1. Introduction
Stroke constitutes a challenging multi-factorial
pathological entity engaging several medical and surgical
specialties in its management, while it remains the third cause of
mortality and disability in the western hemisphere (1). The incidence of stroke in the USA
alone is 700,000 cases annually, and the incidence of
stroke-related deaths is ~170,000 individuals per year. In Europe,
the annual stroke incidence rates per year per 100,000 individuals
are 141.3 cases among males and 94.6 cases among females (1).
Cerebral infarction is a condition that is
potentially catastrophic and is usually managed in specialized
stroke or neuro-intensive care units (2). Adjusted for the European population,
the annual incidence of cerebral infarction per 100,000 individuals
is 114.7 cases among males and 74.9 cases among females (1). Based on the International Stroke Trial
multi-national randomized control study, the outcome was documented
as highly variable between countries, although recent data state
that stroke remains a major cause of mortality and morbidity, thus
challenging the health care systems in western civilizations
(3).
Middle cerebral artery (MCA) infarctions account for
10-15% of all supratentorial infarctions, while 10-20% of these are
massive and may cause severe brain edema. The reported rates of
mortality with conservative treatment in these extensive MCA
infarcts remain as high as 80%, despite optimal medical treatment
(1,4). The progressive development of
post-infarction edema results in regional venous obstruction and
consequently, in further tissue swelling, which compromises
arterial inflow to the penumbra zone, thus inducing further
ischemic damage and the enlargement of the infarcted area (4). This escalation of edema has as a
sequence the breakdown of the blood-brain barrier, which induces a
vicious cycle of further compromise of cerebral perfusion, tissue
oxygenation and metabolism, inevitably leading to the development
of medically intractable intracranial hypertension. In these cases,
the most common mechanism of mortality is the mechanical shifting
of the intracranial components, and finally, their transtentorial
or transfalcine herniation.
Neurosurgical intervention, in the form of
decompressive craniectomy (DC), may be employed in the treatment of
cerebral infarction when massive brain edema occurs and when
symptoms of medically refractory intracranial pressure are evident
(5). The concept of bone
decompression is the removal of a large part (or parts) of the
skull to increase the potential volume of the cranial cavity and
allow the expansion of the edematous brain. This maneuver, along
with the draining of cerebrospinal fluid, may detonate an increased
intracranial pressure (ICP), prevent or decrease the mechanical
pressure applied by the rigid skull to the edematous brain
parenchyma, reverse or decrease the shift of the intracranial
anatomical structures, and thus may prevent any impeding
herniations. For several decades, however, the performance of DC
was not considered until all the conservative regimens employed
failed and the intracranial pressure was unmanageable. Over the
past decade, several clinical investigators have suggested that the
early employment of DC in patients suffering extensive MCA infarcts
may prevent the catastrophic cascade of intracranial hypertension
and may markedly affect the overall outcomes of these patients
(1,2,4).
The present study reviewed the pertinent literature
evaluating the role of DC in the management of patients with large
MCA infarcts and aimed to identify those factors that may favorably
or unfavorably affect the success of the employed DC. The
literature regarding the occurrence of any complications associated
with DC in patients suffering MCA infarcts was also reviewed.
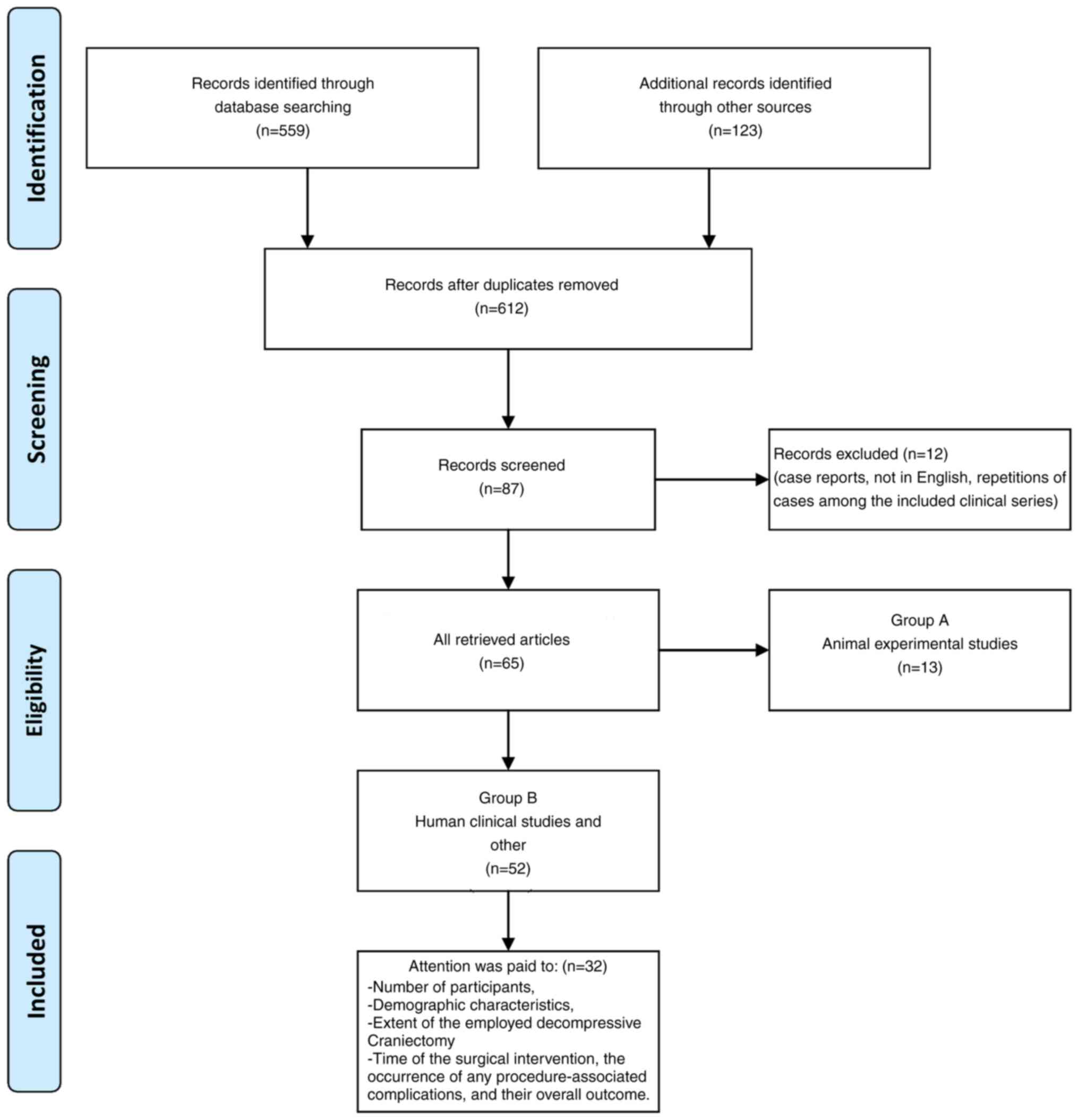
2. Data extraction
An extensive literature search of the PubMed medical
database was performed using the terms ‘malignant cerebral
infarction’, ‘middle cerebral artery’, ‘surgery’, ‘ischemic
stroke’, ‘decompressive craniectomy’ and ‘cerebral infarction’, as
well as all their possible combinations. The search was not limited
by language, journal, or type of publication. In addition, the
reference lists of all the retrieved articles were carefully
examined to identify any further relevant articles. Only articles
written in the English language were reviewed. The retrieved
articles were carefully reviewed and were categorized into two
groups as follows: Animal experimental studies and human clinical
studies. Articles referring to case reports were excluded. Every
possible effort was made to identify any repetition of cases among
the published clinical series and/or reports in different journals.
In these cases, only the original clinical series was included in
the present review (Fig. 1).
During the process of reviewing the human studies,
particular attention was paid to the number of participants, their
demographic characteristics, the extent of the employed DC, the
time of the surgical intervention, the occurrence of any
procedure-associated complications and their overall outcome. Data
referring to psychological or social performance were also
examined.
3. Animal experimental studies
In 1995, Forsting et al (6) reported their results of employing DC
in an experimental model of induced cerebral ischemia. They
performed DC in 30 rats with focal cerebral ischemia, and they
suggested that DC not only decreased mortality, but also
significantly improved outcomes and reduced the infarct size,
probably by increasing perfusion pressure through a leptomeningeal
vascular network. Similarly, in their studies, Doerfler et
al (7,8) found that DC reduced mortality and
improved the outcomes of experimental animals suffering cerebral
ischemia following endovascular occlusion of the MCA. Moreover,
Doerfler et al (9) and
Jieyong et al (10) reported
that after inducing permanent focal ischemia in rats, the
combination of DC and mild hypothermia ameliorated the infarct
volume and thus improved the neurological outcomes of the
experimental animals.
Engelhorn et al (11,12),
by performing perfusion and diffusion-weighted magnetic resonance
imaging (MRI) following MCA occlusion in rats, revealed that early
re-perfusion and craniectomy were effective in decreasing the
infarct volume by improving cerebral perfusion pressure. They
postulated that re-perfusion remains the optimal therapy for
malignant hemispheric stroke, since combined treatments yield no
additional benefit. In 2015, Slotty et al (13) examined the hemodynamic effects of DC
in comparison to the employment of re-perfusion techniques. They
evaluated their results by performing perfusion-weighted MRI, and
they concluded that both craniectomy and re-perfusion increased
cerebral perfusion in the acute phase of cerebral ischemia;
however, re-perfusion resulted in a homogeneous improvement of
perfusion in the cortex and the basal ganglia, while DC improved
only cortical perfusion under the craniectomy site (13).
4. Decompressive craniectomy in humans:
Historical evolution
Since Kocher, DC has been employed in various forms
and with significantly varying results for traumatic brain injury
(TBI) by several clinical investigators (14-16).
There are numerous retrospective studies, as well as prospective,
multi-center clinical trials examining the role of DC in the
management of patients with TBI (17,18).
The treatment of cerebral ischemic stroke entered a
new era with the development and evolution of specialized stroke
units and the improvement of neuro-intensive care units.
In previous studies, the mortality rate due to an
unmanageable elevated ICP by conservative treatment, and
subsequently, brain herniation, was reported to be 80%; following
DC, this decreased to 20-30% (19-21).
Soon, the necessity for designing and performing prospective,
multi-center, large-scale, randomized clinical trials became more
apparent. Thus, five multi-center, randomized clinical trials have
been initiated since 2000 in an attempt to prove undisputedly the
decrease in mortality and also to set new standards for treating
patients suffering large MCA infarcts (22-26).
Moreover, these studies aimed to address issues, such as the
functional outcomes and the intermediate and long-term quality of
life (QOL) of these patients (27,28).
5. Technical aspects of decompressive
craniectomy
Although there are numerous variations in the
technique of DC, the surgical procedure that is most widely
employed is the typical unilateral fronto-temporo-parietal
craniectomy. The patient is positioned in a supine position, with
his head rotated away from the surgeon by 60˚ and slightly flexed,
so the temporal area would be the most superior part. A roll may be
necessary to be placed under the ipsilateral shoulder in order to
elevate it. The head is usually secured with a three-point fixation
device. Special attention needs to be paid to the patient's neck
after positioning him or her in order to prevent any venous outflow
obstruction (29).
The skin incision is in the form of a reverse
question mark, beginning at the zygoma in front of the ear,
preserving the ipsilateral superficial temporal artery, extending
backwards toward the inion for ~5 cm, and then continuing
anteriorly (running 1-2 cm laterally and parallel to the sagittal
sinus) and ending just behind the frontal hair line. The skin flap
is reflected laterally, and a large craniectomy is performed
involving the frontal, parietal, and temporal bones using a
high-speed craniotome. A key point for a successful DC is the
removal of the temporal bone all the way to the floor of the middle
cranial fossa to minimize the uncal pressure on the adjacent
brainstem. The removed bone flap is generally considered to be at
least 12 cm in its largest diameter. The underlying dura may be
incised in a cruciate or arcuate fashion all the way to the
craniectomy edges. The majority of surgeons are not eager perform a
resection of the underlying infarcted brain tissue. An augmentative
duraplasty is performed by utilizing either a pericranial flap or a
dural substitute. The temporal fascia and the skin are
re-approximated (29).
6. Human clinical trials
Rengachary et al (30) recommended craniectomy for massive
cerebral infarction and published their experience with 3 patients.
Mori et al (31) then
reported good results with early external decompressive craniectomy
with duroplasty, which helps patients with massive hemispheric
embolic infarction recover more functionally.
Hacke et al (32) introduced the term malignant space
occupying middle cerebral infarction in 1995 in a prospective study
recruiting 37 patients, stating that the outcome after craniectomy
was unexpectedly good.
Several retrospective case series were published
after 1995, comparing DC with conservative treatment, reporting
mortality rates up to 35% in surgically treated patients, while in
the mortality rate of the group treated conservatively ranged from
60-100%.
Koh et al (33), in Singapore in 2000, published their
experience of 10 patients, reporting a mortality rate of 20% and
severe disability of 40%, and concluded that decompression should
be considered in young patients who have a rapidly deteriorating
status.
Robertson et al (34), evaluating 12 patients treated with
decompression, stated that large decompression, anterior temporal
lobectomy, the resection of infracted tissue and duraplasty were
beneficial to a significant number of patients, reporting a
mortality rate of 17% and severe disability in 41% of patients.
Pranesh et al (35), assessing 19 patients treated
surgically 20 to 100 h post-onset (mean, 60 h), reported a very low
mortality rate overall and a very promising functional outcome in
patients <50 years of age.
In 2004, Woertgen et al (36) retrospectively analyzed the records
of 48 patients treated with craniectomy, finding a mortality rate
of 26% and reporting a good functional outcome. Furthermore, they
stated that the QOL index did not differ significantly between
patients with left- or right-sided lesions, and concluded with the
result that 83% of the survivors would agree to surgery in the
future (36).
Harscher et al (37) analyzed the charts of 30 consecutive
patients who underwent craniectomy, most of them within the first
96 h following the onset of symptoms, and in 2 cases, craniectomy
was performed as far as 200 h post-onset. In a long-term follow-up,
they reported a low mortality rate immediately post-operatively,
but documented a late mortality rate due to complications (sepsis,
lung embolism). They related mortality to age and other risk
factors and complications (37).
Huh et al (38), in a retrospective study of 24
patients, reported 14 survivors and a Glasgow Outcome Scale (GOS)
of 4-5 in 9 patients. Yang et al (39) compared 10 patients treated
surgically to 14 patients receiving medical treatment alone,
reporting a mortality rate of 10% in the surgical group and 64% in
the group treated conservatively. They assessed functional outcomes
using the Barthel index (BI) and modified Rankin scale (RS), and
revealed better results in the surgical group (39).
Gupta et al (40), analyzing data from 138 patients
screening 15 studies in 2004, reported a mortality rate
post-operatively of 24%. In 2004, Mori et al (31) from Japan retrospectively assessed 71
patients with massive hemispheric infarctions (infarction volume,
>200 cm3), dividing them into three groups:
Conservative (21 pts), early surgery (21 pts) and 29 patients in
the late surgical group who were treated surgically following brain
herniation. The 6-month follow-up mortality rate was 70% in the
conservative group, 27% in the late surgical group and 19% in the
early surgical group (31).
Furthermore, they reported significantly improved GOS scores in the
early surgery group than those in the late group (31).
Schwab et al (41) published the first prospective
non-controlled trial in 1998. They included 63 consecutive patients
and reported a mortality rate of 17%. They also examined the BI and
RS scores of the survivors and reached the conclusion that
hemicraniectomy performed early (<24 h from the onset of
symptoms) leads to an improved functional outcome and a significant
decrease in the length of time spent in the intensive care unit
(41).
In 1998, the Swedish Malignant Cerebral Arrest
Infarction Study (SWEMMIS) was initiated at three Swedish
university hospitals and published its results in 2006(42). Patients were prospectively included
if they were <70 years of age, were previously healthy, and
suffered from an acute malignant MCA infarction (42). After assessing 30 patients who were
operated on with hemicraniectomy, it was concluded that if the
patient survives the acute phase, long-term survival appears to be
favorable in patients treated with hemicraniectomy. The outcome, as
measured by the modified RS score, may be better among younger
patients (42).
Kilincer et al (43) presented a non-randomized prospective
study, performing decompressive craniectomy in 32 patients (age
range, 27 to 77 years) and reported a 6-month mortality rate of up
to 50%. The following were considered prognostic factors of poor
outcomes: An age >60 years, a low pre-operative Glasgow Coma
Scale (GCS; <7/15), anisocoria and early (<72 h)
deterioration (43).
More than 20 centers have published retrospective or
non-randomized prospective case studies assessing neurological
outcomes in patients with DC for malignant MCA infarction. Data
from all groups stated a decreased mortality rate of 20-30%
following decompression compared with 70-80% among patients
receiving maximal conservative treatment (44,45).
The global concern for the functional outcomes of survivors and the
identification of which patients will benefit from DC remains, and
further questions remain to be unanswered. Thus, the need for
prospective randomized multicenter trials arouses among the global
medical community in order to document thoroughly the decrease in
mortality, assess the functional outcome, and determine prognostic
factors.
Randomized clinical trials
Over the past decade, five randomized controlled
trials have been initiated assessing decompressive craniectomy in
acute ischemic stroke with malignant edema.
The Hemicraniectomy and Durotomy Upon Deterioration
From Infarction-Related Swelling Trial (HeADDFIRST) was the first
randomized trial to be conducted in the USA (22). The principal inclusion criteria were
clinical and radiological deterioration within 96 h of stroke
onset, and the researchers aimed to investigate mortality,
functional outcomes, QOL and patient perceptions. In the last
published data, they presented a mortality rate of 26.7% in the
surgical group (mean age, 52.3 years) and a mortality rate of 45.5%
in the standard medical group (mean age, 53.5 years).
Hemicraniectomy for Malignant MCA Infarcts (HeMMI)
is a single-center trial conducted in the Philippines, including
patients with clinical deterioration within 72 h post-ictal,
assessing the modified RS and BI scores (23). However, there is no recent update
available.
Decompressive Surgery for the Treatment of Malignant
Infarction of MCA (DECIMAL) is a multicenter trial conducted in
France, enrolling patients between 18 and 55 years of age suffering
from malignant MCA infarction within 24 h of stroke onset, defined
by the association of three criteria: An NIH Stroke Scale (NIHSS)
score >2, computed tomography (CT) scan findings involving
>50% of MCA territory, and a diffusion-weighted imaging (DWI)
infarct volume >145 ml (24).
Eligible patients were randomly assigned to receive standard
medical therapy alone or conservative treatment plus DC and
durotomy. For patients in the surgical group, DC has to be
performed no later than 6 h following randomization and up to 30 h
post-ictal. Assessing outcomes, the investigators defined favorable
functional outcome as a modified RS score <3 and a BI score
>85 at 1 year. QOL was assessed using the Stroke Impact Scale.
Recruitment was terminated after the inclusion of 38 patients due
to slow enrollment and a significant difference in mortality rates
favoring surgery. It was concluded that early DC increased the
number of patients with moderate disability by more than half, and
significantly reduced the mortality rate compared with that of
medical therapy by more than half (24).
Decompressive Surgery for the Treatment of Malignant
Infarction of the MCA (DESTINY) is a prospective multicenter
randomized controlled clinical trial designed in Germany. The
conductors from the University of Heidelberg defined the inclusion
criteria as follows: An age 18-60 years, a NIHSS score >18 for
the non-dominant hemisphere and a NIHSS score >20 for the
dominant hemisphere, CT scan documented signs including 2/3 of the
territory of MCA and including part of the basal ganglia (25). They assessed mortality rates after
30 days and functional outcomes after 6 months (a modified RS score
of 0-3 indicate a favorable outcome, and a score of 4-6 an
unfavorable outcome). After including 32 patients, DESTINY achieved
a statistically significant reduction of 30 days of mortality (12
vs. 57% of the conservative group), and as for functional outcomes,
after 12 months of follow-up, 47% of the patients in the surgical
group had a modified RS score of 0-3 compared to 27% of the
patients in the conservative group.
Hemicraniectomy after middle cerebral artery
infarction with life-threatening edema trial (HAMLET) is a
multi-center open, randomized treatment trial designed in The
Netherlands (26). The conductors
from the University of Utrecht enrolled patients from 18 to 60
years of age suffering from acute ischemic stroke in the territory
of MCA with an onset of symptoms within 96 h prior to the planned
treatment, an NIHSS score >16 for right-sided lesions, >21
for left-sided lesions, and a GCS score of 13/15 or less for
right-sided lesions or 9/15 or less for left-sided lesions. The
radiological inclusion criteria were considered hypodensity on a CT
scan involving at least two-thirds of the territory of MCA and
space-occupying edema (midline shift is not a requirement of
inclusion). In addition, there must be a possibility to start trial
treatment within 3 h following randomization. Following the
randomization of 64 patients, 32 assigned to surgical decompression
and 32 to optimal medical treatment, the conductors concluded that
surgical decompression reduced fatality and poor outcomes in
patients with space-occupying infarctions who are treated within 48
h from stroke onset. There is no evidence that this operation
improves functional outcomes when it is delayed after 96 h, and the
decision to perform the procedure should depend on the importance
patients and relatives attribute to survival and dependency.
The three European randomized controlled trials, the
French DECIMAL, the German DESTINY and the Dutch HAMLET, have a
similar design and share the same primary outcome measures:
Favorable functional outcomes, as determined by the modified RS
score; thus, a collaborative protocol for a pooled analysis of
individual patient data from the three trials was planned. At the
time of the analysis, DECIMAL and DESTINY had been interrupted,
whereas HAMLET was still ongoing. The principal aim of the pooled
analysis was to obtain sufficient data to reliably estimate the
effects of DC and avoid unnecessary and unethical continuation of
randomization in the individual trials. A total of 93 patients were
included in the pooled analysis: A total of 38 patients from
DECIMAL, 32 patients from DESTINY and 23 patients from HAMLET, of
whom 51 patients were randomized to DC and 42 patients to
conservative treatment. The pooled analysis yielded three
conclusions as follows: i) Significantly fewer patients after
decompression had an unfavorable outcome defined as a modified RS
score of 5 or mortality at 12 months compared to patients receiving
conservative treatment; ii) significantly fewer patients following
surgical treatment had a modified RS score >3 at 12 months than
patients following conservative treatment; iii) the survival rate
at 12 months was higher following surgical treatment than following
conservative treatment.
In order to assess the survival rates and functional
outcomes of elderly patients, in 2001, Holtkamp et al
(46), after analyzing 12 patients
aged 55-75 years, stated that although craniectomy improved
survival rates, the functional outcomes and level of independence
were poor. However, since in the randomized trials the upper age
limit was 60 years, the question of the benefit of DC in elderly
patients remains unresolved. A prospective randomized controlled
open multicentre trial is aiming to fill this gap.
DESTINY II is investigating the efficacy of early
hemicraniectomy in patients >60 years suffering malignant MCA
infarcts (25). The inclusion
criteria are an age ≥61 years, either sex, with clinical signs and
symptoms of a unilateral MCA infarct, an NIHSS score >14 for
infarcts of the non-dominant hemisphere and >19 for infarcts of
the dominant hemisphere, symptom onset prior to 48 h, treatment
within 6 h following randomization, neuroradiological findings and
informed consent. Apart from mortality and morbidity, DESTINY II
will assess neurological status (NIHSS score), disability (modified
RS), activities of daily living (BI), QOL, speech and language
disturbance and depression.
As for the pediatric population, the first published
data were obtained in 2011 by Smith et al (47), who analyzed 7 pediatric patients
suffering malignant middle cerebral artery infarction. They
reported a moderately good neurological outcome after DC in
children with ischemic stroke, regardless of etiology, GCS, or
other aspects. They also suggested that ICP monitoring may delay
surgical treatment (47).
7. Other
Statistical data are mainly derived from the USA.
Adeoye et al (48), after
queuing the Premier data base, identified almost 600,000 admissions
for acute ischemic stroke during the study period 2005-2008. Only
420 DC (0.072%) were reported, although the rate of DC increased
linearly by 20% per year; however, the rate of hemicraniectomy did
not increase further following the publication of the pooled
analysis in 2007. These patients tended to be younger,
non-Caucasian, and male; 28% of these patients were >65 years of
age (48).
In addition, from the National Stroke Association,
Alshekhlee et al (49)
identified 500,000 cases of acute ischemic stroke, of which 250
underwent DC (0.05%) and 1.5% were treated with thrombolysis. The
mortality rate was significantly lower than in previous cohorts;
however, hemicraniectomy remains associated with a high hospital
mortality rate (49).
In contrast to the promising data for survival,
neuropsychological segregation received little attention until the
present day. In 2011, Schmidt et al (50), after assessing cognition and the
impairment of higher cortical functions in 20 patients at 1 year
post-surgery, noted that patients are at a high risk of the onset
of depression, apart from severe cognitive impairment that
resembles even dementia in a number of cases.
Functional MRI (f-MRI) is used to assess the extent
and location of functional recovery. Cheung et al (51) documented their experience in 2005,
analyzing f-MRI in 3 patients at 13-25 months post-surgery. The
examination was performed on a 3.0 Tesla device, and the image
acquisition was done using a gradient-echo T2 weighted sequence
based on the blood-oxygen-level-dependent (BOLD) contrast
technique. Brain activation was triggered by hand gripping or foot
movement tasks. Activation was observed in the contralateral
hemisphere and less in the infarcted hemisphere; the authors
documenting the functional recovery in peri-infarct regions suggest
that DC alone may be preferable to strokectomy (51).
8. Predicting malignant evolution
In order to identify which patients will benefit
from DC among patients suffering hemispheric infarction,
researchers state that certain steps need be taken into
consideration. The first step is to identify which patient is at a
high risk of developing malignant cerebral edema. Different
clinical and radiological factors have been proposed as predictors.
An NIHSS score of at least 20 for dominant or 15 for non-dominant
strokes, a younger age, an early hypodensity >50% of the MCA
territory, including the basal ganglia, a midline shift >5 mm,
and an infarct volume on DWI of at least 145 ml. The impressive
progress in neuroimaging over the past few years is very promising
as a prognostic factor in treating acute ischemic stroke.
CT scan signs of ischemia may be quite subtle, but
still, an infarction of >50% of the MCA territory, as well as
compression of basal cisterns and sulcal effacement are
neuroradiological evidence of brain swelling before a midline shift
occurs. However, the identification of an acute ischemic stroke on
the initial CT scan is observed in ~70% of cases. An MRI, or
perfusion CT is superior in predicting malignant evolution and the
definition of infarct size (52).
Diffusion and perfusion MRI as early as 6 h post-ictal can predict
malignant evolution with high specificity. A threshold of ≥145 ml
of the infarct volume in diffusion MRI has a sensitivity of 100%;
however, the majority of researchers treat malignant infarction
volumes >80 ml (53).
9. Prognostic factors for favorable
outcomes
Age limit
When 60 years of age is selected as a cut-off point,
statistical analysis provides one of the strongest predictors of
the optional outcome. The majority of surgeons are not eager to
perform DC on elderly patients (54). Previous studies have noted the
association of poor outcomes with patient's age and comorbidities
(54,55). In addition, data from surgical
experience suggest that a favorable outcome is anticipated more
when decompression is performed at an early stage and definitely
prior to the onset of herniation. Low initial scores on GCS, the
involvement of additional vascular territories, and infarction in
the dominant hemisphere are considered predictors of a worse
outcome (56). The surgical
experience and knowledge state that the younger the patient, the
earlier the decompression, and the smaller the infarct, the better
the post-operative neurological status.
Hemispheric dominance
A dominant hemispheric infarction should not be an
exclusion criterion (57). The loss
of the capability to communicate in combination with severe motor
symptoms is often considered to be too disabling; thus,
decompression over the dominant hemisphere was viewed with
skepticism. There is no indication that patients with dominant
malignant infarction do not benefit from treatment; neither
mortality nor functional outcome or QOL have been found to be
associated with the hemisphere in the pooled analysis (57).
Timing of surgical procedure
An aggressive and early approach may lead to
unnecessary surgical interventions (considering the cranioplasty)
for a patient who could recover with conservative treatment
(58). On the other hand, if DC is
performed too late, the patient is at risk of irreversible brain
stem damage due to herniation (59). A pre-operative GCS of at least 8/15
is critical for a positive outcome (60-63).
ICP monitoring is not goal-standard, since herniation signs precede
the elevation of ICP (64).
Monitoring ICP is not standard of care in cerebral infarction,
since there is no supporting evidence of improving outcomes or
facilitating medical treatment (64). The majority of treatments are aimed
at direct or indirect signs of a raised ICP (64).
Surgical complications
The complication rate is higher. Craniectomy
violates dural and bony tissue planes, and creates abnormal
communication among cranial spaces, predisposing post-operative
fluid or cerebrospinal fluid collections, such as subdural hygromas
and external hydrocephalus (63).
Current data state that the extra axial fluid collection rates are
lower than those of DC due to TBI, and of note, they appear to
exhibit a trend to resolve spontaneously (58-60).
Ropper et al (65) reported
rates of 18%, while rates ranged from 14-60% after DC for MCA
stroke, compared to 60% that Aarabi et al (66) reported after reviewing DC for TBI.
Severe cardiac events, including life-threatening arrhythmias,
myocardial ischemia, cardiac failure and cardiac arrest are common
in the acute period following stroke and DC and is a key
contributor to mortality (65). The
complications reported are common for DC: Epidural subdural
hemorrhage, infections, and cases of post-op hydrocephalus, which
are very rare (67). The most
common complication is external brain herniation through
insufficient decompression, which exaggerates the vicious circle of
ischemia and swelling (67). A
summary of the outcomes and factors influencing the success of
employed DC, and the complications associated with DC in patients
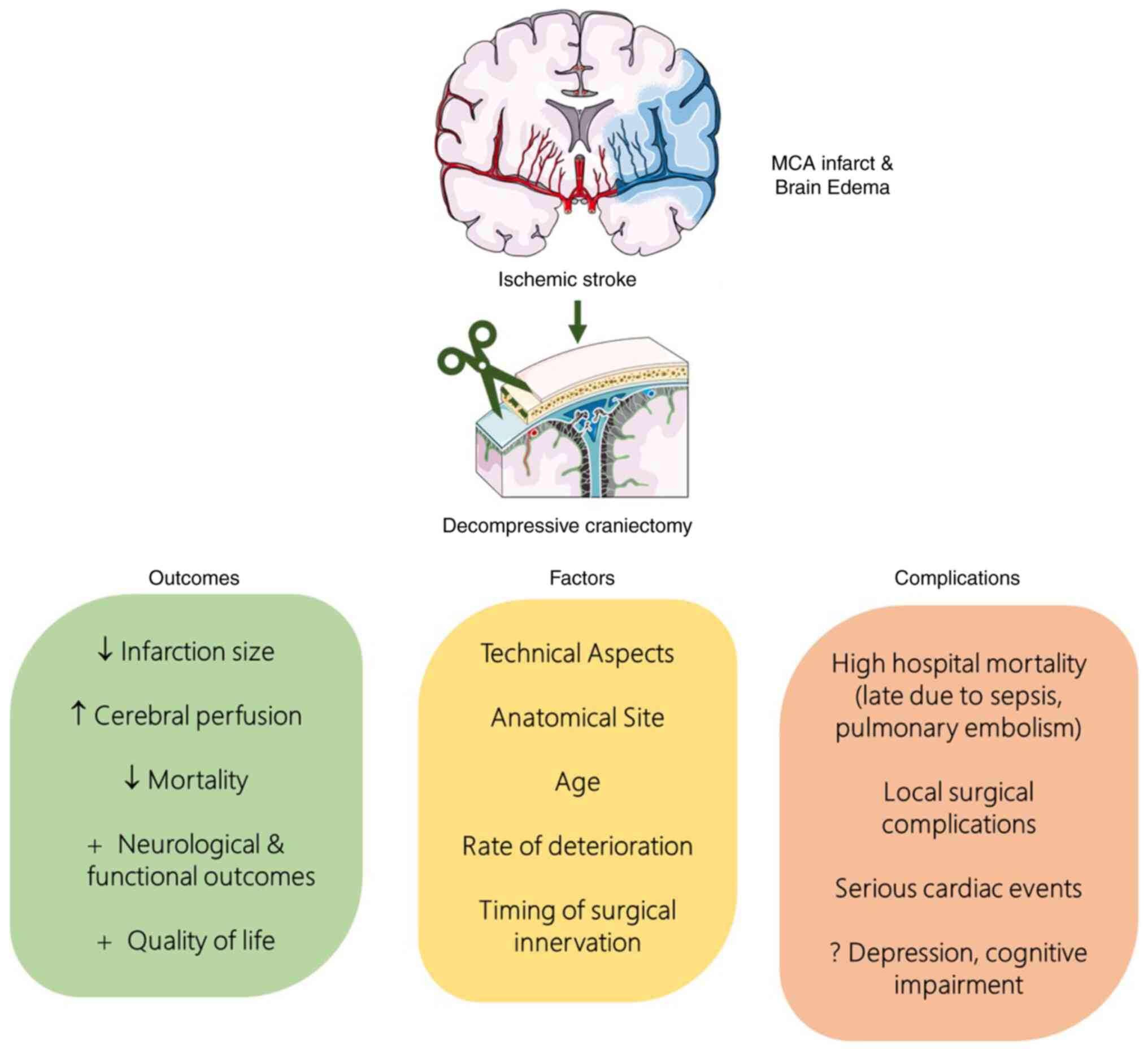
with MCA infarcts is illustrated in Fig. 2.
10. Conclusions and future perspectives
There is no doubt that DC decreases mortality rates,
as shown in all clinical trials. Functional outcome appears to be
the goal standard in modern-era neurosurgery, and QOL should be
further discussed among the medical community and with patient
consent. Future studies are required to analyze the role of factors
potentially contributing to post-operative hydrocephalus, external
hydrocephalus and external brain herniation through insufficient
decompression, which exaggerates the vicious circle of ischemia and
swelling, remained unclear. In addition, it would be helpful to
analyze the ICP level and course following DC in patients with
cerebral infarction and TBI and to associate these parameters with
the neurological outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GF and KNF conceptualized the study. VEG, GF, NT,
PS, IGL, DAS, CG and KNF analyzed the data, and wrote and prepared
the draft of the manuscript. KNF and GF provided critical
revisions. All authors contributed to manuscript revision, and have
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Ingall T: Stroke-incidence, mortality,
morbidity and risk. J Insur Med. 36:143–152. 2004.PubMed/NCBI
|
|
2
|
Polivka J Jr, Polivka J, Pesta M, Rohan V,
Celedova L, Mahajani S, Topolcan O and Golubnitschaja O: Risks
associated with the stroke predisposition at young age: Facts and
hypotheses in light of individualized predictive and preventive
approach. EPMA J. 10:81–99. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
No authors listed. The international
stroke trial (IST): A randomised trial of aspirin, subcutaneous
heparin, both, or neither among 19435 patients with acute ischaemic
stroke. international stroke trial collaborative group. Lancet.
349:1569–1581. 1997.PubMed/NCBI
|
|
4
|
Kürten S, Munoz C, Beseoglu K, Fischer I,
Perrin J and Steiger HJ: Decompressive hemicraniectomy for
malignant middle cerebral artery infarction including patients with
additional involvement of the anterior and/or posterior cerebral
artery territory-outcome analysis and definition of prognostic
factors. Acta Neurochir (Wien). 160:83–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moscote-Salazar LR, Alvis-Miranda HRL,
Ramos-Villegas Y, Quintana-Pajaro L, Rubiano AM, Alcalá-Cerra G,
González-Torres JB and Narváez-Rojas AR: Refractory traumatic
intracranial hypertension: The role of decompressive craniectomy.
Cir Cir. 87:358–364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Forsting M, Reith W, Schäbitz WR, Heiland
S, von Kummer R, Hacke W and Sartor K: Decompressive craniectomy
for cerebral infarction. An experimental study in rats. Stroke.
26:259–264. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Doerfler A, Schwab S, Hoffmann TT,
Engelhorn T and Forsting M: Combination of decompressive
craniectomy and mild hypothermia ameliorates infarction volume
after permanent focal ischemia in rats. Stroke. 32:2675–2681.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Doerfler A, Engelhorn T, Heiland S, Benner
T and Forsting M: Perfusion- and diffusion-weighted magnetic
resonance imaging for monitoring decompressive craniectomy in
animals with experimental hemispheric stroke. J Neurosurg.
96:933–940. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Doerfler A, Forsting M, Reith W, Staff C,
Heiland S, Schäbitz WR, von Kummer R, Hacke W and Sartor K:
Decompressive craniectomy in a rat model of ‘malignant’ cerebral
hemispheric stroke: Experimental support for an aggressive
therapeutic approach. J Neurosurg. 85:853–859. 1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jieyong B, Zhong W, Shiming Z, Dai Z, Kato
Y, Kanno T and Sano H: Decompressive craniectomy and mild
hypothermia reduces infarction size and counterregulates Bax and
Bcl-2 expression after permanent focal ischemia in rats. Neurosurg
Rev. 29:168–172. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Engelhorn T, Doerfler A, Kastrup A,
Beaulieu C, de Crespigny A, Forsting M, Moseley ME and Faraci FM:
Decompressive craniectomy, reperfusion, or a combination for early
treatment of acute ‘malignant’ cerebral hemispheric stroke in rats?
Potential mechanisms studied by MRI. Stroke. 30:1456–1463.
1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Engelhorn T, Doerfler A, de Crespigny A,
Beaulieu C, Forsting M and Moseley ME: Multilocal magnetic
resonance perfusion mapping comparing the cerebral hemodynamic
effects of decompressive craniectomy versus reperfusion in
experimental acute hemispheric stroke in rats. Neurosci Lett.
344:127–131. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Slotty PJ, Kamp MA, Beez T, Beenen H,
Steiger HJ, Turowski B and Hänggi D: The influence of decompressive
craniectomy for major stroke on early cerebral perfusion. J
Neurosurg. 123:59–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kocher T: Hirnerschütterung, hirndruck und
chirurgische eingriffe bei hirnkrankheiten. Wien: Alfred Hölder,
1901.
|
|
15
|
Sahuquillo J and Dennis JA: Decompressive
craniectomy for the treatment of high intracranial pressure in
closed traumatic brain injury. Cochrane Database Syst Rev.
12(CD003983)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu G, Zhu L, Wang X, Zhang H and Li Y:
Decompressive craniectomy for patients with traumatic brain injury:
A pooled analysis of randomized controlled trials. World Neurosurg.
133:e135–e148. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Iaccarino C, Lippa L, Munari M, Castioni
CA, Robba C, Caricato A, Pompucci A, Signoretti S, Zona G, Rasulo
FA, et al: Management of intracranial hypertension following
traumatic brain injury: A best clinical practice adoption proposal
for intracranial pressure monitoring and decompressive craniectomy.
Joint statements by the traumatic brain injury section of the
Italian society of neurosurgery (SINch) and the neuroanesthesia and
neurocritical care study group of the italian society of
anesthesia, analgesia, resuscitation and intensive care (SIAARTI).
J Neurosurg Sci. 65:219–238. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Garg K, Singh PM, Singla R, Aggarwal A,
Borle A, Singh M, Chandra PS, Kale SS and Mahapatra AK: Role of
decompressive craniectomy in traumatic brain injury-a meta-analysis
of randomized controlled trials. Neurol India. 67:1225–1232.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hacke W, Schwab S, Horn M, Spranger M, De
Georgia M and von Kummer R: ‘Malignant’ middle cerebral artery
territory infarction: Clinical course and prognostic signs. Arch
Neurol. 53:309–315. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Maia IHM, Melo TP, Lima FO, Carvalho JJF,
Mont'alverne FJA, Lopes JÚnior E, DiÓgenes MB, Cunha TSL, Queiroz
BMA, Tamietti MF and Maia FM: Decompressive craniectomy versus
conservative treatment: Limits and possibilities in malignant
stroke. Arq Neuropsiquiatr. 78:349–355. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mattos JP, Joaquim AF, Almeida JP,
Albuquerque LA, Silva EG, Marenco HA and Oliveira ED: Decompressive
craniectomy in massive cerebral infarction. Arq Neuropsiquiatr.
68:339–345. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Frank JI, Schumm LP, Wroblewski K, Chyatte
D, Rosengart AJ, Kordeck C and Thisted RA: HeADDFIRST Trialists.
Hemicraniectomy and durotomy upon deterioration from
infarction-related swelling trial: Randomized pilot clinical trial.
Stroke. 45:781–787. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Staykov D and Gupta R: Hemicraniectomy in
malignant middle cerebral artery infarction. Stroke. 42:513–516.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vahedi K, Hofmeijer J, Juettler E, Vicaut
E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell
PM, et al: Early decompressive surgery in malignant infarction of
the middle cerebral artery: A pooled analysis of three randomised
controlled trials. Lancet Neurol. 6:215–222. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jüttler E, Bösel J, Amiri H, Schiller P,
Limprecht R, Hacke W and Unterberg A: DESTINY II Study Group.
DESTINY II: DEcompressive surgery for the treatment of malignant
infarction of the middle cerebral arterY II. Int J Stroke. 6:79–86.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hofmeijer J, Kappelle LJ, Algra A, Amelink
GJ, van Gijn J and van der Worp HB: HAMLET investigators. Surgical
decompression for space-occupying cerebral infarction [the
hemicraniectomy after middle cerebral artery infarction with
life-threatening edema trial (HAMLET)]: A multicentre, open,
randomised trial. Lancet Neurol. 8:326–333. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li YP, Hou MZ, Lu GY, Ciccone N, Wang XD,
Dong L, Cheng C and Zhang HZ: Neurologic functional outcomes of
decompressive hemicraniectomy versus conventional treatment for
malignant middle cerebral artery infarction: A systematic review
and meta-analysis. World Neurosurg. 99:709–725.e3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kiphuth IC, Köhrmann M, Lichy C, Schwab S
and Huttner HB: Hemicraniectomy for malignant middle cerebral
artery infarction: Retrospective consent to decompressive surgery
depends on functional long-term outcome. Neurocrit Care.
13:380–344. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang X and Wen L: Technical
considerations in decompressive craniectomy in the treatment of
traumatic brain injury. Int J Med Sci. 7:385–390. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rengachary SS, Batnitzky S, Morantz RA,
Arjunan K and Jeffries B: Hemicraniectomy for acute massive
cerebral infarction. Neurosurgery. 8:321–328. 1981.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mori K, Nakao Y, Yamamoto T and Maeda M:
Early external decompressive craniectomy with duroplasty improves
functional recovery in patients with massive hemispheric embolic
infarction: Timing and indication of decompressive surgery for
malignant cerebral infarction. Surg Neurol. 62:420–430.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hacke W, Stingele R, Steiner T, Schuchardt
V and Schwab S: Critical care of acute ischemic stroke. Intensive
Care Med. 21:856–862. 1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Koh MS, Goh KY, Tung MY and Chan C: Is
decompressive craniectomy for acute cerebral infarction of any
benefit? Surg Neurol. 53:225–230. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Robertson SC, Lennarson P, Hasan DM and
Traynelis VC: Clinical course and surgical management of massive
cerebral infarction. Neurosurgery. 55:55–62. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pranesh MB, Dinesh Nayak S, Mathew V,
Prakash B, Natarajan M, Rajmohan V, Murali R and Pehlaj A:
Hemicraniectomy for large middle cerebral artery territory
infarction: Outcome in 19 patients. J Neurol Neurosurg Psychiatry.
74:800–802. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Woertgen C, Erban P, Rothoerl RD, Bein T,
Horn M and Brawanski A: Quality of life after decompressive
craniectomy in patients suffering from supratentorial brain
ischemia. Acta Neurochir (Wien). 146:691–695. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Harscher S, Reichart R, Terborg C,
Hagemann G, Kalff R and Witte OW: Outcome after decompressive
craniectomy in patients with severe ischemic stroke. Acta Neurochir
(Wien). 148:31–37. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huh JS, Shin HS, Shin JJ, Kim TH, Hwang YS
and Park SK: Surgical management of massive cerebral infarction. J
Korean Neurosurg Soc. 42:331–336. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang XF, Yao Y, Hu WW, Li G, Xu JF, Zhao
XQ and Liu WG: Is decompressive craniectomy for malignant middle
cerebral artery infarction of any worth? J Zhejiang Univ Sci B.
6:644–649. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gupta R, Connolly ES, Mayer S and Elkind
MSV: Hemicraniectomy for massive middle cerebral artery territory
infarction: A systematic review. Stroke. 35:539–543.
2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schwab S, Steiner T, Aschoff A, Schwarz S,
Steiner HH, Jansen O and Hacke W: Early hemicraniectomy in patients
with complete middle cerebral artery infarction. Stroke.
29:1888–1893. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Malm J, Bergenheim AT, Enblad P, Hårdemark
HG, Koskinen LO, Naredi S, Nordström CH, Norrving B, Uhlin J and
Lindgren A: The Swedish malignant middle cerebral artery infarction
study: Long-term results from a prospective study of
hemicraniectomy combined with standardized neurointensive care.
Acta Neurol Scand. 113:25–30. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kilincer C, Asil T, Utku U, Hamamcioglu
MK, Turgut N, Hicdonmez T, Simsek O, Ekuklu G and Cobanoglu S:
Factors affecting the outcome of decompressive craniectomy for
large hemispheric infarctions: A prospective cohort study. Acta
Neurochir (Wien). 147:587–594. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rossini Z, Nicolosi F, Kolias AG,
Hutchinson PJ, De Sanctis P and Servadei F: The history of
decompressive craniectomy in traumatic brain injury. Front Neurol.
10(458)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Das S, Mitchell P, Ross N and Whitfield
PC: Decompressive hemicraniectomy in the treatment of malignant
middle cerebral artery infarction: A meta-analysis. World
Neurosurg. 123:8–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Holtkamp M, Buchheim K, Unterberg A,
Hoffmann O, Schielke E, Weber JR and Masuhr F: Hemicraniectomy in
elderly patients with space occupying media infarction: Improved
survival but poor functional outcome. J Neurol Neurosurg
Psychiatry. 70:226–228. 2001.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Smith SE, Kirkham FJ, Deveber G, Millman
G, Dirks PB, Wirrell E, Telfeian AE, Sykes K, Barlow K and Ichord
R: Outcome following decompressive craniectomy for malignant middle
cerebral artery infarction in children. Dev Med Child Neurol.
53:29–33. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Adeoye O, Hornung R, Khatri P, Ringer A
and Kleindorfer D: The rate of hemicraniectomy for acute ischemic
stroke is increasing in the United States. J Stroke Cerebrovasc
Dis. 20:251–254. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Alshekhlee A, Horn C, Jung R, Alawi AA and
Cruz-Flores S: In-hospital mortality in acute ischemic stroke
treated with hemicraniectomy in US hospitals. J Stroke Cerebrovasc
Dis. 20:196–201. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schmidt H, Heinemann T, Elster J, Djukic
M, Harscher S, Neubieser K, Prange H, Kastrup A and Rohde V:
Cognition after malignant media infarction and decompressive
hemicraniectomy-a retrospective observational study. BMC Neurol.
11(77)2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cheung A, Telaghani CK, Wang J, Yang Q,
Mosher TJ, Reichwein RK and Cockroft KM: Neurological recovery
after decompressive craniectomy for massive ischemic stroke.
Neurocrit Care. 3:216–223. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Alshoabi S, Alnajmani R, Shamsuddin M and
Gameraddin M: Early signs of middle cerebral artery infarction on
multidetector computed tomography: Review of 20 cases. Brain Circ.
5:27–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Simard JM, Sahuquillo J, Sheth KN, Kahle
KT and Walcott BP: Managing malignant cerebral infarction. Curr
Treat Options Neurol. 13:217–229. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
De Bonis P, Pompucci A, Mangiola A,
Paternoster G, Festa R, Nucci CG, Maviglia R, Antonelli M and Anile
C: Decompressive craniectomy for elderly patients with traumatic
brain injury: It's probably not worth the while. J Neurotrauma.
28:2043–2048. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Marshall GT, James RF, Landman MP, O'Neill
PJ, Cotton BA, Hansen EN, Morris JA Jr and May AK: Pentobarbital
coma for refractory intra-cranial hypertension after severe
traumatic brain injury: Mortality predictions and one-year outcomes
in 55 patients. J Trauma. 69:275–283. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Barthélemy EJ, Melis M, Gordon E, Ullman
JS and Germano IM: Decompressive craniectomy for severe traumatic
brain injury: A systematic review. World Neurosurg. 88:411–420.
2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kamal Alam B, Bukhari AS, Assad S,
Muhammad Siddique P, Ghazanfar H, Niaz MJ, Kundi M, Shah S and
Siddiqui M: Functional outcome after decompressive craniectomy in
patients with dominant or non-dominant malignant middle cerebral
infarcts. Cureus. 9(e997)2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Shah A, Almenawer S and Hawryluk G: Timing
of decompressive craniectomy for ischemic stroke and traumatic
brain injury: A review. Front Neurol. 10(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dasenbrock HH, Robertson FC, Vaitkevicius
H, Aziz-Sultan MA, Guttieres D, Dunn IF, Du R and Gormley WB:
Timing of decompressive hemicraniectomy for stroke: A nationwide
inpatient sample analysis. Stroke. 48:704–711. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tsai CL, Chu H, Peng GS, Ma HI, Cheng CA
and Hueng DY: Preoperative APACHE II and GCS scores as predictors
of outcomes in patients with malignant MCA infarction after
decompressive hemicraniectomy. Neurol India. 60:608–612.
2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Fotakopoulos G, Tsianaka E, Siasios G,
Vagkopoulos K and Fountas K: Posttraumatic hydrocephalus after
decompressive craniectomy in 126 patients with severe traumatic
brain injury. J Neurol Surg A Cent Eur Neurosurg. 77:88–92.
2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Fotakopoulos G, Tsianaka E, Vagkopoulos K
and Fountas KN: According to which factors in severe traumatic
brain injury craniectomy could be beneficial. Surg Neurol Int.
7(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pachatouridis D, Alexiou GA, Zigouris A,
Michos E, Drosos D, Fotakopoulos G and Voulgaris S: Management of
hydrocephalus after decompressive craniectomy. Turk Neurosurg.
24:855–858. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Poca MA, Benejam B, Sahuquillo J, Riveiro
M, Frascheri L, Merino MA, Delgado P and Alvarez-Sabin J:
Monitoring intracranial pressure in patients with malignant middle
cerebral artery infarction: Is it useful? J Neurosurg. 112:648–657.
2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ropper AE, Nalbach SV, Lin N, Dunn IF and
Gormley WB: Resolution of extra-axial collections after
decompressive craniectomy for ischemic stroke. J Clin Neurosci.
19:231–234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Aarabi B, Hesdorffer DC, Ahn ES, Aresco C,
Scalea TM and Eisenberg HM: Outcome following decompressive
craniectomy for malignant swelling due to severe head injury. J
Neurosurg. 104:469–479. 2006.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gopalakrishnan MS, Shanbhag NC, Shukla DP,
Konar SK, Bhat DI and Devi BI: Complications of decompressive
craniectomy. Front Neurol. 9(977)2018.PubMed/NCBI View Article : Google Scholar
|