The management of human immunodeficiency virus (HIV)
infection has evolved over the past decades, with antiretroviral
therapy (ART) serving a critical role in achieving virological
suppression and improving immune function. Despite the efficacy of
various ART regimens, the need for optimizing treatment persists to
enhance patient outcomes, minimize adverse effects and treat
comorbidities and associated infection (1-3).
Novel ART regimens are designed to be highly potent
while maintaining a better safety profile. Integrase strand
inhibitors (INSTIs) have emerged as a cornerstone in first-line
treatment regimens. These include first-generation INSTIs such as
raltegravir and elvitegravir and second-generation agents such as
dolutegravir, bictegravir and cabotegravir (4,5-7).
Bictegravir, available as a fixed-dose combination tablet with the
nucleos(t)ide reverse transcriptase inhibitors (NRTIs)
emtricitabine and tenofovir alafenamide (B/F/TAF), has been
approved both by the U.S. Food and Drug Administration and European
Medicines Agency for once-daily treatment. Landmark phase III
trials have demonstrated its efficacy in both treatment-naïve
people living with HIV (PLWH) and as a switch regimen (4-7).
B/F/TAF does not require pre-treatment molecular detection of Human
Leukocyte Antigen B allele, which has already been associated with
hypersensitivity to the nucleoside-reverse transcriptase inhibitor
(NRTI) abacavir (8). The B/F/TAF
regimen exhibits activity against hepatitis B virus and HIV-1 non-B
subtypes and is suitable for ‘rapid start’ treatment, since it is
has a high genetic barrier against resistance development (9). Additionally, it has low drug-drug
interactions, comes as a fixed-dose combination and has simple
administration guidelines regarding meals, making it a convenient
option for PLWH (4,10). As an effective switch strategy,
B/F/TAF has shown non-inferiority to continuing a number of
regimens, including those with pre-existing NRTI mutations, in
several randomized controlled trials (4-7,9,10).
Real-world studies further support the virological efficacy and
safety of B/F/TAF as a switch regimen (4-7,9,10).
The aim of the present retrospective study was to
evaluate the efficacy of B/F/TAF in a real-world setting, focusing
on virological, immunological, safety and metabolic profiles.
Data from a cohort of 127 PLWH enrolled from April
2019 to May 2022 at ARNAS Garibaldi in Catania, Italy, were
statistically analyzed to determine a safety and efficacy profile
of B/F/TAF. Cohort's age ranged from 23 to 72 years, with a mean
age of 46.8 (±10.7) years. The majority of the cohort (75,6%) was
composed by male individuals, and sexual transmission was the main
cause of infection (88.1%).
The inclusion criteria were as follows: i) HIV-1
infection, ii) age >18 years and iii) written informed consent.
Exclusion criteria were pregnancy and other ongoing treatments
incompatible with B/F/TAF.
Epidemiological and clinical data were collected
from 107 patients. The epidemiological data gathered at baseline
included age, sex, ethnicity, previous regimen, height, weight,
comorbidities (such as viral co-infection, hypertension, obesity,
diabetes, dyslipidaemia, cancer, osteoporosis, hyperthyroidism,
hypothyroidism and psychiatric disease), acquired immune deficiency
syndrome (AIDS)-associated diseases (oesophageal candidiasis,
Kaposi's sarcoma, cerebral toxoplasmosis, lymphoma, Pneumocystis
jirovecii pneumonia, progressive multifocal
leukoencephalopathy, atypical mycobacterial infection, cervical
carcinoma, hepatitis B or C and other co-infection), time since
onset of HIV, CD4 nadir, sexual orientation, transmission mode,
recreational habits, cigarette smoking and alcohol consumption.
Clinical parameters were assessed using clinical laboratory
automated instrumentation: High- and low-density lipoproteins (HDL
and LDL), triglycerides, Total cholesterol, creatinine,
Glutamate-Pyruvate transaminase (GPT) and Glutamate-Oxalate
Transaminase (GOT) were assessed using the DxC 500 AU Chemistry
Analyzer by Beckman Coulter; HIV Viral Load (VL) was analyzed using
the HIV-1 COBAS test on COBAS 4800 (Roche Diagnostics Corporation);
CD4+ and CD8+ T lymphocytes levels were assessed using
cytofluorimetric automated instrumentation BD Facs Canto II (BD
Biosciences) using the BD Multitest™ CD3 FITC/CD8 PE/CD45 PerCP/CD4
APC with BD Trucount™ Tubes (BD Biosciences), a specific four-color
reagent useful to determine the absolute count of CD4+ and CD8+
lymphocytes per microliter by gating all mature lymphocytes (CD3+)
using a specific fluorophore (Fluorescein Isothiocyanate, FITC).
Mature lymphocytes expressing both CD3+ and CD8+
glycoproteins are gated by assessing the fluorescence of both the
respective fluorophores (FITC and Phycoerithrin). The same process
is performed also for the CD4+ T lymphocytes, using Allophycocyanin
(APC) as a specific fluorophore. The total leukocytes count is
performed by targeting CD45+ cluster of differentiation, expressed
on all white blood cells. Peridinin-Chlorophyll-Protein (PerCP) was
used as the specific fluorophore for CD45+ glycoprotein. This
allows to obtain also the relative quantification of both CD4+ and
CD8+ cells.
Qualitative data were summarized using absolute and
relative frequencies. Quantitative variables are presented as
either mean and SD or medians and interquartile range. The normal
distribution was evaluated using Shapiro-Wilk test. The McNemar
test was used to compare qualitative variables between study
time-points. Comparisons of biochemical parameters were evaluated
using either one-way ANOVA for repeated measures followed by post
hoc Bonferroni's test or the non-parametric Friedman test.
P<0.05 was considered to indicate a statistically significant
difference. STATA17 software (2021. Stata: Release 17; StataCorp
LLC.) was used for statistical computations.
A total of 127 patients were enrolled, 96 (75.6%)
male and 31 (24.4%) female, with a mean age of 46.8±10.7 years
(Tables I and II), at the Infectious Disease Unit of the
Hospital Azienda di Rilievo Nazionale ed Alta Specializzazione
Garibaldi, University of Catania (Catania, Italy). Patients were
predominantly Caucasian (88.1%), heterosexual (54.7%), men who wave
sex with men (37.6%), with some bisexual (7.7%); thus, the mode of
HIV transmission was primarily sexual (88.1%; Table I). A total of 49.6% of patients were
smokers, 12.6% used alcohol, 9.5% were drug users and 11% reported
substance abuse (Table I). The
primary therapeutic regimens were elvitegravir + cobicistat +
Emtricitabine (FTC) + TAF (53.54%), dolutegravir + TAF + FTC
(7.88%), raltegravir + TAF + FTC (7.09%) and darunavir + cobicistat
+ FTC + TAF (7.09%). The primary reasons for treatment switch were
proactive strategy (34.52%), simplification (27.38%) or adverse
events (21.42%).
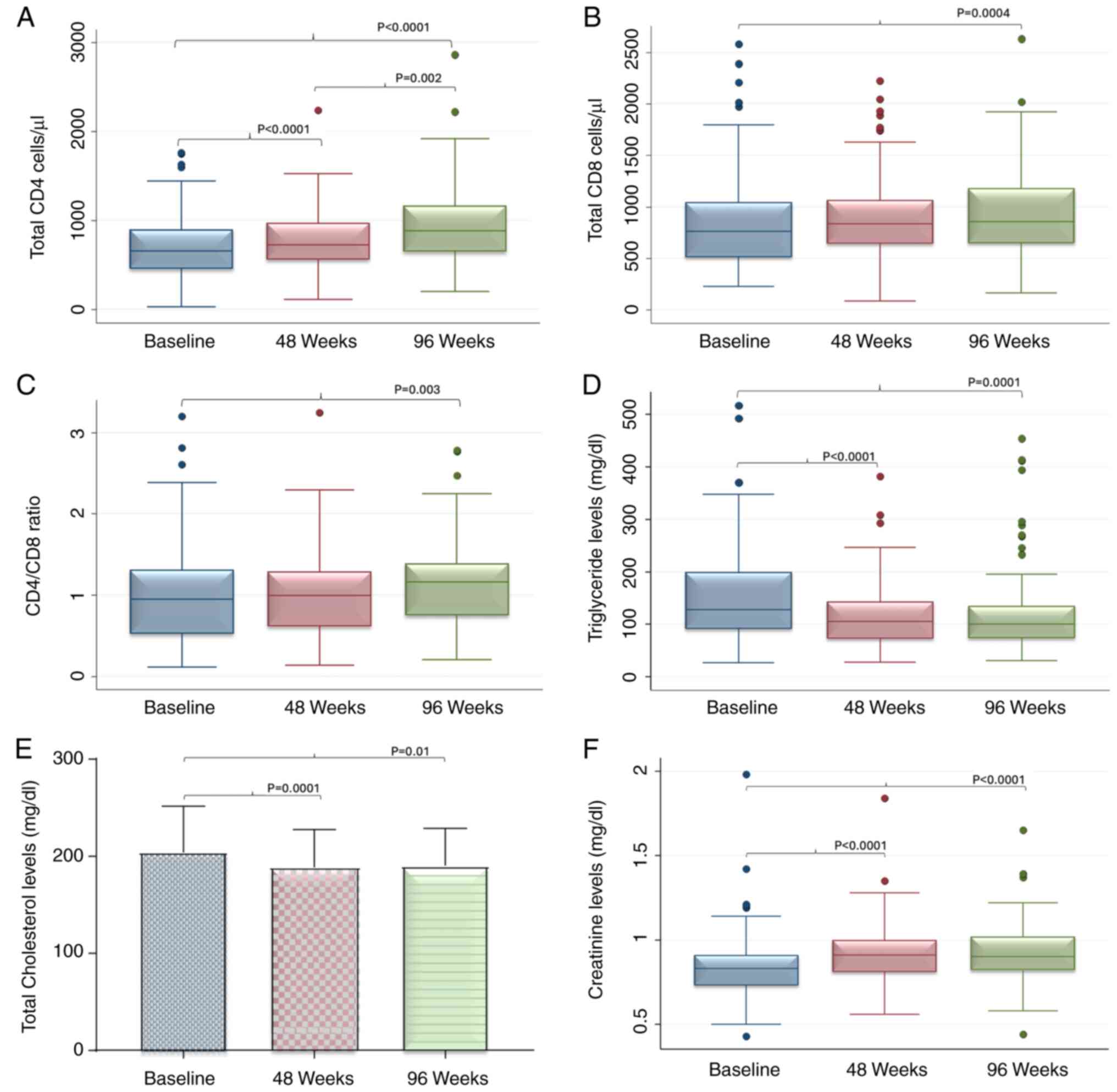
A significant increase in absolute CD4 cell count
was observed from 658 (459.5-900.5) to 726 (562-974) after 48 weeks
and to 882.5 (647.5-1169) cells/µl after 96 weeks (Table IV; Fig.
1A). Furthermore, the CD8 lymphocyte count significantly
increased. Baseline values of 760.5 (511.0-1044.5) were recorded,
while after 48 weeks the values were 837.6 (641-1066) and after 96
weeks 859.5 (648.5-1181; Fig. 1B)
cells/µl. Finally, there was a significant increase in CD4/CD8
ratio from 0.95 (0.52-1.31) to 1.00 (0.61-1.29) after 48 weeks and
1.16 (0.75-1.39) after 96 weeks (Fig.
1C).
At a metabolic level, there was a significant
decrease in the median values of triglyceride levels from a median
value of 128 (91-200) at baseline to 105 (73-143.5) at 48 and 100
(74-135) mg/dl at the 96 weeks (Table
V; Fig. 1C). A significant
decrease was also observed for total cholesterol levels from a mean
value of 204.2±47.5 at baseline to 188.6±38.5 after 48 weeks and
190.1±38.8 mg/dl after 96 weeks (Fig.
1E). Serum creatinine showed a significant increase from 0.83
(0.73-0.91) to 0.91 (0.81-1.02) after 48 weeks and to 0.90
(0.82-1.02) mg/dl after 96 weeks (Fig.
1F). Finally, significant changes in median GPT values were
found from 26.5 (18.5-38.5) at baseline to 29 (20-38.5) U/l after
48 weeks. The value decreased to 25.5 (20-40) U/l after 96 weeks.
Median GPT values remained between normal ranges of 5 to 35
U/l.
The present study confirmed the virological and
immunological efficacy of B/F/TAF, as well as a favorable metabolic
profile. B/F/TAF led to a statistically significant increase in
CD4/CD8 ratio >1, which is clinically associated with a lower
probability of serious non-AIDS events (11,12).
Success of ART is measured by virological
suppression (viral load <50/copies/ml) or the ability to
maintain viral load <200 copies/ml (8,13,14).
ART can lower the viral load not only in blood, but also in the
semen and cervicovaginal and anorectal secretions, reducing the
risk of sexual transmission (14,15).
Hence, viral suppression is crucial in HIV treatment. Switching to
B/F/TAF has been shown to maintain viral load <50 copies in
previous studies (16-31).
The present study confirmed the aforementioned data, showing a
higher percentage of patients who were virus-suppressed at 96
weeks.
HIV exhibits a specific tropism for CD4 lymphocytes,
targeting and killing these cells and decreasing their plasma
concentration (32,33). ART lowers viral load, which leads to
an increase in CD4 count (34,35). A
higher CD4 cell count is associated with decreased risk of clinical
progression in PLWH (12). In the
literature, patients who switch to B/F/TAF maintained the CD4 count
induced by previous therapy (18,25,26).
Absolute CD4 count increases but to the best of our knowledge, only
Chen et al (18), Lazzaro
et al (25) and Lazzaro
et al (26) found a
significant increase in CD4 count in patients who switched to
B/F/TAF.
During the asymptomatic stage of HIV infection,
naive CD8 T cells are progressively depleted (36,37).
Although the total number of CD8 T cells increases during this
stage, the number of naive CD8 T cells decreases in parallel with
overall CD4 T cell count (36,37).
The use of ART during primary HIV infection helps preserve
HIV-specific CD8+ T cells both physically and
functionally, while sustaining HIV-specific T helper cells
(38). Patients who maintain high
CD8 lymphocyte count (>1,500 cells/µl) despite ART are at a
higher risk of developing adverse events (12). Conditions with increased incidence
in these patients include myocardial infarction (39), restenosis following coronary
stenting (40), cancer (41) and non-AIDS-associated mortality
(42). CD4/CD8 ratio is an
important marker of inflammation and associated with serious
non-AIDS events (12). As
aforementioned, ART typically increases CD4 count and CD4/CD8 ratio
(11,12). Low CD4/CD8 ratio is associated with
older age in PLWH (43). Chronic
HIV infection has been observed to cause immunological changes
similar to those seen in natural aging (44). Low CD4/CD8 ratio is associated with
impaired vaccine response (45),
bacterial (46), fungal (47) and zoonotic infections (48), myocardial infarction (43), cancer (49) and non-AIDS mortality (12). The present study found a significant
increase in both CD4 and CD8 T cell count and CD4/CD8 ratio in
patients who switched to B/F/TAF. Despite the increase in CD8 T
cells, the simultaneous increase in CD4 T cells increased CD4/CD8
ratio. Similar results were observed in studies that measured both
CD8 count and CD4/CD8 ratio, with both metrics showing significant
increases (25,26).
HIV-1, via its accessory protein Nef, impairs the
efflux of cholesterol from macrophages (50). This virus-mediated shift redirects
cholesterol transport from normal physiological efflux to
virus-controlled transport, thereby decreasing the cell ability to
export excess cholesterol (50).
ART is not always successful in keeping triglyceride or LDL levels
low (51). This is due to several
factors, including continuous inflammation and immune activation
(as ART achieves viral suppression but not elimination),
mitochondrial dysfunction and altered distribution of adipose
tissue (52). Frequent dyslipidemia
leads to increased cardiovascular risk in PLWH (52,53).
Switching to B/F/TAF has been shown to be effective in maintaining
lipid levels in the normal range: Most studies have found no
significant changes in lipid profile (17,20,27,28,31,54,55).
However, certain studies have showed significant decreases in
triglyceride (18,19,21,22,56),
total cholesterol (18,25,30,55)
and LDL levels (18,25,30)
and total cholesterol/HDL ratio (19,25,56)
and increased HDL levels (18,21,25).
Chen et al (18) revealed
also a significant increase in patients taking lipid lowering
agents. Lazzaro et al (26)
confirmed that after 96 weeks, lipid values remained constant.
Here, significant decreases in triglyceride and total cholesterol
levels were recorded, suggesting that switching to B/F/TAF may lead
to an improvement in lipid profile.
Serum creatinine concentration is serves as a
measure of the glomerular filtration rate and is commonly used as
an index of renal function in clinical practice (57,58).
In HIV infection, renal damage can occur due to both the infection
and the therapy (59), particularly
with the use of TAF (60).
Significant increases in serum creatinine were recorded by Lazzaro
et al (25,26), a small increase was reported by
Kityo et al (22), while
Molina et al (28) did not
record significant changes. Here, a significant increase in serum
creatinine levels was reported in patients switching to B/F/TAF.
However, values remained within normal range (0.63-1.16 for male
and 0.48-0.93 mg/dl for female patients) (61).
GPT is an enzyme used as a marker of hepatocyte
lysis, systemic inflammation and oxidative stress (62). The persistent activation of immune
cells by HIV, along with ongoing inflammation and oxidative stress
related to HIV infection, leads to increased GPT levels, even in
patients on Highly Active Antiretroviral Therapy (HAART) (63). Lazzaro et al (25,26)
showed a decrease in GPT levels at 48 weeks and increase at 96
weeks in patients who switched to B/F/TAF, however, these changes
were not significant. Another study found that naïve patients on
B/F/TAF experienced decrease in GPT levels, while patients who
switched to B/F/TAF treatment showed an increase in GPT levels
(56). The present study showed
significant changes in GPT levels: At 48 weeks there was an
increase, but at 96 weeks there was a decrease. However, the median
value did not exceed the cut-off threshold of 40 U/l, a value
beyond which it becomes an indicator of liver damage (64,65).
One of the primary limitations of the present study
is the small samples size (127 patients). This is mainly due to the
real-world nature of the study, with patients enrolled and followed
by only one center. Additionally, lipid levels showed a significant
reduction, but it was not possible to retrieve data on
administration of statins and dietary supplements to lower lipid
levels, thus is not possible to determine the effects of these
molecules on lipid levels. Due to the real-world nature of the
cohort, it was not possible to obtain data regarding adherence to
treatment, which may have been useful to determine the impact of
therapeutic adherence on outcomes.
In real life, switching to B/F/TAF was safe and
highly effective both virologically and immunologically. The
present study reported a decrease in cholesterol and triglyceride
levels, showing a favorable metabolic profile, which may reduce
inflammation, leading to a healthier state and less organ
damage.
Not applicable.
Funding: No funding was received.
The data generated in the present study may be
requested from the corresponding author.
BC, SS, GNC and AM conceived the study. VF, GF, VR,
BMC and GFP designed the study. MVP, GS and RB analyzed data. EVN,
EVR, GN, NV, AM, VC, MSPR, EP and VB performed experiments. EVR and
GN wrote the manuscript. VF and GF reviewed the manuscript. BC, EVN
and GN supervised the study. All authors have read and approved the
final manuscript. SS and GN confirm the authenticity of all the raw
data.
All participants provided written informed consent
to partake in the study. The study was conducted in accordance with
the Declaration of Helsinki and approved by the Provincial Ethics
Committee of Messina (approval no. 34/17 of the 22/03/2017).
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Trickey A, Sabin CA, Burkholder G, Crane
H, d'Arminio Monforte A, Egger M, Gill MJ, Grabar S, Guest JL,
Jarrin I, et al: Life expectancy after 2015 of adults with HIV on
long-term antiretroviral therapy in Europe and North America: A
collaborative analysis of cohort studies. Lancet HIV. 10:e295–e307.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marino A, Zafarana G, Ceccarelli M,
Cosentino F, Moscatt V, Bruno G, Bruno R, Benanti F, Cacopardo B
and Celesia BM: Immunological and clinical impact of DAA-Mediated
HCV Eradication in a Cohort of HIV/HCV Coinfected Patients:
Monocentric Italian Experience. Diagnostics (Basel).
11(2336)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
D'Andrea F, Venanzi Rullo E, Marino A,
Moscatt V, Celesia BM, Cacopardo B, Condorelli F, La Rocca G, Di
Rosa M, Pellicanò GF, et al: Hepatitis B virus infection and
hepatocellular carcinoma in PLWH: Epidemiology, pathogenesis and
treatment. World Cancer Res J. 7(e1537)2020.
|
|
4
|
Peters E and Iwuji AC: Efficacy, safety
and tolerability of Biktarvy in HIV-1 infection: A scoping review.
Antivir Ther. 28(13596535231159030)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Celesia BM, Marino A, del Vecchio RF,
Bruno R, Palermo F, Gussio M, Nunnari G and Cacopardo B: Is it Safe
and Cost Saving to Defer the CD4+ cell count monitoring in stable
patients on art with more than 350 or 500 cells/µl? Mediterr J
Hematol Infect Dis. 11(e2019063)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Celesia BM, Marino A, Borracino S,
Arcadipane AF, Pantò G, Gussio M, Coniglio S, Pennisi A, Cacopardo
B and Panarello G: Successful extracorporeal membrane oxygenation
treatment in an acquired immune deficiency syndrome (AIDS) patient
with acute respiratory distress syndrome (ARDS) Complicating
Pneumocystis jirovecii Pneumonia: A Challenging Case. Am J Case
Rep. 21(e919570)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Micali C, Russotto Y, Facciolà A, Marino
A, Celesia BM, Pistarà E, Caci G, Nunnari G, Pellicanò GF and
Venanzi Rullo E: Pulmonary kaposi sarcoma without respiratory
symptoms and skin lesions in an HIV-Naïve Patient: A case report
and literature review. Infect Dis Rep. 14:228–242. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ambrosioni J, Levi L, Alagaratnam J, Van
Bremen K, Mastrangelo A, Waalewijn H, Molina JM, Guaraldi G,
Winston A, Boesecke C, et al: Major revision version 12.0 of the
European AIDS Clinical Society guidelines 2023. HIV Med.
24:1126–1136. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Marino A, Cosentino F, Ceccarelli M,
Moscatt V, Pampaloni A, Scuderi D, D'Andrea F, Rullo EV, Nunnari G,
Benanti F, et al: Entecavir resistance in a patient with
treatment-naïve HBV: A case report. Mol Clin Oncol.
14(113)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marino A, Pavone G, Martorana F, Fisicaro
V, Motta L, Spampinato S, Celesia BM, Cacopardo B, Vigneri P and
Nunnari G: Navigating the Nexus: HIV and Breast Cancer-A Critical
Review. Int J Mol Sci. 25(3222)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ron R, Martínez-Sanz J, Herrera S,
Ramos-Ruperto L, Díez A, Sainz T, Álvarez-Díaz N, Correa-Pérez A,
Muriel A, López-Alcalde J, et al: CD4/CD8 ratio and CD8+ T-cell
count as prognostic markers for non-AIDS mortality in people living
with HIV. A systematic review and meta-analysis. Front Immunol.
15(1343124)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Serrano-Villar S, Wu K, Hunt PW, Lok JJ,
Ron R, Sainz T, Moreno S, Deeks SG and Bosch RJ: Predictive value
of CD8+ T cell and CD4/CD8 ratio at two years of successful ART in
the risk of AIDS and non-AIDS events. EBioMedicine.
80(104072)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
World Health Organization (WHO):
Consolidated guidelines on HIV prevention, testing, treatment,
service delivery and monitoring: Recommendations for a public
health approach. WHO, Geneva, 2021.
|
|
14
|
Baeten JM, Donnell D, Ndase P, Mugo NR,
Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira
E, et al: Antiretroviral prophylaxis for HIV prevention in
heterosexual men and women. N Engl J Med. 367:399–410.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Baeten JM, Kahle E, Lingappa JR, Coombs
RW, Delany-Moretlwe S, Nakku-Joloba E, Mugo NR, Wald A, Corey L,
Donnell D, et al: Genital HIV-1 RNA predicts risk of heterosexual
HIV-1 transmission. Sci Transl Med. 3(77ra29)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Acosta RK, Willkom M, Andreatta K, Liu H,
Martin R, Parvangada A, Martin H, Collins S and White KL: Switching
to Bictegravir/Emtricitabine/Tenofovir Alafenamide (B/F/TAF) From
Dolutegravir (DTG)+F/TAF or DTG+F/Tenofovir Disoproxil Fumarate
(TDF) in the Presence of Pre-existing NRTI Resistance. J Acquir
Immune Defic Syndr. 85:363–371. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chang HM, Chou PY, Chou CH and Tsai HC:
Outcomes After Switching to BIC/FTC/TAF in patients with
virological failure to protease inhibitors or non-nucleoside
reverse transcriptase inhibitors: A real-world cohort study. Infect
Drug Resist. 14:4877–4886. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen LY, Sun HY, Chuang YC, Huang YS, Liu
WD, Lin KY, Chang HY, Luo YZ, Wu PY, Su YC, et al: Patient-reported
outcomes among virally suppressed people living with HIV after
switching to Co-formulated bictegravir, emtricitabine and tenofovir
alafenamide. J Microbiol Immunol Infect. 56:575–585.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Daar ES, DeJesus E, Ruane P, Crofoot G,
Oguchi G, Creticos C, Rockstroh JK, Molina JM, Koenig E, Liu YP, et
al: Efficacy and safety of switching to fixed-dose bictegravir,
emtricitabine, and tenofovir alafenamide from boosted protease
inhibitor-based regimens in virologically suppressed adults with
HIV-1: 48 week results of a randomised, open-label, multicentre,
phase 3, non-inferiority trial. Lancet HIV. 5:e347–e356.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
De Socio GV, Tordi S, Altobelli D, Gidari
A, Zoffoli A and Francisci D: Dolutegravir/Lamivudine versus
Tenofovir Alafenamide/Emtricitabine/Bictegravir as a Switch
Strategy in a Real-Life Cohort of Virogically Suppressed People
Living with HIV. J Clin Med. 12(7759)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hagins D, Kumar P, Saag M, Wurapa AK, Brar
I, Berger D, Osiyemi O, Hileman CO, Ramgopal MN, McDonald C, et al:
Switching to Bictegravir/Emtricitabine/Tenofovir Alafenamide in
Black Americans With HIV-1: A Randomized Phase 3b, Multicenter,
Open-Label Study. J Acquir Immune Defic Syndr. 88:86–95.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kityo C, Hagins D, Koenig E, Avihingsanon
A, Chetchotisakd P, Supparatpinyo K, Gankina N, Pokrovsky V,
Voronin E, Stephens JL, et al: Switching to Fixed-Dose Bictegravir,
Emtricitabine, and Tenofovir Alafenamide (B/F/TAF) in Virologically
Suppressed HIV-1 Infected Women: A Randomized, Open-Label,
Multicenter, Active-Controlled, Phase 3, Noninferiority Trial. J
Acquir Immune Defic Syndr. 82:321–328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Knobel H, Cañas-Ruano E, Guelar A, Knobel
P, Villar-García J, González-Mena A, Canepa C, Arrieta-Aldea I,
Marcos A, Abalat-Torrres A and Güerri-Fernández R: Switching to
Dolutegravir/lamivudine or Bictegravir/Emtricitabine/Tenofovir
alafenamide. A comparative real-world study. HIV Res Clin Pract.
24(2239564)2023.PubMed/NCBI
|
|
24
|
Lake JE, Hyatt AN, Feng H, Debroy P,
Kettelhut A, Miao H, Peng L, Bhasin S, Bell S, Rianon N, et al: A
randomized clinical trial of transgender women switching to
B/F/TAF: The (mo)BETTA Trial. Open Forum Infect Dis.
10(ofad178)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lazzaro A, Cacciola EG, Borrazzo C,
Innocenti GP, Cavallari EN, Mezzaroma I, Falciano M, Fimiani C,
Mastroianni CM, Ceccarelli G and d'Ettorre G: Switching to a
Bictegravir Single Tablet Regimen in Elderly People Living with
HIV-1: Data Analysis from the BICTEL Cohort. Diagnostics (Basel).
12(76)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lazzaro A, Bianchini D, Gentilini Cacciola
E, Mezzaroma I, Falciano M, Andreoni C, Fimiani C, Santinelli L,
Maddaloni L, Bugani G, et al: Immune Reconstitution and Safe
Metabolic Profile after the Switch to
Bictegravir/Emtricitabine/Tenofovir Alafenamide Fumarate among
Virologically Controlled PLWH: A 96 Week Update from the BICTEL
Cohort. Viruses. 15(1222)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Maggiolo F, Rizzardini G, Molina JM,
Pulido F, De Wit S, Vandekerckhove L, Berenguer J, D'Antoni ML,
Blair C, Chuck SK, et al: Bictegravir/Emtricitabine/Tenofovir
Alafenamide in Virologically Suppressed People with HIV Aged ≥ 65
Years: Week 48 Results of a Phase 3b, Open-Label Trial. Infect Dis
Ther. 10:775–788. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Molina JM, Ward D, Brar I, Mills A,
Stellbrink HJ, López-Cortés L, Ruane P, Podzamczer D, Brinson C,
Custodio J, et al: Switching to fixed-dose bictegravir,
emtricitabine, and tenofovir alafenamide from dolutegravir plus
abacavir and lamivudine in virologically suppressed adults with
HIV-1: 48 week results of a randomised, double-blind, multicentre,
active-controlled, phase 3, non-inferiority trial. Lancet HIV.
5:e357–e365. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tsai MS, Sun HY, Chen CP, Lee CH, Lee CY,
Liu CE, Tang HJ, Hung TC, Li CW, Lee YT, et al: Switching to
coformulated bictegravir, emtricitabine, and tenofovir alafenamide
maintained viral suppression in adults with historical virological
failures and K65N/R mutation. Int J Infect Dis. 126:39–47.
2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rolle CP, Nguyen V, Patel K, Cruz D,
DeJesus E and Hinestrosa F: Real-world efficacy and safety of
switching to bictegravir/emtricitabine/tenofovir alafenamide in
older people living with HIV. Medicine (Baltimore).
100(e27330)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sax PE, Rockstroh JK, Luetkemeyer AF,
Yazdanpanah Y, Ward D, Trottier B, Rieger A, Liu H, Acosta R,
Collins SE, et al: Switching to bictegravir, emtricitabine, and
tenofovir alafenamide in virologically suppressed adults with human
immunodeficiency virus. Clin Infect Dis. 73:e485–e493.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Klatzmann D, Barré-Sinoussi F, Nugeyre MT,
Danquet C, Vilmer E, Griscelli C, Brun-Veziret F, Rouzioux C,
Gluckman JC, Chermann JC, et al: Selective tropism of
lymphadenopathy associated virus (LAV) for helper-inducer T
lymphocytes. Science. 225:59–63. 1984.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Doitsh G and Greene WC: Dissecting How CD4
T Cells Are Lost During HIV Infection. Cell Host Microbe.
19:280–291. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bishop JD, DeShields S, Cunningham T and
Troy SB: CD4 count recovery after initiation of antiretroviral
therapy in patients infected with human immunodeficiency virus. Am
J Med Sci. 352:239–244. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ho DD, Neumann AU, Perelson AS, Chen W,
Leonard JM and Markowitz M: Rapid turnover of plasma virions and
CD4 lymphocytes in HIV-1 infection. Nature. 373:123–126.
1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Roederer M, Dubs JG, Anderson MT, Raju PA
and Herzenberg LA and Herzenberg LA: CD8 naive T cell counts
decrease progressively in HIV-infected adults. J Clin Invest.
95:2061–2066. 1995.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Favre D, Stoddart CA, Emu B, Hoh R, Martin
JN, Hecht FM, Deeks SG and McCune JM: HIV disease progression
correlates with the generation of dysfunctional naive CD8(low) T
cells. Blood. 117:2189–2199. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oxenius A, Price DA, Easterbrook PJ,
O'Callaghan CA, Kelleher AD, Whelan JA, Sontag G, Sewell AK and
Phillips RE: Early highly active antiretroviral therapy for acute
HIV-1 infection preserves immune function of CD8+ and CD4+ T
lymphocytes. Proc Natl Acad Sci USA. 97:3382–3387. 2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Badejo OA, Chang CC, So-Armah KA, Tracy
RP, Baker JV, Rimland D, Butt AA, Gordon AJ, Rinaldo CR Jr, Kraemer
K, et al: CD8+ T-Cells Count in Acute Myocardial Infarction in HIV
disease in a predominantly male cohort. Biomed Res Int.
2015(246870)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schneider S, Spinner CD, Cassese S, Promny
D, Hapfelmeier A, Byrne RA, Baumann M, Jäger H, Steinlechner E,
Laugwitz KL and Kastrati A: Association of increased CD8+ and
persisting C-reactive protein levels with restenosis in HIV
patients after coronary stenting. AIDS. 30:1413–1421.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Borges ÁH, Neuhaus J, Babiker AG, Henry K,
Jain MK, Palfreeman A, Mugyenyi P, Domingo P, Hoffmann C, Read TR,
et al: Immediate antiretroviral therapy reduces risk of
infection-related cancer during early HIV Infection. Clin Infect
Dis. 63:1668–1676. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Helleberg M, May MT, Ingle SM, Dabis F,
Reiss P, Fätkenheuer G, Costagliola D, d'Arminio A, Cavassini M,
Smith C, et al: Smoking and life expectancy among HIV-infected
individuals on antiretroviral therapy in Europe and North America.
AIDS. 29:221–229. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Castilho JL, Shepherd BE, Koethe J, Turner
M, Bebawy S, Logan J, Rogers WB, Raffanti S and Sterling TR:
CD4+/CD8+ ratio, age, and risk of serious non-communicable diseases
in HIV-infected adults on antiretroviral therapy. AIDS. 30:899–908.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Dock JN and Effros RB: Role of CD8 T cell
replicative senescence in human aging and in HIV-mediated
Immunosenescence. Aging Dis. 2:382–397. 2011.PubMed/NCBI
|
|
45
|
Avelino-Silva VI, Miyaji KT, Hunt PW,
Huang Y, Simoes M, Lima SB, Freire MS, Caiaffa-Filho HH, Hong MA,
Costa DA, et al: CD4/CD8 Ratio and KT Ratio Predict Yellow Fever
Vaccine Immunogenicity in HIV-Infected Patients. PLoS Negl Trop
Dis. 10(e0005219)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Collin A, Le Marec F, Vandenhende MA,
Lazaro E, Duffau P, Cazanave C, Gérard Y, Dabis F, Bruyand M and
Bonnet F: ANRS CO3 Aquitaine Cohort Study Group. Incidence and risk
factors for severe bacterial infections in people living with HIV.
ANRS CO3 Aquitaine Cohort, 2000-2012. PLoS One.
11(e0152970)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Vrinceanu D, Dumitru M, Patrascu OM,
Costache A, Papacocea T and Cergan R: Current diagnosis and
treatment of rhinosinusal aspergilloma (Review). Exp Ther Med.
22(1264)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Saydam FN, Erdem H, Ankarali H, El-Arab
Ramadan ME, El-Sayed NM, Civljak R, Pshenichnaya N, Moroti RV,
Mahmuodabad FM, Maduka AV, et al: Vector-borne and zoonotic
infections and their relationships with regional and socioeconomic
statuses: An ID-IRI survey in 24 countries of Europe, Africa and
Asia. Travel Med Infect Dis. 44(102174)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sigel K, Wisnivesky J, Crothers K, Gordon
K, Brown ST, Rimland D, Rodriguez-Barradas MC, Gibert C, Goetz MB,
Bedimo R, et al: Immunological and infectious risk factors for lung
cancer in US veterans with HIV: A longitudinal cohort study. Lancet
HIV. 4:e67–e73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mujawar Z, Rose H, Morrow MP, Pushkarsky
T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM,
Bobryshev YV, et al: Human immunodeficiency virus impairs reverse
cholesterol transport from macrophages. PLoS Biol.
4(e365)2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lu L, Yang Y, Yang Z, Wu Y, Liu X, Li X,
Chen L, Han Y, Song X, Kong Z, et al: Altered plasma metabolites
and inflammatory networks in HIV-1 infected patients with different
immunological responses after long-term antiretroviral therapy.
Front Immunol. 14(1254155)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Papantoniou E, Arvanitakis K, Markakis K,
Papadakos SP, Tsachouridou O, Popovic DS, Germanidis G, Koufakis T
and Kotsa K: Pathophysiology and clinical management of
dyslipidemia in people living with HIV: Sailing through Rough Seas.
Life (Basel). 14(449)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Stanley TL and Grinspoon SK: Body
composition and metabolic changes in HIV-Infected Patients. J
Infect Dis. 205 (Suppl 3):S383–S390. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Avihingsanon A, Chetchotisakd P,
Kiertiburanakul S, Ratanasuwan W, Siripassorn K, Supparatpinyo K,
Martin H, Wang H, Wong T and Wang HY: Efficacy and safety of
switching to bictegravir, emtricitabine, and tenofovir alafenamide
in virologically suppressed Asian adults living with HIV: A pooled
analysis from three international phase III randomized trials. HIV
Med. 24:290–300. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Maggiolo F, Rizzardini G, Molina JM,
Pulido F, De Wit S, Vandekerckhove L, Berenguer J, D'Antoni ML,
Blair C, Chuck SK, et al: Bictegravir/emtricitabine/tenofovir
alafenamide in older individuals with HIV: Results of a 96-week,
phase 3b, open-label, switch trial in virologically suppressed
people ≥65 years of age. HIV Med. 24:27–36. 2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Squillace N, Ricci E, Maggi P, Taramasso
L, Menzaghi B, De Socio GV, Piconi S, Maurizio Celesia B, Orofino
G, Sarchi E, et al: Real-life safety of Emtricitabine/Tenofovir
Alafenamide/Bictegravir. PLoS One. 18(e0289132)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Perrone RD, Madias NE and Levey AS: Serum
creatinine as an index of renal function: new insights into old
concepts. Clin Chem. 38:1933–1953. 1992.PubMed/NCBI
|
|
58
|
Kashani K, Rosner MH and Ostermann M:
Creatinine: From physiology to clinical application. Eur J Intern
Med. 72:9–14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ando M and Yanagisawa N: Epidemiology,
clinical characteristics, and management of chronic kidney disease
in human immunodeficiency virus-infected patients. World J Nephrol.
4:388–395. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ryom L, Mocroft A, Kirk O, Worm SW, Kamara
DA, Reiss P, Ross M, Fux CA, Morlat P, Moranne O, et al:
Association between antiretroviral exposure and renal impairment
among HIV-positive persons with normal baseline renal function: The
D:A:D study. J Infect Dis. 207:1359–1369. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pottel H, Vrydags N, Mahieu B,
Vandewynckele E, Croes K and Martens F: Establishing age/sex
related serum creatinine reference intervals from hospital
laboratory data based on different statistical methods. Clin Chim
Acta. 396:49–55. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yamada J, Tomiyama H, Yambe M, Koji Y,
Motobe K, Shiina K, Yamamoto Y and Yamashina A: Elevated serum
levels of alanine aminotransferase and gamma glutamyltransferase
are markers of inflammation and oxidative stress independent of the
metabolic syndrome. Atherosclerosis. 189:198–205. 2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Nixon DE and Landay AL: Biomarkers of
immune dysfunction in HIV. Curr Opin HIV AIDS. 5:498–503.
2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Siest G, Schiele F, Galteau MM, Panek E,
Steinmetz J, Fagnani F and Gueguen R: Aspartate aminotransferase
and alanine aminotransferase activities in plasma: statistical
distributions, individual variations, and reference values. Clin
Chem. 21:1077–1087. 1975.PubMed/NCBI
|
|
65
|
Sookoian S and Pirola CJ: Liver enzymes,
metabolomics and genome-wide association studies: From systems
biology to the personalized medicine. World J Gastroenterol.
21:711–725. 2015.PubMed/NCBI View Article : Google Scholar
|