Introduction
Physalis genus (Solanaceae family) comprises
~71 species distributed in America and Asian temperate regions
(1,2). Plants belonging to this genus are
recognized by the presence of calyces that cover the fruit
protecting it from external damage (birds or insects and adverse
climatic conditions) (2). The
edible fruit and beneficial properties of teas prepared with
different parts of the plants confer value within traditional
medicine worldwide to treat inflammation, hepatitis, dermatitis,
cancer and diabetes (2). Their
biological activity is associated with the presence of withanolides
(physalins, neophysalins, withaphysalins), labdane diterpenes,
sucrose esters, flavonoids and ceramides (2-4).
Among species of the genus Physalis, ground cherry (P.
angulata) is used in Colombian folk medicine, as well in Asian
and African countries, for its antimicrobial, antimalarial,
anti-inflammatory, antinociceptive, anti-proliferative and
immunomodulatory properties (4-7).
It is effective against cervix, skin, lung, liver, gastric and
colorectal cancer (CRC) (4,7). To the best of our knowledge, the
biological properties of P. angulata calyces have been
studied scarcely (2,4-6,8-10)
despite their high levels of secondary metabolites for defense. In
our previous research, fractionation of total ethanolic extract
obtained from calyces yielded P. angulata dichloromethane
fraction (PADF), which exhibited maintained or improved bioactivity
particularly when evaluating its anti-inflammatory effect (6,8,9). PADF
demonstrated a promising anti-inflammatory profile in experimental
models [lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages,
12-O-tetradecanoylphorbol-13-acetate-induced acute ear edema and
dextran sulfate sodium (DSS)-induced colitis] (6,9). The
present study aimed to evaluate the effect of PADF on CRC cell
lines and the azoxymethane (AOM)/DSS-induced colitis-associated
cancer (CAC) mouse model. CRC is a serious public health problem
that in 2020 caused worldwide nearly 1.9 million new cases and
915,880 deaths, being the third most common cancer diagnosed and
the second leading cause of cancer death (11). Chronic inflammation, such as that
found in inflammatory bowel disease, which includes ulcerative
colitis and Crohn's disease, is considered one of the most common
risk factors for the development of CRC, as it promotes tumor
growth and development (12). CRC
chemotherapy confronts serious challenges such as resistance, toxic
reactions and side effects (13,14).
Plants with biological activity may contain metabolites that
facilitate development of novel therapy (14).
Materials and methods
Reagents and chemicals
AOM, crystal violet, Eagle's Minimal Essential
Medium (EMEM), DMEM, hematoxylin-eosin (H&E), McCoy's 5A
medium, protease inhibitor cocktail, and phenylmethylsulfonyl
fluoride (PMSF) were purchased from Sigma-Aldrich (Merck KGaA). MTT
was obtained from Calbiochem. DSS (36-50 kDa) was obtained from MP
Biomedicals. Tissue Protein Extraction Reagent (TPER®)
was obtained from Thermo Fisher Scientific, Inc. and fetal bovine
serum (FBS) from Gibco (Thermo Fisher Scientific, Inc.). All
solvents (ethanol, methanol, dichloromethane, hexane and DMSO) were
reagent or HPLC grade.
Plant collection and preparation of
PADF
P. angulata calyces were collected at Loma de
Arena, Bolívar, Colombia. A sample of the whole plant was sent to
Herbarium of the Universidad de Antioquia, Medellín, Colombia and
authenticated according to a voucher previously deposited (no.
HUA213028) (6). Clean and dried
calyces were used for preparation of PADF according to the
procedure described in a previous article (6) (Data
S1).
Cell culture
Colorectal adenocarcinoma (HT-29 and Caco-2) and
healthy colonic epithelial cells (CCD 841 CoN) were obtained from
the American Type Culture Collection (ATCC) and maintained at 37˚C
and 5% CO2 in McCoy's 5A medium (HT-29) or EMEM (Caco-2
and CCD 841 CoN) supplemented with 10% FBS and 1.5 g/l sodium
bicarbonate (Sigma-Aldrich; Merck KGaA).
MTT assay
HT-29, Caco-2 or CCD 847 CoN cells were seeded
(5,000-15,000 cells/well) in 96-well plates (2D culture) or
Perfecta3D® Hanging Drop Plate (3D culture; 3D
Biomatrix) and incubated at 37˚C for 24 and 72 h, respectively, to
allow attachment or spheroid formation, respectively. After that,
cells were treated for 48 h at 37˚C with PADF (3.13, 6.25, 12.50
and 25.00 µg/ml), vehicle (MeOH), or Triton X-100 (1%) as a
positive control. Cell viability was measured using MTT (0.25-5.00
mg/ml). After 4 h, formazan crystals were dissolved in DMSO, and
the optical density (OD) at 550 nm was measured using a plate
reader (Multiskan GO; Thermo Fisher Scientific, Inc.). Selective
index (SI) was calculated as SI=IC50 of PADF in CCD 847
CoN cells/IC50 of the same compound in HT-29 or Caco-2
cells.
Clonogenic assay
The replicative capacity of HT-29 cells was
confirmed as previously described (15,16).
Cells (750 cells/well) were cultured into a 6-well plate at 37˚C
for 6 h and treated with PADF (3.13, 6.25, 12.50 and 25.00 µg/ml)
or vehicle for 48 h. Subsequently, the culture medium was refreshed
and colonies were allowed to form for 7 days at 37˚C. Finally,
colonies were fixed with 7:1 acetic acid-methanol at 24˚C for 30
min, stained with 0.5% crystal violet at 24˚C for 2 h, and counted
manually using light stereomicroscope (EZ4 HD, Leica Microsystems
GmbH; magnification, x10). A colony was considered to contain at
least 50 cells.
Gap closure assay
HT-29 cells (2x105 cells/well) were grown
with McCoy's 5A medium supplemented with 10% FBS (17,18),
on µDish35 mm,high plates (Ibidi) for 24 h (100%
confluence). The insert was removed to generate a scratch in the
monolayer, which was then treated with PADF (6.91 and 13.82 µg/ml)
or vehicle. Images were captured at 24, 48 and 72 h with an
inverted light microscope (Nikon Corporation; magnification x10).
The open area in each image was measured using ImageJ 1.47v
software (National Institutes of Health) and values were normalized
to the open area at 0 h.
Cell cycle analysis
The impact of PADF on cell cycle distribution was
determined by propidium iodide (PI) staining (cat. no. ab139418;
Abcam) and analyzed using a FACS Aria III flow cytometer (BD
Biosciences) and FlowJo X10.0.7 software (FlowJo LLC). HT-29 cells
(2x105 cells/ml) were exposed to PADF (13.8, 18.0 and
27.6 µg/ml) for 24-48 h at 37˚C. Cells were harvested by
trypsinization, fixed with 66% ethanol for 2 h at 4˚C, stained with
PI and incubated with RNase A for 30 min at 37˚C in the dark.
Doxorubicin (Doxo) was employed as a positive control.
Early and late apoptosis
detection
HT-29 cells (1-2x105 cells/ml) were
exposed to PADF (0, 13.8, 18.0 and 27.6 µg/ml) for 24-48 h at 37˚C.
In the first assay, cells were stained with Annexin V-FITC (cat.
no. BMS500FI-100; eBioscience) and analysis was performed using a
flow cytometer (FACS Aria III, BD Biosciences) and the images were
analyzed using FlowJo X10.0.7 software. For the second assay, DNA
electrophoresis was used to detect DNA fragmentation using the
apoptotic DNA ladder detection kit (cat. no. ab66093; Abcam)
following the manufacturer's instructions.
Mitochondrial membrane potential
(ΔΨm)
ΔΨm was monitored by JC-1 Mitochondrial Membrane
Potential kit (Item No. 10009172; Cayman Chemical Company). Cells
were incubated at 37˚C with JC-1 staining solution for 15 min and
rinsed according to the manufacturer's instructions. Measurement
was performed using a microplate fluorometer (Infinite 200 PRO,
Tecan Group Ltd.) to quantify the fluorescence of J-aggregates
(excitation 535 and emission 595 nm) and -monomers (excitation 485
and emission 585 nm).
Animals
Female Balb/c mice (mean weight, 19.41±0.13 g) were
obtained from the Instituto Nacional de Salud (Bogotá, Colombia).
Animals were housed in filtered-capped polycarbonate cages and kept
in a controlled environment (19.48±0.10˚C, 68.63±1.15% humidity
under a 12/12-h light/dark cycle) with access to food and water
ad libitum. All experiments were designed and conducted
following local and international regulations [European Union
regulations (CEC council 86/809), EU Directive 2010/63/EU,
protocols of the Organisation for Economic Cooperation and
Development] and approved by the Committee of Ethics in Research of
the University of Cartagena (Project Approval Minute No. 81 from
August 13, 2015).
AOM/DSS-induced CRC
The total experiment duration was 77 days (11
weeks). In brief, 5-6-week-old animals were randomized into control
(vehicle), AOM + DSS and treatment groups (PADF 10 and 20 mg/kg,
6/group). Carcinogenesis was induced by intraperitoneal (IP)
injection of AOM 10 mg/kg. One week later, animals received 2-4%
DSS in drinking water for 7 days. To promote chronic
inflammation-driven tumor progression, three DSS cycles at 2 weeks
intervals (normal drinking water) were performed (19,20).
Two doses of PADF (10 and 20 mg/kg, IP), selected according to
previous study (6), and the acute
toxicity test (Data S1), were
administered for 3 weeks after the final DSS cycle, while control
groups were treated with vehicle (saline). To check the safety of
PADF, toxicity control groups administered PADF daily (10 and 20
mg/kg/day) without AOM or DSS. Body weight was monitored daily,
while macroscopic parameters such as colon length and weight/length
ratio and tumor progression (tumor number, size and load) were
measured following sacrifice. Tumor load value was calculated as
the sum of the diameters of all the tumors in each animal. A
portion of the colon was rolled from the proximal to distal end.
Samples of colonic tissue containing tumors and adjacent normal
control tissue were also harvested for further analysis. The
experiments had two sets of replications using 197 experimental
animals in total. Sacrifice was performed by cervical dislocation
following anesthesia by 2% inhaled sevoflurane. Animal death was
verified by loss of heartbeat, reflexes and breathing. Humane
endpoints were excessive weight loss (>4 g), diarrhea or
hemorrhage. However, no animal was euthanized according to these
endpoints.
Histology analysis
Colon samples were fixed with 4% buffered formalin
for 30 days at 24˚C and embedded in paraffin; 5 µm sections were
cut and stained with H&E and examined by two blinded
researchers using light microscopy (cat. no. DM500, Leica
Microsystems GmbH; magnification x10). Severity of mucosal
inflammation, dysplasia and cancer were assessed. The epithelial
damage and cellular infiltration were scored from 0-4 as previously
described (21). Dysplastic
proliferation was graded as low- or high-grade and scored
considering the area of mucosal surface affected as absent (0),
low- (1), medium- (2) or high-grade (3). Cancer was scored as absent (0), early
invasive (1) or advanced (2).
Cell viability assay using conditioned
media
RAW 264.7 macrophages (ATCC; cat. no. TIB-71)
maintained in DMEM with 10% FBS at 37˚C and 5% CO2, were
cultured in 24-well plates (200,000 cells/well). After 48 h,
macrophages were treated with 12.5 µg/ml PADF for 1 h and then
stimulated for 6 h with LPS (1 µg/ml) or IL-4 (40 ng/ml) to induce
an inflammatory M(LPS) or alternative M(IL-4) profile,
respectively. Culture supernatant was collected and added to HT-29
cells seeded in 96-well plates (10,000 cells/well), which were
incubated for 48 h at 37˚C. HT-29 cell viability was measured using
the MTT method as aforementioned.
Protein expression analysis
Colonic tissue and HT-29 cells were homogenized
using TPER® extraction reagent supplemented with
protease inhibitor cocktail and PMFS for western blotting (Data S1) or cOmplete tablets Mini,
EDTA-free (Roche Diagnostics GmbH) for ELISA. The extracted
proteins were quantified with Bradford assay (Bio-Rad Laboratories,
Inc.) using BSA as standard. The levels of IL-1β (cat. no.
88-7261-88), IL-6 (ref 88-7066-88; both Invitrogen), and TNF-α (cat
no. 430204; BioLegend) in supernatant were measured using mouse
ELISA kits following the manufacturer's instructions.
Statistical analysis
All data are presented as the mean ± SEM of ≥2
independent experiments. The half-maximal inhibitory concentration
50 (IC50) was calculated using non-linear regression.
Statistical analysis was performed by one-way ANOVA followed by
Dunnett's multiple comparisons test. Statistical analyses were
performed using GraphPad Prism 6 demo version (GraphPad Software,
Inc.; Dotmatics). P<0.05 was considered to indicate a
statistically significant difference.
Results
PADF extraction and chemical
characterization
A total of 3.15 g PADF was obtained from 114 g dried
calyces (2.76% yield) of P. angulata. Based on the
information previously reported (6), four types of metabolites
(withanolides, sucrose esters, phenolic compounds and flavonoids)
were quantified for PADF chemical characterization. Only sucrose
derivatives, most likely sucrose esters, were detected in
significant amounts (Table SI;
Data S1). There were low levels of
withanolides and phenolic compounds and flavonoids were not
detected.
Cytotoxic effect of PADF
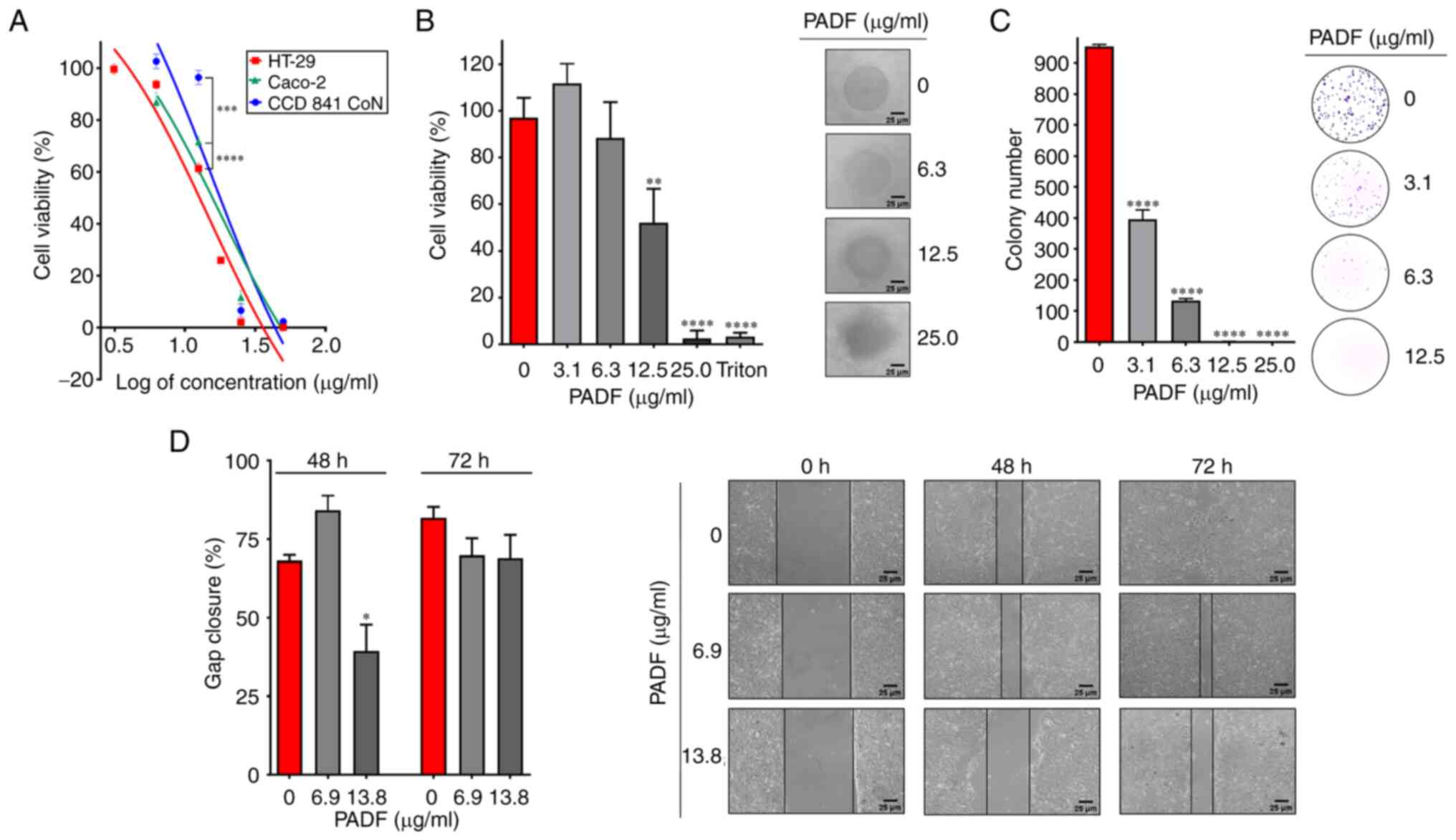
Fig. 1A shows the
inhibitory effect of PADF on the viability of colorectal
adenocarcinoma cell lines. HT-29 and Caco-2 cells were notably
sensitive to PADF, exhibiting IC50 values of 13.82±0.42
and 29.56±3.73 µg/ml, respectively. By contrast, normal colon
epithelial cells (CCD 841 CoN) were not significantly affected by
PADF at cytotoxic concentrations (12.5 µg/ml), displaying an
IC50 of 44.80±1.77 µg/ml and selectivity index of 3.24
(HT-29) and 1.52 (Caco-2).
Due to the higher susceptibility of HT-29 cells,
this cell line was selected to study the effect of PADF. Initially,
its cytotoxic effect was tested at different exposure times; PADF
exerted significant cytotoxicity at 6 h, which increased to a
maximum at 48 h (Fig. S1A;
Data S1). Due to the ability of
HT-29 cells to form spheroids, 3D culture was also employed to
mimic the cancer microenvironment better since it reflects
physiological relevant cell-cell interactions (22-24).
PADF showed a concentration-dependent effect, preserving the
cytotoxic effect (IC50=13.31±4.50 µg/ml; Fig. 1B) observed in the 2D model. Thus,
this fraction demonstrates its ability to affect HT-29 cell
viability in a tumor environment.
Effect of PADF on clonogenicity and
migration of HT-29 cells
HT-29 cells formed multiple colonies after 7 days
(Fig. 1C). This was inhibited by
PADF treatment in a concentration-dependent manner. Similarly, PADF
decreased gap closure of treated cells in comparison with control
cells. (Fig. 1D). Therefore, PADF
affected not only the clonogenicity but also the migration of CRC
cells.
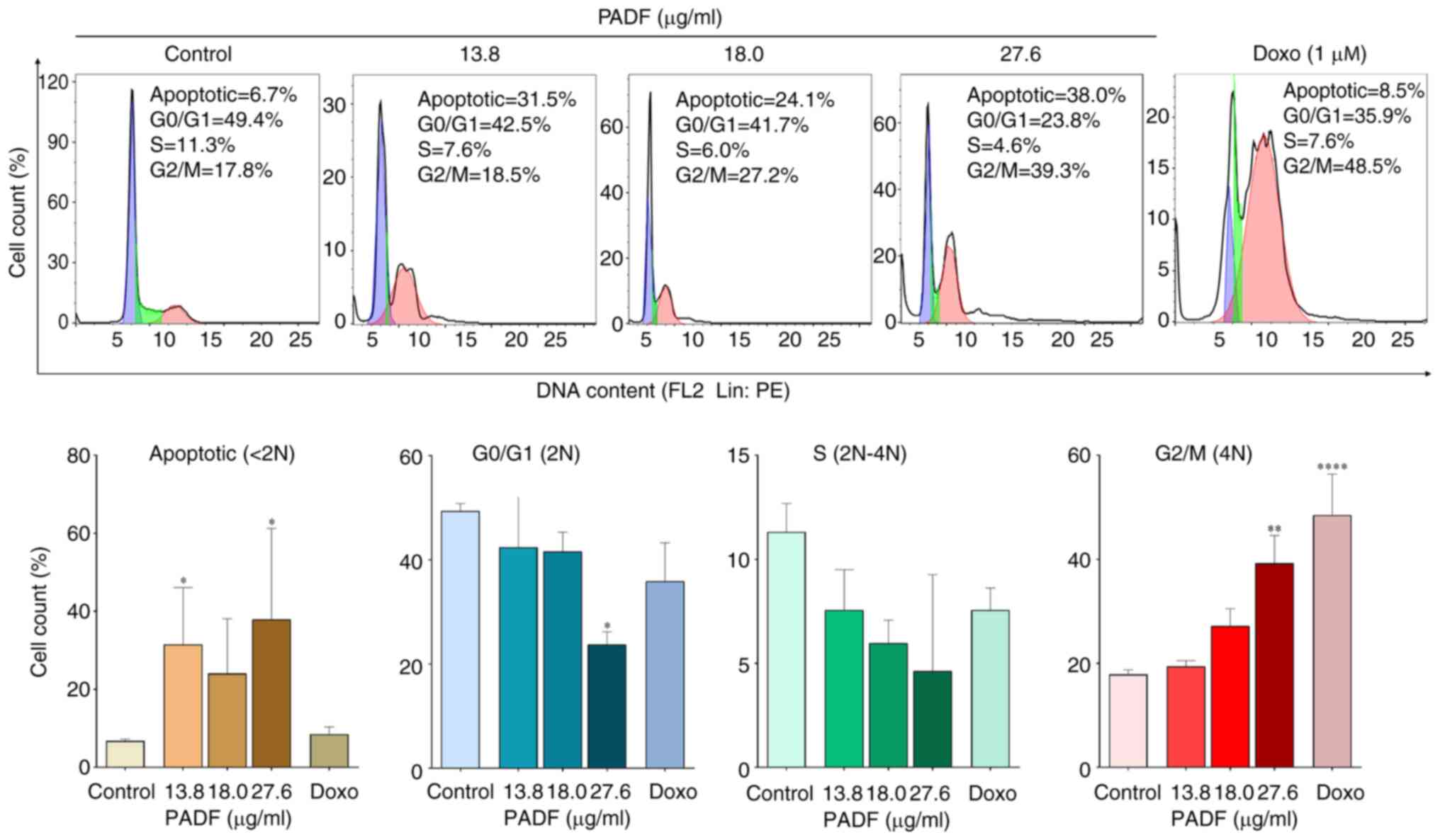
Cell cycle and apoptosis analysis
Flow cytometry was employed to detect changes in
cell cycle distribution. PADF induced cell cycle arrest in G2/M
phase and significantly increased the proportion of sub-G1 cells,
suggesting apoptosis induction (Fig.
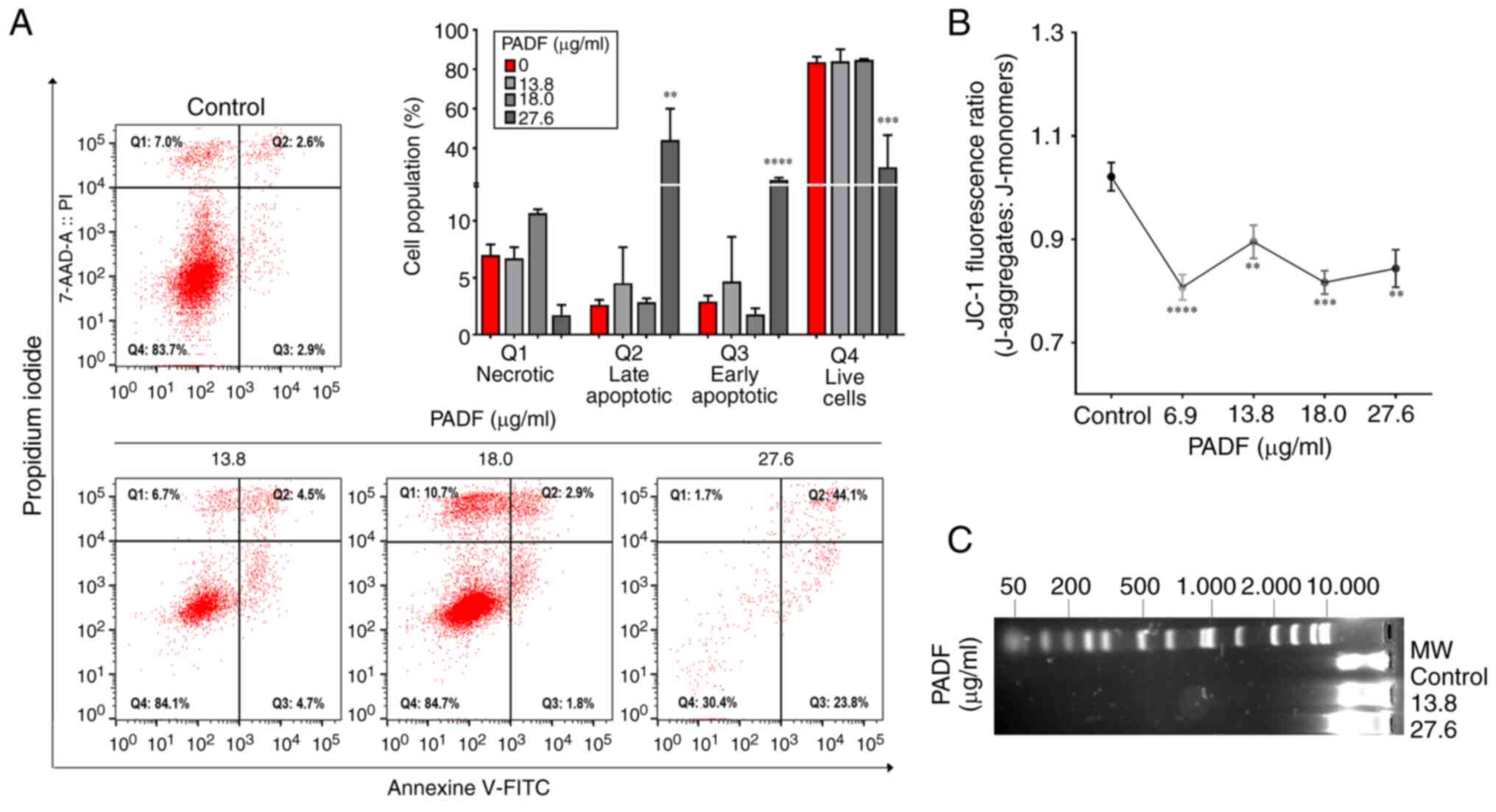
2), which was corroborated by ΔΨM, Annexin V-staining and
detection of DNA fragmentation. Annexin V-staining demonstrated
that PADF induced a significant increase in the proportion of
apoptotic cells (early and late), especially at the highest
concentration (Fig. 3A).
Consistently, this effect was accompanied by a significant
reduction of ΔΨM, expressed as JC-1 fluorescence ratio, compared
with control cells (Fig. 3B), as
well as the degradation of DNA in PADF-treated HT-29 cells
(Fig. 3C). To verify if apoptosis
was associated with induction of oxidative stress by PADF,
induction of reactive oxygen species (ROS) was measured; no change
in ROS production was detected (Fig.
S1B; Data S1).
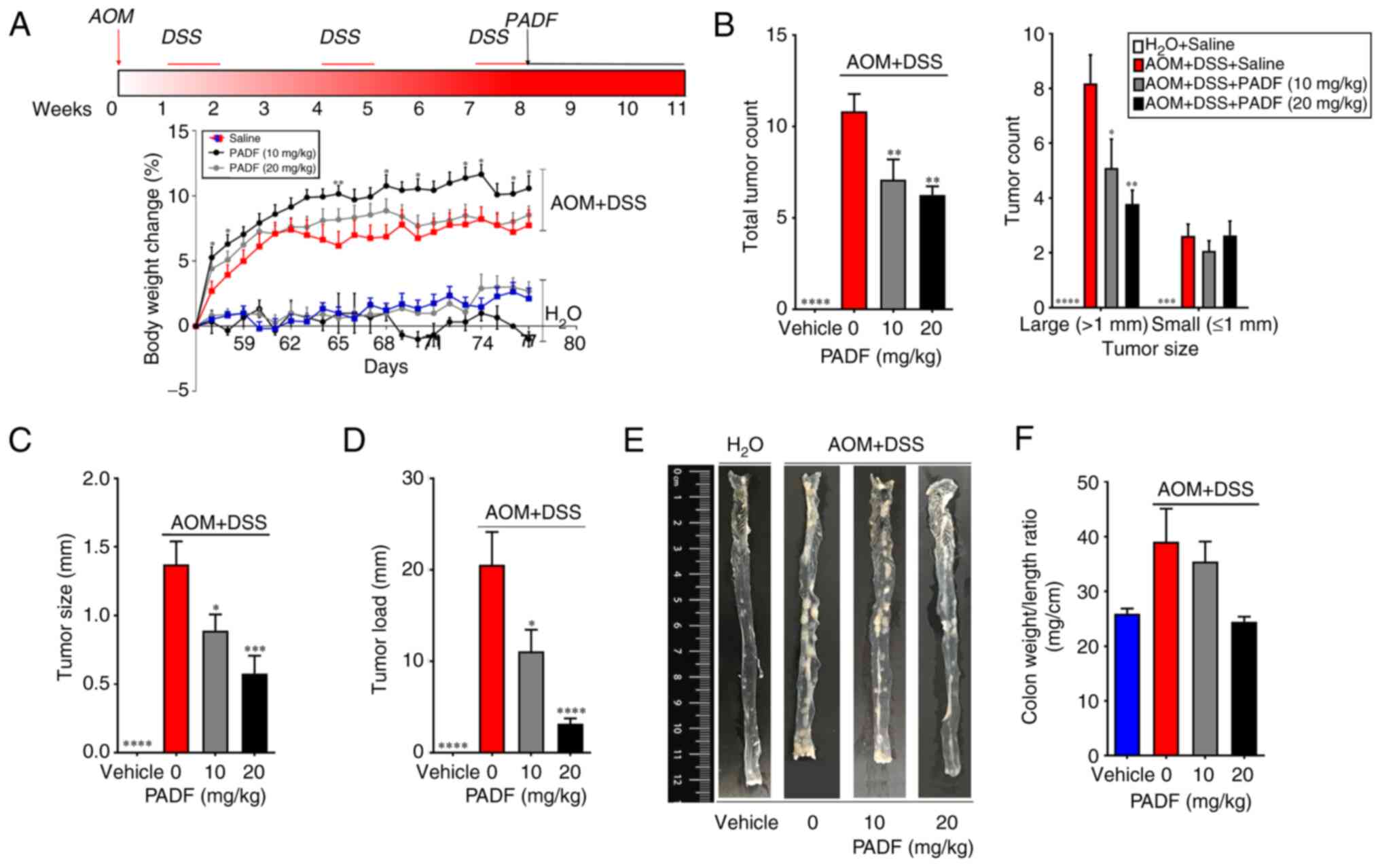
In vivo assay
AOM-DSS produced a notable decrease in body weight
on day 56; this loss of weight was significantly improved by the
treatment with PADF at 10 mg/kg/day, while the higher dose (20
mg/kg/day) did not reverse this (Fig.
4A). Macroscopic assessment showed that 100% of animals in the
AOM + DSS developed tumors (mean, 10.82±0.96 tumors in the middle
and distal colon); by contrast, vehicle group showed no tumors
(Fig. 4B). PADF (10 and 20
mg/kg/day) markedly reduced the total tumor load, specifically
diminishing the number of tumors >1 mm in diameter. Furthermore,
PADF significantly reduced the tumor size and tumor load by 35-85%
(Fig. 4C-E). To assess the severity
of the disease, colon weight/length ratio was measured; this was
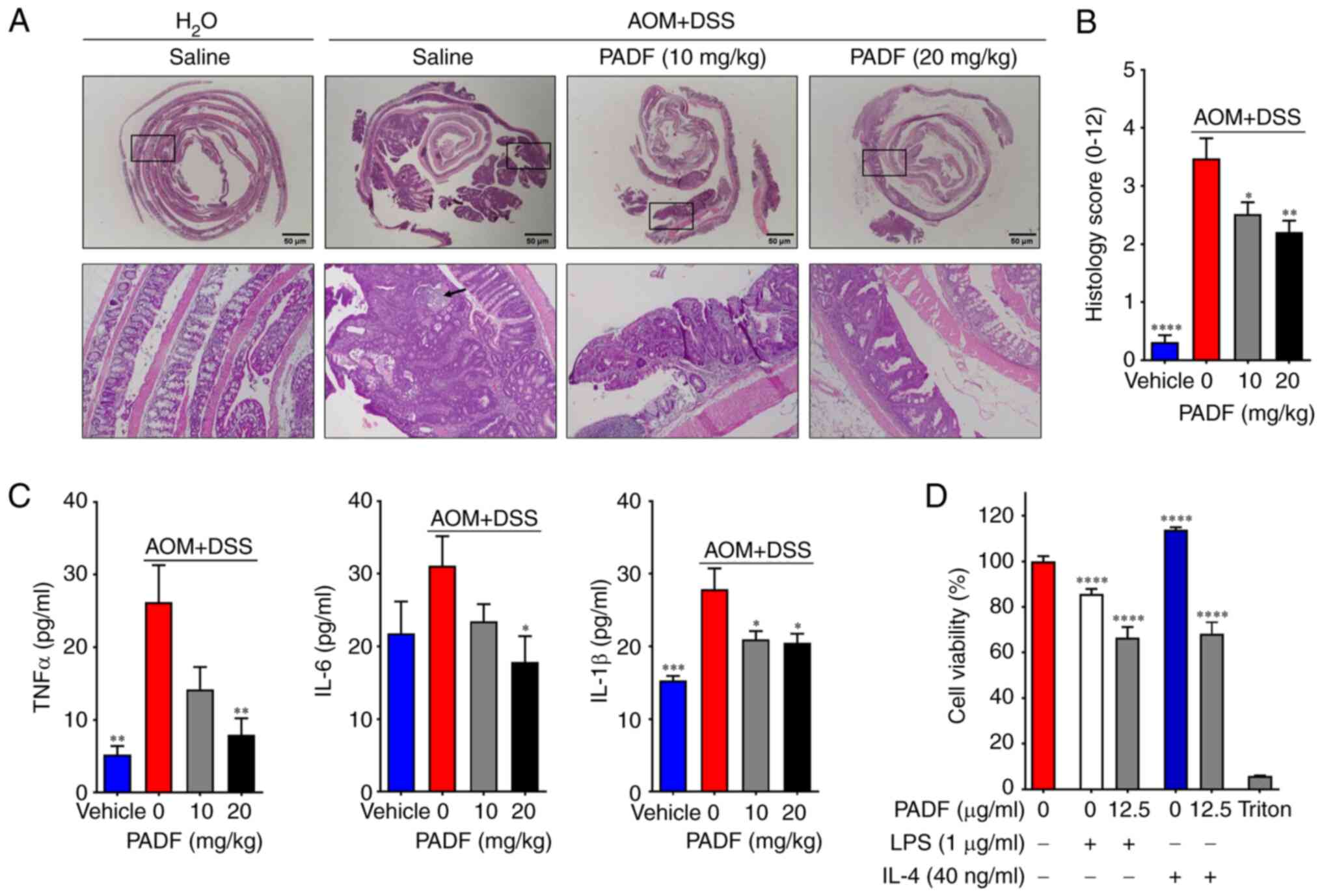
reduced in animals treated with PADF. Histological examination of
colon sections stained with H&E of the AOM + DSS group
confirmed large adenomas or adenocarcinomas inside the lumen and
prominent extension of dysplasia (both high- and low grade)
accompanied by slight inflammation of the epithelium and cellular
infiltration (Fig. 5A and B). Conversely, PADF (10 and 20 mg/kg)
promoted the recovery of tissue architecture by reducing tumor
growth and dysplasia, thus lowering the histology score from
3.48±0.35 for AOM + DSS to 2.21±0.19 for PADF 20 mg/kg group
(Fig. 5B).
During the study, the toxicity control group was
monitored to verify that the administration of PADF did not alter
the body weight of healthy mice; no signs of toxicity were detected
at any dose (Table SII; Fig. S2; Data
S1). Moreover, a detailed post-mortem analysis revealed no
notable changes during necropsy and hematological analysis
(Tables SII and SIII; Data
S1). Likewise, PADF did not induce genotoxic effects, as
established by the Ames test, micronucleus assay and comet assay
(Fig. S2; Data S1), indicating the safety of PADF at
effective dosage levels.
Protein expression analysis
PADF significantly reduced the levels of
pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) in colon tissue
compared with the AOM + DSS group (Fig.
5C). Based on the immunomodulatory effect on macrophages of
PADF (6), it was assessed whether
PADF inhibited viability of CRC cells treated with
macrophage-conditioned media. Treatment with supernatants from
LPS-stimulated macrophages decreases viability (Fig. 5D). By contrast, treatment with
supernatants from IL-4-stimulated macrophages increased viability.
However, when PADF (12.5 µg/ml) was administered, the viability of
HT-29 cells was always significantly reduced, regardless of the
conditional media.
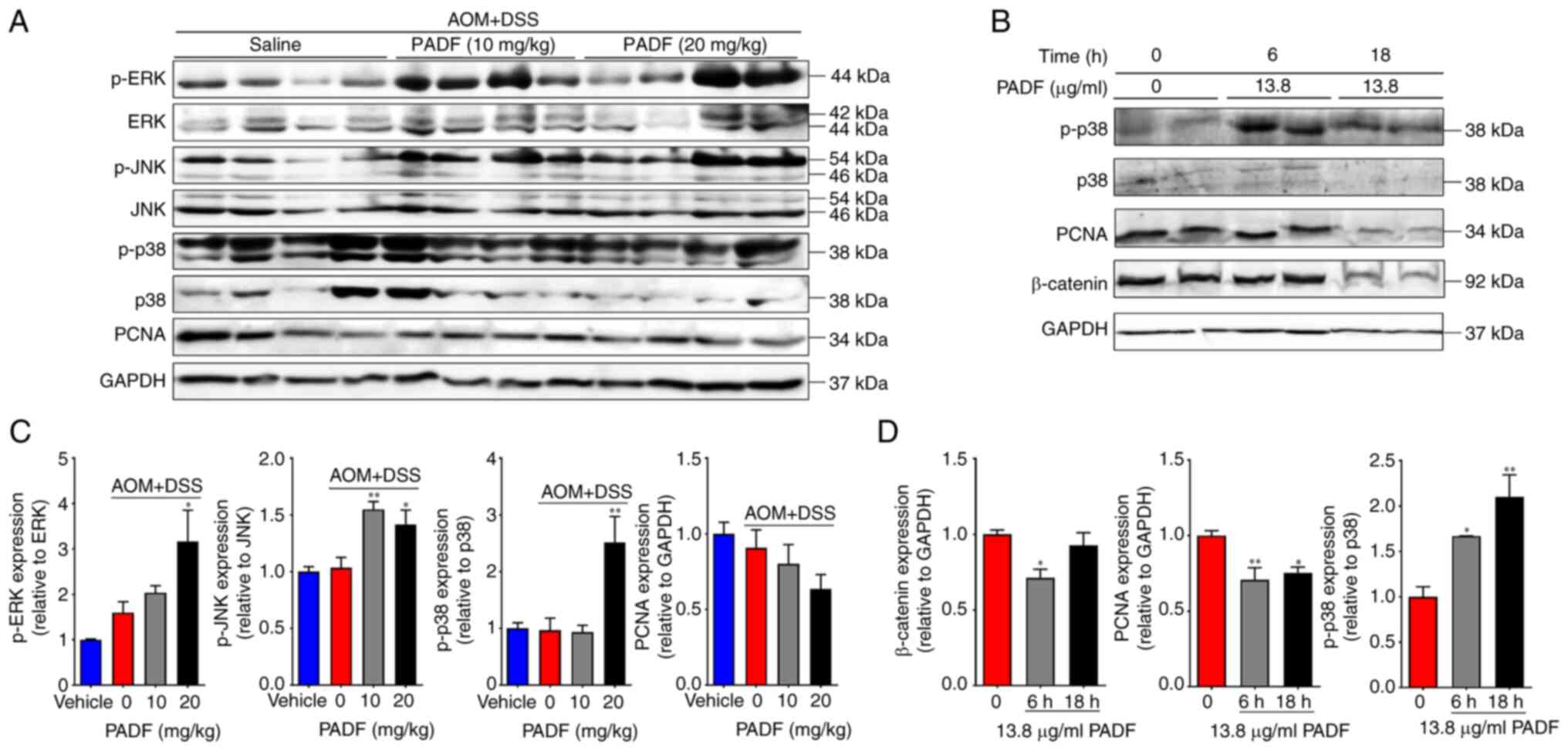
Western blot analysis of colonic samples showed that
PADF (10 and 20 mg/kg/day) significantly reduced the expression of
proliferating cell nuclear antigen (PCNA) while enhancing that of
phosphorylated MAPK in comparison with the AOM + DSS group
(Fig. 6A and B). The decreased levels of PCNA and
increased of p-p38 were confirmed in human CRC cells by western
blotting (Fig. 6C and D).
Discussion
The present study demonstrated therapeutic potential
of P. angulata calyces in CAC. Although the present study is
not the first to identify the promising anti-tumor effect of
derivatives from calyces of Physalis species (25), the present study combined in
vitro and in vivo experiments to assess the
effectiveness of PADF, as well as its mechanisms of action.
PADF chemical characterization revealed an enriched
fraction in sucrose esters, metabolites detected in other
Physalis species that exhibit anti-inflammatory properties
(3,26,27).
Although studies have reported sucrose esters with cytotoxic
activity against human cancer cells in Prunus tomentosa
(Rosaceae) and Echium angustifolium (Boraginaceae) (28-30),
to the best of our knowledge, the present study is the first to
report an anti-tumoral effect by a fraction enriched in sucrose
esters in the Physalis genus.
The present results support the anticancer potential
of PADF. PADF maintained cytotoxic activity on HT-29 cells in 2D
(cell culture in a monolayer) and 3D models (spheroids). In
addition, PADF demonstrated low cytotoxicity on CCD 841 CoN and
another non-cancerous cell line (PCS-201-012;
IC50=30.71±1.92 µg/ml), reported in a previous study
(8), further supporting its
selective inhibitory activity against CRC cell lines. Although
single-cell spheroids are a simple 3D approach to model CRC, the
present results suggested that PADF may be effective in more
complex experimental settings. The effectiveness as a cytotoxic
agent was confirmed by colony formation assay (31), which demonstrated an inhibitory
effect on the colony-forming ability at concentrations four times
lower than the IC50. Another technique used for
screening antitumor drugs is the gap closure assay, which is used
to evaluate the effects of selected drugs on cell migration, a
feature of tumor metastasis that allows neoplastic cells to invade
surrounding blood and lymphatic vessels and spread to other organs
(31,32). PADF showed a potent inhibitory
effect on HT-29 migration, making it a potentially useful
alternative to be used in advanced stages of CRC where cells tend
to invade other tissue.
To determine the mechanism of PADF underlying its
cytotoxic activity on HT-29 cells, flow cytometry was employed. The
results showed that PADF treatment induced apoptosis and G2/M
arrest. G2/M checkpoint blocks the entry into mitosis when DNA is
damaged to allow activation of repair mechanisms or the induction
of programmed cell death (33). To
confirm whether PADF triggered apoptosis, effects on early
apoptosis were assessed by ΔΨM quantify J-aggregates and monomers,
early/mid apoptosis assessing phosphatidylserine translocation by
staining with Annexin-V, and late apoptosis by DNA fragmentation
through electrophoresis (34). The
present results confirmed that apoptosis occurred after PADF
treatment of CRC cells.
Given the promising results in vitro as well
as the strong anti-inflammatory activity observed previously
(6), PADF was evaluated using a
model of CAC, a disease resulting from the
inflammation/dysplasia/carcinoma sequence where malignant cell
proliferation, pro-inflammatory cytokines [IL-6, IL-1β and tumor
necrosis factor (TNF)-α] and several signaling pathways (MAPK,
NF-κB and Wnt/β-catenin) are hyperactivated (22). Animal models are key to identify
carcinogens and pathogenesis and progression of cancer and screen
drug candidates during pre-clinical development. Despite its
limitations, the combination of AOM and DSS as a model of CAC has
popularity for its reproducibility, potency, low price and ease of
use (35-39).
The sustained release of pro-inflammatory cytokines and mediators
(IL-1β, IL-6, TNF-α, nitric oxide and prostaglandin E2) can
accelerate the process of colon carcinogenesis in both humans and
mice (40-43).
Therefore, these are important targets to assess when modeling CAC.
PADF significantly decreased the levels of pro-inflammatory
cytokines (TNF-α, IL-6 and IL-1β) in colon biopsies when compared
with the AOM + DSS group. This anti-inflammatory effect, which
promotes tissue recovery and mucosal regeneration, combined with
the harmful effect against CRC cell lines, makes PADF an attractive
alternative to treat CAC.
To assess the mechanism underlying with the
beneficial effect of PADF, expression of PCNA and MAPKs (ERK, JNK,
and p38) was determined as markers of abnormal cellular
proliferation, inflammation, and stress response (44,45).
PADF decreased PCNA phosphorylation and increased MAPK
phosphorylation. As PCNA is involved with DNA synthesis and
initiation of cell proliferation (40,43),
this supported the inhibitory effect of PADF against malignant
cells in CRC. However, increased MAPK phosphorylation by P.
angulata appears contradictory since these proteins are
traditionally associated with cancer pathogenesis (42,46,47).
Nonetheless, activation of this pathway is dynamic during the
inflammation/dysplasia/carcinoma sequence in the AOM-DSS model. For
example, p-p38 is highly expressed during inflammation, but when
carcinoma is established, expression of p-p38 decreases to normal
levels. The elevated levels of p-p38 observed after administration
of PADF in the high-grade dysplasia/carcinoma phase could be
related to the induction of apoptosis in malignant cells, similar
to that behavior observed with cisplatin, doxorubicin, and
camptothecin (48,49), while protecting normal epithelial
cells, a key role in the maintenance of epithelial homeostasis
(50). Numerous naturally derived
compounds (e.g., Phenethyl isothiocyanate, Evodiamine, Triptolide,
Quercetin, and Honokiol) have been described as apoptotic inducers
by increasing phosphorylation of JNK and ERK (49,51,52).
The inhibition of PCNA and activation of p-p38 was confirmed using
human CRC cells. β-catenin expression decreased, suggesting the
inhibition of the Wnt signaling pathway, activation of which
contributes to the initiation and progression of CRC (51).
In conclusion, PADF demonstrated potent cytotoxic,
anti-tumor and anti-inflammatory activity in experimental models of
CRC where viability of malignant cells was suppressed through
apoptosis, cell cycle arrest, inhibition of cytokine expression and
the modulation of key signaling pathways involving p38, PCNA, MAPKs
and β-catenin. This bioactivity may be related to the high content
of sucrose esters, metabolites with anti-inflammatory and
antibacterial properties. Further investigations are needed to
identify the compounds responsible for the activity.
Supplementary Material
Materials and methods
Biologic effects of PADF. (A) HT-29
cell viability. (B) Intracellular levels of reactive oxygen species
of HT-29 cells after PADF treatment. n=10/group.
*P<0.05, **P<0.01,
***P<0.001, and ****P<0.0001 vs. cells
without treatment. PADF, Physalis angulata dichloromethane
fraction.
Effect of PADF on genotoxic and
mutagenic parameters of BALB/c mice. (A) Proportion of MN-PCE,
MN-NCE and PCE/NCE ratio of micronucleated erythrocytes of
peripheral blood and bone marrow. (B) DNA damage measured by comet
assay in peripheral blood cells. (C) Mutagenesis in Salmonella
typhimurium TA100 measured by Ames test. n=10/group.
*P<0.05, ***P<0.001 and
****P<0.0001 vs. vehicle or control. PADF,
Physalis angulata dichloromethane fraction; MN,
Micronucleus; PCE, Polychromatic erythrocytes; NCE, Normochromatic
erythrocytes; MMC, Mitomycin c; 2-AA, 2-Aminoanthracene.
Content of representative secondary
metabolites of PADF.
Effect of PADF on weight of BALB/c
mice.
Effect of PADF on hematological
parameters of BALB/c mouse serum.
Acknowledgements
The authors would like to thank Professor Josefina
Zakzuk for support with flow cytometry and Ms. Jennifer Vazquez,
Ms. Catherine Meza, and Ms. Laura Ospina for their assistance with
the micronucleus assay (all Universidad de Cartagena, Cartagena,
Colombia).
Funding
Funding: The present study was supported by Minciencias and
Universidad de Cartagena (grant nos. 878-2015, 057-2018, and
025-2019).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LF conceived, designed and supervised the study and
revised the manuscript. YO and RS designed the study. YO wrote the
manuscript, constructed figures and supervised the study. LF, YO,
IP, and RS confirm the authenticity of all the raw data. DC, DR, IP
and JC analyzed data. DC performed experiments and wrote the
manuscript. DR and JC performed experiments and revised the
manuscript. IP and RS revised the manuscript. DR and IP constructed
figures. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal study protocol was approved by
Institutional Ethics Committee of the Universidad de Cartagena
(protocol Minute No. 81 from August 13, 2015). All the experiments
were designed and conducted following local and international
regulations (European Union regulations (CEC council 86/809), EU
Directive 2010/63/EU, protocols of the Organisation for Economic
Cooperation and Development). In addition, tumor burden did not
exceed the recommended dimensions according to University of
Pennsylvania guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Physalis-The Plant List. Version 1,
2010 [cited 2020 Feb 5]. Available from: http://www.theplantlist.org/browse/A/Solanaceae/Physalis/.
|
|
2
|
Mazova N, Popova V and Stoyanova A:
Phytochemical composition and biological activity of
Physalis spp.: A mini-review. Food Sci Appl Biotechnol.
3:56–70. 2020.
|
|
3
|
Zhang WN and Tong WY: Chemical
constituents and biological activities of plants from the genus
Physalis. Chem Biodivers. 13:48–65. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rengifo-Salgado E and Vargas-Arana G:
Physalis angulata L. (Bolsa Mullaca): A review of its
traditional uses, chemistry and pharmacology. Bol Latinoam Caribe
Plant Med Aromat. 12:431–445. 2013.
|
|
5
|
Cobaleda-Velasco M, Alanis-Bañuelos RE,
Almaraz-Abarca N, Rojas-López M, González-Valdez LS, Ávila-Reyes JA
and Rodrigo S: Phenolic profiles and antioxidant properties of
Physalis angulata L. as quality indicators. J Pharm
Pharmacogn Res. 5:114–128. 2017.
|
|
6
|
Rivera D, Ocampo Y and Franco LA:
Physalis angulata calyces modulate macrophage polarization
and alleviate chemically induced intestinal inflammation in mice.
Biomedicines. 8(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iwansyah AC, Luthfiyanti R, Ardiansyah
RCE, Rahman N, Andriana Y and Hamid HA: Antidiabetic activity of
Physalis angulata L. fruit juice on streptozotocin-induced
diabetic rats. S Afr J Bot. 145:313–319. 2022.
|
|
8
|
Rivera DE, Ocampo YC, Castro JP, Caro D
and Franco LA: Antibacterial activity of Physalis angulata
L., Merremia umbellata L., and Cryptostegia grandiflora Roxb. Ex
R.Br. -medicinal plants of the Colombian Northern Coast. Orient
Pharm Exp Med. 15:95–102. 2015.
|
|
9
|
Rivera DE, Ocampo YC, Castro JP, Barrios
L, Diaz F and Franco LA: A screening of plants used in Colombian
traditional medicine revealed the anti-inflammatory potential of
Physalis angulata calyces. Saudi J Biol Sci. 26:1758–1766.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chouhan S and Guleria S: Anti-inflammatory
activity of medicinal plants: Present status and future
perspectives. In: Singh B (ed) Botanical Leads for Drug Discovery.
Springer Singapore, pp67-92, 2020.
|
|
11
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nebbia M, Yassin NA and Spinelli A:
Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal
Surg. 33:305–317. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Makin G: Principles of chemotherapy.
Paediatr Child Heal. 28:183–188. 2018.
|
|
14
|
Safarzadeh E, Shotorbani SS and Baradaran
B: Herbal medicine as inducers of apoptosis in cancer treatment.
Adv Pharm Bull. 4 (Suppl 1):S421–S427. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Franken NAP, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang X: Clonogenic assay to test cancer
therapies. Bio Protocol. 2:1–3. 2012.
|
|
17
|
HT-29 | ATCC. Atcc.org, 2020.
Available from: https://www.atcc.org/products/htb-38#detailed-product-information.
|
|
18
|
Weisser H, Göbel T, Melissa Krishnathas G,
Kreiß M, Angioni C, Sürün D, Thomas D, Schmid T, Häfner AK and
Kahnt AS: Knock-out of 5-lipoxygenase in overexpressing tumor
cells-consequences on gene expression and cellular function. Cancer
Gene Ther. 30:108–123. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Neufert C, Becker C and Neurath MF: An
inducible mouse model of colon carcinogenesis for the analysis of
sporadic and inflammation-driven tumor progression. Nat Protoc.
2:1998–2004. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Deng J, Zhao L, Yuan X, Li Y, Shi J, Zhang
H, Zhao Y, Han L, Wang H, Yan Y, et al: Pre-administration of
berberine exerts chemopreventive effects in AOM/DSS-induced
colitis-associated carcinogenesis mice via modulating inflammation
and intestinal microbiota. Nutrients. 14(726)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Obermeier F, Kojouharoff G, Hans W,
Schölmerich J, Gross V and Falk W: Interferon-gamma (IFN-gamma)-
and tumour necrosis factor (TNF)-induced nitric oxide as toxic
effector molecule in chronic dextran sulphate sodium (DSS)-induced
colitis in mice. Clin Exp Immunol. 116:238–245. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kimlin LC, Casagrande G and Virador VM: In
vitro three-dimensional (3D) models in cancer research: An update.
Mol Carcinog. 52:167–182. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Atat O El, Farzaneh Z, Pourhamzeh M, Taki
F, Abi-Habib R, Vosough M and El-Sibai M: 3D modeling in cancer
studies. Hum Cell. 35:23–36. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zibaei Z, Babaei E, Rezaie Nezhad Zamani
A, Rahbarghazi R and Azeez HJ: Curcumin-enriched Gemini surfactant
nanoparticles exhibited tumoricidal effects on human 3D spheroid
HT-29 cells in vitro. Cancer Nanotechnol. 12(3)2021.
|
|
25
|
Ballesteros-Vivas D, Alvarez-Rivera G,
León C, Morantes SJ, Ibánez E, Parada-Alfonso F and Valdés A:
Anti-proliferative bioactivity against HT-29 colon cancer cells of
a withanolides-rich extract from golden berry (Physalis
peruviana L.) calyx investigated by foodomics. J Funct Foods.
63(103567)2019.
|
|
26
|
Ocampo YC, Caro DC, Rivera DE and Franco
LA: Safety of sucrose esters from Physalis peruviana L. in a
28-day repeated-dose study in mice. Biomed Pharmacother.
90:850–862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang CR, Khan W, Bakht J and Nair MG: New
antiinflammatory sucrose esters in the natural sticky coating of
tomatillo (Physalis philadelphica), an important culinary
fruit. Food Chem. 196:726–732. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mora Vargas JA, Orduña Ortega J, Metzker
G, Larrahondo JE and Boscolo M: Natural sucrose esters:
Perspectives on the chemical and physiological use of an under
investigated chemical class of compounds. Phytochemistry.
177(112433)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y, Wang Z, Sun Y, Zhu M, Jiang Y, Bai
H, Yang B and Kuang H: Isovaleryl sucrose esters from Atractylodes
japonica and their cytotoxic activity. Molecules.
29(3069)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Teng Y, Lan P, White LV and Banwell MG:
The useful biological properties of sucrose esters: Opportunities
for the development of new functional foods. Crit Rev Food Sci
Nutr. 64:8018–8035. 2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hatta MNA, Mohamad Hanif EAM, Chin SF, Low
TY and Neoh HM: Parvimonas micra infection enhances proliferation,
wound healing, and inflammation of a colorectal cancer cell line.
Biosci Rep. 43(BSR20230609)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Silva Nunes JP and Martins Dias AA: ImageJ
macros for the user-friendly analysis of soft-agar and
wound-healing assays. Biotechniques. 62:175–179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Stark GR and Taylor WR: Analyzing the G2/M
checkpoint. In: Checkpoint Controls and Cancer; Methods in
Molecular Biology™. Vol. 280. Humana Press: Totowa, NJ,
USA, pp51-82, 2004.
|
|
34
|
Chinnasamy S, Zameer F and Muthuchelian K:
Molecular and biological mechanisms of apoptosis and its detection
techniques. J Oncol Sci. 6:49–64. 2020.
|
|
35
|
Thaker AI, Shaker A, Suprada Rao M and
Ciorba MA: Modeling colitis-associated cancer with azoxymethane
(AOM) and dextran sulfate sodium (DSS). J Vis Exp.
4100:2012.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
De Robertis M and Signori E:
Azoxymethane/dextran sodium sulfate (AOM/DSS) model of colorectal
cancer. In: Čemažar M, Jesenko T and Lampreht Tratar U (eds) Mouse
Models of Cancer. Methods in Molecular Biology. Vol. 2773. Humana,
New York, NY, pp51-58, 2024.
|
|
37
|
Chen X, Ding Y, Yi Y, Chen Z, Fu J and
Chang Y: Review of animal models of colorectal cancer in different
carcinogenesis pathways. Dig Dis Sci. 69:1583–1592. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lin R, Piao M, Song Y and Liu C: Quercetin
suppresses AOM/DSS-induced colon carcinogenesis through its
anti-inflammation effects in mice. J Immunol.
2020(9242601)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee YP, Chiu CC, Lin TJ, Hung SW, Huang
WC, Chiu CF, Huang YT, Chen YH, Chen TH and Chuang HL: The
germ-free mice monocolonization with Bacteroides fragilis improves
azoxymethane/dextran sulfate sodium induced colitis-associated
colorectal cancer. Immunopharmacol Immunotoxicol. 41:207–213.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
De Robertis M, Massi E, Poeta M, Carotti
S, Morini S, Cecchetelli L, Signori E and Fazio VM: The AOM/DSS
murine model for the study of colon carcinogenesis: From pathways
to diagnosis and therapy studies. J Carcinog. 10(9)2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Giner E, Recio MC, Ríos JL, Cerdá-Nicolás
JM and Giner RM: Chemopreventive effect of oleuropein in
colitis-associated colorectal cancer in c57bl/6 mice. Mol Nutr Food
Res. 60:242–255. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Takahashi M, Mutoh M, Kawamori T, Sugimura
T and Wakabayashi K: Altered expression of beta-catenin, inducible
nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced
rat colon carcinogenesis. Carcinogenesis. 21:1319–1327.
2000.PubMed/NCBI
|
|
43
|
Tang A, Li N, Li X, Yang H, Wang W, Zhang
L, Li G, Xiong W, Ma J and Shen S: Dynamic activation of the key
pathways: Linking colitis to colorectal cancer in a mouse model.
Carcinogenesis. 33:1375–1383. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ranganathan AC, Adam AP and Aguirre-Ghiso
JA: Opposing roles of mitogenic and stress signaling pathways in
the induction of cancer dormancy. Cell Cycle. 5:1799–1807.
2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang L and Gu X: 4-hydroxysesamin
protects rat with right ventricular failure due to pulmonary
hypertension by inhibiting JNK/p38 MAPK signaling. Aging (Albany
NY). 16:8142–8154. 2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
KRAS gene-Genetics Home Reference-NIH,
2020 [cited 2020 Mar 9]. Available from: https://ghr.nlm.nih.gov/gene/KRAS.
|
|
47
|
Vivona AA, Shpitz B, Medline A, Bruce WR,
Hay K, Ward MA, Stern HS and Gallinger S: K-ras mutations in
aberrant crypt foci, adenomas and adenocarcinomas during
azoxymethane-induced colon carcinogenesis. Carcinogenesis.
14:1777–1781. 1993.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bragado P, Armesilla A, Silva A and Porras
A: Apoptosis by cisplatin requires p53 mediated p38alpha MAPK
activation through ROS generation. Apoptosis. 12:1733–1742.
2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cheung KL, Khor TO, Yu S and Kong ANT:
PEITC induces G1 cell cycle arrest on HT-29 cells through the
activation of p38 MAPK signaling pathway. AAPS J. 10:277–281.
2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gupta J and Nebreda AR: Roles of p38α
mitogen-activated protein kinase in mouse models of inflammatory
diseases and cancer. FEBS J. 282:1841–1857. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li W, Li C, Zheng H, Chen G and Hua B:
Therapeutic targets of Traditional Chinese Medicine for colorectal
cancer. J Tradit Chinese Med. 36:243–249. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hu R, Kim BR, Chen C, Hebbar V and Kong
ANT: The roles of JNK and apoptotic signaling pathways in
PEITC-mediated responses in human HT-29 colon adenocarcinoma cells.
Carcinogenesis. 24:1361–1367. 2003.PubMed/NCBI View Article : Google Scholar
|