Introduction
Stroke is a serious global health problem and the
second leading cause of disability worldwide (1,2). An
estimated 80-87% of cases are ischemic (3). Over the past few decades, it has been
established that inflammatory reaction has a vital
pathophysiological role in the development of ischemic stroke
(4,5). In the acute phase, inflammation may
aggravate secondary brain damage through activation of glial cells,
upregulation of pro-inflammatory cytokines and disruption of the
blood-brain barrier (6). This
suggests, to a certain extent, that inflammation may be a target
for the prevention of secondary strokes (7).
Plasma C-reactive protein (CRP) is predominantly
synthesized by the liver after tissue damage and is a sensitive
marker of inflammation (8).
According to previous research, CRP-based inflammatory markers were
associated with various inflammatory conditions, such as diabetic
nephropathy (9), subacute
thyroiditis (10) and hepatitis
(11). As stroke is also associated
with inflammation, it is worthwhile studying CRP in subjects who
have suffered a stroke. Although the association between CRP and
clinical outcomes in patients with stroke has been under intense
investigation, it has remained inconclusive. Certain studies
suggested that CRP was related to post-stroke functional outcome
(12-15),
whereas other studies reported no significant association (8,16,17).
In addition, factors that may be related to CRP levels in patients
with acute ischemic stroke have rarely been explored.
In the present study, the association between plasma
CRP levels and the functional outcomes of patients with acute
ischemic stroke were investigated and relevant factors that may
influence CRP expression levels were analyzed.
Subjects and methods
Patients
The study included 218 consecutive patients with
acute ischemic stroke who were hospitalized and treated at the
affiliated hospital of Soochow University (Suzhou, China) within 24
h after the onset of symptoms between June 2019 and January 2020.
Acute ischemic stroke was defined as a sudden global or focal
neurological deficit persisting for >24 h, which occurred owing
to global or focal brain dysfunction as confirmed by computed
tomography and/or magnetic resonance imaging at first presentation
(18). Patients with hemorrhagic
stroke, stroke mimics (such as hypoglycemia or complicated
migraine), transient ischemic attack, a history of recent infection
within 1 month prior to the stroke, infection as a secondary
complication of stroke within 24 h after admission, history of
surgery or trauma within 3 months, statin use, corticosteroids or
anti-inflammatory drug use prior to hospitalization were excluded
from the present study. Infection, such as pneumonia, urinary tract
infections or pressure sore, was diagnosed by chest X-ray, routine
urine analysis or comprehensive physical examination. All
participants were given standardized antithrombotic treatment
following the 2018 China Guidelines for the Diagnosis and Treatment
of Acute Ischemic Stroke (19).
Written informed consent was obtained from the patients or their
relatives. The present study was approved by the ethics committee
of the Affiliated Hospital of Soochow University (Suzhou, China;
no. 2018104).
Stroke-related clinical data
The patients' data, including age, sex, stroke
severity evaluated by the initial National Institutes of Health
Stroke Scale (NIHSS) score, past medical history (hypertension,
diabetes, coronary heart disease, atrial fibrillation, smoking and
alcohol consumption), the laboratory data (low-density lipoprotein,
high-density lipoprotein, triglycerides, total cholesterol, CRP and
fasting glucose), were retrospectively reviewed. Venous fasting
blood samples were obtained from each patient on the morning after
admission. The level of CRP was measured by immunoturbidimetry
assay (Olympus Au2700 automatic biochemical analyzer; Orion
Diagnostics Oy). Hypertension was defined based on history of high
blood pressure and/or two or more recordings exceeding 140/90 mmHg
during the hospital stay. Type 2 diabetes was defined based on any
history of diabetes and/or a fasting glucose level of ≥7.0 mmol/l
or random blood glucose ≥11 mmol/l measured at >2 different
time-points during the hospital stay. Atrial fibrillation was
defined when at least one electrocardiogram indicated the presence
of atrial fibrillation during the hospital stay or if a history of
atrial fibrillation was present. Smoking was defined as smoking of
at least one cigarette a day for at least six months prior to
hospitalization. Alcohol consumption was defined as drinking
alcohol more than once a week on average during the past year.
Functional outcome measurement
Trained neurologists determined the modified Rankin
Scale (mRS) scores [ranging from 0 (no symptoms at all) to 6
(death)] to evaluate functional outcomes after 3 months. The mRS
score was usually recorded in the outpatient department or via a
standardized telephone follow-up interview if the former was not
feasible. A poor functional outcome was defined as an mRS score
>2 at 3 months after stroke (20).
Statistical analysis
The Kolmogorov-Smirnov test was used to analyze
whether the data for the study variables followed a normal
distribution. Continuous variables conforming to a normal
distribution are expressed as the mean ± standard deviation (SD)
and continuous variables with a non-normal distribution as the
median [interquartile range (IQR)]. Categorical variables are
expressed as n (%). Differences between the two groups were
examined using χ2, t-tests and Mann-Whitney U-tests as
appropriate. Furthermore, multivariate logistic regression models
were used to determine factors independently associated with
clinical outcome at 3 months. Odds ratios (OR) and 95% confidence
intervals (CI) were calculated to estimate the association between
CRP levels and functional outcome. Receiver operating
characteristic (ROC) curve analysis and Youden's J statistic were
used to determine the best cut-off value of CRP for predicting poor
outcome. A Spearman correlation analysis was used to explore the
correlation between the stroke-related variables and CRP levels.
All tests were two-sided and P<0.05 was considered to indicate
statistical significance. All statistical analyses were performed
using SPSS for Windows (version 23.0; IBM Corporation).
Results
Baseline characteristics
The clinical characteristics, laboratory data and
outcomes of the patients were summarized in Table I. Of the 218 patients, 133 (61.0%)
were male and 85 (39.0%) were female. The mean age was 66.7±12.3
years. The median NIHSS score on admission was 10.0 (IQR,
6.0-14.0). The median CRP level was 3.7 (IQR, 1.7-10.6) mg/l. The
3-month mRS scores were obtained by the outpatient department
follow-up for 48 (22.0%) patients and standardized telephone
follow-up interview for 170 (78.0%) patients. In total, 85 (39.0%)
patients had a poor functional outcome and 35 (16.1%) died at 3
months after their acute ischemic stroke (data not shown).
 | Table IResults of the univariate analysis of
factors affecting the 3-month functional outcome (modified ranking
scale at the third month after stroke) of patients with acute
ischemic stroke. |
Table I
Results of the univariate analysis of
factors affecting the 3-month functional outcome (modified ranking
scale at the third month after stroke) of patients with acute
ischemic stroke.
| Variable | All patients
(n=218) | Poor outcome
(n=85) | Favorable outcome
(n=133) | P-value |
|---|
| Age, years | 66.7±12.3 | 70.0±12.4 | 64.7±11.9 | 0.002 |
| Male sex | 133 (61.0) | 57 (67.1) | 76 (57.1) | 0.143 |
| Initial NIHSS
score | 10 (6-14) | 16 (12-20) | 8 (5-9) | <0.001 |
| Past medical
history | | | | |
|
Hypertension | 149 (68.3) | 54 (70.0) | 95 (71.4) | 0.221 |
|
Diabetes | 37 (17.0) | 16 (16.7) | 21 (17.0) | 0.560 |
|
Coronary
heart disease | 68 (31.2) | 26 (30.6) | 42 (31.6) | 0.878 |
|
Atrial
fibrillation | 33 (15.1) | 9 (10.6) | 24 (18.0) | 0.134 |
|
Smoking | 59 (27.0) | 20 (23.5) | 39 (29.3) | 0.348 |
|
Alcohol
consumption | 39 (17.9) | 11 (12.9) | 28 (21.1) | 0.127 |
| Laboratory
results | | | | |
|
Low-density
lipoprotein, mg/dl | 2.5 (2.1-3.3) | 2.5 (2.1-3.3) | 2.5 (2.1-3.2) | 0.939 |
|
High-density
lipoprotein, mg/dl | 1.0 (0.9-1.2) | 1.0 (0.9-1.3) | 1.0 (0.9-1.2) | 0.549 |
|
Triglycerides,
mg/dl | 1.3 (1.0-2.0) | 1.3 (0.9-1.8) | 1.4 (1.0-2.1) | 0.245 |
|
Total
cholesterol, mg/dl | 4.3 (3.4-5.0) | 4.3 (3.6-5.1) | 4.2 (3.4-5.0) | 0.817 |
|
CRP,
mg/l | 3.7 (1.7-10.6) | 10.7
(5.1-13.6) | 1.9 (1.0-4.8) | <0.001 |
|
Fasting
glucose, mmol/l | 5.6 (5.0-7.0) | 6.4 (5.5-7.9) | 5.3 (4.9-6.4) | <0.001 |
|
Telephone
follow-up | 170 (78.0) | 62 (72.9) | 108 (81.2) | 0.375 |
In the univariate analysis, patients with poor
functional outcomes were more likely to have an older age (mean,
70.0 vs. 64.7 years; P=0.002), higher NIHSS score (median, 16.0 vs.
8.0; P<0.001), higher level of fasting glucose (median, 6.4 vs.
5.3 mmol/l; P<0.001) and higher levels of CRP (median, 10.7 vs.
1.9 mg/l; P<0.001) compared to patients with good functional
outcomes (Table I).
Multivariate model for poor
outcome
After adjusting for age and fasting blood glucose,
multivariate logistic regression analysis indicated that the CRP
level (OR=1.146, 95%CI: 1.012-1.297, P=0.031; Table II) and NIHSS score (OR=2.056,
95%CI: 1.615-2.619, P<0.001; Table
II) were independently associated with a poor outcome at 3
months after the stroke.
 | Table IIResults of the multivariate logistic
regression analysis of factors affecting 3-month functional outcome
of patients with acute ischemic stroke. |
Table II
Results of the multivariate logistic
regression analysis of factors affecting 3-month functional outcome
of patients with acute ischemic stroke.
| Variable | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age | 1.025 | 0.977-1.076 | 0.310 |
| Initial NIHSS
score | 2.056 | 1.615-2.619 | <0.001 |
| CRP | 1.146 | 1.012-1.297 | 0.031 |
| Fasting
glucose | 1.116 | 0.851-1.463 | 0.428 |
Predictive value of CRP level for poor
outcome
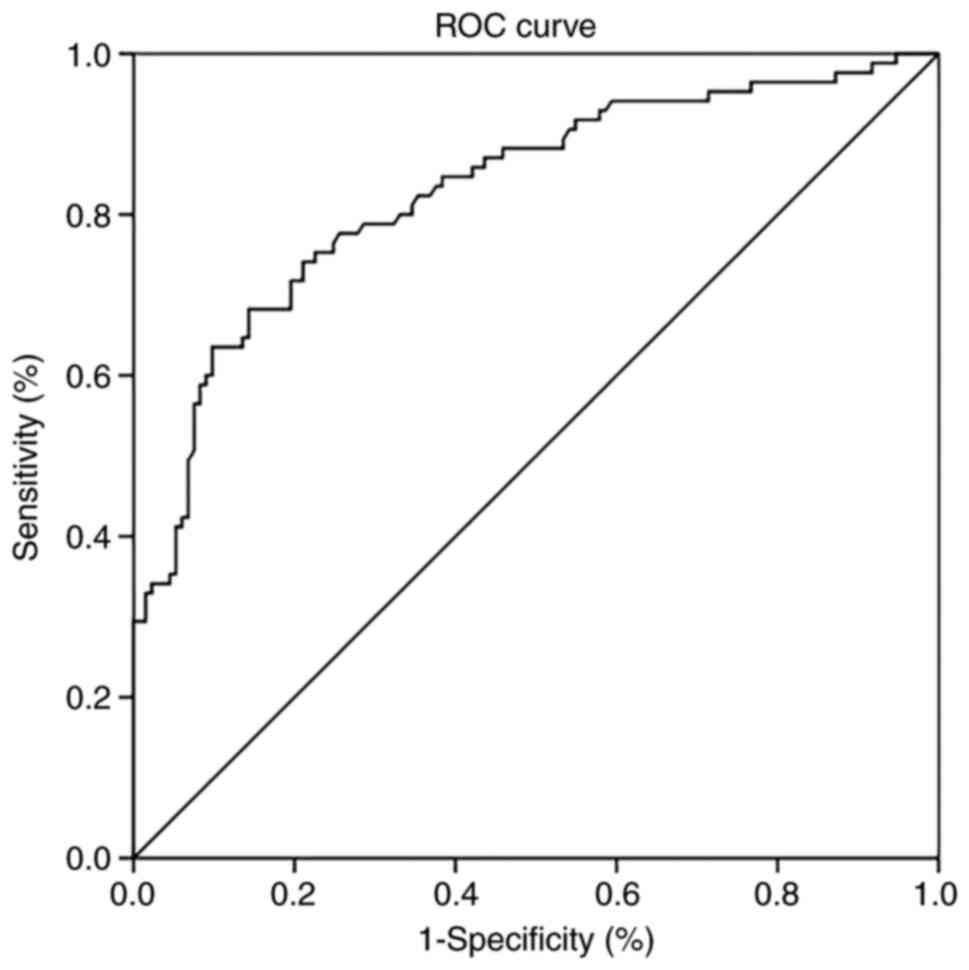
The ROC curve analysis indicated that the area under
the ROC curve to distinguish favorable from poor outcome at 3
months was 0.829 (95% CI: 0.772-0.887, P<0.001). When the Youden
index value was the highest (0.539), the optimal cut-off value of
CRP found from the ROC curve was 6.34 mg/l, with 68.2% sensitivity
and 85.7% specificity (Fig. 1).
Baseline characteristics and relevant
factors associated with different CRP levels
According to the optimal cut-off value of CRP, the
patients were divided into two groups. Subsequently, χ2,
t-tests or Mann-Whitney U-tests were performed as appropriate to
explore stroke-related clinical data that may be related to the CRP
expression level. Univariate analysis indicated that patients with
CRP ≥6.34 mg/l were older (mean, 70.4 vs. 64.8 years; P=0.002), had
a higher baseline NIHSS score (median, 14.5 vs. 8.0; P<0.001)
and higher levels of fasting glucose (median, 6.4 vs. 5.3 mmol/l;
P<0.001; Table III).
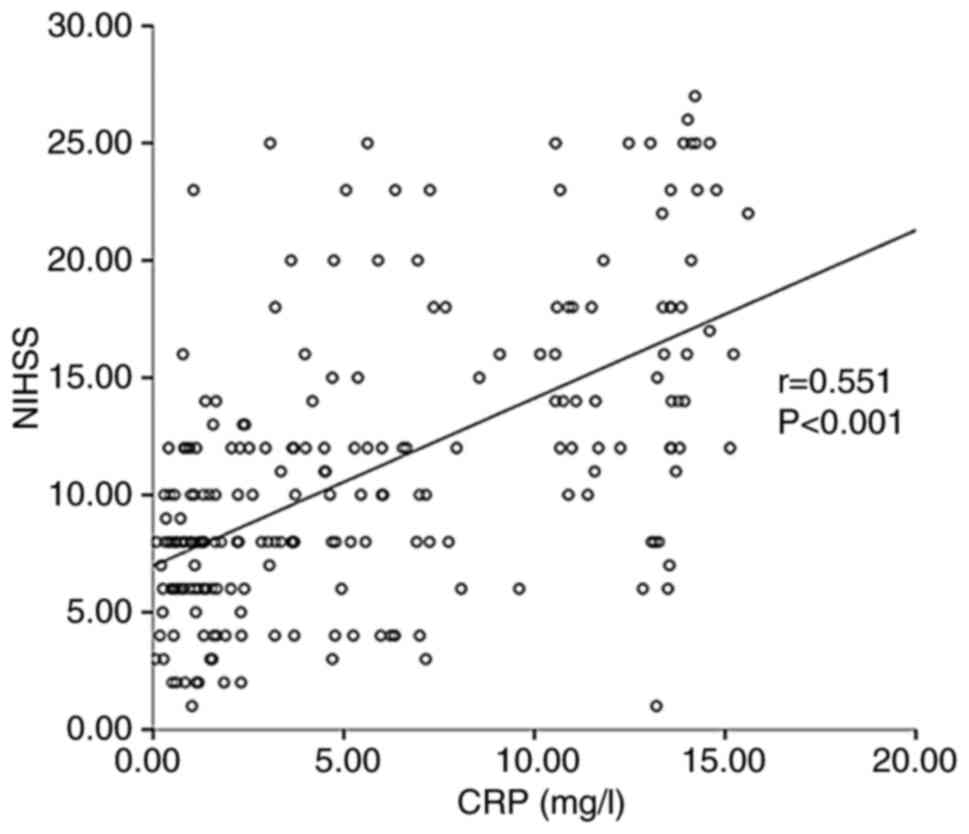
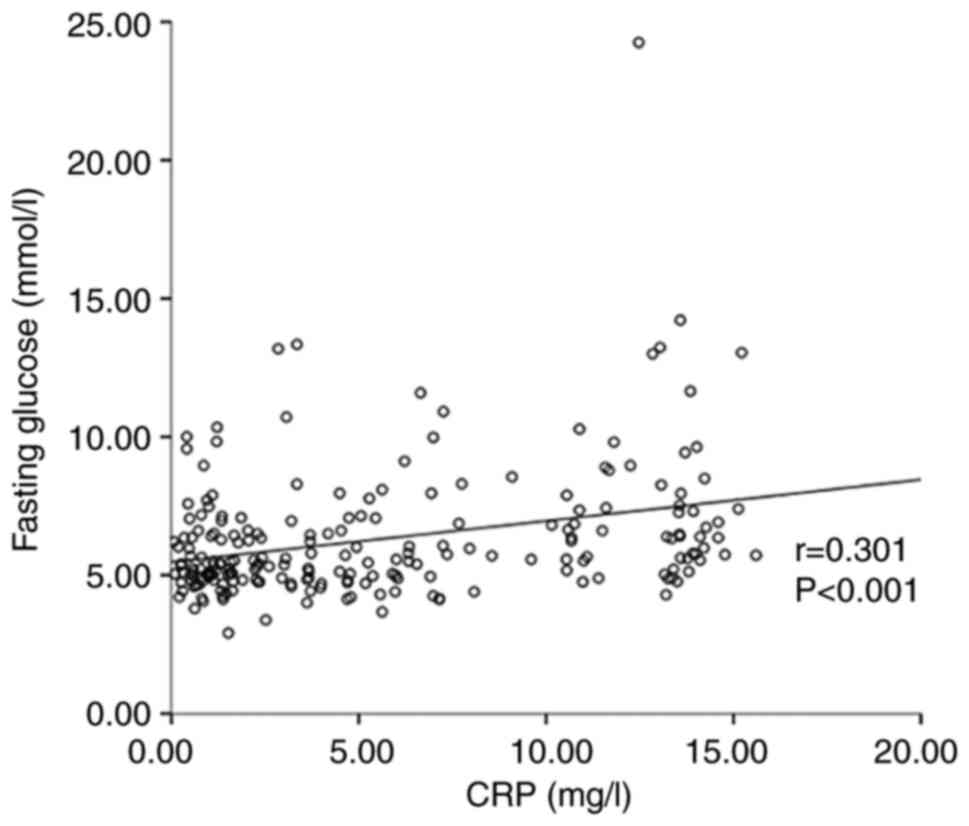
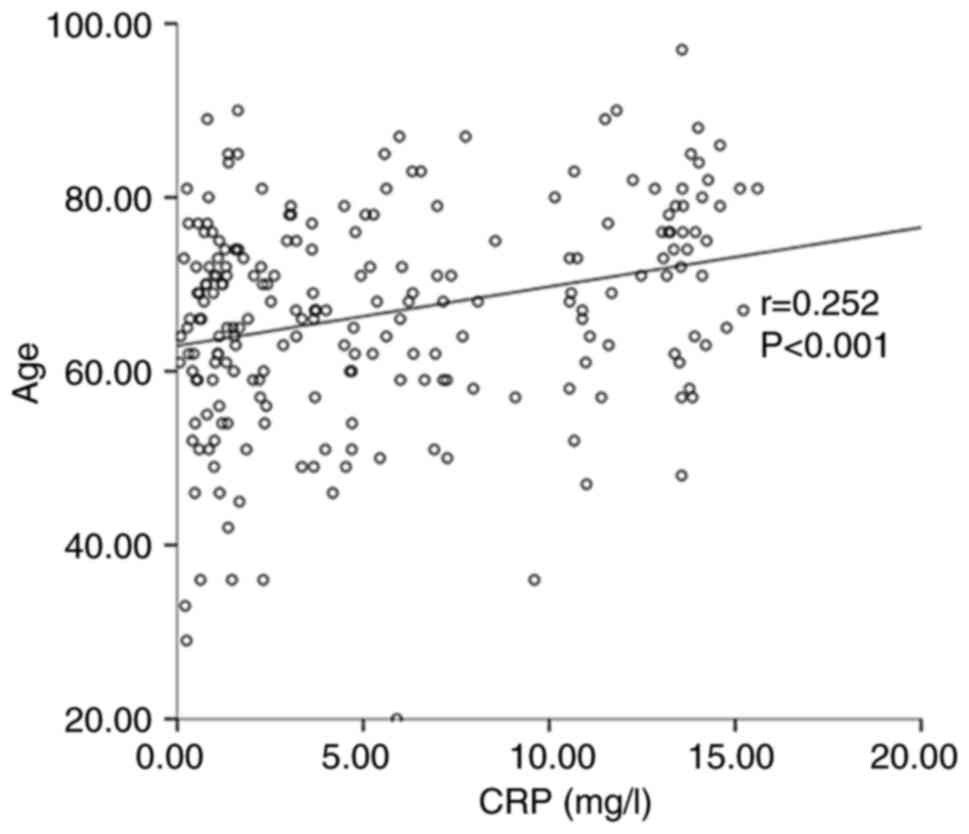
Furthermore, a Spearman correlation analysis was used to
investigate the association between stroke-related clinical data
and CRP levels. The results indicated that the CRP level was
positively related to the baseline NIHSS score (r=0.551,
P<0.001) (Fig. 2), fasting
glucose (r=0.301, P<0.001) (Fig.
3) and age (r=0.252, P<0.001) (Fig. 4).
 | Table IIIComparison of baseline
characteristics and outcome of patients with different CRP
levels. |
Table III
Comparison of baseline
characteristics and outcome of patients with different CRP
levels.
| Variable | CRP <6.34 mg/l
(n=142) | CRP ≥6.34 mg/l
(n=76) | P-value |
|---|
| Age, years | 64.8±12.2 | 70.4±11.5 | 0.002 |
| Male sex | 88 (62.0) | 45 (59.2) | 0.690 |
| Initial NIHSS
score | 8 (6-11.8) | 14.5 (11-18.5) | <0.001 |
| Past medical
history | | | |
|
Hypertension | 95 (66.9) | 54 (71.1) | 0.530 |
|
Diabetes | 24 (16.9) | 13 (17.1) | 0.970 |
|
Coronary
heart disease | 43 (30.3) | 25 (32.9) | 0.691 |
|
Atrial
fibrillation | 19 (13.4) | 14 (18.4) | 0.322 |
|
Smoking | 37 (26.1) | 22 (28.9) | 0.647 |
|
Alcohol
consumption | 22 (15.5) | 17 (22.4) | 0.207 |
| Laboratory
results | | | |
|
Low-density
lipoprotein, mg/dl | 2.5 (2.1-3.2) | 2.4 (2.0-3.3) | 0.213 |
|
High-density
lipoprotein, mg/dl | 1.0 (0.9-1.2) | 1.0 (0.9-1.3) | 0.363 |
|
Triglycerides,
mg/dl | 1.4 (1.0-2.1) | 1.2 (0.9-1.7) | 0.065 |
|
Total
cholesterol, mg/dl | 4.4 (3.7-5.0) | 3.9 (3.3-4.9) | 0.116 |
|
Fasting
glucose, mmol/l | 5.2 (4.8-6.4) | 6.4 (5.6-8.3) | <0.001 |
|
3-month mRS
>2 | 25 (17.6) | 60 (78.9) | <0.001 |
Discussion
The present study indicated that elevated CRP levels
within the first 24 h after stroke were significantly associated
with poor functional outcome 3 months after stroke In addition, CRP
levels were found to be likely correlated with stroke severity at
admission, fasting glucose levels and age.
Similar to the findings reported in most studies
(12-15),
the present data indicated that CRP levels were independently
associated with the functional outcomes of stroke. CRP is a
glycoprotein produced by the liver, which may be rapidly
upregulated by inflammatory cytokines, thereby potentiating
ischemic brain injury (21).
Furthermore, CRP may increase cerebral cell damage by promoting the
development and progression of atherosclerosis, activating the
complement system, inhibiting the fibrinolytic system and promoting
thrombosis (22). Thus, an increase
in CRP levels may reflect a greater degree of cerebral necrosis
(22). Certain studies have
indicated that CRP levels are not significantly associated with
stroke outcomes. In the studies of Topakian et al (16) and Karlinski et al (17), CRP assessed within 24 h from symptom
onset was not associated with the 3-month outcome; however, all of
their patients were treated with intravenous thrombolysis
treatment. The reason may be that early thrombolytic treatment may
reduce systemic inflammation due to inhibition of brain tissue
necrosis (23). Wang et al
(8) performed a study comprising
368 patients and a systematic review of 18 studies involving 15,238
patients. They determined that CRP was not associated with poor
outcome in patients with infection. Previous research has
demonstrated that infection may prolong patients' hospital stay and
delay recovery, thereby affecting functional outcomes (24). Furthermore, infection may
significantly increase CRP levels, weakening the effect of CRP
alone on stroke outcomes.
The present study determined that the best cutoff
value of CRP to distinguish between good and poor outcomes was 6.34
mg/l, which was higher than the 3.0 mg/l reported by Li et
al (15). This discrepancy is
likely due to the fact that in this previous study, the cohort had
mild ischemic stroke and transient ischemic attack with lower NIHSS
scores than those in the present study.
In accordance with previous studies (25,26),
the present study demonstrated that an elevated CRP level was
correlated with stroke severity at admission, although the studies
included different populations. Increased CRP levels may emphasize
enhanced cerebral ischemia or a continuous increase in inflammation
(27). There is increasing evidence
that the inflammatory mechanism after cerebral ischemia may lead to
secondary neuronal damage (25,27).
Otherwise, stroke severity is usually related to the extent of
brain tissue necrosis. When necrotic tissue is cleared by cells,
body fluids and metabolic mechanisms, a part of the inflammatory
response may be attributed to the increase in CRP (28). Therefore, it is not surprising that
elevated CRP levels are positively correlated with stroke
severity.
The present study also indicated that CRP levels
were positively correlated with fasting blood glucose. Chinsky
(29) reported that hyperglycemia
may lead to insulin resistance and impaired insulin secretion due
to inflammatory mediators and cytokines, such as interleukin-6 and
TNF-α, as well as excessive CRP release, leading to uncontrolled or
even systemic inflammatory reactions. Intensive insulin therapy by
the strict control of blood glucose levels may help reduce the
incidence of infection and systemic inflammatory response (30).
The present study indicated that CRP levels were
positively related to age. Stephan et al (31) and Woloshin and Schwartz (32) reported that younger individuals
tended to have lower CRP values due to higher homeostatic reserves
and more sports activities, which reduces systemic inflammation and
lowers CRP levels (33). This
supports the present results.
However, the present study has certain limitations.
This retrospective, single-center study had a limited sample size.
Further studies with a larger sample size across multiple centers
are required. Infarct volume and rehabilitation treatment are
important factors for stroke outcome that should be considered.
However, due to the retrospective nature of the present study,
these data were not included in the present analysis; these data
will be included in a subsequent study. CRP levels were measured
only once per patient and the exact time of collecting the blood
samples was unknown, although all blood samples were collected
within 24 h in this study. Additional studies with serial CRP
measurements and a precise blood collection time are required.
Finally, important conditions influencing CRP levels were not
excluded (e.g., tissue injury, neoplastic disease, acute coronary
syndrome and other inflammatory diseases); therefore, further
studies with strict inclusion and exclusion criteria are
warranted.
In conclusion, the present results suggest that CRP
levels were associated with functional outcomes 3 months after the
acute ischemic event; furthermore, CRP levels were related to older
age, higher baseline NIHSS score and the fasting glucose levels on
the next morning after admission.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Natural Science Research
Project of Universities of Anhui Province in China (grant no.
2022AH051244), the Health Research Program of Anhui in China (grant
no. AHWJ2022b090) and the Scientific Research Fund Project for
Talent Introduction of Yijishan Hospital in China (grant no.
YR202111).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL was involved in all aspects of research,
including study design, data analysis and revision of the
manuscript. JB and SG analyzed the data and prepared the
manuscript. TH and XL collected and analyzed general patient data.
SZ and ZC interpreted the images and evaluated the clinical data of
stroke patients. SG and ZC checked and approved the authenticity of
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Affiliated Hospital of Soochow University (Suzhou,
China; no. 2018104). All participants provided written informed
consent in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to
declare.
References
|
1
|
GBD 2019 Diseases and Injuries
Collaborators. Global burden of 369 diseases and injuries in 204
countries and territories, 1990-2019: A systematic analysis for the
Global Burden of Disease Study 2019. Lancet. 396:1204–1222.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ojaghihaghighi S, Vahdati SS, Mikaeilpour
A and Ramouz A: Comparison of neurological clinical manifestation
in patients with hemorrhagic and ischemic stroke. World J Emerg
Med. 8:34–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mu SW, Dang Y, Wang SS and Gu JJ: The role
of high mobility group box 1 protein in acute cerebrovascular
diseases. Biomed Rep. 9:191–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Godinho J, de Oliveira RMW, de
Sa-Nakanishi AB, Bacarin CC, Huzita CH, Longhini R, Mello JCP,
Nakamura CV, Previdelli IS, Dal Molin Ribeiro MH and Milani H:
Ethyl-acetate fraction of Trichilia catigua restores long-term
retrograde memory and reduces oxidative stress and inflammation
after global cerebral ischemia in rats. Behav Brain Res.
337:173–182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu X, Yuan L, Wang W, Xu J, Yang Q, Zhu Y,
Xu Y, Yang K, Ge L, Huang X and Zhou Z: Systemic inflammatory
response syndrome and outcomes in ischemic patients treated with
endovascular treatment. Clin Interv Aging. 15:2331–2340.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton
MT and Shi FD: Global brain inflammation in stroke. Lancet Neurol.
18:1058–1066. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang L, Li Y, Wang C, Guo W and Liu M:
C-reactive protein, infection, and outcome after acute ischemic
stroke: A registry and systematic review. Curr Neurovasc Res.
16:405–415. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bilgin S, Kurtkulagi O, Atak BM, Duman TT,
Kahveci G, Khalid A and Aktas G: Does C-reactive protein to serum
Albumin Ratio correlate with diabEtic nephropathy in patients with
Type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes.
15:1071–1074. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Baruah MP, Bhattacharya B and Baruah UM:
C-Reactive protein level can be a better indicator than erythrocyte
sedimentation rate in assessing the severity of inflammation and
guiding glucocorticoid therapy in subacute thyroiditis. Indian J
Endocrinol Metab. 26:328–333. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Demirkol ME, Aktas G, Bilgin S, Kahveci G,
Kurtkulagi O, Atak BM and Duman TT: C-reactive protein to
lymphocyte count ratio is a promising novel marker in hepatitis C
infection: The clear hep-c study. Rev Assoc Med Bras (1992).
68:838–841. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
VanGilder RL, Davidov DM, Stinehart KR,
Huber JD, Turner RC, Wilson KS, Haney E, Davis SM, Chantler PD,
Theeke L, et al: C-reactive protein and long-term ischemic stroke
prognosis. J Clin Neurosci. 21:547–553. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rocco A, Ringleb PA, Grittner U, Nolte CH,
Schneider A and Nagel S: Follow-up C-reactive protein level is more
strongly associated with outcome in stroke patients than admission
levels. Neurol Sci. 36:2235–2241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Geng HH, Wang XW, Fu RL, Jing MJ, Huang
LL, Zhang Q, Wang XX and Wang PX: The Relationship between
C-Reactive Protein level and discharge outcome in patients with
acute ischemic stroke. Int J Environ Res Public Health.
13(636)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li J, Zhao X, Meng X, Lin J, Liu L, Wang
C, Wang A and Wang Y and Wang Y: CHANCE Investigators.
High-Sensitive C-Reactive protein predicts recurrent stroke and
poor functional outcome: Subanalysis of the clopidogrel in
high-risk patients with acute nondisabling cerebrovascular events
trial. Stroke. 47:2025–2030. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Topakian R, Strasak AM, Nussbaumer K,
Haring HP and Aichner FT: Prognostic value of admission C-reactive
protein in stroke patients undergoing iv thrombolysis. J Neurol.
255:1190–1196. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Karlinski M, Bembenek J, Grabska K,
Kobayashi A, Baranowska A, Litwin T and Czlonkowska A: Routine
serum C-reactive protein and stroke outcome after intravenous
thrombolysis. Acta Neurol Scand. 130:305–311. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee S, Song IU, Na SH, Jeong DS and Chung
SW: Association between long-term functional outcome and change in
hs-CRP level in patients with acute ischemic stroke. Neurologist.
25:122–125. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu L, Chen W, Zhou H, Duan W, Li S, Huo
X, Xu W, Huang L, Zheng H, Liu J, et al: Chinese Stroke Association
guidelines for clinical management of cerebrovascular disorders:
Executive summary and 2019 update of clinical management of
ischaemic cerebrovascular diseases. Stroke Vasc Neurol. 5:159–176.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang XG, Xue J, Yang WH, Xu XS, Sun HX,
Hu L, Liu LY and Yue YH: Inflammatory markers as independent
predictors for stroke outcomes. Brain Behav.
11(e01922)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ye Z, Zhang H, Sun L, Cai H, Hao Y, Xu Z,
Zhang Z and Liu X: GWAS-Supported CRP gene polymorphisms and
functional outcome of large artery atherosclerotic stroke in han
Chinese. Neuromolecular Med. 20:225–232. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Abubakar SA, Okubadejo NU, Ojo OO, Oladipo
O, Ojini FI and Danesi MA: Relationship between admission serum
C-reactive protein and short term outcome following acute ischaemic
stroke at a tertiary health institution in Nigeria. Niger J Clin
Pract. 16:320–324. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ye L, Cai R, Yang M, Qian J and Hong Z:
Reduction of the systemic inflammatory induced by acute cerebral
infarction through ultra-early thrombolytic therapy. Exp Ther Med.
10:1493–1498. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Suda S, Aoki J, Shimoyama T, Suzuki K,
Sakamoto Y, Katano T, Okubo S, Nito C, Nishiyama Y, Mishina M and
Kimura K: Stroke-associated infection independently predicts
3-month poor functional outcome and mortality. J Neurol.
265:370–375. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye Z, Zhang Z, Zhang H, Hao Y, Zhang J,
Liu W, Xu G and Liu X: Prognostic Value of C-Reactive protein and
homocysteine in large-artery atherosclerotic stroke: A prospective
observational study. J Stroke Cerebrovasc Dis. 26:618–626.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Irimie CA, Varciu M, Irimie M, Ifteni PI
and Minea DI: C-Reactive Protein and T3: New prognostic factors in
acute ischemic stroke. J Stroke Cerebrovasc Dis. 27:2731–2737.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arenillas JF, Alvarez-Sabin J, Molina CA,
Chacon P, Montaner J, Rovira A, Ibarra B and Quintana M: C-reactive
protein predicts further ischemic events in first-ever transient
ischemic attack or stroke patients with intracranial large-artery
occlusive disease. Stroke. 34:2463–2468. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Audebert HJ, Rott MM, Eck T and Haberl RL:
Systemic inflammatory response depends on initial stroke severity
but is attenuated by successful thrombolysis. Stroke. 35:2128–2133.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chinsky K: The evolving paradigm of
hyperglycemia and critical illness. Chest. 126:674–676.
2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Robinson LE and van Soeren MH: Insulin
resistance and hyperglycemia in critical illness: Role of insulin
in glycemic control. AACN Clin Issues. 15:45–62. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Stephan Y, Sutin AR and Terracciano A:
Younger subjective age is associated with lower C-reactive protein
among older adults. Brain Behav Immun. 43:33–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Woloshin S and Schwartz LM: Distribution
of C-reactive protein values in the United States. N Engl J Med.
352:1611–1613. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Caudroit J, Stephan Y, Chalabaev A and Le
Scanff C: Subjective age and social-cognitive determinants of
physical activity in active older adults. J Aging Phys Act.
20:484–496. 2012.PubMed/NCBI View Article : Google Scholar
|