Introduction
The medicinal plant Andrographis paniculata
(Burm. f.) Wall. ex Nees (A. paniculata) is indigenous to
Southeast Asia, China, North America, and the West Indies (1). This herb is widely used in herbal
remedies to treat malaria, viral hepatitis and liver cancer
(2). Numerous natural compounds
have been identified in A. paniculata, including
andrographolide and its derivatives. These compounds are well-known
for their biological activities, including anti-SARS CoV-2 activity
(3,4). Anticancer studies on this plant have
begun a good many years ago, using B16F0 melanoma syngenic and
HT-29 xenograft models (2003), colon cancer cell line HT-29 (2004),
human lymphocytes and albino mice (2014), the Caco-2 model (2017),
and acute myeloid leukemia (AML) cell line U937 (2018) (5-9).
The anticancer effect of A. paniculata has been demonstrated
in a variety of ways, including cell cycle arrest, induction of
apoptosis, anti-angiogenic behavior, and suppression of IL-6
expression (10). Andrographolide
was reported to inhibit PI3K/AKT signaling in the A549 cell model
(11).
The neurotrophic receptor tyrosine kinase
(NTRK)1, NTRK2, and NTRK3 genes code for members of
the tropomyosin receptor kinase (Trk) family, an upstream molecule
in the PI3K/AKT signaling pathway (12). Native Trk must be stimulated by an
extracellular signal, such as nerve growth factor, brain-derived
growth factor, or neurotrophin 3, in order to become active
(12). However, due to the fusion
of NTRK3 and ETS variant transcription factor 6
(ETV6), ETV6-NTRK3 (EN) can self-phosphorylate (13). The EN fusion gene has been
identified in congenital fibrosarcoma (14), secretory breast carcinoma (15), AML (16,17),
mammary analog secretory carcinoma of the salivary gland (18), chronic eosinophilic leukemia
(19) congenital mesoblastic
nephroma (20), and thyroid cancer
associated with 131I radiation exposure (21). Due to the already low prevalence of
NTRK fusions in most tumours (<1%) EN cases are even more
uncommon (22). To date, TRK
inhibitors are a viable treatment option for patients whose tumours
test positive for the EN fusion. Both larotrectinib and
entrectinib, which target the NTRK fusion protein, have shown
promising results in recent clinical trials against locally
advanced and metastatic solid tumours (23,24).
EN fusions are typically found in rare diseases, such as primary
renal fibrosarcoma (only six cases have been reported), secretory
carcinoma of the breast and salivary gland (1 case), and AML (4
cases). Few cases have been reported, and thus EN gene fusion
expression requires additional clinical data and fundamental
research to be supported.
The purpose of the present study was to ascertain
the inhibitory effect of A. paniculata methanol extract
(MeAP) on EN-associated cell lines (IMS-M2 and BaF3/EN) as well as
to identify the mechanism of action.
Materials and methods
Materials and plant extraction
preparation
Andrographis paniculata (Burm. f.) Wall. ex
Nees (A. paniculata) was collected in August 2017 and
verified by Dr Dang Van My (Traditional Medicine Center, Tinh Bien,
Vietnam) (voucher no. BNAG-2017-0115). The leaves were cleaned and
dried at 40˚C in a dry oven after collection. Dry samples were
blended into a fine powder and mixed with methanol (1:10 w/v). The
mixture was spun at room temperature for 4 days before filtering
with Whatman filter paper. The obtained extract was evaporated at
40˚C under vacuum. Subsequently, the crude extract continued to be
placed in the drying oven at 40˚C for solvent evaporation and
elevated drying. The extraction efficacy was 4.18%. The 200-mg/ml
stock solution was produced by weighing and dissolving the dry
crude extract in dimethylsulfoxide (DMSO). The stock solution was
stored at -20˚C.
The inhibitor of EN, PKC412 (Sigma-Aldrich; Merck
KGaA) was dissolved in DMSO and was used as a positive reagent
control (13). Control cells were
grown with the same amount of carrier DMSO as in the highest
reagent concentration. In all of the experiments, the amount of
DMSO was kept below 0.1% to keep it from killing cells.
Cell lines and culture conditions
In the present study, the EN-positive human
AML cell line, IMS-M2, provided by Professor Yuko Sato (University
of Tokyo, Tokyo, Japan), the stable transfection with EN,
BaF3/EN (established by HTC), and the Vero
(ATCC-CCL-81™) cells were used (13,25).
Vero cells were used as negative control cells to specify the
selective effect of MeAP on cells with EN. The cells were cultured
at 37˚C in a humidified incubator with 5% CO2 in Roswell
Park Memorial Institute 1640 medium (Sigma-Aldrich; Merck KGaA)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.), 100 IU/ml penicillin, and 0.1 mg/ml streptomycin (both from
Sigma-Aldrich; Merck KGaA).
Cell viability
The cells (1x105 cells/ml) were seeded in
6-well plates with or without MeAP treatment. Following treatment
of the cells, 10 µl of cell suspension and 10 µl of 0.4% Trypan
blue (product no. T8154; Sigma Aldrich; Merck KGaA) were mixed
together. The cells were manually counted with a hemocytometer
(26). After 48 h of incubation,
the cytotoxicity of MeAP was determined dose-dependently, and the
half maximal inhibitory concentration (IC50) was then
calculated.
For dose and cell-density dependent tests, cells
were treated with or without MeAP (12.5 µg/ml) for 48 h. For the
time-dependent test, cells were treated with or without MeAP (12.5
µg/ml) at 24, 48, 72 and 96 h.
The cytotoxic activity of MeAP was determined using
the 3-(4,5-dimethylthiazol)-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) method. DMSO (100 µl) was used to used to dissolve the purple
formazan and the absorbance was read at 570 nm using a universal
microplate reader (27) for the
remaining adherent cell lines. The methods cited were modified to
accommodate the research conditions of the laboratory.
To identify the selective effects of MeAP on
leukemia cells, the Selective Index (SI) values were calculated. SI
values >3 were considered to be highly selective for cells of
interest (28).
Morphological changes in MeAP-treated
cells
IMS-M2 and BaF3/EN cells were seeded overnight at a
density of 1x105 cells/well in six-well culture plates.
The cells were then treated with varying concentrations of MeAP
(6.25, 12.5, 25, and 50 µg/ml) or PKC412 (60 nM) and maintained at
37˚C and 5% CO2 for 72 h. The untreated cells served as
the control. To detect the morphological changes in the cells, an
inverted light microscope was used at a magnification of x10.
Western blot analysis
The IMS-M2 and BaF3/EN cells were plated at a
density of 1x105 cells/ml on a 10-cm dish with varying
concentrations of MeAP (12.5, 25, or 50 µg/ml). After the indicated
time points (4, 8 or 24 h) of incubation, the cells were removed
and washed twice with PBS (-) (TBR Technology Corporation). The
cells were then lysed in ice-cold protein lysis buffer (10 mM
disodium diphosphate, 50 mM sodium fluoride, 5 mM
ethylenediaminetetraacetic acid, 1 mM sodium orthovanadate, 5 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1 mM
phenylmethylsulfonyl fluoride, 0.01% Triton X-100, 150 mM sodium
chloride, and 75 µg/ml aprotinin) (29). After centrifuging the cells at
15,000 x g for 10 min (4˚C), total protein cell lysates were
obtained. Protein samples (20 µg, as measured by the BCA protein
assay kit) were loaded onto wells, and the proteins were resolved
on a 12.5% polyacrylamide gel electrophoresis, and then
electroblotted onto a Hypond-P membrane (Amersham; Cytiva). The
membrane was then blocked at room temperature for 1 h with 5% skim
milk buffer. Following a wash, primary antibodies were used to
probe the membrane, and ECL was used to detect antibody binding
(Amersham; Cytiva). Anti-TrkC (C-14) (1:500; cat. no. sc-11; Santa
Cruz Biotechnology, Inc.), anti-actin (1:1,000; cat. no. A2066;
Sigma-Aldrich; Merck KGaA), anti-phosphotyrosine 4G10 (1:1,000;
cat. no. 05-321MG; Upstate Biotechnology, Inc.; Merck KGaA),
caspase-3 (1:1,000; cat. no. 9662; Cell Signaling Technology, Inc.)
and anti-PARP (1:1,000; cat. no. 016-16831; FUJIFILM Wako Pure
Chemical Corporation) were used as primary antibodies. The primary
antibodies were incubated at room temperature for 1 h, or overnight
at 4˚C. The membranes were then washed twice for 15 min each time
and incubated with a horseradish peroxidase (HRP)-conjugated
secondary antibody for 1 h at room temperature (1:1,000; anti-mouse
IgG HRP (cat. no. sc-2031) or anti-rabbit IgG HRP (cat. no.
sc-2317; both from Santa Cruz Biotechnology, Inc.).
Immunoprecipitation (IP)
IMS-M2 or BaF3/EN cells were treated with MeAP
(12.5, 25 or 50 g/ml) for 8 h and then harvested for IP. The cells
were lysed as described above. A total of 500 mg of total cell
lysates were immunoprecipitated overnight at 4˚C with anti-TrkC
(C-14) (1:500; cat. no. sc-11; Santa Cruz Biotechnology, Inc.).
Subsequently, Protein G Sepharose 4 Fast Flow (Amersham Pharmacia
Biosciences; Cytiva) was added and all the procedures were carried
out in accordance with the manufacturer's instructions. The
immunoprecipitates were washed with Tris-buffered saline with
Tween-20 three times. Using SDS-PAGE and western blotting, the
bound proteins were separated and analysed.
Statistical analysis
Data were compiled from three independent
experiments and are presented as the mean ± SEM. Data were compared
using a paired Student's t-test or a one-way ANOVA with Tukey's
post hoc test in GraphPad Prism version 8.3.0. (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of MeAP on EN-positive cells in
a dose-dependent manner
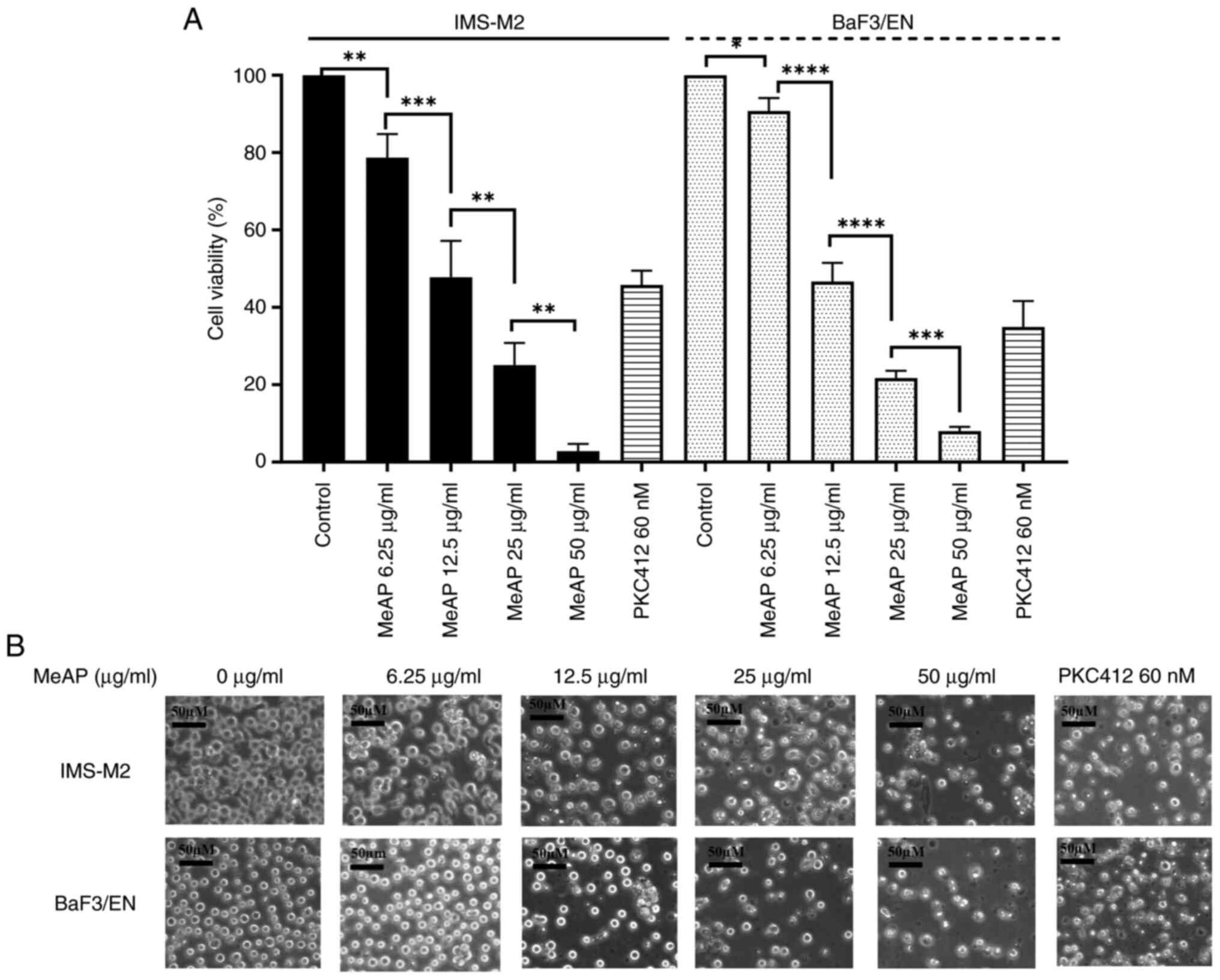
The inhibitory potential of MeAP was determined by
assessing its cytotoxic effects on IMS-M2 and BaF3/EN cells at
varying concentrations. MeAP (0, 6.25, 12.5, 25 and 50 µg/ml) was
added to the 6-well plates and results were collected after 48 h.
PKC412 at 60 nM (13) was used as a
positive control. MeAP was found to inhibit the viability of these
cells (Fig. 1A). The ANOVA method
was used to determine whether there were any statistically
significant differences in the toxic tendency of the extract across
these cell lines. The IC50 values for IMS-M2 and BaF3/EN
were 12.38±0.57 µg/ml and 13.06±0.49 µg/ml, respectively.
Morphological assessment was conducted 48 h after
treatment (Fig. 1B). The
observations indicated that the cells grew normally in the
untreated group, with normal morphology such as round shape,
uniform size, intact membrane and nucleus (IMS-M2 and BaF3/EN). In
comparison to the control group, when the extract concentration was
increased, the number and size of the treated cells significantly
decreased. The shape of the cells was altered and cells began to
shrink. By contrast to organelle dilation caused by early membrane
permeability, cell shrinkage is a frequent and prominent
morphological property of the apoptotic cell death process
(30,31). The observational experiment revealed
that the assessed cell lines exhibited apoptotic tendencies.
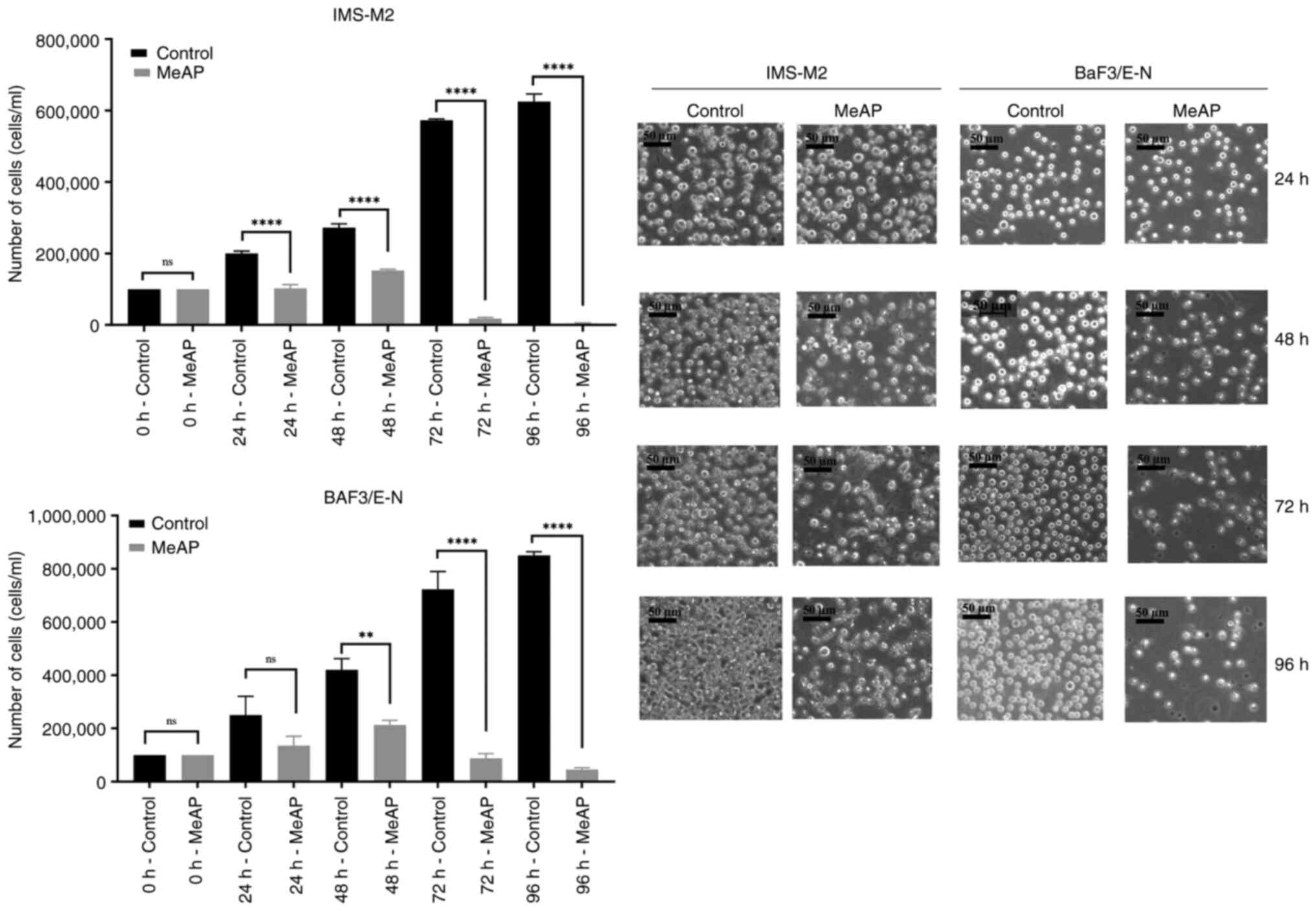
Effect of MeAP on AML cells in
time-dependent manner
For up to 96 h, IMS-M2 and BaF3/EN cells were
examined for viability with or without MeAP (12.5 µg/ml) treatment.
At five time points (0, 24, 48, 72 and 96 h), the number of viable
cells was observed and calculated. As revealed in Fig. 2, MeAP was able to inhibit the
viability of cells in time-dependent manner. After 24 h of exposure
to the extract, the cells grew very slowly and the number of cells
was not significantly altered (Fig.
2, left panels). Between 48 and 72 h, the number of viable
cells began to markedly decline. After 72 h of treatment, only 15%
of the cells remained viable, and decreased to ~0% 24 h later. The
cell density decreased as the culture time was extended. Fig. 2, right panel shows how cell
morphology changed after MeAP co-cultured, with cell shrinkage,
cell debris, and membrane blebbing.
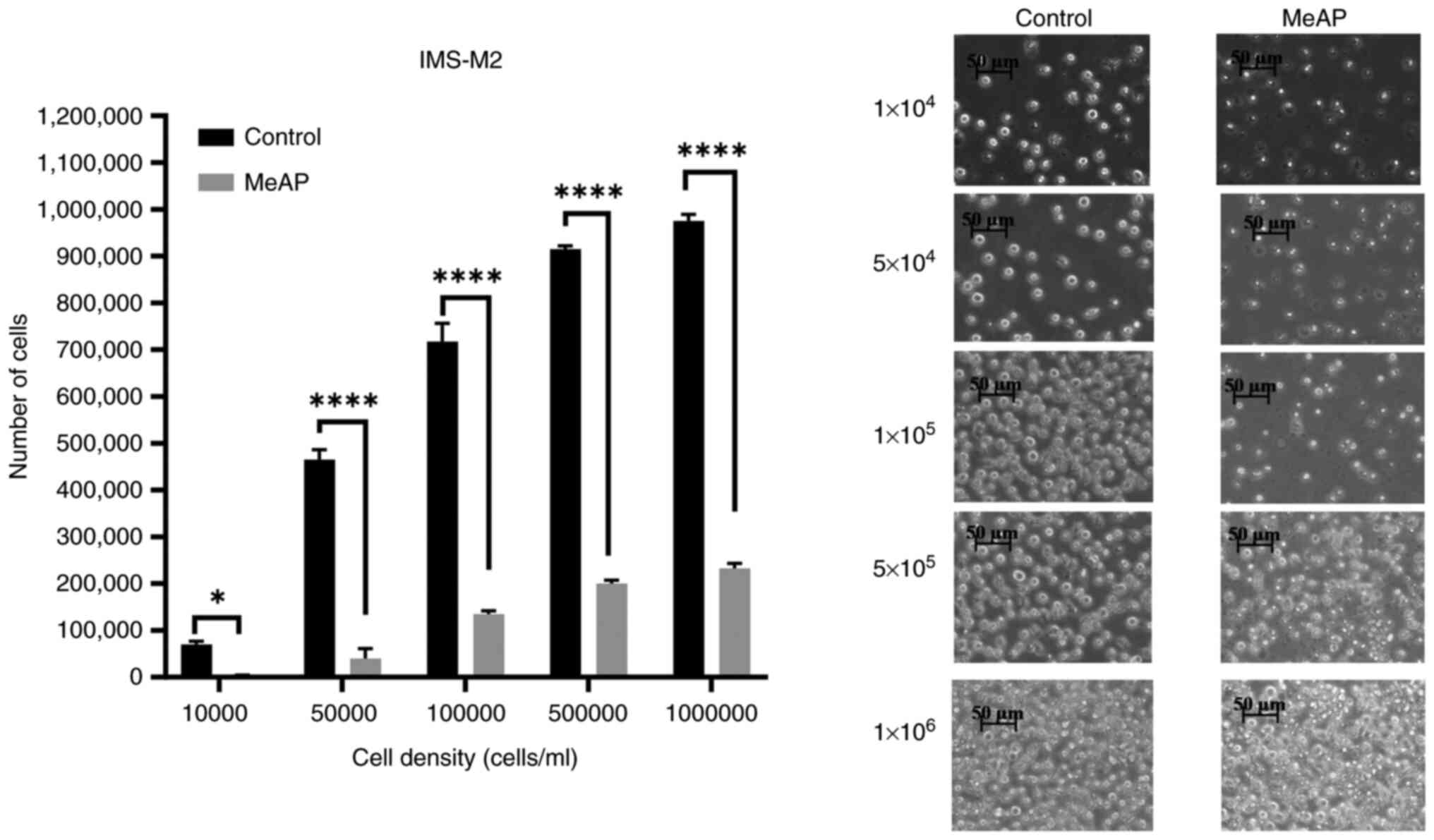
Effect of MeAP on AML cells in a cell
density-dependent manner
Statistical analysis of the effect of MeAP (12.5
µg/ml) revealed statistical differences between the control and
experimental groups at the same cell density, as well as
differences between the groups (Fig.
3). Experiments with various densities (P<0.0001;
<α=0.05) were performed. When treated with MeAP, almost all
cells died at a density of 104 cells/ml. When the cell
density increased from 5x104 to 5x105
cells/ml, the percentage of viable cells increased from 8.72 to
21.86%. The percentage of viable cells in the experimental group
was 23.84±0.58% at a density of 106 cells/ml. The
percentage of cells in the MeAP-treated group was significantly
lower than that in the control group, decreasing from 100 to 76.15%
at a density of 104-106 cells/ml. In summary,
the inhibitory effect of MeAP on IMS-M2 and BaF3/EN cell viability
at the IC50 concentrations was cell
density-dependent.
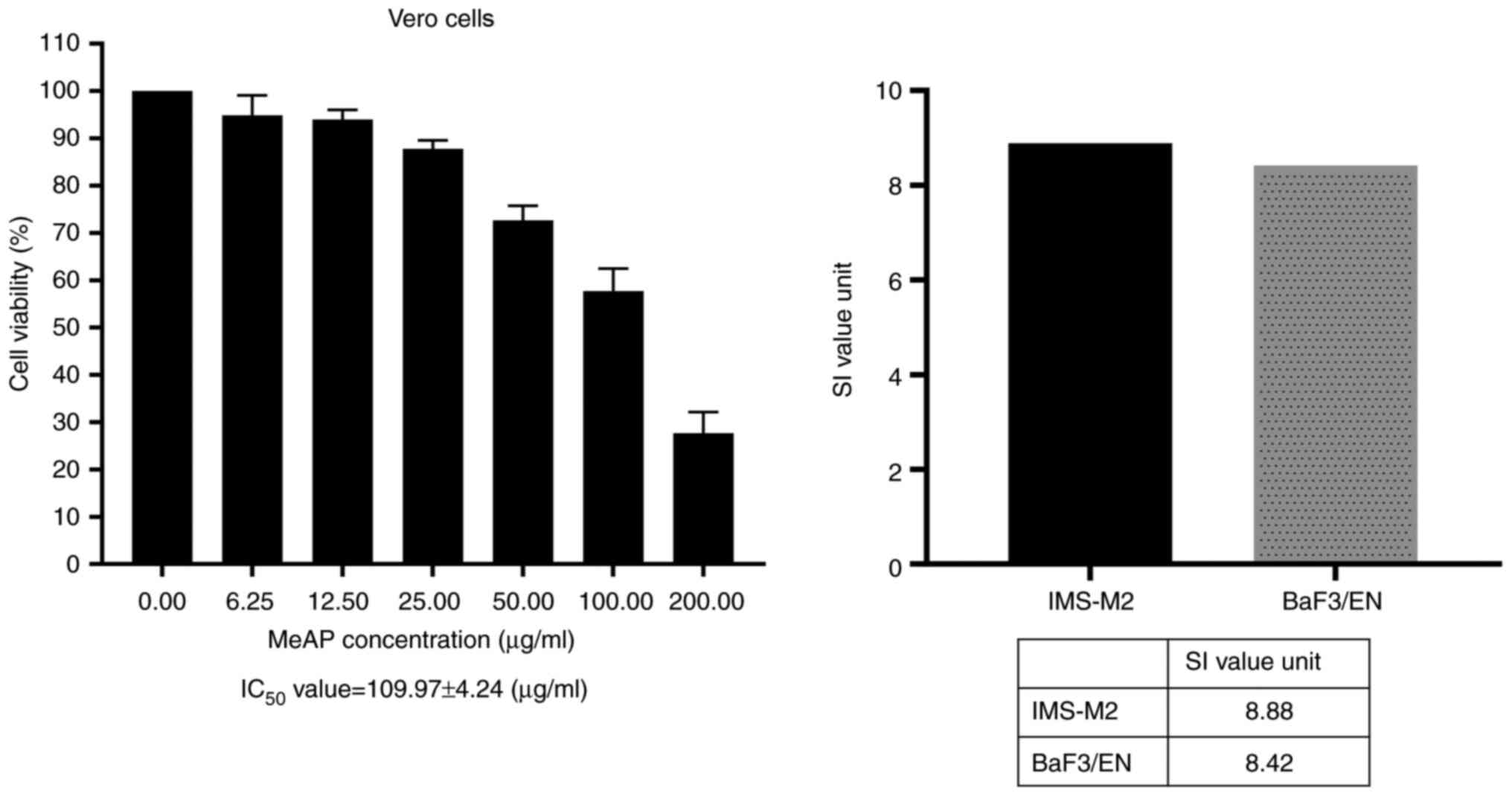
Selective index
As illustrated in Fig.
4, MeAP exerted a significantly less sensitive effect on Vero
cells, with an IC50 value of 109.97±4.24 (µg/ml). It was
also demonstrated that MeAP had an SI value >3, which indicated
that it had marked cytotoxic potential and was very efficient at
killing leukemia cells (Fig. 4,
right panel).
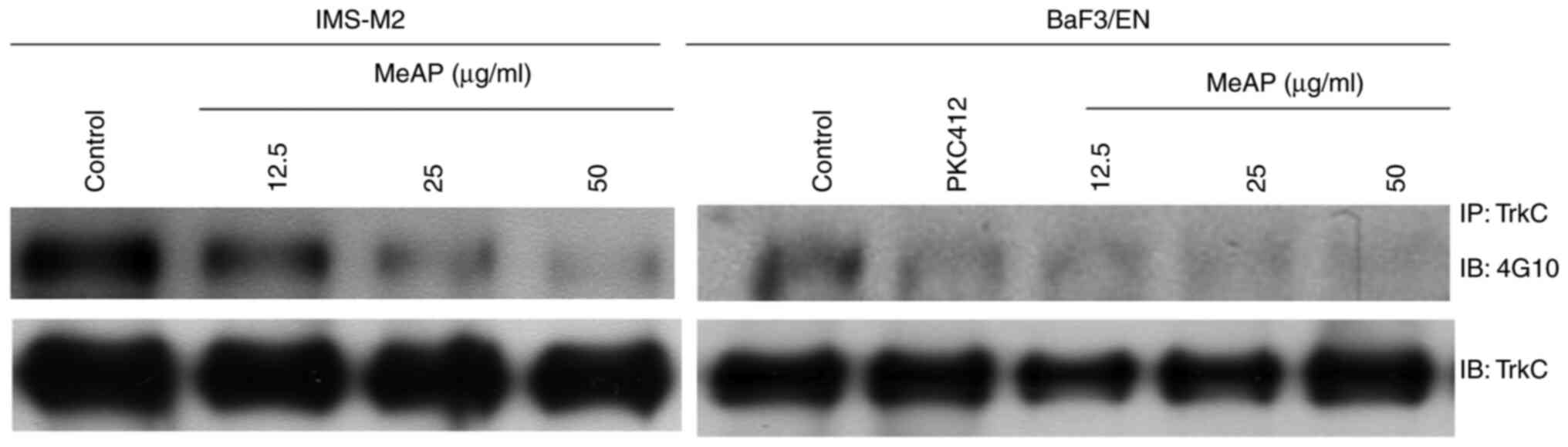
MeAP inhibits the activity of EN
protein
Although it is unknown how EN leads to cancer, the
fusion protein has been considered as a significant target for
cancer treatment (9,32). Additionally, previous research using
an EN-expressing cell model demonstrated that PKC412 is an
effective inhibitor of EN-associated leukemia (13).
It was hypothesized that the viability inhibition
observed in both cell lines is due to the fusion protein's
phosphorylation being inhibited. To elucidate the mechanism of
MeAP-mediated viability inhibition in IMS-M2 and BaF3/EN cells, the
phosphorylation status of EN in these cells was examined after
treatment with or without MeAP. To determine the status of EN
tyrosine phosphorylation, the total protein of EN was
immunoprecipitated with TrkC antibody and immunoblotted with 4G10
antibody. As predicted, MeAP treatment inhibited the
phosphorylation of EN in a dose-dependent manner, but total EN
protein levels were unaffected (Fig.
5).
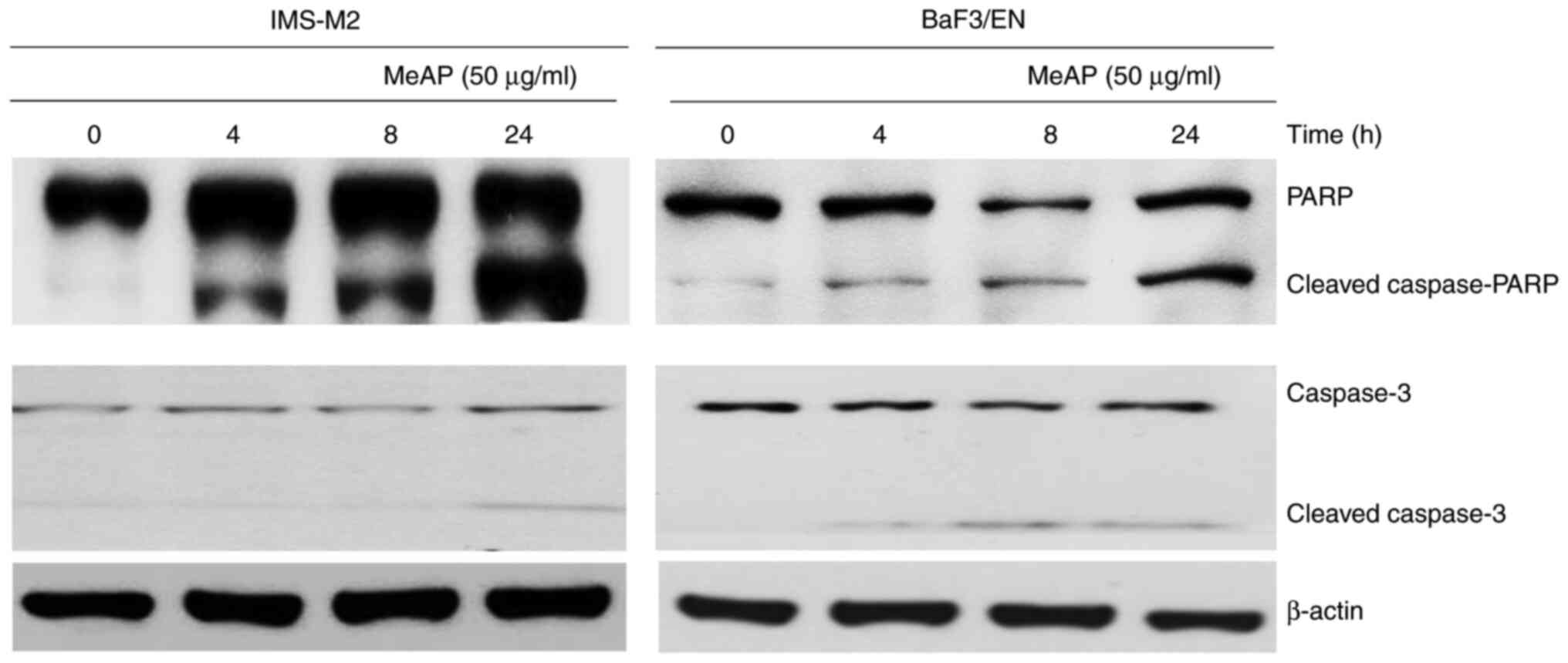
MeAP induces apoptosis in IMS-M2 and
BaF3/EN cells
Cell viability inhibition of IMS-M2 and BaF3/EN
cells following MeAP treatment may result in changes in the shape
and size of cells, as illustrated in Fig. 1, Fig.
2 and Fig. 3. Next, it was
examined whether MeAP treatment could affect the expression of
apoptotic markers. These cells were treated with MeAP at a
concentration of 50 µg/ml for up to 24 h. The results indicated
that MeAP treatment activated the caspase cascade. PARP and
caspase-3 molecules were turned into cleaved forms in the cells
that were exposed to MeAP (50 µg/ml), which indicated that
apoptosis occurred. These findings indicated that MeAP induced
apoptosis in IMS-M2 and BaF3/E-N cells (Fig. 6).
Discussion
NTRK1, NTRK2, and NTRK3 encode
the tyrosine kinases TrkA, TrkB, and TrkC, respectively. In a
variety of tumor types, oncogenic gene fusions involving members of
the NTRK family have been identified and appear to result in
constitutive Trk kinase activity (33). Orally administered larotrectinib is
a highly selective inhibitor that inhibits all three Trk protein
isoforms at nanomolar concentrations (34). Significant tumor regressions have
been reported with larotrectinib in a child with congenital
fibrosarcoma harboring an EN fusion and an adult with a soft
tissue sarcoma harboring an LMNA-NTRK1 fusion (35,36).
Larotrectinib has also demonstrated preliminary activity in a
number of other cancer types with NTRK fusion positivity
(23). In July 2016, the FDA
designated larotrectinib as a breakthrough therapy for the
treatment of unresectable or metastatic solid tumors with
NTRK fusions.
The EN fusion gene is expressed at low levels
in AML, however it exerts a potent transforming effect on numerous
cell lines, including hematopoietic cells, and transformed cells
can induce tumors in nude mice (13,37).
Previous research documented two cases of EN-AML, one with
AML M2 and severe myelofibrosis at the time of diagnosis, as well
as rapidly spreading leukemia cells to multiple organs, and the
other with primary myelofibrosis and progression to AML M7. The
EN fusion gene may be involved in the pathogenesis of acute
myeloid leukemia (37).
Due to the rarity of EN-positive cases in the world
(22), the established cell lines
were also rare and not commercially available. Extreme difficulty
exists in obtaining cell lines with EN fusion. In the present
study, two EN-positive cells were utilised, one from a human
(IMS-M2 cell line) and the other from transfected mouse BaF3 cells
(BaF3/EN). It was demonstrated that BaF3/EN cell growth was
dependent on EN-signalling (13).
Because the number of cases is so low, there is
almost no literature on the use of medicinal herbs to treat
diseases caused by the fusion gene EN. This may be the first
study to mention the use of medicinal herbs to prevent leukemia
cell proliferation caused by the EN fusion gene.
Currently, reports on the ability of inhibition of
the proliferation of cancer cells in A. paniculata have
primarily focused on andrographolide compounds, which are found in
high concentrations in leaves (38). Indeed, this compound was shown to
induce cell cycle arrest and mitochondrial-mediated apoptosis in
the leukemia cell line HL-60 with an IC50 of 14.01 µg/ml
after a 24-h experiment (39).
Other research has demonstrated that the IC50 for this
compound's toxicity to MCF-7 breast cancer cell lines is 500 g/ml.
Furthermore, scientists have investigated the effects of this
compound on many other cancer cell lines and discovered a positive
inhibitory effect on the non-small lung cancer cell line A549, as
well as on the nasopharyngeal cancer cell lines (HSC-2, HSC-3, and
HSC-4) (40,41).
In addition, MeAP has been demonstrated to inhibit
the proliferation of colon cancer (HT-29 cells) (5), human alveolar basal epithelial cell
line (A-549), human breast adenocarcinoma cell line (MCF-7), human
embryonic kidney (HEK), human cervical cancer cell line (HeLa), and
human invasive ductal carcinoma cell line (BT-544), as demonstrated
in previous study (42).
Furthermore, The GC-MS chromatogram of MeAP extracts displayed 21
peaks, indicating the presence of 21 distinct phytochemical
compounds. It was determined that 2(5H)-furanone (14.73%), quinic
acid (QA; 17.32%), and phytol (11.43%) were the most abundant
phytochemicals (42).
In the present study, the IC50 values of
MeAP were determined to be 12.38±0.57 µg/ml (IMS-M2) and 13.06±0.49
µg/ml (BaF3/EN). The SI value helps to evaluate the specificity of
an extract for certain cells. A high SI value indicates a more
selective extract. An SI value >3 units indicates the general
toxicity of a compound (28). The
results in the present study revealed that MeAP had an SI value of
>3, which indicated that it exerted marked cytotoxic potential
and was very effective at killing leukemia cells that had
EN.
Moreover, MeAP treatment inhibited EN
phosphorylation and induced apoptosis in these cells. Therefore, it
was concluded that the inhibitory effect of MeAP on AML cell lines
harboring the EN fusion gene was dependent on dose, time,
and cell density. MeAP can stop the viability of EN-carrying AML
cells by blocking the phosphorylation of EN, which causes the cells
to die.
The limitation of the present study is that no
knockout or overexpression cell line with its parental cell line
was used, such as IMS-M2 vs. IMS-M2 EN knockout or BaF3 vs.
BaF3/EN, to provide solid evidence for the selective activity of
MeAP. Future research should be conducted to address this
issue.
Acknowledgements
We would like to thank Professor Yuko Sato
(University of Tokyo, Japan) for providing the IMS-M2 cell line
used in the present study.
Funding
Funding: The present study was supported by Thu Dau Mot
University under grant no. DT.21.1-058.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HTC and BTKL conceived and designed the study. VNT,
NTQ and HTC performed the experiments; VNT, NTQ, BTKL and HTC
acquired and analyzed the data, as well as wrote and revised the
manuscript critically for important intellectual content. BTKL and
HTC confirm the authenticity of all the raw data. All authors have
read and agreed to the published version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hossain MS, Urbi Z, Sule A and Hafizur
Rahman KM: Andrographis paniculata (Burm. f.) Wall. ex Nees:
A review of ethnobotany, phytochemistry, and pharmacology.
ScientificWorldJournal. 2014(274905)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Davis C, Chan BY, Zhen Ong AS, Koh Y, Wen
Yap AFH, Goh SH and Vidyarthi AR: An evaluation of a medical
student international service-learning experience in Southeast
Asia. Educ Health (Abingdon). 34:3–10. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jadhav AK and Karuppayil SM:
Andrographis paniculata (Burm F) Wall ex Nees: Antiviral
properties. Phytother Res. 35:5365–5373. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Sa-Ngiamsuntorn K, Suksatu A, Pewkliang Y,
Thongsri P, Kanjanasirirat P, Manopwisedjaroen S,
Charoensutthivarakul S, Wongtrakoongate P, Pitiporn S, Chaopreecha
J, et al: Anti-SARS-CoV-2 activity of Andrographis
paniculata extract and its major component andrographolide in
human lung epithelial cells and cytotoxicity evaluation in major
organ cell representatives. J Nat Prod. 84:1261–1270.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kumar RA, Sridevi K, Kumar NV, Nanduri S
and Rajagopal S: Anticancer and immunostimulatory compounds from
Andrographis paniculata. J Ethnopharmacol. 92:291–295.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rajagopal S, Kumar RA, Deevi DS,
Satyanarayana C and Rajagopalan R: Andrographolide, a potential
cancer therapeutic agent isolated from Andrographis
paniculata. J Exp Ther Oncol. 3:147–158. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahmad S, Ahmad S, Arshad M and Afzal M:
Andrographia paniculata a miracle herbs for cancer
treatment: In vivo and in vitro studies against aflatoxin B1
toxicity. Egypt J Med Hum Genet. 15:163–171. 2014.
|
|
8
|
Li L, Yue GG, Lee JK, Wong EC, Fung KP, Yu
J, Lau CB and Chiu PW: The adjuvant value of Andrographis
paniculata in metastatic esophageal cancer treatment-from
preclinical perspectives. Sci Rep. 7(854)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hodroj MH, Jardaly A, Abi Raad S, Zouein A
and Rizk S: Andrographolide potentiates the antitumor effect of
topotecan in acute myeloid leukemia cells through an intrinsic
apoptotic pathway. Cancer Manag Res. 10:1079–1088. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Malik Z, Parveen R, Parveen B, Zahiruddin
S, Aasif Khan M, Khan A, Massey S, Ahmad S and Husain SA:
Anticancer potential of andrographolide from Andrographis
paniculata (Burm.f.) Nees and its mechanisms of action. J
Ethnopharmacol. 272(113936)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA
and Chen JH: Inhibitory effects of andrographolide on migration and
invasion in human non-small cell lung cancer A549 cells via
down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol.
632:23–32. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Amatu A, Sartore-Bianchi A and Siena S:
NTRK gene fusions as novel targets of cancer therapy across
multiple tumour types. ESMO Open. 1(e000023)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chi HT, Ly BT, Kano Y, Tojo A, Watanabe T
and Sato Y: ETV6-NTRK3 as a therapeutic target of small molecule
inhibitor PKC412. Biochem Biophys Res Commun. 429:87–92.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Knezevich SR, McFadden DE, Tao W, Lim JF
and Sorensen PH: A novel ETV6-NTRK3 gene fusion in congenital
fibrosarcoma. Nat Genet. 18:184–187. 1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tognon C, Knezevich SR, Huntsman D,
Roskelley CD, Melnyk N, Mathers JA, Becker L, Carneiro F,
MacPherson N, Horsman D, et al: Expression of the ETV6-NTRK3 gene
fusion as a primary event in human secretory breast carcinoma.
Cancer Cell. 2:367–376. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Eguchi M, Eguchi-Ishimae M, Tojo A,
Morishita K, Suzuki K, Sato Y, Kudoh S, Tanaka K, Setoyama M,
Nagamura F, et al: Fusion of ETV6 to neurotrophin-3 receptor TRKC
in acute myeloid leukemia with t(12;15)(p13;q25). Blood.
93:1355–1363. 1999.PubMed/NCBI
|
|
17
|
Setoyama M, Tojo A, Nagamura F, Asano S,
Ishimae M, Eguchi M and Kamada N: A unique translocation of the
gene in a case of acute myelogenous leukemia with inv(12)(p13q15).
Blood. 92:1454–1455. 1998.PubMed/NCBI
|
|
18
|
Skálová A, Vanecek T, Sima R, Laco J,
Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RH,
Passador-Santos F, et al: Mammary analogue secretory carcinoma of
salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto
undescribed salivary gland tumor entity. Am J Surg Pathol.
34:599–608. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Forghieri F, Morselli M, Potenza L,
Maccaferri M, Pedrazzi L, Paolini A, Bonacorsi G, Artusi T,
Giacobbi F, Corradini G, et al: Chronic eosinophilic leukaemia with
ETV6-NTRK3 fusion transcript in an elderly patient affected with
pancreatic carcinoma. Eur J Haematol. 86:352–355. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rubin BP, Chen CJ, Morgan TW, Xiao S,
Grier HE, Kozakewich HP, Perez-Atayde AR and Fletcher JA:
Congenital mesoblastic nephroma t(12;15) is associated with
ETV6-NTRK3 gene fusion: Cytogenetic and molecular relationship to
congenital (infantile) fibrosarcoma. Am J Pathol. 153:1451–1458.
1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Leeman-Neill RJ, Kelly LM, Liu P, Brenner
AV, Little MP, Bogdanova TI, Evdokimova VN, Hatch M, Zurnadzy LY,
Nikiforova MN, et al: ETV6-NTRK3 is a common chromosomal
rearrangement in radiation-associated thyroid cancer. Cancer.
120:799–807. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Solomon JP, Benayed R, Hechtman JF and
Ladanyi M: Identifying patients with NTRK fusion cancer. Ann Oncol.
30 (Suppl 8):viii16–viii22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lapeña LM, Caldas MCS, Ramírez C, Basilio
MS, Junco PT, Rodríguez-Laguna L, Martínez-González V,
Marín-Manzano E, Perez-Martinez A and Lopez-Gutierrez JC:
Larotrectinib as an effective therapy in congenital infantile
fibrosarcoma: Report of two cases. European J Pediatr Surg Rep.
10:e76–e79. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ernst MS, Lysack JT, Hyrcza MD, Chandarana
SP and Hao D: TRK inhibition with entrectinib in metastatic
salivary secretory carcinoma (SC): A case report. Curr Oncol.
29:3933–3939. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chi HT, Thuong NTL and Ly BTK:
Sphagneticola trilobata (L.) Pruski (asteraceae) methanol extract
induces apoptosis in leukemia cells through suppression of BCR/ABL.
Plants (Basel). 10(980)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ly BT, Chi HT, Yamagishi M, Kano Y, Hara
Y, Nakano K, Sato Y and Watanabe T: Inhibition of FLT3 expression
by green tea catechins in FLT3 mutated-AML cells. PLoS One.
8(e66378)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ala AA, Olotu BB and Ohia CMD: Assessment
of cytotoxicity of leaf extracts of Andrographis paniculata
and Aspilia africana on murine cells in vitro. Arch Basic Appl Med.
6:61–65. 2018.PubMed/NCBI
|
|
28
|
Mahavorasirikul W, Viyanant V,
Chaijaroenkul W, Itharat A and Na-Bangchang K: Cytotoxic activity
of Thai medicinal plants against human cholangiocarcinoma,
laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern
Med. 10(55)2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Iijima Y, Okuda K, Tojo A, Tri NK,
Setoyama M, Sakaki Y, Asano S, Tokunaga K, Kruh GD and Sato Y:
Transformation of Ba/F3 cells and Rat-1 cells by ETV6/ARG.
Oncogene. 21:4374–4383. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ziegler U and Groscurth P: Morphological
features of cell death. News Physiol Sci. 19:124–128.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Doonan F and Cotter TG: Morphological
assessment of apoptosis. Methods. 44:200–204. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tognon C, Garnett M, Kenward E, Kay R,
Morrison K and Sorensen PH: The chimeric protein tyrosine kinase
ETV6-NTRK3 requires both Ras-Erk1/2 and PI3-kinase-Akt signaling
for fibroblast transformation. Cancer Res. 61:8909–8916.
2001.PubMed/NCBI
|
|
33
|
Vaishnavi A, Le AT and Doebele RC: TRKing
down an old oncogene in a new era of targeted therapy. Cancer
Discov. 5:25–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vaishnavi A, Capelletti M, Le AT, Kako S,
Butaney M, Ercan D, Mahale S, Davies KD, Aisner DL, Pilling AB, et
al: Oncogenic and drug-sensitive NTRK1 rearrangements in lung
cancer. Nat Med. 19:1469–1472. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nagasubramanian R, Wei J, Gordon P,
Rastatter JC, Cox MC and Pappo A: Infantile fibrosarcoma with
NTRK3-ETV6 fusion successfully treated with the tropomyosin-related
kinase inhibitor LOXO-101. Pediatr Blood Cancer. 63:1468–1470.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Doebele RC, Davis LE, Vaishnavi A, Le AT,
Estrada-Bernal A, Keysar S, Jimeno A, Varella-Garcia M, Aisner DL,
Li Y, et al: An oncogenic NTRK fusion in a patient with soft-tissue
sarcoma with response to the tropomyosin-related kinase inhibitor
LOXO-101. Cancer Discov. 5:1049–1057. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou F and Chen B: Acute myeloid leukemia
carrying ETV6 mutations: Biologic and clinical features.
Hematology. 23:608–612. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chao WW and Lin BF: Isolation and
identification of bioactive compounds in Andrographis
paniculata (Chuanxinlian). Chin Med. 5(17)2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cheung HY, Cheung SH, Li J, Cheung CS, Lai
WP, Fong WF and Leung FM: Andrographolide isolated from
Andrographis paniculata induces cell cycle arrest and
mitochondrial-mediated apoptosis in human leukemic HL-60 cells.
Planta Med. 71:1106–1111. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lin HH, Tsai CW, Chou FP, Wang CJ, Hsuan
SW, Wang CK and Chen JH: Andrographolide down-regulates
hypoxia-inducible factor-1α in human non-small cell lung cancer
A549 cells. Toxicol Appl Pharmacol. 250:336–345. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Suzuki R, Matsushima Y, Okudaira N,
Sakagami H and Shirataki Y: Cytotoxic components against human oral
squamous cell carcinoma isolated from Andrographis
paniculata. Anticancer Res. 36:5931–5935. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Anoor PK, Yadav AN, Rajkumar K, Kande R,
Tripura C, Naik KS and Burgula S: Methanol extraction revealed
anticancer compounds quinic acid, 2(5H)-furanone and phytol in
Andrographis paniculata. Mol Clin Oncol.
17(151)2022.PubMed/NCBI View Article : Google Scholar
|