Introduction
Nanotechnology, a prominent domain in modern
materials science, involves manipulation and comprehension of
matter at atomic and molecular scales (1). Nanotechnology and nanoscience center
around nanoparticles (NPs) 1-100 nm in diameter that exhibit unique
physical, chemical and biological attributes (2). NPs have potential applications in
medicine, electronics, chemistry, pharmaceuticals, agriculture and
the food industry (3).
Historically, the creation of NPs has necessitated costly and
time-consuming physical and chemical processes demanding
specialized equipment (4).
The chemical synthesis route for NPs has concerns
over the incorporation of chemical compounds, use of toxic solvents
and the emergence of noxious by-products (5). Consequently, a more eco-friendly,
efficient and economical method termed ‘green synthesis’ has
emerged, using natural biological entities such as plants, fungi
and bacteria for NP creation (6).
Empirical studies underscore plants as the optimal choice for
large-scale biosynthesis (6,7). Green
synthesis of metal NPs, predominantly with plants as the reductive
agents, has gained traction (6).
This methodology coats NPs, enhancing their biological effect.
Furthermore, plant-derived NPs demonstrated enhanced stability and
diversified morphological features compared with conventional
synthesis (8).
Metal NPs with distinct physical and chemical
properties have garnered scientific attention (2). Owing to high surface-to-volume ratio,
they serve as potential drug carriers, and can cross the
blood-brain barrier and epithelial cell junctions to access remote
targets (9). Their antiangiogenic
capabilities have been demonstrated in numerous in vitro
models, including chick embryo chorioallantoic membrane (CAM),
aortic ring and Matrigel-endothelial culture assay (4,6).
Angiogenesis, the formation of new blood vessels
from pre-existing ones, plays a pivotal role in tissue development,
wound healing and the prognostic evaluation of cancer (10). Various angiogenic promoters and
suppressors regulate this process (11). The equilibrium between
pro-angiogenic molecules, such as vascular endothelial growth
factor (VEGF) and fibroblast growth factor-2(12), and their antagonists, such as
angiostatin and angiopoietin 2, is crucial. Disruptions in this
balance instigate pathological conditions (13). Numerous studies have aimed to
modulate angiogenesis as its excess can cause cancer, arthritis,
asthma, psoriasis and diabetic blindness (13,14).
Given the role of angiogenesis in tumor evolution and metastasis,
therapeutic interventions targeting its inhibition have been
identified such as strategies blocking the VEGF pathway, pivotal
for cancer cell dynamics (15).
Metal oxide and carbon-based nanomaterials with reduced toxicity
have shown potential in suppressing angiogenic pathways, making
them suitable candidates for therapeutic applications in cancer and
other disorders (16).
Historically, medicinal plants have been
repositories of bioactive compounds. At present, 40% of
prescription drugs owe their origins to herbs (17-19).
The medicinal plant Elaeagnus angustifolia (E. ang), a
member of the Elaeagnaceae family commonly termed oleaster or
Russian olive, has numerous therapeutic applications (20). For example, the leaf extract of
E. ang has efficacy in managing chronic bronchitis (21), expediting wound healing (22) and serving as a muscle relaxant
(23). Its fruit extract
ameliorates pain in rheumatoid arthritis, asthma, nausea and
vomiting and aids wound healing in skin tissues (24,25).
Pharmacological studies have shown that E. ang L. has
anti-inflammatory, antimicrobial, antinociceptive and anti-oxidant
effects that might be used for treating a number of distresses
(26,27). E. ang is commonly found
naturally in Jordan (28).
Synthesis of zinc oxide NPs using E. ang
combines the therapeutic potentials of both entities, especially in
wound healing and cellular migration (16). The fusion of zinc mineral and plant
extract might potentiate synergistic effects on angiogenesis and
wound healing. The present study aimed to elucidate the influence
of green composite bimetallic NPs on angiogenesis through in
vitro aortic assay and wound healing experiments.
Materials and methods
Plant collection
E. ang L. was collected during its blossoming
phase in April, 2021, from a cultivated region on Istiqlal Street,
Amman, Jordan. The identification of the plant was verified by
Professor Barakat Abu Irmaileh (Faculty of Agriculture, The
University of Jordan, Amman, Jordan). A voucher specimen (no.
ELEA-1FMJ) was deposited in the Department of Pharmaceutical
Sciences, School of Pharmacy, The University of Jordan. Following
collection, the leaves were passively air-dried in the shade for ~1
week until a consistent weight was observed. Once dried, the leaves
were ground to achieve a fine consistency. This powdered form was
then utilized to prepare the aqueous extract.
Preparation of the aqueous leaf
extract of E. ang L
Leaves of E. ang L. were carefully rinsed
with deionized water, then dried at 30˚C in a contamination-free
setting. A total of ~200 g dried leaves were finely ground into a
powdery consistency using a pestle and mortar. This powdered
material was combined with 500 ml deionized water. The mixture was
subjected to reflux for 4 h in an oil bath at 90˚C. After the
reflux period, the solution was allowed to return to room
temperature and filtered through Whatman No.1 filter paper to
remove any solid residues. The resulting extract of E. ang
L. was used as a reducing agent in NP synthesis.
Synthesis and characterization of
bimetallic ZnO4 NPs (Fe2ZnO4)
The aqueous extract was mixed with an equivalent
volume of 1 mM iron (III) chloride hexahydrate
(FeCl3•6H2O) and 1 mM zinc acetate
[Zn(OAc)2]. This mixture was stirred for ~1 h and was
then exposed to ultrasonic waves in an ultrasonic bath for 30 min
at room temperature. pH of the reaction mixture was maintained at
10-12 using a NaOH solution. NPs were separated by centrifugation
at 7,500 x g for 10-15 min at room temperature, and dried in an
oven at 80˚C for 4 h. NPs were washed multiple times with water
until a neutral pH was achieved, as previously described (24).
Synthesis of bimetallic
Fe2ZnO4 NPs
Metal salt solutions (1 mM) (silver nitrate and zinc
acetate) were combined and heated to 80˚C for ~30 min. Following
this, 50 ml E. ang L. extract was gradually added using a
burette until the total volume reached 90 ml. This blend was
stirred continuously at 80˚C for 1 h. After stirring, the solution
was allowed to settle, leading to the formation of a precipitate.
This precipitate underwent sonication in an ultrasonic chamber for
30 min, as previously described (25). The pH was then adjusted to 10-12
using a NaOH solution. The NPs were isolated by centrifugation at
7,500 x g for 10-15 min at room temperature. NPs were dried in an
oven set at 80˚C for 4 h and the remaining solid was washed several
times with water until a neutral pH was reached, as previously
described (29).
Characterization of bimetallic
NPs
The composition of the particles was confirmed via
Fourier-transform infrared spectroscopy (FTIR) analysis. The
crystallinity of the bimetallic NPs was investigated using x-ray
diffraction (XRD) analysis, covering a range of 25-65˚.
Crystallographic structure of the prepared NPs was
determined by powder X-ray diffraction pattern. The infrared (IR)
spectrum was acquired using a Perkin Elmer RX-FTIR spectrometer
with potassium bromide disc and scanning range of 4,000-300
cm-1.
Cell culture
Cell lines were sourced from the American Type
Culture Collection (ATCC), including the breast cancer cell line
MCF-7 (cat. no. ATCC® HTB-22TM) and a standard dermal fibroblast
cell line (BJ; cat. no. ATCC® CRL-2522). The cell lines were
cultured in RPMI-1640 medium (HyClone; Cytiva) in vented 75
cm2 culture flasks. The medium was supplemented with 10%
(v/v) heat-inactivated fetal bovine serum (Gibco, Thermo Fisher
Scientific) antibiotics (100 U/ml penicillin and 100 g/ml
streptomycin), 2 mM L-glutamine and 25 µM HEPES. These flasks were
kept in an atmosphere of 5% CO2 and 95% air and a set
temperature of 37˚C. All cell experiments were performed in a class
II biological safety cabinet to ensure sterility. MCF-7 breast
cancer and the standard fibroblast cells were seeded in 96-well
plates at densities of 7,000 and 9,000 cells/well, respectively,
and were then incubated at 37˚C overnight adhere to the plate well
surface.
In vitro anti-proliferative and
cytotoxicity assay
The cytotoxicity of bimetallic NPs on MCF-7 and
fibroblast cells was evaluated by MTT assay, as previously
described (30). A total of 7,000
MCF-7 cells were seeded in duplicate in each well of a 96-well
plate and incubated for 24 h at 37˚C. A total of 10 mg/ml
bimetallic E. ang-Fe2ZnO4 and
Fe2ZnO4 NPs and 10 mM aqueous leaf extract of
E. ang L. was dissolved in 1% dimethyl sulfoxide (DMSO).
Seven distinct concentrations, ranging from 200, 100, 50, 25, 12.5,
6.25 and 3.125 µg/ml, were tested. After 72 h incubation at 37˚C,
the viability of the cells was assessed. Doxorubicin was used as a
positive control. Spectrophotometric measurements were employed to
ascertain the antiproliferative activity across varied
concentrations of E. ang-Fe2ZnO4. The
absorbance of these solutions was determined at 570 nm.
Dose-response curves were analyzed by regression analysis using
sigmoidal curves [log(concentration) vs. normalized absorbance].
The half-maximal inhibitory concentration (IC50) was
determined with GraphPad Prism software version 8.0.0 (La Jolla,
CA).
Ex vivo aortic ring assay
Rat aortic ring assay was conducted using eight
adult (8-10 weeks) male Wister albino rats (Rattus
norvegicus; weight, 200-250 g; Animal House Unit, The
University of Jordan). Rats were housed in The University of Jordan
Animal House Unit at 22±1˚C under a 12/12-h light/dark cycle, and
the humidity range was 50-60% with free access to food and water.
Experimental procedures were approved by the scientific research
committee (The Institutional Review Board of the University of
Jordan) at the University of Jordan (approval no. 47-2022). All
experiments were performed according to the Animal (Scientific
Procedure) Act 1986 and International Association for the Study of
Pain guidelines (13). Rats were
euthanized using CO2 overdose followed by decapitation.
A fill rate of 30-70% of the chamber volume/min with CO2
was used to induce rapid unconsciousness with minimal distress to
the animals.
Immediately post-extraction, the aortae were
submerged in a cold sterile 1% PBS solution in a Petri dish. Using
a dissecting microscope for precision, extraneous connective tissue
was removed. The aortae, after cleaning with PBS, were sectioned
into ~1 mm thick rings using a surgical scalpel. These segments
were immersed in cold PBS solution and stored on ice until use.
Each aortic ring was positioned within a 48-well
plate using cold pipette tips. Rings were immersed in 25 µl low
growth factor Matrigel™ (Corning, Inc.) and were placed in pairs.
Following 30 min incubation at 37˚C to allow coagulation, aliquots
of 250 µl, with concentrations 100, 50, 25, 12.5, 6.25 and 3.125
µg/ml, of the three different extracts diluted in RPMI-1640 were
added. For the control group, DMSO was used at a concentration of
100 µg/ml. The plate was then incubated at 37˚C. On day 4, medium
was refreshed. On day 5, images were captured using an inverted
light microscope at 4x magnification.
The angiogenic response was quantified by measuring
the extension of blood vessels from the primary ring explants using
ImageJ software1.29 (National Institutes of Health). For each ring,
≥35 comparable structures, evenly spaced around the ring, were
measured (26).
Data were analyzed with GraphPad Prism 8 software.
The elongation distance of the emerging vessels for each ring was
recorded as the mean of percentage inhibition relative to the
unaltered control (n=2) (27).
Vessel lengths were expressed in arbitrary units and the inhibition
of vessel formation was as follows: Angiogenic
inhibition=[1-(A0/A)] x100, where A0 represents vessel growth
distance in treated rings and A signifies the vessel growth
distance in control rings.
Treatment of MCF-7 with E.
ang-Fe2ZnO4 and Fe2ZnO4
NPs and organic extract
A total of ~1x105 MCF-7 cells were seeded
in a 24-well plate and incubated overnight at 37˚C in 5%
CO2 to ensure adhesion. A 10 mg/ml stock solution of
E. ang-Fe2ZnO4 and
Fe2ZnO4 NPs and organic extract was then
prepared using DMSO. Serial dilutions were made in RPMI-1640
(HyClone; Cytiva) complete media at 200.00, 100.00, 50.00, 25.00,
12.50 and 6.25 µg/ml E. ang-Fe2ZnO4
NPs and organic extract. For Fe2ZnO4 NPs,
dilutions were made to 200, 100, 50 and 25 µg/ml based on
cytotoxicity and anti-angiogenic effects. DMSO concentration was
<1% for all treatments. Quadruplicate samples were used for
every concentration. Control wells, containing only the complete
medium, were also included in each assay. Following 72 h incubation
at 37˚C with the treatments, conditioned media was harvested and
stored at -80˚C until use.
Measurement of VEGF concentration
using ELISA
Secretion levels of VEGF in conditioned media from
both treated and control (untreated) cells were quantified by
ELISA, as previously described (31). Human VEGF (cat. no. #DY293B05;
R&D Systems, Inc.) ELISA kit was used to measure the
concentration of VEGF secreted in conditioned media, following the
manufacturer's protocol. Concentrations were calculated using
Microsoft Excel software (Microsoft Office professional plus 2016)
using the standard curve equation and further analyzed using Prism
8 statistical analysis software (GraphPad Software, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software, version 8.0.0. IC50 values for each tested NP were
derived by fitting the observed data to a logarithmic trend line
depicted on the cytotoxicity graphs (log concentration against
inhibition percentage). Data are presented as the mean ± SEM. Each
experiment was performed in triplicate. To assess number and length
of blood vessels, images were analyzed using the ImageJ 1.29
(National Institutes of Health). Data were analyzed using one-way
ANOVA followed by Bonferroni's multiple comparisons post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
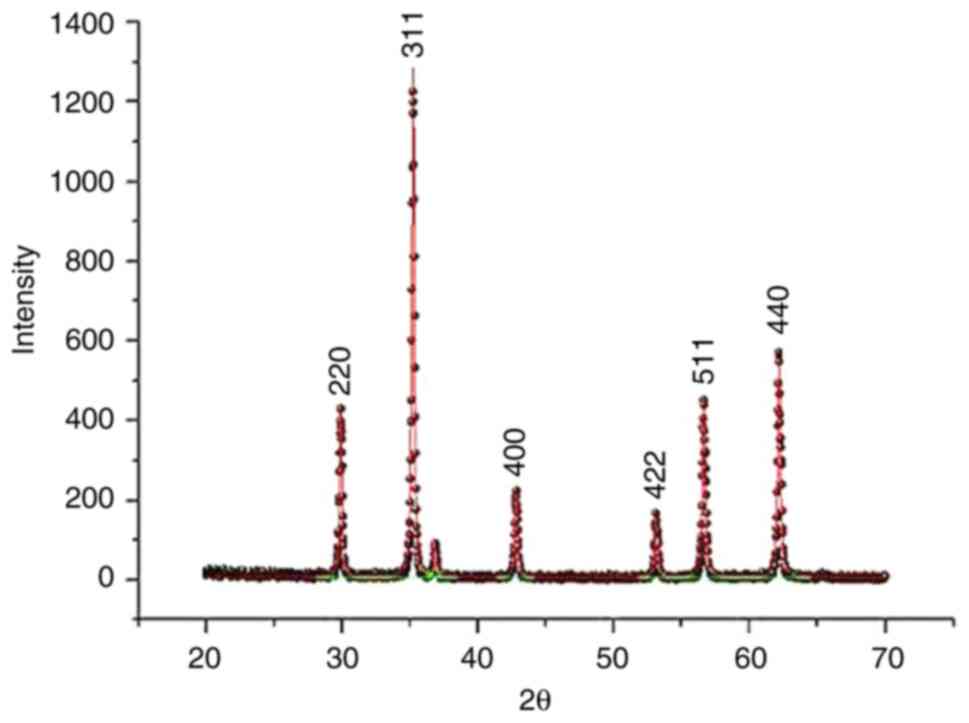
To characterize synthesized NPs, XRD, FTIR
spectroscopy and ζ potential measurements were performed. Formation
of the bimetallic ZnO NPs was confirmed by the XRD patterns
(Fig. 1). Distinct peaks consistent
with the standard data for the spinel (Franklinite) phase (ICSD
card no. 30,860) were demonstrated (8). Furthermore, the characterization data
obtained for spinel Fe2ZnO4 were consistent
with previously documented XRD pattern for NPs (29), exhibiting mean particle size of 18
nm (Fig. 1).
Structure of E.
ang-Fe2ZnO4 was determined by the X-ray
diffraction pattern. Fe2ZnO4 NPs had mean
particle size of 90.22 nm. A lattice constant value of 299.96 and a
corresponding d-spacing value of 90.22 nm were also identified,
indicating the crystalline disposition and intrinsic interplanar
spacing of Fe2ZnO4 NPs (Fig. 1).
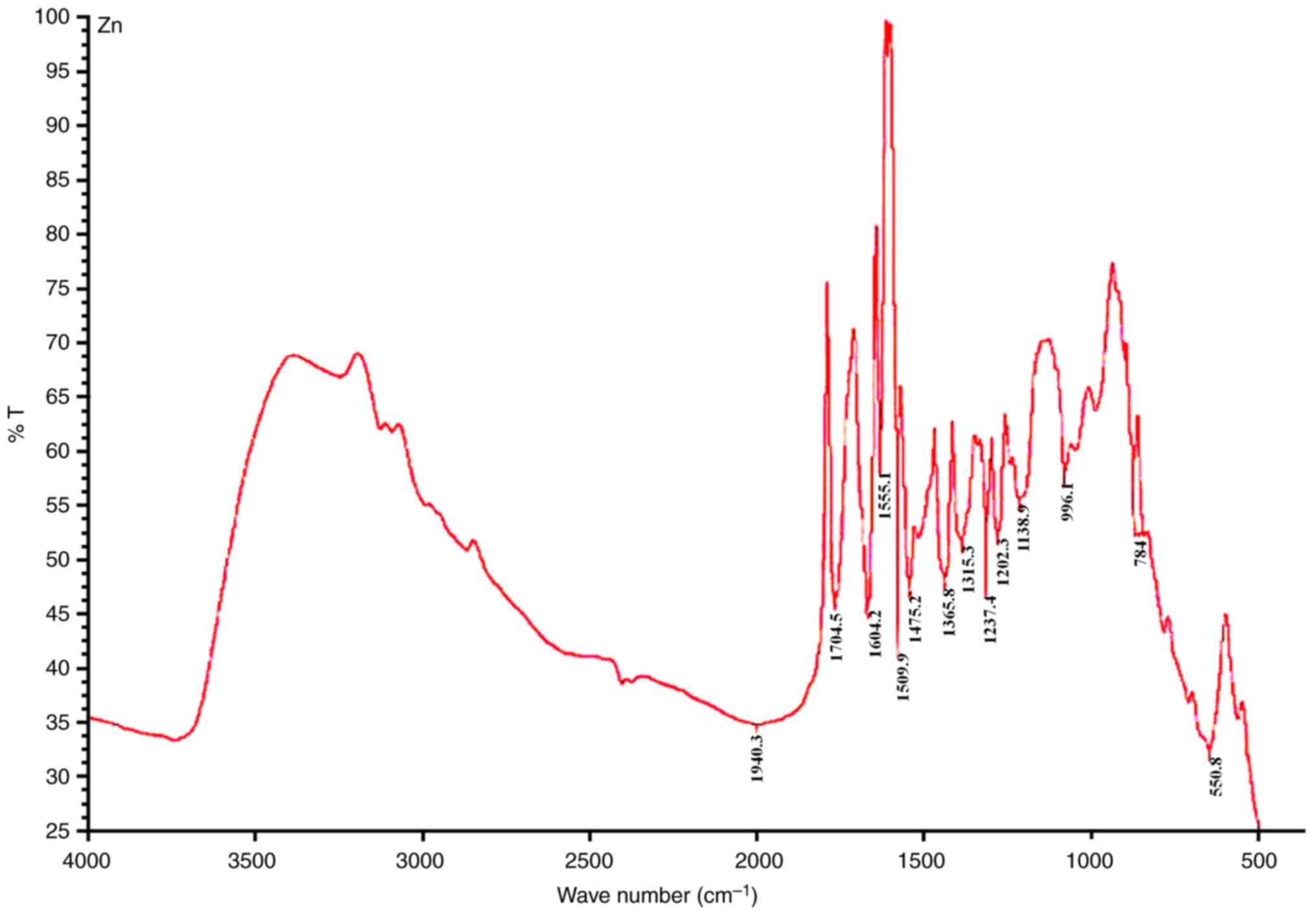
To elucidate potential interactions between
bimetallic ZnO NPs and bioactive constituents of E. ang L. aqueous
leaf extract, FTIR spectrum of the bimetallic NPs was analyzed and
it shows the confrmed the vibrational stretching modes of
metal-oxygen bonds in ZnFe2O4 nanoparticles. (Fig. 2). Additionally, the charging
characteristic of the prepared NPs were inspected by zeta potential
with -21 mV which indicate that the prepared nanoparticles are
likely to exhibit good stability and resist agglomeration.
(Fig. 2).
Anti-proliferative effect and
cytotoxicity of bimetallic E. ang-Fe2ZnO4 and
ZnO NPs
IC50 of E.
ang-Fe2ZnO4 and metallic ZnO NPs and
E. ang L. aqueous leaf extract was evaluated in MCF-7 and
fibroblast cell lines using MTT assay. Cytotoxicity of E.
ang-Fe2ZnO4 NPs and
Fe2ZnO4 NPs in MCF-7 cells were assessed in
comparison with the fibroblast cell line. Further, the cytotoxicity
exhibited by E. ang-Fe2ZnO4 was
determined in both MCF-7 and fibroblast cell lines using
IC50. Fe2ZnO4 NPs had
IC50 values of 3.574 µg/ml for MCF-7 cells and 61.290
µg/ml for fibroblasts (Figs. S1
and S2). Conversely, E.
ang-Fe2ZnO4 NPs demonstrated an augmented
resilience, producing an IC50 value >200 for MCF-7
and 67.15 µg/ml for fibroblasts. The isolated E. ang L.
aqueous extract had IC50 values >200 for MCF-7 and
78.65 µg/ml for fibroblasts. Doxorubicin, a chemotherapeutic agent,
had IC50 values of 0.350±0.025 for MCF-7 and 7.037±2.960
µM for fibroblasts, underscoring its cytotoxic effect.
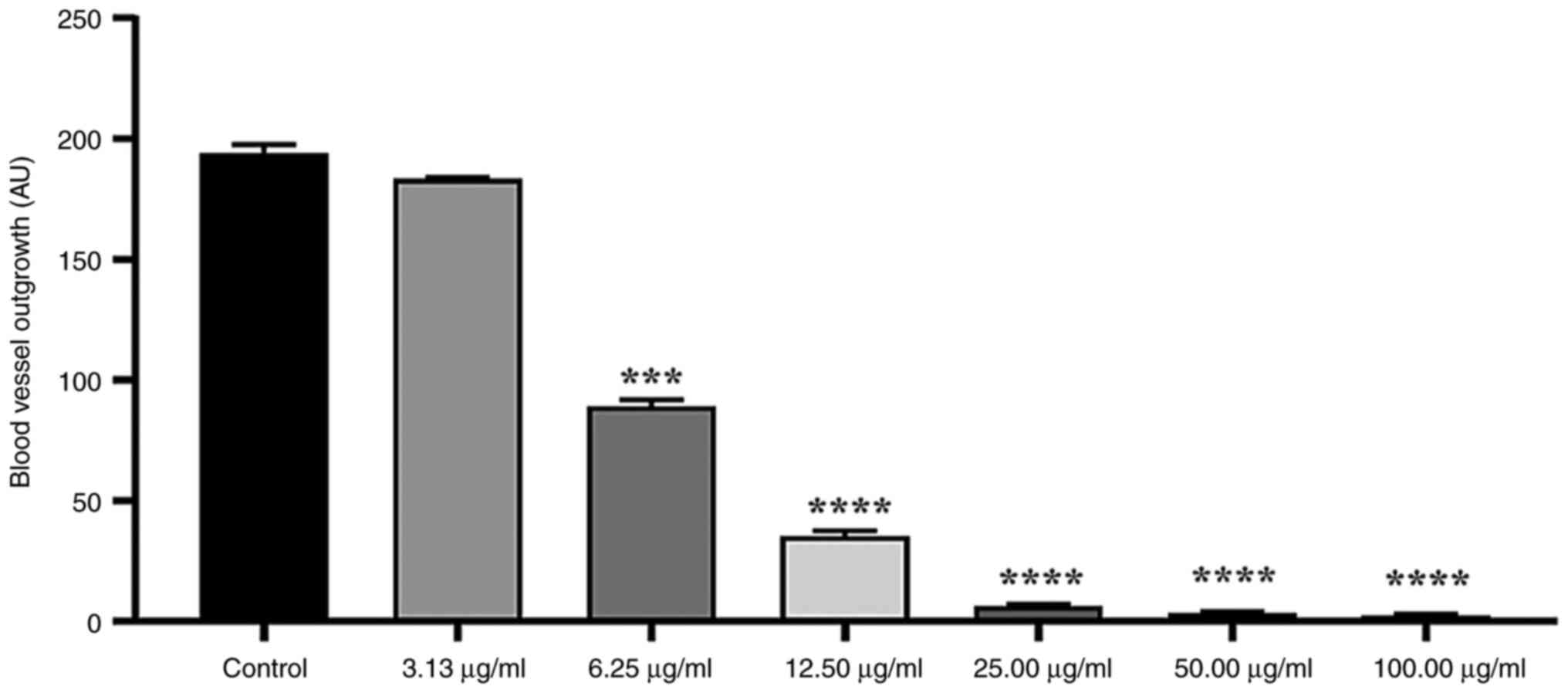
Anti-angiogenic activity
The angiogenic potential of E.
ang-Fe2ZnO4 and
Fe2ZnO4 NPs and the aqueous extract of E.
ang L. leaf was evaluated utilizing the rat aortic ring assay.
Angiogenesis was quantified by measuring the extension of vessels
emanating from the primary ring (Fig.
S3). A significant inhibition of micro-vessel outgrowth from
the aortic rings was observed when treated with E.
ang-Fe2ZnO4 NPs in a dose-dependent
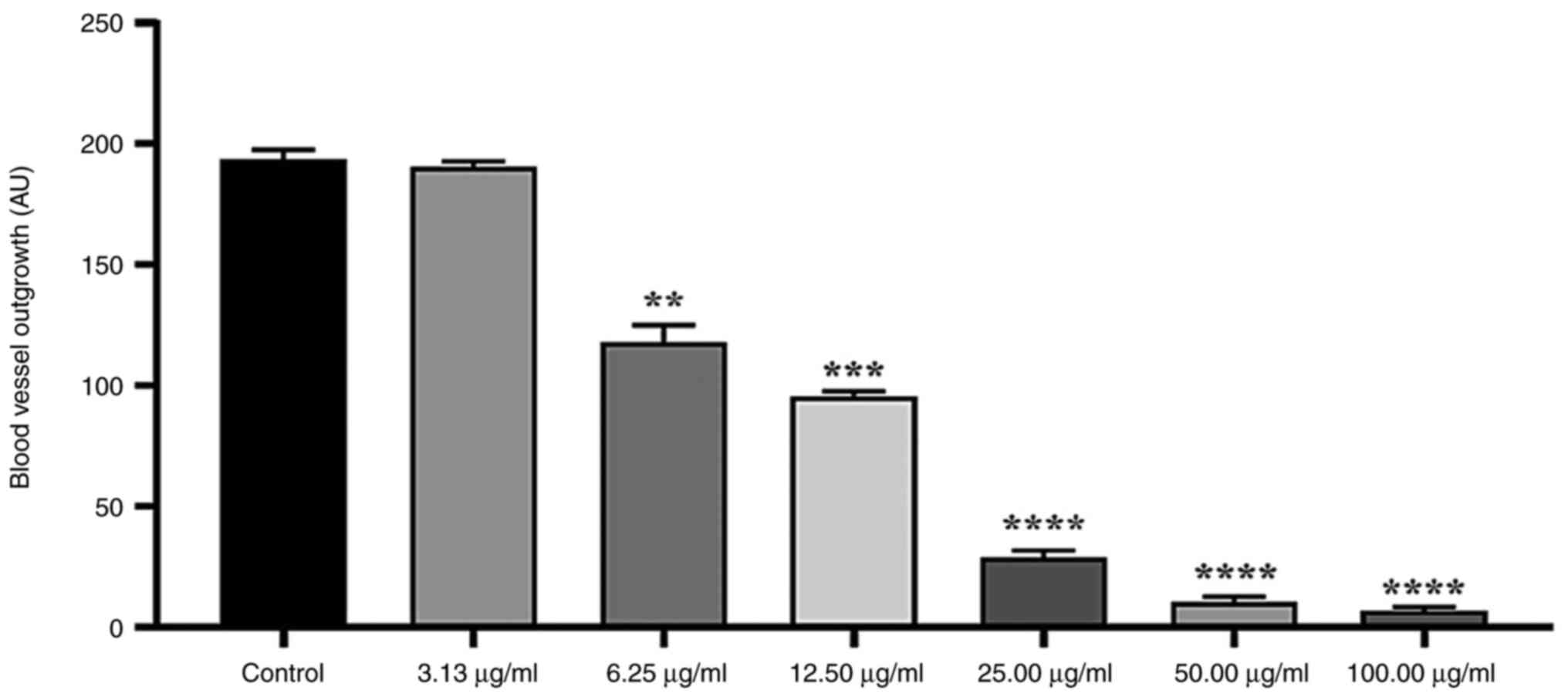
manner (Fig. 3). At a concentration
of 100 µg/ml, the mean inhibition of 98.71±0.36% was noted compared
with the control group. At 50.0, 25.0 and 12.5 µg/ml, the growth of
new blood vessels was significantly decreased by 98.190±0.365,
96.650±10.365 and 81.680±1.090%, respectively. Moreover, the
concentration 6.25 µg/ml significantly decreased the growth of new
blood vessels by 47%±1.075. However, 3.125 µg/ml did not
significantly inhibit micro-vessel outgrowth (5.30±0.36%).
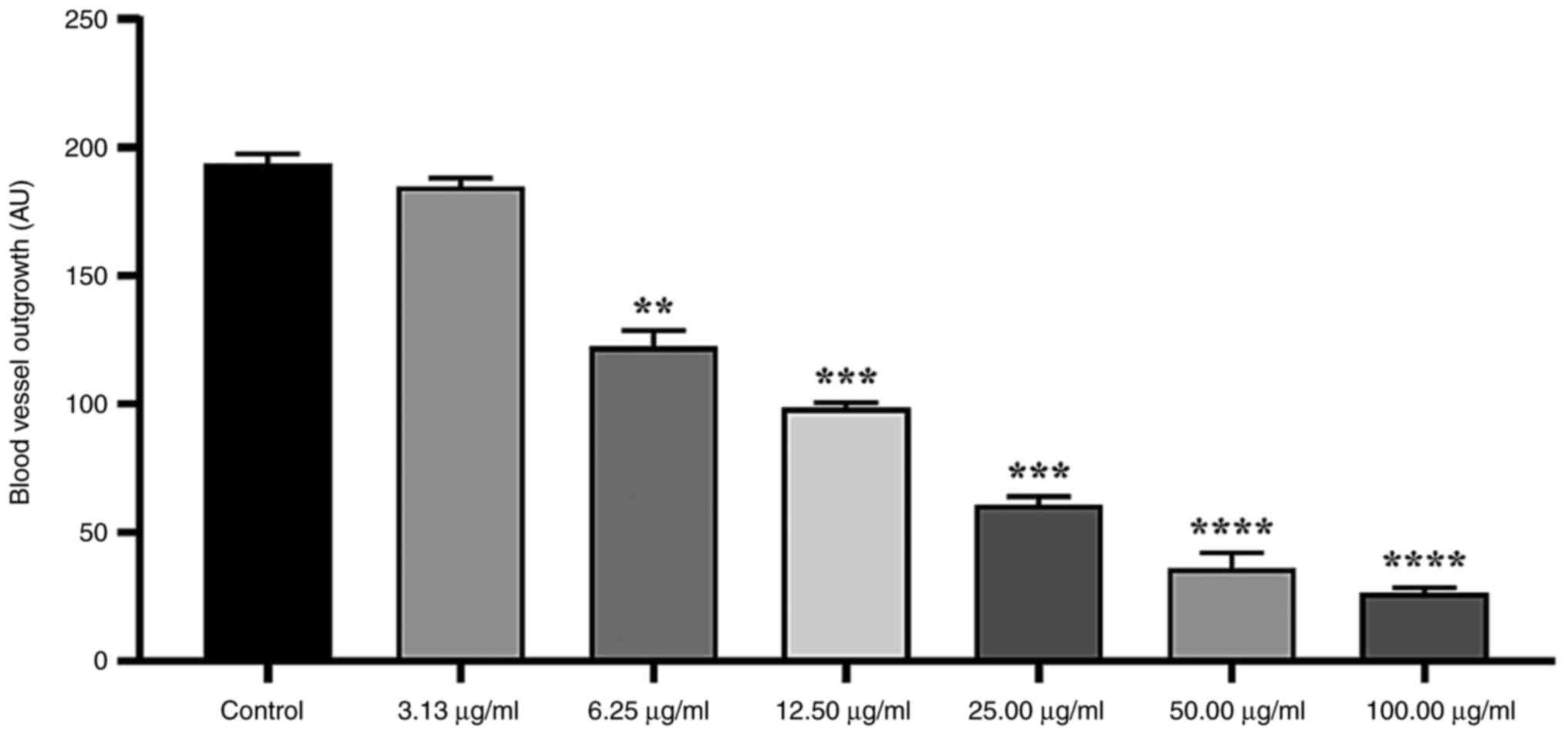
Significant inhibition of micro-vessel outgrowth
from the aortic rings was observed when treated with
Fe2ZnO4 NPs in a dose-dependent manner
(Fig. 4). At 100 µg/ml, a
significant inhibition of 86.32±1.09% was noted in comparison with
the control group. Moreover, concentrations of 50.0, 25.0 and 12.5
µg/ml attenuated the growth of new blood vessels by 81.300±3.102,
67.740±1.094 and 48.640±1.095%, respectively. A concentration of
3.125 µg/ml did not significantly inhibit micro-vessel outgrowth
(3.741±1.095%).
Micro-vessel outgrowth from the aortic rings was
observed to be significantly inhibited following exposure to E.
ang L. extract at 100 µg/ml (96.38±0.73% compared with the
control; Fig. 5). E. ang L.
aqueous extract also significantly decreased micro-vessel outgrowth
by ~94.580±1.090, 85.030±1.450 and 50.710±1.094 at concentrations
of 50.0, 25.0 and 12.5 µg/ml, respectively. At 3.125 µg/ml, E.
ang L. extract resulted in growth inhibition of
1.680±1.095%.
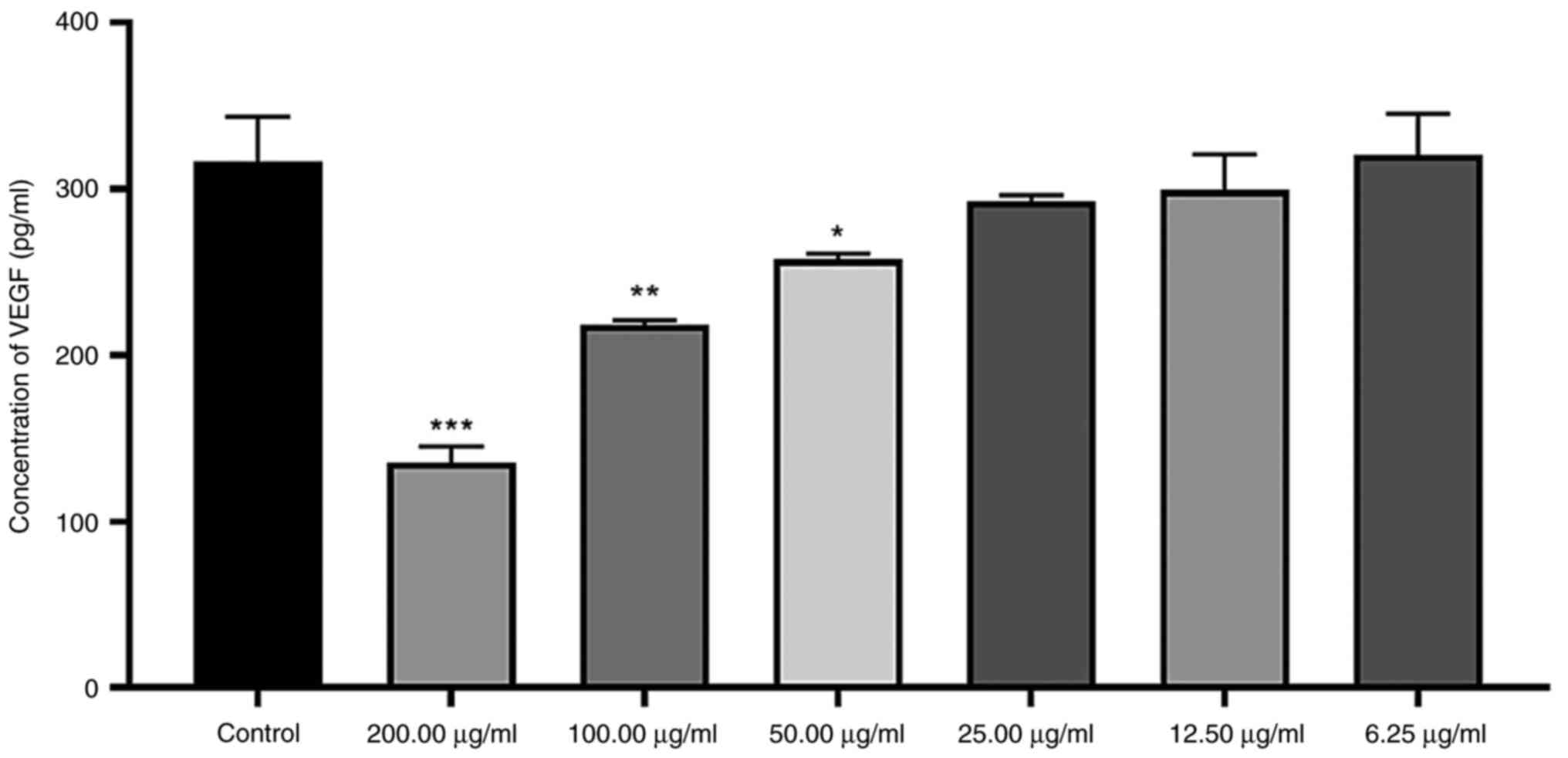
VEGF secretion by MCF-7 cells
The secretion of VEGF was significantly inhibited by
200 µg/ml E. ang-Fe2ZnO4 NP, reducing
to ~0.427 times that of the control. Also, significant 0.690 and
0.814 times suppression of VEGF protein was observed upon treatment
with 50 and 25 µg/ml, respectively; other concentrations showed
insignificant reduction of protein levels of VEGF (Fig. 6).
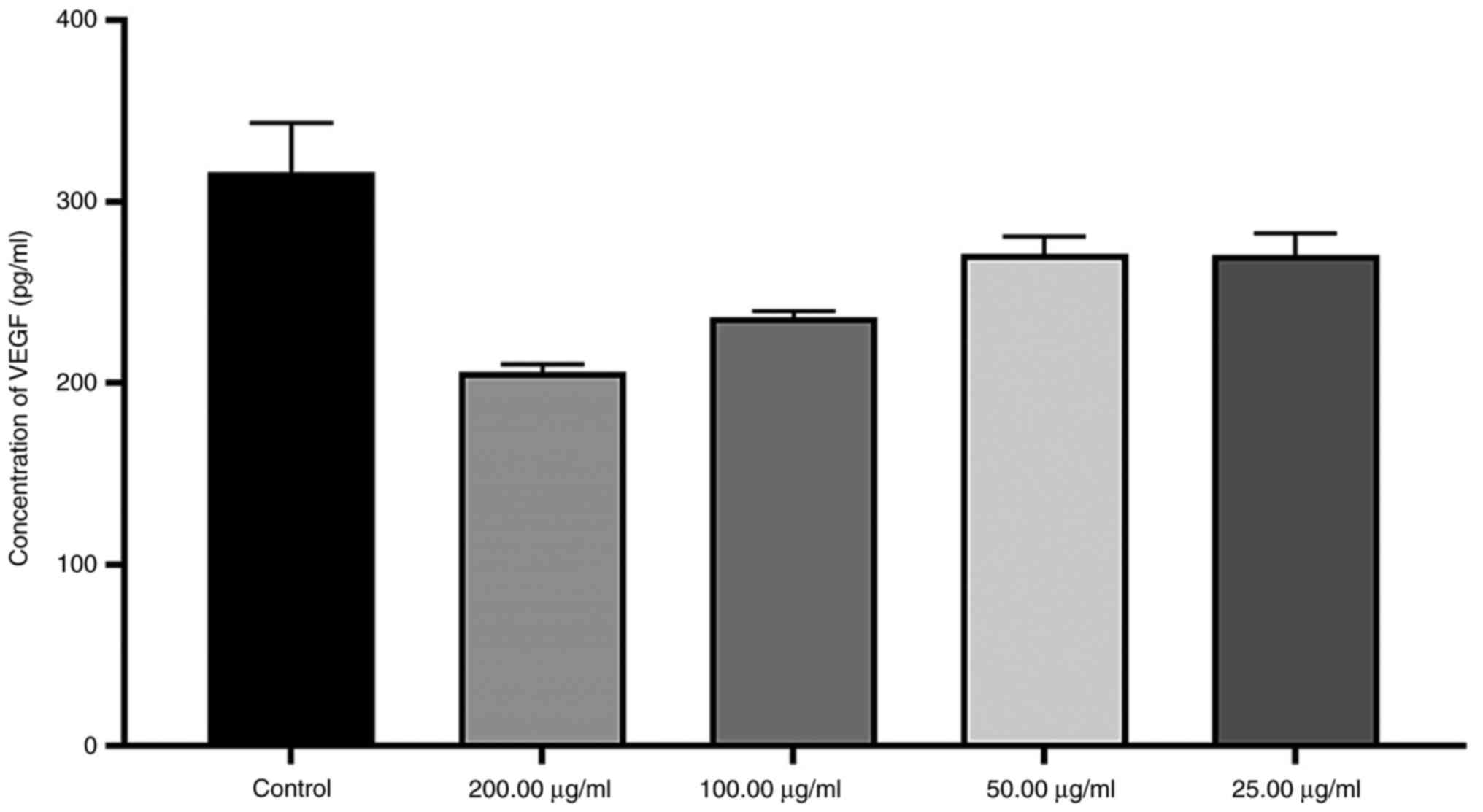
At a concentration of 200 µg/ml, VEGF secretion in
response to Fe2ZnO4 -NPs decreased to ~0.348
times that observed in the control group, but this reduction was
not statistically significant. The other concentrations also did
not cause any significant change in VEGF secretion (Fig. 7).
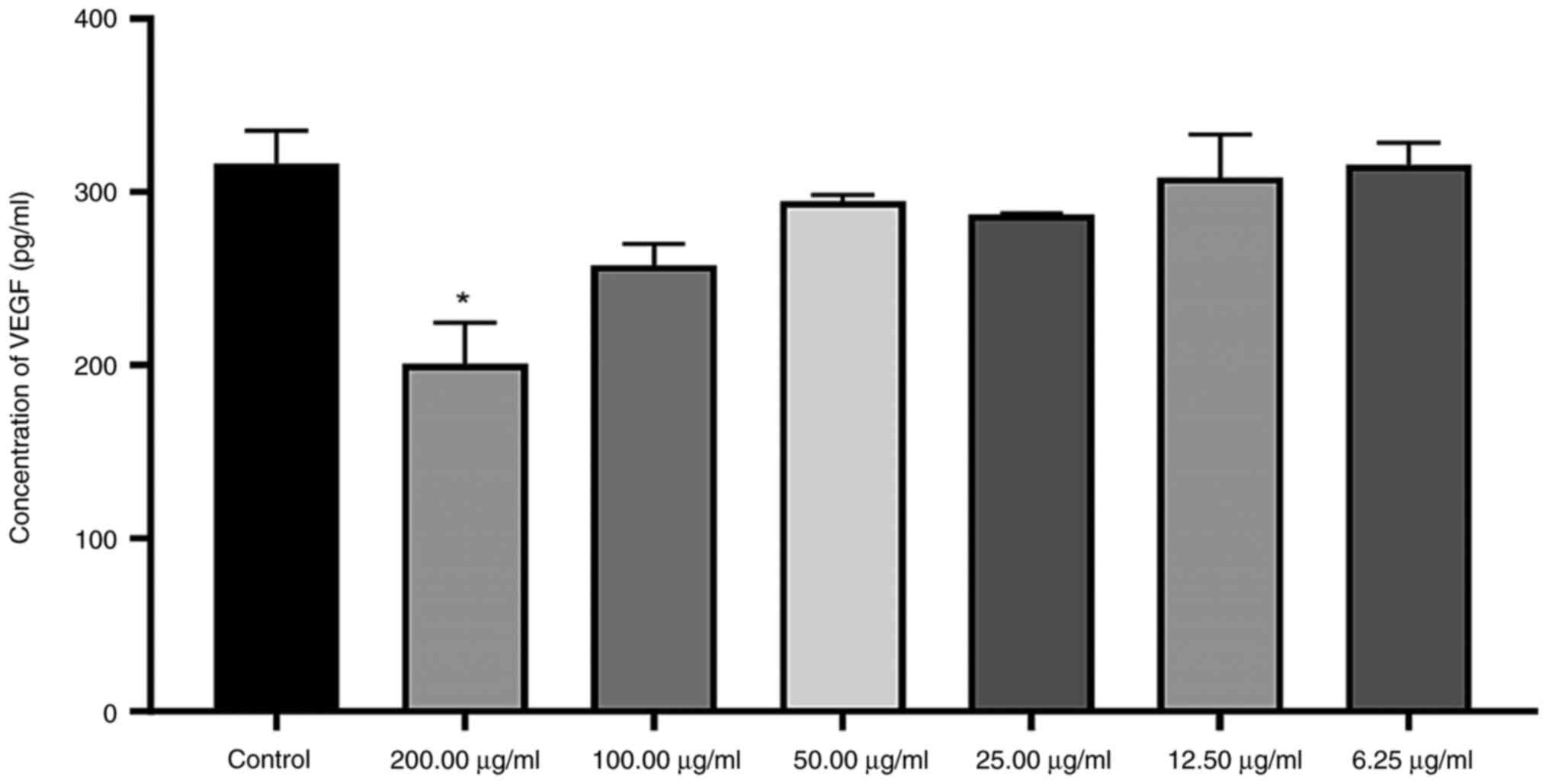
VEGF secretion, upon exposure to 200 µg/ml organic
extract, was observed to be significantly decreased by 0.366 times
relative to the levels in the control. However, no other
concentrations caused a significant decline in VEGF protein levels
(Fig. 8).
Discussion
Research is focused on green synthesis of metallic
NPs, a low-cost, environmentally friendly method that uses natural
organisms as a reduction source to produce safer, more biologically
functional NPs (32). Here,
Fe2ZnO4 bimetallic NPs were phytosynthesized
using an aqueous extract of E. ang. It is hypothesized that
these bimetallic nanoparticles have anti-inflammatory and
antioxidant properties.
X-ray patterns displayed sharp peaks, aligning with
the standard data for all E.
ang-Fe2ZnO4 (29). The mean of crystallite size was
between 5.10 and 114.41 nm (29).
Particle size serves a key role in cellular transport. Smaller
particles more readily penetrate the plasma membrane, making NPs
with diameter <100 nm suitable for various drug delivery
systems. Studies indicate that due to their small size, ZnO NPs can
traverse blood capillaries and interact with multiple cells in
different tissues (21,22).
The present results align with prior research,
demonstrating that green synthesis of bimetallic ZnO NPs via plant
extracts produces stable, nano-sized ZnO NPs (8). The present study investigated the
anti-angiogenesis and cytotoxic effects of E.
ang-Fe2ZnO4 and zinc-iron oxide,
nickel-zinc oxide, copper-zinc oxide, and manganese-zinc oxide NPs.
The cytotoxicity of these NPs and E. ang L. extract on MCF-7
and fibroblast cells was assessed. Bimetallic NPs exhibited notable
cytotoxic effects, while E.
ang-Fe2ZnO4 NPs and E. ang L. leaf
extract did not demonstrate toxicity against MCF-7 cells at the
highest concentration (200 µg/ml). Prior study found low
cytotoxicity of Fe2ZnO4 coated with
Boswellia carteri resin against Raw 264.7 macrophage cells
(29). A previous study also
highlighted the anticancer potential of green-synthesized silver
NPs derived from Fagonia indica extract against MCF-7 cells
(33). Similarly, at concentrations
>175 µg/ml, green-synthesized ZnO NPs decrease human hepatocyte
(HepG2) cell viability to <40%; at the maximum concentration
(2,800 µg/ml), >95% of the cells die, in addition to
anti-angiogenic effects demonstrated by CAM assay (32).
The present findings suggest that green NP
synthesis, may provide a protective effect against cell toxicity by
masking metallic NPs, which results in lower cytotoxic effect on
cells (34).
Angiogenesis, a key physiological process
responsible for novel blood vessel formation, is vital in embryonic
development, ovulation (10) and
wound healing (3). Two primary
methods for modulating angiogenesis exist: Direct and indirect
pathways. The direct pathway involves modulating the ability of
vascular endothelial cells to proliferate, migrate and respond to
angiogenic factors such as VEGF. The indirect pathway is based on
the capacity to affect the expression and activity of angiogenic
factors that induce angiogenesis. This includes controlling
expression of receptors on endothelial cells such as tyrosine
kinase receptor IGF-IR and chemokine receptor CCR7(3).
The present study investigated anti-angiogenic
potential via rat aortic ring assay. Bimetallic
Fe2ZnO4 and E.
ang-Fe2ZnO4 NPs and E. ang L.
extract exhibited high inhibitory activity at 100 µg/ml, resulting
in >85% mean inhibition. E.
ang-Fe2ZnO4 NPs continued to show a
significant inhibitory activity at 6.25 µg/ml with 39.10±3.65%
vessel outgrowth inhibition. By comparison with
Fe2ZnO4 NPs and the extract of E. ang
L., E. ang-Fe2ZnO4 NPs significantly
inhibited growth of vessels, indicating an additive effect for
green synthesis of Fe2ZnO4 NPs.
Anti-angiogenic properties of green-synthesized ZnO
NPs generated using extract of Hyssops officinalis L. have
been investigated via CAM assay and show considerable decrease in
the number and length of blood vessels and suppression of vessel
formation by inducing death in endothelial cells (4). In vivo and in vitro
assays have been used to identify angiogenic activators and
inhibitors (10,14). Typically, CAM and aortic ring in
vivo assays are used to investigate angiogenic activity. Rat
aorta rings offer a sensitive assay for the investigation of
angiogenic activators and inhibitors. E.
ang-Fe2ZnO4 NPs had a significant
inhibitory activity on VEGF expression in MCF-7 cells. Bimetallic
Fe2ZnO4-NPs and E. ang L. extract
showed inhibitory effects at 200 µg/ml, while lower concentrations
elevated the secretion of VEGF compared with E.
ang-Fe2ZnO4 NPs. It was hypothesized that
E. ang-Fe2ZnO4 had an additive impact
in reducing VEGF secretion. These data are consistent with a
previous study that demonstrated that ZnO NPs suppress the
expression of genes encoding VEGF and VEGF receptor (35).
Nevertheless, the present study has limitations
that must be addressed. The present study only investigated a
limited number of these angiogenesis biomarkers; the effect of
E. ang water extract on different angiogenesis biomarkers
should be assessed in the future, including the cell cycle
regulators.
The present current investigation used leaf extract
of E. ang L. for the green synthesis of bimetallic zinc
oxide NPs, which is a method that is both environmentally friendly
and cost-effective. The synthesized NPs, particularly E.
ang-Fe2ZnO4 NPs, exhibited potent
anti-angiogenic and cytotoxic effects against MCF-7 breast cancer
cells, while maintaining minimal toxicity toward healthy cells.
These findings suggest NPs are promising candidates for anti-cancer
therapy, especially in targeting angiogenesis. Bimetallic NPs
require in vivo validation to ensure their efficacy and
safety for potential clinical applications. Furthermore,
standardization of E. ang aqueous extract should be
performed to obtain uniform products for experimental testing, with
phytochemical analyses to isolate and determine the quantity of the
most potent active ingredients.
Supplementary Material
Inhibitory effect of E.
ang-Fe2ZnO4 and Fe2ZnO4 nanoparticles and E. ang L.
aqueous extract in MCF7 cancer cell line. Cell viability was
determined by MTT assay relative to untreated cells. Data is the
mean ± SEM. E. lang, Elaeagnus angustifolia
L.
Inhibitory effect of E.
ang-Fe2ZnO4 and Fe2ZnO4 nanoparticles and E. ang L.
aqueous extract in fibroblast cell line. Cell viability was
determined by MTT assay relative to untreated cells. Data are
presented as the mean ± SEM E. lang, Elaeagnus
angustifolia L.
Representative images of the
inhibitory effect of E. ang-Fe2ZnO4 NPs on angiogenesis in
the rat aortic ring assay. Rings were treated with (A) DMSO or (B)
100.000, (C) 50.000, (D) 25.000, (E) 12.50 and (F) 6.250
μg/ml E. ang-Fe2ZnO4 NPs. Magnification, x4. E.
ang, Elaeagnus angustifolia L.; NP, nanoparticle.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Deanship of Academic
Research at the University of Jordan (grant no. 19/2021/528).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
AI and AAZ conceived the study, designed and
performed the experiments, analyzed data and wrote the manuscript.
TAT designed and performed the experiments and wrote the
manuscript. WAA designed the experiments and wrote the manuscript.
HAA designed the experiments and analyzed data. MZ analyzed data.
AA designed and performed the experiments, analyzed data and wrote
the manuscript. AI and AA confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Animal Ethics Committee at the University of Jordan (approval no.
47-2022; Amman, Jordan).
Patient consent for publication
Not applicable.
Authors' information
Asma' Al-Zabin, orcid.org/0009-0008-4795-4019 Amer Imraish,
orcid.org/0000-0003-1191-2905 Malik
Zihlif, orcid.org/0000-0002-8005-3908 Tuqa Abu Thiab
orcid.org/0000-0002-3054-4047 Wajdy
Al-Awaida, orcid.org/0000-0003-3095-2224 Hamzeh J. Al-Ameer,
orcid.org/0000-0002-1681-6747 Afnan
Al-Hunaiti, orcid.org/0000-0002-0740-1179.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rao MD and Gautam P: Synthesis and
characterization of ZnO nanoflowers using Chlamydomonas
reinhardtii: A green approach. Environmental Progress &
Sustainable Energy. 35:1020–1026. 2016.
|
|
2
|
Khan S, Mansoor S, Rafi Z, Kumari B,
Shoaib A, Saeed M, Alshehri S, Ghoneim MM, Rahamathulla M, Hani U
and Shakeel F: A review on nanotechnology: Properties,
applications, and mechanistic insights of cellular uptake
mechanisms. J Mol Liquids. 348(118008)2022.
|
|
3
|
Sogno I, Venè R, Ferrari N, De Censi A,
Imperatori A, Noonan DM, Tosetti F and Albini A: Angioprevention
with fenretinide: Targeting angiogenesis in prevention and
therapeutic strategies. Crit Rev Oncol Hematol. 75:2–14.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mohammad GRKS, Tabrizi MH, Ardalan T,
Yadamani S and Safavi E: Green synthesis of zinc oxide
nanoparticles and evaluation of anti-angiogenesis,
anti-inflammatory and cytotoxicity properties. J Biosci.
44(30)2019.PubMed/NCBI
|
|
5
|
Adeola FO: Global impact of chemicals and
toxic substances on human health and the environment. Handbook of
Global Health, 2020: p. 1-30.
|
|
6
|
Vimalraj S, Ashokkumar T and Saravanan S:
Biogenic gold nanoparticles synthesis mediated by Mangifera indica
seed aqueous extracts exhibits antibacterial, anticancer and
anti-angiogenic properties. Biomed Pharmacother. 105:440–448.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Imade EE, Ajiboye TO, Fadiji AE, Onwudiwe
DC and Babalola OO: Green synthesis of zinc oxide nanoparticles
using plantain peel extracts and the evaluation of their
antibacterial activity. Sci African. 16(e01152)2022.
|
|
8
|
Mohammadian M, Es'haghi Z and Hooshmand S:
Green and chemical synthesis of zinc oxide nanoparticles and size
evaluation by UV-vis spectroscopy. J Nanomed Res. 7(00175)2018.
|
|
9
|
Sukhanova A, Bozrova S, Sokolov P,
Berestovoy M, Karaulov A and Nabiev I: Dependence of nanoparticle
toxicity on their physical and chemical properties. Nanoscale Res
Lett. 13(44)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 (6 Suppl 16):S15–S18.
2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lacerda JZ, Ferreira LC, Lopes BC,
Aristizábal-Pachón AF, Bajgelman MC, Borin TF and Zuccari DAPC:
Therapeutic potential of melatonin in the regulation of MiR-148a-3p
and angiogenic factors in breast cancer. Microrna. 8:237–247.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
13
|
Tahvilian R, Zangeneh MM, Falahi H,
Sadrjavadi K, Jalalvand AR and Zangeneh A: Green synthesis and
chemical characterization of copper nanoparticles using Allium
saralicum leaves and assessment of their cytotoxicity, antioxidant,
antimicrobial, and cutaneous wound healing properties. Applied
Organometallic chemistry. 33(e5234)2019.
|
|
14
|
Shadmehri AA, Namvar F, Miri H, Yaghmaei P
and Moghaddam MN: Anti-Angiogenesis effect of graphene-loaded green
synthesized zinc oxide nanoparticles on chick chorioalantoic
membrane. J Biotechem Tec. 17-22:2018.
|
|
15
|
Cui L, Liang J, Liu H, Zhang K and Li J:
Nanomaterials for angiogenesis in skin tissue engineering. Tissue
Eng Part B Rev. 26:203–216. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ahtzaz S, Nasir M, Shahzadi L, Amir W,
Anjum A, Iqbal F, Chaudhry AA, Yar M and ur Rehman I: A study on
the effect of zinc oxide and zinc peroxide nanoparticles to enhance
angiogenesis-pro-angiogenic grafts for tissue regeneration
applications. Materials & Design. 132:409–418. 2017.
|
|
17
|
Newman DJ, Cragg GM and Snader KM: Natural
products as sources of new drugs over the period 1981-2002. J Nat
Prod. 66:1022–1037. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
El-Shafey ES and Elsherbiny ES: The role
of apoptosis and autophagy in the insulin-enhancing activity of
oxovanadium (IV) bipyridine complex in streptozotocin-induced
diabetic mice. Biometals. 33:123–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
El Seedy GM, El-Shafey ES and Elsherbiny
ES: Ziziphus spina-christi (L.) fortified with Camellia sinensis
mediates apoptosis, Notch-1 signaling, and mitigates
obesity-induced non-alcoholic fatty liver. J Food Biochem: Jul 9,
2021 (Epub ahead of print).
|
|
20
|
Sahan Y, Dundar AN, Aydin E, Kilci A,
Dulger D, Kaplan FB, Gocmen D and Celik G: Characteristics of
cookies supplemented with oleaster (Elaeagnus angustifolia L.)
Flour. I physicochemical, sensorial and textural properties.
Journal of Agricultural Science, 2013. 5: 160, 2013.
|
|
21
|
Ogunyemi SO, Abdallah Y, Zhang M, Fouad H,
Hong X, Ibrahim E, Masum MMI, Hossain A, Mo J and Li B: Green
synthesis of zinc oxide nanoparticles using different plant
extracts and their antibacterial activity against Xanthomonas
oryzae pv. oryzae. Artif Cells Nanomed Biotechnol. 47:341–352.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choi HS, Ashitate Y, Lee JH, Kim SH,
Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV and
Tsuda A: Rapid translocation of nanoparticles from the lung
airspaces to the body. Nat Biotechnol. 28:1300–1303.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gürbüz I, Ustün O, Yesilada E, Sezik E and
Kutsal O: Anti-ulcerogenic activity of some plants used as folk
remedy in Turkey. J Ethnopharmacol. 88:93–97. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chakraborty AJ, Mitra S, Tallei TE, Tareq
AM, Nainu F, Cicia D, Dhama K, Emran TB, Simal-Gandara J and
Capasso R: Bromelain a potential bioactive compound: A
comprehensive overview from a pharmacological perspective. Life
(Basel). 11(317)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yanez M, Blanchette J and Jabbarzadeh E:
Modulation of inflammatory response to implanted biomaterials using
natural compounds. Curr Pharm Des. 23:6347–6357. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Y, Guo T, Li JY, Zhou SZ, Zhao P and
Fan MT: Four flavonoid glycosides from the pulps of Elaeagnus
angustifolia and their antioxidant activities. Adv Mater Res.
756-759:16–20. 2013.
|
|
27
|
Ahmadiani A, Hosseiny J, Semnanian S,
Javan M, Saeedi F, Kamalinejad M and Saremi S: Antinociceptive and
anti-inflammatory effects of Elaeagnus angustifolia fruit extract.
J Ethnopharmacol. 72:287–292. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mohammed FI, Al-Essa MK, Shafagoj YA and
Afifi FU: Investigation of the direct effects of the alcoholic
extract of Elaeagnus angustifolia L.(Elaeagnaceae) on dispersed
intestinal smooth muscle cells of guinea pig. Sci Pharm. 74:21–30.
2006.
|
|
29
|
Imraish A, Abu Thiab T, Al-Awaida W,
Al-Ameer HJ, Bustanji Y, Hammad H, Alsharif M and Al-Hunaiti A: In
vitro anti-inflammatory and antioxidant activities of
ZnFe2O4 and CrFe2O4
nanoparticles synthesized using Boswellia carteri resin. J Food
Biochem. 45(e13730)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Scherbakov AM, Vorontsova SK, Khamidullina
AI, Mrdjanovic J, Andreeva OE, Bogdanov FB, Salnikova DI, Jurisic
V, Zavarzin IV and Shirinian VZ: Novel pentacyclic derivatives and
benzylidenes of the progesterone series cause anti-estrogenic and
antiproliferative effects and induce apoptosis in breast cancer
cells. Invest New Drugs. 41:142–152. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sanaeimehr Z, Javadi I and Namvar F:
Antiangiogenic and antiapoptotic effects of green-synthesized zinc
oxide nanoparticles using Sargassum muticum algae extraction.
Cancer Nanotechnol. 9(3)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abdalla AME, Xiao L, Ullah MW, Yu M,
Ouyang C and Yang G: Current challenges of cancer anti-angiogenic
therapy and the promise of nanotherapeutics. Theranostics.
8:533–548. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kumar B, Smita K, Cumbal L and Debut A:
Green approach for fabrication and applications of zinc oxide
nanoparticles. Bioinorg Chem Appl. 2014(523869)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tada-Oikawa S, Ichihara G, Suzuki Y,
Izuoka K, Wu W, Yamada Y, Mishima T and Ichihara S: Zn (II)
released from zinc oxide nano/micro particles suppresses
vasculogenesis in human endothelial colony-forming cells. Toxicol
Rep. 2:692–701. 2015.PubMed/NCBI View Article : Google Scholar
|