Introduction
SARS-CoV-2, the virus responsible for the global
pandemic of Coronavirus Disease 2019 (COVID-19), first identified
in Wuhan, China, in December 2019, has led to unprecedented
challenges in public health, clinical management and medical
research. This coronavirus primarily targets human cells from
several tissues by binding to the angiotensin-converting enzyme II
(ACE2) receptor (1). The virus
predominantly spreads through respiratory droplets that are
released during activities such as coughing, sneezing or even
talking, explaining the high transmissibility and infectious nature
of the virus (2-4).
The clinical spectrum of COVID-19 is remarkably
diverse, ranging from asymptomatic or mild flu-like symptoms in
~70% of patients, to severe respiratory distress and multi-organ
failure in more critical cases (2).
Commonly reported symptoms include fever, malaise, dry cough and
sore throat. However, the pandemic has unveiled a myriad of
atypical presentations that deviate from the classical respiratory
symptoms, thereby complicating the diagnostic process (5). These atypical manifestations include,
but are not limited to, ocular symptoms, cardiac complications such
as myocarditis, dermatological signs, gastrointestinal and hepatic
disturbances, cerebrovascular incidents and various neurological
symptoms (6).
The recognition of these unusual presentations is
pivotal in ensuring timely diagnosis and appropriate management of
COVID-19. Such awareness also aids in implementing effective
isolation measures to curtail the spread of SARS-CoV-2. Among these
atypical manifestations, oral symptoms have garnered attention,
ranging from simple taste disturbances to more complex conditions
such as mucosal lesions and salivary gland infections (7).
The impact of SARS-CoV-2 on children, although
initially perceived as minor, has evolved into a subject of major
concern. In paediatric patients, the virus often manifests in forms
markedly different from adults (from asymptomatic diseases, which
represent the most common form, to multisystem inflammatory
syndrome), which can lead to underdiagnosis or misdiagnosis. This
is particularly crucial as children, being active social agents in
schools and family units, can be key vectors in the transmission of
the virus. Furthermore, the emergence of multisystem inflammatory
syndrome in children associated with COVID-19 underscores the
unpredictable nature of the virus in the younger population
(8). Atypical manifestations in
children can be more cumbersome than those involving adults,
resulting in differential diagnosis and clinical management that
may pose challenges for clinicians (9).
As such, expanding our knowledge on the diverse
clinical presentations of COVID-19 in children, such as the case of
SARS-CoV-2 induced parotitis presented in the present case, is not
only pivotal for the paediatric care but also for the broader
public health strategy. This case report aims to shed light on the
less explored aspects of COVID-19 in paediatrics, illustrating how
even non-respiratory symptoms can herald the presence of the virus
in this demographic, thus necessitating a broader clinical
vigilance among healthcare providers.
The present case report contributed to this growing
body of literature by reporting SARS-CoV-2 infection presenting
primarily with unilateral parotitis and sialadenitis in a
12-year-old girl, further expanding our understanding of the
virus's clinical heterogeneity.
Case report
In November 2023, a 12-year-old female patient
visited our clinic (Polyclinic University Hospital ‘G. Rodolico’,
Catania, Italy) due to the sudden onset of right-sided unilateral
parotitis, accompanied by sialadenitis, hyperaemia of the skin and
pain upon touch. She was up-to-date on her immunizations, including
the measles-mumps-rubella vaccine. The patient had not previously
been vaccinated for SARS-CoV-2. The parents of the patient reported
no history of taking medications such as propylthiouracil,
phenothiazines, iodides or phenylbutazone. The patient had no
chronic diseases. Upon admission, her temperature was 36.3˚C, heart
rate was 78 beats per minute, blood pressure was 110/69 mmHg and
oxygen saturation in room air was 99%. The parents of the patient
reported intermittent fevers (up to 38˚C) for 3 days prior to
admission without respiratory symptoms or facial swelling. Fever
was treated with paracetamol.
Clinical examination was normal for the age and sex
of the patient. Blood tests revealed in range haemoglobin and
platelet counts. The total white count was 8,440 cells/ml, with
neutrophils at 6,380 cells/ml (75.6%) and lymphocytes at 1,588
cells/ml (18.8%). C-reactive protein levels were raised (15 mg/dl;
normal range, 0-0.5 mg/dl) as well as erythrocyte sedimentation
rate (47 mm/h; normal range, <10 mm/h), ferritin and lactate
dehydrogenase levels were within the normal range.
Mumps serology was negative for IgM and positive for
IgG. The main viral infectious disease screenings, including
serology and a respiratory panel on a pharyngeal swab
(BioFire® Respiratory 2.1 Panel; BioFire Diagnostics,
Inc.; bioMérieux), were negative. This included tests for
Epstein-Barr virus, human herpesvirus 6, human immunodeficiency
virus, respiratory syncytial virus, parainfluenza virus types 2 and
3, influenza A and B viruses, adenovirus, coxsackie viruses,
parvovirus B19, lymphocytic choriomeningitis virus, human bocavirus
and paramyxovirus RNA. Overall, two sets of blood cultures were
negative. A nasopharyngeal swab tested positive for SARS-CoV-2,
which was subsequently confirmed by the BioFire®
respiratory panel. A chest radiograph showed no parenchymal lung
alterations suggestive of a pneumonic process. The patient had no
upper or lower respiratory symptoms.
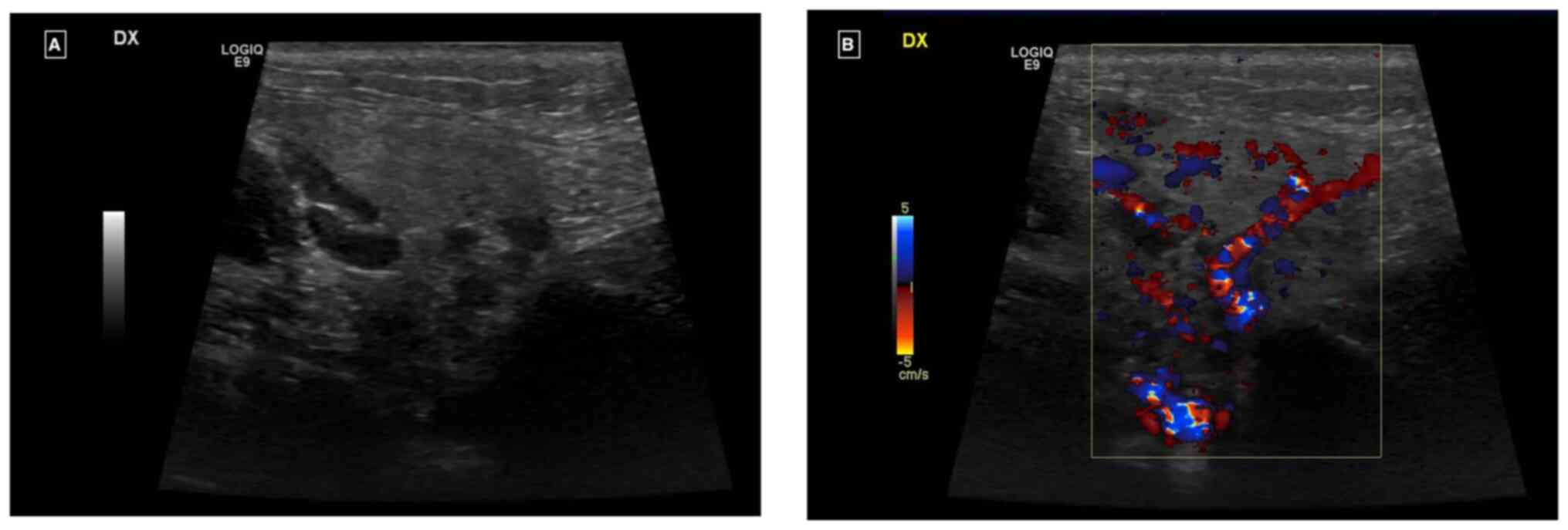
Ultrasound examination revealed an enlarged right
parotid gland and right submandibular gland, both with a
homogeneous echostructure and a diffused increase in echogenicity
of the glandular parenchyma. There was also a diffuse increase in
intraparenchymal vascularity and reactive adenitis. No fluid
collection, obstructing stones, dilatation of salivary ducts,
masses or abscesses were observed (Fig.
1A and B).
Non-steroidal anti-inflammatory drugs therapy
(NSAID) with ibuprofen was started at a dosage of 200 mg every 12
h. Although clinical symptoms did not significantly improve, within
48 h clear saliva without pus was observed to discharge from the
parotid glands. The patient was discharged with a prescription for
analgesic therapy to be used as needed. SARS-CoV-2 swab tested
negative after 7 days and the patient did not develop other
symptoms or manifestations. Parents reported they administered
ibuprofen for a total of 6 days.
At a follow-up visit 2 weeks later, we noted the
complete resolution of the clinical condition of the patient.
Discussion
The present case highlights the novelty of
SARS-CoV-2-associated unilateral parotitis in a 12-year-old girl, a
rare presentation within the paediatric population. Diverging from
typical respiratory symptoms of COVID-19, this instance emphasizes
the capability of the virus to cause glandular manifestations such
as parotitis. The effective management with NSAIDs, without
preceding respiratory symptoms or common viral infections,
underscores the importance of considering COVID-19 in differential
diagnoses for acute parotitis in children, expanding our
understanding of the virus's diverse clinical manifestations.
Similar cases have emerged in the literature since
the onset of the COVID-19 pandemic, predominantly in adult
populations (10-12).
The clinical spectrum of SARS-CoV-2 infection typically ranges from
minimally symptomatic disease to severe pneumonia and critical
illness, with ~70% of patients either asymptomatic or exhibiting
mild flu-like symptoms, such as fever, dry cough and sore throat
(9).
The atypical presentations of SARS-CoV-2 infection
are diverse, including myocarditis, stroke, liver damage,
gastrointestinal symptoms, ocular manifestations, dermatological
lesions and a range of neurological symptoms, observed in both mild
and severe cases (13).
The parotitis seen in SARS-CoV-2 infections can be
attributed to several interacting pathological mechanisms (11,12).
Direct viral invasion is a primary suspect, where SARS-CoV-2
infiltrates the salivary gland epithelium by binding to ACE2
receptors, causing local inflammation and cellular damage (14). Concurrently, the host's immune
response, while attempting to combat the virus, may overshoot,
releasing a deluge of cytokines in a detrimental ‘cytokine storm’,
leading to further tissue inflammation and gland swelling (15,16).
Compounding this, SARS-CoV-2 affects endothelial cells, causing
dysfunction that manifests as increased vascular permeability and
oedema, which is a condition conducive to parotitis (17). The propensity of the virus to induce
a hypercoagulable state could also precipitate microthrombi within
the tiny blood vessels of the salivary glands, impairing blood flow
and oxygen delivery, exacerbating inflammation (18). Moreover, the concept of molecular
mimicry may offer insight into prolonged or recurrent gland
inflammation, as the immune system's production of antibodies
against the virus could inadvertently target structurally similar
antigens within the salivary glands (19). This complex interplay of direct
viral effects, immune response dysregulation, vascular pathology
and autoimmune phenomena not only elucidates the occurrence of
parotitis in COVID-19 but also reflects the multisystemic impact of
SARS-CoV-2, warranting a broad spectrum of considerations in the
clinical management of these patients (2,20,21).
The timeline for the development of parotitis during
SARS-CoV-2 infection is not well-defined. In several reports,
parotitis was noted 1-3 days after the onset of coronavirus
symptoms (11,22,23).
Parotitis not related to mumps has been associated with various
viruses, including enteroviruses, adenoviruses, cytomegalovirus,
influenza, parainfluenza, Epstein-Barr, herpes simplex and herpes
virus 6(24).
In the present case, the patient presented with
acute mumps-like symptoms and unilateral sialadenitis following a
3-day history of fever, without any chronic diseases, trauma and
with normal blood tests. The positive SARS-CoV-2 swab, combined
with ultrasound findings which ruled out oncological or obstructive
conditions, along with normal blood samples and physical
examination, facilitated a non-invasive approach. This approach
allowed us to reassure the parents and monitor the patient over
time, avoiding the immediate use of more invasive imaging
techniques such as CT scans, which involve ionizing radiation.
To the best of our knowledge the scientific
literature report before the present study reports only six
patients who developed a parotitis which could be ascribed to
SARS-CoV-2 infection (Table I). The
age of the patient in the present case is somewhat aligned with the
range observed in the literature, with reported cases varying from
2 months to 7 years of age (25,26).
While most cases involved males, the present patient is female,
suggesting no strong sex predilection for SARS-CoV-2 associated
parotitis.
 | Table ICharacteristics of SARS-CoV-2
associated parotitis in children. |
Table I
Characteristics of SARS-CoV-2
associated parotitis in children.
| Author | No. of patients | Age | Sex | Symptoms | Lab findings | SARS-CoV-2 test
performed | Respiratory
symptoms | Treatment | Duration (days) | Outcome | (Refs.) |
|---|
| Brehm et
al | 1 | 10 weeks | M | Facial swelling,
decreased appetite | Leucocytosis, high
platelets | PCR | Rhinorrhoea,
cough |
Amoxicillin/clavulanic acid | 7 | Resolved | (27) |
| Likitnukul | 1 | 4 years | M | Facial swelling, low
grade fever, decreased appetite | High CRP, high
amylase | PCR | Mild cough | Analgesic (not
specified) | 3 | Resolved | (28) |
| Keles et
al | 1 | 4 years | M | Facial swelling and
pain, difficulty swallowing | High amylase | PCR | None | NSAID (not
specified) | 3 | Resolved | (26) |
| Sharma et
al | 3 | 7 years | F | Facial swelling,
partial maloc- clusion, trismus, decreased appetite | High CRP and ESR,
high amylase | PCR | Cough (4 weeks
before) | Prednisolone 1
mg/kg/day | 5 | Resolved | (25) |
| | | 3.5 years | M | Facial swelling,
fever, poor appetite | Leucocytosis, high
ESR and CRP | PCR | None | Prednisolone 1
mg/kg/day | 5 | Resolved | |
| | | 2 months | M | Facial swelling,
irritability, decreased appetite, fever | Leucocytosis, high
CRP | PCR | None | Metilpredni- solone
2 mg/kg/day | 5 | Resolved | |
Unlike other cases, the present patient had no
preceding respiratory symptoms, which is atypical given that
respiratory symptoms were present in most literature cases, albeit
mild in some (27), highlighting
the variable presentation of the disease. All patients had
undergone neck ultrasounds. The laboratory parameters in the
current case were mostly within normal ranges, contrasting with
other reports where leucocytosis and elevated acute-phase reactants
were common (28). This discrepancy
may point to a less aggressive inflammatory response in the present
patient.
Our patient was treated only with NSAID therapy,
without the need of either antibiotics (often overprescribed by
clinicians aiming to prevent bacterial superinfections) or
corticosteroid therapy.
The pathogenesis of SARS-CoV-2-associated
sialadenitis is yet to be fully understood. However, SARS-CoV-2
should be considered in the differential diagnosis of
parotitis/sialadenitis, particularly if it presents unilaterally.
Prompt isolation measures are crucial to mitigate the spread of
infection (29).
The epidemiological landscape of COVID-19 in the
paediatric population presents a multifaceted challenge. Although
children account for a significant portion of total cases, their
clinical manifestations often differ from adults, leading to unique
considerations in public health strategies (30). Notably, the prevalence of COVID-19
among children varies with age, showing higher incidence in
school-aged children and adolescents compared with younger children
(31,32). Additionally, the impact of
socioeconomic factors is pronounced in this demographic, as
children from lower socioeconomic backgrounds face increased
exposure risks and potential health disparities (33). Understanding these dynamics is
crucial, not only for direct paediatric care but also for broader
community health policies, especially considering the role of
children in viral transmission within schools and households
(34). This complex epidemiological
profile underscores the need for tailored approaches in both
prevention and treatment strategies for COVID-19 among
children.
The present case underscores the importance of
including SARS-CoV-2, and coronaviruses in general, in the list of
pathogens responsible for parotitis and sialadenitis, alongside
mumps and influenza. Notably, a comprehensive respiratory panel and
serology are essential for accurate diagnosis in cases presenting
with parotitis-like symptoms.
Effective COVID-19 prevention in children hinges on
a multi-faceted approach that balances public health guidelines
with the unique needs of the paediatric population. Vaccination,
when available and approved for specific age groups, plays a
crucial role in reducing transmission and severity of the disease
among children (35).
In conclusion, the present case highlights the need
for heightened vigilance and a broader diagnostic perspective
during the ongoing COVID-19 pandemic. Understanding the diverse
clinical manifestations of SARS-CoV-2, including rare presentations
such as unilateral parotitis, is crucial for prompt diagnosis,
effective patient management and prevention of virus
transmission.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no
datasets were generated or analysed during the current study.
Authors' contributions
AM, GC, SS, MP, AB, EL, SC, EVR, GN and PP
contributed to the study conception and design. GC and AM
conceptualised the study. SS designed the methodology. MP, AB, EL
and SC performed the investigation. EVR and GN wrote the original
draft preparation. GN reviewed and edited the manuscript. PP
supervised the study. All authors have read and approved the final
manuscript. GN and PP confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Written informed consent for data collection was
obtained from the parents of the subject involved in the study.
Patient consent for publication
Informed consent for publication was obtained from
the parents of the subject involved in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marino A, Munafò A, Augello E, Bellanca
CM, Bonomo C, Ceccarelli M, Musso N, Cantarella G, Cacopardo B and
Bernardini R: Sarilumab administration in COVID-19 patients:
Literature review and considerations. Infect Dis Rep. 14:360–371.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zimmermann P and Curtis N: Coronavirus
infections in children including COVID-19: An overview of the
epidemiology, clinical features, diagnosis, treatment and
prevention options in children. Pediatr Infect Dis J. 39:355–368.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang
J, Shen J, Zhu LR, Chen Y, Iacucci M, et al: Manifestations and
prognosis of gastrointestinal and liver involvement in patients
with COVID-19: A systematic review and meta-analysis. Lancet
Gastroenterol Hepatol. 5:667–678. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cosentino F, Moscatt V, Marino A,
Pampaloni A, Scuderi D, Ceccarelli M, Benanti F, Gussio M, Larocca
L, Boscia V, et al: Clinical characteristics and predictors of
death among hospitalized patients infected with SARS-CoV-2 in
Sicily, Italy: A retrospective observational study. Biomed Rep.
16(34)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pagliari D, Marra A and Cosentini R:
Atypical manifestations of COVID-19: To know signs and symptoms to
recognize the whole disease in the emergency department. Intern
Emerg Med. 16:1407–1410. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang Z, Chen X, Huang R, Li S, Lin D, Yang
Z, Sun H, Liu G, Qiu J, Tang Y, et al: Atypical presentations of
coronavirus disease 2019 (COVID-19) from onset to readmission. BMC
Infect Dis. 21(127)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Binmadi NO, Aljohani S, Alsharif MT,
Almazrooa SA and Sindi AM: Oral manifestations of COVID-19: A
cross-sectional study of their prevalence and association with
disease severity. J Clin Med. 11(4461)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zachariah P: COVID-19 in children. Infect
Dis Clin North Am. 36:1–14. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kachru S and Kaul D: COVID-19
manifestations in children. Curr Med Res Pract. 10:186–188.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Friedrich RE, Droste TL, Angerer F, Popa
B, Koehnke R, Gosau M and Knipfer C: COVID-19-associated parotid
gland abscess. In Vivo. 36:1349–1353. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maegawa K and Nishioka H:
COVID-19-associated parotitis and sublingual gland sialadenitis.
BMJ Case Rep. 15(e251730)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brandini DA, Takamiya AS, Thakkar P,
Schaller S, Rahat R and Naqvi AR: Covid-19 and oral diseases:
Crosstalk, synergy or association? Rev Med Virol.
31(e2226)2021.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Sayed MA and Abdelhakeem M: Typical and
atypical clinical presentation of COVID-19 infection in children in
the top of pandemic in el-minia governorate (two center
experience). Mediterr J Hematol Infect Dis.
14(e2022002)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng
X, Li T and Chen Q: High expression of ACE2 receptor of 2019-nCoV
on the epithelial cells of oral mucosa. Int J Oral Sci.
12(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Montazersaheb S, Hosseiniyan Khatibi SM,
Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, Farahzadi R
and Ghasemnejad T: COVID-19 infection: An overview on cytokine
storm and related interventions. Virol J. 19(92)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Campanella E, Marino A, Ceccarelli M,
Gussio M, Cosentino F, Moscatt V, Icali C, Nunnari G, Celesia BM
and Acopardo B: Pain crisis management in a patient with sickle
cell disease during SARS-CoV-2 infection: A case report and
literature review. World Acad Sci J. 4(14)2022.
|
|
17
|
Wu X, Xiang M, Jing H, Wang C, Novakovic
VA and Shi J: Damage to endothelial barriers and its contribution
to long COVID. Angiogenesis. 27:5–22. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Spampinato S, Pavone P, Cacciaguerra G,
Cocuzza S, Venanzi Rullo E, Marino S, Marino A and Nunnari G:
Coronavirus OC43 and influenza H3N2 concomitant unilateral
parotitis: The importance of laboratory tests in mumps-like
parotitis. Pathogens. 12(1309)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nunez-Castilla J, Stebliankin V, Baral P,
Balbin CA, Sobhan M, Cickovski T, Mondal AM, Narasimhan G,
Chapagain P, Mathee K and Siltberg-Liberles J: Potential
autoimmunity resulting from molecular mimicry between SARS-CoV-2
spike and human proteins. Viruses. 14(1415)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pavone P, Ceccarelli M, Taibi R, La Rocca
G and Nunnari G: Outbreak of COVID-19 infection in children: Fear
and serenity. Eur Rev Med Pharmacol Sci. 24:4572–4575.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pavone P, Marino S, Marino L, Cacciaguerra
G, Guarneri C, Nunnari G, Taibi R, Marletta L and Falsaperla R:
Chilblains-like lesions and SARS-CoV-2 in children: An overview in
therapeutic approach. Dermatol Ther. 34(e14502)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rayan MA, Bader JA, Feras AA and Shaikha
JA: COVID-19 associated parotitis in pediatrics. Glob J Pediatr
Neonatal Care. 2:2020.
|
|
23
|
Riad A, Kassem I, Badrah M and Klugar M:
Acute parotitis as a presentation of COVID-19? Oral Dis. 28 (Suppl
1):S968–S969. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wilson M and Pandey S: Parotitis.
Emergency Management of Infectious Diseases: Second edition.
pp126-128, 2023.
|
|
25
|
Sharma S, Mahajan V and Gupta R:
Unilateral acute parotitis: A novel manifestation of pediatric
coronavirus disease. Indian Pediatr Case Rep. 2:200–203. 2022.
|
|
26
|
Ekemen Keles Y, Karadag Oncel E, Baysal M,
Kara Aksay A and Yılmaz Ciftdogan D: Acute parotitis in a
4-year-old in association with COVID-19. J Paediatr Child Health.
57:958–959. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brehm R, Narayanam L and Chon G:
COVID-19-associated parotitis in a 10-week-old male. Cureus.
14(e31054)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Likitnukul S: COVID-19 associated
parotitis in a 4-year-old boy. J Paediatr Child Health.
58:1911–1912. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Erdem H, Hargreaves S, Ankarali H,
Caskurlu H, Ceviker SA, Bahar-Kacmaz A, Meric-Koc M, Altindis M,
Yildiz-Kirazaldi Y, Kizilates F, et al: Managing adult patients
with infectious diseases in emergency departments: International
ID-IRI study. J Chemother. 33:302–318. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ludvigsson JF: Systematic review of
COVID-19 in children shows milder cases and a better prognosis than
adults. Acta Paediatr. 109:1088–1095. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Götzinger F, Santiago-García B,
Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI,
Gabrovska N, Velizarova S, Prunk P, Osterman V, et al: COVID-19 in
children and adolescents in Europe: A multinational, multicentre
cohort study. Lancet Child Adolesc Health. 4:653–661.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bialek S, Gierke R, Hughes M, McNamara LA,
Pilishvili T and Skoff T: Coronavirus disease 2019 in
children-United States, February 12-April 2, 2020. MMWR Morb Mortal
Wkly Rep. 69:422–426. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zachariah P, Johnson CL, Halabi KC, Ahn D,
Sen AI, Fischer A, Banker SL, Giordano M, Manice CS, Diamond R, et
al: Epidemiology, clinical features, and disease severity in
patients with coronavirus disease 2019 (COVID-19) in a children's
hospital in New York City, New York. JAMA Pediatr.
174(e202430)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Parri N, Lenge M and Buonsenso D:
Coronavirus Infection in Pediatric Emergency Departments
(CONFIDENCE) Research Group. Children with Covid-19 in pediatric
emergency departments in Italy. N Engl J Med. 383:187–190.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cag Y, Al Madadha ME, Ankarali H, Cag Y,
Demir Onder K, Seremet-Keskin A, Kizilates F, Čivljak R, Shehata G,
Alay H, et al: Vaccine hesitancy and refusal among parents: An
international ID-IRI survey. J Infect Dev Ctries. 16:1081–1088.
2022.PubMed/NCBI View Article : Google Scholar
|