Introduction
The multiple endocrine neoplasia type 1 gene was
identified in 1997, and consists of 10 exons encoding a 610-amino
acid protein referred to as menin (1). Menin is predominantly localized in the
cell nucleus, although it is also found in the cytosol and membrane
(2). The distribution of menin is
gene-related, and it is associated with the two nuclear
localization signals present in its carboxy-terminal region. Menin,
as a critical tumor suppressor protein, has been proposed to play
important roles in transcriptional regulation, genome stability,
cell division, proliferation and apoptosis, and its emerging roles
in cancer development have attracted large attention (3-6).
IQ motif-containing GTPase-activating protein 1
(IQGAP1) is a scaffold protein that participates in several
cellular functions, including cell-cell adhesion (7), migration (8), transcription (9) and signal transduction (10). IQGAP1 has been implicated in the
tumorigenesis and progression of a variety of human cancer types,
including aggressive lung (11),
breast (12) and pancreatic
(9) cancer, as well as colorectal
carcinoma (13) and gastric cancer
(14).
Yan et al (15) revealed that menin reduced the
interaction of GTP-Rac1 with IQGAP1 and enhanced the intercellular
adhesion of β cells. The roles of IQGAP1 and menin in tumor
progression have been confirmed in previous research (15). However, the mechanisms by which
menin inhibits tumor occurrence, and the association of menin with
IQGAP1 remain elusive. In the present study, the expression of
menin and IQGAP1 was examined in gastric cancer tissues and cells,
and the association between these two molecules and the
proliferation of gastric cancer cells was investigated.
Materials and methods
Tissue samples
A total of 108 samples of tumor and adjacent tissue
specimens from patients with gastric cancer who were diagnosed and
received surgery at the Department of Surgery, Affiliated Hospital
of Jiangnan University (Wuxi, China) were obtained between June
2012 and July 2014. None of the patients received preoperative
chemotherapy or radiotherapy. Cases were excluded if they had been
diagnosed with previous, recurrent or metastasized cancer from
another origin. The samples were ground for protein extraction. The
corresponding non-neoplasia mucosa tissues, which were resected ≥5
cm away from the tumor margin, served as controls.
Clinicopathological data were obtained from a retrospectively
constructed medical database, which had been reviewed and confirmed
by two pathologists. The present study was approved by the Research
Ethics Committee of Jiangnan University (approval no.
JDFY20220801-1), and all patients provided written informed consent
for the use of their data in research.
Reagents and antibodies
The adenoviral vectors pAd-LacZ (encoding
β-galactosidase), pAd-IQGAP1 and pAd-Menin were kindly gifted by Dr
Yong-Chang Chen (Jiangsu University, Zhenjiang, China). Mouse
anti-menin antibody (EPR3986; product code ab92443) was obtained
from Abcam, while mouse anti-IQGAP1 (cat. no. sc-376021), mouse
anti-phosphorylated (p)-Akt (cat. no. sc-377556), mouse anti-Akt
(cat. no. sc-5298), mouse anti-NF-κB (cat. no. sc-8414) and goat
anti-β-actin antibodies (cat. no. sc-8432), as well as
short-interfering RNAs (siRNAs) for IQGAP1
(5'-AAGTTCTACGGGAAGTAATTG-3') and menin
(5'-CCACCUUUCUUGUGCAGUCCCUA-3'), and negative control siRNAs (RNA
sequence, 5'-AUGAACGUGAAUUGCUCAA-3') were purchased from Santa Cruz
Biotechnology, Inc. The horseradish peroxidase (HRP)-conjugated
secondary antibody (cat. no. KCB002) was purchased from Rockland
Immunochemicals Inc. Immunohistochemical and ECL reagents were
acquired from EMD Millipore. All other reagents were of analytical
grade.
Cell culture, transfection and plasmid
construction
The AGS cell line (cat. no. TCHu232) was purchased
from the Institute of Cell Biology of the Chinese Academy of
Sciences. The human gastric epithelial cell line GES-1 was a kind
gift from Dr Yong-Chang Chen (Jiangsu University, Zhenjiang,
China). The cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) with 10% new-born calf serum (NBCS; Lanzhou
Minhai Bio-Engineering Co., Ltd.) in an incubator with 5%
CO2 at 37˚C. The culture medium was changed every 2
days, and the cells were sub-cultured until reaching
confluence.
Adenovirus encoding menin or IQGAP1 gene at an MOI
of 100 pfu/cell was used to infect human gastric cancer AGS cells.
The expression level of target protein in AGS cells infected with
the above recombinant adenovirus was detected by western blotting.
For transfection, a complex of Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and siRNA was prepared
according to the manufacturer's instructions. Briefly, cells were
seeded to 80% confluence and infected at 37˚C for 6 h using 100
pmol siRNA. After 6 h, the siRNA/lipid complexes were removed, and
the cells were maintained in complete medium for an additional 48
h. The protein expression was then determined by western
blotting.
Western blot analysis
The target proteins were extracted and detected by
western blotting. Frozen tissue samples and cells were homogenized
in RIPA buffer (Sigma-Aldrich; Merck KGAa). The homogenates were
heated in boiling water for 5 min, and the proteins in the
supernatant were quantified by Bio-Rad Protein Assay (Bio-Rad
Laboratories, Inc.). In total, 20 µg protein extracts were
separated using 10% SDS PAGE and transferred onto a PVDF membrane.
After blocking with 10% non-fat milk in TBS with 0.1% Tween-20 for
1 h at room temperature (RT), the membrane was incubated with
specific antibodies against menin (1:500), IQGAP1 (1:1,000), p-Akt
(1:1,000), Akt (1:1,000), NF-κB (1:1,000) and β-actin (1:1,000 as a
loading control) overnight at 4˚C. Subsequently, the membrane was
incubated with the corresponding HRP-conjugated secondary
antibodies (1:1,000) for 1 h at room temperature, followed by three
washes. The proteins bands were visualized using ECL. Images were
analyzed using the imaging system (iBright™ CL750 Imaging System;
Thermo Fisher Scientific Inc.).
MTT assay
AGS cells were infected with pAd-IQGAP1 or pAd-Menin
to overexpress IQGAP1 or menin, respectively, whereas siRNAs were
transfected into AGS cells to knock down the expression of menin or
IQGAP1. The cells were serum-starved for 12 h and then trypsinized,
washed, counted and re-suspended in 96-well plates (10,000 cells in
100 µl DMEM) for various time periods (12, 24, 48 and 72 h). Cell
proliferation was measured using MTT assay. Briefly, 20 µl of MTT
dye (5 mg/ml) was added to each well, and the plate was incubated
for 4 h. Dimethylsulfoxide (DMSO; 150 µl) was added to the wells to
dissolve the formazan crystals. The absorbance value at 450 nm was
measured for each sample, and all the experiments were repeated
three times with ≥3 replicates.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Unpaired two-tailed Student's t-test followed by Bonferroni's post
hoc test were used to analyze differences among the variables.
One-way ANOVA followed by Dunnett's post hoc test was employed to
analyze differences between multiple sets of data. The association
between each independent clinicopathological variable and menin or
IQGAP1 was examined by χ2 test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of menin and IQGAP1 in
gastric cancer tissues and cell lines
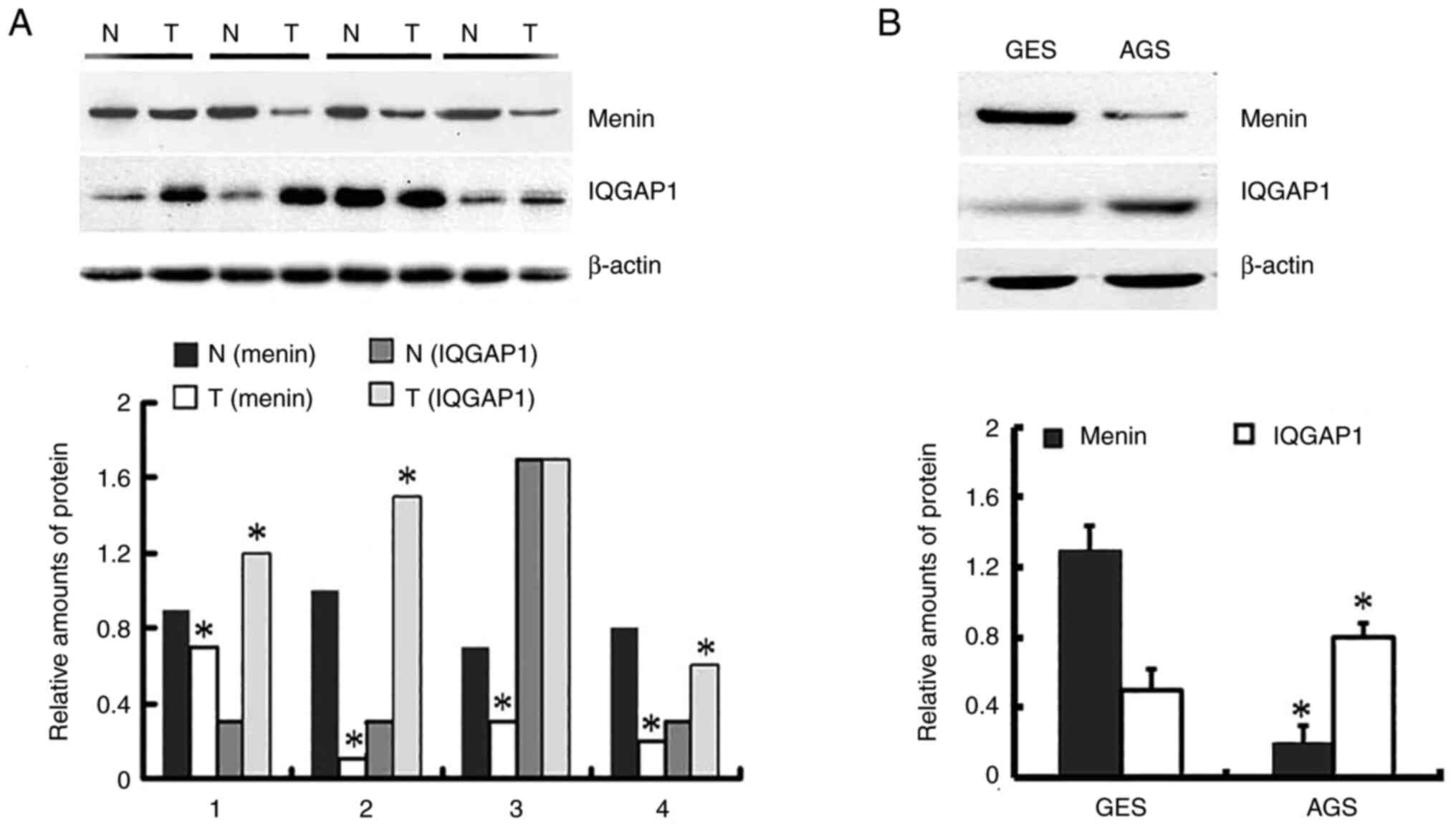
Total proteins were extracted from 108 gastric
cancer and paired normal tissues. Western blotting was used to
detect the expression of menin and IQGAP1 proteins. According to
the relative intensity of the protein bands (calculated as the
ratio of the median of the gray scale bands of target proteins to
β-actin), the samples were separated into an expression group
(≥0.467 for menin and ≥0.57 for IQGAP1) and a no-expression group
(<0.467 for menin and <0.57 for IQGAP1). The median of the
gray scale bands of menin protein/β-actin was 0.315 in 108 gastric
cancer tissues and 0.800 in paired normal tissues. The median of
the gray scale bands of IQGAP1 protein/β-actin was 0.852 in 108
gastric cancer tissues and 0.371 in paired normal tissues (Fig. S1). As shown in Fig. 1, and Table I, menin expression was significantly
lower in 23.1% (25 out of 108) gastric cancer tissues than in 76.9%
(83 out of 108) paired non-neoplastic mucosa tissues (P<0.001).
However, IQGAP1 was positively expressed in 66.7% (72 out of 108)
gastric cancer tissues and 33.3% (36 out of 108) paired normal
tissues (P<0.001). A negative association was identified between
menin and IQGAP1 expression in gastric cancer tissues.
 | Table IExpression of menin and IQGAP1 in 108
gastric cancer tissues and paired normal tissues. |
Table I
Expression of menin and IQGAP1 in 108
gastric cancer tissues and paired normal tissues.
| | Menina | | IQGAP1b | |
|---|
| Gastric tissues | No. | >0.467 | ≤0.467 | P-value | >0.57 | ≤0.57 | P-value |
|---|
| Gastric cancer
tissues | 108 | 25 | 83 | <0.001 | 72 | 36 | <0.001 |
| Paired normal
tissues | 108 | 83 | 25 | | 36 | 72 | |
The protein expression of menin in AGS cells was
significantly lower than that in GES-1 cells. However, the
expression of IQGAP1 in AGS cells was significantly higher than
that in GES-1 cells (Fig. 1B). The
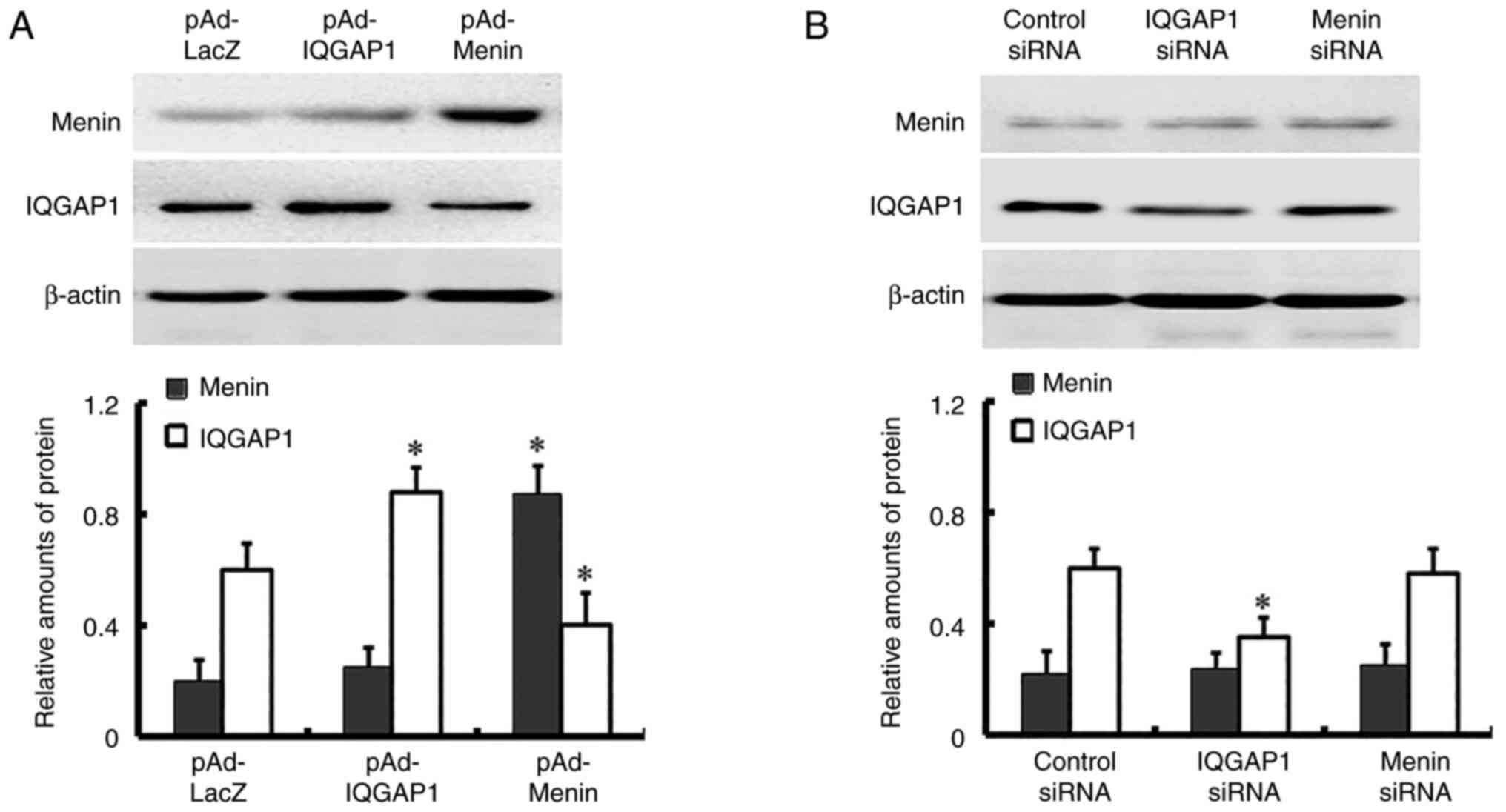
expression of menin and IQGAP1 was overexpressed in AGS cells
infected with pAd-IQGAP1 and pAd-Menin, while the expression of
IQGAP1 was knocked down in AGS cells transfected with IQGAP1 siRNAs
(Fig. 2). The expression of menin
and IQGAP1 in cells infected with pAd-IQGAP1 and pAd-Menin or
transfected with IQGAP1 siRNAs for 12, 24, 48 and 72 h was also
detected, and the expression of menin and IQGAP1 was observed to be
increased or decreased in a time-dependent manner. The expression
reached the highest or lowest level at 48 h post-infection, and
remained at a stable level thereafter; thus, 48 h was used in
subsequent experiments (Fig. 3). Of
note, the expression levels of menin were not significantly altered
over the period of time assessed in the menin siRNA groups compared
with those in the control group. (Figs.
2 and 3).
Menin and IQGAP1 expression levels are
associated with clinicopathological characteristics in 108 patients
with gastric cancer
The association between menin and IQGAP1 expression
levels and clinicopathological parameters is summarized in Table II. The results showed that menin
and IQGAP1 expression were associated with histological grade
(P=0.047 and P=0.044, respectively), distant metastasis (P=0.033
and P=0.028, respectively), lymph node (LN) metastasis (P=0.006 and
P=0.04, respectively) and TNM stage (P=0.014 and P=0.038,
respectively). However, no significant associations were observed
with patient sex (P=0.662 and P=0.561, respectively) or age
(P=0.349 and P=0.395, respectively).
 | Table IIAssociation of menin and IQGAP1
expression with clinicopathological parameters in 108 gastric
cancer patients. |
Table II
Association of menin and IQGAP1
expression with clinicopathological parameters in 108 gastric
cancer patients.
| | Menin | | IQGAP1 | |
|---|
| Clinicopathological
parameters | No. | ≥0.467 | <0.467 | P-value | ≥0.57 | <0.57 | P-value |
|---|
| Sex | | | | | | | |
|
Male | 73 | 16 | 57 | 0.662 | 50 | 23 | 0.561 |
|
Female | 35 | 9 | 26 | | 22 | 13 | |
| Age (years) | | | | | | | |
|
<60 | 39 | 11 | 28 | 0.349 | 24 | 15 | 0.395 |
|
≥60 | 69 | 14 | 55 | | 48 | 21 | |
| Histological
grade | | | | | | | |
|
WD and
MD | 59 | 18 | 41 | 0.047 | 35 | 24 | 0.044 |
|
PD | 49 | 7 | 42 | | 38 | 11 | |
| Distant
metastasis | | | | | | | |
|
Absence | 47 | 17 | 30 | 0.033 | 28 | 19 | 0.028 |
|
Presence | 61 | 11 | 50 | | 44 | 17 | |
| LN metastasis | | | | | | | |
|
Absence | 34 | 15 | 19 | 0.006 | 18 | 16 | 0.040 |
|
Presence | 74 | 14 | 60 | | 54 | 20 | |
| TNM stage | | | | | | | |
|
I and
II | 48 | 18 | 30 | 0.014 | 26 | 22 | 0.038 |
|
III and
IV | 60 | 10 | 50 | | 44 | 16 | |
Menin influences IQGAP1
expression
To determine whether the increase or decrease in one
protein would affect the expression of the other protein, menin and
IQGAP1 were overexpressed with pAd-Menin and pAd-IQGAP1,
respectively. siRNAs targeting menin and IQGAP1 were transfected
into AGS cells to knock down the expression of menin and IQGAP1.
Western blotting was used to detect the expression of menin and
IQGAP1. The results revealed that exogenous expression of menin
repressed IQGAP1 expression. However, increased or knocked down
expression of IQGAP1 did not affect the expression of menin. There
was no change in menin or IQGAP1 expression levels in the menin
siRNA group compared with those in the control group (Fig. 2).
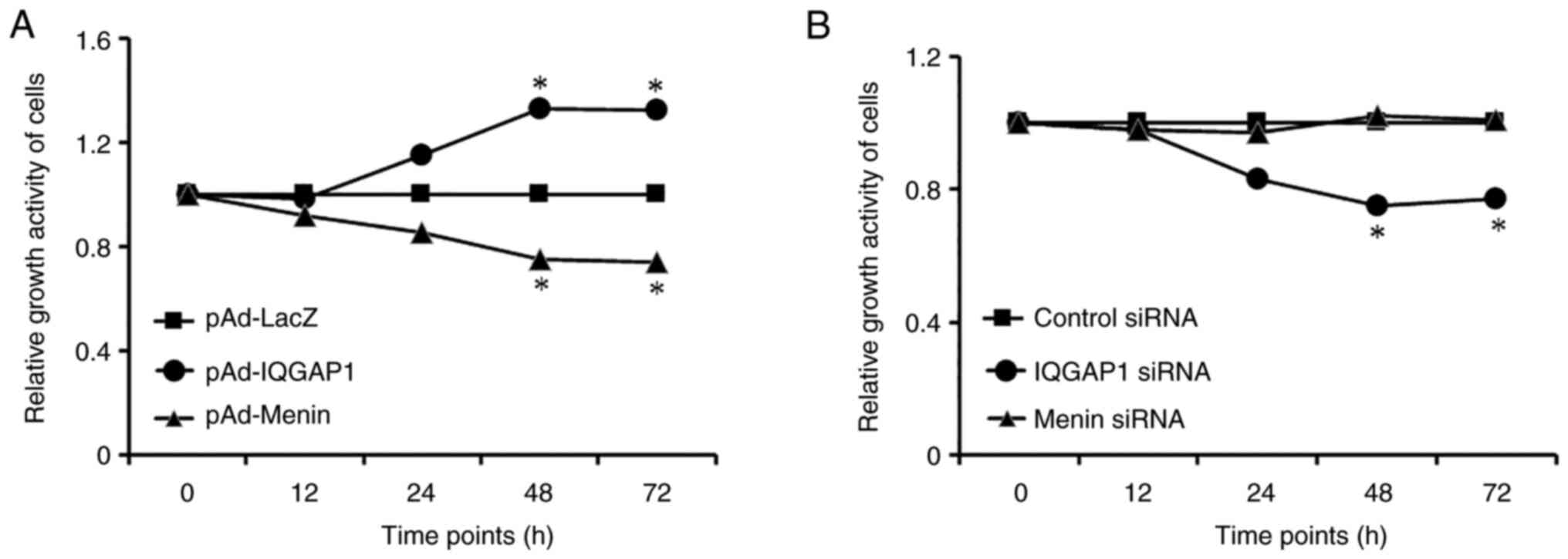
Effects of menin and IQGAP1 on the
proliferation of gastric cancer cells
The effects of menin and IQGAP1 on cell
proliferation were evaluated by MTT assay. AGS cells were
transfected with siRNAs, which interfered with the expression of
menin and IQGAP1. In addition, AGS cells were infected with an
adenovirus vector encoding IQGAP1 or menin for overexpression. The
results revealed that ectopic expression of IQGAP1 promoted,
whereas silencing of IQGAP1 expression inhibited, gastric cancer
cell proliferation. On the other hand, increased expression of
menin suppressed cell proliferation. Because menin protein is less
expressed in AGS cells, menin siRNA could further reduce menin
expression in AGS cells (Fig. 3).
The results revealed that menin suppressed, whereas IQGAP1
stimulated, the proliferation of gastric cancer cells.
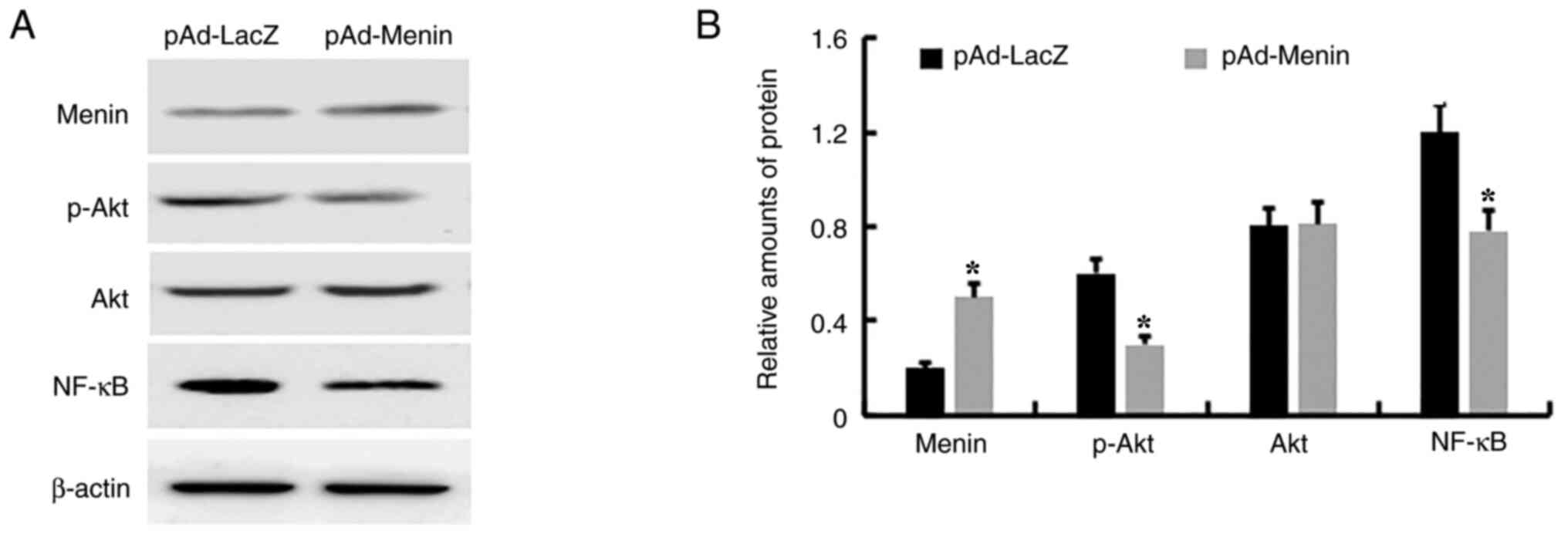
Menin inhibits the PI3K/Akt and NF-κB
signaling pathways
To explore the potential mechanism by which menin
suppressed the proliferation of gastric cancer cells, the effects
of menin on the expression of crucial proteins of the PI3K and
NF-κB signaling pathways were examined. The levels of p-Akt and
NF-κB were notably downregulated in menin-overexpressing AGS cells,
while no changes in Akt were observed (Fig. 4).
Discussion
In gastric cancer, the role of the expression of
IQGAP1 is well understood; however, less is known about the
expression of menin or the association between menin and IQGAP1 in
this cancer type. The present study analyzed the protein expression
of menin and IQGAP1 in gastric cancer tissues and AGS cell lines,
since these proteins appeared to be implicated in the development
and progression of gastric cancer according to previous studies
(2,14). The present results revealed that
menin expression was significantly lower in gastric cancer tissues
than in paired non-neoplastic mucosa tissues. However, IQGAP1 was
highly expressed in gastric cancer tissues compared with its
expression in paired non-neoplastic mucosa tissues. The protein
expression of IQGAP1 in AGS cells was significantly higher than
that in GES-1 cells. However, the expression of menin in AGS cells
was significantly lower than that in GES cells.
In the present study, various clinicopathological
factors were analyzed for their potential association with menin
and IQGAP1 protein expression in gastric cancer tissues. Low menin
expression in gastric cancer was found to be significantly
associated with poor clinicopathological factors, including poor
differentiation, distant metastasis, LN metastasis and advanced
clinicopathological stage, while high expression of IQGAP1 was
associated with these poor clinicopathological characteristics. No
significant associations of menin or IQGAP1 expression with sex or
age were observed.
Menin has been identified as a tumor suppressor in a
variety of endocrine neoplasia, including pituitary adenomas
(16) and parathyroid tumors
(17), as well as adrenal (18) and islet (19) tumors. Menin is predominantly
expressed in the cell nucleus, and may act as a scaffold protein to
regulate gene transcription through the coordination of various
chromatin-associating proteins (20). Menin also interacts with
cytoskeletal proteins such as glial fibrillary acid protein,
vimentin and IQGAP1 (15,21). A previous study confirmed the
co-localization of menin and IQGAP1 in β cell lines (15). Therefore, it could be speculated
that menin may interact with IQGAP1 to affect the proliferation of
gastric cancer cells.
To study the effects of menin and IQGAP1 on the
proliferation of gastric cancer cells, menin and IQGAP1 were
overexpressed with pAd-Menin and pAd-IQGAP1, respectively. siRNAs
targeting IQGAP1 and menin were transfected into gastric cancer
cells to knock down the expression of these proteins. The effects
of menin and IQGAP1 on the proliferation of gastric cancer cells
were studied using MTT assay. The results revealed that ectopic
expression of IQGAP1 promoted, whereas silencing of IQGAP1
expression inhibited, the proliferation of gastric cancer cells.
However, increased expression of menin inhibited cell
proliferation. Notably, exogenous menin inhibited the expression of
IQGAP1, while increasing or interfering with the expression of
IQGAP1 did not inhibit the expression of menin. In the present
study, no significant changes in menin expression were observed in
the menin knockout group, possibly because menin is poorly
expressed in AGS cells.
Menin inhibits cell proliferation via various
mechanisms. For example, menin interacts with NF-кB and suppresses
NF-кB-mediated cyclin D1 transcription, thus inhibiting cell
proliferation (22). Through
interacting with Akt1, menin suppressed both Akt1-induced
proliferation and anti-apoptosis in non-endocrine and endocrine
cells (23). Further studies should
be carried out to detect the expression of transcription factors
such as PI3K and NF-κB, which are involved in regulating cell
proliferation and repressing IQGAP1-mediated transcription
(24). It was observed that the
level of p-AKT and NF-κB were significantly downregulated in
menin-overexpressing AGS cells. However, Akt levels were not
altered in menin-overexpressing AGS cells. The pre-experimental
results revealed that menin siRNA could not further reduce the
expression of menin in gastric cancer cells. Thus, the altered
effect on phosphorylated protein (p-AKT) and NF-κB expression by
reducing menin was not determined in the present study. This
suggested that the function of menin was partly accomplished by the
regulation of PI3K/Akt and NF-κB. However, the detailed mechanism
by which menin inhibits PI3K/Akt and NF-κB in gastric cancer needs
to be investigated in future studies.
In conclusion, the present study confirmed that the
protein levels of menin in gastric cancer tissues and AGS cells was
lower than those in paired normal tissues and GES-1 cells. To the
best of our knowledge, the present study has demonstrated for the
first time that menin significantly inhibits the proliferation of
gastric cancer cells. The inhibition is partly achieved by
suppressing the expression of IQGAP1, which is accompanied by
reduced expression of PI3K and NF-κB. How menin inhibits the
expression of IQGAP1 will be investigated in a future study. The
results of the present study indicated that menin may be a
potential molecular marker and target in gastric cancer
therapy.
Supplementary Material
Relative protein expression levels of
menin and IQGAP1 in 108 gastric cancer and paired normal tissues.
The distribution of protein expression in 108 tissues is shown as a
scatter plot. The median of the gray scale bands of menin
protein/β-actin was 0.315 in 108 gastric cancer tissues and 0.800
in paired normal tissues. The median of the gray scale bands of
IQGAP1 protein/β-actin was 0.852 in 108 gastric cancer tissues and
0.371 in paired normal tissues. IQGAP1, IQ motif-containing
GTPase-activating protein 1.
Acknowledgements
The authors would like to thank Dr Yong-Chang Chen
(Jiangsu University, Zhenjiang, China) for providing the adenoviral
vectors pAd-LacZ (encoding β-galactosidase), pAd-IQGAP1 and
pAd-Menin, as well as the human gastric epithelial cell line,
GES-1.
Funding
Funding: The present study was supported by the Medical Science
Foundation of Wuxi, Jiangsu Province (grant nos. QNRC047, HB2020049
and Q201953), and Wuxi Medical Key Discipline (grant no.
ZDXK2021002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FR and HZ conceived the study, wrote the manuscript
and generated the figures. FR and QG conducted the experiments and
analyzed the data. All authors examined and verified all the raw
data. FR and HZ confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
JDFY20220801-1) by the Research Ethics Committee of Jiangnan
University (Wuxi, China), and written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lemos MC and Thakker RV: Multiple
endocrine neoplasia type 1 (MEN1): Analysis of 1336 mutations
reported in the first decade following identification of the gene.
Hum Mutat. 29:22–32. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ren F, Xu HW, Hu Y, Yan SH, Wang F, Su BW
and Zhao Q: Expression and subcellular localization of menin in
human cancer cells. Exp Ther Med. 3:1087–1091. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Katona BW, Glynn RA, Hojnacki TA and Hua
X: Menin: Expanding and dichotomous roles in cancer. Oncoscience.
6:368–370. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Klossowski S, Miao H, Kempinska K, Wu T,
Purohit T, Kim E, Linhares BM, Chen D, Jih G, Perkey E, et al:
Menin inhibitor MI-3454 induces remission in MLL1-rearranged and
NPM1-mutated models of leukemia. J Clin Invest. 130:981–997.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marx SJ: Recent topics around multiple
endocrine neoplasia type 1. J Clin Endocrinol Metab. 103:1296–1301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Matkar S, Thiel A and Hua X: Menin: A
scaffold protein that controls gene expression and cell signaling.
Trends Biochem Sci. 38:394–402. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carmon KS, Gong X, Yi J, Wu L, Thomas A,
Moore CM, Masuho I, Timson DJ, Martemyanov KA and Liu QJ: LGR5
receptor promotes cell-cell adhesion in stem cells and colon cancer
cells via the IQGAP1-Rac1 pathway. J Biol Chem. 292:14989–15001.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi S and Anderson RA: IQGAP1 is a
phosphoinositide effector and kinase scaffold. Adv Biol Regul.
60:29–35. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hu W, Wang Z, Zhang S, Lu X, Wu J, Yu K,
Ji A, Lu W, Wang Z, Wu J and Jiang C: IQGAP1 promotes pancreatic
cancer progression and epithelial-mesenchymal transition (EMT)
through Wnt/β-catenin signaling. Sci Rep. 9(7539)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Abel AM, Schuldt KM, Rajasekaran K, Hwang
D, Riese MJ, Rao S, Thakar MS and Malarkannan S: IQGAP1: Insights
into the function of a molecular puppeteer. Mol Immunol.
65:336–349. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chuang HC, Chang CC, Teng CF, Hsueh CH,
Chiu LL, Hsu PM, Lee MC, Hsu CP, Chen YR, Liu YC, et al: MAP4K3/GLK
promotes lung cancer metastasis by phosphorylating and activating
IQGAP1. Cancer Res. 79:4978–4993. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zeng F, Jiang W, Zhao W, Fan Y, Zhu Y and
Zhang H: Ras GTPase-Activating-Like Protein IQGAP1 (IQGAP1)
promotes breast cancer proliferation and invasion and correlates
with poor clinical outcomes. Med Sci Monit. 24:3315–3323.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fan J, Zhang W, Wu Y, Wan P, Guo Q and
Zhang Y: miR124 inhibits cell growth through targeting IQGAP1 in
colorectal cancer. Mol Med Rep. 18:5270–5278. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu Y, Tao Y, Chen Y and Xu W: RhoC
regulates the proliferation of gastric cancer cells through
interaction with IQGAP1. PLoS One. 7(e48917)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yan J, Yang Y, Zhang H, King C, Kan HM,
Cai Y, Yuan CX, Bloom GS and Hua X: Menin interacts with IQGAP1 to
enhance intercellular adhesion of beta-cells. Oncogene. 28:973–982.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Theodoropoulou M, Cavallari I, Barzon L,
D'Agostino DM, Ferro T, Arzberger T, Grübler Y, Schaaf L, Losa M,
Fallo F, et al: Differential expression of menin in sporadic
pituitary adenomas. Endocr Relat Cancer. 11:333–344.
2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bhuiyan MM, Sato M, Murao K, Imachi H,
Namihira H and Takahara J: Expression of menin in parathyroid
tumors. J Clin Endocrinol Metab. 85:2615–2619. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Patocs A, Balogh K and Racz K: Adrenal
tumors in MEN1 syndrome and the role of menin in adrenal
tumorigenesis. Adv Exp Med Biol. 668:97–103. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Song TY, Lim J, Kim B, Han JW, Youn HD and
Cho EJ: The role of tumor suppressor menin in IL-6 regulation in
mouse islet tumor cells. Biochem Biophys Res Commun. 451:308–313.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hendy GN, Kaji H and Canaff L: Cellular
functions of menin. Adv Exp Med Biol. 668:37–50. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lopez-Egido J, Cunningham J, Berg M, Oberg
K, Bongcam-Rudloff E and Gobl A: Menin's interaction with glial
fibrillary acidic protein and vimentin suggests a role for the
intermediate filament network in regulating menin activity. Exp
Cell Res. 278:175–183. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu T and Hua X: Menin represses
tumorigenesis via repressing cell proliferation. Am J Cancer Res.
1:726–739. 2011.PubMed/NCBI
|
|
23
|
Wang Y, Ozawa A, Zaman S, Prasad NB,
Chandrasekharappa SC, Agarwal SK and Marx SJ: The tumor suppressor
protein menin inhibits AKT activation by regulating its cellular
localization. Cancer Res. 71:371–382. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zong C, Zhang X, Xie Y and Cheng J:
Transforming growth factor-β inhibits IQ motif containing guanosine
triphosphatase activating protein 1 expression in lung fibroblasts
via the nuclear factor-κB signaling pathway. Mol Med Rep.
12:442–448. 2015.PubMed/NCBI View Article : Google Scholar
|