Introduction
Pancreatic cancer is the 8th most common cause of
cancer-related deaths worldwide due to its poor prognosis and
difficulty of diagnosis and treatment (1). Mortality rates have remained constant
over the past three decades. Chemotherapy is the mainstay treatment
for the 15-20% of patients whose pancreatic tumours can be
effectively removed surgically, as well as for patients with
tumours that are not surgically resectable (2). However, >90% of pancreatic cancer
cases are resistant to current chemotherapies (3,4). At
present, the DNA synthesis inhibitor, gemcitabine,
(2',2'-difluoro-2'-deoxycytidine) is used as the preferred
chemotherapeutic agent (5,6), despite the fact that even when
combined with other chemo- or radio-therapeutic agents, it exhibits
limited efficacy, and produces severe adverse reactions (5,7).
Gemcitabine can be toxic to healthy cells, resulting in
unpredictable severe toxic effects. The inherent biological
characteristics of pancreatic cancer, as well as the blood-pancreas
barrier, possibly contribute to the unsatisfactory therapeutic
effects of gemcitabine (8).
Thus, there is a need for more effective anticancer
drugs which have lesser toxic side effects to improve clinical
efficiency. In the present study, fucoxanthin, a natural product
first isolated from the marine seaweeds Fucus,
Dictyota, and Laminaria in 1914, and thus one of the
most abundant carotenoids, was examined (9). Fucoxanthin is found in both macroalgae
such as Undaria pinnatifida or Laminaria japonica, as
well as microalgae such as Phaeodactylum tricornutum or
Cylindrotheca closterium (10). Fucoxanthinol, the main fucoxanthin
metabolite, has been detected in human plasma after the daily
intake of wakame (Japanese common name for U. pinnatifida).
Its bioavailability and metabolism are higher in humans than in
mice (11) and does not exhibit any
significant adverse effects in vivo (12). It has been regarded as a potential
natural substance for cancer treatment (9). Although human experiments on the
effects of fucoxanthin are lacking, animal experiments have shown
evidence of anticancer effects. Oral administration of fucoxanthin
has revealed no toxicity and mutagenicity (13-17).
Apart from anticancer effects, fucoxanthin has also been
demonstrated to have other health beneficial effects (18-22).
Fucoxanthin uses various mechanisms to suppress tumour formation;
inducing autophagy (21,22), arresting the cell cycle at the G1/G0
phase, inducing apoptosis, enhancing gap junctional intercellular
communication, and involving different regulatory events in
SAPK/JNK, Akt/mTOR, Bcl-2, JAK/STAT, MAPK and NF-κB pathways
(23,24). Fucoxanthin has been shown to enhance
chemotherapeutic efficacy of cisplatin in vitro (25). Recently, the combination therapy
(chemotherapy in combination with fucoxanthin) has been evaluated
in colon and liver cancers (25-29),
lung cancer (30), breast cancer
(31), and cervical cancer
(32).
Pancreatic ductal adenocarcinoma accounts for
>90% of all pancreatic cancers (33), and MIA PaCa-2 and PANC-1 cell lines,
derived from primary tumours, are currently used as in vitro
models to study pancreatic ductal adenocarcinoma carcinogenesis
(34,35).
The aim of the present study was to investigate the
suppressive effect of fucoxanthin on the growth of pancreatic
cancer cell lines, MIA PaCa-2 and PANC-1, and the effect of
combining it with gemcitabine. The 293 cell line, as a reference
line for potential toxicity towards a non-cancer cell line, and MIA
PaCa-2 and PANC-1 cell lines were utilised in the present study to
determine the various cytotoxic effects of treatment between
pancreatic cancer cells and a control. Flow cytometry was used to
determine the possible mechanism of action by analysing the cell
cycle and apoptosis.
Materials and methods
Materials
Fucoxanthin (cat. no. F6932) and MTT (cat. no.
M2128) were purchased from Sigma-Aldrich NZ; Merck KgaA. Human
pancreatic cancer cell lines, MIA PaCa-2 (cat. no. CRL-1420TM) and
PANC-1 (cat. no. CRL-1469TM), and human cell line, 293 (cat. no.
CRL-1573TM) were purchased from American Type Culture Collection
(ATCC). Cell culture medium (RPMI-1640), penicillin-streptomycin
and L-glutamine were purchased from Thermo Fisher Scientific, Inc.
Fetal bovine serum was purchased from Moregate BioTech. All three
cell lines were stored in liquid nitrogen. Following thawing, the
cell lines were maintained in a tissue culture flask containing
complete growth culture medium (RPMI-1640) with 1% of L-glutamine,
1% of penicillin-streptomycin and 10% of fetal bovine serum. All
cells were cultured in an incubator at 37˚C, with 5% carbon dioxide
humidified air.
MTT assay
MTT assays were used to determine cell viability
(36). Cells were seeded at
densities of 5,000 cells/well in 96-well plates, for 6-24 h and
then cultured in the incubator at 37˚C for the following
experiment. A total of 100 µl of fresh complete culture medium
containing various concentrations of drugs (i.e., gemcitabine 25,
50 and 500 nM) was added to corresponding wells. Following
incubation for 0, 24, 48 and 72 h, the medium was carefully removed
and replaced with 100 µl of fresh complete culture medium. An
aliquot of 10 µl of MTT stock solution (0.2 mg/ml) was added to
each well and the plates were placed in the 37˚C incubator for 2 h.
The supernatant was gently removed from the wells. An aliquot of
150 µl of DMSO was added to each well and mixed thoroughly using an
orbit plate shaker. After incubating at 37˚C for 20-30 min, the
plate was shaken briefly and the absorbance was measured by a plate
reader (FLUOstar Omega; Alphatech Systems, Ltd.), at 540 nm.
Background subtraction was utilised for generation of final
values.
Determination of the effect of
fucoxanthin
The cell density selected in this study for MIA
PaCa-2, PANC-1 and 293 cells was 5,000 cells/well, according to our
preliminary culture study. Following proper dilution, a
multi-channel pipette was used to seed 100 µl of cells/well into
the 96-well plate. The fucoxanthin stock solution was prepared by
dissolving fucoxanthin in absolute ethanol with its concentration
as 5 mM. Fucoxanthin (prepared in absolute ethanol) was diluted
with cell culture medium. The range of concentrations of
fucoxanthin were different for the three cell lines: MIA PaCa-2
(fucoxanthin concentration from 0.02 to 1 µM); PANC-1 (from 0.5 to
100 µM) and 293 (from 1 to 100 µM). Since fucoxanthin was dissolved
in ethanol, the effect of ethanol alone on the cells was
determined. The results revealed that 2, 1.6 and 1.0% fucoxanthin
inhibited the growth of MIA PaCa-2 cells after incubating for 24,
48 and 72 h. Ethanol at a concentration of ≤0.5% did not
significantly affect the growth of MIA PaCa-2 cells. No effect on
the growth of PANC-1 cells cultured with ethanol controls was
noted. The study was then carried out using fucoxanthin
concentrations under 25 µM, and 100 µl of each diluted fucoxanthin
solution was added to wells immediately for analysis.
Determination of the colour effect of
fucoxanthin on the absorbance value (OD value)
As fucoxanthin is an orange-coloured pigment, its
colour may impact the final absorbance value of each well when
performing MTT assays. Different concentrations of fucoxanthin
solution were prepared to assess respective absorbance values. The
highest fucoxanthin concentration used was 100 µM and two test
methods were used for this study. The first was performed by
testing 100 µl of each fucoxanthin solution under the plate reader
directly; and the other method was carried out by following the MTT
assay protocol. A total of 10 µl MTT solution was added to each
well and incubated at 37˚C for 4 h, then 150 µl DMSO was added and
finally the absorbance was read at 540 nm. According to the
results, fucoxanthin solution was replaced with fresh cell culture
medium before the addition of the MTT solution in the present
study.
Determination of optimum concentration
of gemcitabine
According to previous single treatment experiment
results, the optimum concentrations of fucoxanthin and gemcitabine
were determined. In the combination treatment experiment,
fucoxanthin at concentrations of 150, 250 and 300 nM, and
gemcitabine, at 25 and 50 nM were concurrently combined with each
other to treat MIA PaCa-2 cells. In addition, fucoxanthin
concentrations (10 and 20 µM) were combined with gemcitabine (50
and 500 nM, respectively) to treat PANC-1 cells. An MTT assay was
applied to determine the cell viability, and the experimental steps
were the same as those aforementioned.
Synergism analysis
The nature of the combination between gemcitabine
and fucoxanthin was quantified by synergism quotient (SQ) (37-39).
SQ is defined as the net growth inhibitory effect of the
combination treatment by the sum of the net individual treatment
effect on growth inhibition. A quotient of >1.1 indicates a
synergistic effect, a quotient between 0.9 and 1.1 indicates an
additive effect, while a quotient of <0.9 indicates an
antagonistic effect (38).
Cell cycle assay
After detaching, counting and diluting, cells were
seeded onto 6-well plates. The seeding density for all cell lines
in this study was 50,000 cells/ml and a 2-ml cell solution was
seeded in each well. The plates were maintained in a 37˚C incubator
for 6-24 h to ensure that almost all of the cells were detached
from the walls of the wells.
When the cell attachment rate was high, cells were
treated with 2 ml of serum-free medium (no FBS, with 1% penicillin
and 1% L-glutamine) in each well, for synchronizing cell
proliferation. One plate set in this study was a control group and
the other plates were designed as treatment groups. In Plate 1
(control group), wells A, B and C were treated with 2 ml of
serum-free medium (no FBS), and wells D, E and F were filled with 2
ml of complete culture (10%) medium. Before adding the fresh medium
(serum-free medium or 10% FBS medium), the old medium was removed
first. The fresh medium was added slowly and gently in circles
along the wall of the well. For Plate 2 (treatment group), all the
wells were treated with 2 ml of serum-free medium. The plates were
maintained in the 37˚C incubator for 24 h.
All treatments were maintained in 15 ml centrifuge
tubes which were prepared with complete culture (10% FBS) medium.
The old serum-free medium was first removed gently, and then 2 ml
of well-mixed treatment solution was carefully added into the
designated wells. A total of 2 ml complete culture medium was added
to the control well. All plates were maintained in the incubator
for 48 h (day 2) and 72 h (day 3). Control cells were collected
from Plate 1 with day 0 samples. Namely, fucoxanthin, 150, 250 and
300 nM, and gemcitabine, 25 and 50 nM, were used to treat MIA
PaCa-2 solely and jointly. For PANC-1 cells, fucoxanthin
concentrations were 10 and 20 µM and gemcitabine were 50 and 500
nM.
Cell cycle analysis
The permeabilizing solution of (total volume) 1 ml
per test tube consisted of: 0.1% Triton X-100 (1 µl for each 1 ml)
+ RNAse A (50 µg/ml from stock solution of 1 mg/ml). For example,
20 tubes were formulated and dispensed as follows: 20 µl Triton
X-100 + 1,000 µl RNAse +19,980 µl PBS. After centrifuging all tubes
at 125 x g for 2 min at 0-4˚C, the ethanol was gently removed, and
3 ml ice cold PBS was added to each tube before the second
centrifugation step. Following the second centrifugation (125 x g
for 2 min at 0-4˚C), another 3 ml of ice-cold PBS was added to each
tube to replace the initial wash. In total, the cells were washed
twice with 3 ml ice cold PBS. In the process of PI staining, the
supernatant in each well was gently removed and then 1 ml of
well-mixed permeabilizing solution was added to each tube. All
cells in the tubes were carefully mixed before being transferred to
test tubes and then incubated at 37˚C for 30-45 min. Following
permeabilization, 5 µg/ml of PI (5 µl to 1 ml in each tube) was
added to each test tube and incubated for 5 min. Finally, all the
tubes were analysed under a flow cytometer (MoFlo XDP Beckman
Coulter, Inc.).
The IC50 values were calculated using
PRISM® software (version 6.0; GraphPad Software, Inc.),
and the IC50 values were obtained using dose-response inhibition,
nonlinear regression (curve fit): Log (inhibitor) vs.
response-variable slope (four parameters). Kaluza® Flow
Cytometry Analysis Software (version 1.3; Beckman Coulter, Inc.)
was applied in cell cycle results analysis to measure the cell
cycle distributions in the present study.
Statistical analysis
All experiments in the present study were performed
at least three times. Data were assessed for normal distribution
before analysis. Statistical differences in multiple groups were
determined by one-way analysis of variance (ANOVA) with
PRISM® software (version 6.0; GraphPad Software, Inc.)
and SPSS (version 22.0; IBM Corp.). Statistical comparisons were
performed using Tukey's post hoc test. Analysis between two groups
was determined using unpaired Student's t-test. Data are expressed
as the means ± standard deviation (SD; sample n=3 with triplicate
analysis performed on each sample). P<0.05 was considered to
indicate statistically significant differences.
Results
In order to analyse the treatment effect of
gemcitabine or fucoxanthin on pancreatic cancer cell lines MIA
PaCa-2, PANC-1, and 293 cell growth, cells were treated in
vitro in a dose or time-and-dose course experiment. Experiments
were performed in triplicate and repeated at least three times
independently (Table I).
 | Table ICytotoxicity (IC50) of
treatment in MIA PaCa-2 cells, PANC-1 and 293 cells detected at
various time points: gemcitabine (72 h) or fucoxanthin (24, 48 and,
72 h). |
Table I
Cytotoxicity (IC50) of
treatment in MIA PaCa-2 cells, PANC-1 and 293 cells detected at
various time points: gemcitabine (72 h) or fucoxanthin (24, 48 and,
72 h).
| | IC50
(µM) aftergem-citabine treatment | IC50
(µM) after fucoxanthin treatment |
|---|
| Cell line | 72 h | 24 h | 48 h | 72 h |
|---|
| MIA PaCa-2 | 25.00±0.47 | 17.72±1.04 | 10.68±0.64 | 8.74±0.28 |
| PANC-1 | 48.55±2.30 | N/A | N/A | 10.58±0.56 |
| 293 Cells | 48.82±3.27 | 28.97±1.58 | 8.70±1.17 | 8.28±0.30 |
Inhibitory effect of gemcitabine or
fucoxanthin on MIA PaCa-2 pancreatic cancer cells
Culture of cells with various concentrations of
gemcitabine for 72 h resulted in significant suppression of cell
viability in a dose-dependent manner. ANOVA analysis revealed that
cell viability was statistically and significantly decreased with
the increasing concentration of gemcitabine, thus the concentration
of gemcitabine was a significant factor in altering cell viability
(P<0.01); the IC50 was 25.00±0.47 nM after 72 h of
treatment (Table I). Gemcitabine at
25 and 50 nM exhibited approximately 63.45 and 42.18% cell
viability of MIA PaCa-2 cells at 48 h, respectively (Table II). These two concentrations were
selected in the following combination study.
 | Table IICell viability percentages of MIA
PaCa-2 cells after 72 h of incubation with gemcitabine,
fucoxanthin, or gemcitabine-fucoxanthin combination. |
Table II
Cell viability percentages of MIA
PaCa-2 cells after 72 h of incubation with gemcitabine,
fucoxanthin, or gemcitabine-fucoxanthin combination.
| | Gemcitabine
(nM) |
|---|
| Compound
Fucoxanthin (nM) | 0 (%) | 25 (%) | 50 (%) |
|---|
| 0 | 100 | 63.45±0.54 | 42.18±0.48 |
| 150 | 99.8±0.19 |
59.76±1.63a |
39.81±0.69a |
| 250 | 98.99±0.89 |
53.06±0.70a |
38.32±0.52a |
| 300 | 99.83±0.61 |
50.40±0.50a |
36.72±0.12a |
Fucoxanthin inhibited the proliferation of MIA
PaCa-2 cells in a concentration-dependent manner (Table II) at 72 h. One-way ANOVA revealed
that cell viability was statistically and significantly decreased
with the increasing concentration of fucoxanthin treatment
(P<0.01) after treatment for 72 h. The IC50 values
within the three days of treatment significantly and statistically
decreased to 17.72±1.04 (24 h), 10.68±0.64 (48 h) and 8.74±0.28 µM
(72 h) (Table I). Morphological
changes observed microscopically became more marked with increasing
culture time, in the presence of fucoxanthin concentrations
>6.25 µM. Cells diminished in size and were scattered and more
easily detached. After 72 h, the volume of cells was significantly
reduced, and the edges of the cells were rough (data not
shown).
Inhibitory effect of gemcitabine or
fucoxanthin on PANC-1 pancreatic cancer cells
Gemcitabine was effective in inhibiting PANC-1 cells
at only 72 h of incubation in a dose-dependent manner, yielding an
IC50 value of 48.55±2.30 nM (Table I). ANOVA revealed that cell
viability was statistically and significantly decreased with the
increasing concentration of gemcitabine treatment, thus, the
alteration of treatment concentration was associated with a change
in cell viability.
Furthermore, fucoxanthin inhibited proliferation of
PANC-1 cells in a dose-dependent manner after 72 h incubation. Only
72-h incubations were performed in view of the aforementioned
result of gemcitabine. The IC50 value was 10.58±0.56 µM
(Table I). ANOVA revealed that
treatment with fucoxanthin was a significant factor in the
inhibition of cell growth with increasing treatment concentrations
and exposure times.
Inhibitory effect of gemcitabine and
fucoxanthin on 293 cell line
The same gemcitabine concentrations used for MIA
PaCa-2 cells were used for 293 cells. Gemcitabine significantly
inhibited cell viability in a concentration-dependent manner. The
IC50 value was 48.82±3.27 nM (72 h) (Table I). Fucoxanthin inhibited 293 cell
growth at concentrations in a dose- and time-dependent manner. The
IC50 values showed a significant decrease after three
days of treatment to 28.97±1.58 (24 h), 8.70±1.17 (48 h), and
8.28±0.30 µM (72 h), respectively (Table I). ANOVA indicated that the
treatment with gemcitabine or fucoxanthin was a significant factor
in the inhibition of cell growth with increasing treatment
concentrations and exposure times.
Inhibitory effect of
gemcitabine-fucoxanthin combination
Based on single drug experiments, 72 h of incubation
time for combination drug treatment was used for MIA PaCa-2 cells.
The cell viability in groups treated with combination of
gemcitabine and fucoxanthin for 72 h, is presented in Table II. The cell viability was not
markedly altered with fucoxanthin treatment alone (concentrations
from 0 nM to 300 nM). However, gemcitabine 25 nM combined with 150,
250 and 300 nM fucoxanthin significantly reduced the cell viability
by approximately 4, 10 and 13%, respectively, as compared with
gemcitabine alone (cell viability, 63.45±0.54%). Similarly, in the
gemcitabine 50-nM treatment group, the enhanced inhibitory effect
of the combination treatment with fucoxanthin was also observed:
Gemcitabine treatment 50 nM alone (42.18±0.48%) vs. gemcitabine
treatment with increasing concentrations of fucoxanthin at 150
(39.81±0.69%), 250 (38.32±0.52%) and 300 nM (36.72±0.12%).
According to the statistical analysis, single treatment with
fucoxanthin alone could not significantly decrease the cell
viability (P>0.01) at treatment concentrations of 150, 250 and
300 nM. However, gemcitabine treatment alone at 25 and 50 nM
significantly decreased the cell viability (P<0.01). Combination
treatment also significantly decreased the cell viability
(P<0.01). Additionally, in the presence of fucoxanthin, the cell
viability was significantly decreased in a dose-dependent manner as
compared to gemcitabine treatment alone (P<0.01). As revealed in
Table III, treatment with
gemcitabine at 25 nM with increasing concentrations of fucoxanthin
from 150 to 300 nM, yielded an SQ value which increased from 1.079
(additivity) to 1.316 (synergism). Gemcitabine, at 50 nM, with
increasing concentrations of fucoxanthin, increased the SQ value
from 1.034 (additivity) to 1.085 (additivity). These results
indicated that treatment with fucoxanthin generated a synergistic
effect at a combined treatment with 25 nM of gemcitabine. In all
groups, with increasing concentrations of fucoxanthin, the cell
viability of MIA PaCa-2 cells decreased. Hence, fucoxanthin was
able to enhance the inhibitory effect of gemcitabine on cell
viability of MIA PaCa-2 cells in a concentration-dependent manner,
even at a low concentration range. The single treatment results
revealed that PANC-1 cells were not sensitive to gemcitabine as
compared to MIA PaCa-2 cells. Therefore, gemcitabine at 50 and 500
nM was used in combination with fucoxanthin at 10 and 20 µM. The
treatment time was also 72 h.
 | Table IIISynergism analysis of MIA PaCa-2
cells after 72 h of incubation with combination treatment of
gemcitabine-fucoxanthin. |
Table III
Synergism analysis of MIA PaCa-2
cells after 72 h of incubation with combination treatment of
gemcitabine-fucoxanthin.
| | Gemcitabine
(nM) |
|---|
| Compound
Fucoxanthin (nM) | 0 | 25 | 50 |
|---|
| 0 | N/A | N/A | N/A |
| 150 | N/A | 1.079 | 1.034 |
| 250 | N/A | 1.205 | 1.033 |
| 300 | N/A | 1.316 | 1.085 |
The PANC-1 cell viability values under different
concentrations of drugs are listed in Table IV. After 72 h of incubation, with
10 and 20 µM fucoxanthin alone, the number of viable cells
decreased to 56.91±3.00 and 18.54±1.10%, respectively. Treatment
with gemcitabine alone decreased the number of viable cells.
However, even at a concentration of gemcitabine increased 10 times
from 50 to 500 nM, the cell viability was minimally affected (from
~10 to 18%, respectively). This effect was observed in all
combination treatments. In the combination treatment group
(fucoxanthin, 10 µM), the addition of gemcitabine at concentrations
of 50 and 500 nM did not significantly reduce (~3%) the viability
of cancer cells. Once increased, a fucoxanthin concentration of 20
µM, demonstrated only a 2% increase in the inhibition rate at
gemcitabine concentrations of 50 and 500 nM. The single treatment
of either fucoxanthin alone or gemcitabine alone decreased the cell
viability. However, the combination treatment did not generate a
significant effect for reduced cell viability (P>0.01). As noted
in Table V, the concentration of
fucoxanthin (from 10 to 20 µM) increased the SQ values in this
stated range: 50 nM gemcitabine [0.889 (antagonistic effect) to
0.902 (additive effect), respectively] and 500 nM [0.823
(antagonistic effect) to 0.840 (antagonist effect)].
 | Table IVCell viability percentages of PANC-1
cells after 72 h of incubation with gemcitabine, fucoxanthin, or
gemcitabine-fucoxanthin combination. |
Table IV
Cell viability percentages of PANC-1
cells after 72 h of incubation with gemcitabine, fucoxanthin, or
gemcitabine-fucoxanthin combination.
| | Gemcitabine
(nM) |
|---|
| Compound
Fucoxanthin (nM) | 0 (%) | 50 (%) | 500 (%) |
|---|
| 0 | 100 | 90.20±0.67 | 82.15±0.88 |
| 10 |
56.91±3.00a |
52.92±0.63a |
49.42±2.51a |
| 20 |
18.54±1.10b |
17.42±1.20b |
16.01±0.36b |
 | Table VSynergism analysis of PANC-1 cells
after 72 h of incubation with combination treatment of
gemcitabine-fucoxanthin. |
Table V
Synergism analysis of PANC-1 cells
after 72 h of incubation with combination treatment of
gemcitabine-fucoxanthin.
| | Gemcitabine
(nM) |
|---|
| Compound
Fucoxanthin (nM) | 0 | 50 | 500 |
|---|
| 0 | N/A | N/A | N/A |
| 10 | N/A | 0.889 | 0.823 |
| 20 | N/A | 0.902 | 0.840 |
Gemcitabine significantly inhibited the cell
viability of 293 cells by approximately 23, 49 and 76% after 72 h
of incubation at 25, 50 and 500 nM concentrations, respectively
(Table VI; P<0.05). In the
fucoxanthin alone groups (150, 250, 300, 10,000 and 20,000 nM),
there was no significant suppression by fucoxanthin at ≤300 nM, but
there was significant inhibition at high concentrations of 10 and
20 µM, as compared to the control (P<0.01). In the combination
treatment groups, adding fucoxanthin at concentrations of 0 to 150
nM, slightly increased cell viability (2.58 and 4.05%; P<0.05)
at gemcitabine treatment concentrations of 25 and 50 nM,
respectively. No significant differences in cell viability were
observed at gemcitabine treatment concentrations of 25 and 50 nM
combined with fucoxanthin treatment concentrations of 150 to 300 nM
(P<0.01). However, combination with high concentrations of
fucoxanthin (10 and 20 µM), induced significantly greater
inhibition than the gemcitabine alone group (P<0.01). This may
be due to the cytotoxicity of fucoxanthin used alone at high
concentrations (Table VI). In
Table VII, gemcitabine (25 nM)
had an SQ value increase from 0.833 (antagonistic effect) to 1.087
(additive effect) as the concentration of fucoxanthin increased
from 150 to 300 nM. Consistently, the SQ value increased from 0.900
(additive effect) to 0.918 (additive effect) with increasing
fucoxanthin concentrations of 150 to 300 nM in the gemcitabine
50-nM groups. However, once the concentration of fucoxanthin
increased to 10 mM, the SQ value decreased below 0.700
(antagonistic effect).
 | Table VICell viability percentages of 293
cells after 72 h of incubation with gemcitabine, fucoxanthin, or
gemcitabine-fucoxanthin combination. |
Table VI
Cell viability percentages of 293
cells after 72 h of incubation with gemcitabine, fucoxanthin, or
gemcitabine-fucoxanthin combination.
| | Gemcitabine
(nM) |
|---|
| Compound
Fucoxanthin (nM) | 0 (%) | 25 (%) | 50 (%) | 500 (%) |
|---|
| 0 | 100 |
77.42±2.34a |
51.27±0.88b |
23.60±1.79c |
| 150 | 99.08±1.71 | 80.00±5.80 | 55.32±2.20 | |
| 250 | 99.06±1.23 | 79.02±4.49 | 55.80±2.49 | |
| 300 | 100.04±1.99 | 75.92±4.77 | 55.73±2.31 | |
| 10000 |
30.51±2.92b | |
22.47±2.30b | 20.04±1.45 |
| 20000 |
12.72±0.66c | |
11.25±1.03c |
9.89±0.93b |
 | Table VIISynergism analysis of 293 cells after
72 h of incubation with combination treatment of
gemcitabine-fucoxanthin. |
Table VII
Synergism analysis of 293 cells after
72 h of incubation with combination treatment of
gemcitabine-fucoxanthin.
| | Gemcitabine
(nM) |
|---|
| Compound
Fucoxanthin (nM) | 0 | 25 | 50 | 500 |
|---|
| 0 | N/A | N/A | N/A | N/A |
| 150 | N/A | 0.833 | 0.900 | |
| 250 | N/A | 0.875 | 0.900 | |
| 300 | N/A | 1.087 | 0.918 | |
| 10000 | N/A | | 0.655 | 0.559 |
| 20000 | N/A | | 0.649 | 0.565 |
Combination of gemcitabine and fucoxanthin on the
cell cycle. The cell cycle distribution of MIA PaCa-2 cells is
presented in Fig. S1. According to
Fig. S1 the G0-G1 phase was
significantly increased from 41.04% (control) to 60.37% (24-h
serum-starved cells) and the G2-M and S phases were decreased from
27.00% (control) to 15.75% (24-h serum-starved cells) and 17.28%
(control) to 10.62% (24-h serum-starved cells), respectively. The
distribution of the cell cycle was not altered by incubation with
150 or 250 nM fucoxanthin alone for either 48 or 72 h (Table SI). However, fucoxanthin at a
concentration of 300 nM has a slight effect: The percentage of the
G0-G1 phase increased ~1.9 and 3.75% at both
48 and 72 h, respectively. Treatment with 10 µM fucoxanthin for 48
h, resulted in an increase of MIA PaCa-2 cells in
G0-G1 phase (from 48.78 to 59.51%), and the
percentage of sub-G1 was increased (from 8.0 to 12.83%).
At 72 h, fucoxanthin (10 µM) not only increased the cells in
sub-G1 phase, but also increased the percentage of cells
in the G2-M phase with a concomitant decrease in the
number of cells in the S phase (Table
SI). Gemcitabine (at 25 and 50 nM) caused a significant
accumulation of cells in the S phase, as compared to the control at
both 48 and 72 h, while the number of cells in the
G0-G1 phase was reduced (Table SI). Additionally, sub-G1
phase accumulation increased in a time- and dose-dependent manner,
particularly with 50-nM gemcitabine treatment at 72 h.
In the combination treatment groups, the results
indicated that fucoxanthin (150, 250 and 300 nM) combined with
gemcitabine in MIA PaCa-2 cells, for 48 h, increased the percentage
of cells in the S phase, but the cells in the sub-G1
phase were not altered (Table SI),
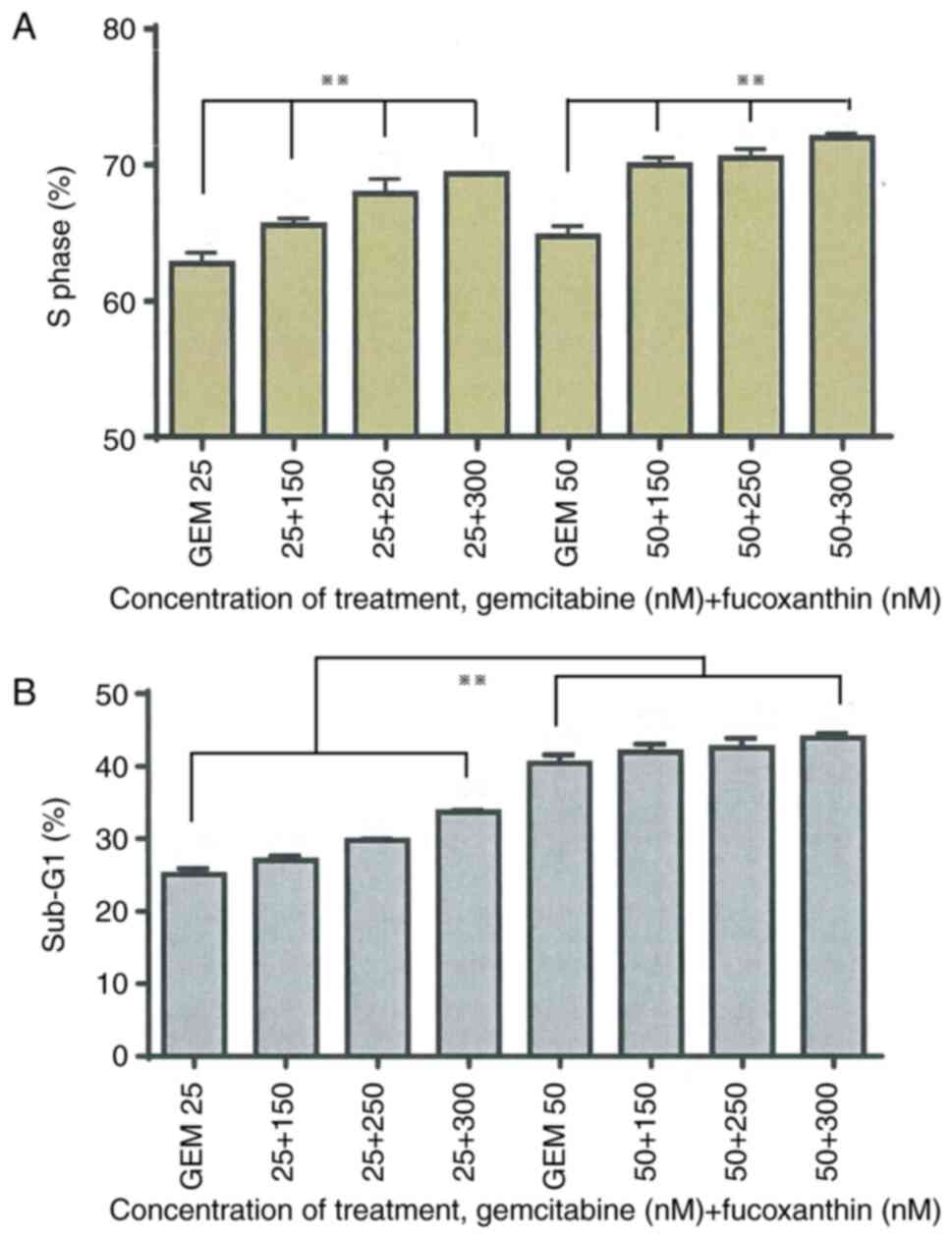
as compared to gemcitabine (25 and 50 nM) alone (Fig. 1A). At the 72-h combination
treatment, the sub-G1 percentage was enhanced with the
corresponding increase in fucoxanthin concentration. However,
sub-G1 phase accumulation in the 25-nM gemcitabine
combination groups was more apparent than the 50-nM gemcitabine
groups (Fig. 1B).
Effects of gemcitabine and fucoxanthin
on PANC-1 cell cycle progression
The cell cycle distribution of PANC-1 cells is
presented Table SII. In addition,
cell starvation (medium-only, without serum) for 24 h, synchronised
and blocked the PANC-1 cells in G0-G1 phase
(Fig. S2). The percentage of cells
in the G0-G1 phase was increased by ~19%,
while the number of cells in the S phase decreased from 20.73 to
9.62% and the number of cells in the G2-M decreased from
24.34 to 18.03%. In the groups treated only with fucoxanthin (10 or
20 µM) for 48 h, the results revealed that the accumulation of
cells in the G0-G1 phase was significantly
increased compared to the control (Table SII). Furthermore, the percentage of
cells in the S phase decreased with the increase of fucoxanthin
concentration. At 72 h, fucoxanthin 20 µM did not block the cells
in the G0-G1 phase. However, fucoxanthin
induced the increase of the percentage of cells in the
sub-G1 phase (24.70%) compared with the control cells
(9.00%) at 72 h (Table SII).
Fucoxanthin decreased the proportion of cells in the
G2-M phase and increased the percentage of
sub-G1 cells in a time- and dose-dependent manner.
Gemcitabine, 50 nM, 500 nM and 50 µM blocked the cells in the
G0-G1 phase at 48 h, and the percentage of
cells in the sub-G1 phase was increased in a time- and
dose-dependent manner. Similar results were obtained at 72 h,
except for gemcitabine at a concentration of 50 µM which induced
cell apoptosis [56.65 (control) vs. 25.99% (50 µM gemcitabine);
Table SII)].
As revealed in Table
SII, in the combination treatment groups (50 and 500 nM
gemcitabine combined with 10 µM fucoxanthin), an increase in the
percentage of cells in the G0-G1 phase as
well as in the sub-G1 phase was observed, compared with
the cells treated only with 10 µM fucoxanthin for 48 h. The
accumulation of cells in the S phase increased with higher
gemcitabine concentrations (50 to 500 nM), and changes in the
G2-M phase were negligible. However, cells treated with
the combination drugs for 72 h did not exhibit increased arrest of
the G0-G1 phase, with respect to cells
treated only with fucoxanthin 10 µM. By contrast, the percentage of
cells in the sub-G1 phase increased after treatment for
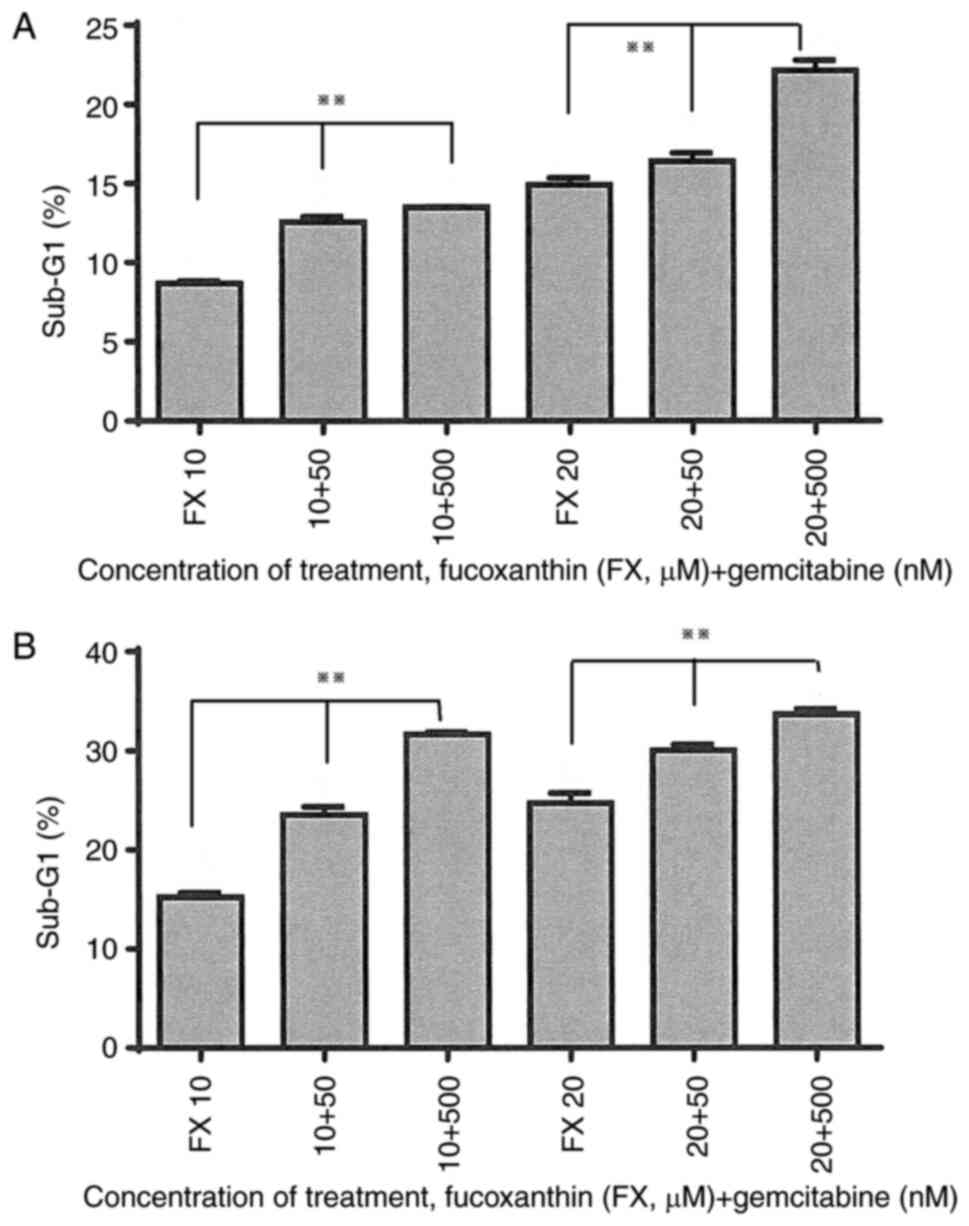
48 and 72 h (Fig. 2A and B). As for cells treated with 20 µM
fucoxanthin combined with gemcitabine (50 and 500 nM), the combined
treatment induced increased accumulation of cells in the S phase
and cell apoptosis, as compared to cells treated only with 20 µM
fucoxanthin, at both at 48 and 72 h (Table SII). G2-M phase
detection was negligible in the fucoxanthin 20-µM combination
treatment groups at both time points, similar to the 10-µM
fucoxanthin combination treatment groups (Table SII).
Effects of gemcitabine and fucoxanthin
on 293 cell cycle progression
The results of cell cycle distribution revealed that
24-h starvation (medium-only, without serum) did not arrest 293
cells in the G0-G1 phase; it was demonstrated
that the percentage of serum-starved cells in the
G0-G1 phase was slightly decreased compared
with the control (Fig. S3). The
cell cycle distribution of 293 cells is presented in Table SIII. In the single treatment
groups, the distribution of cell cycle phases in
fucoxanthin-treated (150, 250 and 300 nM) 293 cells was similar to
that in control cells at both 48 and 72 h after treatment. Cells
treated only with fucoxanthin at a concentration of 10 µM at 48 h
induced a 1.53% higher cell apoptosis value than the control cells,
and the percentage of cells in the G0-G1
phase was even lower than that of control cells. The
G2-M and S phases were not markedly altered in relation
to the controls. However, at 72 h, 10 µM fucoxanthin was
responsible for a higher accumulation of cells in the
G2-M phase, and its percentage of sub-G1 cells was only
~2% higher than the same concentration at 48 h. There were no
significant differences in the cell cycle distribution of 25-nM
gemcitabine-treated cells, at 48 h, in relation to the control, and
apoptosis in sub-G1 was increased by only ~6%. However, treatment
with 50-nM gemcitabine induced apoptosis from sub-G1 (25.28%), and
most of the cells were arrested at the S phase. At 72 h,
gemcitabine at a concentration of 25 nM increased the percentage of
sub-G1 over a small range as compared with control
group. Gemcitabine, at a concentration of 50 nM, induced cell
apoptosis (sub-G1) of up to 38.57% and no cell phase distribution
could be observed, implying a high level of apoptotic induction in
these cells (Table SIII).
In the combination treatment groups [fucoxanthin
(150, 250 and 300 nM) combined with gemcitabine (25 and 50 nM), for
48 and 72 h], no significant differences in the distribution of all
phases in the cell cycle were revealed, as compared to cells
treated only with gemcitabine. Combination treatments induced a
slightly lower percentage of cell apoptosis compared to that
observed with single-gemcitabine treatment.
Discussion
To the best of our knowledge, this is the first
study, investigating the effects of fucoxanthin and its combination
with gemcitabine in pancreatic cancer cells. The characteristics of
high anticancer efficacy, with mild or no toxicity to normal cells,
is the primary objective in cancer research. Fucoxanthin used alone
in treatment has exhibited significant anticancer efficacy
(31), however, when used in
combination with gemcitabine as determined in the present study,
was more effective with less harmful effects to normal cells.
The results obtained in the present study indicate
that the inhibitory effect of gemcitabine on MIA PaCa-2, PANC-1 and
293 cells occurs in a dose-dependent manner. This is consistent
with previous studies of gemcitabine on, pancreatic ductal
adenocarcinoma cell lines MIA PaCa-2 and PANC-1 (39,40),
breast adenocarcinoma cell lines MCF-7 and MDA-MB-231(41), colorectal adenocarcinoma cell line
HT-29(42), and cervical carcinoma
cell lines (43). The
IC50 values for gemcitabine determined in this study
were 25.00±0.47 nM for MIA PaCa-2 cells, 48.55±2.30 µM for PANC-1
cells and 48.82±3.27 nM for 293 cells. These three different
IC50 values indicated a different sensitivity of the
three cell lines to gemcitabine. MIA PaCa-2 cell line was the most
sensitive to treatment of gemcitabine. PANC-1 and 293 cell lines
both showed strong chemo-resistance with 293 cells displaying
slightly stronger chemo-resistance than PANC-1. Previous studies
also revealed the same results as concluded in this study (38,39,44).
PANC-1 was found to have low sensitivity to gemcitabine in a
previous study (40). MIA PaCa-2
and PANC-1 are both primary tumour cells (35). The difference in the IC50
values between these cell lines could be due to the fact that
PANC-1 cells are more resistant as compared to MIA PaCa-2 cells.
Moreover, in a previous study it was reported that even at very low
doses (5-10 nM), gemcitabine was able to induce nuclear factor-κB
(NF-κB) activity, which can promote gemcitabine chemo-resistance
(45). Hence, it can be surmised
that doses (>50 nM) used in this study could significantly
activate the NF-κB activity.
In the present study, fucoxanthin inhibited the
viability of human pancreatic cancer cell lines, MIA PaCa-2 and
PANC-1, as well as 293 cells in a dose-dependent manner.
Fucoxanthin time-dependently suppressed the proliferation of MIA
PaCa-2 and 293 cells. In the higher fucoxanthin concentration
treatment groups of each cell line, formation of nuclear
condensation was evidently clear when observed under the inverted
microscope (data not shown). The antioxidant property of
fucoxanthin was considered to be one of the major reasons for the
anticancer effect of fucoxanthin (46-48).
NF-κB activity has been demonstrated to be inhibited by
antioxidants (49,50). It has been suggested that some part
of fucoxanthin is hydrolysed to fucoxanthinol during the uptake
(51). Fucoxanthinol is then
further converted into amarouciaxanthin A in HepG2 cells. These two
fucoxanthin metabolites were found to reduce the viability of human
prostate cancer cell line PC-3 (27,52).
Fucoxanthinol was also found to have more efficient
anti-proliferative effects than fucoxanthin (23,53,54).
The difference in sensitivity for different cell lines may be due
to the different content of hydrolytic enzymes in the different
cells.
In the present investigation, gemcitabine was used
with fucoxanthin simultaneously, to explore their combined effects
on pancreatic cancer cells. To the best of our knowledge, applying
a fucoxanthin concentration range under 1 µM in anticancer research
was performed for the first time. The aim of using low fucoxanthin
concentrations was to determine whether fucoxanthin is able to
effectively improve the cytotoxicity of gemcitabine even at low
concentrations. The carotenoid fucoxanthin is known to sensitize
multidrug-resistant cancer cells, and a proposed mechanism for
fucoxanthin overcoming multiple drug resistance in cancer cells and
increasing efficacy of chemotherapy in targeted cells has been
reported (55).
The findings of the present study may potentially
increase the benefits of fucoxanthin-coupled treatments. The
combined effect of fucoxanthin and gemcitabine on the reduction of
MIA PaCa-2 cell viability was significantly higher than either
gemcitabine (25 and 50 nM) or fucoxanthin (150, 250 and 300 nM)
used alone. A synergistic effect was only observed in the 25-nM
gemcitabine combined with fucoxanthin at concentrations higher than
250-nM groups. In addition, gemcitabine (50 nM) with fucoxanthin
only demonstrated an additive effect.
A possible explanation for this observation may be
that gemcitabine at a concentration of 50 nM is highly cytotoxic,
and therefore low doses of fucoxanthin cannot provide additional
effects beyond the chemotherapeutic dose. PANC-1 cells were found
to be resistant to gemcitabine and sensitive to fucoxanthin
treatment. Thus, fucoxanthin (10 and 20 µM) played a leading role
in the combination effect in the suppression of PANC-1 cells.
Fucoxanthin was shown to significantly enhance the inhibitory
effect of gemcitabine. The cell viability under the combination
treatment was significantly lower than that in cells treated only
with fucoxanthin or gemcitabine. However, the combination effect
was not synergistically improved by these two drugs.
In the present study, it was found that low doses of
fucoxanthin treatment (150, 250, 300 nM) could not inhibit the
growth of 293 cells for 72 h. However, high concentrations of the
fucoxanthin (10 and 20 µM) significantly suppressed the
proliferation of 293 cells. Low concentrations of fucoxanthin did
not promote the inhibitory effect of gemcitabine (25 nM).
Interestingly, in the 50-nM gemcitabine groups, low doses of
fucoxanthin aided in reducing the cytotoxicity of gemcitabine. In
addition, there was more 293 cell survival under the incubation
with combination treatment for 72 h in relation to cells cultured
only with gemcitabine (50 nM). Fucoxanthin was demonstrated to have
no inhibitory effect on human lymphocyte cells, uninfected
leukaemia cell lines and human peripheral blood mononuclear cells,
over a certain concentration range (23). These literature studies potentially
indicate that fucoxanthin is still toxic to non-cancer cells when
used at high doses, although its cytotoxicity is selective. Through
its mechanism of action, gemcitabine has been determined as a
pyrimidine nucleotide analog to be involved in DNA synthesis,
thereby inhibiting the DNA synthesis and relating to cell division
from the whole process of cell mitosis (56-59).
Conversely, fucoxanthin exerts it anti-proliferative and
cancer-preventing effects via different molecules and pathways
including the Bcl-2 proteins, MAPK, apoptosis, or metastasis
(51). Kumar et al reported
that the anti-proliferative effects of fucoxanthin are selective,
i.e., fucoxanthin has the capability to target cancer cells only,
leaving normal physiological cells unaffected or less affected
(51). Therefore, similar to the
results of this previous study, the results of the present study
indicate that fucoxanthin selectively exerts its effect only on
pancreatic cancer cells.
A suitable concentration range of fucoxanthin and
gemcitabine has to be established in order to create synergistic
effects in cancer cell growth inhibition and neutralize toxicity to
non-cancer cells. The arrest of cell cycle progression, induction
of apoptosis, or both, are factors in the inhibition of cancer cell
proliferation (60). The anticancer
activity of gemcitabine is primarily performed by impairing DNA
synthesis. It results in the cytostasis owing to the block of the
cell cycle in the G0-G1 or S phases (61). Subsequently, cells may undergo
apoptosis or mitotic catastrophe upon escaping the cell cycle
blockage, which will finally lead to cell death (62). Gemcitabine, depending on its
exposure time and concentration, has been demonstrated to block
certain human solid tumour cells in the S phase (63). However, a previous study indicated
that gemcitabine arrests cells in the G0-G1,
G1, early S or S phases only depending upon the
concentration of gemcitabine (64).
In the present study, gemcitabine was demonstrated to
dose-dependently arrest the MIA PaCa-2 cells in the S phase after
both 48- and 72-h exposure. Sub-G1 is an index of
apoptotic DNA fragmentation (65).
The results of the present study indicated that gemcitabine first
blocked MIA PaCa-2 cells in the S phase and then induced cell
apoptosis. The same result was obtained in 293 cells. However,
gemcitabine was revealed to arrest PANC-1 cells in the
G0-G1 phase after culture for 48 and 72 h.
Gemcitabine induced the apoptosis of PANC-1 cells in a time- and
dose-dependent manner. Taken together, the results of the present
study on MIA PaCa-2, PANC-1 and 293 cells are consistent with
previous research aforementioned, namely that gemcitabine induces
G0-G1 and S phase arrest and subsequently
undergoes apoptosis. Moreover, all these results are consistent
with the results of the cytotoxicity analysis.
Carotenoids have been demonstrated to inhibit tumour
cell growth by inducing cell cycle arrest at the G1
phase and/or apoptosis (66,67).
Fucoxanthin has been suggested to accumulate cells in the
G0-G1 phase of different cell lines as
previously reported (68). In the
present study, higher concentrations of fucoxanthin were found to
arrest the cells in the G0-G1 phase.
Fucoxanthin has been observed to block the human gastric
adenocarcinoma cell line, MGC-803, in the G2-M phase
(69). By contrast, it has been
suggested that carotenoids cannot arrest cells in the
G2-M phase (70). Thus,
whether fucoxanthin induces the arrest of cells in the
G2-M phase requires further research. Low doses of
fucoxanthin (150, 250 and 300 nM) could not markedly alter the cell
cycle distribution. This is consistent with the cytotoxicity
analysis.
Fucoxanthin combined with gemcitabine was found to
help induce pancreatic cancer cell arrest in the
G0-G1 or S phase. The lack of cell cycle
arrest by fucoxanthin on 293 cells implies that fucoxanthin has
selective toxicity. Both gemcitabine and fucoxanthin block cells in
the G1/S phase and the effects of each compound on
cellular metabolism are different. From literature reported,
carotenoids reverse multidrug resistance and enhance sensitivity of
cancer treatment in in vitro and in vivo models
(55,71,72).
However, in the present study only additive effects were observed.
A plausible explanation for this observation may be the sequence of
drug administration. The administration of scheduled medications
has been demonstrated to be very important for the antitumor
effect. The same drugs with different treatment sequences were
found to produce differing results (73). Gemcitabine and fucoxanthin were
added simultaneously to the cell lines in the present study.
Therefore, pre-treating the cells with one of the compounds and
later treating these cells with the second one should be assessed
in further studies. Nevertheless, low doses of fucoxanthin were
revealed to help improve the anti-proliferative efficacy of
gemcitabine by inducing growth arrest, while high doses of
fucoxanthin inhibited proliferation by inducing apoptosis. The
synergistic effect could be observed by using the best combination
concentrations and optimal treatment sequence of fucoxanthin and
gemcitabine.
In conclusion, fucoxanthin effectively improved the
cytotoxicity of gemcitabine even at low concentrations, and as a
type of carotenoid, fucoxanthin generated a synergistic effect
increasing the sensitivity of chemotherapy in certain pancreatic
cancer cell lines. Fucoxanthin also exhibited selective toxicity
against cancer cells even at low concentrations. Thus, fucoxanthin,
the most abundant carotenoid found in marine algae, at low
therapeutic concentrations, may be considered as a potential
adjunct treatment for pancreatic cancer in combination with other
clinical cancer chemotherapy drugs. A limitation of the present
study is identified in the MTT assay. This assay was used as a
single method for cell viability determination, while other methods
may also be applied. The color of fucoxanthin itself may be a
potential risk that could affect the actual result of the
colorimetric assay. Hence other cell viability assays such as Cell
Counting Kit-8 or crystal violet assays may be used to validate the
cytotoxic results. In addition, using non-cancerous pancreas-origin
cell lines may be a more appropriate choice for future study.
Therefore, the present results are not conclusive. Furthermore,
more data should be generated, such as changes in cell morphology,
and mechanism studies detailing the effect of fucoxanthin on
caspase-3 or caspase-9 should be performed to demonstrate the
effectiveness of fucoxanthin treatment. In addition, the
selectivity of observed fucoxanthin bioactivity should be examined
using in vivo studies.
Supplementary Material
Cell cycle distribution of MIA PaCa-2
treated with gemcitabine in the presence and absence of
fucoxanthin.
Cell cycle distribution of PANC-1
cells after treatment with gemcitabine in the presence and absence
of fucoxanthin.
Cell cycle distribution of 293 cells
after treatment with gemcitabine in the presence and absence of
fucoxanthin.
Cell cycle distribution of MIA PaCa-2
cells after treatment with gemcitabine in the presence and absence
of fucoxanthin.
Cell cycle distribution of PANC-1
cells after treatment with gemcitabine in the presence and absence
of fucoxanthin.
Cell cycle distribution of 293 cells
after treatment with gemcitabine in the presence and absence of
fucoxanthin.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the New
Zealand-China Tripartite Partnership Fund (to JLu, JLi, BZ and TY)
of the New Zealand Ministry of Education, the Royal Society of New
Zealand Catalyst Seeding Fund (grant no. 21-AUT-005-CSG), and the
Shanghai Engineering Research Center of Plant Germplasm Resources
(grant no. 17DZ2252700).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding authors.
Authors' contributions
JLu, JLi, TY, BZ and TF conceived the study. JLu,
KSW, XJW, YL, LC, YZ, MJ, JLiu designed the study and acquired the
data. AH, YH, JLu, XJW and KSW analyzed the data. JLu, JLi, TY, BZ
and TF provided experimental materials. XJW, AH, KSW and JLu wrote
the original draft. YL, LC, YZ, MJ, JLiu, JLu, JLi, TY, BZ, TF and
YH revised the work critically for important intellectual content.
TF, KSW and JLu confirm the authenticity of all the raw data. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society: Cancer Facts and
Figures. American Cancer Society, Atlanta, pp1-56, 2017.
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neoptolemos JP, Urrutia R, Abbruzzese JL
and Büchler MW: Pancreatic cancer. New York, NY, Springer,
2010.
|
|
4
|
Ministry of Health. Cancer programme.
2015. Retrieved March 19, 2015, from http://www.health.govt.nz/our-work/diseases-and-conditions/cancer-programme.
|
|
5
|
Bosetti C, Bertuccio P, Negri E, La
Vecchia C, Zeegers MP and Boffetta P: Pancreatic cancer: Overview
of descriptive epidemiology. Mol Carcinog. 51:3–13. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goodman KA and Hajj C: Role of radiation
therapy in the management of pancreatic cancer. J Surg Oncol.
107:86–96. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Morton JP, Timpson P, Karim SA, Ridgway
RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG,
et al: Mutant p53 drives metastasis and overcomes growth
arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA.
107:246–251. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mulcahy MF, Wahl AO and Small W Jr: The
current status of combined radiotherapy and chemotherapy for
locally advanced or resected pancreas cancer. J Natl Compr Canc
Netw. 3:637–642. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Peng J, Yuan JP, Wu CF and Wang JH:
Fucoxanthin, a marine carotenoid present in brown seaweeds and
diatoms: Metabolism and bioactivities relevant to human health. Mar
Drugs. 9:1806–1828. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang H, Tang Y, Zhang Y, Zhang S, Qu J,
Wang X, Kong R, Han C and Liu Z: Fucoxanthin: A promising medicinal
and nutritional ingredient. Evid Based Complement Alternat Med.
2015(723515)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Beppu F, Niwano Y, Sato E, Kohno M, Tsukui
T, Hosokawa M and Miyashita K: In vitro and in vivo evaluation of
mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol
(FXOH). J Toxicol Sci. 34:693–698. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zorofchian Moghadamtousi S, Karimian H,
Khanabdali R, Razavi M, Firoozinia M, Zandi K and Abdul Kadir H:
Anticancer and antitumor potential of fucoidan and fucoxanthin, two
main metabolites isolated from brown algae. ScientificWorldJournal.
2014(768323)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haefner B: Drugs from the deep: Marine
natural products as drug candidates. Drug Discov Today. 8:536–544.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aravindan S, Delma CR, Thirugnanasambandan
SS, Herman TS and Aravindan N: Anti-pancreatic cancer deliverables
from sea: First-hand evidence on the efficacy, molecular targets
and mode of action for multifarious polyphenols from five different
brown-algae. PLoS One. 8(e61977)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gammone MA and D'Orazio N: Anti-obesity
activity of the marine carotenoid fucoxanthin. Mar Drugs.
13:2196–2214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
López-Rios L, Vega T, Chirino R, Jung JC,
Davis B, Pérez-Machín R and Wiebe JC: Toxicological assessment of
Xanthigen® nutraceutical extract combination:
Mutagenicity, genotoxicity and oral toxicity. Toxicol Rep.
9:1021–1031. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Delma CR, Thirugnanasambandan S,
Srinivasan GP, Raviprakash N, Manna SK, Natarajan M and Aravindan
N: Fucoidan from marine brown algae attenuates pancreatic cancer
progression by regulating p53-NFκB crosstalk. Phytochemistry.
167(112078)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beppu F, Niwano Y, Tsukui T, Hosokawa M
and Miyashita K: Single and repeated oral dose toxicity study of
fucoxanthin (FX), a marine carotenoid, in mice. J Toxicol Sci.
34:501–510. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hashimoto T, Ozaki Y, Mizuno M, Yoshida M,
Nishitani Y, Azuma T, Komoto A, Maoka T, Tanino Y and Kanazawa K:
Pharmacokinetics of fucoxanthinol in human plasma after the oral
administration of kombu extract. Br J Nutr. 107:1566–1569.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dang TT, Bowyer MC, Van Altena IA and
Scarlett CJ: Comparison of chemical profile and antioxidant
properties of the brown algae. Int J Food Sci Technol. 53:174–181.
2008.
|
|
21
|
Guan B, Chen K, Tong Z, Chen L, Chen Q and
Su J: Advances in fucoxanthin research for the prevention and
treatment of inflammation-related diseases. Nutrients.
14(4768)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mumu M, Das A, Emran TB, Mitra S, Islam F,
Roy A, Karim MM, Das R, Park MN, Chandran D, et al: Fucoxanthin: A
promising phytochemical on diverse pharmacological targets. Front
Pharmacol. 13(929442)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ishikawa C, Tafuku S, Kadekaru T, Sawada
S, Tomita M, Okudaira T, Nakazato T, Toda T, Uchihara JN, Taira N,
et al: Anti-adult T-cell leukemia effects of brown algae
fucoxanthin and its deacetylated product, fucoxanthinol. Int J
Cancer. 123:2702–2712. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu CL, Liang AL and Hu ML: Protective
effects of fucoxanthin against ferric nitrilotriacetate-induced
oxidative stress in murine hepatic BNL CL.2 cells. Toxicol In
Vitro. 25:1314–1319. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu CL, Lim YP and Hu ML: Fucoxanthin
enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway
and downregulates DNA repair gene expression in human hepatoma
HepG2 cells. Mar Drugs. 11:50–66. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang SK, Li Y, White WL and Lu J: Extracts
from New Zealand Undaria pinnatifida containing fucoxanthin
as potential functional biomaterials against cancer in vitro. J
Funct Biomater. 5:29–42. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Martin LJ: Fucoxanthin and its metabolite
fucoxanthinol in cancer prevention and treatment. Mar Drugs.
13:4784–4798. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takahashi K, Hosokawa M, Kasajima H,
Hatanaka K, Kudo K, Shimoyama N and Miyashita K: Anticancer effects
of fucoxanthin and fucoxanthinol on colorectal cancer cell lines
and colorectal cancer tissues. Oncol Lett. 10:1463–1467.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Terasaki M, Kubota A, Kojima H, Maeda H,
Miyashita K, Kawagoe C, Mutoh M and Tanaka T: Fucoxanthin and
colorectal cancer prevention. Cancers (Basel).
13(2379)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ming JX, Wang ZC, Huang Y, Ohishi H, Wu
RJ, Shao Y, Wang H, Qin MY, Wu ZL, Li YY, et al: Fucoxanthin
extracted from Laminaria japonica inhibits metastasis and
enhances the sensitivity of lung cancer to Gefitinib. J
Ethnopharmacol. 265(113302)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Malhão F, Macedo AC, Costa C, Rocha E and
Ramos AA: Fucoxanthin holds potential to become a drug adjuvant in
breast cancer treatment: Evidence from 2D and 3D cell cultures.
Molecules. 26(4288)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ye GL, Du DL, Jin LJ and Wang LL:
Sensitization of TRAIL-resistant cervical cancer cells through
combination of TRAIL and fucoxanthin treatments. Eur Rev Med
Pharmacol Sci. 21:5594–5601. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hidalgo M, Cascinu S, Kleeff J, Labianca
R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL and Heinemann V:
Addressing the challenges of pancreatic cancer: Future directions
for improving outcomes. Pancreatology. 15:8–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiang PH, Motoo Y, Sawabu N and Minamoto
T: Effect of gemcitabine on the expression of apoptosis-related
genes in human pancreatic cancer cells. World J Gastroenterol.
12:1597–1602. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gradiz R, Silva HC, Carvalho L, Botelho MF
and Mota-Pinto A: MIA PaCa-2 and PANC-1-pancreas ductal
adenocarcinoma cell lines with neuroendocrine differentiation and
somatostatin receptors. Sci Rep. 6(21648)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ghasemi M, Turnbull T, Sebastian S and
Kempson I: The MTT assay: Utility, limitations, pitfalls, and
interpretation in bulk and single-cell analysis. Int J Mol Sci.
22(12827)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cho YS and Cho-Chung YS: Antisense protein
kinase A RIalpha acts synergistically with hydroxycamptothecin to
inhibit growth and induce apoptosis in human cancer cells:
Molecular basis for combinatorial therapy. Clin Cancer Res.
9:1171–1178. 2003.PubMed/NCBI
|
|
38
|
Bocci G, Fioravanti A, Orlandi P,
Bernardini N, Collecchi P, Del Tacca M and Danesi R: Fluvastatin
synergistically enhances the antiproliferative effect of
gemcitabine in human pancreatic cancer MIAPaCa-2 cells. Br J
Cancer. 93:319–330. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yeo D, Huynh N, Beutler JA, Christophi C,
Shulkes A, Baldwin GS, Nikfarjam M and He H: Glaucarubinone and
gemcitabine synergistically reduce pancreatic cancer growth via
down-regulation of P21-activated kinases. Cancer Lett. 346:264–272.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rathos MJ, Joshi K, Khanwalkar H, Manohar
SM and Joshi KS: Molecular evidence for increased antitumor
activity of gemcitabine in combination with a cyclin-dependent
kinase inhibitor, P276-00 in pancreatic cancers. J Transl Med.
10(161)2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu S, Guo J, Wei W, Zhang J, Fang J and
Beebe SJ: Enhanced breast cancer therapy with nsPEFs and low
concentrations of gemcitabine. Cancer Cell Int.
14(98)2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kornmann M, Butzer U, Blatter J, Beger HG
and Link KH: Pre-clinical evaluation of the activity of gemcitabine
as a basis for regional chemotherapy of pancreatic and colorectal
cancer. Eur J Surg Oncol. 26:583–587. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hernández P, Olivera P, Dueñas-Gonzalez A,
Pérez-Pastenes MA, Zárate A, Maldonado V and Meléndez-Zajgla J:
Gemcitabine activity in cervical cancer cell lines. Cancer
Chemother Pharmacol. 48:488–492. 2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yong-Xian G, Xiao-Huan L, Fan Z and
Guo-Fang T: Gemcitabine inhibits proliferation and induces
apoptosis in human pancreatic cancer PANC-1 cells. J Cancer Res
Ther. 12 (Suppl):S1–S4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Iwase R, Haruki K, Fujiwara Y, Furukawa K,
Shiba H, Uwagawa T, Misawa T, Ohashi T and Yanaga K: Combination
chemotherapy of nafamostat mesylate with gemcitabine for
gallbladder cancer targeting nuclear factor-κB activation. J Surg
Res. 184:605–612. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Rousseau EJ, Davison AJ and Dunn B:
Protection by beta-carotene and related compounds against
oxygen-mediated cytotoxicity and genotoxicity: Implications for
carcinogenesis and anticarcinogenesis. Free Radic Biol Med.
13:407–433. 1992.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bertram JS and Vine AL: Cancer prevention
by retinoids and carotenoids: Independent action on a common
target. Biochim Biophys Acta. 1740:170–178. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pádua D, Rocha E, Gargiulo D and Ramos A:
Bioactive compounds from brown seaweeds: Phloroglucinol,
fucoxanthin and fucoidan as promising therapeutic agents against
breast cancer. Phytochem Lett. 14:91–98. 2015.
|
|
49
|
Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ,
Ha KS, Han JA, Yim SV, Chang K, Kwon YG, et al: Astaxanthin
inhibits nitric oxide production and inflammatory gene expression
by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol
Cells. 16:97–105. 2003.PubMed/NCBI
|
|
50
|
Campo GM, Avenoso A, Campo S, D'Ascola A,
Traina P, Samà D and Calatroni A: The antioxidant effect exerted by
TGF-1beta-stimulated hyaluronan production reduced NF-kB activation
and apoptosis in human fibroblasts exposed to FeSo4 plus ascorbate.
Mol Cell Biochem. 311:167–177. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kumar SR, Hosokawa M and Miyashita K:
Fucoxanthin: A marine carotenoid exerting anti-cancer effects by
affecting multiple mechanisms. Mar Drugs. 11:5130–5147.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fan M, Nath AK, Tang Y, Choi YJ, Debnath
T, Choi EJ and Kim EK: Investigation of the anti-prostate cancer
properties of marine-derived compounds. Mar Drugs.
16(160)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Asai A, Sugawara T, Ono H and Nagao A:
Biotransformation of fucoxanthinol into amarouciaxanthin A in mice
and HepG2 cells: Formation and cytotoxicity of fucoxanthin
metabolites. Drug Metab Dispos. 32:205–211. 2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Maeda H, Hosokawa M, Sashima T, Takahashi
N, Kawada T and Miyashita K: Fucoxanthin and its metabolite,
fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells.
Int J Mol Med. 18:147–152. 2006.PubMed/NCBI
|
|
55
|
Eid SY, Althubiti MA, Abdallah ME, Wink M
and El-Readi MZ: The carotenoid fucoxanthin can sensitize multidrug
resistant cancer cells to doxorubicin via induction of apoptosis,
inhibition of multidrug resistance proteins and metabolic enzymes.
Phytomedicine. 77(153280)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Baker CH, Banzon J, Bollinger JM, Stubbe
J, Samano V, Robins MJ, Lippert B, Jarvi E and Resvick R:
2'-Deoxy-2'-methylenecytidine and 2'-deoxy-2',2'-difluorocytidine
5'-diphosphates: Potent mechanism-based inhibitors of
ribonucleotide reductase. J Med Chem. 34:1879–1884. 1991.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Jones RM, Kotsantis P, Stewart GS, Groth P
and Petermann E: BRCA2 and RAD51 promote double-strand break
formation and cell death in response to gemcitabine. Mol Cancer
Ther. 13:2412–2421. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Li Y, Wang LR, Chen J, Lou Y and Zhang GB:
First-line gemcitabine plus cisplatin in nonsmall cell lung cancer
patients. Dis Markers. 2014(960458)2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ke Z, Fu T, Wang X, Xuan M, Yin H, Zhou J,
Liu Y and Liang A: CHK1 inhibition overcomes gemcitabine resistance
in non-small cell lung cancer cell A549. Res Sq, 2022.
|
|
60
|
Du L, Lyle CS, Obey TB, Gaarde WA, Muir
JA, Bennett BL and Chambers TC: Inhibition of cell proliferation
and cell cycle progression by specific inhibition of basal JNK
activity: Evidence that mitotic Bcl-2 phosphorylation is
JNK-independent. J Biol Chem. 279:11957–11966. 2004.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bildstein L, Pili B, Marsaud V, Wack S,
Meneau F, Lepêtre-Mouelhi S, Desmaële D, Bourgaux C, Couvreur P and
Dubernet C: Interaction of an amphiphilic squalenoyl prodrug of
gemcitabine with cellular membranes. Eur J Pharm Biopharm.
79:612–620. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mc Gee MM: Targeting the mitotic
catastrophe signaling pathway in cancer. Mediators Inflamm.
2015(146282)2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Montano R, Thompson R, Chung I, Hou H,
Khan N and Eastman A: Sensitization of human cancer cells to
gemcitabine by the Chk1 inhibitor MK-8776: Cell cycle perturbation
and impact of administration schedule in vitro and in vivo. BMC
Cancer. 13(604)2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Guo JR, Chen QQ, Lam CW, Wang CY, Wong VK,
Chang ZF and Zhang W: Profiling ribonucleotide and
deoxyribonucleotide pools perturbed by gemcitabine in human
non-small cell lung cancer cells. Sci Rep. 6(37250)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kajstura M, Halicka HD, Pryjma J and
Darzynkiewicz Z: Discontinuous fragmentation of nuclear DNA during
apoptosis revealed by discrete ‘sub-G1’ peaks on DNA content
histograms. Cytometry A. 71:125–131. 2007.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Haddad NF, Teodoro AJ, Leite de Oliveira
F, Soares N, de Mattos RM, Hecht F, Dezonne RS, Vairo L, Goldenberg
RC, Gomes FC, et al: Lycopene and beta-carotene induce growth
inhibition and proapoptotic effects on ACTH-secreting pituitary
adenoma cells. PLoS One. 8(e62773)2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Milani A, Basirnejad M, Shahbazi S and
Bolhassani A: Carotenoids: Biochemistry, pharmacology and
treatment. Br J Pharmacol. 174:1290–1324. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Das SK, Hashimoto T, Shimizu K, Yoshida T,
Sakai T, Sowa Y, Komoto A and Kanazawa K: Fucoxanthin induces cell
cycle arrest at G0/G1 phase in human colon carcinoma cells through
up-regulation of p21WAF1/Cip1. Biochim Biophys Acta. 1726:328–335.
2005.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yu RX, Hu XM, Xu SQ, Jiang ZJ and Yang W:
Effects of fucoxanthin on proliferation and apoptosis in human
gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway.
Eur J Pharmacol. 657:10–19. 2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Koklesova L, Liskova A, Samec M, Buhrmann
C, Samuel SM, Varghese E, Ashrafizadeh M, Najafi M, Shakibaei M,
Büsselberg D, et al: Carotenoids in cancer apoptosis-the road from
bench to bedside and back. Cancers (Basel). 12(2425)2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
García-Olmo DC, Riese HH, Escribano J,
Ontañón J, Fernandez JA, Atiénzar M and García-Olmo D: Effects of
long-term treatment of colon adenocarcinoma with crocin, a
carotenoid from saffron (Crocus sativus L.): An experimental
study in the rat. Nutr Cancer. 35:120–126. 1999.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Eid SY, El-Readi MZ and Wink M:
Carotenoids reverse multidrug resistance in cancer cells by
interfering with ABC-transporters. Phytomedicine. 19:977–987.
2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kwon M, Jung H, Nam GH and Kim IS: The
right timing, right combination, right sequence, and right delivery
for cancer immunotherapy. J Control Release. 331:321–334.
2021.PubMed/NCBI View Article : Google Scholar
|