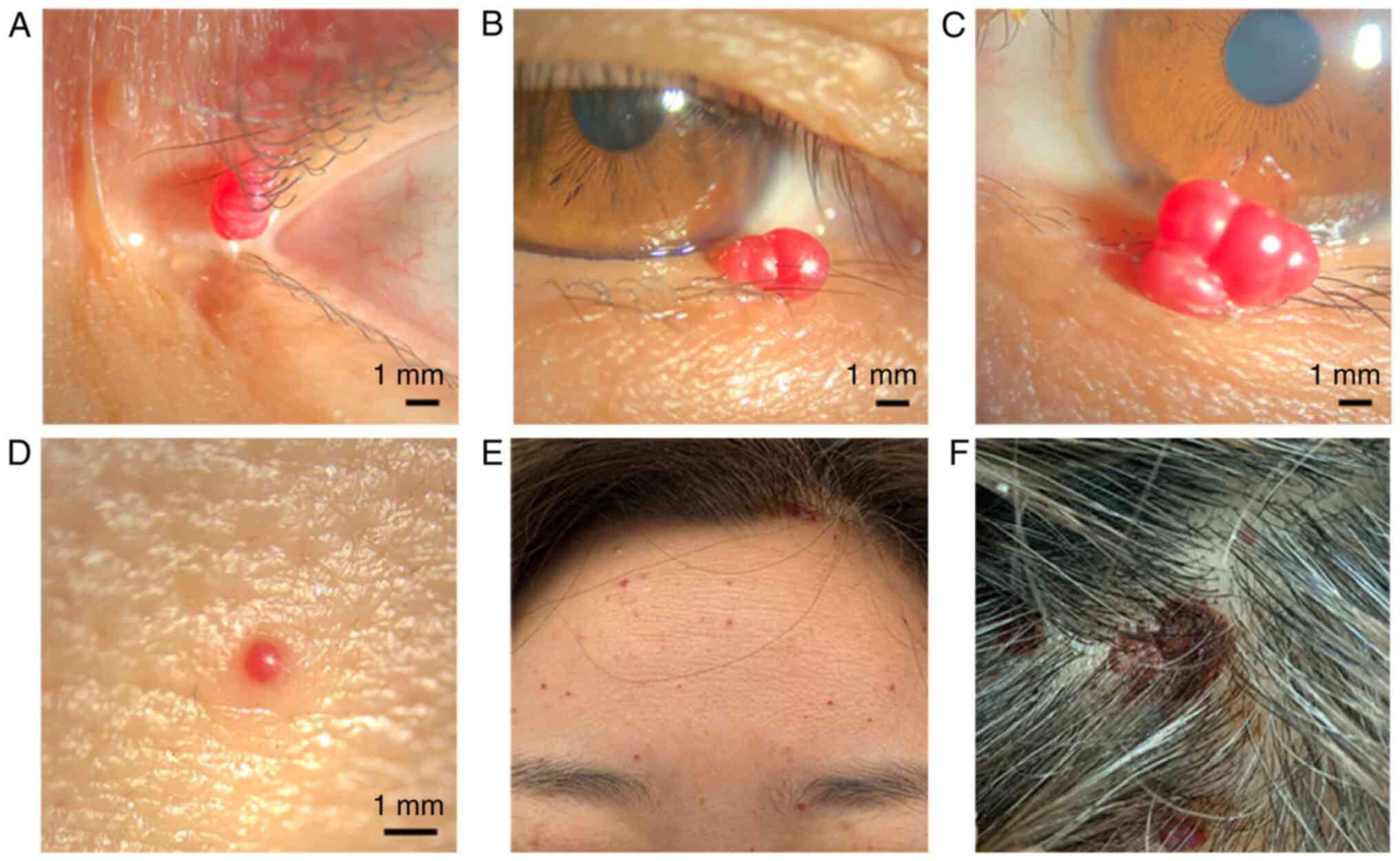
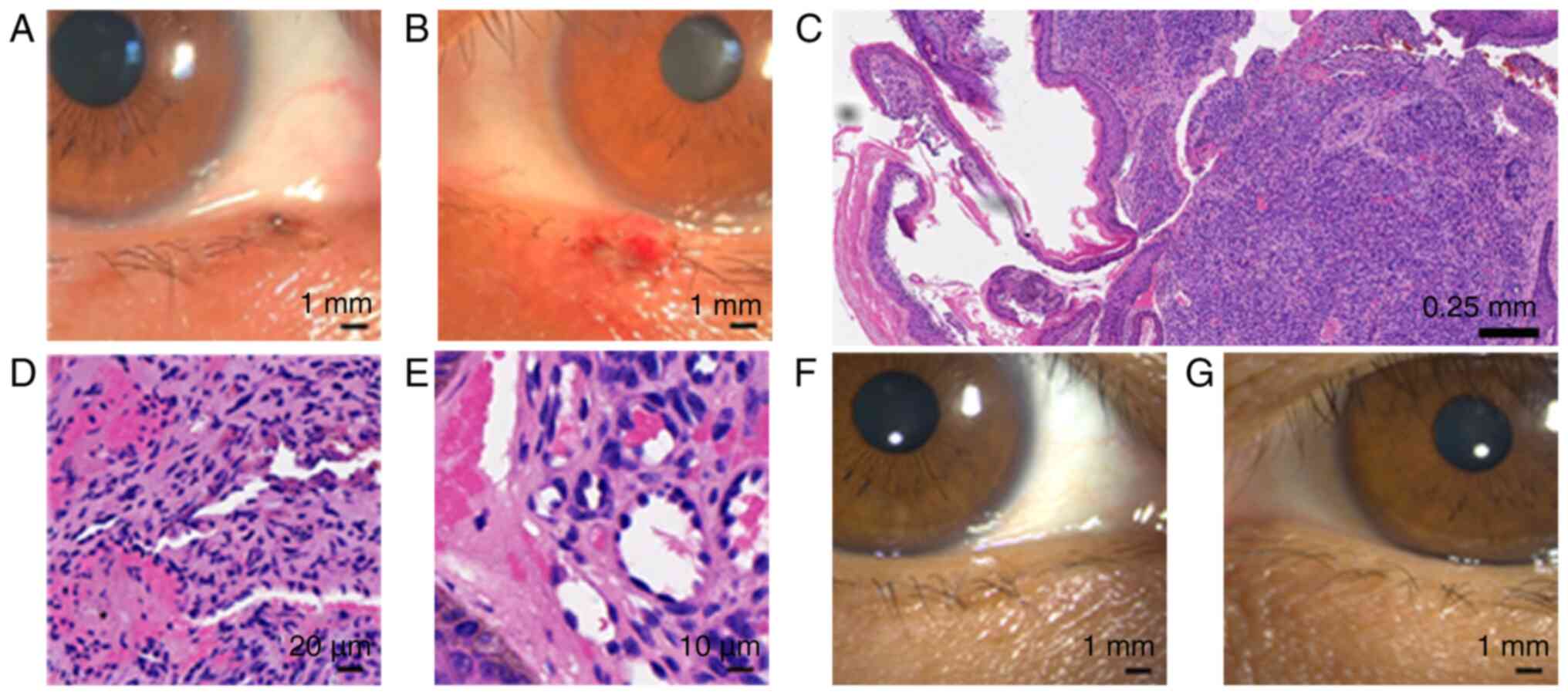
|
1
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang G, Liu S, Dong J, Xi X, Kong R, Li W
and Du Q: PD-1 inhibitor-based adverse events in solid tumors: A
retrospective real-world study. Front Pharmacol.
13(974376)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Villadolid J and Amin A: Immune checkpoint
inhibitors in clinical practice: Update on management of
immune-related toxicities. Transl Lung Cancer Res. 4:560–575.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7(306)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moslehi JJ, Salem JE, Sosman JA,
Lebrun-Vignes B and Johnson DB: Increased reporting of fatal immune
checkpoint inhibitor-associated myocarditis. Lancet.
391(933)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mahmood SS, Fradley MG, Cohen JV, Nohria
A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R,
Chen CL, Gupta D, et al: Myocarditis in patients treated with
immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–1764.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ma K, Lu Y, Jiang S, Tang J, Li X and
Zhang Y: The relative risk and incidence of immune checkpoint
inhibitors related pneumonitis in patients with advanced cancer: A
meta-analysis. Front Pharmacol. 9(1430)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Markham A and Keam SJ: Camrelizumab: First
global approval. Drugs. 79:1355–1361. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mo H, Huang J, Xu J, Chen X, Wu D, Qu D,
Wang X, Lan B, Wang X, Xu J, et al: Safety, anti-tumour activity,
and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody
in advanced solid tumours: A dose-escalation, phase 1 study. Br J
Cancer. 119:538–545. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Wang C, Li X, Dong L, Yang Q, Chen
M, Shi F, Brock M, Liu M, Mei Q, et al: Improved clinical outcome
in a randomized phase II study of anti-PD-1 camrelizumab plus
decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother
Cancer. 9(e002347)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J,
Shao G, Zhang Y, Xu L, Yin T, et al: Camrelizumab in combination
with apatinib in patients with advanced hepatocellular carcinoma
(RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer
Res. 27:1003–1011. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu
R, Zhang G, Zhao C, Zhang Y, Chen C, et al: Anti-PD-1 antibody
SHR-1210 combined with apatinib for advanced hepatocellular
carcinoma, gastric, or esophagogastric junction cancer: An
open-label, dose escalation and expansion study. lin Cancer Res.
25:515–523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ren C, Mai ZJ, Jin Y, He MM, Wang ZQ, Luo
HY, Zhang DS, Wu CY, Wang F and Xu RH: Anti-PD-1 antibody SHR-1210
plus apatinib for metastatic colorectal cancer: A prospective,
single-arm, open-label, phase II trial. Am J Cancer Res.
10:2946–2954. 2020.PubMed/NCBI
|
|
18
|
Fang W, Yang Y, Ma Y, Hong S, Lin L, He X,
Xiong J, Li P, Zhao H, Huang Y, et al: Camrelizumab (SHR-1210)
alone or in combination with gemcitabine plus cisplatin for
nasopharyngeal carcinoma: Results from two single-arm, phase 1
trials. Lancet Oncol. 19:1338–1350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie L, Xu J, Sun X, Guo W, Gu J, Liu K,
Zheng B, Ren T, Huang Y, Tang X, et al: Apatinib plus camrelizumab
(anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO)
progressing after chemotherapy: A single-arm, open-label, phase 2
trial. J Immunother Cancer. 8(e000798)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lan C, Shen J, Wang Y, Li J, Liu Z, He M,
Cao X, Ling J, Huang J, Zheng M, et al: Camrelizumab plus apatinib
in patients with advanced cervical cancer (CLAP): A multicenter,
open-label, single-arm, phase II trial. J Clin Oncol. 38:4095–4106.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cohen AC, Roane BM and Leath CA III: Novel
therapeutics for recurrent cervical cancer: Moving towards
personalized therapy. Drugs. 80:217–227. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Wang Y, Tian Z, Lin Y, Li H, Zhu Z,
Liu Q, Su S, Zeng Y, Jia W, et al: Multicenter phase II trial of
camrelizumab combined with apatinib and eribulin in heavily
pretreated patients with advanced triple-negative breast cancer.
Nat Commun. 13(3011)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen X, Ma L, Wang X, Mo H, Wu D, Lan B,
Qu D, Zhang H, Huang J and Xu B: Reactive capillary hemangiomas: A
novel dermatologic toxicity following anti-PD-1 treatment with
SHR-1210. Cancer Biol Med. 16:173–181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang J, Xu B, Mo H, Zhang W, Chen X, Wu
D, Qu D, Wang X, Lan B, Yang B, et al: Safety, activity, and
biomarkers of SHR-1210, an Anti-PD-1 antibody, for patients with
advanced esophageal carcinoma. Clin Cancer Res. 24:1296–1304.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen
J, Fang Y, Duan J, Zhou C, Hu Y, et al: Camrelizumab plus apatinib
in extensive-stage SCLC (PASSION): A multicenter, two-stage, phase
2 trial. J Thorac Oncol. 16:299–309. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Teng Y, Guo R, Sun J, Jiang Y and Liu Y:
Reactive capillary hemangiomas induced by camrelizumab (SHR-1210),
an anti-PD-1 agent. Acta Oncol. 58:388–389. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu B and Sun HC: Camrelizumab: An
investigational agent for hepatocellular carcinoma. Expert Opin
Investig Drugs. 31:337–346. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen
Z, Chai X, Xiong J, Bai Y, Yang L, et al: Reactive cutaneous
capillary endothelial proliferation in advanced hepatocellular
carcinoma patients treated with camrelizumab: Data derived from a
multicenter phase 2 trial. J Hematol Oncol. 13(47)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li K, Chen J, Hu Y, Wang YZ, Shen Y, Chen
G, Peng W, Fang Z, Xia B, Chen X, et al: Neoadjuvant chemotherapy
plus camrelizumab for locally advanced cervical cancer (NACI
study): A multicentre, single-arm, phase 2 trial. Lancet Oncol.
25:76–85. 2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen L, Lucas E, Zhang X, Liu Q, Zhuang Y,
Lin W, Chen H and Zhou F: Programmed death-ligand 1 expression in
human papillomavirus-independent cervical adenocarcinoma and its
prognostic significance. Histopathology. 80:338–347.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Belum VR, Benhuri B, Postow MA, Hellmann
MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD
and Lacouture ME: Characterisation and management of dermatologic
adverse events to agents targeting the PD-1 receptor. Eur J Cancer.
60:12–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou J, Mao Q, Li Y, Li Z, He H, Chen Q
and Liu C: Oral reactive capillary hemangiomas induced by SHR-1210
in the treatment of non-small cell lung cancer: A case report and
literature review. BMC Oral Health. 21(559)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yu Q and Wang WX: Camrelizumab (SHR-1210)
leading to reactive capillary hemangioma in the gingiva: A case
report. World J Clin Cases. 8:624–629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Finlay WJJ, Coleman JE, Edwards JS and
Johnson KS: Anti-PD1 ‘SHR-1210’ aberrantly targets pro-angiogenic
receptors and this polyspecificity can be ablated by paratope
refinement. MAbs. 11:26–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu J, Cao G, Zhang G, Liu S and Shi D:
Nasal alar metastasis of advanced hepatocellular carcinoma
misdiagnosed as reactive cutaneous capillary endothelial
proliferation in a patient treated with camrelizumab and apatinib:
A case report. J Gastrointest Oncol. 14:1643–1649. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xie C, Zhou X, Liang C, Li X, Ge M, Chen
Y, Yin J, Zhu J and Zhong C: Apatinib triggers autophagic and
apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62
signaling in lung cancer. J Exp Clin Cancer Res.
40(266)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi
D, Yu D, Gao P, Chen C, et al: Neoadjuvant therapy with immune
checkpoint blockade, antiangiogenesis, and chemotherapy for locally
advanced gastric cancer. Nat Commun. 14(8)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang J, Li S, Zhang L and Zhang X: A
combination of anti-PD-1 therapy and apatinib successfully treated
a patient with EGFR mutation-negative advanced lung adenocarcinoma:
A case report. J Cancer Res Ther. 19:141–143. 2023.PubMed/NCBI View Article : Google Scholar
|