Introduction
Diabetic macular edema is a clinical condition that
causes severe visual impairment. The vision of patients with
diabetic retinopathy (DR) can be maintained through various topical
treatments, including intravitreal administration of anti-vascular
endothelial growth factor (VEGF) drugs, retinal photocoagulation,
sub-Tenon injection of triamcinolone acetonide (STTA) or
intravitreal injection of triamcinolone acetonide, and pars plana
vitrectomy (1). However, some DR
patients may be resistant to standard treatment, leading to
refractory cystoid macular edema (CME). Vitrectomy with incision of
cystoid lesions has been reported as an alternative treatment for
treatment-resistant CME secondary to DR (2-4).
In 2020, Imai et al (5) reported on cystoid lesion components in
CME caused by diabetic macular edema or branch retinal vein
occlusion using transmission electron microscopy (TEM) and mass
spectrometry (MS) analysis. TEM revealed that the cystoid lesion
components were non-cellular structures composed mainly of
microfibrils wrapped in collagen fibers. MS analysis also revealed
that the component contains fibrinogen α, β and γ (5). To date, to the best of our knowledge,
there have been no histopathological or immunohistochemical studies
examining the expression of proteins in cystoid lesion components
in patients with diabetes.
Fibrinogen is known to undergo various
post-translational modifications (6). Post-translational modifications of
fibrinogen affect its function, subsequently contributing to
various pathological conditions. For example, it has been reported
that glycation and methylglyoxal (MGO)-derived advanced glycation
end-product (AGE) modification of fibrinogen occurs in patients
with diabetes (6,7).
The present study describes the case of a patient
with refractory diabetic CME who underwent vitrectomy with en
bloc removal of the cystoid lesion component. The present study
also performed histopathological and immunohistochemical analysis
of the cystoid lesion content to assess its immunoreactivity for
fibrin/fibrinogen and AGE.
Case report
Clinical presentation
A 69-year-old Japanese man complained of visual loss
and visual field distortion in the left eye. In July 2021, the
patient was referred to Hokkaido University Hospital (Sapporo,
Japan) due to residual diabetic CME despite receiving intravitreal
anti-VEGF injections of aflibercept (IVA) a total of eight times
for 3 years prior to referral. The patient had a medical history of
diabetes mellitus, dyslipidemia and hypertension, and was diagnosed
with diabetes at the age of 56 years. At the time of referral, the
patient was being treated for diabetes with subcutaneous injection
of dulaglutide, a weekly glucagon-like peptide-1 receptor agonist,
1.5 mg/week and insulin lispro 10 U/morning and 12 U/evening. The
serum HbA1c levels were well controlled at 6.0%. The patient
underwent cataract surgery on their right eye at the age of 66
years. The best-corrected visual acuity (BCVA) was 1.2 in the right
eye (OD) and 0.5 in the left eye (OS). Intraocular pressure was
normal in both eyes. Slit-lamp examination demonstrated an
intraocular lens (OD) and mild cataracts (OS). Fundus examination
showed dot hemorrhages and hard exudates in the peri-macular region
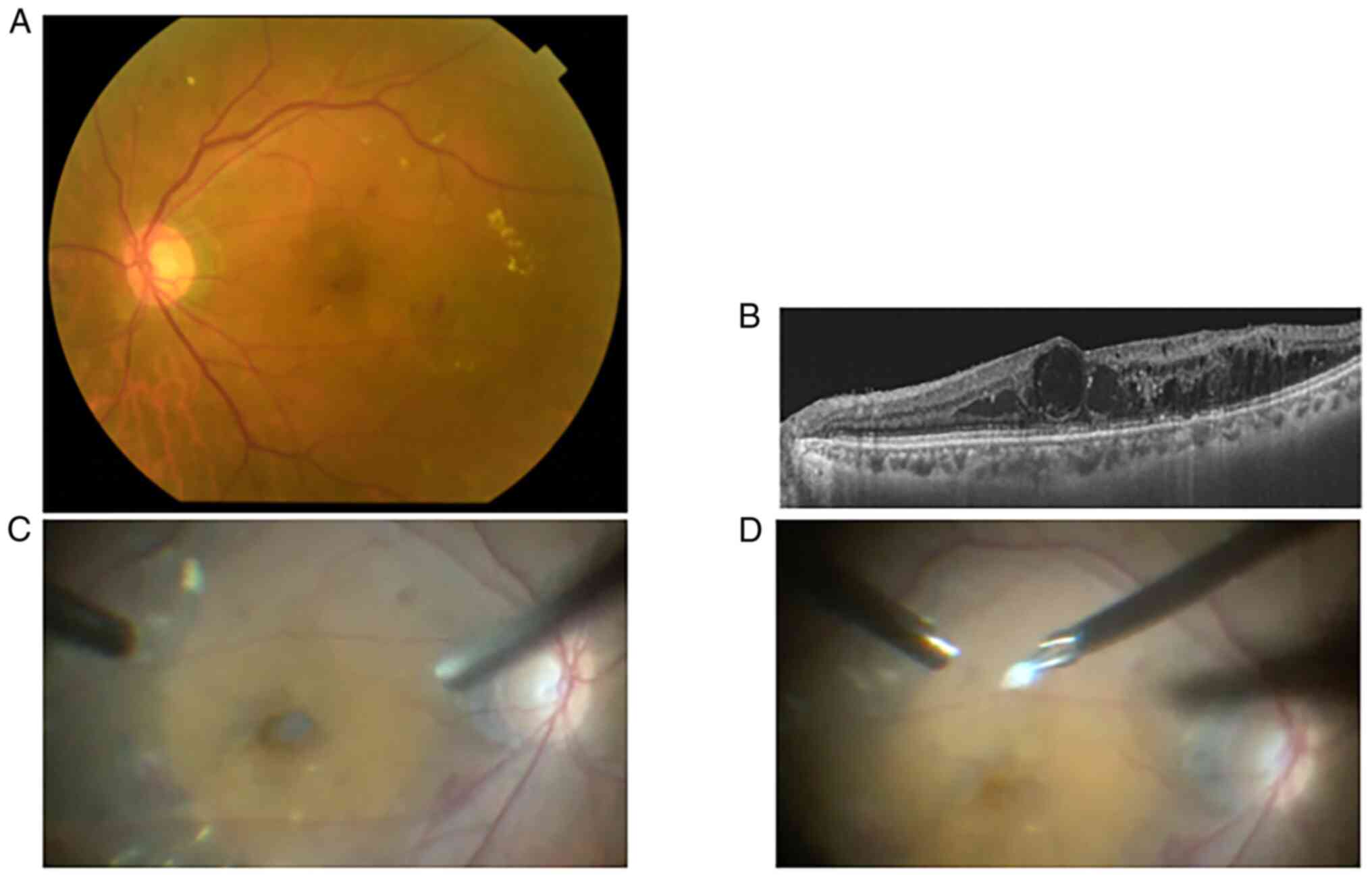
with pan-retinal photocoagulation scars in both eyes (Fig. 1A). Swept-source optical coherence
tomography demonstrated macular edema with foveal cystoid lesions,
where the reflectivity was slightly higher than that of vitreous
fluids OS (Fig. 1B). A total of 3
months after the initial visit, the patient underwent pars plana
vitrectomy of the left eye with cataract surgery, internal limiting
membrane peeling and removal of the cystoid lesion component. The
cystoid lesion component was a translucent soft solid (Fig. 1C and D), which was then fixed in 4% formalin at
room temperature overnight immediately after removal and embedded
in paraffin for hematoxylin-eosin staining at room temperature for
a few minutes each, and fluorescence immunohistochemical staining.
The BCVA of the left eye 1 month after surgery was 0.3.
Postoperatively, the CME subsided but soon recurred. Therefore, the
left eye was further treated with STTA, direct photocoagulation for
microaneurysms and IVA during the next year. A total of 1 year
after surgery, the BCVA of the left eye had decreased to 0.2.
Methods
The formalin-fixed, paraffin-embedded tissue
sections (5 µm) underwent pathological diagnosis and
immunohistochemical analysis. Immunohistochemical analysis was
performed as follows: The sections were dewaxed in xylene,
dehydrated in various concentrations of ethanol and rinsed in
phosphate-buffered saline after rinsing in Milli-Q water for 5 min.
As a pretreatment, microwave-based antigen retrieval was conducted
in 10 mM citrate buffer (pH 6.0) for 10 min after boiling. The
sections were then incubated with 5.0% normal goat serum (cat. no.
50062Z; Thermo Fisher Scientific, Inc.) for 1 h at room temperature
and with the following primary antibodies: Rabbit anti-human
fibrin/fibrinogen polyclonal antibody (1:200 dilution; cat. no.
A0080; Agilent Technologies, Inc.), rabbit anti-AGEs polyclonal
antibody (1:100 dilution; cat. no. ab23722; Abcam), rabbit
anti-collagen type 1 polyclonal antibody (1:100 dilution; cat. no.
600-401-103-0.1; Rockland Immunochemicals, Inc.), mouse anti-glial
fibrillary acidic protein (GFAP) monoclonal antibody (1:100
dilution; cat. no. 14-9892-82; Thermo Fisher Scientific, Inc.),
mouse anti-human receptor for AGE (RAGE) monoclonal antibody (1:100
dilution; cat. no. MAB11451; R&D Systems, Inc.), normal rabbit
IgG (1:70 dilution; cat. no. AB-105-C; R&D Systems, Inc.) and
normal mouse IgG (1:20 dilution; cat. no. X0931; Agilent
Technologies, Inc.) at 4˚C overnight. Data on immunoreactivity for
normal mouse IgG are not shown. The sections were then incubated
with Alexa Fluor 488-conjugated (1:500 dilution; cat. no. A32723;
Thermo Fisher Scientific, Inc.) or Alexa Fluor 546-conjugated
(1:500 dilution; cat. no. A11035; Thermo Fisher Scientific, Inc.)
secondary antibodies at room temperature for 1 h. Sections were
visualized with an inverted fluorescence-phase contrast
microscope.
Histopathological findings
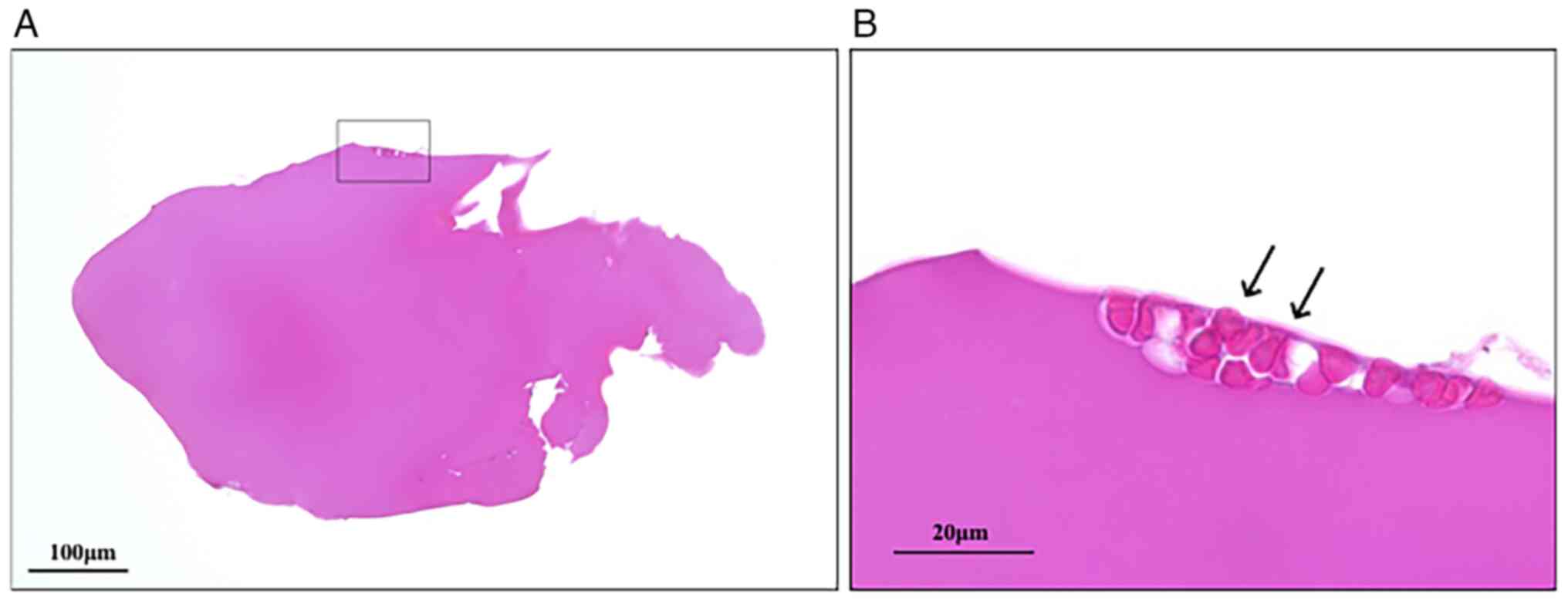
Microscopic examination of the excised tissue
revealed it to be elliptical in shape, measuring 0.7x0.4 mm. It
displayed a homogeneous structure comprising eosinophilic material
without cellular components (Fig.
2A). No membranous structure was observed surrounding the
component, but a few erythrocyte aggregates were detected at the
margin (Fig. 2B, arrows).
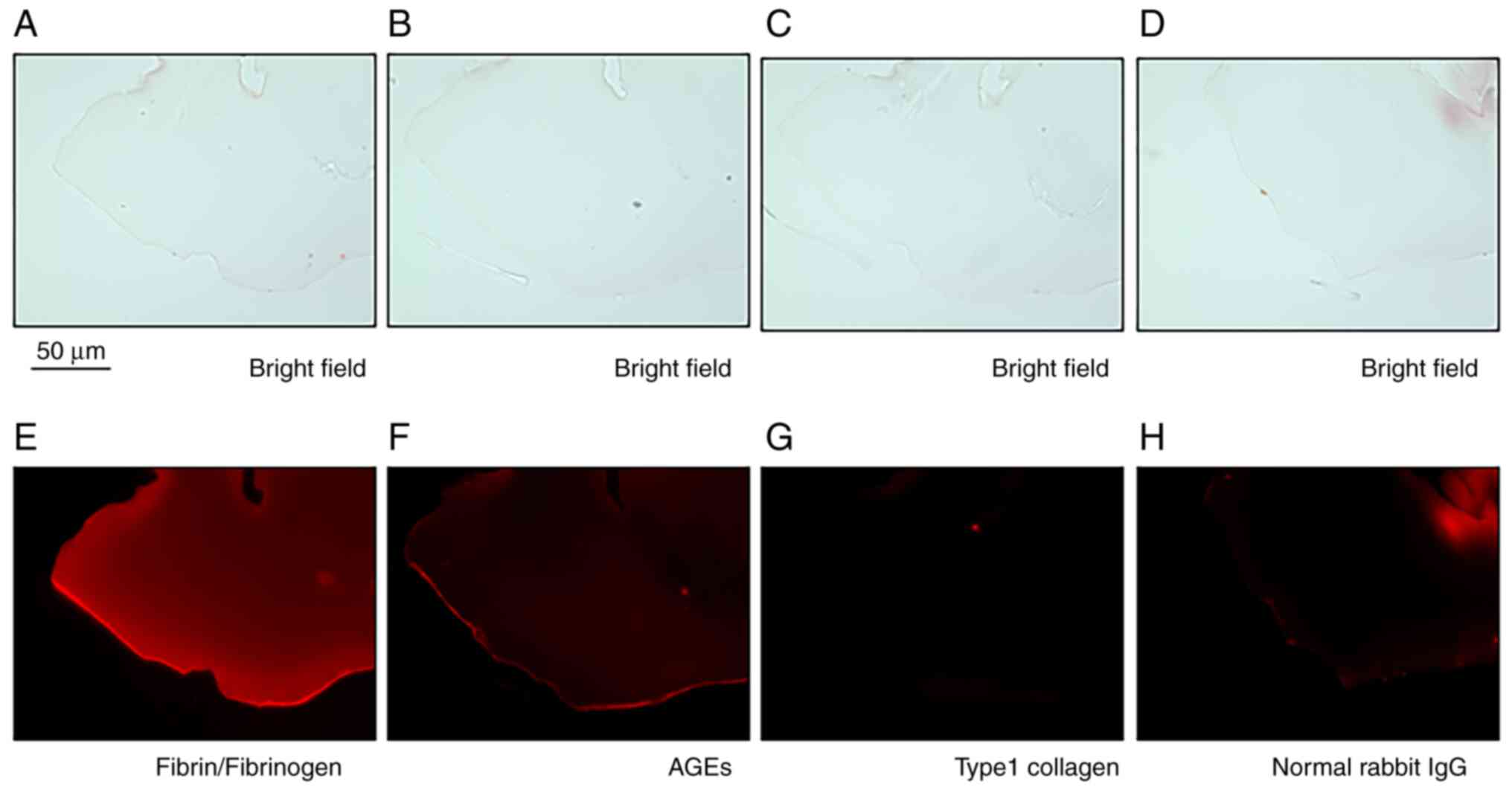
Immunohistochemical analysis demonstrated that the tissue was
positive for fibrin/fibrinogen and weakly positive for AGEs
(Fig. 3E and F). By contrast, no immunoreactivity for
GFAP, RAGE (data not shown) or type 1 collagen (Fig. 3G) was observed. Immunoreactivity for
normal rabbit IgG was shown as a negative control (Fig. 3H). In addition, bright-field
microscopic images consistent with Fig.
3E-H are shown in Fig. 3A-D.
Histopathological images were obtained using the Biorevo light and
fluorescence microscope system (BZ-9000; Keyence Corporation).
Discussion
The present study demonstrated clinicopathological
findings of the cystoid lesion component in refractory diabetic
CME. The pathological features of the cystoid lesion were
homogeneous structures that consisted of acellular eosinophilic
material with positive immunoreactivity for fibrin/fibrinogen and
weakly positive immunoreactivity for AGEs. Moreover, the excised
tissue was a solid material, suggesting that it was insoluble
fibrin, not soluble fibrinogen.
In a previous report, TEM for the cystoid lesion
component depicted microfibrils wrapped in collagen fibrils
(5). In the present case,
membranous collagen structures were not microscopically observed.
Furthermore, the immunohistochemical analysis revealed no staining
for type I collagen or GFAP, a representative retinal intermediate
filament, in the component. In contrast to a previous report
(5), there is a possible reason why
a membranous structure was not observed around the excised
components in this case. It is conceivable that the membranous
structure was not present from the outset. There are instances
where the membranous structure may or may not be present; in this
case, it was absent. Furthermore, the erythrocytes interspersed
within the tissue were thought to originate from retinal
hemorrhages and microaneurysms with high viscosity, suggesting the
possibility of fibrinogen leakage from these microvascular
lesions.
In the present case, immunohistochemistry of the
tissue for AGEs showed weakly positive staining. These results
suggested that AGEs may post-transcriptionally modify fibrin clots
of the cystoid lesion in diabetic CME. Fibrinogen is a 340-kDa
glycoprotein synthesized in hepatocytes. It is secreted from
hepatocytes into the blood, with plasma concentrations ranging from
1.5 to 3.0 g/l and a half-life of ~3 days (8,9). The
high plasma concentrations and the long half-life allow fibrinogen
to undergo various post-translational modifications that affect its
function, including susceptibility to fibrinolysis (6). AGEs modification progressively occurs
in patients with diabetes and AGEs are considered a significant
pathogenic factor for diabetic complications (7,10).
Furthermore, studies investigating fibrinogen
glycation and MGO-derived AGE modification have shown that the
fibrinolysis of the clot is reduced due to modification of the
plasmin-cleavage sites (6,7). Therefore, based on the current study,
fibrin clots in the cystoid lesion may be fibrinolytic-resistant
due to glycation and AGEs modification in patients with diabetes.
In addition, AGEs modification may also cause cellular dysfunction
(7), possibly leading to retinal
damage. Therefore, various post-translational modifications of
fibrin/fibrinogen, such as glycation and AGEs modification, may
influence the pathogenesis of diabetic CME.
There are several limitations to the present report.
First, the glycation of the excised tissue could not be confirmed,
although this study discussed post-transcriptional modification by
glycation and AGEs. Nevertheless, additional studies could not be
performed because the excised tissue was too small to allow further
analysis. Second, since this is a single case report, it remains
unclear whether this phenomenon generally happens in diabetic
CME.
In conclusion, to the best of our knowledge, the
current study is the first to provide evidence that the cystoid
lesion component in diabetic CME is a fibrin clot
post-translationally modified by AGEs. In patients with diabetes,
post-translational modifications, such as AGE modification, may
lead to resistance to fibrinolysis by plasmin. These findings
indicated that it is important to know how the components of the
cystoid lesion undergo post-transcriptional modifications, since it
may induce alterations in the characteristics of the lesion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TT, MS, and SK substantially contributed to the
conceptualization of the present study and confirmed the
authenticity of all the raw data. TT drafted the original
manuscript. SK supervised the conduct of the study and contributed
to the revision of the manuscript draft. MS performed the surgery
in this case and removed the component. IH and MM significantly
contributed to the immunohistochemical staining. ET made
significant contributions to the pathological diagnosis. SI
contributed to interpretation of the results and the revision of
the manuscript draft. All authors critically reviewed and revised
the manuscript draft, and read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written, retrospective informed
consent for publication following detailed explanation of the
purpose of the manuscript and understanding that no identifiable
information was going to be released.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tomkins-Netzer O, Ismetova F, Bar A,
Seguin-Greenstein S, Kramer M and Lightman S: Functional outcome of
macular edema in different retinal disorders. Prog Retin Eye Res.
48:119–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tachi N, Hashimoto Y and Ogino N:
Cystotomy for diabetic cystoid macular edema. Doc Ophthalmol.
97:459–463. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Asahina Y, Tachi N, Asahina Y, Yoshimura
K, Ueta Y and Hashimoto Y: Six-month postoperative outcomes of
intraoperative OCT-guided surgical cystotomy for refractory cystoid
macular edema in diabetic eyes. Clin Ophthalmol. 11:2099–2105.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Imai H, Tetsumoto A, Yamada H, Hayashida
M, Otsuka K, Miki A, Kusuhara S and Nakamura M: Long-term effect of
cystotomy with or without the fibrinogen clot removal for
refractory cystoid macular edema secondary to diabetic retinopathy.
Retina. 41:844–851. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Imai H, Otsuka K, Tetsumoto A, Miki A and
Nakamura M: Effectiveness of en bloc removal of fibrinogen-rich
component of cystoid lesion for the treatment of cystoid macular
edema. Retina. 40:154–159. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
De Vries JJ, Snoek CJM, Rijken DC and De
Maat MPM: Effects of post-translational modifications of fibrinogen
on clot formation, clot structure, and fibrinolysis: A systematic
review. Arterioscler Thromb Vasc Biol. 40:554–569. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lund T, Svindland A, Pepaj M, Jensen AB,
Berg JP, Kilhovd B and Hanssen KF: Fibrin(ogen) may be an important
target for methylglyoxal-derived AGE modification in elastic
arteries of humans. Diab Vasc Dis Res. 8:284–294. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jennewein C, Tran N, Paulus P, Ellinghaus
P, Eble JA and Zacharowski K: Novel aspects of fibrin(ogen)
fragments during inflammation. Mol Med. 17:568–673. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stein TP, Leskiw MJ and Wallace HW:
Measurement of half-life human plasma fibrinogen. Am J Physiol.
234:D504–D510. 1978.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Singh R, Barden A, Mori T and Beilin L:
Advanced glycation end-products: A review. Diabetologia.
44:129–146. 2001.PubMed/NCBI View Article : Google Scholar
|