-HT, GABAA and Glu were
assessed in mCPP-induced anxious mice and the results revealed that
the level of 5-HT was significantly reduced (P<0.001), the level
of GABAA was also reduced (P<0.01) and the level of
Glu was enhanced (P<0.05) in the model mice. AEO treatment
significantly increased the levels of 5-HT and GABAA and
decreased the levels of Glu in a dose-dependent manner (Fig. 5A-C; P<0.05 or P<0.01). These
results indicated that AEO potentially serves a role in regulating
neurotransmitter levels.
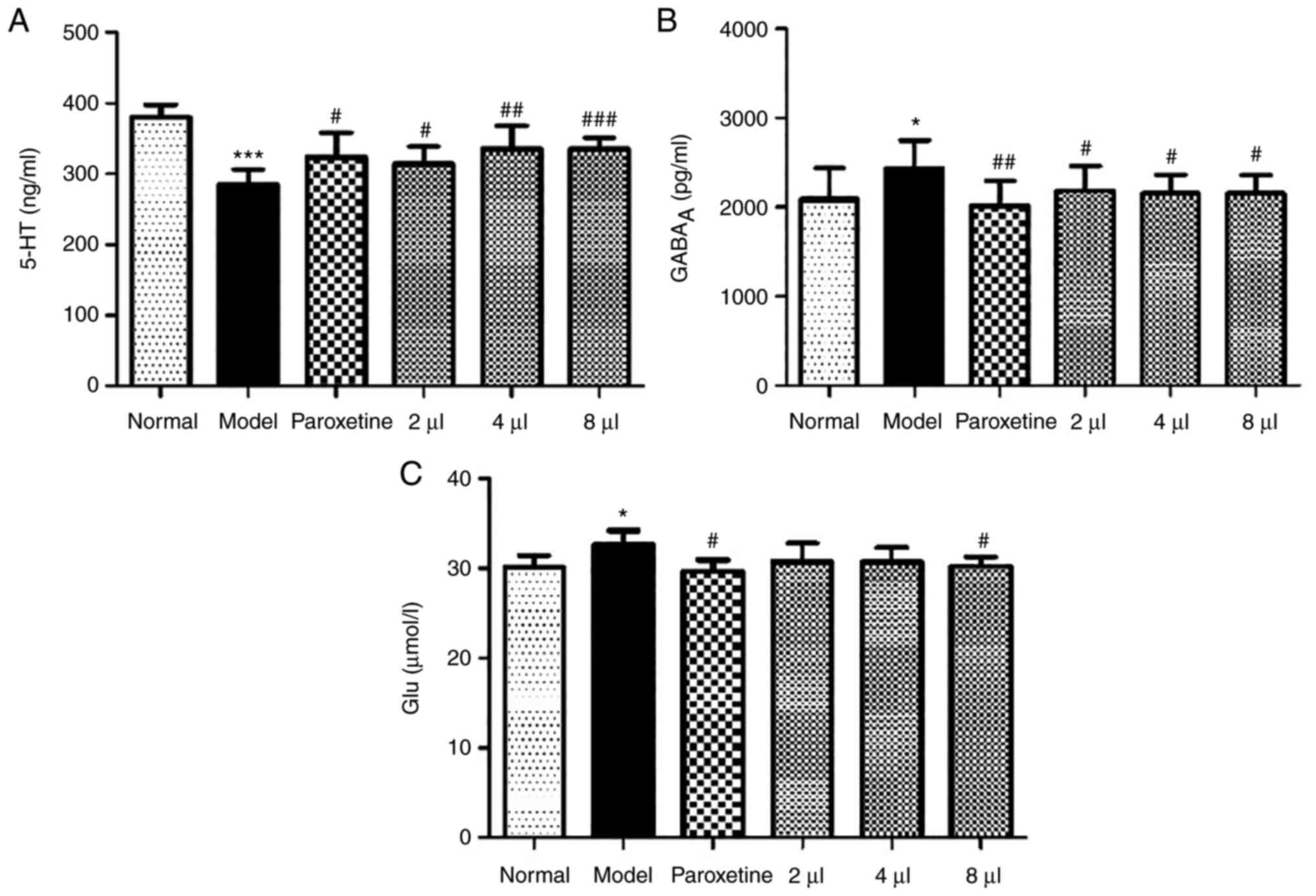
Effects of AEO on the 5-HT,
GABAA and Glu levels in CUMS-induced depressant
mice
The levels of 5-HT, GABAA and Glu were
assessed in CUMS-induced depressant mice and the results revealed
that the levels of 5-HT were significantly reduced (P<0.001) and
the levels of GABAA and Glu were enhanced (P<0.05) in
model mice. AEO significantly increased the levels of 5-HT and
decreased the levels of GABAA and Glu (Fig. 6A-C; P<0.05 or P<0.01).
Furthermore, the effect of AEO treatment was the same as that of
treatment with paroxetine.
Effects of AEO on protein expression
levels in mCPP-induced anxious mice
The protein expression levels of GABAT, GRM5, GluR1
and VGluT1 were assessed in mCPP-induced anxious mice and the
results revealed that AEO inhalation significantly upregulated the
protein expression levels of GABAT, GRM5, GluR1 and VGluT1
(P<0.05, P<0.01 and P<0.001). Furthermore, the results
demonstrated that AEO treatment potentially served a role in
relieving anxiety via regulating the protein expression levels and
transport of GABA and Glu, and therefore the balance of the
Glu/GABA system (Fig. 7).
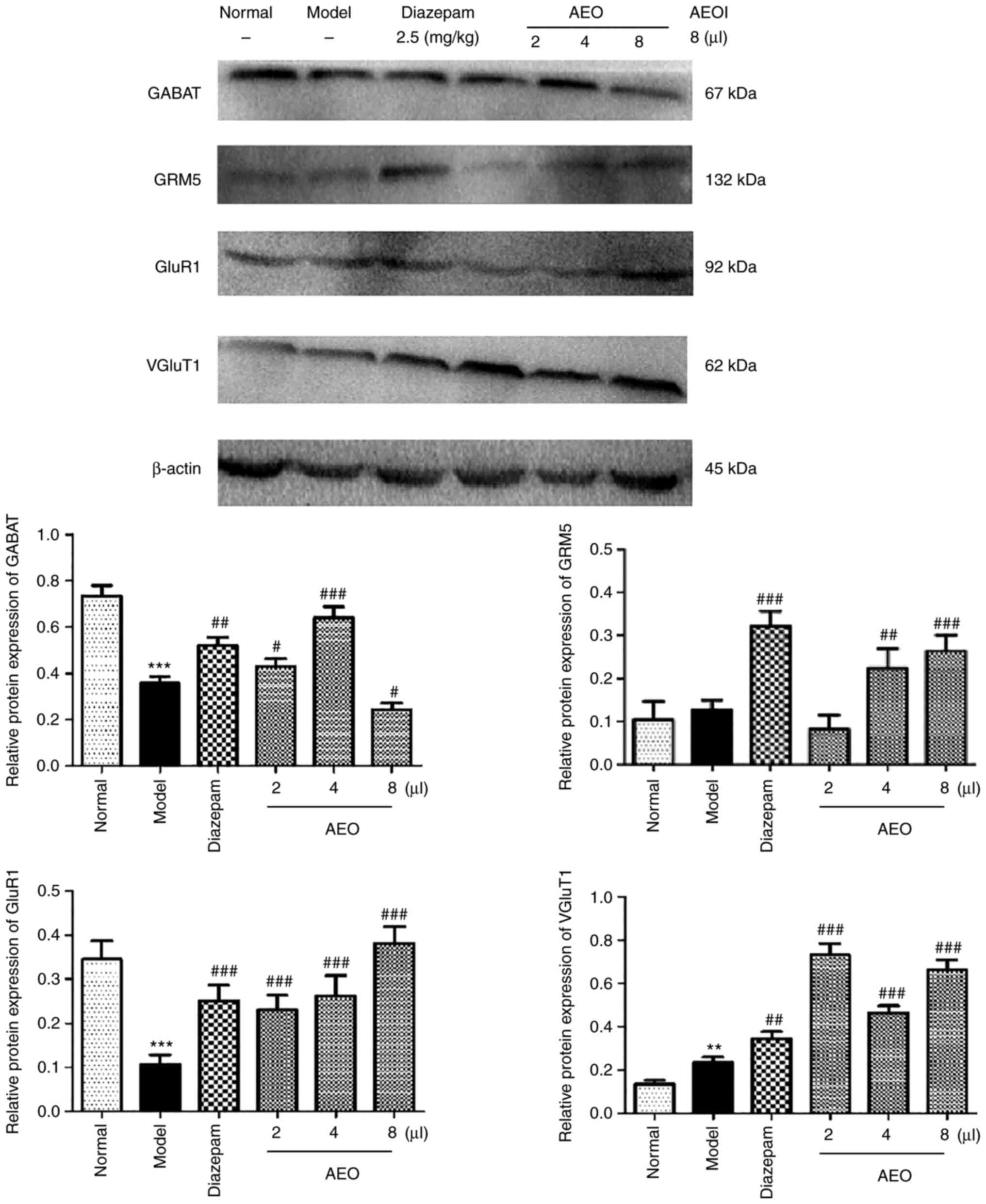 | Figure 7Effects of inhalation of agarwood
essential oil on the protein levels of GABAT, GRM5, GluR1 and
VGluT1 in M-chlorophenylpiperazine-induced anxious mice. The
protein levels of GABAT, GRM5, GluR1 and VGluT1 were assessed using
western blotting. All values are expressed as the means ± SD (n=3).
**P<0.01 and ***P<0.001 vs. the normal
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the model group. GABAT, GABA
transaminase; GRM5, Glu metabotropic receptor 5; GluR1, Glu
ionotropic receptor AMPA type subunit 1; VGluT1, vesicular Glu
transporter 1; AEOI, agarwood essential oil inhalation. |
Effects of AEO on protein expression
levels in CUMS-induced depressant mice
The protein expression levels of GABAT, GRIN2B,
GRM5, GluR1 and VGluT1 were assessed in CUMS-induced depressant
mice and the results revealed that AEO inhalation markedly
decreased the protein expression levels of GABAT, GRIN2B and GRM5,
and increased the protein expression levels of GluR1 and VGluT1.
The results demonstrated that AEO treatment potentially served an
antidepressant role via the regulation of the protein expression
levels of GABA and Glu and the balance of the Glu/GABA system
(Fig. 8).
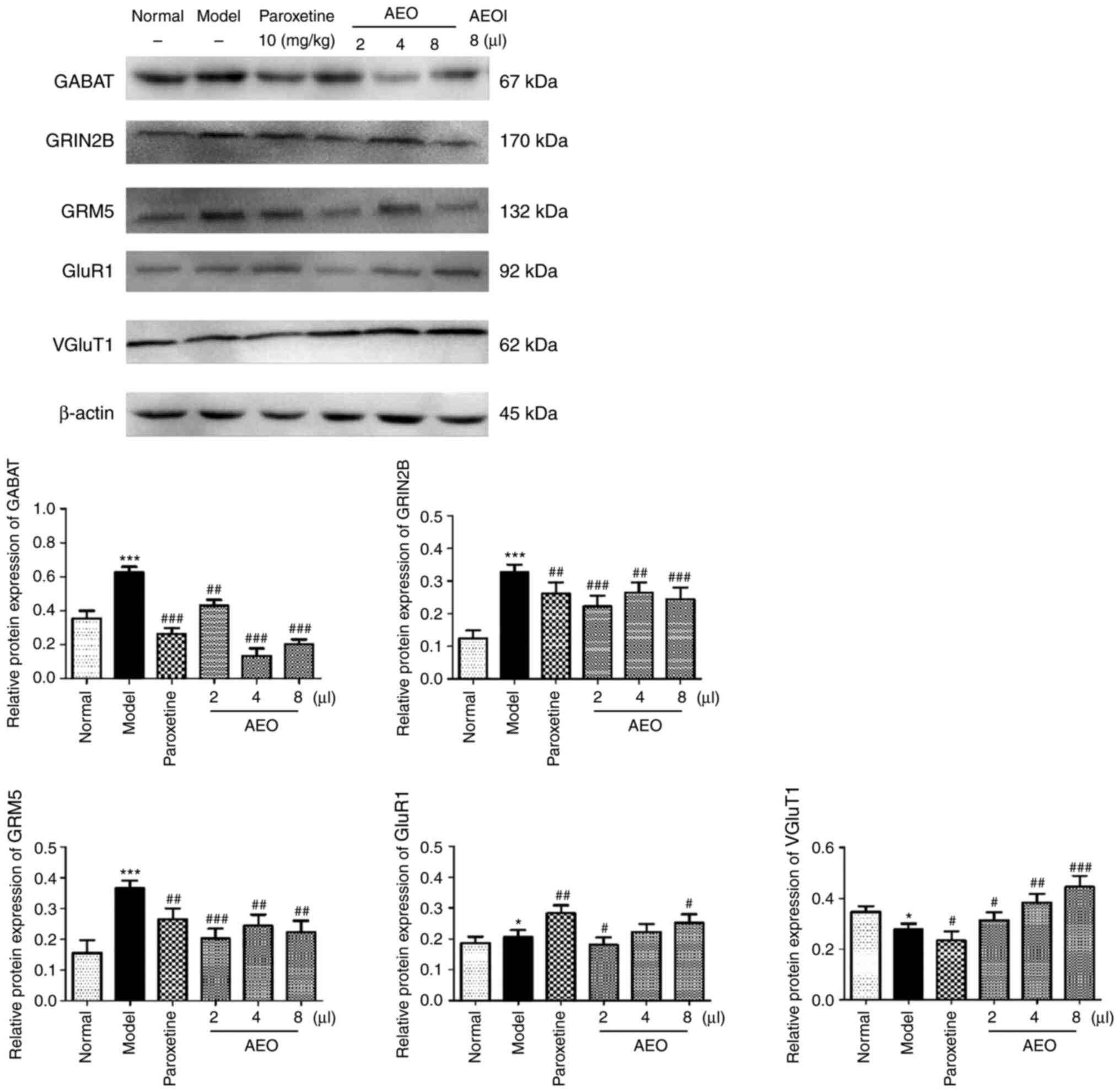 | Figure 8Effects of inhalation of agarwood
essential oil on the protein levels of GABAT, GRIN2B, GRM5, GluR1
and VGluT1 in chronic unpredictable mild stress-induced depressant
mice. The protein levels of GABAT, GRM5, GluR1 and VGluT1 were
assessed using western blotting. All values are expressed as the
means ± SD (n=3). *P<0.05 and
***P<0.001 vs. the normal group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the model group. GABAT, GABA
transaminase; GRIN2B, glutamate receptor N2B subunit; GRM5, Glu
metabotropic receptor 5; GluR1, Glu ionotropic receptor AMPA type
subunit 1; VGluT1, vesicular Glu transporter 1; AEOI, agarwood
essential oil inhalation. |
Discussion
The present study suggested that AEO displays
anxiolytic and antidepressant effects via balancing the E/I Glu and
GABA neurotransmissions in anxious and depressant mice. Behavioral
evaluation further confirmed that AEO not only significantly
inhibited the anxiety of mice as assessed by LDET and OFT, but also
exerted a significant antidepressant effect by decreasing the
immobility time of mice as determined using TST and FST. In
summary, the results indicated that AEO may represent a potential
treatment of anxiety and depression by regulating the homeostasis
of the Glu-GABA system.
Spontaneous locomotor activity tests, including the
LDET and OFT, are used to generate general parameters to study the
effects of a drug (29). A decrease
in locomotor activity indicates that the drug exerts an inhibitory
effect on the central nervous system (CNS) (30). The results of a previous study by
the authors demonstrated that AEO exerted significant antianxiety
and antidepression effects via inhibition of the HPA axis (19). It was also determined that AEO
exerted sleep-promoting effects via increasing GABAA
receptor function (20), indicating
that AEO exerted antidepressant or anxiolytic effects. In the
present study, it was confirmed that AEO exerted anxiolytic and
antidepressant effects by inhibiting the locomotor activity as
assessed using LDET and OFT, and prolonging the immobility time as
determined using TST and FST, which indicated that AEO may prevent
and treat neurological disorder diseases by regulating both E/I
bidirectional effects.
The abnormal secretion of neurotransmitters is
important in the pathogenesis of anxiety, depression and comorbid
disorders (31). The GABA
counterbalance with Glu serves an important role in anxiety and
depression (32). Glu is the main
excitatory neurotransmitter in the CNS and is a precursor of GABA,
whose role in the pathogenesis of anxiety and depression remains
unclear. Previous studies have reported that increases in Glu
levels are closely associated with anxiety in the clinic, which
suggests that glutamatergic neurons serve an important role in the
etiology of anxiety and depression (33,34).
In the present study, AEO increased the levels of 5-HT and
GABAA and decreased the levels of Glu in anxiety-induced
mice, and decreased the levels Glu and GABAA in
depression-induced mice. These results suggested that AEO
potentially affected the two most important amino acids with regard
to anxiety, depression and other neurological disorders, which was
consistent with a previous study (35).
The hippocampus may play a critical role in the
pathophysiology and treatment of anxiety and depression. Its
functions correspond to those altered in anxiety and depression
(36-38).
The hippocampus, involved in the regulation of multiple
neurotransmitter systems, including GABA, Glu, 5-HT, DA and NE, is
a common target of antianxiety and antidepressant treatments
(20,39-41).
The balance of E/I synaptic transmission is an important factor in
regulating normal physiological functions of the CNS and in
regulating neurological disorders. Alterations of Glu, GABA and
their corresponding receptors could reflect the balance of mental
processing. Enhanced excitatory transmission and reduced inhibitory
transmission was revealed to result in anxiety-like behaviors
(42,43). In addition, increased and decreased
GABA may result in depression-like behaviors (44). In previous studies it was reported
that the level of vesicular glutamate transporter 1 (VGluT1) was
decreased in the CUMS model, and ketamine injection alleviated this
abnormality (45,46). The N-methyl-D-aspartate
receptor actively induced anxiety through its antagonists (47). Concurrently, the GABAA
receptor, as the main inhibitory neurotransmitter of the CNS,
mainly modulates the behavioral responses deriving from stressful
conditions (48). Neurotransmitters
and their receptors play different roles in various neurological
disorders. In the present study, the expression levels of GABA and
Glu receptors and transporters including GABAT, GRIN2B, GRM5, GluR1
and VGluT1 were assessed. AEO inhalation increased the expression
of GABAT and GRM5 in anxiety-induced mice, but decreased the levels
of GABAT, GRIN2B and GRM5 in depression-induced mice. Concurrently,
AEO also increased the levels of GluR1 and VGluT1 in anxiety- and
depression-induced mice. These results suggested that AEO played an
antianxiety and antidepressant role by regulating excitatory Glu
and inhibitory GABA (E/I) neurotransmitter secretion balance.
In conclusion, the present study demonstrated that
AEO incense exerted potential antianxiety and antidepressant
effects via the regulation of their receptors and via the GABA/Glu
system. These effects were similar to those of treatment with
diazepam and paroxetine. The inhalation of AEO resulted in two-way
effects in regulating the balance of the GABA/Glu system, which
suggested that AEO could serve as a potential therapeutic candidate
aiding in the treatment of anxiety, depression and CNS diseases.
Furthermore, the present study may also provide a theoretical basis
for the development and utilization of agarwood. However, the
specific underlying molecular mechanism of AEO needs to be further
explored.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Research
Project of Hainan Province (grant nos. ZDYF2022SHFZ030 and
ZDYF2020111), the National Natural Science Foundation of China
(grant no. 82204657), the National Key R&D Program of China
(grant no. 2018YFC1706403), the High-level Talents Program of
Hainan Province (grant no. 2019RC345), and the CAMS Innovation Fund
for Medical Sciences (CIFMS; grant no. 2021-I2M-1-032).
Availability of data and materials
The datasets used during the present study are
available from the first author or corresponding author upon
reasonable request.
Authors' contributions
CW and JW designed the study. CW, BG and YW
performed the experiments. YL and DC extracted the AEO, and
assisted with the experiments. CW contributed to the preparation of
the manuscript. CW and JW revised the manuscript. CW and BG
analyzed the data and confirm the authenticity of all the raw data.
All authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal care and experimental protocol was
approved by the Institutional Animal Care and Use Ethics Committee
of Hainan Institute of Materia Medica Co., Ltd., Haikou, China
(approval no. 2021HL014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization. depression and
other common mental disorders: Global health estimates. World
Health Organization, Geneva, 2017. https://doi.org/CC
BY-NC-SA 3.0 IGO.
|
|
2
|
Guze SB and Robins E: Suicide and primary
affective disorders. Br J Psychiatry. 117:437–438. 1970.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duman RS and Aghajanian GK: Synaptic
dysfunction in depression: Potential therapeutic targets. Science.
338:68–72. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anisman H and Matheson K: Stress,
depression, and anhedonia: Caveats concerning animal models.
Neurosci Biobehav Rev. 29:525–546. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grillo L: A possible role of anhedonia as
common substrate for depression and anxiety. Dep Res Treat.
2016(1598130)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miller BR and Hen R: The current state of
the neurogenic theory of depression and anxiety. Curr Opin
Neurobiol. 30:51–58. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu W, Wang L, Yang L, Li YJ, Wang M, Qiu
C, Yang Q, Li XB, Huang YL, Liu R and Wu YM: Activation of LXRβ
signaling in the amygdala confers anxiolytic effects through
rebalancing excitatory and inhibitory neurotransmission upon acute
stress. Neurotherapeutics. 17:1253–1270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Murrough JW, Abdallah CG and Mathew SJ:
Targeting glutamate signalling in depression: Progress and
prospects. Nat Rev Drug Discov. 16:472–486. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie W, Cai L, Yu Y, Gao L, Xiao L, He Q,
Ren Z and Liu Y: Activation of brain indoleamine 2,3-dioxygenase
contributes to epilepsy-associated depressive-like behavior in rats
with chronic temporal lobe epilepsy. J Neuroinflammation.
11(41)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chaves EMC, Honório-Júnior JER, Sousa CNS,
Monteiro VS, Nonato DTT, Dantas LP, Lúcio ASSC, Barbosa-Filho JM,
Patrocínio MCA, Viana GSB and Vasconcelos SMM: The anxiolytic-like
effect of 6-styryl-2-pyrone in mice involves GABAergic mechanism of
action. Metab Brain Dis. 33:139–149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pham TH, Defaix C, Nguyen TML,
Mendez-David I, Tritschler L, David DJ and Gardier AM: Cortical and
raphe GABAA, AMPA receptors and glial GLT-1 glutamate
transporter contribute to the sustained antidepressant activity of
ketamine. Pharmcol Biochem Beha. 192(172913)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun H, Guan L, Zhu Z and Li H: Reduced
levels of NR1 and NR2A with depression-like behavior in different
brain regions in prenatally stressed juvenile offspring. PLoS One.
8(e81775)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gasull-Camós JL, Martínez-Torres SL,
Tarrés-Gatius M, Ozaita A, Artigas F and Castañé A: Serotonergic
mechanisms involved in antidepressant-like responses evoked by
GLT-1 blockade in rat infralimbic cortex. Neuropharmacology.
139:41–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
CITES: Amendments to appendices I and II
of the convention - adopted by the Conference of the Parties at its
13th meeting, Bangkok Thailand, 2-14 October, 2004.
|
|
15
|
Hashim YZ, Kerr PG, Abbas P and Mohd
Salleh H: Aquilaria spp. (agarwood) as source of health beneficial
compounds: A review of traditional use, phytochemistry and
pharmacology. J Ethnopharmacol. 189:331–360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Korinek M, Wagh VD, Lo IW, Hsu YM, Hsu HY,
Hwang TL, Wu YC, Cheng YB, Chen BH and Chang FR: Antiallergic
phorbol ester from the seeds of Aquilaria malaccensis. Int J
Mol Sci. 17(398)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. 1st edition.
Chinese Medical Science and Technology Press, Beijing, pp185-186,
2015.
|
|
18
|
Dahham SS, Tabana YM, Iqbal MA, Ahamed
MBK, Ezzat MO, Majid ASA and Majid AMSA: The anticancer,
antioxidant and antimicrobial properties of the sesquiterpene
β-caryophyllene from the essential oil of Aquilaria crassna.
Molecules. 20:11808–11829. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang S, Wang C, Peng D, Liu X, Wu C, Guo P
and Wei J: Agarwood essential oil displays sedative-hypnotic effect
through GABAergic system. Molecules. 22(2190)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang S, Wang C, Yu Z, Wu C, Peng D, Liu X,
Liu Y, Yang Y, Guo P and Wei J: Agarwood essential oil ameliorates
restrain stress-induced anxiety and depression by inhibiting HPA
axis hyperactivity. Int J Mol Sci. 19(3468)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bahrani H, Mohamad J, Paydar MJ and Rothan
HA: Isolation and characterisation of acetylcholinesterase
inhibitors from Aquilaria subintegra for the treatment of
Alzheimer's disease (AD). Curr Alzheimer Res. 11:206–214.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huo HX, Zhu ZX, Pang DR, Li YT, Huang Z,
Shi SP, Zheng J, Zhang Q, Zhao YF, Tu PF and Li J:
Anti-neuroinflammatory sesquiterpenes from Chinese eaglewood.
Fitoterapia. 106:115–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang L, Qiao L, Xie D, Yuan Y, Chen N, Dai
J and Guo S: 2-(2-phenylethyl)chromones from Chinese eaglewood.
Phytochemistry. 76:92–97. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takemoto H, Ito M, Shiraki T, Yagura T and
Honda G: Sedative effects of vapor inhalation of agarwood oil and
spikenard extract and identification of their active components. J
Nat Med. 62:41–46. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yi Z, Wang W and Zhang J: Effects of novel
anxiolytic 4-butyl-alpha-agarofuran on levels of monoamine
neurotransmitters in rats. Eur J Pharmacol. 504:39–44.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo JY, Wang WJ, Fang HJ, Yin DL, Sun SJ,
Liu RW and Li C: Agarofuan derivatives, their preparation,
pharmaceutical composition containing them and their use as
medicine. US Patent 6, 486, 201. Filed November 19, 1999; issued
November 26, 2002.
|
|
27
|
Yang L, Qiao L, Ji C, Xie D, Gong NB, Lu
Y, Zhang J, Dai J and Guo S: Antidepressant abietane diterpenoids
from Chinese eaglewood. J Nat Prod. 76:216–222. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu Y, Chen H, Yang Y, Zhang Z, Wei J,
Meng H, Chen W, Feng J, Gan B, Chen X, et al: Whole-tree
agarwood-inducing technique: an efficient novel technique for
producing high-quality agarwood in cultivated Aquilaria sinensis
trees. Molecules. 18:3086–3106. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Treit D and Fundytus M: Thigmotaxis as a
test for anxiolytic activity in rats. Pharmacol Biochem Behav.
31:959–962. 1988.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mezzomo NJ, Silveira A, Giuliani GS,
Quadros VA and Rosemberg DB: The role of taurine on anxiety-like
behaviors in zebrafish: A comparative study using the novel tank
and the light-dark tasks. Neurosci Lett. 613:19–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xie W, Meng X, Zhai Y, Zhou P, Ye T, Wang
Z, Sun G and Sun X: Panax notoginseng saponins: A review of its
mechanisms of antidepressant or anxiolytic effects and network
analysis on phytochemistry and pharmacology. Molecules.
23(940)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wisłowska-Stanek A, Lehner M, Skórzewska
A, Krząścik P, Maciejak P, Szyndler J, Ziemba A and Płaźnik A:
Changes in the brain expression of alpha-2 subunits of the GABA-A
receptor after chronic restraint stress in low- and high-anxiety
rats. Behav Brain Res. 253:337–345. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Modi S, Rana P, Kaur P, Rani N and Khushu
S: Glutamate level in anterior cingulate predicts anxiety in
healthy humans: A magnetic resonance spectroscopy study. Psychiatry
Res. 224:34–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mathews DC, Henter ID and Zarate CA:
Targeting the glutamatergic system to treat major depressive
disorder: Rationale and progress to date. Drugs. 72:1313–1333.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nuss P: Anxiety disorders and GABA
neurotransmission: A disturbance of modulation. Neuropsychiatr Dis
Treat. 11:165–175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen S, Huang X, Zeng XJ, Sieghart W and
Tietz EI: Benzodiazepine-mediated regulation of alpha1, alpha2,
beta1-3 and gamma2 GABA(A) receptor subunit proteins in the rat
brain hippocampus and cortex. Neurosciences. 93:33–44.
1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dremencov E, Gur E, Lerer B and Newman ME:
Effects of chronic antidepressants and electroconvulsive shock on
serotonergic neurotransmission in the rat hippocampus. Prog
Neuropsychopharmacol Biol Psychiatry. 27:729–739. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tóth K: Glutamatergic neurotransmission in
the hippocampus. Springer, New York, 2010.
|
|
39
|
Mokler D, Morgane PJ, Luebke J and Galler
JR: Serotonin release and modulation of GABA neurotransmission in
the hippocampus of rats exposed to prenatal protein malnutrition.
Serotonin: From the Molecule to the ClinicSerotonin: From the
Molecule to the Clinic, 2000.
|
|
40
|
Pinheiro AC, da Silva AJ, Prado MA,
Cordeiro Mdo N, Richardson M, Batista MC, de Castro Junior CJ,
Massensini AR, Guatimosim C, Romano-Silva MA, et al:
Phoneutria spider toxins block ischemia-induced glutamate
release, neuronal death, and loss of neurotransmission in
hippocampus. Hippocampus. 19:1123–1129. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ferreira-Junior NC, Lagatta DC and Resstel
LBM: Glutamatergic, GABAergic, and endocannabinoid
neurotransmissions within the dorsal hippocampus modulate the
cardiac baroreflex function in rats. Pflugers Arch. 470:395–411.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Stefanits H, Milenkovic I, Mahr N,
Pataraia E, Hainfellner JA, Kovacs GG, Sieghart W, Yilmazer-Hanke D
and Czech T: GABAA receptor subunits in the human
amygdala and hippocampus: Immunohistochemical distribution of 7
subunits. J Comp Neurol. 526:324–348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Guerriero RM, Giza CC and Rotenberg A:
Glutamate and GABA imbalance following traumatic brain injury. Curr
Neurol Neurosci Rep. 15(27)2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Deidda G, Bozarth IF and Cancedda L:
Modulation of GABAergic transmission in development and
neurodevelopmental disorders: Investigating physiology and
pathology to gain therapeutic perspectives. Front Cell Neurosci.
8(119)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yu H, Li M, Zhou D, Lv D, Liao Q, Lou Z,
Shen M, Wang Z, Li M, Xiao X, et al: Vesicular glutamate
transporter 1 (VGLUT1)-mediated glutamate release and membrane
GluA1 activation is involved in the rapid antidepressant-like
effects of scopolamine in mice. Neuropharmacology. 131:209–222.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zink M, Vollmayr B, Gebicke-Haerter PJ and
Henn FA: Reduced expression of glutamate transporters vGluT1, EAAT2
and EAAT4 in learned helpless rats, an animal model of depression.
Neuropharmacology. 58:465–473. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bi LL, Wang J, Luo ZY, Chen SP, Geng F,
Chen YH, Li SJ, Yuan CH, Lin S and Gao TM: Enhanced excitability in
the infralimbic cortex produces anxiety-like behaviors.
Neuropharmacology. 72:148–156. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Poulter MO, Du L, Zhurov V, Merali Z and
Anisman H: Plasticity of the GABA(A) receptor subunit cassette in
response to stressors in reactive versus resilient mice.
Neuroscience. 165:1039–1051. 2010.PubMed/NCBI View Article : Google Scholar
|