Introduction
Atherosclerosis (AS) is a chronic and progressive
vascular wall disease caused primarily by plaques formed by lipid
deposition under intima artery linings and fibers, mediated by
local inflammatory responses of the vessels. The plaques
accumulate, resulting in arterial stenosis and decreased elasticity
(1). Diet containing high-fat
foods, which is one of the main causes of abnormalities in lipid
metabolism, as well as changes in poor lifestyle habits such as
smoking and reduced exercise, all of which have led to increasing
the morbidity and mortality of AS-based cardiovascular disease,
with ~16 million people dying from cardiovascular diseases every
year worldwide, making AS one of the leading contributing diseases
to the global mortality rate (2).
AS is a dynamic process, and disorders of lipid metabolism trigger
AS, leading to damage of endothelial cells and eventual formation
of atherosclerotic plaques (3).
It is well-known that abnormal lipid metabolism and
endothelial cell injury are associated with occurrence and
progression of AS (4,5). Abnormal lipid metabolism is primarily
caused by increased levels of lipids and certain lipoproteins in
plasma, leadings to damage of the vascular endothelium and
accumulation of adhesion factors on endothelial cells (6). The function of endothelial cells is
weakened, causing inflammation and formation of AS (7). Vascular homeostasis depends on the
integrity and normal function of endothelial cells (8). Therefore, regulation of blood-lipid
disorder and protection of endothelial cells are key for
anti-atherosclerosis therapy. Endothelial cell function and
atherosclerosis can be regulated by the
phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) signaling
pathway (9).
Rubia yunnanensis is the dried root and
rhizome of R. yunnanensis Diels, a Rubiaceae plant
and a unique indigenous medicine in Yunnan, China. R.
yunnanensis has pharmacological actions, such as
anti-myocardial ischemia, anti-oxidation and anti-platelet activity
(10). The application of R.
yunnanensis has been recorded in the ‘Southern Yunnan Materia
Medica’ for treatment of cardiovascular and gastrointestinal
disease, menstrual disorder and trauma for hundreds of years
(11).
The present study aimed to determine the impact of
R. yunnanensis alcohol extract in a mouse model of AS,
analyze the main components of R. yunnanensis alcohol
extract and its effects on carotid AS and blood lipid levels, as
well as PI3K and AKT protein expression. The present study aimed to
determine whether R. yunnanensis regulates the PI3K/AKT
pathway to inhibit carotid AS in ApoE-/- mice on
a high-fat diet (HFD).
Materials and methods
Animals
A total of six C57BL/6J and 18
ApoE-/- mice with C57BL/6J genetic background
were used to detect blood lipids and pathological indexes. In
addition, three C57BL/6J mice and nine ApoE-/-
mice with C57BL/6J genetic background were used for protein
detection. All mice (male; age, 8 weeks; weight, 18-22 g) were
purchased from SPF (Beijing) Biotechnology Co., Ltd. [experimental
animal certificate no. SCXK (Jing) 2019-0010]. Mice were kept in a
cage with stainless steel top and plastic bottom with free access
to water and food, and 12/12-h light/dark cycle, temperature of
20-24˚C and humidity of ~50%. All animals received appropriate care
in compliance with the ‘Guide for the Care and Use of Laboratory
Animals’ (12). All experiments
were approved by the Animal Ethics Committee of Yunnan University
of Chinese Medicine and meet the standards of Yunnan University of
Chinese Medicine (Kunming, China; approval no. R-062022053).
Mouse model
The mice were randomly divided into four groups
(n=6/group): Normal control (C57BL/6J mice), model
(ApoE-/- mice), ethanol extract of high-dose
R. yunnanensis (HRY; 2.5 g/kg, ApoE-/-
mice) and ethanol extract of low-dose R. yunnanensis (LRY;
1.25 g/kg, ApoE-/- mice). The experimental mice
used for WB assay were grouped into 4 groups (n=3/group):. Normal
control (C57BL/6J mice), model (ApoE-/- mice),
ethanol extract of high-dose R. yunnanensis (HRY; 2.5 g/kg,
ApoE-/- mice) and HRY + LY group (HRY; 2.5 g/kg,
LY294002; 10 mg/kg, ApoE-/- mice). The control
group received normal diet; all other groups received HFD diet (40%
fat and 1.25% cholesterol). HRY, LRY and HRY + LY group were orally
given ethanol extract of R. yunnanensis every day for 12
weeks; the other groups were orally given equal volume of normal
saline. To investigate whether the PI3K/AKT signaling pathway can
be activated by R. yunnanensis, we administered ethanol
extract of high-dose R. yunnanensis along with the PI3K/AKT
inhibitor LY294002. HRY + LY group was intraperitoneally injected
with LY294002 (Med Chem Express, 10 mg/kg, twice/week for 2 weeks)
beginning from the tenth week. The experiment lasted for 12 weeks
and no premature death was observed.
Preparation of alcohol extract of R.
yunnanensis and analysis of main components by high-performance
liquid chromatography (HPLC)
R. yunnanensis was acquired from Yunnan Huide
Pharmaceutical Co., Ltd., China and identified by Professor Yin
Zili of Yunnan University of Chinese Medicine (Yunnan). R.
yunnanensis extract was obtained after soaking 500 g R.
yunnanensis powder in 95% ethanol for 24 h, with the extraction
heated at 60˚C by reflux. Soaking and heating reflux was repeated
four times to obtain the extract. Then, 95% ethanol extract of
R. yunnanensis was freeze-dried into powder, 0.5 g was
taken, methanol was added for ultrasonic dissolution and sample was
filtered (0.22 µm microporous membrane filter) and processed by
HPLC (Agilent 1260) gradient elution as follows: Mobile phases A
and B were acetonitrile and gradient elution, respectively, with
0.1% formic acid solution (10 min, 5-24% A; 10 min, 24% A; 10 min,
24-34% A and 15 min, 34% A and 0~10 min, 95%B→76%B; 10~20 min,
76%B; 20~30 min, 76%B→66%B; 30~45 min, 66%B). The detection
wavelength was 280 nm. R. yunnanensis and standard products
were dissolved in methanol at a flow rate of 1 ml/min and the
sample size is 10 µl and column (Agilent TC-C18 (4.6x250 mm, 5 µm))
temperature of 30˚C.
Acute toxicity experiment and
effective dose selection of alcohol extract from R.
yunnanensis
To study the adverse effects of ethanol extract of
R. yunnanensis on mice, acute toxicity test was performed
(Data S1).
Blood lipid level determination
The mice were anesthetized by intraperitoneal
injection of pentobarbital sodium (40 mg/kg). When mice had no
response to tail pinching, 1.5ml blood was collected and serum was
obtained by centrifugation at 4˚C and 680 x g for 15 min.
Subsequently, total cholesterol (TC; cat. no. A111-1-1),
triglyceride (TG; cat. no. A110-1-1), low-density lipoprotein
cholesterol (LDL-C; cat. no. A113-1-1) and high-density lipoprotein
cholesterol (HDL-C; cat. no. A112-1-1) were measured according to
total cholesterol test kit instructions,. triglyceride test kit
instructions, low density lipoprotein cholesterol test kit
instructions and high-density lipoprotein cholesterol test kit
instructions. All kits were bought from Nanjing Jiancheng
Bioengineering Institute.
Histological examination of carotid
AS
Mice were sacrificed by guillotine decapitation.
Death was verified by absence of corneal and eyelash reflex
(response to touch) and breathing. The carotid artery was removed
under operating microscopes, adipose tissue was removed and carotid
artery tissue was fixed at room temperature with 4%
paraformaldehyde for 24 h. Samples were stored in -80˚C in liquid
nitrogen for protein detection.
For hematoxylin and eosin staining, fixed carotid
artery was dehydrated with ascending ethanol gradient, then washed
with xylene, paraffin-embedded and cut into translateral sections
~4 µm thick, then stained with hematoxylin and eosin for 5 min each
at room temperature. Finally, observations were made using light
microscopy (magnification, x40).
For Masson's staining, fixed carotid artery was
dehydrated with ascending sucrose gradient. The optimal cutting
temperature-embedded carotid artery was transversally cut into
sections ~8 µm thick, then slices were soaked in Masson's A
solution overnight, rinsed with tap water and soaked for 1 min in
Masson's B and C mixed dye. The sections were immersed in Masson's
D solution for 6 min, rinsed in tap water, soaked in Masson's E
solution for 1 min without rinsing, then soaked in Masson F
solution for 30 sec. All steps are performed at room temperature.
Finally, stained collagen in carotid artery tissue was examined by
light microscopy (magnification, x40). Analysis was performed using
Image J software, and the collagen area ratio was calculated as
collagen area divided by total area.
For Oil red O staining, fixed carotid artery was
dehydrated with ascending sucrose gradient. The OCT-embedded
carotid artery was transversally cut into slices ~8 µm thick,
immersed in oil red O dye solution for 10 min (away from light) and
then the slices were differentiated twice (2 and 5 sec) in 60%
isopropyl alcohol. Sections were soaked in distilled water twice
(10 sec each time), then soaked with hematoxylin dye for 5 min,
washed with distilled water three times (5, 10 and 30 sec),
differentiated for 8 sec, washed in distilled water twice (10 sec
each), dipped in blue solution for 1 sec, then immersed in tap
water twice (5 and 10 sec). All steps are performed at room
temperature. Finally, the carotid artery was examined by light
microscopy (magnification, x40). Analysis was performed using Image
J software (version 1.45S, National Institutes of Health), and
lipid area ratio was calculated as lipid area divided by total
area.
For Verhoeff's Van Gieson (EVG) staining, fixed
carotid artery was dehydrated with increasing sucrose gradient. The
OCT-embedded carotid artery was transversally cut into sections ~8
µm thick, then sections were placed in EVG dye solution (EVG dye
solution A:B: EVG dye solution C=5:2:2) for 5 min. Sections were
stained with EVG dye solution B, then washed with tap water after
differentiation until elastic fibers were a purplish black color,
and then VG dye solution(EVG dye solution E:D=9:1) was applied the
sections were immersed for 3min. All steps are performed at room
temperature. Finally, elastic and collagen fibers of carotid artery
tissue was examined by light microscopy (magnification, x40).
Western blot analysis
Western blotting was used to measure total and
phosphorylated (p-) levels of AKT and PI3K. Samples were prepared
used RIPA cracking buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology) and lysate was centrifuged at 4˚C, 7,992 x g for 5
min and the supernatant was collected into a pre-cooled Eppendorf
tube. BCA protein assay kit (cat. no. P0010; Beyotime Institute of
Biotechnology) was used for protein determination and protein (50
µg/lane) were separated via 10% SDS-PAGE. The proteins were then
transferred to polyvinylidene difluoride membranes, which were then
incubated in 5% buttermilk for 2 h at room temperature. Membranes
were rinsed with TBST buffer with 0.1% Tween. Then, the membrane
was incubated overnight at 4˚C with primary antibodies (all
1:1,000; all Cell Signaling Technology, Inc.) as follows: Anti-PI3K
(cat. no. 4257), anti-p-PI3K (cat. no. 4228), anti-AKT (cat. no.
9272), anti-p-AKT (cat. no. 9271) and anti-β-actin (cat. no. 4967).
After the first antibody incubation, the membrane was washed with
TBST three times, then incubated with horseradish
peroxidase-conjugated polyclonal goat anti-rabbit IgG (1:5,000;
cat. no. ab6721; Abcam) at ambient temperature for 1 h, then washed
three times in TBST. Finally, chemiluminescence reagent (cat. no.
A38555; Cell Signaling Technology, Inc.) was used to observe bands
with a Bio-Rad ChemiDoc™ XRS Gel Imaging System (Bio-Rad
Laboratories, Inc.). Image Lab™ V4.0 software (Bio-Rad
Laboratories, Inc.) was used to detect optical signals and quantify
protein levels. All experimental groups were divided into three
groups and the experiment was repeated three times.
Statistical analysis
Data were analyzed by GraphPad Prism 8.0 software
(GraphPad Software, Inc.; Dotmatics). The measurement data are
expressed as mean ± standard deviation. All experiments were
repeated three times. If data conformed to normal distribution and
the variance was homogeneous, one-way ANOVA was used followed by
Tukey's post hoc correction. If the data were normally distributed
but with uneven variance, Welch's ANOVA test was used, followed by
Tamhane's T2. P<0.05 was considered to indicate a statistically
significant difference.
Results
HPLC analysis of components of alcohol
extract of R. yunnanensis
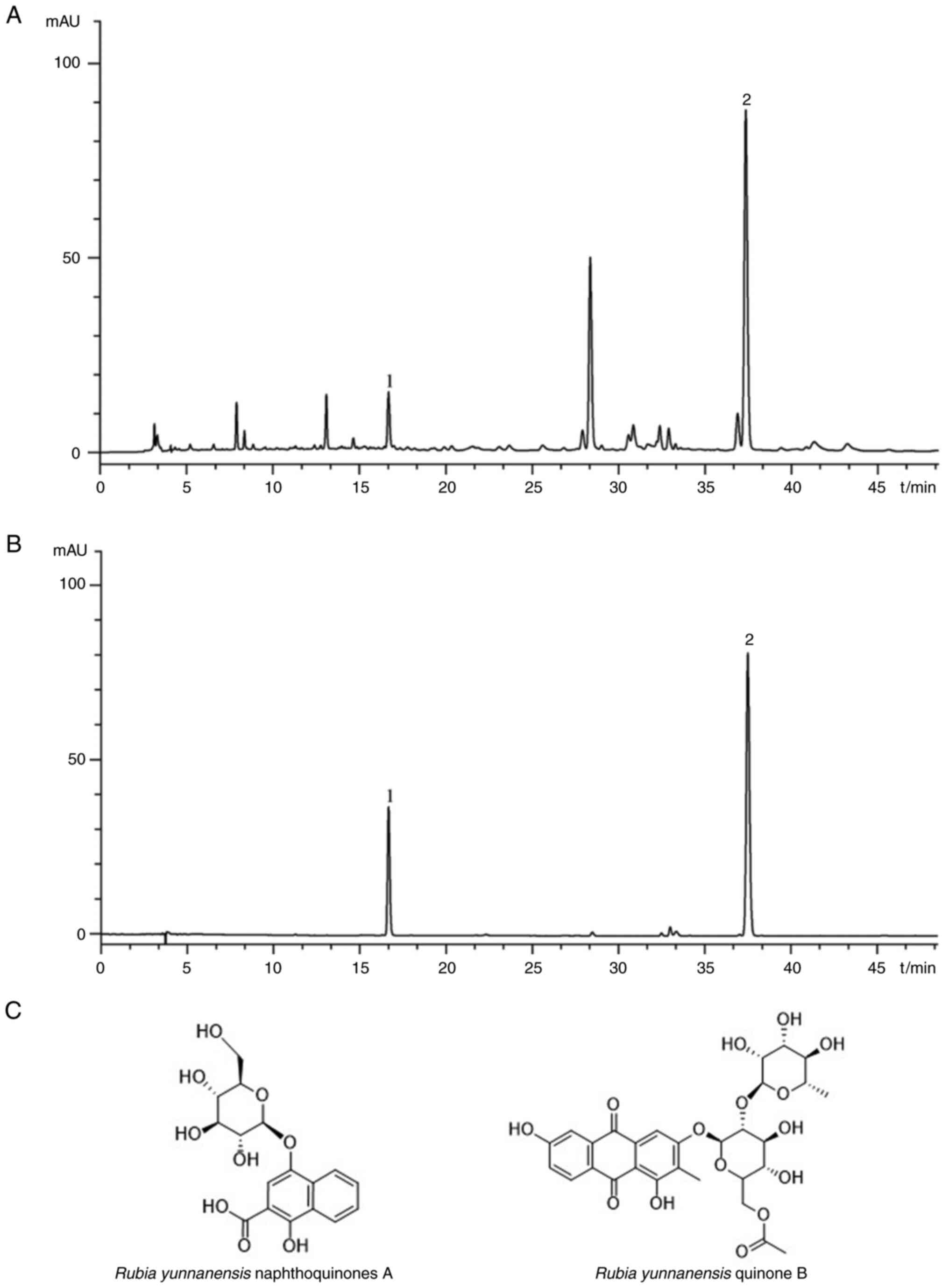
A total of two primary compounds, namely, R.
yunnanensis naphthoquinones A and R. yunnanensis quinone
B (Fig. 1A and B), was detected in the alcohol extract of
R. yunnanensis. Both compounds have a benzene ring in their
structure (Fig. 1C).
Acute toxicity experiment and
effective dose selection of alcohol extract from R.
yunnanensis
To study the adverse effects of ethanol extract of
R. yunnanensis on mice, acute toxicity test was performed.
Ethanol extract of R. yunnanensis had no adverse effects on
mice when used in conventional doses (Tables SI and SII). Alcoholic extract of R.
yunnanensis (2.500, 1.250 and 0.625 g/kg) showed that 0.625
g/kg had no effect on the pathological indices of carotid artery in
mice (Fig. S1). Therefore, 2.50
and 1.25 g/kg were selected for this study.
Effect of alcohol extract of R.
yunnanensis on blood lipid levels
Compared with the normal control group, serum levels
of TC, TG and LDL-C in the model group were significantly
increased, while levels of HDL-C (all P<0.0001; Fig. 2A-D) were significantly decreased.
Compared with the model group, levels of serum TC (P<0.001), TG
(P<0.01) and LDL-C (P<0.0001) were decreased in the HRY
group, while levels of HDL-C were significantly increased
(P<0.01). The levels of serum TC, TG and LDL-C were decreased
and HDL-C (all P<0.05) levels were increased in the LRY
group.
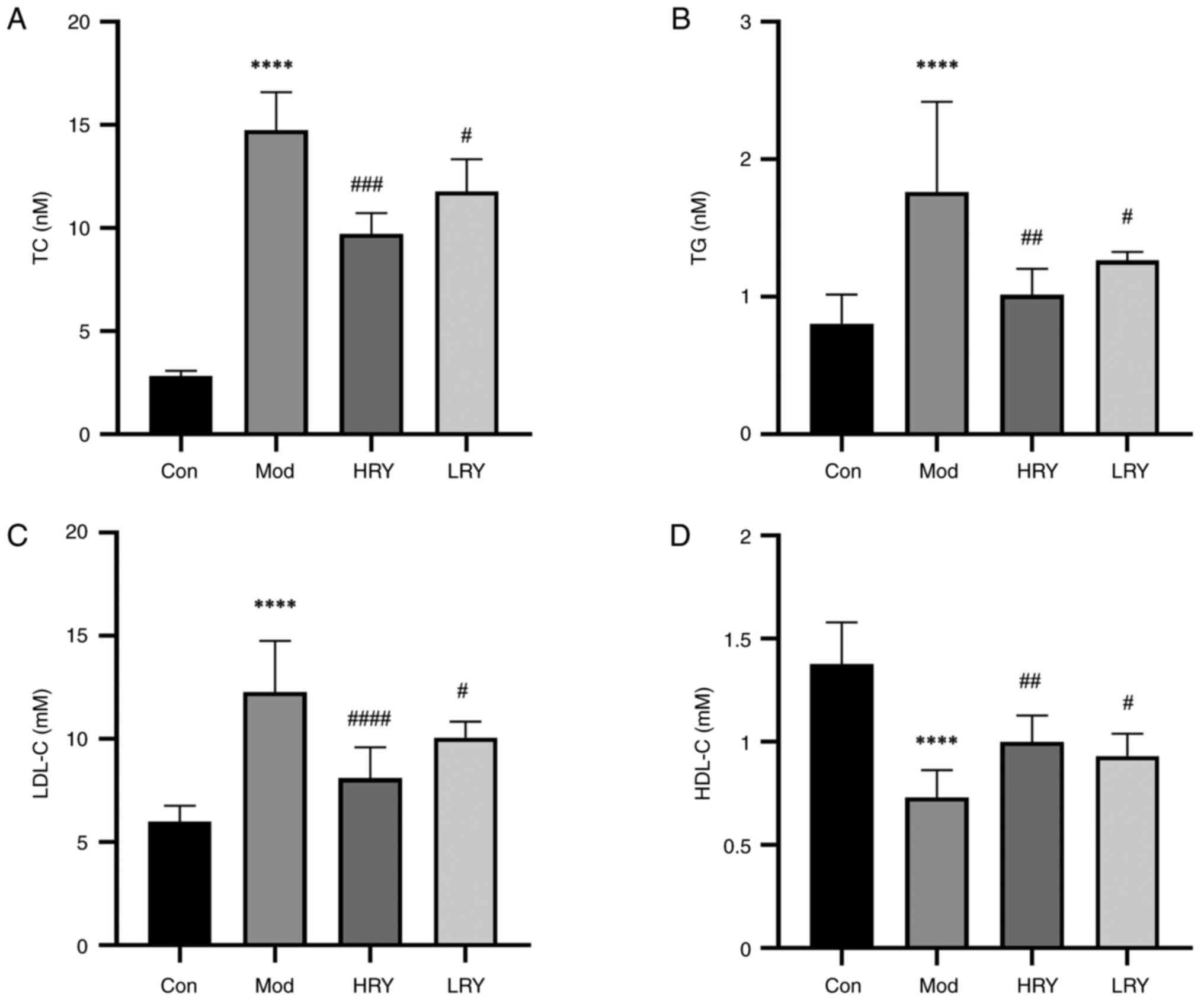 | Figure 2Ethanol extract of RY decreases the
levels of blood lipids in a carotid atherosclerosis model. (A) TC,
(B) TG, (C) LDL-C and (D) HDL-C levels. n=6.
****P<0.0001 vs. Con. #P<0.05,
##P<0.01, ###P<0.001 and
####P<0.0001 vs. Mod. RY, Rubia yunnanensis;
H, high; L, low; TC, total cholesterol; TG, triglyceride; DL-C,
density lipoprotein cholesterol; Con, control; Mod, model. |
Alcohol extract of R. yunnanensis
improves the intima of blood vessels
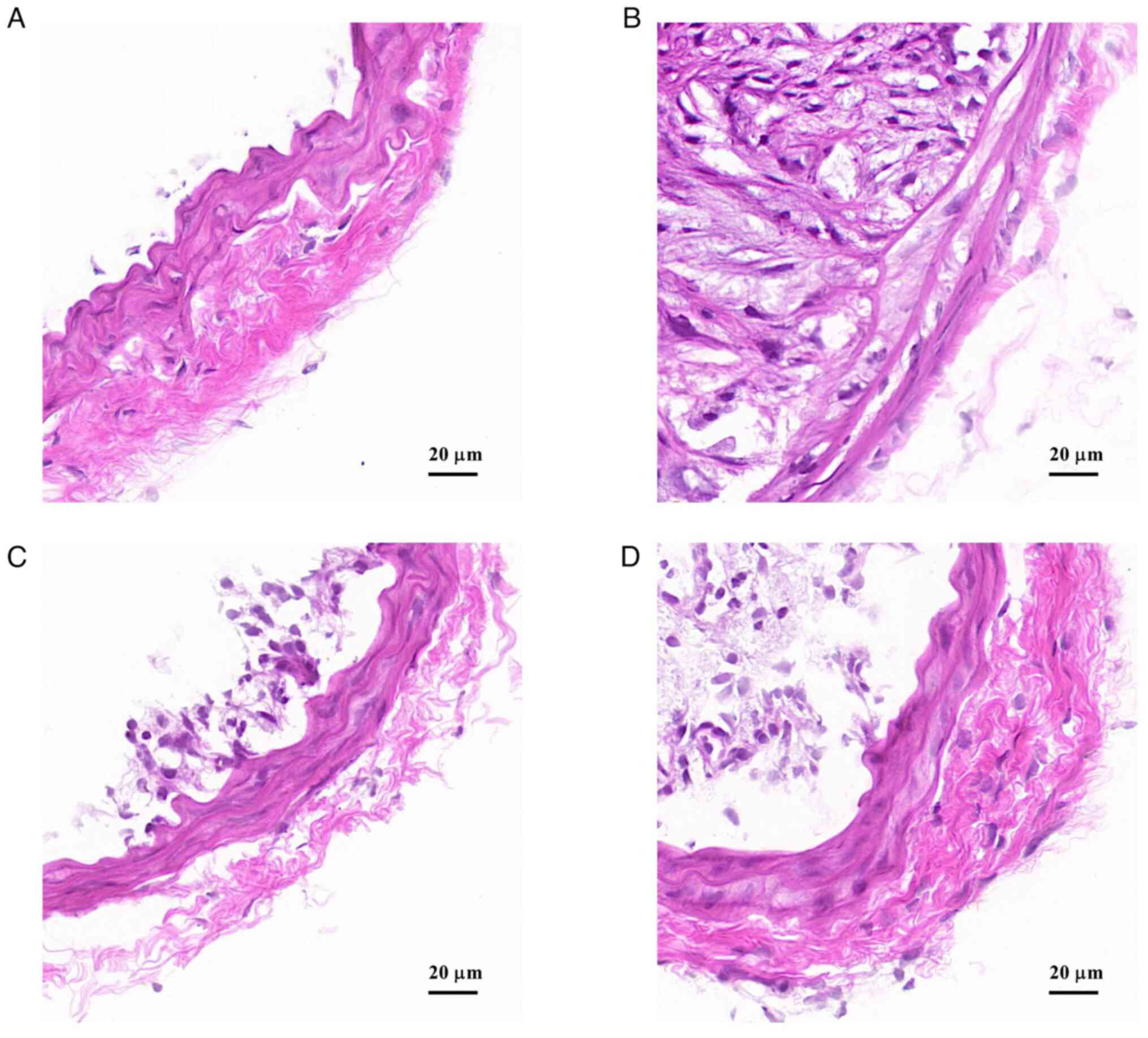
Hematoxylin and eosin staining results showed that
in the control group, the vascular structure was complete, smooth
and the hierarchy was clear, with no obvious damage or stenosis of
the lumen (Fig. 3A). In the model
group, vascular endothelium was damaged, the structure and
morphology were disorganized, plaque area and the number of lipid
nuclei in the plaque was large and the lumen was severely narrowed
(Fig. 3B). Compared with the model
group, carotid vascular injury were notably reduced in the HRY and
LRY groups (Fig. 3C and D).
Ethanol extract of R. yunnanensis
reduces the content of collagen fiber
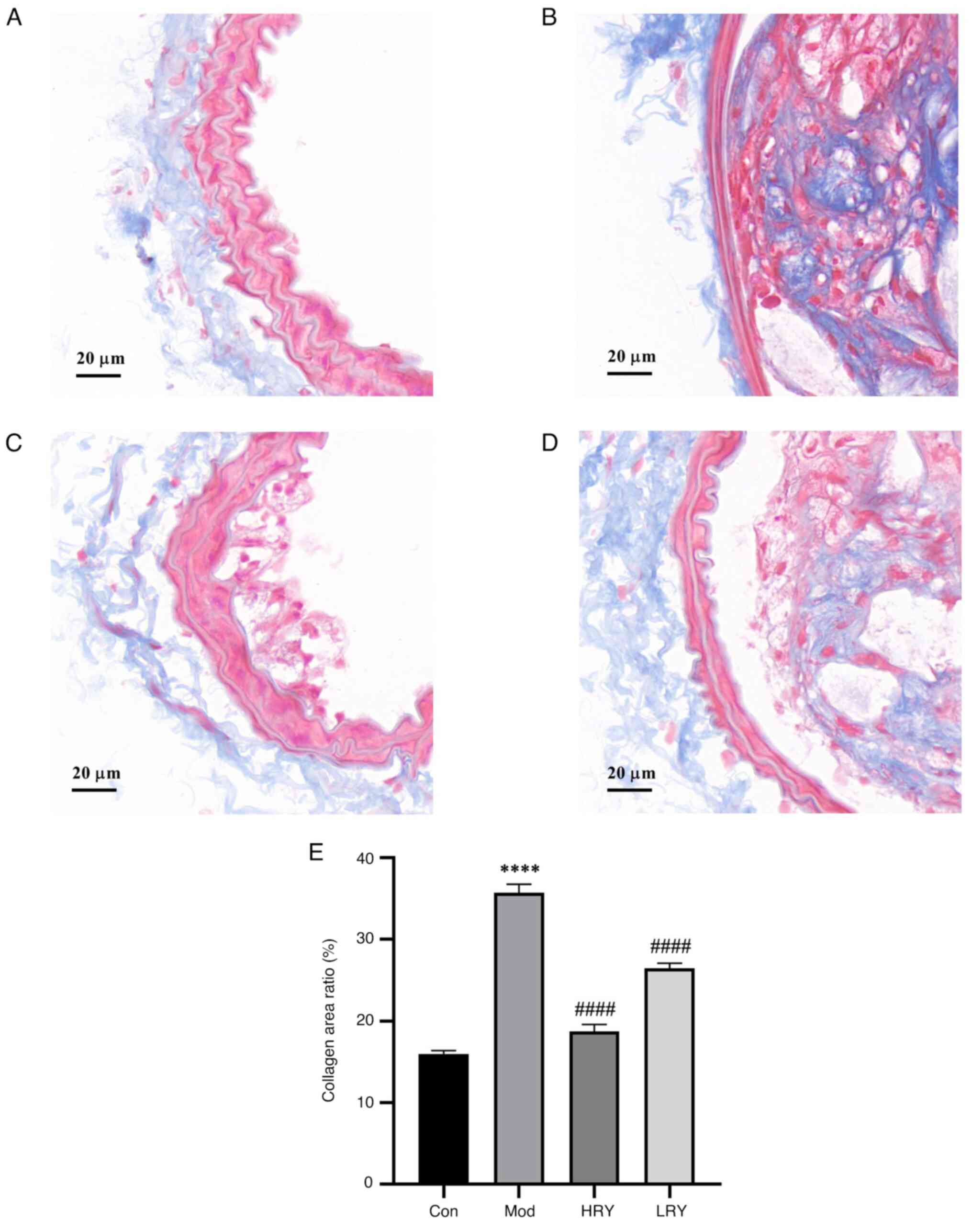
After Masson staining, collagen fibers were blue and
smooth muscle cells were red. Levels of aortic blue staining in the
model group was significantly higher than that in the control group
and the degree of fibrosis was significantly increased
(P<0.0001; Fig. 4A, B and E).
Compared with the model, the collagen fiber content in HRY was
significantly decreased (P<0.0001; Fig. 4C and E). The collagen fiber content of LRY was
also significantly decreased (P<0.0001; Fig. 4D and E).
Ethanol extract of R. yunnanensis
inhibits lipid accumulation in carotid artery
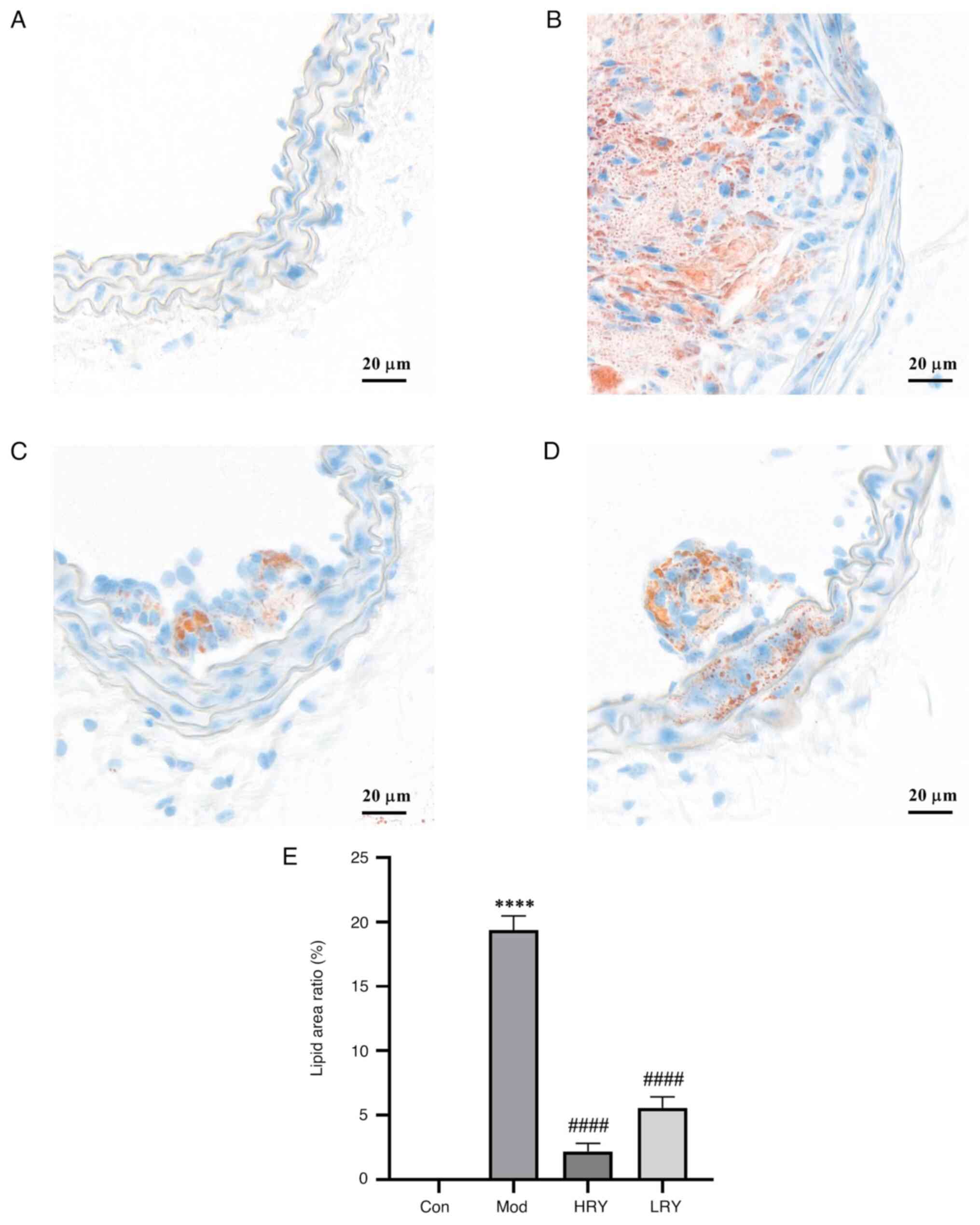
Oil red O staining showed no lipid deposition in the
carotid artery of the control group (Fig. 5A and E). Compared with the control group, there
was a large amount of lipid deposition in the carotid artery of the
model group (P<0.0001; Fig. 5B
and E). Compared with the model,
the area of carotid artery lipid content in HRY was significantly
reduced (P<0.0001; Fig. 5C and
E). The area of carotid artery
lipid content also decreased significantly in the LRY group
(P<0.0001; Fig. 5D and E).
Ethanol extract of R. yunnanensis
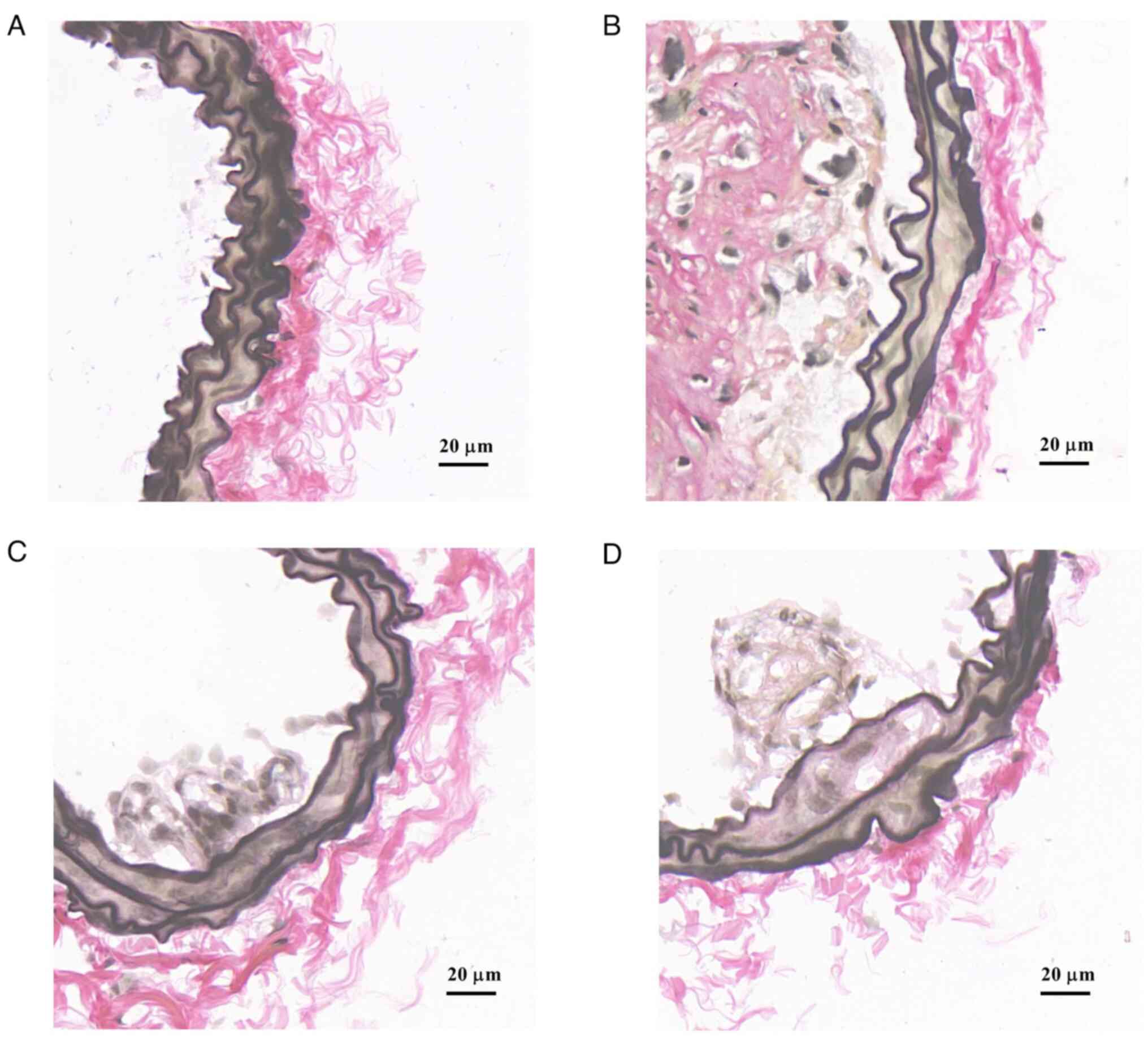
decreases content of elastic fiber
EVG staining showed that elastic fibers of the inner
wall of blood vessels in the normal control group were intact and
without interruption (Fig. 6A). In
the model group, a large number of elastic fibers were clustered in
the intima of blood vessels (Fig.
6B). The aggregation of elastic fibers in the HRY and L groups
was less than that in the model (Fig.
6C and D).
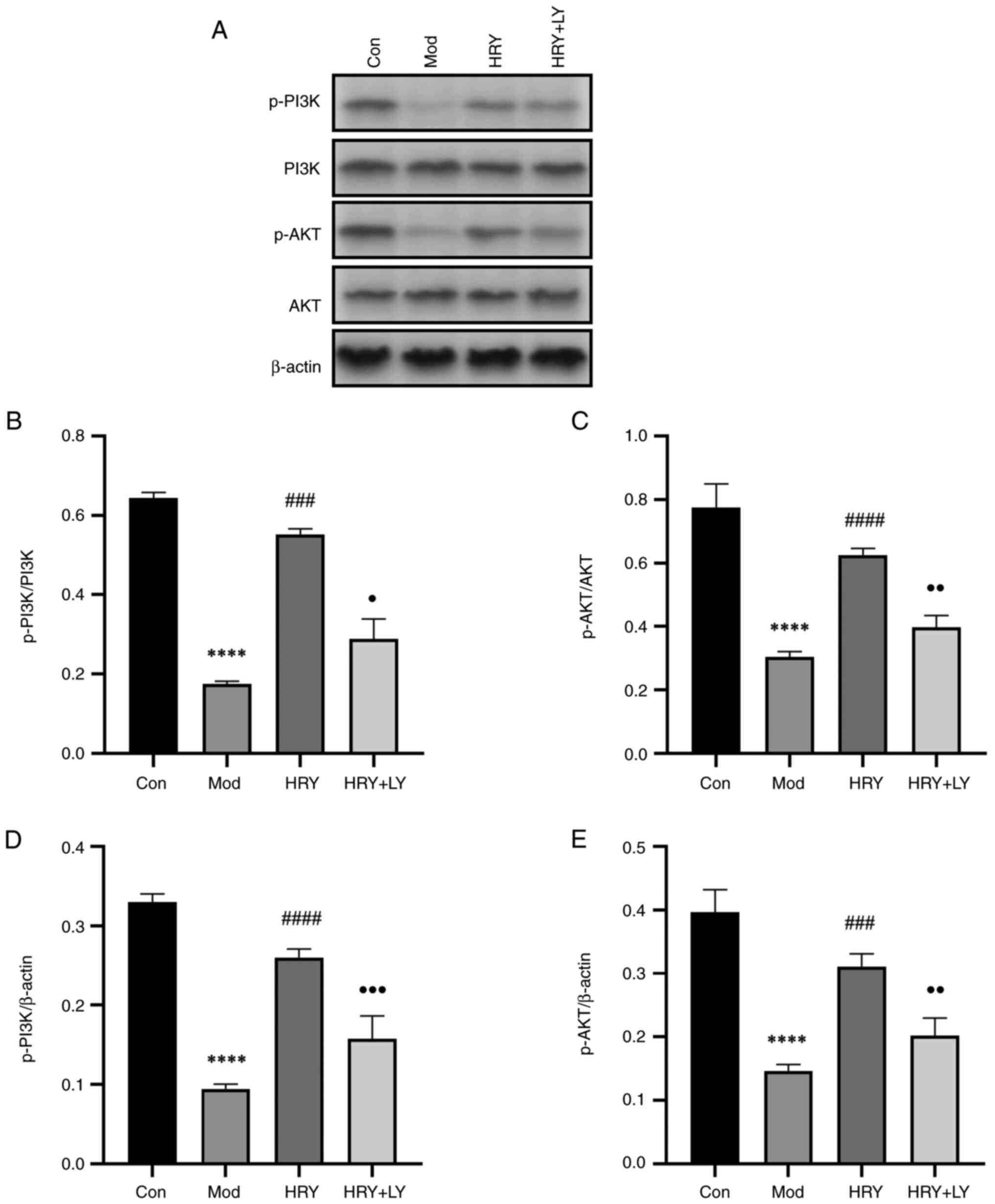
Protein expression of PI3K, AKT and
p-PI3K and p-AKT is upregulated in carotid artery
The phosphorylation of PI3K and AKT was measured by
western blotting. Mice were treated with LY294002 dissolved in
ethanol extract of R. yunnanensis. Western blotting showed
that compared with the normal control group, the protein ratios of
p-PI3K/PI3K and p-AKT/AKT in the carotid tissues of the model group
were significantly decreased and p-PI3K and p-AKT (all P<0.0001;
Fig. 7B-E) protein expression also
decreased significantly. Compared with the model group, the protein
ratios of p-PI3K/PI3K (P<0.001; Fig.
7B) and p-AKT/AKT (P<0.0001; Fig. 7C) in carotid tissue in HRY were
significantly increased and p-PI3K (P<0.0001; Fig. 7D) and p-AKT (P<0.001; Fig. 7E) protein expression was
significantly increased. LY294002 inhibitor eliminated this effect,
and compared with HRY group, the protein ratios of p-PI3K/PI3K
(P<0.05; Fig. 7B), and p-AKT/AKT
(P<0.01, Fig. 7C) in carotid
tissue in HRY + LY were significantly decreased and p-PI3K
(P<0.001; Fig. 7D) and P-AKT
(P<0.01; Fig. 7E) protein
expression was significantly decreased. HRY activated the PI3K/AKT
signaling pathway after carotid endothelial cell injury and
LY294002 inhibited the PI3K/AKT signaling pathway.
Discussion
Carotid AS is widely recognized as a complex
pathophysiological disease associated with abnormal lipid
metabolism and endothelial dysfunction (13,14).
The AS mouse model of ApoE-/- induced mice on HFD is
frequently used in animal experiments to assess drug efficacy and
mechanisms in atherosclerosis; this model resembles the disease
course of atherosclerosis (15,16).
The aim of the present study was to use an established model of
carotid AS in ApoE-/- mice induced by HFD and to
explore the mechanism of inhibition of carotid AS by an ethanol
extract of R. yunnanensis.
Chinese herbal medicine has been used to prevent and
treat disease for >100 years. R. yunnanensis has been
used by Yunnan peoples to treat cardiovascular disease (17). Previous studies have shown that
R. yunnanensis extract has significant pharmacological
effects such as lowering blood lipids and antiplatelet aggregation
(17,18). Ren et al (19) conducted HPLC on R.
yunnanensis samples and identified two primary components,
R. yunnanensis naphthoquinones A and R. yunnanensis
quinone side B (19). Therefore, in
the present study, the chemical composition of ethanol extract of
R. yunnanensis was analyzed; R. yunnanensis
naphthoquinones A and R. yunnanensis quinone B were the main
compounds.
Lipid metabolism disorder affects initiation and
progression of carotid AS (20).
Lipids include TG and TC. Elevated TG will cause functional damage
to vascular endothelial cells, strong lipid metabolism, excessive
generation of oxygen free radicals, promotion of adhesion molecule
expression, oxidative damage, foam cell formation, inflammation and
the development of carotid AS (21,22).
Increased TC promotes the adhesion of white blood cells and the
aggregation of platelets, causing release of growth factors and
monocyte infiltration, facilitates the multiplication of smooth
muscle cells, accelerating their entry into the intima, and
promotes the production of collagen fibers, making fatty plaques
change into fibrous plaques (23,24).
Lipid can combine with apolipoprotein to form lipoprotein, which is
the primary form of lipid transported in the body (25). Most TC is found in LDL-C. High
levels of LDL-C are associated with inflammation and lipid
accumulation, which stimulates plaque formation (26). HDL-C reverses the transport of TC to
the liver, thereby decreasing TC content in the body. When HDL-C
levels are significantly decreased, reverse transport of TC will be
inhibited, which increases the TC content in the body and increases
the risk of AS (27). Here, the
lipid levels and carotid artery pathology were examined in a mouse
model of carotid AS. Ethanol extract of R. yunnanensis
lowered levels of TC, TG and LDL-C, whereas the levels of HDL-C
increased and damage to endothelial cells and accumulation of
lipids, collagen and elastic fibers decreased. These findings
indicated that induced disruption of lipid metabolism in the mouse
model could be regulated by ethanol extract of R.
yunnanensis, which reduced plaques in the carotid artery.
Damage to vascular endothelial cells caused by lipid
metabolism disorders is involved in the formation of carotid AS
(28). The innermost layer of the
blood vessel wall is attached by the vascular endothelium, which
has a single layer of endothelial cells acting as a physical wall
between blood flow and the blood vessel wall, regulating the
exchange of molecules (29).
Intravascular environmental homeostasis is associated with
endothelial cells. Endothelial cells regulate vascular active
factors, such as adhesion and chemotactic molecules, to maintain
their balance and stability (30).
Studies have found that vascular endothelial cells prevent the
accumulation of inflammatory factors to prevent AS (31,32).
When the vascular endothelium is damaged, LDL enters the
subendothelial layer and is oxidized, resulting in secretion of
various cell adhesion molecules and inflammation, prompting
monocytes to adhere to the endothelial surface, resulting in
formation of foam cells and accelerating the formation of AS
(33). The PI3K/AKT pathway is one
of the most common signaling pathways in living organisms and it is
involved in AS, the proliferation and migration of epithelial
cells, endothelial dysfunction, lipid metabolism and other
processes required for maintaining normal body function (34-36).
PI3K is an important kinase that promotes cell proliferation and
inhibits apoptosis by activating many downstream factors. AKT is a
key downstream target enzyme in PI3K activation that acts in a
number of cellular processes (37,38).
AKT activates downstream factors by conducting the signal sent by
PI3K, thereby regulating cell proliferation and apoptosis (39). Investigations have shown that
upregulation of PI3K/AKT pathway activity promotes vascular
regeneration and inhibits vascular endothelial apoptosis, the
multiplication of smooth muscle cells and development of AS
(40,41).
The present study investigated the effects of
ethanol extract of R. yunnanensis on protein expression
levels of p-AKT/AKT and p-PI3K/PI3K in carotid arteries of the AS
mouse model. The aim was to determine whether the ethanol extract
of R. yunnanensis upregulates phosphorylation of PI3K and
AKT protein in the AS model to alleviate endothelial cell damage.
As expected, a HFD induced abnormal blood lipid levels in
ApoE-/- mice and caused severe pathological
carotid AS, with hematoxylin and eosin staining showing endothelial
cells were irregularly arranged and a large number of AS plaques
had formed, indicating that carotid AS had caused serious
endothelial cell damage. Concurrently, p-PI3K and p-AKT protein
content and p-PI3K/PI3K and p-AKT/AKT ratios were decreased. These
results indicated that phosphorylation of PI3K and AKT was
inhibited in carotid AS, suggesting that the activity of the
PI3K/AKT signaling pathway was inhibited. However, following
treatment with R. yunnanensis extract, the expression of
p-PI3K and p-AKT and the ratios of p-PI3K/PI3K and p-AKT/AKT
increased, indicating that R. yunnanensis alcohol extract
activated the PI3K/AKT signaling pathway. To evaluate the role of
the PI3K/AKT signaling pathway in inhibition of AS by R.
yunnanensis alcohol extract, PI3K/AKT inhibitor
LY294002(42) was used to treat AS
induced by HFD following intervention with RY + H. LY294002 blocked
the PI3K/AKT signaling, suggesting that activity of PI3K/AKT
signaling pathway could be upregulated by the ethanol extract of
R. yunnanensis.
In summary, in ApoE-/- mice fed
HFD for 12 weeks, R. yunnanensis extract lowered blood lipid
levels, inhibited carotid artery lipid accumulation and decreased
elastic fiber thinning loss and intimal hyperplasia. R.
yunnanensis extract decreased blood lipid levels and alleviated
endothelial damage, inhibiting the occurrence and development of AS
via upregulation of the PI3K/AKT pathway. The extract of R.
yunnanensis mainly contains R. yunnanensis
naphthoquinones A and R. yunnanensis quinone B. Overall,
R. yunnanensis is a promising treatment for AS. Future
research should investigate the mechanism of action of R.
yunnanensis in the treatment of AS to provide a theoretical
basis for its potential drug use.
Supplementary Material
Acute toxicity test of ethanol extract
of Rubia yunnanensis by oral administration in mice (maximum
dose)
Hematoxylin and eosin staining.
Carotid artery vessels in (A) control, (B) model and ethanol
extract of (C) high., (D) medium. and (E) low-dose Rubia
yunnanensis group.
yH2AX
yH2AX
Acknowledgements
The authors would like to thank Professor Yin Zili
(Yunnan University of Chinese Medicine, Yunnan) for assistance with
the identification service.
Funding
Funding: The present study was supported by the Xingdian Talent
Support Program-Special for Young Talent (grant no.
XDYC-QNRC-2022-0284) and National Administration of Traditional
Chinese Medicine High-level Key Discipline Construction Project
‘Dai Medicine’.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL, LY and XD designed the experiments and analyzed
data. JC and PC analyzed data. XD and GL wrote and revised the
manuscript. JC and PC confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Yunnan University of Traditional Chinese
Medicine (approval no. R-062022053).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen J, Zhang X, Millican R, Sherwood J,
Martin S, Jo H, Yoon YS, Brott BC and Jun HW: Recent advances in
nanomaterials for therapy and diagnosis for atherosclerosis. Adv
Drug Deliv Rev. 170:142–199. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Legein B, Temmerman L, Biessen EA and
Lutgens E: Inflammation and immune system interactions in
atherosclerosis. Cell Mol Life Sci. 70:3847–3869. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Geovanini GR and Libby P: Atherosclerosis
and inflammation: Overview and updates. Clin Sci (Lond).
132:1243–1252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Usai MV, Bosiers MJ, Bisdas T, Torsello G,
Beropoulis E, Kasprzak B, Stachmann A and Stavroulakis K: Surgical
versus endovascular revascularization of subclavian artery
arteriosclerotic disease. J Cardiovasc Surg (Torino). 61:53–59.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sun Y, Gao Y, Zhou L, Lu Y, Zong Y, Zhu H,
Tang Y, Zheng F, Sun Y and Li Y: A multi-target protective effect
of Danggui-Shaoyao-San on the vascular endothelium of
atherosclerotic mice. BMC Complement Med Ther.
23(60)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695.
2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Williams IL, Wheatcroft SB, Shah AM and
Kearney MT: Obesity, atherosclerosis and the vascular endothelium:
Mechanisms of reduced nitric oxide bioavailability in obese humans.
Int J Obes Relat Metab Disord. 26:754–764. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xing SS, Yang XY, Zheng T, Li WJ, Wu D,
Chi JY, Bian F, Bai XL, Wu GJ, Zhang YZ, et al: Salidroside
improves endothelial function and alleviates atherosclerosis by
activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway.
Vascul Pharmacol. 72:141–152. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang R, Miao Y, Chen L, Yi S and Tan N:
De Novo transcriptome analysis reveals putative genes involved in
anthraquinone biosynthesis in Rubia yunnanensis. Genes (Basel).
13(521)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yi S, Lin Q, Zhang X, Wang J, Miao Y and
Tan N: Selection and validation of appropriate reference genes for
quantitative RT-PCR analysis in Rubia yunnanensis diels based on
transcriptome data. Biomed Res Int. 2020(5824841)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Larmann J, Jurk K, Janssen H, Müller M,
Herzog C, Lorenz A, Schmitz M, Nofer JR and Theilmeier G: Hepatic
overexpression of soluble urokinase receptor (uPAR) suppresses
diet-induced atherosclerosis in low-density lipoprotein
receptor-deficient (LDLR-/-) mice. PLoS One.
10(e0131854)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu S, Liu Y, Liu Z, Hu Y and Jiang M: A
review of the signaling pathways of aerobic and anaerobic exercise
on atherosclerosis. J Cell Physiol. 238:866–879. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou ZX, Ren Z, Yan BJ, Qu SL, Tang ZH,
Wei DH, Liu LS, Fu MG and Jiang ZS: The role of ubiquitin E3 ligase
in atherosclerosis. Curr Med Chem. 28:152–168. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kolovou G, Anagnostopoulou K, Mikhailidis
DP and Cokkinos DV: Apolipoprotein E knockout models. Curr Pharm
Des. 14:338–351. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakashima Y, Plump AS, Raines EW, Breslow
JL and Ross R: ApoE-deficient mice develop lesions of all phases of
atherosclerosis throughout the arterial tree. Arterioscler Thromb.
14:133–140. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao Y, Su Y, Huo Y, Mi J, Wang X, Wang Z,
Liu Y and Zhang H: Identification of antihyperlipidemic
constituents from the roots of Rubia yunnanensis Diels. J
Ethnopharmacol. 55:1315–1321. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liou MJ and Wu TS: Triterpenoids from
Rubia yunnanensis. J Nat Prod. 65:1283–1287. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ren H, Wang X, Gao L, Niu L and Li J:
Characteristic spectrum and quantitative study of the index
components of the Yi medicine Rubia yunnanensis. Chin Med Mater.
45:1400–1404. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Yang R, Powell-Braxton L, Ogaoawara AK,
Dybdal N, Bunting S, Ohneda O and Jin H: Hypertension and
endothelial dysfunction in apolipoprotein E knockout mice.
Arterioscler Thromb Vasc Biol. 19:2762–2768. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nader MA, el-Agamy DS and Suddek GM:
Protective effects of propolis and thymoquinone on development of
atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res.
33:637–643. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Calan M, Calan O, Gonen MS, Bilgir F,
Kebapcilar L, Kulac E, Cinali T and Bilgir O: Examination of
adhesion molecules, homocysteine and hs-CRP in patients with
polygenic hypercholesterolemia and isolated hypertriglyceridemia.
Intern Med. 50:1529–1535. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meyer-Lindemann U, Mauersberger C, Schmidt
AC, Moggio A, Hinterdobler J, Li X, Khangholi D, Hettwer J, Gräßer
C, Dutsch A, et al: Colchicine Impacts leukocyte trafficking in
atherosclerosis and reduces vascular inflammation. Front Immunol.
13(898690)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Massberg S, Brand K, Gruner S, Page S,
Müller E, Müller I, Bergmeier W, Richter T, Lorenz M, Konrad I, et
al: A critical role of platelet adhesion in the initiation of
atherosclerotic lesion formation. J Exp Med. 196:887–896.
2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vega GL and Grundy SM:
Hypercholesterolemia with cholesterol-enriched LDL and normal
levels of LDL-apolipoprotein B. Effects of the step I diet and bile
acid sequestrants on the cholesterol content of LDL. Arterioscler
Thromb Vasc Biol. 16:517–522. 1996.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Badimon L, Vilahur G and Padro T:
Lipoproteins, platelets and atherothrombosis. Rev Esp Cardiol.
62:1161–1178. 2009.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
27
|
Calabresi L, Gomaraschi M, Simonelli S,
Bernini F and Franceschini G: HDL and atherosclerosis: Insights
from inherited HDL disorders. Biochim Biophys Acta. 1851:13–18.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gimbrone MA Jr and Garcia-Cardena G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aird WC: Endothelium as an organ system.
Crit Care Med. 32 (5 Suppl):S271–S279. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Corre I, Paris F and Huot J: The p38
pathway, a major pleiotropic cascade that transduces stress and
metastatic signals in endothelial cells. Oncotarget. 8:55684–55714.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rafieian-Kopaei M, Setorki M, Doudi M,
Baradaran A and Nasri H: Atherosclerosis: Process, indicators, risk
factors and new hopes. Int J Prev Med. 5:927–946. 2014.PubMed/NCBI
|
|
32
|
Poznyak A, Grechko AV, Poggio P,
Myasoedova VA, Alfieri V and Orekhov AN: The diabetes
mellitus-atherosclerosis connection: The role of lipid and glucose
metabolism and chronic inflammation. Int J Mol Sci.
21(1835)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Marchio P, Guerra-Ojeda S, Vila JM,
Aldasoro M, Victor VM and Mauricio MD: targeting early
atherosclerosis: A focus on oxidative stress and inflammation. Oxid
Med Cell Longev. 2019(8563845)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li Y, Xu Q, Shi M, Gan P, Huang Q, Wang A,
Tan G, Fang Y and Liao H: Low-level laser therapy induces human
umbilical vascular endothelial cell proliferation, migration and
tube formation through activating the PI3K/Akt signaling pathway.
Microvasc Res. 129(103959)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen L, Qin L, Liu X and Meng X: CTRP3
Alleviates Ox-LDL-Induced inflammatory response and endothelial
dysfunction in mouse aortic endothelial cells by activating the
PI3K/Akt/eNOS Pathway. Inflammation. 42:1350–1359. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou YJ, Xu N, Zhang XC, Zhu YY, Liu SW
and Chang YN: Chrysin improves glucose and lipid metabolism
disorders by regulating the AMPK/PI3K/AKT signaling pathway in
insulin-resistant HepG2 cells and HFD/STZ-Induced C57BL/6J mice. J
Agric Food Chem. 69:5618–5627. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Q, Li N, Cui HH, Tian XQ, Jin C, Chen
GH and Yang YJ: Tongxinluo exerts protective effects via
anti-apoptotic and pro-autophagic mechanisms by activating AMPK
pathway in infarcted rat hearts. Exp Physiol. 102:422–435.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jing R, Zhong QQ, Long TY, Pan W and Qian
ZX: Downregulated miRNA-26a-5p induces the apoptosis of endothelial
cells in coronary heart disease by inhibiting PI3K/AKT pathway. Eur
Rev Med Pharmacol Sci. 23:4940–4947. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu J, Xu P, Liu D, Wang R, Cui S, Zhang
Q, Li Y, Yang W and Zhang D: TCM Regulates PI3K/Akt signal pathway
to intervene atherosclerotic cardiovascular disease. Evid Based
Complement Alternat Med. 2021(4854755)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Luo L, Liang H and Liu L: Myristicin
regulates proliferation and apoptosis in oxidized low-density
lipoprotein-stimulated human vascular smooth muscle cells and human
umbilical vein endothelial cells by regulating the PI3K/Akt/NF-κB
signalling pathway. Pharm Biol. 60:56–64. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Guo J, Jie W, Shen Z, Li M, Lan Y, Kong Y,
Guo S, Li T and Zheng S: SCF increases cardiac stem cell migration
through PI3K/AKT and MMP-2/-9 signaling. Int J Mol Med. 34:112–118.
2014.PubMed/NCBI View Article : Google Scholar
|