Introduction
Lung cancer is the most common type of cancer death
globally with an estimated 1.6 million fatalities each year
(1). Non-small cell lung cancer
(NSCLC) constitutes ~85% of lung cancer cases (2). Doxorubicin is a chemotherapeutic
frequently prescribed to treat a variety of solid tumors (3). An imbalance between the oxygen supply
and demand for cancer cells is known as hypoxia (4). Hypoxia, or the lack of oxygen, is a
characteristic of solid tumors that encourages genomic instability,
increased aggressiveness, and metastasia; hypoxia plays a
significant role in treatment resistance and poor survival
(5,6).
A total of two types of hypoxia have been identified
according to the duration of exposure: acute hypoxia and chronic
hypoxia; each with distinct biological effects (7). Normal human lung tissue has an oxygen
concentration of ~5.6%, whereas NSCLC has an oxygen concentration
of 1.9-2.2% (8,9). Marhuenda et al (10) demonstrated that lung cancer cell
growth is differentially enhanced by intermittent and continuous
hypoxia regimes. Acute hypoxia is considered to be a sudden and
quick exposure to short-term hypoxic circumstances when the blood
vessel closure lasts for several minutes (11). Accordingly, A549 cells were
subjected to continuous hypoxia in vitro for a few minutes
to 72 h (12). Thus, hypoxia will
lead to a number of adaptive processes such as autophagy that
reduce oxidative metabolism to enable the cell to survive under
these conditions (12). Notably,
the resulting reactive oxygen species can lead to tumor cell
survival and development (13).
Acute hypoxia makes tumors more aggressive because
it promotes spontaneous metastasis (14). Acute hypoxia also results in genomic
instability due to a delayed reaction to DNA damage (15). A rise in radiation and/or
chemotherapy resistance is seen in both chronic and acute hypoxia.
The increase in resistance may be brought on by hypoxia, which can
shorten senescence, produce disorganized and dysfunctional blood
vessels and promote metastasis (16).
Hypoxia can result in chemoresistance and thus it is
crucial to investigate the genetics of hypoxic cells for an
improved understanding of how the hypoxic microenvironment affects
the evolution of genetic changes. Accordingly, the present study
used a 96-well PCR array to determine the alterations in gene
expression linked to the emergence of doxorubicin-resistant
phenotype in non-small cell lung cancer cell line. The present
study used doxorubicin as a model drug because the hypoxic
phenotype is often resistant to doxorubicin as shown previously
(17).
To the to the best of the authors' knowledge, this
is the first study to measure how hypoxic cells develop resistance
at the genomic level. The cell line was exposed to cyclical and
intermittent hypoxia.
Materials and methods
Cell culture NSCLC cell line A549 was
purchased from the American Type Culture Collection. A549 cells
were maintained in Dulbecco's modified Eagle's medium (DMEM), high
glucose (Euroclone SpA), supplemented with 10% (v/v)
heat-inactivated bovine fetal blood (FBS) from Biowest, 1% 2 mM
L-glutamine, and 100 U/ml streptomycin and 100 g/ml penicillin from
Euroclone SpA. Cells were cultured at 37˚C in an incubator (NuAire,
Inc.) with 5% CO2 and 95% air. Under a class II
biological safety cabinet (Heal Force Bio-meditech Holdings Ltd.),
all cell culture processes were carried out sterile conditions.
Prior to use, 76% ethanol was used to disinfect all used items
including disposables.
Exposure to hypoxia
In order to create hypoxic conditions, an anaerobic
atmospheric generator, Oxoid AnaeroGen Compact (Thermo Fisher
Scientific, Inc.), was employed. To create and sustain anaerobic
conditions, the system comprised a tightly packed bag (sealed with
a plastic clip) and a gas-generating sachet. The vented culture
flasks were located inside the plastic bags, and the paper sachets
were taken out and put inside the bag before it was closed with a
plastic zip. A549 cells were exposed to 10 hypoxia events, each
lasting 72 h. The normoxic cells were cultured alongside the
hypoxic cells as a control group.
Cell proliferation assay
Following the manufacturer's instructions, a cell
proliferation assay was used to track the anti-proliferative
effects of doxorubicin on A549 cells and discover the resistance
pattern brought on by the hypoxia injections. Instead of a
clinically comparable medicine, doxorubicin was employed in this
work as an example because it is known to lose some of its activity
throughout the development of the hypoxic phenotype (18).
The assay was a colorimetric experiment that relies
on the reduction of MTT from a yellow tetrazole to a purple
formazan, a process that takes place in the mitochondria of live
cells. To summarize, a coated 96-well plate (Greiner Bio-One
International GmbH) was seeded with ~5x103 cells per
well. The hypoxic and normoxic cells were seeded in duplicate for
at least 24 h. Then the wells were used to aspirate the media.
Various concentrations of doxorubicin were added to each well. The
cells were then cultured at 37˚C for 72 h. After that, each well
received ~15 µl of MTT reagent. Then 100 µl of DMSO was added to
each well after the plates had been incubated for 4 h at 37˚C.
After that, plates were kept at room temperature in the dark
overnight.
The absorbance was recorded using a 96-well plate
reader (Elx808 Absorbance Microplate Reader; Biotek; Agilent
Technologies, Inc.) at a wavelength of 570 nm. The results of the
MTT cell proliferation assay were evaluated using GraphPad Prism
5.0 software (Dotmatics). From the logarithmic trend line of
cytotoxicity graphs, the inhibitory concentration (IC50)
values, which is the drug concentration at which 50 percent of
cells are viable, were calculated.
Ribonucleic acid sample
preparation
Following the manufacturer's instructions, total RNA
was extracted from normoxic cells and from A549 cells after 10
cycles of acute hypoxia using the TrizolLS Single-step RNA reagent;
Bio Basic, Inc.) The RNA was kept at -80˚C to prevent degradation
and the RNA concentration was measured using a NanoDrop 2000c
spectrophotometer (Thermo Scientific, Inc.). By comparing the
optical densities of the samples at 260 and 280 nm (Elx808
Absorbance Microplate Reader; Biotek; Agilent Technologies, Inc.),
the purity of extracted RNA was ascertained. For all samples, the
optical density (OD) 260/280 ratio ranged from 1.9-2.2.
Complementary deoxyribonucleic acid
synthesis
The RT2 First Strand kit (Qiagen GmbH) was used to
create complementary DNA strands in line with the manufacturer's
instructions. From each sample, aliquots containing 1 µg of total
RNA were used. Peak resistance, which occurred at the 10th episode
in the acute hypoxia model of the MTT colorimetric test, was used
to select the RNA for cDNA production. For comparison, the RNA of
normoxic cells was also used during the cDNA production process. A
NanoDrop 2000c spectrophotometer (Thermo Scientific, Inc.) was used
to assess cDNA quantity and quality.
Gene expression profiling using
PCR
The effect of hypoxia on gene expression of A549
cell lines was investigated using a 96-well Real Time 2 Profiler
PCR array (PAHS-004Z-D; Human Cancer Drug Resistance; cat. no.
330231; Qiagen, GmbH). (cat. no. 330231; Qiagen, GmbH) at 37˚C. In
this array, 96-well plates contain primers assays for 84 genes
known to respond to low oxygen concentration, in addition to 12
genes for quality control. Primers were provided by Qiagen GmbH
(sequences not available). cDNA was mixed with RT2 SYBR green
master mix (Qiagen GmbH) and nuclease-free water (Bio Basic, Inc.).
Then, 20 µl mix was placed in every well and the plate was
centrifuged (Hettich Holding GmbH & Co.) at 1,000 x g for 1 min
at room temperature to remove air bubbles. qPCR was performed using
the CFX (Bio-Rad Laboratories, Inc.) thermocycler as follows:
Initial denaturation at 95˚C for 10 min, followed by 40 cycles of
95˚C for 15 sec and 60˚C for 1 min. Fold change is calculated by
using the 2-∆∆Cq method (19) β-actin, β-2-microglobulin, GAPDH,
hypoxanthine phosphoribosyltransferase 1 and ribosomal protein
lateral stalk subunit P0 (RPLP0) were used as a control for
normalization.
The threshold for gene expression change was set at
two folds of regulation of gene expression for consideration of
significance. For the examination of the PCR arrays used in the
present study, such a cut off is advised. Therefore, it was
regarded to have a substantial effect if the level of gene
expression/repression is increased/decreased by two folds.
Fold change calculation
Fold change was calculated by using the ∆∆Cq method
originally published by Livak and Schmittgen (19). It is the ratio of the relative gene
expression of the control group to the test group. Numbers >1
indicate upregulated or increased gene expression, numbers between
0 and 1 indicate downregulated or decreased gene expression, and a
fold change of 1 indicates no change.
First, ∆Cq, also called the normalized raw data, for
each gene is calculated by subtracting the selected normalization
factor from the Cq value of each gene of interest. If one
housekeeping gene is selected, then its Cq value is used as the
normalization factor. The five housekeeping genes (ACTB, B2M,
GAPDH, HPRT1 and RPLP0) was used as a control for normalization.
The five different housekeeping genes are used in the fold of
changes calculation to increase the accuracy of normalization.
ΔCq = Cq (Gene of Interest) - Cq
(Housekeeping/Reference Gene)
Or+
ΔCq=Cq (Gene of Interest)-Average [Cq
(Housekeeping/Reference Genes)]
Next, gene-specific ∆Cq values in samples in the
same group were averaged.
Average ΔCTq = [ΔCTq (sample 1) + ΔCq (sample 2) +
ΔCq (sample 3) + … ΔCq (Sample n)]/n samples.
The ΔΔCq value was calculated by subtracting the ΔCq
value of each test group from the control group.
ΔΔCq = ΔCq (test group n) - ΔCq (control group)
The fold-change value is then calculated by
converting the ΔΔCq from a log2 scale to a linear scale using the
following equation:
Fold change = 2-ΔΔCq …. (5).
Statistical analysis
The replicate 2-Cq values for each gene in each
treatment group were compared with the control group to get the
P-values (two-tail distribution, equal variances between the two
samples). RT2 Profiler PCR Array Data Analysis Webportal was used
for the statistical analysis (https://geneglobe.qiagen.com/jo/analyze). It only
calculates P-values for groups that have at least three samples
(including the control group). The RT2 Profiler PCR Array Data
Analysis Handbook contains a complete description of the analysis.
Unpaired Student's t-test were been used were applicable.
The following formula was used to determine the
viability of cells based on the MTT experimental findings.
[(B-A)/A]*100 = viability%
Where A is the absorbance under the negative control
at 570 nm and B is the absorbance during the treatment at 570 nm.
Data sets from MTT tests were examined using GraphPad Prism
software version 9 (Dotmatics) to determine IC50
values.
The data were represented as the mean of the
calculated IC50 ± standard deviation. The experiments
were performed in three different biological replicates. P<0.05
was considered to indicate a statistically significant
difference.
Results
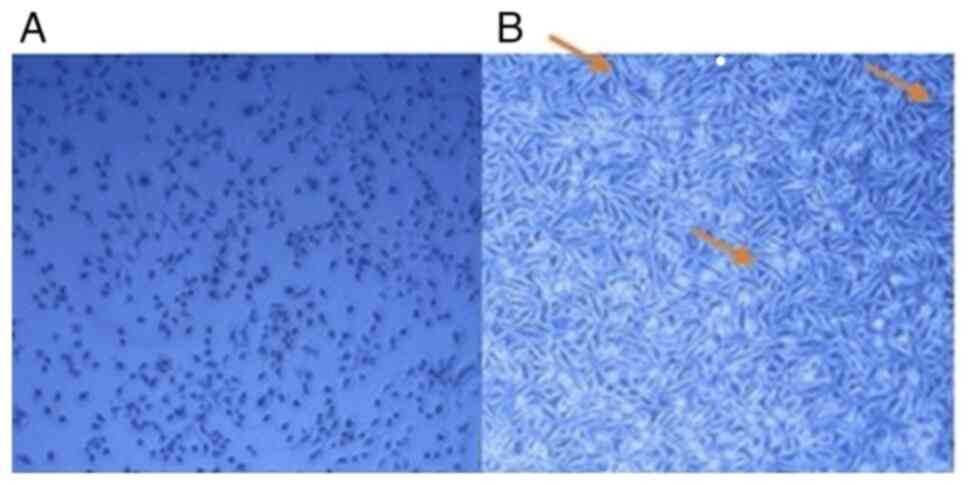
Effect of hypoxia on A549 cell line
morphology and proliferation
After receiving acute doses of hypoxia, the A549
cell line underwent changes in morphology and proliferation
relative to normoxic cells. The typical shape of the A549 cell line
is depicted in Fig. 1. Acute
hypoxia leads to a slower proliferation rate and increased
mortality of NSCLC cells (17). The
A549 cells developed an elongated spindle-shape morphology
characteristic of the epithelial-mesenchymal transition (EMT)
phenotype (Fig. 1). Longer hypoxic
episodes that simultaneously caused growth arrest resulted in more
significant morphological changes. Control cells doubled within 22
h, and the number of A549 cells were reduced when exposed to
hypoxia.
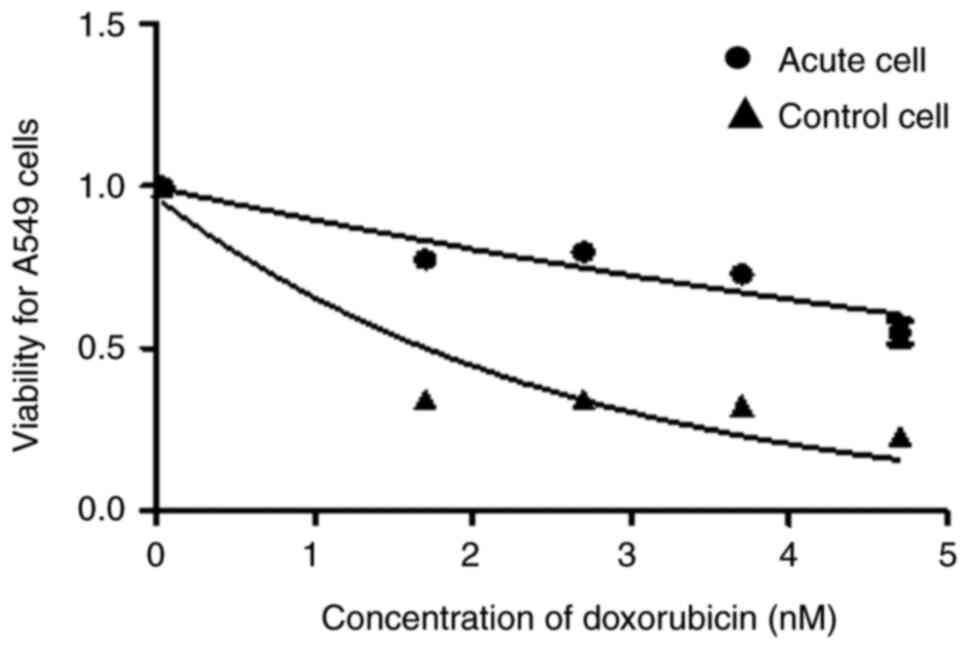
Effect of hypoxia on A549 cells
resistant to doxorubicin
The effect of doxorubicin on the growth of NSCLC
cells was evaluated by determining cell viability using the MTT
assay to test the development of drug resistance to the hypoxic
phenotype. The IC50 increased after 10 episodes of acute
hypoxia compared with normoxic cells (Table I). The viability of A549 cells was
higher than the control cells after 10 episodes of acute hypoxia
(Fig. 2).
 | Table IIC50 of doxorubicin in
acute hypoxic A549 cells measured at hypoxia episode 10. |
Table I
IC50 of doxorubicin in
acute hypoxic A549 cells measured at hypoxia episode 10.
|
IC50 | Mean | Standard deviation
of the mean |
|---|
| Control normoxic
A549 cells | 0.059 µM | ±0.0007 |
| Acute hypoxia A549
cells (10 episodes) | 8.164 µM | ±2.0000 |
| Fold change | 138,372 | |
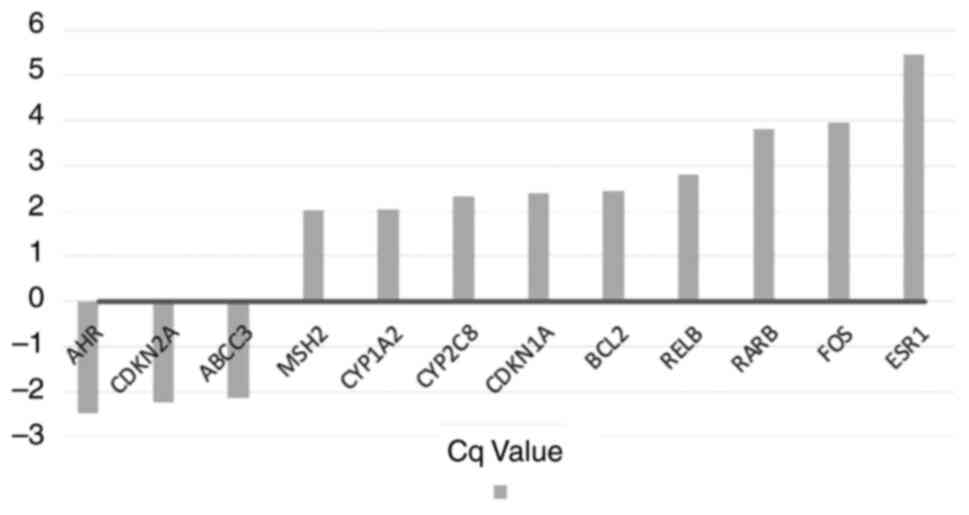
Gene expression in A549 cells exposed
to the acute hypoxia
For the purposes of the present study, a cut-off
value of two-fold was chosen to demonstrate marked up- and
downregulation of the genes due to hypoxia. the role of hypoxia in
altering the genetic expression and its relationship to drug
resistance could then be assessed. A total of nine genes were
notably upregulated compared with normoxic cells (Table II and Fig. 3) after 10 sessions of 72 h of acute
hypoxia; three were markedly downregulated (Table III and Fig. 3).
 | Table IIGenes upregulated in acute
hypoxia-A549 cells. |
Table II
Genes upregulated in acute
hypoxia-A549 cells.
| Number | Gene Symbol | Gene
description | Fold
upregulation | Gene function
(Qiagen, Inc.) | P-value |
|---|
| 1 | MSH2 | MutS homolog 2,
colon cancer, nonpolyposis type 1 (E. coli) | 2.021 | Protein Coding
gene | 0.001908 |
| 2 | CYP1A2 | Cytochrome P450,
family 1, subfamily A, polypeptide 2 | 2.027 | Drug
metabolism | 0.48241 |
| 3 | CDKN1A | Cyclin-dependent
kinase inhibitor 1A (p21, Cip1 | 2.39 | Regulator of cell
cycle progression at G1 | 0.048339 |
| 4 | BCL2 | B-cell CLL/lymphoma
2 | 2.45 | Anti- or
pro-apoptotic regulators | 0.032504 |
| 5 | RELB | V-rel
reticuloendotheliosis viral oncogene homolog B | 2.8 | Transcription
Factor | 0.000008 |
| 6 | CYP2C8 | Cytochrome P450,
family 2, subfamily C, polypeptide 8 | 2.32 | Drug
metabolism | 0.206225 |
| 7 | RARB | Retinoic acid
receptor, beta | 3.81 | DNA-binding
transcription factor activity | 0.001668 |
| 8 | FOS | FBJ murine
osteosarcoma viral oncogene homolog | 3.96 | Regulators of cell
proliferation, differentiation, and transformation | 0.002811 |
| 9 | ESR1 | Estrogen receptor
1 | 5.45 | Hormone nuclear
binding, DNA binding and activation of transcription | 0.040855 |
 | Table IIIGenes downregulated in acute
hypoxia-A549 cells. |
Table III
Genes downregulated in acute
hypoxia-A549 cells.
| Number | Gene symbol | Gene
description | Fold
downregulation | Gene function
(Qiagen, Inc.) | P-value |
|---|
| 1 | ABCC3 | ATP-binding
cassette, sub-family C (CFTR/MRP), member 3 | -2.15 | ATP-dependent drug
efflux pump | 0.01293 |
| 2 | CDKN2A | Cyclin-dependent
kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | -2.26 | Tumor suppressor
gene | 0.401826 |
| 3 | AHR | Aryl hydrocarbon
receptor | -2.49 | DNA-binding
transcription factor activity | 0.061225 |
Discussion
The A549 cells were subjected to 10 cycles of 72 h
of hypoxic episodes each in an effort to overcome cancer resistance
under these conditions. Doxorubicin chemo-resistance was evaluated
using the MTT proliferation assay. A gene expression profile was
then performed after 10 sessions of acute hypoxia at which point
resistance developed. The results confirmed that hypoxia was
implicated in the chemo-resistant phenotype that developed in the
lung cancer cell line.
Genes upregulated in acute
hypoxia
The genes upregulated in acute hypoxia belonged to a
number of crucial drug resistance mechanisms. Acute hypoxia
increased the expression of two genes involved in the drug
inactivation of the A549 cell line: CYP1A2 increased up to two-fold
and CYP2C8 increased by 2.32-fold. These genes are responsible for
the metabolism of hydrocarbon substances such as doxorubicin
(19,20) and might be the cause of the reported
doxorubicin resistance. The main way to activate CYP1A2 is via
nuclear translocation and ligand-mediated transactivation of
aromatic hydrocarbon receptors (21). The expression and activity of this
gene have been linked to malignancies and chemotherapy resistance
(22). The overexpression and
polymorphisms of CYP2C8 were also significantly connected with the
basal enzymatic activity, which can result in chemo-resistance
(22). Similarly, a 1.88-fold
increase in expression of CYP2B6 was observed.
The results of the present study showed that acute
hypoxia caused the estrogen receptor alpha gene (ESR1) to be
upregulated by 5.45-fold. This gene produces the estrogen receptor,
a nuclear ligand-inducible transcriptional receptor (23) protein. Upregulation of ESR1 has been
linked to the development of EMT in lung cancer cells, which
contributes to tumor resistance in immune-mediated cytotoxicity and
lung cancer chemotherapy. Various studies have shown that ESR1 is
dysregulated in cancer, which results in therapeutic resistance and
metastatic biology (23,24).
A total of four genes were upregulated in the cell
cycle and cell death inhibition mechanisms: cyclin dependent kinase
inhibitor 1A (CDKN1A; also known as p21), c-Fos, BcL2, and RARβ.
CDKN1A experienced a 2.39-fold increase in regulation. It functions
as a cell cycle regulator and mediates cell proliferation,
differentiation, death, and growth. CDKN1A is known to have a key
inhibitory function in p53-dependent apoptosis (23). Cells that express it are protected
from doxorubicin-induced apoptosis (25). The relevance of CDKN1A in tumor
development and its role in mediating a drug-resistant phenotype,
including chemotherapeutic medicines, tamoxifen and trastuzumab
resistance, has been suggested previously (26).
c-Fos is a proto-oncogene that occurs in different
cancer types. The main functions of the protein c-Fos are signal
transduction, cell differentiation and proliferation. The role of
c-Fos overexpression in boosting EMT state and CSC marker
expression has been demonstrated in a number of studies. In head
and neck squamous cell carcinoma (HNSCC) cells, the unique function
of c-Fos has been suggested to be a regulator of EMT and cancer
stem cell reprogramming (26). In
mammary epithelial cells, overexpression of c-Fos has been shown to
promote the development of a drug resistant phenotype resulting in
cell polarity loss and EMT, which promotes invasive and metastatic
growth (26). These results
convincingly demonstrated that c-Fos promotes stemness in HNSCC
cancer and plays a crucial role in tumor development (27).
The Bcl-2 family of proteins can either suppress or
enhance apoptosis and is an important regulator of cell death
(28). Bcl-2 proteins that are both
pro-survival and pro-apoptotic are commonly up- and downregulated
in various types of cancer cell. The Bcl-2 family plays a role in
carcinogenesis and cancer cell resistance to anticancer therapies
(29). The transcriptional
expression of this gene is significantly increased in response to
hypoxia in an HIF-1-dependent manner (28). These findings are consistent with
the discovery of BCL-2 overexpression in doxorubicin-resistant
A549(30).
Lung cancer is characterized by promoter
methylation, also known as hypermethylation, which is an early
event in the carcinogenic process and inactivates tumor suppressor
genes. The promoter methylation increases with neoplastic
progression from hyperplasia to adenocarcinoma. Retinoic acid
receptor β (RARβ) is frequently investigated in the context of
promoter methylation in lung cancer (30). RARβ is a member of the RAR
superfamily and is frequently suppressed in a variety of malignant
tumors (30,31). Research has indicated that drug
resistance may be caused by a lack of RARβ expression in clinical
studies (32-34).
Studies have revealed that the cholangiocarcinoma QBC939 cell
line's reduced RARβ expression makes it much more resistant to
chemotherapeutic drugs (32-34).
Additional research demonstrates a strong correlation between
elevated risk in NSCLC patients and hypermethylation of RARβ
(35). A potential risk factor,
diagnostic marker and potential therapeutic target for NSCLC is the
inactivation of the RARβ gene brought on by RARβ methylation
(36). Unexpectedly, the present
study discovered that the RARβ gene was upregulated in A549 cells
by a factor of 3.81. More research is thus needed to examine the
role of RARβ in lung cancer. One explanation of such debate can be
taken from the observation that RARβ can be re-expressed in
dormancy models which is related to the hypoxic model represented
in the present study (35).
The NF-κb family consists of five members: p105/p50
(NF-b1), p100/p52 (NF-b2), p65 (RelA), RelB, and c-Rel. These
members regulate the transcription of target genes (37). Studies have investigated how RelB
works in solid tumors and hematologic malignancies. Interleukin
(IL)-8 regulation and the anti-apoptotic response in breast cancer
cells are significantly regulated by RelB and the aryl hydrocarbon
receptor (38). RelB is a helpful
regulator in multiple myeloma cell survival (39). High RelB expression in NSCLC is
associated with low differentiation, deep tumor invasion, positive
lymph node metastasis, distant metastasis, and advanced clinical
stages (35). These results suggest
that increased RelB expression is essential for the development and
metastasis of NSCLC tumors. Thus, resistance to chemotherapy and
ionizing radiation have been associated with activation of the
transcription factor NF-κB during treatment (40). These results are consistent with the
findings of the present study, which showed that RelB was increased
by 2.8-fold. This partly explains doxorubicin resistance and
underscores the value of targeting NF-κB as a viable therapeutic
approach to combat chemo-resistance and radiation-resistance in the
treatment of cancer (41).
Genes downregulated in acute
hypoxia
The present study identified three genes
downregulated in acute hypoxia for NSCLC: CDKN2A by a factor of
2.26, AHR by a factor of 2.49, and ABCC3 by a factor of 2.15. A
member of the family of ABCC3 is actively effluxed in a wide range
of anticancer medications from tumor cells. This in turn
contributes to multidrug resistance (17). The 2.15-fold downregulation of this
gene in NSCLC suggests that it plays no part in the resistance
mechanism.
An investigation into the role of CDKN2A in gastric
cancer has revealed that CDKN2A depletion results in unchecked cell
proliferation, which causes neoplastic transformation (42,43).
This explains why CDKN2A was downregulated in the present study.
However, there is still debate over CDKN2A's role in lung cancer
and thus more research is required.
Limitations of the present study include the absence
of histological analysis, the absence of data on additional cell
lines, and the absence of data on clinically relevant drugs
In conclusion, the A549 lung cancer cell line
exhibits alterations in gene expression levels after acute hypoxia.
The results shed significant light on the potential pleiotropic
pathways including the ESR1 pathway and nucleic transcription
receptors such as CDKN1A, which are crucial in doxorubicin
resistance induction. Targeting the modulated genes in NSCLC cases
resistant to chemotherapy may have clinical value.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Deanship of
Scientific Research at The University of Jordan, Amman, Jordan
(grant no. 21/2017/2255).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MZ conceived the present study. RA and MZ were
responsible for methodology, formal analysis, and investigation. MZ
and NA were responsible for resources. MS was responsible for
writing the original draft preparation. MZ, MS and NA were
responsible for project administration. All the authors were
responsible for writing, reviewing and editing the manuscript and
for supervision. RA, MZ and NA confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer Statistics, 2008. CA Cancer J Clin.
58:71–96. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sinha BK, Mimnaugh EG, Rajagopalan S and
Myers CE: Adriamycin activation and oxygen free radical formation
in human breast tumor cells: Protective role of glutathione
peroxidase in adriamycin resistance 1. Cancer Res. 49:3844–3848.
1989.PubMed/NCBI
|
|
4
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9 (Suppl 5):S4–S9. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Das B, Tsuchida R, Malkin D, Koren G,
Baruchel S and Yeger H: Hypoxia enhances tumor stemness by
increasing the invasive and tumorigenic side population fraction.
Stem Cells. 26:1818–1830. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen Z, Han F, Du Y, Shi H and Zhou W:
Hypoxic microenvironment in cancer: Molecular mechanisms and
therapeutic interventions. Sig Transduct Target Ther.
8(70)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bayer C and Vaupel P: Acute versus chronic
hypoxia in tumors : Controversial data concerning time frames and
biological consequences. Strahlenther Onkol. 188:616–627.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Le QT, Chen E, Salim A, Cao H, Kong CS,
Whyte R, Donington J, Cannon WA, Wakelee H, Tibshirani R, et al: An
evaluation of tumor oxygenation and gene expression in patients
with early stage non-small cell lung cancers. Clin. Cancer Res.
12:1507–1514. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ziółkowska-Suchanek I: Mimicking tumor
hypoxia in non-small cell lung cancer employing three-dimensional
in vitro models. Cells. 10(141)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marhuenda E, Campillo N, Gabasa M,
Martínez-García MA, Campos-Rodríguez F, Gozal D, Navajas D, Alcaraz
J, Farré R and Almendros I: Effects of Sustained and Intermittent
Hypoxia on Human Lung Cancer Cells. Am J Respir Cell Mol Biol.
61:540–544. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rouschop KM, Ramaekers CH, Schaaf MB,
Keulers TG, Savelkouls KG, Lambin P, Koritzinsky M and Wouters BG:
Autophagy is required during cycling hypoxia to lower production of
reactive oxygen species. Radiother Oncol. 92:411–416.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hsieh CH, Shyu WC, Chiang CY, Kuo JW, Shen
WC and Liu RS: NADPH oxidase subunit 4-mediated reactive oxygen
species contribute to cycling hypoxia-promoted tumor progression in
glioblastoma multiforme. PLoS One. 6(e23945)2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rofstad EK, Gaustad JV, Egeland TA,
Mathiesen B and Galappathi K: Tumors exposed to acute cyclic
hypoxic stress show enhanced angiogenesis, perfusion and metastatic
dissemination. Int J Cancer. 127:1535–1546. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pires IM, Bencokova Z, Milani M, Folkes
LK, Li JL, Stratford MR, Harris AL and Hammond EM: Effects of acute
versus chronic hypoxia on DNA damage responses and genomic
instability. Cancer Res. 70:925–935. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Erler JT, Cawthorne CJ, Williams KJ,
Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C,
Stratford IJ and Dive C: Hypoxia-Mediated Down-Regulation of Bid
and Bax in Tumors Occurs via Hypoxia-Inducible Factor 1-Dependent
and -Independent Mechanisms and Contributes to Drug Resistance. Mol
Cell Biol. 24:2875–2889. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vaupel P, Kelleher DK and Höckel M:
Oxygenation status of malignant tumors: Pathogenesis of hypoxia and
significance for tumor therapy. Semin Oncol. 28 (2 Suppl 8):29–35.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Al-Najjar LM, Zihlif M and Jarrar Y:
Differential molecular alterations promoting non-small cell
lungcancer under hypoxia. Genome Instab Dis. 3:108–121. 2022.
|
|
18
|
Chen YL, Yang TY, Chen KC, Wu CL, Hsu SL
and Hsueh CM: Hypoxia can impair doxorubicin resistance of
non-small cell lung cancer cells by inhibiting MRP1 and P-gp
expression and boosting the chemosensitizing effects of MRP1 and
P-gp blockers. Cell Oncol (Dordr). 39:411–433. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chou CW, Wang CC, Wu CP, Lin YJ, Lee YC,
Cheng YW and Hsieh CH: Tumor cycling hypoxia induces
chemoresistance in glioblastoma multiforme by upregulating the
expression and function of ABCB1. Neuro Oncol. 14:1227–1238.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Green SL and Giaccia AJ: Tumor hypoxia and
the cell cycle: Implications for malignant progression and response
to therapy. Cancer J Sci Am. 4:218–223. 1998.PubMed/NCBI
|
|
21
|
Zhou SF, Wang B, Yang LP and Liu JP:
Structure, function, regulation and polymorphism and the clinical
significance of human cytochrome P450 1A2. Drug Metab Rev.
42:268–354. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
AbuHammad S and Zihlif M: Gene expression
alterations in doxorubicin resistant MCF7 breast cancer cell line.
Genomics. 101:213–220. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lei JT, Gou X, Seker S and Ellis MJ: ESR1
alterations and metastasis in estrogen receptor positive breast
cancer. J Cancer Metastasis Treat. 5(38)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fialka I, Schwarz H, Reichmann E, Oft M,
Busslinger M and Beug H: The estrogen-dependent C-junER protein
causes a reversible loss of mammary epithelial cell polarity
involving a destabilization of adherens junctions. J Cell Biol.
132:1115–1132. 1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|
|
26
|
Bunz F, Hwang PM, Torrance C, Waldman T,
Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW and
Vogelstein B: Disruption of p53 in human cancer cells alters the
responses to therapeutic agents. J Clin. Invest. 104:263–269.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Muhammad N, Bhattacharya S, Steele R,
Phillips N and Ray RB: Involvement of c-Fos in the promotion of
cancer stem-like cell properties in head and neck squamous cell
carcinoma. Clin Cancer Res. 23:3120–3128. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sermeus A, Genin M, Maincent A, Fransolet
M, Notte A, Leclere L, Riquier H, Arnould T and Michiels C:
Hypoxia-Induced modulation of apoptosis and BCL-2 family proteins
in different cancer cell types. PLoS One. 7(e47519)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brtko J: Role of retinoids and their
cognate nuclear receptors in breast cancer chemoprevention. Cent
Eur J Public Health. 15:3–6. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hua F, Fang N, Li X, Zhu S, Zhang W and Gu
J: A meta-analysis of the relationship between RARβ gene promoter
methylation and non-small cell lung cancer. PLoS One.
9(e96163)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu Z, Zhang L, Ding F, Li J, Guo M, Li W,
Wang Y, Yu Z, Zhan Q, Wu M and Liu Z: 5-Aza-2'-deoxycytidine
induces retinoic acid receptor-beta(2) demethylation and growth
inhibition in esophageal squamous carcinoma cells. Cancer Lett.
230:271–283. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pérez RJ, Benoit YD and Gudas LJ: Deletion
of retinoic acid receptor β (RARβ) impairs pancreatic endocrine
differentiation. Exp Cell Res. 319:2196–204. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ren HY, Chen B, Huang GL, Liu Y and Shen
DY: Upregulation of retinoic acid receptor-β reverses drug
resistance in cholangiocarcinoma cells by enhancing susceptibility
to apoptosis. Mol Med Rep. 14:3602–3608. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Y, Lu DG, Ma YM and Liu H: Association
between Retinoic acid receptor-β hypermethylation and NSCLC risk: A
meta-analysis and literature review. Oncotarget. 8:5814–5822.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Demchenko YN, Glebov OK, Zingone A, Keats
JJ, Bergsagel PL and Kuehl WM: Classical and/or alternative
NF-kappaB pathway activation in multiple myeloma. Blood.
115:3541–3552. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Qin H, Zhou J, Zhou P, Xu J, Tang Z, Ma H
and Guo F: Prognostic significance of RelB overexpression in
non-small cell lung cancer patients. Thorac. Cancer. 7:415–421.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Adam AP, George A, Schewe D, Bragado P,
Iglesias BV, Ranganathan AC, Kourtidis A, Conklin DS and
Aguirre-Ghiso JA: Computational identification of a
p38SAPK-regulated transcription factor network required for tumor
cell quiescence. Cancer Res. 69:5664–5672. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao Y, Lu H, Yan A, Yang Y, Meng Q, Sun
L, Pang H, Li C, Dong X and Cai L: ABCC3 as a marker for multidrug
resistance in non-small cell lung cancer. Sci Rep.
3(3120)2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fukuda Y and Schuetz JD: ABC transporters
and their role in nucleoside and nucleotide drug resistance.
Biochem Pharmacol. 83:1073–1083. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kirovski G, Stevens AP, Czech B, Dettmer
K, Weiss TS, Wild P, Hartmann A, Bosserhoff AK, Oefner PJ and
Hellerbrand C: Down-regulation of methylthioadenosine phosphorylase
(MTAP) induces progression of hepatocellular carcinoma via
accumulation of 5'-deoxy-5'-methylthioadenosine (MTA). Am J Pathol.
178:1145–1152. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: Prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267.
2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Semenza GL: Intratumoral hypoxia and
mechanisms ofimmune evasion mediated by hypoxia-inducible factors.
Physiology (Bethesda). 36:73–83. 2021.PubMed/NCBI View Article : Google Scholar
|