1. Historical insights on HBV vaccines
The discovery of hepatitis B virus (HBV) and the
development of the HBV vaccine involved serendipity and luck. In
his book entitled ‘Hepatitis B: The hunt for a killer virus’
(1), Professor Baruch S. Blumberg
(1925-2011), winner of the 1976 Nobel Prize, states that: ‘The most
significant outcome of our research has, probably been the
invention and the introduction of the HBV vaccine’. Professor
Blumberg was awarded the Nobel Prize for ‘discoveries concerning
new mechanisms for the origin and dissemination of infectious
diseases’, and, specifically, for the discovery of HBV. The vaccine
was invented in 1969, 2 years after the recognition of HBV, as one
of the causative agents of hepatitis. The HBV vaccine is the first
vaccine against two viruses, HBV and hepatitis D virus (HDV;
satellite virus of HBV), chronic disease, sexually transmitted
disease and cancer. Professor Blumberg's initial research focussed
on gene distribution and disease susceptibility in different ethnic
groups. In the serum of a patient with transfused haemophilia, he
and his co-workers, discovered an antibody, which reacted with a
protein in the serum of an Australian patient (2). Thus, it was named ‘Australia’ antigen
(Au), which was associated with hepatitis and found to be identical
to serum hepatitis antigen (SHAg) (3), now known as HBsAg.
As early as 1908, a virus was implicated as being
the causative agent of liver disease. In the late 1940s and early
1950s, two different forms of virus were proposed to cause
infective hepatitis (virus A: foecal-oral route of transmission)
and serum hepatitis (virus B: blood-borne), respectively (4-6).
The whole virus, named HBV, was only isolated and visualized
following transmission and electron microscopy studies in
1970(7). In addition to viral
particles, the plasma of infected individuals also carries
subviral, non-infectious particles, composed of HBsAg, at a ratio
of 1:1,000, respectively (8).
The infectious particles can be separated from
non-infectious particles using ultracentrifugation and the subviral
particles enriched by enzymatic treatment and column separation, to
eliminate viable virus. This was fundamentally the process used for
the production of the first HBV vaccine by Professor Blumberg and
his team. The first crude demonstration of active immunization and
59% protection was performed by the New York paediatrician, Dr Saul
Krugman, who inoculated children with a boiled, inactivated
preparation of a 1:10 dilution of infectious serum in distilled
water and then challenged them with infectious serum (9). In 1975, Merck was licensed to develop
the vaccine, after the safety of the vaccine was demonstrated in
chimpanzees (10-12)
and consequently the first placebo-controlled, randomized,
double-blind clinical trial demonstrated its efficacy in reducing
the incidence of HBV infection (13).
The large-scale roll-out of these first-generation
HBV vaccines began in 1984. Taiwan, where HBV was endemic, was the
first country to introduce the nation-wide vaccination of all
neonates in July, 1984(14). In the
first 15-month period, 78% of ~450,000 pregnant women were screened
and 18% were HBsAg-positive, with half of them considered to be
highly infectious. The schedule for the vaccination of infants born
HBsAg-positive was 5 µg of plasma-derived vaccine at 1, 5 and 9
weeks, with a booster dose at 1 year (15). Negligible adverse side effects were
experienced (15). In sub-Saharan
Africa (SSA), which is also a geographical region where HBV is
endemic, the first large-scale trial of HBV vaccination was
performed in Gambia (16,17). The Gambia Hepatitis Intervention
Study (GHIS) was established in 1986 to evaluate the protective
effectiveness of infant HBV immunization in the prevention of
chronic liver disease, particularly, hepatocellular carcinoma (HCC)
and cirrhosis later in adult life. In both regions, the vaccination
strategy, with plasma-derived HBsAg, led to the desired outcomes,
namely in the reduction of the incidence and chronic carriage of
HBV, with a concomitant decrease in the number of HCC cases, both
in childhood and later in adulthood (8,14).
Other countries, which adopted and implemented universal HBV
vaccination early on, included Bulgaria (1989), Malaysia (1990),
Italy, Spain, USA (1991) and Israel (1992) (18). On the other hand, several
high-income countries, including Japan, Denmark, Finland, Norway,
Sweden and the UK did not routinely vaccinate children until more
recently (19). Sweden introduced
infant vaccination in 2003; Japan, Norway and UK in 2019, whereas
Denmark and Finland do not yet vaccinate children. Instead, the
latter countries target immigrant groups from areas of a high HBV
endemicity and adolescents with high-risk factors for HBV
infection. In addition, they practice selective vaccination plus
the administration of hepatitis B immune globulin (HBIG) to
neonates born to HBsAg-/hepatitis B e antigen (HBeAg)-positive
mothers following the screening of pregnant women (20).
Early on, a number of disadvantages of using
patient-derived HBsAg for vaccine production were identified
(8). Firstly, in the context of
acquired immune deficiency syndrome (AIDS), whose infectious agent
had not yet been identified, the safety of plasma-derived HBsAg was
not guaranteed, particularly when considering that HBV was most
often isolated from patients with AIDS. Secondly, the anticipated
decrease in the prevalence of chronic hepatitis B, following
widespread vaccination, would eventually lead to the supply of
HBsAg from patient sera being limited.
Following the cloning of the HBV genome in 1978 by
three groups independently (21-23),
the road was opened for the production of HBsAg, HBcAg and HBV DNA,
in large amounts in vitro using gene technology. Although
glycosylated HBsAg can be expressed in mammalian cells, the process
is costly and the yield is relatively low (8). The expression of HBsAg in yeast cells
(Saccharomyces cerevisiae) provided sufficient HBsAg, which
was glycosylated (24,25) and shown to be immunogenic both in
chimpanzees (12) and in infants of
HBsAg/HBeAg-positive mothers in Thailand (26). By 1986, the recombinant yeast
vaccines became the accepted vaccines and in 1992, the World Health
Organization (WHO) recommended global universal childhood HBV
vaccination (27), considering that
vaccination is an economically attractive option, both in terms of
cost-effectiveness and benefit-cost ratios (28).
In the 1990s, third-generation vaccines were
developed, following the transfection of mammalian cells with
plasmids coding for pre-S/S proteins. These cells expressed and
secreted the LHBsAg (large), MHBsAg (middle) and HBsAg (small)
(29), either individually or in
combination. Such a glycosylated preS1/preS2/S-containing vaccine,
is licensed in Israel for the universal vaccination of infants and
in some countries in East Asia. Together with new adjuvants
developed, these vaccines have proved to be immunogenic in
non-responders to the earlier vaccines (28). However, the yeast derived vaccines
continue to be the HBV vaccine of choice.
By the year 2000, over a billion doses of vaccines
had been administered globally (1).
By December, 2021 (21 years later), globally, 190 countries had
introduced the HBV vaccine with three doses (HepB3) for infants,
with an 80% global coverage and different WHO regions demonstrating
different coverages over the years. In addition, 111 WHO member
states introduced the hepatitis B birth dose (HepB-BD), given
within the first 24 h of life. Global coverage is 42%; 78% in the
WHO Western Pacific Region, although considerably lower, at 17%, in
the WHO African Region (30). The
global coverage of both HepB3 and HepB-BD are below the targets set
by the WHO for 2020. The targets set by the WHO for 2030 are ≥90%
coverage for both HepB3 and HepB-BD (31). Mathematical modelling has predicted
that with 90% vaccination coverage with the inclusion of HepB-BD
would prevent 84% of global deaths associated with HBV, as opposed
to a 68% reduction with HepB3 only (32). A more recent model has demonstrated
that scaling up infant vaccination from 80 to 90% globally would
avert 4.3 million deaths between 2015 and 2030, whereas a further
18.7 million new cases would be prevented by the administration of
HepB-BD compared to HepB3(33).
This will require commitment from both policy makers and agencies
involved in the implementation of successful HBV vaccination.
2. Dosages, schedules and injection
routes
The age of infection is a determining factor for the
development of chronic hepatitis B infection. In total, 90% of
neonates born to HBeAg-positive mothers and 20-60% of children
<5 years of age will become chronic carriers of HBV, as opposed
to only 5% of adults, who are infected (34,35).
Globally, HBV infection is acquired at birth or early childhood.
Thus, it is crucial to implement the vaccination of infants as
early as possible, ideally at birth, in order to: i) Prevent the
perinatal mother-to-child transmission (PMCT) of HBV from
HBsAg-positive mothers; ii) prevent the horizontal transmission of
HBV in early childhood; iii) confer long-term immunity against
future exposures later on in life, thus leading to protection
against acute hepatitis B in adolescents and adults, following
either sexual or nosocomial transmission; iv) prevent HCC; and v)
protect against HDV infection.
The vaccination schedules have varied over the
course of the years and are dependent on local epidemiological
criteria and national programmes. For optimal efficacy, the vaccine
should be administered within 12 to 24 h after birth, as the levels
of antibodies against HBsAg (anti-HBs) decline over time when the
vaccine is administered >24 h (36). The most frequently recommended
schedule is three doses of the vaccine administered at 0, 1 and 6
months to infants; however, there are a range of alternative
schedules administered in different countries. The latest HBV
vaccine WHO position paper states: ‘The birth dose should be
followed by 2 or 3 additional doses to complete the primary series.
Both of the following options are considered appropriate: (i) a
3-dose schedule of HBV vaccine, with the first dose (monovalent)
being given at birth and the second and third (monovalent or as
part of a combined vaccine) given at the same time as the first and
third doses of the diphtheria-tetanus-pertussis
(DTP)-containing vaccine; or (ii) 4 doses, where a monovalent birth
dose is followed by 3 (monovalent or combined vaccine) doses,
usually given with other routine infant vaccines; the additional
dose does not cause any harm. The interval between doses should be
at least 4 weeks.’ (37). The
standard paediatric dose is 5 to 10 µg HBsAg, whereas the standard
adult dose is 10 to 20 µg, with 40 µg administered to patients who
are immunocompromised and in those undergoing dialysis. An
accelerated schedule of three doses of the vaccine administered at
0, 1 and 2 months, followed by a booster dose administered at 12
months, is recommended for health care workers exposed to HBV or a
susceptible sexual partner of a patient with acute hepatitis B in
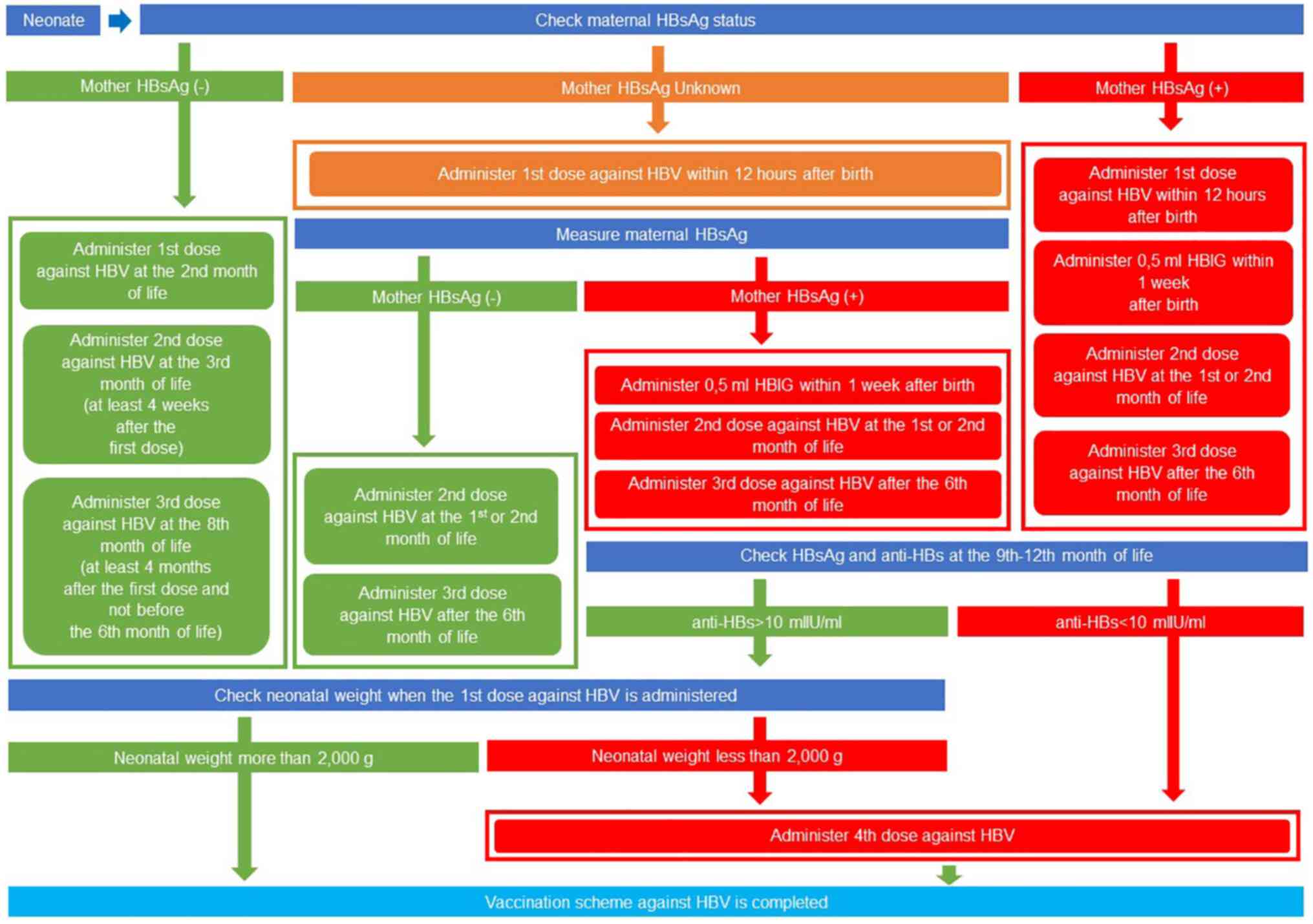
order to provide rapid protection (28). The algorithm of the vaccination
scheme against HBV, based on the 2023 Hellenic National Vaccination
Programme (https://www.moh.gov.gr), is presented in
Fig. 1.
To achieve the optimal response, the vaccine is
administered intramuscularly into the anterolateral thigh in
neonates and infants <1 year of age, or into the deltoid region
in children >1 year of age and in adults. Buttock administration
and the intradermal route are not recommended (28). Administration into the gluteal
muscle results in reduced immunogenicity and in the risk of sciatic
nerve injury (37). The HBV vaccine
(monovalent or combined) and other vaccines administered during the
same visit should be administered at different injection sites
(37).
Infants and children exhibit a 100% response to the
vaccine, with anti-HB levels being >10 mIU/ml, the levels
necessary for immunity. Furthermore, 95% of adults are protected
following the administration of three doses. Thus, booster doses of
the HBV vaccine are not required for immunocompetent individuals,
whereas for immunocompromised patients regular testing is required,
with boosters administered if anti-HB titres decrease to <10
mIU/ml (18,38).
3. Contraindications for HBV
vaccination
The safety of the HBV vaccines has been demonstrated
by both clinical trials and post-approval follow-up. Between 1 to
7.5% of vaccinated children were shown to develop adverse events, a
percentage lower than that observed in adults, with the frequency
of adverse events decreasing with each additional injection
[(39) and references cited
therein]. The most commonly reported adverse events include pain
and swelling at the injection site, and fever and mild systemic
reactions, such as fever, irritability, poor appetite, diarrhoea
and vomiting, generally lasting for 1 to 2 days (39,40).
There is no evidence to associate an increase in the number of
febrile episodes, sepsis evaluations, allergic or neurological
reactions or medical procedures in neonates and infants (41). The frequency of severe vaccine
reactions related to HBV vaccination is extremely low (42). Epidemiological studies have found no
association between HBV vaccination and sudden infant death (SID)
(43,44), diabetes mellitus (45) and demyelinating diseases (46), including Guillain-Barre syndrome
(47). Although certain studies
have suggested that HBV vaccination may be a risk factor for the
development of multiple sclerosis (48), a previous systematic review and
meta-analysis did not establish such a link (49). The WHO specifies only two
contraindications to the HBV vaccination: An allergic reaction to
any vaccine component and anaphylaxis to a previous dose (37). For pre-term neonates, a low birth
weight (<2 kg) is not a contraindication to HBV vaccination. HBV
vaccination is safe, although with possible reduced immunogenicity;
therefore, pre-term babies should receive HepB-BD within 24 h,
followed by three (and not two) subsequent doses. HBV vaccination
is not contraindicated for pregnant and lactating women (37).
4. Challenges with multivalent vaccines
Multivalent vaccines, introduced in the early 1990s,
provided a notable advancement in immunizations. In multivalent
formulations, the HBV vaccine is combined with the DTP,
Haemophilus influenzae type B (Hib) and inactivated polio
(IPV) vaccines. Multivalent vaccines include Pediarix
[GlaxoSmithKline Biologicals (GSK)], used in the USA (50), with three hexavalent vaccines
currently licensed in Europe: Vaxelis (Sanofi Pasteur and
MSD), Hexyon (Sanofi Pasteur) and Infanrix Hexa (GSK)
(51). The multivalent vaccines
have different formulations and/or schedules (50,51)
but can be used interchangeably if necessary. A vaccine containing
recombinant HBsAg and inactivated hepatitis A virus Twinrix
(GSK) is used for the vaccination of individuals aged ≥18 years.
The obvious advantage of combined vaccines is the reduced number of
injections required for paediatric immunization, with fewer doctor
visits, less pain, increased safety with fewer syringes, less
wastage and thus, reduced immunization programme costs. The use of
multivalent vaccines leads to improved coverage and compliance
rates and timeliness, as well as benefits in shipment and storage
(51,52). However, the monovalent HBV vaccine
must be used at birth, as multivalent vaccines containing HBV, DTP
and Hib antigens have a reduced immunogenicity when administered
before the age of 6 weeks. Monovalent vaccines include
Engerix-B (GSK) and Recombivax HB (Merck & Co.,
Inc.). Compared to the monovalent HBV vaccine, slight increases in
short-term adverse events (fever, redness, swelling, etc.) have
been documented for the multivalent formulations. Another
disadvantage of using multivalent vaccines is that production
issues may arise due to the complexity of combined vaccines; this
therefore requires contingence planning in the event of vaccine
shortages.
Routine vaccination with pentavalent and hexavalent
combinations, including DTP, Hib, HepB3 and IPV has been used in
vaccination programmes for >15 years, with Europe taking the
lead compared to other regions worldwide (51). In total, 20 of 33 European countries
routinely used hexavalent vaccines in children (51). Multivalent vaccines have a good
immunogenicity, safety and tolerability and can be administered
with other paediatric vaccines, including pneumococcal conjugate
vaccines, rotavirus, meningococcal conjugate, measles, mumps,
rubella and varicella vaccines (51). Different schedules have been used
with good antibody titres obtained, regardless of the posology used
(51).
5. Long immunogenicity and long-term
protection
The cut-off value, which measures the vaccine
response, at 1 to 2 months after receiving a complete HepB3 scheme,
is a concentration of anti-HB B >10 mIU/ml and considered to be
protective. This was determined from a study that demonstrated that
children who did not mount this level of antibodies could become
infected with HBV (53). Response
to the vaccine is considered a surrogate marker for clinical
protection. Following vaccination, anti-HB levels decline over time
at a rate dependent on the age of vaccination. Findings
extrapolated from previous research (50) have shown that at 18 years
post-vaccination, only 16% of individuals who had received HepB3
prior to the age of 1 year had anti-HB levels >10 mIU/ml
(54-56)
compared to 74%, who had been vaccinated when they were >1 year
of age (57-63).
However, even for previously vaccinated individuals, whose anti-HB
levels decline to <10 mIU/ml, an anamnestic response can be
elicited following a booster for up to 30 years following initial
vaccination (62-64).
Both the antibody and cellular response have been shown to persist
(65).
Thus, an initial vaccine response can protect
against hepatitis B, either acute or chronic, for as long as 30
years post-vaccination, regardless of whether anti-HB levels
persist at levels ≥10 mIU/ml or not (64-66).
Thus, immunocompetent individuals do not require boosters, provided
they had received the complete vaccine series at the recommended
schedules either as children or adults. However, there have been
exceptions and HBV infection has been documented infrequently in
vaccinated individuals. In a study on 3.7 million blood donors,
nine HBV DNA-positive, HBsAg/anti-HBc-negative donors were
identified (1 in 410,540 donations) and evaluated for vaccination
status. In total, six of the nine HBV-DNA-positive donations were
from vaccinated individuals, whose infections were subclinical and
resolved (67). In addition, four
of the nine donors contracted the HBV infection from their sexual
partners (67). In an Italian
study, 362 of 11,311 (3.2%) cases with acute hepatitis B were
vaccinated (68). The possible
reasons cited for the breakthrough HBV infections in vaccinated
individuals include incomplete vaccination (68); infection with vaccine-escape S
mutant strains of HBV (68); and
infection with genotypes expressing serological subtype different
to the vaccine strain, belonging to subgenotype A2, which expresses
adw2(67). Thus,
individuals, who are at high risk of HBV infection or
immunocompromised, should be monitored and given a booster,
especially if the anti-HBs levels decline below 10 mIU/ml.
6. Selective HBV vaccination
Neonates, born to HBsAg-/HBeAg-positive mothers, are
at a high risk of being infected perinatally i.e., PMCT. Thus, it
is critical to offer additional temporary protection until the
neonates respond to the HepB-BD. The immediate administration of
HBIG can provide such protection. As previously demonstrated, the
HBsAg-carrier rate was high, at 92%, when neonates were
administered the placebo, whereas it decreased to 26% in those who
received three HBIG doses at birth, and at 3 and 6 months, and 54%
among infants who received a single dose of HBIG at birth only
(69). HBIG should be administered
in conjunction with HepB-BD and not instead of the birth dose.
Importantly, the co-administration of HBIG does not suppress the
anti-HB response (37). As
previously demonstrated, compared to the administration of the
vaccine alone, the additional administration of HBIG enhanced the
protection of neonates born to HBsAg-positive mothers (70). However, the improvement in
protection is not significant in full-term neonates born to
HBsAg-positive/HBeAg-negative mothers (71). Infants who have received
post-exposure prophylaxis may be safely breastfed beginning
immediately after birth (50).
Despite the administration of HepB-BD and/or HBIG, a
low frequency of PMTC can occur in neonates born to
HBsAg-positive/HBeAg-positive mothers with high viral loads
(71). Thus, the prevention of PMTC
requires an incremental approach (72) as follows: i) At least three doses of
the HBV vaccine, including a timely birth dose within 24 h; ii)
HBsAg testing and linkage-to-care of mothers with follow-up of
infants; iii) the administration of HBIG for children born to
HBsAg-positive mothers; and iv) finally, antiviral treatment (high
viral load).
In Africa, the resource-limited settings use of HBIG
may not be feasible due to safety, supply and cost restraint issues
(73). In addition, in the WHO
Africa region, the coverage of HepB-BD is the lowest globally
(74). In 1995, the HBV vaccine was
introduced into the Expanded Programme on Immunisation (EPI) in
South Africa. The HBsAg-based vaccine is administered as 6, 10 and
14 weeks after birth, with a booster at 18 months, all as part of a
multivalent vaccine (75).
Universal coverage is relatively low at 74% and individuals born
prior to 1995 are excluded. Even though the South African National
Guidelines recommend the HepB-BD, the introduction of catch-up
vaccination and maternal HBV screening, these recommendations are
yet to be implemented. These limitations allow PMCT to occur in
infants born to HBsAg-positive mothers with high viral loads.
Nevertheless, the introduction of HepB-B3 has resulted in decrease
in the chronic carriage of HBV (76-78).
Novel HBV prevention strategies that are being explored, include
mRNA and viral vector-based vaccines by researchers in South Africa
(79).
7. Effectiveness of HBV vaccination
The major impact that universal HBV vaccination has
had on HBV-associated morbidity and mortality was demonstrated by
the outcomes in Taiwan, which was the first country to introduce
universal HBV vaccination in 1984. By 1986, universal infant
coverage was achieved, with preschool children vaccinated by 1987,
and older children and adults by 1990. Following the introduction
of universal infant vaccination, the HBV carriage in infants, born
to highly viraemic HBsAg-positive mothers, decreased from 90 to
15%, with a 10-fold decrease in incidence in these infants and
children infected later by horizontal vaccination. This decrease in
chronic HBV carriage translated to a marked decrease in the
incidence of HCC in HBV vaccinees (80). The incidence of HCC was
statistically significantly lower in vaccinated children compared
to unvaccinated birth cohorts, with the relative risk of a vaccinee
developing HCC being 0.31 (P<0.001), compared with an
unvaccinated child (81). Despite
the high coverage of infant immunization in Taiwan, a small number
of children still developed HCC (82). The reasons for this include vaccine
failure and neonates born to highly viraemic mothers not receiving
HBIG. Furthermore, during the early days of HBV vaccination, the
first dose was administered at 6 weeks, allowing for infection to
occur in early life. This realization led to the introduction of
HepB-BD. Similarly, universal neonate vaccination coupled with mass
screening and the immunization of susceptible Alaska Natives has
eliminated HCC and acute symptomatic HBV infection among Alaska
Native children (83).
Unfortunately, the situation has not been as
efficient in SSA and the implementation of the HBV vaccine
programme has been suboptimal, even though the vaccine was
introduced early in the 1980s in some countries (84,85).
There are a number of reasons for this, including financial and
human resource constraints, the high percentage of babies born in
rural communities, poor delivery services, as well as hepatitis B
being overshadowed by the ‘blockbusters’ HIV/AIDS, malaria and
tuberculosis (73). This has led to
only a 71% coverage of HepB3 in the WHO Africa region compared to
an 80% global coverage as of July 15, 2015, with different
continents, WHO regions and countries exhibiting different levels
of coverage (30). Moreover, until
2001, coverage was very low in the Africa region, with the coverage
levels increasing thereafter, being higher or equal to those in
South-East Asia between 2001 and 2011, thereafter plateauing at
levels below those of South-East Asia; please refer to the
administered and official HBV vaccination coverage (3rd dose, %) in
the different WHO regions reported annually through the WHO/UNICEF
Joint Reporting Form on Immunization (JRF) (86). Full protection requires that babies
receive all three doses of the vaccine. In a number of countries in
SSA, this is not the case, due to the lack of access to health
services in rural areas, the lack of funding from governments, and
the education of both health care workers and the public in
general. At 17%, HepB-BD coverage remains dismally low in SSA. A
large number of births take place at home outside health
facilities, and thus the administration of HepB-BD is not
possible.
8. Conclusions and future perspectives
In order to eliminate HBV, a multiprong approach is
required (73,87). The interventions necessary include:
i) Prevention, involving the timely and universal HepB3 and HepB-BD
vaccination of neonates and susceptible individuals, with full
coverage and completion of the course; ii) the interruption of
transmission by ensuring blood and injection safety, the prevention
of PMCT and harm prevention; and iii) population-based testing and
treatment. In addition to these interventions, it is critical that
advocacy and education are improved. Governments and public health
officials need to be encouraged to fund, implement and administer
the vaccine schedules, including the HBV-BD. Moreover, both health
care workers and the public need to be educated about the
importance of timely vaccination and completion of all doses of the
vaccination schedules. Pregnant women are a key target population,
and they thus need to be further educated about the importance of
prevention of HBV by vaccination of their neonates soon after
birth.
However, there are several challenges faced in
resource-limited settings in SSA (73), including the paucity of good data
due to of lack of surveillance; limited access to laboratories and
health services; the suboptimal interruption of HBV transmission
routes, in particular, PMCT; no population-based testing and
treatment; resource-limited settings in SSA are within the
epicentre of the HIV pandemic; and inadequate public health
responses.
Efficient vaccination against HBV, with universal
coverage, including the Hep-BD and completion of the full course of
vaccination is the first step to the elimination of HBV and the
consequent clinical manifestations of the infection, including HCC.
Although this may appear to be a challenge only in resource-limited
settings, such as SSA, hepatitis B remains a global concern.
Increasing migration from areas where HBV continues to be endemic
to areas of low endemicity exposes an increasing number of
individuals to the virus, and thus a concerted, global strategy is
required.
Acknowledgements
The present study was published in the context of
the 8th Workshop on Paediatric Virology organised virtually on
October 20, 2022, by the Institute of Paediatric Virology
(https://www.paediatricvirology.org)
based on the island of Euboea (Greece), under the auspices of the
World Academy of Sciences (WAS) and the support of the Department
of Clinical Virology of the University of Crete School of Medicine
and the First Department of Paediatrics of the University of Athens
School of Medicine. The authors would like to thank Professor Maria
Theodoridou, Professor Emerita of Paediatric Infectious Diseases at
the University of Athens School of Medicine and President of the
Hellenic Vaccination Committee for her valuable comments.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AK contributed to the conception and design of the
study, wrote the original draft, and edited and critically revised
the manuscript. INM and DAS edited and critically revised the
manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
AK is a member of the Academic Board of the Institute of Paediatric
Virology. INM and DAS are co-founders of the Institute of
Paediatric Virology.
References
|
1
|
Blumberg BS: Hepatitis B: The hunt for a
killer vaccine. Princeton and Oxford: Princeton University Press,
2002.
|
|
2
|
Alter HJ and Blumberg BS: Further studies
on a ‘new’ human isoprecipitin system (Australia antigen). Blood.
27:297–309. 1966.PubMed/NCBI
|
|
3
|
Prince AM: Relation of Australia and SH
antigens. Lancet. 2:462–463. 1968.PubMed/NCBI View Article : Google Scholar
|
|
4
|
MacCallum FO: Early studies of viral
hepatitis. Br Med Bull. 28:105–108. 1972.PubMed/NCBI View Article : Google Scholar
|
|
5
|
MacCallum FO: Hepatitis. Am J Dis Child.
123:332–335. 1972.PubMed/NCBI View Article : Google Scholar
|
|
6
|
MacCallum FO: 1971 International symposium
on viral hepatitis. Historical perspectives. Can Med Assoc J.
106(Spec Issue): (Suppl):S423–S426. 1972.PubMed/NCBI
|
|
7
|
Dane DS, Cameron CH and Briggs M:
Virus-like particles in serum of patients with
Australia-antigen-associated hepatitis. Lancet. 1:695–698.
1970.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gerlich WH: Medical virology of hepatitis
B: How it began and where we are now. Virol J.
10(239)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Krugman S, Giles JP and Hammond J: Viral
hepatitis, type B (MS-2 strain). Studies on active immunization.
JAMA. 217:41–45. 1971.PubMed/NCBI
|
|
10
|
Purcell RH and Gerin JL: Hepatitis B
subunit vaccine: A preliminary report of safety and efficacy tests
in chimpanzees. Am J Med Sci. 270:395–399. 1975.PubMed/NCBI
|
|
11
|
Hilleman MR, Buynak EB, Roehm RR, Tytell
AA, Bertland AU and Lampson GP: Purified and inactivated human
hepatitis B vaccine: Progress report. Am J Med Sci. 270:401–404.
1975.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Buynak EB, Roehm RR, Tytell AA, Bertland
AU II, Lampson GP and Hilleman MR: Development and chimpanzee
testing of a vaccine against human hepatitis B. Proc Soc Exp Biol
Med. 151:694–700. 1976.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Szmuness W, Stevens CE, Harley EJ, Zang
EA, Oleszko WR, William DC, Sadovsky R, Morrison JM and Kellner A:
Hepatitis B vaccine: Demonstration of efficacy in a controlled
clinical trial in a high-risk population in the United States. N
Engl J Med. 303:833–841. 1980.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gerlich WH: Prophylactic vaccination
against hepatitis B: Achievements, challenges and perspectives. Med
Microbiol Immunol. 204:39–55. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen DS, Hsu NH, Sung JL, Hsu TC, Hsu ST,
Kuo YT, Lo KJ and Shih YT: A mass vaccination program in Taiwan
against hepatitis B virus infection in infants of hepatitis B
surface antigen-carrier mothers. JAMA. 257:2597–2603.
1987.PubMed/NCBI
|
|
16
|
Whittle HC, Inskip H, Hall AJ, Mendy M,
Downes R and Hoare S: Vaccination against hepatitis B and
protection against chronic viral carriage in The Gambia. Lancet.
337:747–750. 1991.PubMed/NCBI View Article : Google Scholar
|
|
17
|
The Gambia Hepatitis Intervention Study
The Gambia Hepatitis Study Group. Cancer Res. 47:5782–5787.
1987.PubMed/NCBI
|
|
18
|
Shepard CW, Simard EP, Finelli L, Fiore AE
and Bell BP: Hepatitis B virus infection: Epidemiology and
vaccination. Epidemiol Rev. 28:112–125. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kane MA: Status of hepatitis B
immunization programmes in 1998. Vaccine. 16 (Suppl):S104–S108.
1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Komatsu H: Hepatitis B virus: Where do we
stand and what is the next step for eradication? World J
Gastroenterol. 20:8998–9016. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pasek M, Goto T, Gilbert W, Zink B,
Schaller H, MacKay P, Leadbetter G and Murray K: Hepatitis B virus
genes and their expression in E. coli. Nature. 282:575–579.
1979.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Valenzuela P, Gray P, Quiroga M, Zaldivar
J, Goodman HM and Rutter WJ: Nucleotide sequence of the gene coding
for the major protein of hepatitis B virus surface antigen. Nature.
280:815–819. 1979.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Charnay P, Pourcel C, Louise A, Fritsch A
and Tiollais P: Cloning in Escherichia coli and physical structure
of hepatitis B virion DNA. Proc Natl Acad Sci USA. 76:2222–2226.
1979.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Valenzuela P, Medina A, Rutter WJ, Ammerer
G and Hall BD: Synthesis and assembly of hepatitis B virus surface
antigen particles in yeast. Nature. 298:347–350. 1982.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Harford N, Cabezon T, Crabeel M, Simoen E,
Rutgers A and De Wilde M: Expression of hepatitis B surface antigen
in yeast. Dev Biol Stand. 54:125–130. 1983.PubMed/NCBI
|
|
26
|
Poovorawan Y, Sanpavat S, Pongpunlert W,
Chumdermpadetsuk S, Sentrakul P and Safary A: Protective efficacy
of a recombinant DNA hepatitis B vaccine in neonates of HBe
antigen-positive mothers. JAMA. 261:3278–3281. 1989.PubMed/NCBI
|
|
27
|
McGregor A: WHO: World Health Assembly.
Lancet. 339(1287)1992.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zanetti AR, Van Damme P and Shouval D: The
global impact of vaccination against hepatitis B: A historical
overview. Vaccine. 26:6266–6273. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gerlich WH, Glebe D, Kramvis A and Magnius
LO: Peculiarities in the designations of hepatitis B virus genes,
their products, and their antigenic specificities: A potential
source of misunderstandings. Virus Genes. 56:109–119.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
World Health Organization (WHO):
Immunization Coverage. WHO, Geneva, 2023. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
Accessed Oct 10, 2022.
|
|
31
|
World Health Organization (WHO): Interim
guidance for country validation of viral hepatitis elimination.
WHO, Geneva, 2021. Accessed Oct 10, 2022.
|
|
32
|
Goldstein ST, Zhou F, Hadler SC, Bell BP,
Mast EE and Margolis HS: A mathematical model to estimate global
hepatitis B disease burden and vaccination impact. Int J Epidemiol.
34:1329–1339. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nayagam S, Thursz M, Sicuri E, Conteh L,
Wiktor S, Low-Beer D and Hallett TB: Requirements for global
elimination of hepatitis B: a modelling study. Lancet Infect Dis.
16:1399–1408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hoofnagle JH, Doo E, Liang TJ, Fleischer R
and Lok AS: Management of hepatitis B: Summary of a clinical
research workshop. Hepatology. 45:1056–1075. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McMahon BJ: The natural history of chronic
hepatitis B virus infection. Semin Liver Dis. 24 (Suppl 1):S17–S21.
2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Marion SA, Tomm Pastore M, Pi DW and
Mathias RG: Long-term follow-up of hepatitis B vaccine in infants
of carrier mothers. Am J Epidemiol. 140:734–746. 1994.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hepatitis B vaccines: WHO position paper -
July 2017. Wkly Epidemiol Rec. 92:369–392. 2017.PubMed/NCBI(In English, French).
|
|
38
|
Are booster immunisations needed for
lifelong hepatitis B immunity? European Consensus Group on
Hepatitis B Immunity. Lancet. 355:561–565. 2000.PubMed/NCBI
|
|
39
|
Niu MT, Rhodes P, Salive M, Lively T,
Davis DM, Black S, Shinefield H, Chen RT and Ellenberg SS:
Comparative safety of two recombinant hepatitis B vaccines in
children: Data from the Vaccine Adverse Event Reporting System
(VAERS) and Vaccine Safety Datalink (VSD). J Clin Epidemiol.
51:503–510. 1998.PubMed/NCBI View Article : Google Scholar
|
|
40
|
McMahon BJ, Helminiak C, Wainwright RB,
Bulkow L, Trimble BA and Wainwright K: Frequency of adverse
reactions to hepatitis B vaccine in 43,618 persons. Am J Med.
92:254–256. 1992.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lewis E, Shinefield HR, Woodruff BA, Black
SB, Destefano F, Chen RT and Ensor R: Vaccine Safety Datalink
Workgroup. Safety of neonatal hepatitis B vaccine administration.
Pediatr Infect Dis J. 20:1049–1054. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu Y, Zhang M, Yang M and Chen Q: Adverse
Events of Vaccination against Hepatitis B Virus in Post-Marketing
Surveillance from 2005 to 2017 in Guangdong Province, China.
Vaccines (Basel). 10(1087)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Niu MT, Salive ME and Ellenberg SS:
Neonatal deaths after hepatitis B vaccine: The vaccine adverse
event reporting system, 1991-1998. Arch Pediatr Adolesc Med.
153:1279–1282. 1999.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Eriksen EM, Perlman JA, Miller A, Marcy
SM, Lee H, Vadheim C, Zangwill KM, Chen RT, DeStefano F, Lewis E,
et al: Lack of association between hepatitis B birth immunization
and neonatal death: A population-based study from the vaccine
safety datalink project. Pediatr Infect Dis J. 23:656–662.
2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
DeStefano F, Mullooly JP, Okoro CA, Chen
RT, Marcy SM, Ward JI, Vadheim CM, Black SB, Shinefield HR, Davis
RL, et al: Childhood vaccinations, vaccination timing, and risk of
type 1 diabetes mellitus. Pediatrics. 108(E112)2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
DeStefano F, Verstraeten T, Jackson LA,
Okoro CA, Benson P, Black SB, Shinefield HR, Mullooly JP, Likosky
W, Chen RT, et al: Vaccinations and risk of central nervous system
demyelinating diseases in adults. Arch Neurol. 60:504–509.
2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Duclos P: Safety of immunisation and
adverse events following vaccination against hepatitis B. Expert
Opin Drug Saf. 2:225–231. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hernan MA, Jick SS, Olek MJ and Jick H:
Recombinant hepatitis B vaccine and the risk of multiple sclerosis:
A prospective study. Neurology. 63:838–842. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sestili C, Grazina I and La Torre G: HBV
vaccine and risk of developing multiple sclerosis: A systematic
review and meta-analysis. Hum Vaccin Immunother. 17:2273–2278.
2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schillie S, Vellozzi C, Reingold A, Harris
A, Haber P, Ward JW and Nelson NP: Prevention of Hepatitis B Virus
Infection in the United States: Recommendations of the Advisory
Committee on Immunization Practices. MMWR Recomm Rep. 67:1–31.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Obando-Pacheco P, Rivero-Calle I,
Gómez-Rial J, Rodríguez-Tenreiro Sánchez C and Martinón-Torres F:
New perspectives for hexavalent vaccines. Vaccine. 36:5485–5494.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Maman K, Zöllner Y, Greco D, Duru G,
Sendyona S and Remy V: The value of childhood combination vaccines:
From beliefs to evidence. Hum Vaccin Immunother. 11:2132–2141.
2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Jack AD, Hall AJ, Maine N, Mendy M and
Whittle HC: What level of hepatitis B antibody is protective? J
Infect Dis. 179:489–492. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
54
|
Dentinger CM, McMahon BJ, Butler JC,
Dunaway CE, Zanis CL, Bulkow LR, Bruden DL, Nainan OV, Khristova
ML, Hennessy TW and Parkinson AJ: Persistence of antibody to
hepatitis B and protection from disease among Alaska natives
immunized at birth. Pediatr Infect Dis J. 24:786–792.
2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hammitt LL, Hennessy TW, Fiore AE, Zanis
C, Hummel KB, Dunaway E, Bulkow L and McMahon BJ: Hepatitis B
immunity in children vaccinated with recombinant hepatitis B
vaccine beginning at birth: A follow-up study at 15 years. Vaccine.
25:6958–6964. 2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Samandari T, Fiore AE, Negus S, Williams
JL, Kuhnert W, McMahon BJ and Bell BP: Differences in response to a
hepatitis B vaccine booster dose among Alaskan children and
adolescents vaccinated during infancy. Pediatrics. 120:e373–e381.
2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
McMahon BJ, Dentinger CM, Bruden D, Zanis
C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP and Hennessy
TW: Antibody levels and protection after hepatitis B vaccine:
Results of a 22-year follow-up study and response to a booster
dose. J Infect Dis. 200:1390–1396. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
58
|
Funderburke PL and Spencer L: Hepatitis B
immunity in high risk health care workers. Seven years post
vaccination. AAOHN J. 48:325–330. 2000.PubMed/NCBI
|
|
59
|
McMahon BJ, Bruden DL, Petersen KM, Bulkow
LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H and
Margolis HS: Antibody levels and protection after hepatitis B
vaccination: Results of a 15-year follow-up. Ann Intern Med.
142:333–341. 2005.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Spradling PR, Williams RE, Xing J, Soyemi
K and Towers J: Serologic testing for protection against hepatitis
B virus infection among students at a health sciences university in
the United States. Infect Control Hosp Epidemiol. 33:732–736.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
61
|
Stevens CE, Toy PT, Taylor PE, Lee T and
Yip HY: Prospects for control of hepatitis B virus infection:
Implications of childhood vaccination and long-term protection.
Pediatrics. 90 (1 Pt 2):170–173. 1992.PubMed/NCBI
|
|
62
|
Watson B, West DJ, Chilkatowsky A, Piercy
S and Ioli VA: Persistence of immunologic memory for 13 years in
recipients of a recombinant hepatitis B vaccine. Vaccine.
19:3164–3168. 2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Williams JL, Christensen CJ, McMahon BJ,
Bulkow LR, Cagle HH, Mayers JS, Zanis CL, Parkinson AJ and Margolis
HS: Evaluation of the response to a booster dose of hepatitis B
vaccine in previously immunized healthcare workers. Vaccine.
19:4081–4085. 2001.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bruce MG, Bruden D, Hurlburt D, Zanis C,
Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow
L, et al: Antibody Levels and Protection After Hepatitis B Vaccine:
Results of a 30-Year Follow-up Study and Response to a Booster
Dose. J Infect Dis. 214:16–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Simons BC, Spradling PR, Bruden DJ, Zanis
C, Case S, Choromanski TL, Apodaca M, Brogdon HD, Dwyer G, Snowball
M, et al: A Longitudinal Hepatitis B vaccine cohort demonstrates
long-lasting hepatitis B Virus (HBV) cellular immunity despite loss
of antibody against HBV surface antigen. J Infect Dis. 214:273–280.
2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Leuridan E and Van Damme P: Hepatitis B
and the need for a booster dose. Clin Infect Dis. 53:68–75.
2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Stramer SL, Wend U, Candotti D, Foster GA,
Hollinger FB, Dodd RY, Allain JP and Gerlich W: Nucleic acid
testing to detect HBV infection in blood donors. N Engl J Med,.
364:236–247. 2011.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Tosti ME, Alfonsi V, Lacorte E, Mele A,
Galli C, Zanetti AR and Romanò L: SEIEVA Collaborating Group. Acute
Hepatitis B After the Implementation of Universal Vaccination in
Italy: Results from 22 years of surveillance (1993-2014). Clin
Infect Dis. 62:1412–1418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Beasley RP, Hwang LY, Stevens CE, Lin CC,
Hsieh FJ, Wang KY, Sun TS and Szmuness W: Efficacy of hepatitis B
immune globulin for prevention of perinatal transmission of the
hepatitis B virus carrier state: Final report of a randomized
double-blind, placebo-controlled trial. Hepatology. 3:135–141.
1983.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lee C, Gong Y, Brok J, Boxall EH and Gluud
C: Hepatitis B immunisation for newborn infants of hepatitis B
surface antigen-positive mothers. Cochrane Database Syst Rev.
(2)(CD004790)2006.PubMed/NCBI View Article : Google Scholar
|
|
71
|
World Health Organization (WHO):
Guidelines for the prevention, care and treatment of persons with
chronic hepatitis B infection. WHO, Geneva, 2015. https://apps.who.int/iris/handle/10665/154590.
Accessed Oct 10, 2022.
|
|
72
|
Viral Hepatitis Prevention Board (VHPB):
Prevention and control of hepatitis B with combined vaccines and
timely birth dose vaccination: Hanoi, Vietnam. VHPB, Belgium, 2018.
Accessed Oct 10, 2022.
|
|
73
|
Kramvis A: Challenges for hepatitis B
virus cure in resource-limited settings in sub-Saharan Africa. Curr
Opin HIV AIDS. 15:185–192. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
World Health Organization (WHO): Hepatitis
B vaccination coverage. WHO, Geneva, 2023. Accessed Oct 10,
2022.
|
|
75
|
Spearman CW, Sonderup MW, Botha JF, van
der Merwe SW, Song E, Kassianides C, Newton KA and Hairwadzi HN:
Division of Hepatology, Department of Medicine, University of Cape
Town, South Africa. South African guideline for the management of
chronic hepatitis B: 2013. S Afr Med J. 103 (5 Pt 2):337–349.
2013.PubMed/NCBI
|
|
76
|
Amponsah-Dacosta E, Lebelo RL, Rakgole JN,
Burnett RJ, Selabe SG and Mphahlele MJ: Evidence for a change in
the epidemiology of hepatitis B virus infection after nearly two
decades of universal hepatitis B vaccination in South Africa. J Med
Virol. 86:918–924. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Tsebe KV, Burnett RJ, Hlungwani NP, Sibara
MM, Venter PA and Mphahlele MJ: The first five years of universal
hepatitis B vaccination in South Africa: Evidence for elimination
of HBsAg carriage in under 5-year-olds. Vaccine. 19:3919–3926.
2001.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Burnett RJ, Kramvis A, Dochez C and Meheus
A: An update after 16 years of hepatitis B vaccination in South
Africa. Vaccine. 30 (Suppl 3):C45–C51. 2012.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Maepa MB, Ely A, Kramvis A, Bloom K,
Naidoo K, Simani OE, Maponga TG and Arbuthnot P: Hepatitis B Virus
Research in South Africa. Viruses. 14(1939)2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Chang MH: Decreasing incidence of
hepatocellular carcinoma among children following universal
hepatitis B immunization. Liver Int. 23:309–314. 2003.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chang MH, You SL, Chen CJ, Liu CJ, Lee CM,
Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, et al: Decreased incidence
of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year
follow-up study. J Natl Cancer Inst. 101:1348–1355. 2009.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Chang MH, Chen TH, Hsu HM, Wu TC, Kong MS,
Liang DC, Ni YH, Chen CJ and Chen DS: Taiwan Childhood HCC Study
Group. Prevention of hepatocellular carcinoma by universal
vaccination against hepatitis B virus: The effect and problems.
Clin Cancer Res. 11:7953–7957. 2005.PubMed/NCBI View Article : Google Scholar
|
|
83
|
McMahon BJ, Bulkow LR, Singleton RJ,
Williams J, Snowball M, Homan C and Parkinson AJ: Elimination of
hepatocellular carcinoma and acute hepatitis B in children 25 years
after a hepatitis B newborn and catch-up immunization program.
Hepatology. 54:801–807. 2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Prozesky OW, Szmuness W, Stevens CE, Kew
MC, Harley EJ, Hoyland JA, Scholtz JE, Mitchell AD, Shabangu A,
Kunene E, et al: Baseline epidemiological studies for a hepatitis B
vaccine trial in Kangwane. S Afr Med J. 64:891–893. 1983.PubMed/NCBI
|
|
85
|
Prozesky OW, Stevens CE, Szmuness W, Rolka
H, Harley EJ, Kew MC, Scholtz JE and Mitchell AD: Immune response
to hepatitis B vaccine in newborns. J Infect. 7 (Suppl 1):S53–S55.
1983.PubMed/NCBI View Article : Google Scholar
|
|
86
|
World Health Organization (WHO): Maternal,
Newborn, Child and Adolescent Health and Ageing: Data Portal. WHO,
Geneva, 2023. Accessed Oct 10, 2022.
|
|
87
|
World Health Organization (WHO): Global
Hepatitis Report 2017. WHO, Geneva, 2023. Accessed Oct 10,
2022.
|