Introduction
Obesity is a significant public health concern that
causes a high disease burden in developed countries (1). According to statistical data from
2015, obesity rates continue to rise worldwide, with 600 million
adults and 100 million children reported to be obese in 195
countries (1). Moreover, obesity is
a well-known risk factor for many chronic diseases such as type 2
diabetes, cardiovascular disease and degenerative brain disease
(2). Obesity is caused by
hereditary characteristics, endocrine disorders, dietary and
environmental factors and an imbalance in energy intake and output
(3). It is documented that abnormal
fat accumulation in adipocytes, also called hypertrophic
adipocytes, leads to the development of obesity. Hence, any
material that suppresses excessive fat accumulation in adipocytes
can be considered a potential anti-obesity agent.
Hypertrophic adipocytes are formed by the
differentiation of pre-adipocytes into adipocytes or fat-laden
cells, also known as adipogenesis. Preadipocyte differentiation is
regulated by expression and activation (phosphorylation) of
numerous transcription factors, enzymes and proteins. For example,
the family members of peroxisome proliferator-activated receptors
(PPARs) and CCAAT enhancer binding proteins (C/EBPs) are essential
regulators of preadipocyte differentiation (4). Members of the signal transducer and
activator of transcription (STAT) family are other important
transcriptional factors that regulate preadipocyte differentiation
(5,6). Notably, the expression and
phosphorylation levels of these transcription factors increase
significantly during adipogenesis (7). In addition, the expression of
lipogenic enzymes such as fatty acid synthase (FAS), acetyl-CoA
carboxylase (ACC) and perilipin A, a lipid droplet (LD)-binding and
stabilizing protein, is required for preadipocyte differentiation
(8,9).
Lipolysis is the metabolic pathway through which
lipids, mainly triglycerides (TG), are hydrolyzed into glycerol and
free fatty acids and is controlled by a group of lipases, including
hormone-sensitive lipase (HSL) expressed in adipocytes (10). Therefore, the promotion of lipolysis
in lipid-laden adipocytes is regarded as another key strategy for
preventing or treating obesity. A wealth of information strongly
supports the link between chronic inflammation in adipose tissue
(AT) and preadipocytes and the development of obesity (11). Tumor necrosis factor (TNF)-α is a
pro-inflammatory cytokine that is expressed and secreted in
preadipocytes (12). It has been
revealed that TNF-α induces and aggravates inflammation in the AT
(13). Cyclooxygenase-2 (COX-2) is
an inducible and inflammatory enzyme responsible for the
biosynthesis of prostaglandins (14). It is further documented that TNF-α
is a strong inducer of COX-2 in various cells, including
preadipocytes (15,16). Indeed, the authors of the present
study as well as other groups have demonstrated that the short-term
exposure of TNF-α leads to induction of COX-2 expression in 3T3-L1
preadipocytes (16-18).
Thus, inhibition of the TNF-α-induced COX-2 expression in
preadipocytes is considered as a possible target to manage obesity
inflammation.
Nutmeg (Myristica fragrans) is a seed derived
from an evergreen tree belonging to the Nutmegaceae family.
It is a dioecious plant that grows to a height of 10-20 m and is
cultivated in the Maluku province of Indonesia (19). Nutmeg is often used as a spice to
enhance food flavor. Notably, nutmeg byproducts, such as seeds and
mace, have been reported to have many potential bioactivities,
including anti-obesity, anti-diabetic, anti-fungal, anti-microbial,
antioxidant and anti-inflammatory (20-25).
Evidence indicates that nutmeg seeds contain several alkylbenzene
derivatives, such as myristicin, elemicin and safrole, which are
toxic to the human organism (26,27).
By contrast, lignans in nutmeg seeds are known to contain bioactive
compounds, including nectandrin B, a nutmeg lignan with anti-aging
and anti-diabetic effects (25,28).
Interestingly, a previous study demonstrated that nutmeg lignans
induce the IGF-1-AKT-mTOR pathway in an aging rat model, indicating
their potential anti-sarcopenic effects (29). These findings illustrated that the
nutmeg extraction method for concentrating bioactive lignan
compounds, while removing toxic substances, has great potential for
the treatment of various metabolic diseases. In previous studies,
the authors prepared lignan-enriched nutmeg extract (LNX), which
would comprise minimal levels of toxic myristicin (<0.5%) and
maximum nectandrin B (28) and
reported that LNX restores muscle proteins in aged mice (30), suggesting the potential of LNX as a
therapeutic agent to overcome sarcopenia. However, the anti-obesity
(antiadipogenic, prolipolytic and anti-inflammatory) effects of LNX
on preadipocytes remain unclear. In the present study, the aim was
to primarily demonstrate the LNX-contained whole active component's
potential effects on inhibition of lipid accumulation to evaluate
it as an anti-obesity drug candidate; therefore the authors focused
on LNX, but not on nectandrin B. Therefore, the regulatory effects
of LNX on lipid accumulation in differentiating preadipocytes,
glycerol release and HSL phosphorylation in differentiated
adipocytes and the TNF-α-induced COX-2 expression in preadipocytes
by using 3T3-L1 cells, a mouse white preadipocyte cell line, were
investigated. In the present study, it was reported that LNX exerts
an anti-adipogenic effect on differentiating 3T3-L1 preadipocytes,
which is mediated by the downregulating of STAT3 phosphorylation
and FAS expression.
Materials and methods
Preparation of LNX
LNX used in the present study was obtained from
Daehan Cell Pharm, Inc. (Guri, Republic of Korea). Briefly, the
original seeds of 100 g nutmeg (Myristica fragrans) were
pulverized and dissolved in 500 ml of 80% ethanol (EtOH). The
optimal extraction conditions were determined by monitoring the
minimal toxicity of myristicin and maximal bioactivity of
nectandrin B. The extract was subjected to a mobile phase of the
methanol (MeOH)-H2O (0-32 min, 63% MeOH; 32-37 min,
63-100% MeOH) mixture. The final extract was then adsorbed through
Diaion HP-20 (Merck KGaA), one of the ion exchange resin, and
eluted in a serial concentration of 30-90% EtOH to monitor the
contents of myristicin and nectandrin B. The nutmeg alcohol extract
was detected at 234 nm at a flow rate of 1.5 ml/min using a Waters
HPLC Optima Pak C18 reverse-phase column (cat. no. OP
C18-51002546; 4.6x250 mm, 5 µm pore size, RS Tech. Corp.). The
mobile phase in the analytical column was composed of the mixture
(the ratio of 4:6; absolute acetonitrile and 0.1% formic acid). In
addition, nectandrin B concentration used in the present study is
4.2 from 91% of dry weight of LNX, which is lower than the
concentration used in the previous study (28) with <0.5% of myristicin in a 91%
of dry weight of LNX.
Chemicals and reagents
Primary antibodies against PPAR-γ (cat. no.
sc-7272), C/EBP-α (cat. no. sc-61), phosphorylated (p)-STAT3 (cat.
no. sc-8059) and total (T)-STAT3 (cat. no. sc-8019) were purchased
from Santa Cruz Biotechnology, Inc. The primary antibody against
FAS (cat. no. 9452) was obtained from BD Biosciences. Primary
antibodies against p-HSL (S563) (cat. no. 4139) and p-HSL (S565)
(cat. no. 4137) were acquired from Cell Signaling Technology, Inc.
Primary antibodies against COX-2 (cat. no. 160106) and T-HSL (cat.
no. 10006371) were purchased from Cayman Chemical Company. Primary
antibody against perilipin A (cat. no. 3948-200) was purchased from
BioVision, Inc. The primary antibody of β-actin (A5441),
iso-butylmethylxanthine (IBMX), dexamethasone, insulin, Oil Red O
solution, Isoproterenol, and Free Glycerol Reagent were obtained
from MilliporeSigma. Dulbecco's modified Eagle's medium (DMEM),
fetal bovine serum (FBS), and penicillin/streptomycin were
purchased from Welgene, Inc. Fetal calf serum (FCS) and trypan blue
dye were purchased from Gibco; Thermo Fisher Scientific, Inc.
Enhanced chemiluminescence (ECL) reagents were purchased from
Advansta Inc.
Cell culture and differentiation
3T3-L1 mouse white preadipocytes (American Type
Culture Collection) were cultured in DMEM containing 10%
heat-inactivated FBS and 1% penicillin/streptomycin at
37˚C in a humidified atmosphere containing 5%
CO2. 3T3-L1 preadipocytes were seeded with DMEM
containing 10% FCS and 1% penicillin/streptomycin mixture and
maintained up to the contact inhibition stage for 2 days. The
differentiation of 3T3-L1 preadipocytes was initiated by replacing
the media with new DMEM containing 10% heat-inactivated FBS plus a
cocktail of hormones (MDI): 0.5 mM IBMX (M), 0.5 µM dexamethasone
(D) and 5 µg/ml insulin (I) either with or without LNX at the
designated concentrations for 2 days (Fig. 1A). After 2 days, the first
differentiation medium was removed from the cells and the cells
were subsequently cultured in new DMEM supplemented with 10% FBS
and 5 µg/ml insulin either with or without LNX at the designated
concentrations for an additional 3 days. After 3 days, the second
differentiation medium was removed from the cells and the cells
were further supplemented with new DMEM containing 10% FBS with or
without LNX at the designated concentrations for another 3
days.
Oil red O staining
On day 8 post the differentiation induction, the
control or LNX-treated 3T3-L1 cells underwent phosphate-buffered
saline (PBS) washing and 10% formaldehyde fixation for 2 h at room
temperature (RT). Following a 60% isopropanol washing, the cells
were thoroughly dried. The fixed cells were treated with Oil Red O
working solution for 1 h at RT, followed by washing with distilled
water. Light microscopy was used to identify the lipids or fats
that accumulated in the conditioned cells (Nikon Corporation).
Cell count analysis
On day 8 post differentiation induction, control and
LNX-treated 3T3-L1 cells were stained with 0.4% trypan blue dye at
RT. Only cells with intact membranes could effectively block the
dye. Once dead cells with damaged membranes were exposed to the
dye, they were stained and counted under a light microscope.
Western blot analysis
At the designated time, the control or LNX-treated
3T3-L1 cells were washed with PBS and lysed in a modified RIPA
buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 0.1% SDS, 0.25% sodium
deoxycholate, 1% Triton X-100, 1% Nonidet P-40, 1 mM EDTA, 1 mM
EGTA, proteinase inhibitor cocktail (1X)]. The whole-cell lysates
were collected and centrifuged at 14,000 x g for 15 min at 4°C. The
supernatant was collected and protein concentrations were
determined using bicinchoninic acid (BCA) protein assay kit (Thermo
Fisher Scientific, Inc.).
An aliquot of protein (40 µg per lane) was separated
using 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and
then transferred to nitrocellulose membranes (MilliporeSigma). The
membranes were washed with Tris-buffered saline (10 mM Tris, 150 mM
NaCl) supplemented with 0.05% (v/v) Tween 20 (TBST) and blocked
with blocking buffer (TBST containing 5% (w/v) non-fat dried milk)
at 4˚C overnight. The membranes were incubated overnight with
corresponding primary antibodies for C/EBP-α (1:2,000), PPAR-γ
(1:2,000), p-STAT3 (1:2,000), STAT3 (1:2,000), phosphorylated
(p)-STAT-5 (1:2,000), STAT-5 (1:2,000), FAS (1:1,000), perilipin A
(1:2,000), p-HSL (S563) (1:2,000), p-HSL (S565) (1:2,000), T-HSL
(1:2,000), COX-2 (1:2,000) or β-actin (1:10,000) at 4˚C. The
membranes were then washed with TBST and incubated with a
horseradish peroxidase-conjugated secondary antibody with either
anti-mouse IgG (115-035-062; 1:5,000) or anti-rabbit IgG
(111-035-045; 1:5,000) (Jackson ImmunoResearch Laboratories, Inc.)
for 2 h at RT. The membranes were washed with TBST. ECL reagents
were used to develop the images. Equal protein loading per lane was
quantified based on the relative intensity of β-actin, an internal
control protein. ImageJ software (v.1.6.0.24; National Institutes
of Health) was used to intensify the bands for proteins that were
standardized to the internal control.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the control and
LNX-treated 3T3-L1 cells after 4 h using RNAiso Plus (Takara Bio,
Inc.). A total of three micrograms of total RNA were used to
prepare complementary DNA using random hexadeoxynucleotide primers,
reverse transcriptase (M-MLV RT), reverse transcriptase buffer
(M-MLV RT 5X Buffer) and dNTPs (Promega Corporation). The cDNA was
amplified by PCR with the following primers (Bioneer Corporation):
COX-2 forward, 5'-TTGAAGACCAGGAGTACAGC-3' and reverse,
5'-GGTACAGTTCCATGACATCG-3'; and β-actin forward,
5'-TCATGAAGTGTGACGTTGACATCCGT-3' and reverse,
5'-CCTAGAAGCACTTGCGGTGCACGATG-3'. The PCR conditions applied for
COX-2 were as follows; 30 cycles of denaturation at 95˚C for 30
sec, annealing at 62˚C for 30 sec and extension at 72˚C for 30 sec
and for β-actin; 30 cycles of denaturation at 95˚C for 30 sec,
annealing at 63˚C for 30 sec and extension at 72˚C for 1 min.
Levels of β-actin mRNA expression were used as an internal
control.
Quantification of glycerol
content
On day 8 of the post-differentiation induction,
3T3-L1 cells were serum-starved for 2 h and further treated without
or with LNX (6 µg/ml) or isoproterenol (ISO; 20 µM), a known
lipolysis inducer for another 3 h. The culture medium from the
control, LNX- or ISO-treated cells was stored and the glycerol
content in the respective culture medium was measured using a Free
Glycerol Reagent according to the manufacturer's instructions. The
absorbance was measured at 540 nm using a microplate reader.
Statistical analysis
Cell counting, western blotting and RT-PCR analysis
were conducted in triplicates and repeated two times. The mean and
SD values were used to express the data. One-way ANOVA was employed
to compare the significance of the differences, followed by a
Bonferroni test for the post hoc analysis. P<0.05 was considered
to indicate a statistically significant difference. The statistical
software used in the present study was IBM SPSS Statistics 25
software (IBM Corp.).
Results
Treatment with LNX leads to a
concentration-dependent suppression of lipid accumulation in
differentiating 3T3-L1 preadipocytes
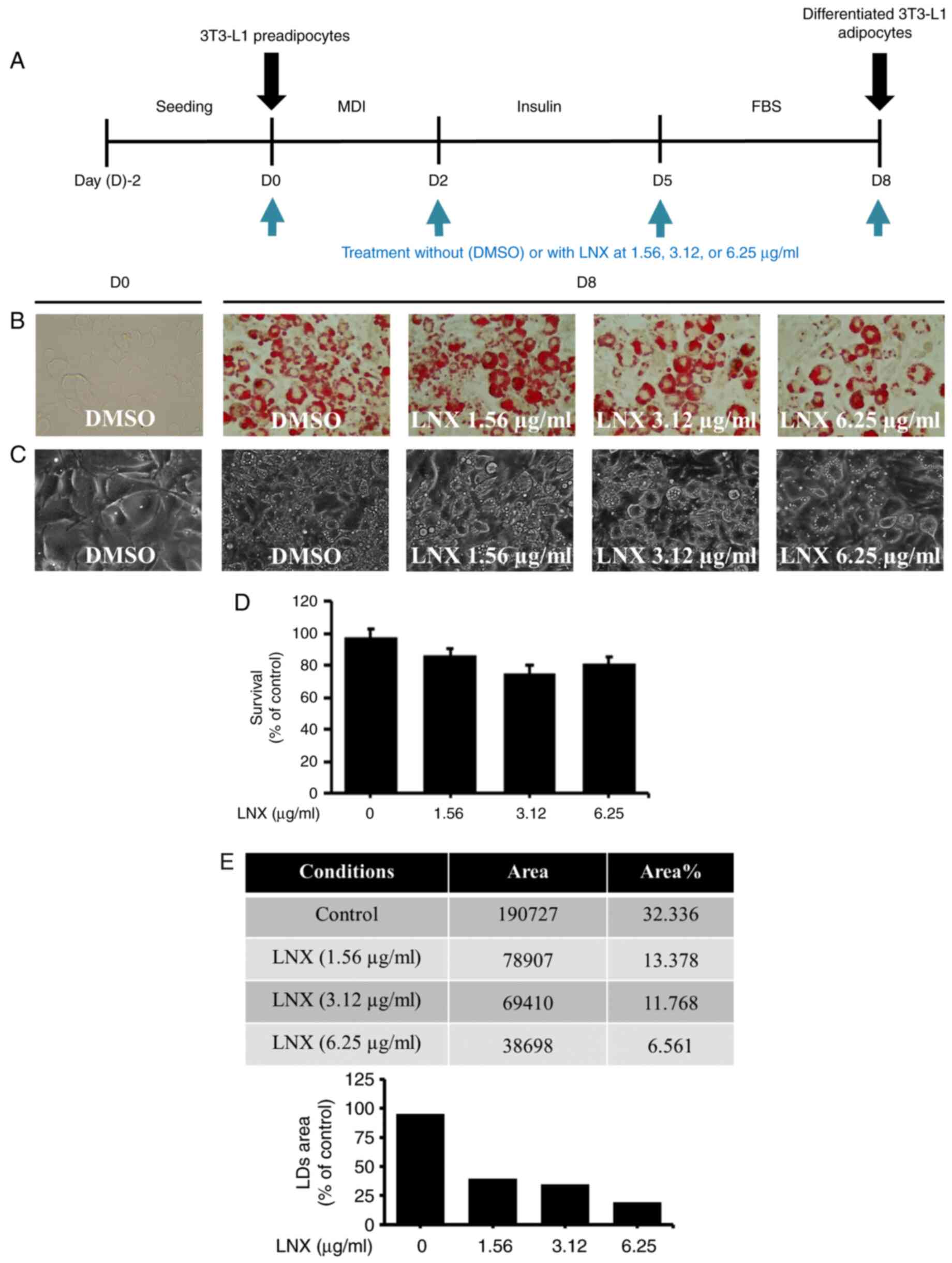
Initially, in the preliminary studies, LNX was
tested at higher concentrations such as 50, 100 and 200 µg/ml for
3T3-L1 preadipocyte differentiation (data not shown). However, LNX
treatment at 100 and 200 µg/ml had been demonstrating cytotoxicity
in differentiating 3T3-L1 cells. Hence, LNX was tested in lower
concentrations as revealed in the present study (6.25, 12.5 and 25
µg/ml) to further check the deposition rate of LDs during 3T3-L1
differentiation. Of interest, it was found that the accumulation of
LDs appeared to be increased in the higher concentration of LNX in
a dose-dependent manner (data not shown). Therefore, since the LNX
effects on the inhibition of lipid accumulation during 3T3-L1
preadipocyte differentiation using Oil Red O staining had to be
assessed, various concentrations of LNX (1.56, 3.12 and 6.25 µg/ml)
have been selected for the present study. The timetable for 3T3-L1
pre-adipocyte differentiation used in the present study is depicted
in Fig. 1A. As demonstrated in
Fig. 1B, Oil Red O staining
revealed markedly higher lipid accumulation in differentiated
3T3-L1 cells (D8) than in undifferentiated cells (D0). Notably,
compared with mock-treated cells, treatment with LNX led to a
dose-dependent inhibition of lipid accumulation in differentiated
3T3-L1 cells. The ability of LNX to downregulate lipid accumulation
in differentiated 3T3-L1 cells was confirmed using phase-contrast
microscopy (Fig. 1C). Additionally,
the effects of 1.56, 3.12 or 6.25 µg/ml LNX on cell growth during
the differentiation of 3T3-L1 preadipocytes into adipocytes, were
examined by using cell count analysis. As illustrated in Fig. 1D, treatment with LNX at the tested
doses did not significantly affect cell growth during 3T3-L1
preadipocyte differentiation. Quantification of the Oil Red O
staining images to determine the LDs percentage using ImageJ
software also revealed that LNX reduced lipid accumulation in a
dose-dependent manner (Fig. 1E).
Hence, based on the maximal lipid-lowering effect on
differentiating 3T3-L1 preadipocytes with no cytotoxicity, the
concentration of 6 µg/ml of LNX was selected for further
experiments.
LNX treatment at 6 µg/ml selectively
downregulates STAT3 phosphorylation and FAS expression in
differentiating 3T3-L1 preadipocytes
Subsequently, to explore the fundamental molecular
and cellular mechanisms underlying this LNX-induced anti-adipogenic
effect, LNX (6 µg/ml) was investigated on the expression and
phosphorylation levels of major adipogenic transcription factors,
including C/EBP-α, PPAR-γ and STAT3/5, using immunoblotting
analysis. As revealed in Fig. 2A,
LNX treatment did not modulate significantly the expression levels
of C/EBP-α and PPAR-γ proteins in 3T3-L1 preadipocyte
differentiation at the assessed timepoints; however, it led to the
slight elevation of the expression levels of C/EBP-α and PPAR-γ on
D5 and D8, respectively. In addition, C/EBP-β was also assessed
during 3T3-L1 differentiation. However, a band for this protein
could not be obtained, which was possibly due to an antibody
error.
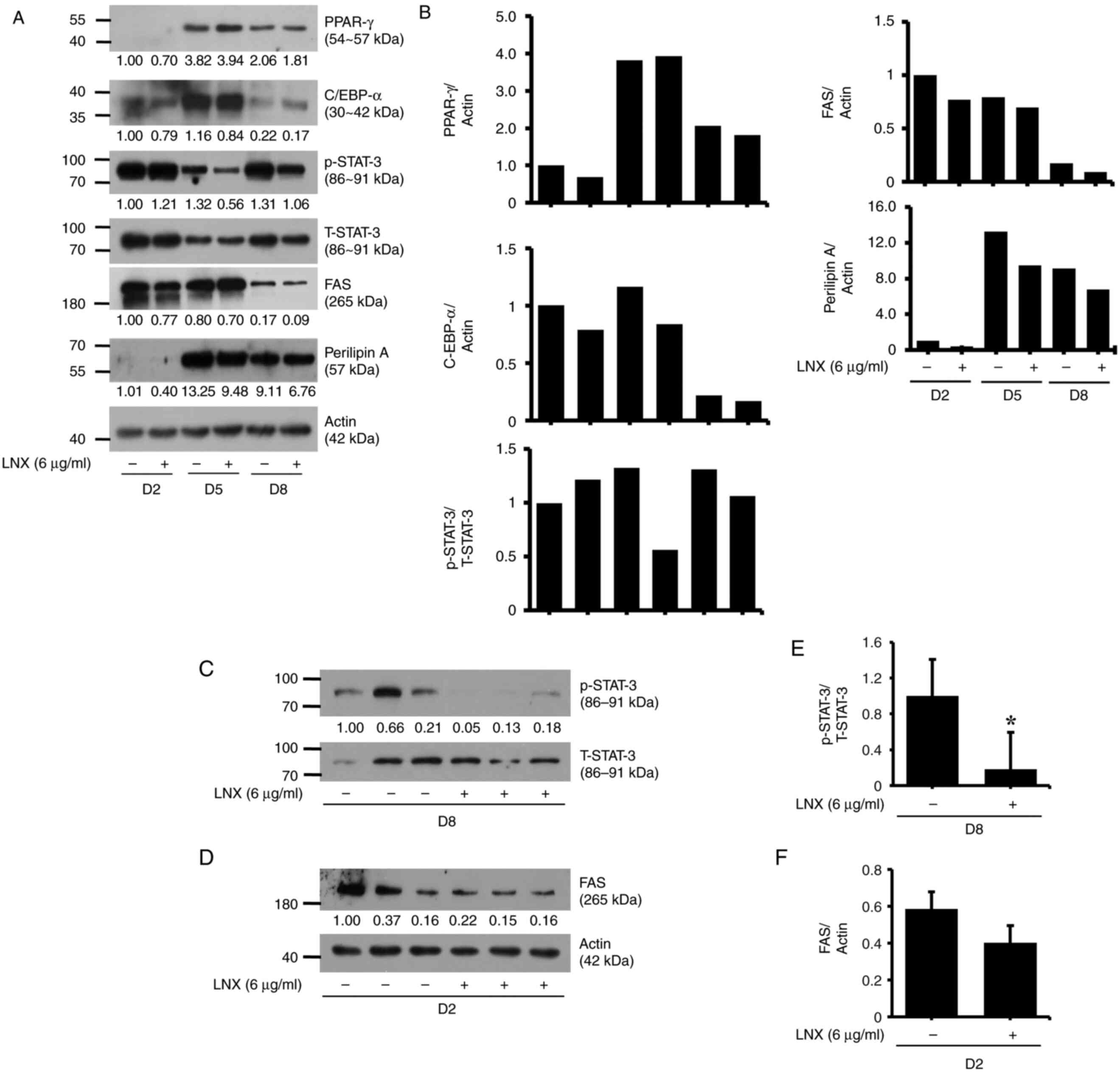 | Figure 2Effects of LNX on the expression
and/or phosphorylation levels of C/EBP-α, PPAR-γ, FAS, Perilipin A
and STAT3 during 3T3-L1 preadipocyte differentiation. (A, C and D)
3T3-L1 preadipocytes were differentiated using induction medium
containing IBMX, dexamethasone and insulin and FBS in the presence
or absence of LNX and harvested at day 2, 5 and 8, respectively.
Cellular proteins at the indicated time points were extracted and
analyzed using western blot analysis. (B) Densitometric analysis of
panel A. (C and D) Western blot analysis in triplicate experiments
on D8. (E and F) The densitometric analysis of (C) and (D),
respectively. *P<0.05 compared with vehicle control.
LNX, lignan-enriched nutmeg extract; PPAR-γ, peroxisome
proliferator-activated receptor gamma; C/EBP-α, CCAAT enhancer
binding protein alpha; p-STAT3, phosphorylated signal transducer
and activator of transcription 3; T-STAT3, total-STAT3; FAS, fatty
acid synthase; D, day. |
In the present study, the day courses such as D2, D5
and D8 in 3T3-L1 preadipocyte differentiation were observed to
determine the p/T-STAT3 and FAS protein expression (Fig. 2A and B). It was revealed that STAT3 was highly
phosphorylated during 3T3-L1 preadipocyte differentiation. Of note,
p-STAT3 expression level was decreased on D5. In addition, on D8,
STAT3 protein levels were strongly phosphorylated again during
differentiation period (as compared with the control group of D5).
Notably, the mid-phase of STAT3 decline affected the adipocyte
differentiation. Meanwhile, FAS protein was greatly attenuated on
D2 compared with D5 or D8. Hence, based on these day course
experiment results, different day courses were investigated on each
target (D8 for STAT3 and D2 for FAS protein expression levels)
(Fig. 2C-F).
Interestingly, while treatment with LNX had no
effect on the phosphorylation and total expression levels of STAT3
on D2 of 3T3-L1 cell differentiation, it markedly reduced the
phosphorylation of STAT3 without altering its total expression
levels on D5 of the cell differentiation. However, LNX treatment
further reduced the phosphorylation and total expression levels of
STAT3 on D8 of cell differentiation. Moreover, LNX treatment
slightly inhibited the expression of FAS on D2, D5 and D8, during
3T3-L1 preadipocyte differentiation. LNX treatment did not
influence perilipin A expression during cell differentiation.
Expression levels of the control actin protein remained constant
under these experimental conditions. The densitometric data from
Fig. 2A are demonstrated in
Fig. 2B. The results of the
triplicate experiments, as demonstrated in Fig. 2C and D, revealed the capability of LNX (6 µg/ml)
to vastly inhibit STAT3 phosphorylation and FAS expression on D8
and D2 of 3T3-L1 preadipocyte differentiation, respectively. The
densitometric data from Fig. 2C and
D are shown in Fig. 2E and F, respectively.
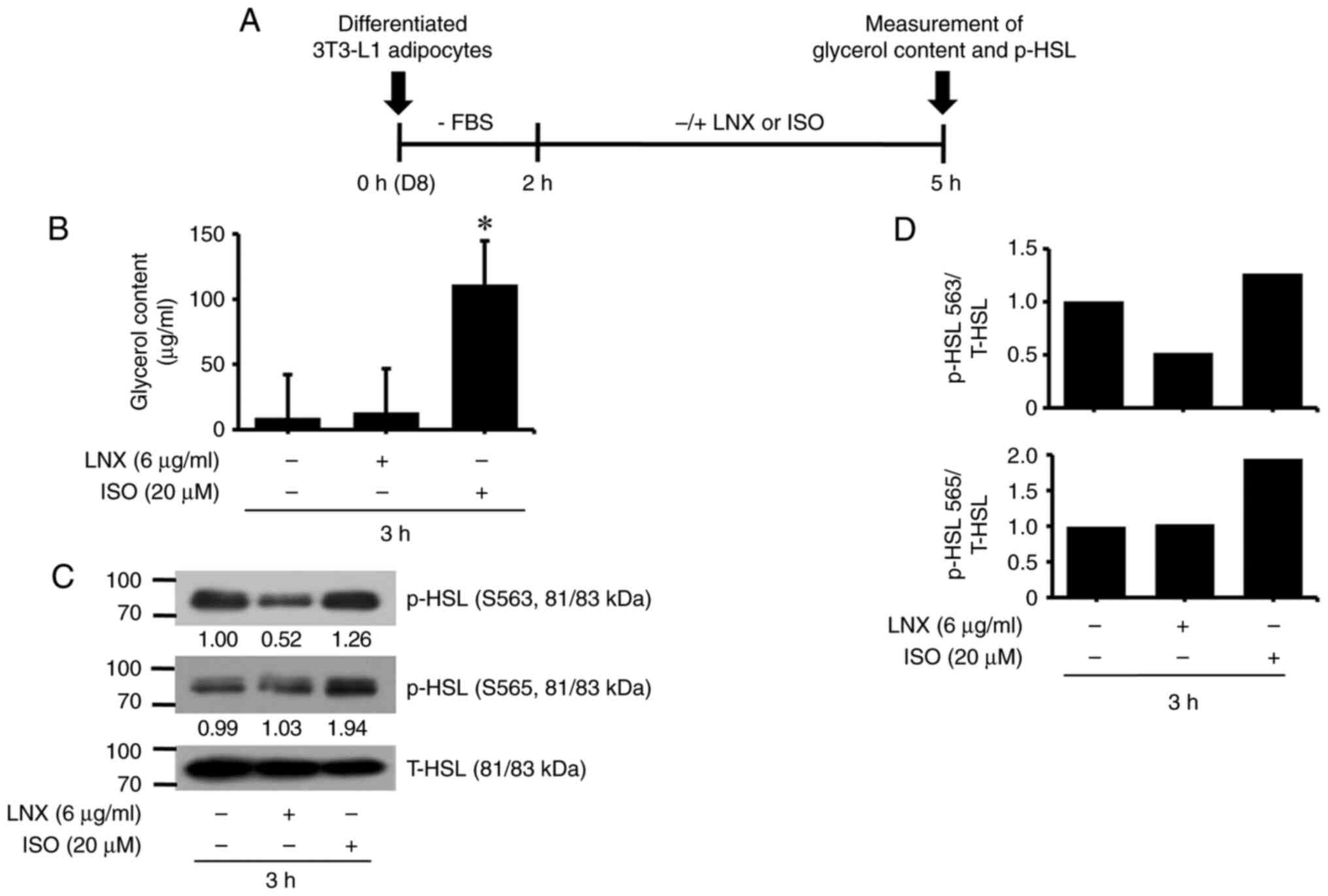
LNX treatment at 6 µg/ml does not
stimulate lipolysis in differentiated 3T3-L1 adipocytes
Subsequently, it was explored whether treatment with
LNX (6 µg/ml) modulated lipolysis in differentiated 3T3-L1
adipocytes by measuring the levels of glycerol content and HSL
phosphorylation, two hallmarks of lipolysis. The experimental
scheme and timescale for measuring glycerol content and HSL
phosphorylation are exhibited in Fig.
3A. To this end, differentiated 3T3-L1 adipocytes on D8 were
serum-starved (0% FBS) for 2 h and incubated in a fresh culture
media containing 10% FBS in the absence or presence of LNX (6
µg/ml) or ISO (20 µM), a known inducer of lipolysis (31), for an additional 3 h. Culture media
and whole-cell lysates from each condition were used to measure the
two lipolysis hallmarks aforementioned. As illustrated in Fig. 3B, ISO treatment for 3 h
significantly enhanced glycerol release from differentiated 3T3-L1
adipocytes, whereas LNX treatment did not. In addition, while ISO
treatment for 3 h significantly elevated p-HSL levels at residues
S563 and S565 in differentiated 3T3-L1 adipocytes, LNX treatment
had no stimulatory effect. The total HSL protein expression levels
remained unchanged under these experimental conditions. The
densitometric data from Fig. 3C are
demonstrated in Fig. 3D.
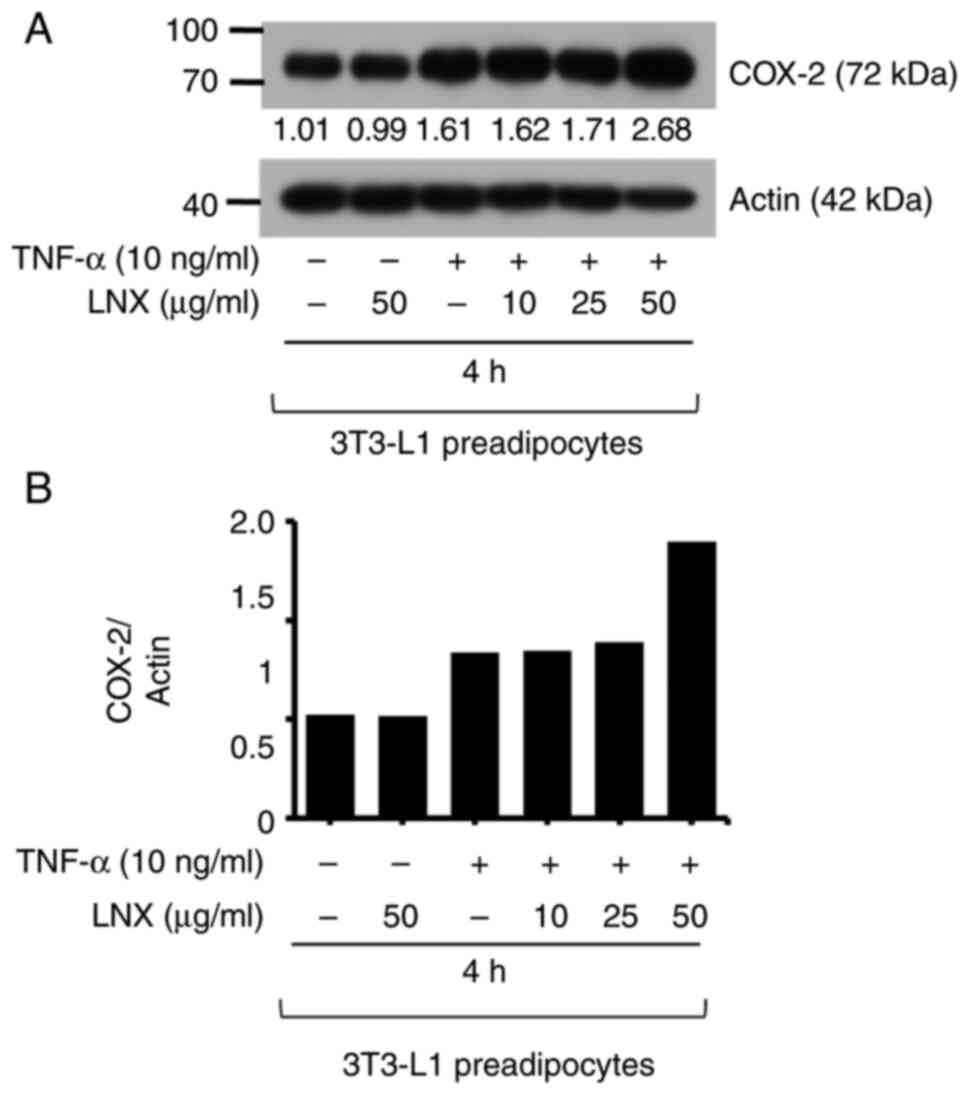
LNX treatment has no effect on the
TNF-α-induced expression of COX-2 in 3T3-L1 preadipocytes
Next, it was further explored whether TNF-α at 10
ng/ml induces COX-2 expression in 3T3-L1 preadipocytes and whether
treatment of LNX at different concentrations (1.5, 3 or 6 µg/ml)
interferes with it by using RT-PCR analysis. As expected, the
exposure of TNF-α for 4 h greatly induced COX-2 mRNA expression in
3T3-L1 preadipocytes. However, LNX treatment at the examined
concentrations did not significantly influence cytokine-induced
COX-2 mRNA expression in these cells (Fig. S1). Using immunoblotting analysis
(Fig. 4A), it was investigated
whether LNX treatment at higher doses (10, 25 and 50 µg/ml) could
modulate cytokine-induced COX-2 expression in 3T3-L1 preadipocytes.
As revealed in Fig. 4, while
treatment with TNF-α for 4 h markedly enhanced COX-2 protein
expression in 3T3-L1 preadipocytes, LNX treatment at such high
doses tested did not interfere with the TNF-α-induced COX-2 protein
expression in 3T3-L1 preadipocytes. Expression levels of the
control actin protein remained constant under these experimental
conditions. The densitometric data from Fig. 4A are illustrated in Fig. 4B.
Discussion
Abnormal lipid accumulation, aggravated inflammation
and the dysregulation of preadipocyte lipolysis are closely linked
to the development of obesity. In the present study, LNX
demonstrated anti-adipogenic, but not pro-lipolytic, and
anti-inflammatory effects in differentiating 3T3-L1 preadipocytes
through the control of STAT3 phosphorylation and FAS
expression.
In the initial experiments, it was revealed that LNX
treatment led to a concentration-dependent suppression of fat
accumulation in differentiating 3T3-L1 preadipocytes with no
cytotoxicity, supporting its anti-adipogenic effect. Mounting
evidence illustrated a pivotal role of adipogenic transcription
factors including C/EBP-α, PPAR-γ and STAT3/5 in adipogenesis of
preadipocytes (32-34).
To date, LNX regulation of the expression and phosphorylation
levels of these transcription factors during the adipocyte
differentiation of 3T3-L1 preadipocytes remains unknown. In the
present study, LNX treatment greatly inhibited STAT3
phosphorylation without affecting total protein expression in the
middle stage (D5) of cell differentiation. Previous studies have
shown that STAT3 phosphorylation is involved in the early stages of
3T3-L1 preadipocyte differentiation (35,36).
These findings suggested that LNX exerts its lipid-lowering effect
on differentiating 3T3-L1 preadipocytes by inhibiting STAT3 and its
downstream components or pathways. Previously, it has also been
reported that STAT3 regulates the upregulation of C/EBP-α and
PPAR-γ at middle or last stage of preadipocyte differentiation
(37). However, in the present
study, it was demonstrated that LNX treatment does not modulate the
expression levels of both C/EBP-α and PPAR-γ throughout 3T3-L1
preadipocyte differentiation. It is therefore conceivable that the
anti-adipogenic effect of LNX on differentiating 3T3-L1
preadipocytes is mediated not through the STAT3-dependent C/EBP-α
and PPAR-γ pathway but via a different STAT3-dependent pathway.
Preadipocyte differentiation is also closely
associated with lipogenesis and lipid or LD stabilization (38,39).
FAS is a key lipogenic enzyme responsible for catalyzing de
novo synthesis of fatty acids and its expression is highly
elevated during preadipocyte differentiation (40,41).
Perilipin A is an LD-binding and stabilizing protein whose
expression is greatly enhanced during preadipocyte differentiation
(42,43). At present, little is known about the
LNX regulation of FAS and perilipin A during preadipocyte
differentiation. Notably, LNX treatment inhibited FAS expression in
the early stages of 3T3-L1 preadipocyte differentiation. However,
the present study demonstrated that LNX treatment does not
interfere with perilipin A expression during 3T3-L1 cell
differentiation. These results suggested that the anti-adipogenic
effect of LNX on differentiating 3T3-L1 preadipocytes is, in part,
mediated through interference with FAS-dependent lipogenesis.
Lipolysis is a biochemical process in which lipids
or TGs stored in adipocytes are broken down, resulting in the
efflux of fatty acids and glycerol (44). Hence, any material that induces
lipolysis in lipid-laden adipocytes can be utilized as a potential
anti-obesity agent. Accordingly, the LNX regulation of lipolysis
and its regulator in differentiated or mature adipocytes were
further tested. However, considering the present findings that LNX
treatment did not enhance glycerol release in differentiated 3T3-L1
adipocytes, it is likely that LNX has no lipolytic activity in
mature 3T3-L1 adipocytes. Cellular lipases which trigger lipolysis
HSL are key for TG degradation in mature adipocytes (45). It has also been revealed that HSL is
phosphorylated at S563 and S565 and becomes active in adipocytes in
response to certain endogenous or exogenous stimuli, such as
norepinephrine or ISO (46,47). Given that, unlike ISO, LNX treatment
does not induce the phosphorylation of HSL at residues S563 and
S565 in differentiated 3T3-L1 adipocytes, it is further evident
that LNX has no lipolytic effect on these cells.
Accumulating evidence strongly indicates that
chronic inflammation contributes to development of obesity
(48). A wealth of information
illustrates that preadipocytes, the predominant cell types present
in the AT, are able to express and secrete numerous
pro-inflammatory cytokines [including TNF-α, interleukin (IL)-1β
and IL-6] and enzymes (COX-2) (49-51),
which can confer and aggravate obese inflammation, via the
autocrine, paracrine and endocrine ways. As aforementioned, the
authors and other groups have previously demonstrated that TNF-α is
an inducer of COX-2 in not only 3T3-L1 preadipocytes but also in
mature or differentiated adipocytes (15-19).
To date, the regulation of pro-inflammatory cytokine-induced
expression of COX-2 by LNX in 3T3-L1 preadipocytes remains unknown.
In the present study, it was observed that LNX treatment at doses
tested does not interfere with the TNF-α-induced COX-2 expression
at the protein and mRNA levels in undifferentiated 3T3-L1 cells.
These results indicated that LNX has no anti-inflammatory effect on
3T3-L1 preadipocytes. In addition, it would be interesting to
investigate whether LNX could alter the cytokine-induced expression
of COX-2 (and other inflammatory mediators) in mature adipocytes.
Moreover, further studies using in vivo experiments will be
able to address the limitations of this study.
In summary, it was firstly reported that LNX has a
potent anti-adipogenic effect on differentiating 3T3-L1
preadipocytes, and this effect is achieved by downregulating STAT3
phosphorylation and FAS expression. The present study advocated
that LNX may be a potential obesity-preventive agent.
Supplementary Material
Effects of LNX on TNF-α-induced COX-2
mRNA expression level in 3T3-L1 preadipocytes. (A) 3T3-L1
preadipocytes were treated with or without TNF-α (10 ng/ml) in the
presence or absence of LNX at the designated concentrations for 4
h. After the aforementioned treatment, total RNA was extracted and
analyzed for COX-2 using reverse transcription PCR analysis. (B)
The densitometric analysis of panel A. LNX, lignan-enriched nutmeg
extract; COX-2, cyclooxygenase-2.
Acknowledgements
The authors would like to thank Daehan-Nupharm Co.,
Ltd. (Hwasung, Republic of Korea) for providing the nutmeg extract
samples and Geron Biotech Ltd., KBSI Research Company, for the help
and support on the current publication.
Funding
Funding: The present study was supported by the Korea Basic
Science Institute (grant. no. C280320).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NP, SD, JL, HY, BJ and JC conceived and designed the
experiments. NP, SD, GB, JL, GK and AM performed the experiments.
HY, BJ, JC, SD, GB and AM analyzed and interpreted the data. HY,
BJ, AM and JC wrote the manuscript; BJ, JL, GB and AM reviewed and
edited the manuscript. All authors read and approved the final
version of the manuscript. HY, BJ and JC confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2015 Obesity Collaborators. Afshin A,
Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L,
Mokdad AH, Moradi-Lakeh M, et al: Health effects of overweight and
obesity in 195 countries over 25 years. N Engl J Med. 377:13–27.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gregg EW and Shaw JE: Global health
effects of overweight and obesity. N Engl J Med. 377:80–81.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543.
2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Madsen MS, Siersbæk Boergesen M, Nielsen R
and Mandrup S: Peroxisome proliferator-activated receptor γ and
C/EBPα synergistically activate key metabolic adipocyte genes by
assisted loading. Mol Cell Biol. 34:939–954. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao P and Stephens JM: Identification of
STAT target genes in adipocytes. JAKSTAT. 2(e23092)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang K, Guo W, Yang Y and Wu J:
JAK2/STAT3 pathway is involved in the early stage of adipogenesis
through regulating C/EBPβ transcription. J Cell Biochem.
112:488–497. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu Z, Rosen ED, Brun R, Hauser S, Adelmant
G, Troy AE, McKeon C, Darlington GJ and Spiegelman BM:
Cross-regulation of C/EBP alpha and PPAR gamma controls the
transcriptional pathway of adipogenesis and insulin sensitivity.
Mol Cell. 3:151–158. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Janovská A, Hatzinikolas G, Staikopoulos
V, Mcinerney J, Mano M and Wittert GA: AMPK and ACC
phosphorylation: Effect of leptin, muscle fibre type and obesity.
Mol Cell Endocrinol. 284:1–10. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Garcia A, Sekowski A, Subramanian V and
Brasaemle DL: The central domain is required to target and anchor
perilipin A to lipid droplets. J Biol Chem. 278:625–635.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schweiger M, Eichmann TO, Taschler U,
Zimmermann R, Zechner R and Lass A: Measurement of lipolysis.
Methods Enzymol. 538:171–193. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hsieh PS, Jin JS, Chiang CF, Chan PC, Chen
CH and Shih KC: COX-2-mediated inflammation in fat is crucial for
obesity-linked insulin resistance and fatty liver. Obesity (Silver
Spring). 17:1150–1157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fuster JJ, Ouchi N, Gokce N and Walsh K:
Obesity-induced changes in adipose tissue microenvironment and
their impact on cardiovascular disease. Circ Res. 118:1786–1807.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim HL, Ha AW and Kim WK: Effect of
saccharin on inflammation in 3T3-L1 adipocytes and the related
mechanism. Nutr Res Pract. 14:109–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng AW, Tan X, Sun JY, Gu CM, Liu C and
Guo X: Catechin attenuates TNF-α induced inflammatory response via
AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS One.
14(e0217090)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Y, Yang P, Chang Q, Wang J, Liu J, Lv
Y, Wang TTY, Gao B, Zhang Y and Yu LL: Inhibitory effect of
piceatannol on TNF-α-mediated inflammation and insulin resistance
in 3T3-L1 Adipocytes. J Agric Food Chem. 65:4634–4641.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kwon HS, Jeong GS and Jang BC:
Cudratricusxanthone A inhibits lipid accumulation and expression of
inducible nitric oxide synthase in 3T3-L1 preadipocytes. Int J Mol
Sci. 22(505)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yadav AK and Jang BC: Inhibition of lipid
accumulation and cyclooxygenase-2 expression in differentiating
3T3-L1 preadipocytes by pazopanib, a multikinase inhibitor. Int J
Mol Sci. 22(4884)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Van Gils C and Cox PA: Ethnobotany of
nutmeg in the spice islands. J Ethnopharmacol. 42:117–124.
1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nguyen PH, Le TVT, Kang HW, Chae J, Kim
SK, Kwon KI, Seo DB, Lee SJ and Oh WK: AMP-activated protein kinase
(AMPK) activators from Myristica fragrans (nutmeg) and their
anti-obesity effect. Bioorg Med Chem Lett. 20:4128–4131.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cho JY, Choi GJ, Son SW, Jang KS, Lim HK,
Lee SO, Sung ND, Cho KY and Kim JC: Isolation and antifungal
activity of lignans from Myristica fragrans against various
plant pathogenic fungi. Pest Manag Sci. 63:935–940. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Zhao W, Guo M, Feng J, Gu Z, Zhao J, Zhang
H, Wang G and Chen W: Myristica fragrans extract regulates
gut microbes and metabolites to attenuate hepatic inflammation and
lipid metabolism disorders via the AhR-FAS and NF-κB signaling
pathways in mice with non-alcoholic fatty liver disease. Nutrients.
14(1699)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao W, Song F, Hu D, Chen H, Zhai Q, Lu
W, Zhao J, Zhang H, Chen W, Gu Z and Wang G: The protective effect
of Myristica fragrans Houtt. Extracts against obesity and
inflammation by regulating free fatty acids metabolism in
nonalcoholic fatty liver disease. Nutrients.
12(2507)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lesmana R, Siannoto M, Nugraha GI,
Goenawan H, Feinisa AK, Pratiwi YS, Veronica F, Tarawan VM,
Susianti S and Supratman U: Nutmeg extract potentially alters
characteristics of white adipose tissue in rats. Vet Med Sci.
7:512–520. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Hien TT, Oh WK, Nguyen PH, Oh SJ, Lee MY
and Kang KW: Nectandrin B activates endothelial nitric-oxide
synthase phosphorylation in endothelial cells: Role of the
AMP-activated protein kinase/estrogen receptor
α/phosphatidylinositol 3-kinase/Akt pathway. Mol Pharmacol.
80:1166–1178. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Smith M: Nutmeg. In: Encyclopedia of
Toxicology. 3rd edition. Elsevier, Amsterdam, pp630-631, 2014.
|
|
27
|
Götz ME, Sachse B, Schäfer B and
Eisenreich A: Myristicin and elemicin: Potentially
toxicalkenylbenzenes in food. Foods. 11(1988)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jang HJ, Yang KE, Oh WK, Lee SI, Hwang IH,
Ban KT, Yoo HS, Choi JS, Yeo EJ and Jang IS: Nectandrin B-mediated
activation of the AMPK pathway prevents cellular senescence in
human diploid fibroblasts by reducing intracellular ROS levels.
Aging (Albany NY). 11:3731–3749. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pratiwi YS, Lesmana R, Goenawan H,
Sylviana N, Setiawan I, Tarawan VM, Lestari K, Abdulah R, Dwipa L,
Purba A and Supratman U: Nutmeg extract increases skeletal muscle
mass in aging rats partly via IGF1-Akt-mTOR pathway and inhibition
of autophagy. Evid Based Complement Alternat Med.
2018(2810840)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee JH, Kang H, Ban KT, Kim BK, Lee JH,
Hwang H, Yoo HS, Cho K and Choi JS: Proteome network analysis of
skeletal muscle in lignan-enriched nutmeg extract fed mice. J Anal
Sci Tech. 14(11)2023.
|
|
31
|
Greenberg AS, Shen WJ, Muliro K, Patel S,
Souza SC, Roth RA and Kraemer FB: Stimulation of lipolysis and
hormone-sensitive lipase via the extracellular signal-regulated
kinase pathway. J Biol Chem. 276:45456–45461. 2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman
MW, Gonzalez FJ and Spiegelman BM: C/EBPalpha induces adipogenesis
through PPARgamma: A unified pathway. Genes Dev. 16:22–26.
2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Stephens JM, Morrison RF and Pilch PF: The
expression and regulation of STATs during 3T3-L1 adipocyte
differentiation. J Biol Chem. 271:10441–10444. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gong Z, Huang C, Sheng X, Zhang Y, Li Q,
Wang M-W, Peng L and Zang YQ: The role of tanshinone IIA in the
treatment of obesity through peroxisome proliferator-activated
receptor gamma antagonism. Endocrinology. 150:104–113.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Richard AJ and Stephens JM: The role of
JAK-STAT signaling in adipose tissue function. Biochim Biophys
Acta. 1842:431–439. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang D, Zhou Y, Lei W, Zhang K, Shi J, Hu
Y, Shu G and Song J: Signal transducer and activator of
transcription 3 (STAT3) regulates adipocyte differentiation via
peroxisome-proliferator-activated receptor gamma (PPARgamma). Biol
Cell. 102:1–12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Collins JM, Neville MJ, Pinnick KE, Hodson
L, Ruyter B, van Dijk TH, Reijngoud DJ, Fielding MD and Frayn KN:
De novo lipogenesis in the differentiating human adipocyte can
provide all fatty acids necessary for maturation. J Lipid Res.
52:1683–1692. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tansey JT, Sztalryd C, Hlavin EM, Kimmel
AR and Londos C: The central role of perilipin a in lipid
metabolism and adipocyte lipolysis. IUBMB Life. 56:379–385.
2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Swierczyński J and Sledziński T: Metabolic
and regulatory function of fatty acid synthase. Postepy Biochem.
58:175–185. 2012.PubMed/NCBI(In Polish).
|
|
41
|
Jensen-Urstad APL and Semenkovich CF:
Fatty acid synthase and liver triglyceride metabolism: Housekeeper
or messenger? Biochim Biophys Acta. 1821:747–753. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Itabe H, Yamaguchi T, Nimura S and Sasabe
N: Perilipins: A diversity of intracellular lipid droplet proteins.
Lipids Health Dis. 16(83)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kern PA, Gregorio GD, Lu T, Rassouli N and
Ranganathan G: Perilipin expression in human adipose tissue is
elevated with obesity. J Clin Endocrinol Metab. 89:1352–1358.
2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Langin D: Control of fatty acid and
glycerol release in adipose tissue lipolysis. C R Biol.
329:598–607. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Althaher AR: An overview of
hormone-sensitive lipase (HSL). ScientificWorldJournal.
2022(1964684)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kraemer FB and Shen WJ: Hormone-sensitive
lipase: control of intracellular tri-(di-)acylglycerol and
cholesteryl ester hydrolysis. J Lipid Res. 43:1585–1594.
2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
McDonough PM, Ingermanson RS, Loy PA, Koon
ED, Whittaker R, Laris CA, Hilton JM, Nicoll JB, Buehrer BM and
Price JH: Quantification of hormone sensitive lipase
phosphorylation and colocalization with lipid droplets in murine
3T3L1 and human subcutaneous adipocytes via automated digital
microscopy and high-content analysis. Assay Drug Dev Technol.
9:262–280. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Roy PK, Islam J and Lalhlenmawia H:
Prospects of potential adipokines as therapeutic agents in
obesity-linked atherogenic dyslipidemia and insulin resistance.
Egypt Heart J. 75(24)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ouchi N, Parker JL, Lugus JJ and Walsh K:
Adipokines in inflammation and metabolic disease. Nat Rev Immunol.
11:85–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chan PC, Liao MT and Hsieh PS: The
dualistic effect of COX-2-mediated signaling in obesity and insulin
resistance. Int J Mol Sci. 20(3115)2019.PubMed/NCBI View Article : Google Scholar
|