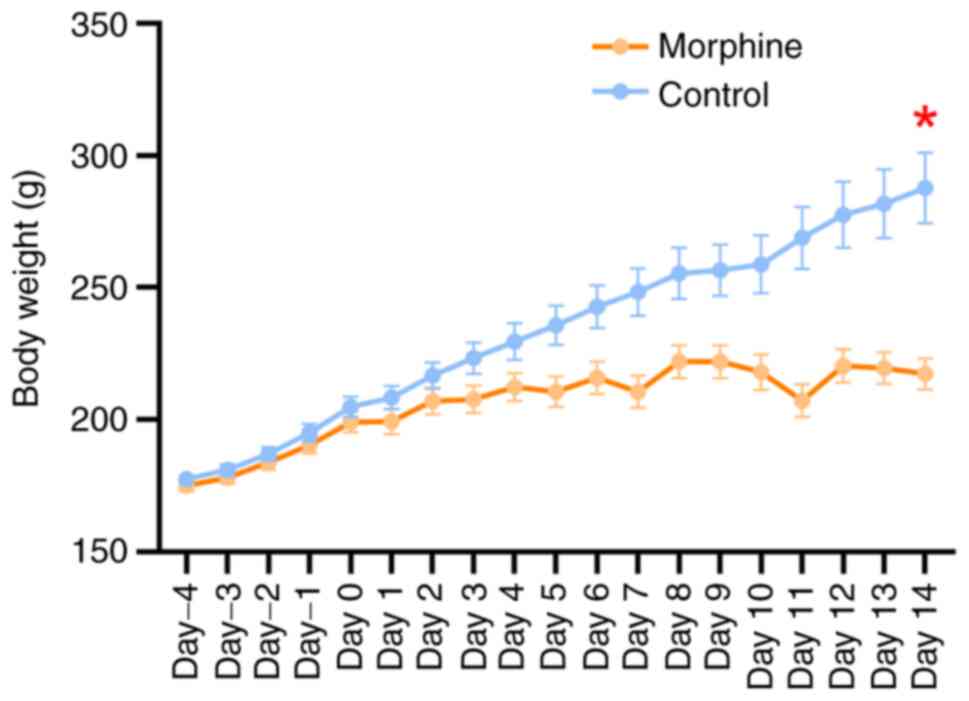
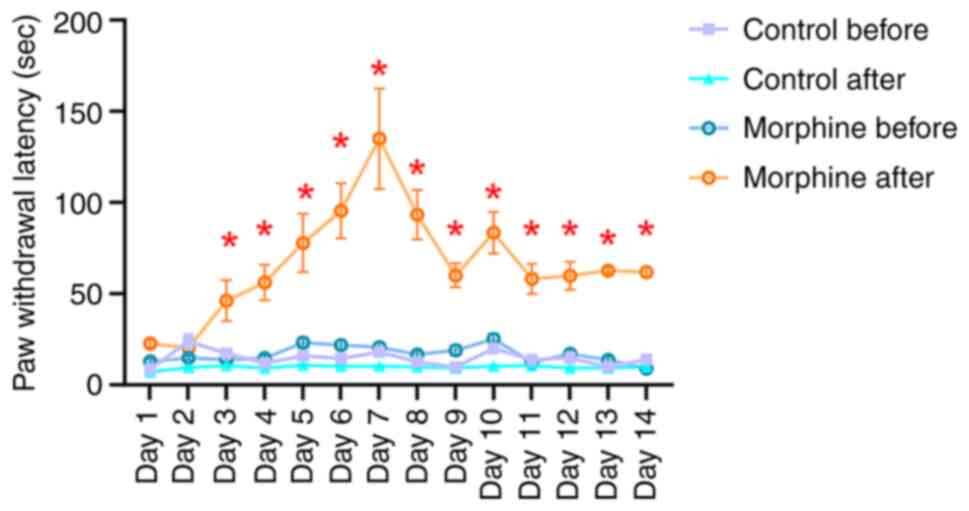
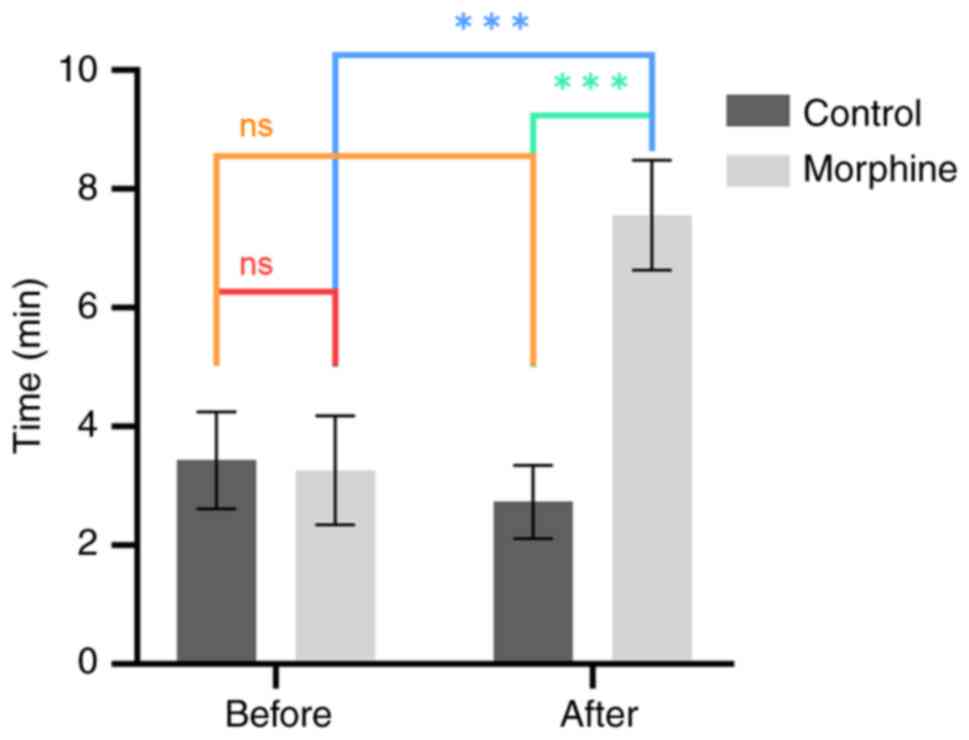
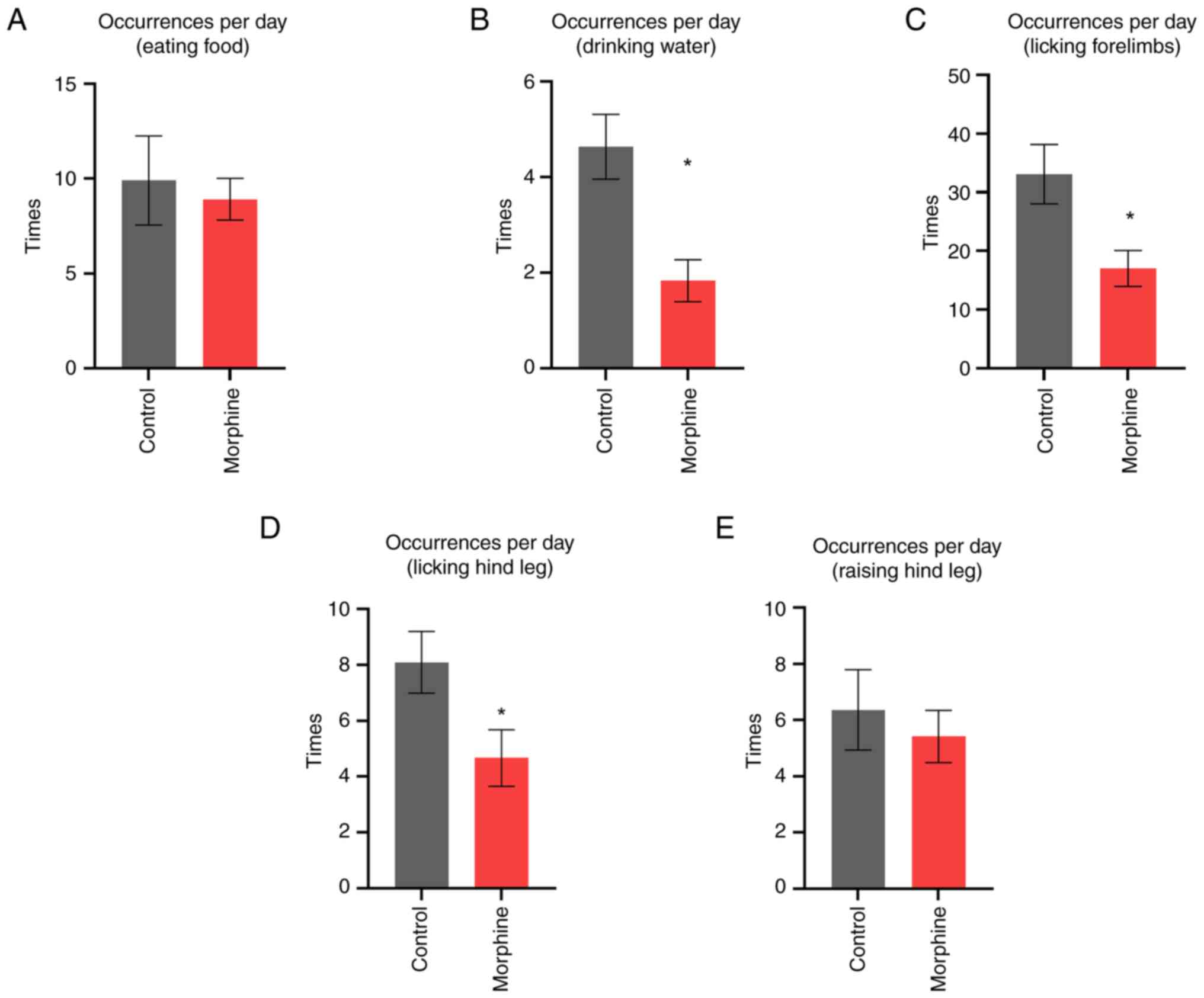
|
1
|
Häuser W, Welsch P, Radbruch L, Fisher E,
Bell RF and Moore RA: Cannabis-based medicines and medical cannabis
for adults with cancer pain. Cochrane Database Syst Rev.
6(Cd014915)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Luger NM, Mach DB, Sevcik MA and Mantyh
PW: Bone cancer pain: From model to mechanism to therapy. J Pain
Symptom Manage. 29 (5 Suppl):S32–S46. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mercadante S: Intravenous morphine for
management of cancer pain. Lancet Oncol. 11:484–489.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nguyen QN, Chun SG, Chow E, Komaki R, Liao
Z, Zacharia R, Szeto BK, Welsh JW, Hahn SM, Fuller CD, et al:
Single-Fraction stereotactic vs conventional multifraction
radiotherapy for pain relief in patients with predominantly
nonspine bone metastases: A Randomized phase 2 trial. JAMA Oncol.
5:872–878. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mantsch JR, Baker DA, Funk D, Lê AD and
Shaham Y: Stress-Induced reinstatement of drug seeking: 20 years of
progress. Neuropsychopharmacology. 41:335–356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reiner DJ, Fredriksson I, Lofaro OM,
Bossert JM and Shaham Y: Relapse to opioid seeking in rat models:
Behavior, pharmacology and circuits. Neuropsychopharmacology.
44:465–477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shalev U, Grimm JW and Shaham Y:
Neurobiology of relapse to heroin and cocaine seeking: A review.
Pharmacol Rev. 54:1–42. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trujillo KA and Akil H: Inhibition of
morphine tolerance and dependence by the NMDA receptor antagonist
MK-801. Science. 251:85–87. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Garofoli M: Adolescent Substance Abuse.
Prim Care. 47:383–394. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lewis DC: Drug overdose, addiction and
binge drinking: Medical problems with public health consequences. R
I Med J (2013). 97:18–19. 2014.PubMed/NCBI
|
|
11
|
McCarty D, Argeriou M, Huebner RB and
Lubran B: Alcoholism, drug abuse, and the homeless. Am Psychol.
46:1139–1148. 1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lu L and Wang X: Drug addiction in China.
Ann N Y Acad Sci. 1141:304–317. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Leshner AI: Addiction is a brain disease,
and it matters. Science. 278:45–47. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu JF and Li JX: Drug addiction: A
curable mental disorder? Acta Pharmacol Sin. 39:1823–1829.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nestler EJ: Molecular basis of long-term
plasticity underlying addiction. Nat Rev Neurosci. 2:119–128.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Chiamulera C, Piva A and Abraham WC:
Glutamate receptors and metaplasticity in addiction. Curr Opin
Pharmacol. 56:39–45. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fourgeaud L, Mato S, Bouchet D, Hémar A,
Worley PF and Manzoni OJ: A single in vivo exposure to cocaine
abolishes endocannabinoid-mediated long-term depression in the
nucleus accumbens. J Neurosci. 24:6939–6945. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gipson CD, Kupchik YM and Kalivas PW:
Rapid, transient synaptic plasticity in addiction.
Neuropharmacology. 76 Pt B:276–286. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hafenbreidel M, Rafa Todd C and Mueller D:
Infralimbic GluN2A-Containing NMDA receptors modulate
reconsolidation of cocaine self-administration memory.
Neuropsychopharmacology. 42:1113–1125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hyman SE: Addiction: A disease of learning
and memory. Am J Psychiatry. 162:1414–1422. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kauer JA and Malenka RC: Synaptic
plasticity and addiction. Nat Rev Neurosci. 8:844–858.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Keralapurath MM, Briggs SB and Wagner JJ:
Cocaine self-administration induces changes in synaptic
transmission and plasticity in ventral hippocampus. Addict Biol.
22:446–456. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lüscher C and Malenka RC: Drug-evoked
synaptic plasticity in addiction: From molecular changes to circuit
remodeling. Neuron. 69:650–663. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mameli M, Bellone C, Brown MT and Lüscher
C: Cocaine inverts rules for synaptic plasticity of glutamate
transmission in the ventral tegmental area. Nat Neurosci.
14:414–416. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Tronson NC and Taylor JR: Molecular
mechanisms of memory reconsolidation. Nat Rev Neurosci. 8:262–275.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Tzschentke TM and Schmidt WJ:
Glutamatergic mechanisms in addiction. Mol Psychiatry. 8:373–382.
2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
van Huijstee AN and Mansvelder HD:
Glutamatergic synaptic plasticity in the mesocorticolimbic system
in addiction. Front Cell Neurosci. 8(466)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ossipov MH, Lai J, King T, Vanderah TW,
Malan TP Jr, Hruby VJ and Porreca F: Antinociceptive and
nociceptive actions of opioids. J Neurobiol. 61:126–148.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mickiewicz AL and Napier TC: Repeated
exposure to morphine alters surface expression of AMPA receptors in
the rat medial prefrontal cortex. Eur J Neurosci. 33:259–265.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boudreau AC and Wolf ME: Behavioral
sensitization to cocaine is associated with increased AMPA receptor
surface expression in the nucleus accumbens. J Neurosci.
25:9144–9151. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hemby SE, Tang W, Muly EC, Kuhar MJ,
Howell L and Mash DC: Cocaine-induced alterations in nucleus
accumbens ionotropic glutamate receptor subunits in human and
non-human primates. J Neurochem. 95:1785–1793. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sutton MA, Schmidt EF, Choi KH, Schad CA,
Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL and Self
DW: Extinction-induced upregulation in AMPA receptors reduces
cocaine-seeking behaviour. Nature. 421:70–75. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bachtell RK, Choi KH, Simmons DL, Falcon
E, Monteggia LM, Neve RL and Self DW: Role of GluR1 expression in
nucleus accumbens neurons in cocaine sensitization and
cocaine-seeking behavior. Eur J Neurosci. 27:2229–2240.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Conrad KL, Tseng KY, Uejima JL, Reimers
JM, Heng LJ, Shaham Y, Marinelli M and Wolf ME: Formation of
accumbens GluR2-lacking AMPA receptors mediates incubation of
cocaine craving. Nature. 454:118–121. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kasanetz F, Deroche-Gamonet V, Berson N,
Balado E, Lafourcade M, Manzoni O and Piazza PV: Transition to
addiction is associated with a persistent impairment in synaptic
plasticity. Science. 328:1709–1712. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
LaLumiere RT and Kalivas PW: Glutamate
release in the nucleus accumbens core is necessary for heroin
seeking. J Neurosci. 28:3170–3177. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Degoulet M, Stelly CE, Ahn KC and Morikawa
H: L-type Ca²+ channel blockade with antihypertensive
medication disrupts VTA synaptic plasticity and drug-associated
contextual memory. Mol Psychiatry. 21:394–402. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lane DA, Lessard AA, Chan J, Colago EE,
Zhou Y, Schlussman SD, Kreek MJ and Pickel VM: Region-specific
changes in the subcellular distribution of AMPA receptor GluR1
subunit in the rat ventral tegmental area after acute or chronic
morphine administration. J Neurosci. 28:9670–9681. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Billa SK, Sinha N, Rudrabhatla SR and
Morón JA: Extinction of morphine-dependent conditioned behavior is
associated with increased phosphorylation of the GluR1 subunit of
AMPA receptors at hippocampal synapses. Eur J Neurosci. 29:55–64.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cai YQ, Wang W, Hou YY, Zhang Z, Xie J and
Pan ZZ: Central amygdala GluA1 facilitates associative learning of
opioid reward. J Neurosci. 33:1577–1588. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun Y, Chen G, Zhou K and Zhu Y: A
conditioned place preference protocol for measuring incubation of
craving in rats. J Vis Exp. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Tzschentke TM: Measuring reward with the
conditioned place preference (CPP) paradigm: Update of the last
decade. Addict Biol. 12:227–462. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lin XJ, Zhang JJ and Yu LC: GluR2-3Y
inhibits the acquisition and reinstatement of morphine-induced
conditioned place preference in rats. Neurosci Bull. 32:177–182.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tzschentke TM: Measuring reward with the
conditioned place preference paradigm: A comprehensive review of
drug effects, recent progress and new issues. Prog Neurobiol.
56:613–672. 1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang X, Bey AL, Katz BM, Badea A, Kim N,
David LK, Duffney LJ, Kumar S, Mague SD, Hulbert SW, et al: Altered
mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3
complete knockout model of autism. Nat Commun.
7(11459)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Domínguez-Salazar E, Naser HF and
Velázquez-Moctezuma J: D1-like antagonist blocks conditioned place
preference induced by ejaculation in male rats. Behav Brain Res.
269:15–19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chang SL, Moldow RL, House SD and Zadina
JE: Morphine affects the brain-immune axis by modulating an
interleukin-1 beta dependent pathway. Adv Exp Med Biol. 402:35–42.
1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Graf JA, Patel JA and Chang SL: Chronic
exposure to morphine, but not ethanol, attenuates the expression of
interleukin-1 beta converting enzyme in rat spleen. Immunol Lett.
58:153–157. 1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
House SD, Mao X, Wu G, Espinelli D, Li WX
and Chang SL: Chronic morphine potentiates the inflammatory
response by disrupting interleukin-1beta modulation of the
hypothalamic-pituitary-adrenal axis. J Neuroimmunol. 118:277–285.
2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lobo MK, Covington HE III, Chaudhury D,
Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW,
Kennedy PJ, et al: Cell type-specific loss of BDNF signaling mimics
optogenetic control of cocaine reward. Science. 330:385–390.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ocasio FM, Jiang Y, House SD and Chang SL:
Chronic morphine accelerates the progression of
lipopolysaccharide-induced sepsis to septic shock. J Neuroimmunol.
149:90–100. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zadina JE, Kastin AJ, Harrison LM, Ge LJ
and Chang SL: Opiate receptor changes after chronic exposure to
agonists and antagonists. Ann N Y Acad Sci. 757:353–361.
1995.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Askari N, Mousavi A and Vaez-Mahdavi MR:
Maternal deprivation effect on morphine-induced CPP is related to
changes in opioid receptors in selected rat brain regions
(hippocampus, prefrontal cortex, and nucleus accumbens). Behav
Processes. 197(104607)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rodgers HM, Lim SA, Yow J, Dinkins ML,
Patton R, Clemens S and Brewer KL: Dopamine D1 or
D3 receptor modulators prevent morphine tolerance and
reduce opioid withdrawal symptoms. Pharmacol Biochem Behav.
194(172935)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liu LW, Lu J, Wang XH, Fu SK, Li Q and Lin
FQ: Neuronal apoptosis in morphine addiction and its molecular
mechanism. Int J Clin Exp Med. 6:540–545. 2013.PubMed/NCBI
|
|
56
|
Papaleo F and Contarino A: Gender- and
morphine dose-linked expression of spontaneous somatic opiate
withdrawal in mice. Behav Brain Res. 170:110–118. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rahmati B and Beik A: Prevention of
morphine dependence and tolerance by Nepeta menthoides was
accompanied by attenuation of Nitric oxide overproduction in male
mice. J Ethnopharmacol. 199:39–51. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Koek W: Morphine-induced conditioned place
preference and effects of morphine pre-exposure in adolescent and
adult male C57BL/6J mice. Psychopharmacology (Berl). 233:2015–2024.
2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Li X, Kshatriya D and Bello NT:
Weight-gain propensity and morphine withdrawal alters locomotor
behavior and regional norepinephrine-related gene expression in
male and female mice. Pharmacol Biochem Behav.
213(173329)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Madayag AC, Gomez D, Anderson EM,
Ingebretson AE, Thomas MJ and Hearing MC: Cell-type and
region-specific nucleus accumbens AMPAR plasticity associated with
morphine reward, reinstatement, and spontaneous withdrawal. Brain
Struct Funct. 224:2311–2324. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
McDevitt DS, McKendrick G and Graziane NM:
Anterior cingulate cortex is necessary for spontaneous opioid
withdrawal and withdrawal-induced hyperalgesia in male mice.
Neuropsychopharmacology. 46:1990–1999. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Nakamura A, Ono H, Ando A, Hinata M,
Niidome K, Omachi S, Sakaguchi G and Shinohara S: Suppression of
the acute upregulation of phosphorylated-extracellular regulated
kinase in ventral tegmental area by a µ-opioid receptor agonist is
related to resistance to rewarding effects in a mouse model of bone
cancer. J Pharmacol Sci. 133:9–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Piccin A and Contarino A: Long-lasting
pseudo-social aggressive behavior in opiate-withdrawn mice. Prog
Neuropsychopharmacol Biol Psychiatry. 97(109780)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Piccin A and Contarino A: The CRF(1)
receptor mediates social behavior deficits induced by opiate
withdrawal. J Neurosci Res. 100:309–321. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Varshneya NB, Walentiny DM, Stevens DL,
Walker TD, Akinfiresoye LR and Beardsley PM: Structurally diverse
fentanyl analogs yield differential locomotor activities in mice.
Pharmacol Biochem Behav. 222(173496)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kalvass JC, Olson ER, Cassidy MP, Selley
DE and Pollack GM: Pharmacokinetics and pharmacodynamics of seven
opioids in P-glycoprotein-competent mice: Assessment of unbound
brain EC50,u and correlation of in vitro, preclinical, and clinical
data. J Pharmacol Exp Ther. 323:346–355. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Melzacka M: Pharmacokinetic aspects of
some behavioral effects of psychotropic drugs. Pol J Pharmacol
Pharm. 36:117–136. 1984.PubMed/NCBI
|
|
68
|
Regenthal R, Krueger M, Koeppel C and
Preiss R: Drug levels: Therapeutic and toxic serum/plasma
concentrations of common drugs. J Clin Monit Comput. 15:529–544.
1999.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sulimai NH, Ko JC, Jones-Hall YL, Weng HY,
Deng M, Breur GJ and Knipp GT: Evaluation of 25% poloxamer as a
slow release carrier for morphine in a rat model. Front Vet Sci.
5(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Santos-Vera B, Vaquer-Alicea ADC,
Maria-Rios CE, Montiel-Ramos A, Ramos-Cardona A, Vázquez-Torres R,
Sanabria P and Jiménez-Rivera CA: Protein and surface expression of
HCN2 and HCN4 subunits in mesocorticolimbic areas after cocaine
sensitization. Neurochem Int. 125:91–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cao DN, Song R, Zhang SZ, Wu N and Li J:
Nucleus accumbens hyperpolarization-activated cyclic
nucleotide-gated channels modulate methamphetamine
self-administration in rats. Psychopharmacology (Berl).
233:3017–3029. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Caruso Brown AE: Treating addiction as a
terminal disease. N Engl J Med. 382:207–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Mu L, Liu X, Yu H, Vickstrom CR, Friedman
V, Kelly TJ, Hu Y, Su W, Liu S, Mantsch JR and Liu QS:
cAMP-mediated upregulation of HCN channels in VTA dopamine neurons
promotes cocaine reinforcement. Mol Psychiatry. 28:3930–3942.
2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Settley C: The physical and psychological
wellbeing of caregivers of individuals suffering from substance
addiction. Arch Psychiatr Nurs. 34:107–109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Xiao ZW, Cao CY, Wang ZX, Li JX, Liao HY
and Zhang XX: Changes of dopamine transporter function in striatum
during acute morphine addiction and its abstinence in rhesus
monkey. Chin Med J (Engl). 119:1802–1807. 2006.PubMed/NCBI
|
|
76
|
Aramjoo H, Riahi-Zanjani B, Farkhondeh T,
Forouzanfar F and Sadeghi M: Modulatory effect of opioid
administration on the activity of cholinesterase enzyme: A
systematic review of mice/rat models. Environ Sci Pollut Res Int.
28:52675–52688. 2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Djabirska I, Delaval L, Tromme A, Blomet
J, Desmecht D and Van Laere AS: Longitudinal quantitative
assessment of TMEV-IDD-induced MS phenotypes in two inbred mouse
strains using automated video tracking technology. Exp Neurol.
379(114851)2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kulbeth HJ, Fukuda S and Brents LK:
Automated quantification of opioid withdrawal in neonatal rat pups
using Ethovision® XT software. Neurotoxicol Teratol.
84(106959)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Richmond-Hacham B, Tseitlin L, Bikovski L
and Pick CG: Investigation of mild traumatic brain injury home cage
behavior: The home cage assay advantages. J Neurotrauma.
41:e1780–e1792. 2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Timotius IK, Roelofs RF, Richmond-Hacham
B, Noldus LPJJ, von Hörsten S and Bikovski L: CatWalk XT gait
parameters: A review of reported parameters in pre-clinical studies
of multiple central nervous system and peripheral nervous system
disease models. Front Behav Neurosci. 17(1147784)2023.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Watkins J, Ghosh A, Keerie AFA, Alix JJP,
Mead RJ and Sreedharan J: Female sex mitigates motor and
behavioural phenotypes in TDP-43Q331K knock-in mice. Sci
Rep. 10(19220)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Lebedev IV, Pleskacheva MG and Anokhin KV:
C57BL/6 mice open field behaviour qualitatively depends on arena
size. Zh Vyssh Nerv Deiat Im I P Pavlova. 62:485–496.
2012.PubMed/NCBI(In Russian).
|
|
83
|
Novati A, Manfré G, Flunkert S, Van der
Harst JE, Homberg JR, Wronski R and Nguyen HP: Validation of
behavioral phenotypes in the BACHD rat model. Behav Brain Res.
393(112783)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Braw Y, Malkesman O, Dagan M, Bercovich A,
Lavi-Avnon Y, Schroeder M, Overstreet DH and Weller A: Anxiety-like
behaviors in pre-pubertal rats of the Flinders Sensitive Line (FSL)
and Wistar-Kyoto (WKY) animal models of depression. Behav Brain
Res. 167:261–269. 2006.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Medvedev IO, Malyshkin AA, Belozertseva
IV, Sukhotina IA, Sevostianova NY, Aliev K, Zvartau EE, Parsons CG,
Danysz W and Bespalov AY: Effects of low-affinity NMDA receptor
channel blockers in two rat models of chronic pain.
Neuropharmacology. 47:175–183. 2004.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Yuan X and Devine DP: The role of anxiety
in vulnerability for self-injurious behaviour: studies in a rodent
model. Behav Brain Res. 311:201–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Le T, Xia M, Jia M, Sarkar N, Chen J, Li
H, Wynn GH, Ursano RJ and Choi KH: Association between initial
morphine intake and body weight change, acoustic startle reflex and
drug seeking in rats. Psychopharmacology (Berl). 231:4569–4577.
2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kon R, Ikarashi N, Hayakawa A, Haga Y,
Fueki A, Kusunoki Y, Tajima M, Ochiai W, Machida Y and Sugiyama K:
Morphine-induced constipation develops with increased aquaporin-3
expression in the colon via increased serotonin secretion. Toxicol
Sci. 145:337–347. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Deroche V, Piazza PV, Casolini P, Maccari
S, Le Moal M and Simon H: Stress-induced sensitization to
amphetamine and morphine psychomotor effects depend on
stress-induced corticosterone secretion. Brain Res. 598:343–348.
1992.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Yunusa S, Müller CP and Hassan Z:
Mitragynine (Kratom)-Withdrawal behaviour and cognitive impairments
can be ameliorated by an epigenetic mechanism. Br J Pharmacol.
181:2070–2084. 2024.PubMed/NCBI View Article : Google Scholar
|