Introduction
Periodontitis is one of the most common diseases
worldwide (1,2) and is caused by chronic bacterial
infection around the teeth (3).
Disease progression is characterized by chronic inflammatory tissue
destruction, especially resorption of the alveolar bone, which
supports the teeth (4).
Furthermore, this disease is associated with numerous systemic
diseases, such as diabetes mellitus and non-alcoholic
steatohepatitis, because of periodontitis-related bacterial
infections and inflammatory responses (5). Thus, numerous attempts have been made
to reduce bacterial infections and chronic inflammation (6). However, these attempts must be made
over a long period, possibly over the entire life stage of the
individual. Phytochemical reagents, including functional foods
and/or dietary supplements, can be suitable for this purpose since
they have mild effects and can be consumed daily.
In our previous studies, some dietary phytochemicals
that can reduce oral biofilm formation (7,8) and
specialized pro-resolving mediators derived from omega-3 fatty
acids, such as resolvin D2, that can regulate chronic inflammation
to induce tissue regenerative phases, were investigated (9). In addition to these direct reactions,
the metabolites of food ingredients have received considerable
attention. The health-contributing effects of garlic (Allium
sativum L.) are well known (10), and a historical review of dietary
supplements for lipid-lowering activity has also highlighted the
effects of garlic (11). Even
snacks containing garlic extracts have been discussed for health
promotion (12), thereby guiding
the industrial utilization of garlic in food products and
indicating its suitability for the development of functional foods
and/or dietary supplements (13).
Numerous studies have supported the health benefits of
garlic-related phytochemical reagents.
Garlic has been shown to improve periodontal disease
in humans (14,15) and dogs (16). Zini et al (14,15)
conducted two clinical intervention studies and showed that oral
intake of aged garlic extract (AGE) prepared using a water-ethanol
extraction method prevented periodontitis. Furthermore, Takahashi
et al (16) showed that AGE
supplementation improved gingivitis and halitosis in Beagle dogs.
The mechanisms underlying the action of AGE include antioxidant
activity (17,18), improvement of peripheral circulation
(19), reduction of inflammation
(20,21) and enhancement of immune reactions
(22,23). Moreover, previous studies have
reported that AGE reduces Porphyromonas gingivalis-derived
lipopolysaccharide (LPS)-induced cellular responses in gingival
epithelium cells (24) and
fibroblasts (25). These mechanisms
of AGE action are considered to be caused by the sulfur components
of AGEs, such as S-allylcysteine (SAC), S-1-propenylcysteine (S1PC)
and S-allyl-mercapto-cysteine (SAMC). AGE can also be expected to
have certain benefits in preventing periodontitis; however, caution
should be exercised when utilizing these purified sulfur
components. Despite studies highlighting the effective utilization
of AGEs containing these compounds in various amounts, the
mechanism underlying the preventive and therapeutic effects of
AGE-containing functional foods and/or dietary supplements for
periodontal disease requires elucidation (26).
The present study aimed to investigate how AGE
modifies periodontal conditions, such as alveolar bone resorption,
inflammatory reactions and histological changes, in a mouse model
of ligature-induced experimental periodontitis.
Materials and methods
Preparation of AGE
AGE was produced by soaking sliced garlic cloves in
ethanol/water for >10 months at room temperature (27).
Animal and experimental
periodontitis
The animal experiment was conducted using 40 male
mice (C57BL/6J: CLEA Japan, Inc.; average weight: 21.5 g at 7 weeks
of age). The housing conditions were constant temperature at 23±1˚C
with a 12/12-h light/dark cycle, and the mice were provided free
access to sterile food (Rodents Diets MF, solid, 12 mmφ pellet,
Oriental Yeast) and water under specific pathogen-free conditions
(28). Each mouse received AGE at a
safe and sufficiently effective dose of 2 g/kg (28-30)
orally using a feeding needle (ball diameter 2.0 mm; As One;
https://www.as-1.co.jp/en/) under
handholding without anesthesia, starting 3 days before induction of
experimental periodontitis and ending 6 days after induction. Pure
water was used as the negative control. Animal care and experiments
were performed in accordance with Okayama University's Guidelines
for the Care and Use of Laboratory Animals, which were reviewed and
approved (approval no. OKU-2022934) by the Animal Care and Use
Committee of Okayama University (Okayama, Japan).
For induction of experimental periodontitis, the
mice were anesthetized with a mixture of 92.48 mg/kg ketamine
hydrochloride (Daiichi Sankyo) and 11.184 mg/kg xylazine
hydrochloride (Bayer Yakuhin, Ltd.) in phosphate-buffered saline
(pH 7.4; Thermo Fisher Scientific, Inc.) by intraperitoneal
injection (31). A 5-0 silk
ligature was tied around the left maxillary second molar (32). The contralateral tooth was not
ligated and served as the baseline control. A total of 7 days after
ligation, mice were euthanized using carbon dioxide gas (volume
displacement rate of CO2 was 30%/min).
Observation of alveolar bone
resorption
A dissecting microscope (1.2; SZ61; Olympus
Corporation) was used to morphologically evaluate the relationship
between the bone and tooth root in the debrided maxilla. After
bleaching with 31% hydrogen peroxide (Santoku Chemical Industries,
Co., Ltd.) followed by staining with 0.5% eosin (Merck KGaA) and 1%
methylene blue (Muto Pure Chemicals, Co., Ltd.), the distance from
the cementoenamel junction to the alveolar bone crest was measured
at three predetermined points on the ligated second molar and two
predetermined points on the affected first molar (32). The third molar was excluded from the
measurements because they had dropped out in some mice.
Reverse transcription-quantitative PCR
(RT-qPCR)
Gingival tissues (1x2 mm) were obtained from the
palatal side of both molars. Total RNA was extracted using a RNeasy
Plus Mini kit (Qiagen GmbH) and quantified using a microvolume
spectrophotometer at 260 and 280 nm (NanoDrop-2000; Thermo Fisher
Scientific, Inc.). The RNA was reverse transcribed to cRNA by using
SuperScript™ IV VILO™ Master Mix (Thermo
Fisher Scientific, Inc.) at 25˚C for 10 min, 42˚C for 60 min, and
85˚C for 5 min. qPCR with the cDNA was performed using Power
SYBR™ Green PCR Master Mix (Thermo Fisher Scientific,
Inc.) and specific primers (Table
SI) using 7300 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
PCR reaction mixture was kept at 50˚C for 2 min, then at 95˚C for
10 min. PCR cycles were 50 cycles of denaturing at 95 ̊C for 15 sec
and annealing and extension at 60˚C for 60 sec. Data were analyzed
using the comparative 2-ΔΔCq method (33). GAPDH was used as a reference
gene.
Histological evaluation
Mouse maxillae and the surrounding periodontal
tissue were fixed in 4% paraformaldehyde phosphate buffer solution
(Nacalai Tesque, Inc.) for 24 h at 4˚C, decalcified in 10%
ethylene-diamine-tetra-acetic acid solution (pH 7.0, Muto Pure
Chemicals Co., Ltd.) for 1 week at 4˚C, and then embedded in
paraffin. Tissue samples were sectioned at 7-µm thickness in the
coronal direction along the long axis of the teeth. Sections were
stained with hematoxylin (Mayer's hemalum solution, Merck KGaA) and
eosin (0.5% aqueous eosin Y-solution, Merck KGaA). Osteoclasts were
visualized using a tartrate-resistant acid phosphatase (TRAP)
staining kit (FUJIFILM Wako Pure Chemical Corporation). Stained
images were observed and captured using a microscope under natural
light (fluorescence microscope BZ-X800, Keyence Corporation;
1,920x1,440 pixels). TRAP-positive cells with large or multiple
nuclei were automatically counted using a microscope (BZ-X800;
Keyence Corporation) in 3-5 sections from each mouse. The average
number of TRAP-positive cells on each mouse's control and ligatured
sides was used for statistical analysis.
Statistical analysis
Data were analyzed using one-way analysis of
variance (ANOVA) followed by Tukey's multiple-comparison test for
multiple-group comparisons. Two-tailed unpaired Student's t-test
was used for two-group comparisons. P<0.05 was considered to
indicate a statistically significant difference. All analyses were
performed using GraphPad Prism (version 8.4.3; GraphPad Software;
Dotmatics).
Results
Effects of AGE feeding on the body
conditions of mice
The body weight of each mouse was monitored daily,
and almost all mice were stable. Only one mouse in the control
group showed a weight reduction of ~5 g 2 days after ligation, but
its body weight recovered 5 days after ligation (Fig. S1). Blood tests for total protein
and albumin levels were also performed. However, no statistically
significant differences were observed between the AGE and control
groups (Fig. S2).
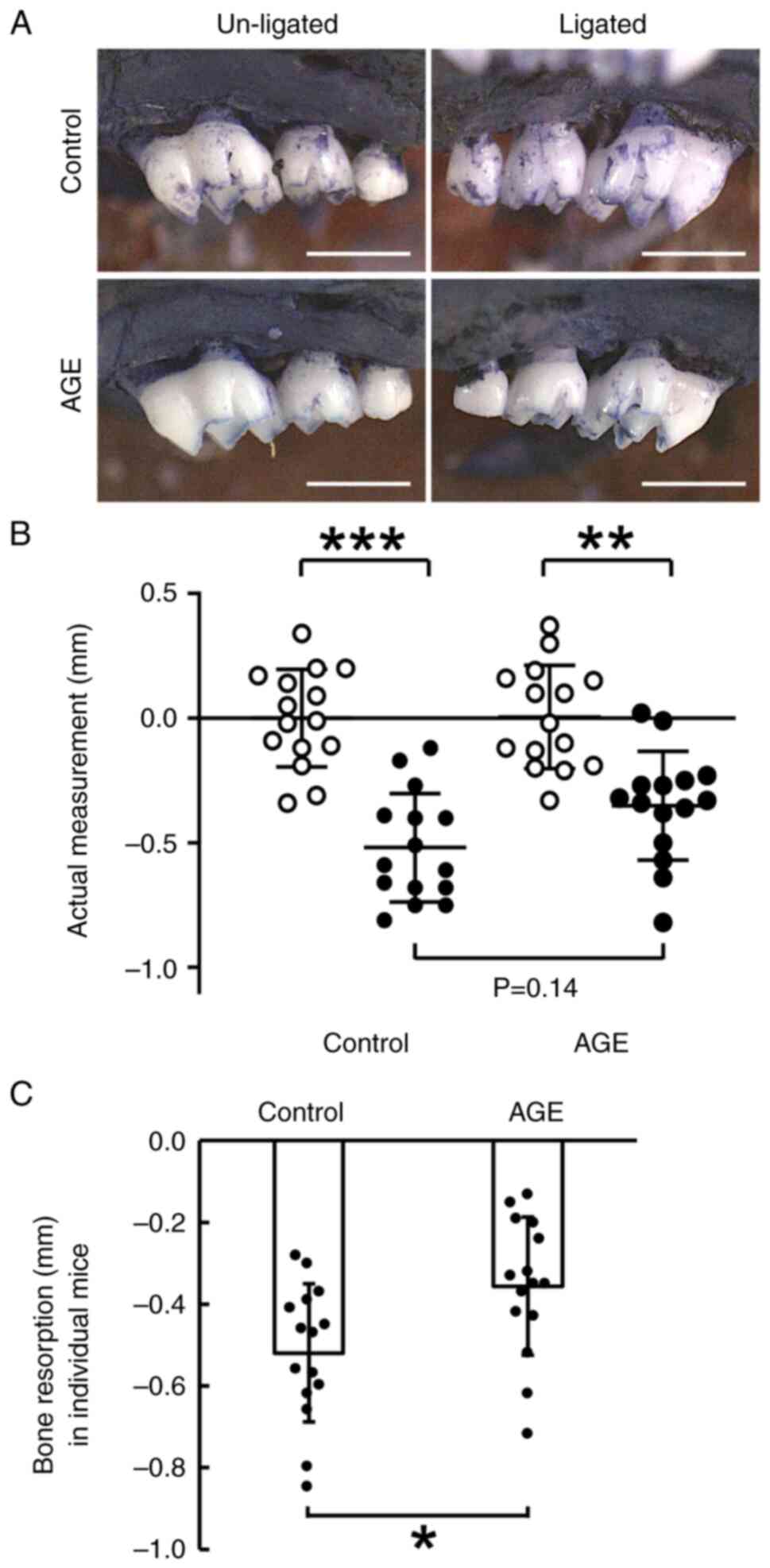
Suppression of alveolar bone
resorption in AGE-fed mice
The actual alveolar bone level reduced significantly
after the 7-day ligation around the second molar in both the AGE
and control groups (Fig. 1A and
B). This reduction was lower in the
AGE group than in the control group (P<0.001 in the AGE group,
P<0.0001 in the control group; Fig.
1B). However, the difference in this reduction between the two
groups was not significant (P=0.14).
Using the difference in actual measurements between
the ligated and un-ligated sites in individual mice,
ligature-induced bone resorption was compared between the AGE group
and the control group. The reduction of bone level in the AGE group
was lower than that in the control group (P<0.05; Fig. 1C).
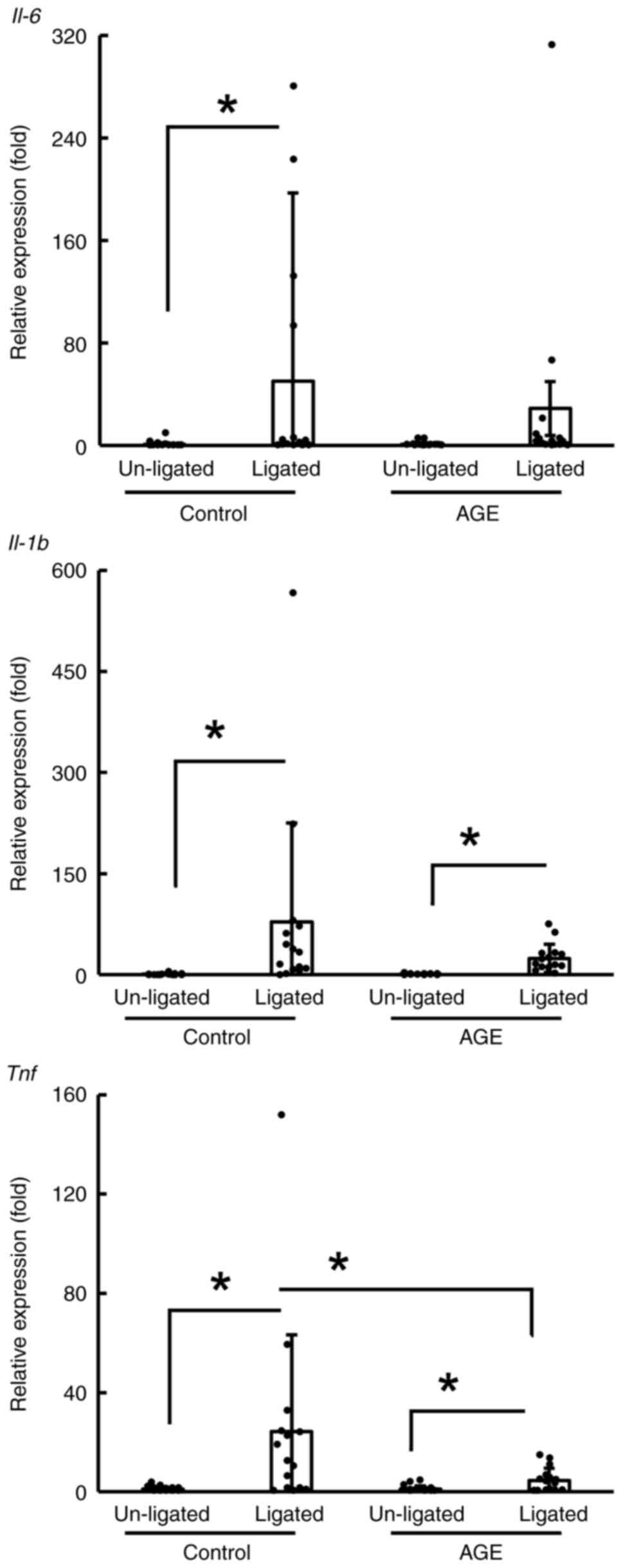
Suppression of mRNA expression of
inflammatory cytokines in AGE-fed mice
The mRNA expression of inflammatory cytokines in the
periodontitis tissue of the control and AGE groups was compared
between the un-ligated and ligated sites (Fig. 2). The expression of these mRNAs at
the ligated sites was greater than that at un-ligated sites,
especially in the control group. Although these mRNAs were also
more highly expressed in the ligated sites than in the un-ligated
sites in the AGE group (P<0.05; Fig.
2), the expression of the tumor necrosis factor gene
(TNF) in the AGE group was lower than that in the control
group (P<0.05; Fig. 2).
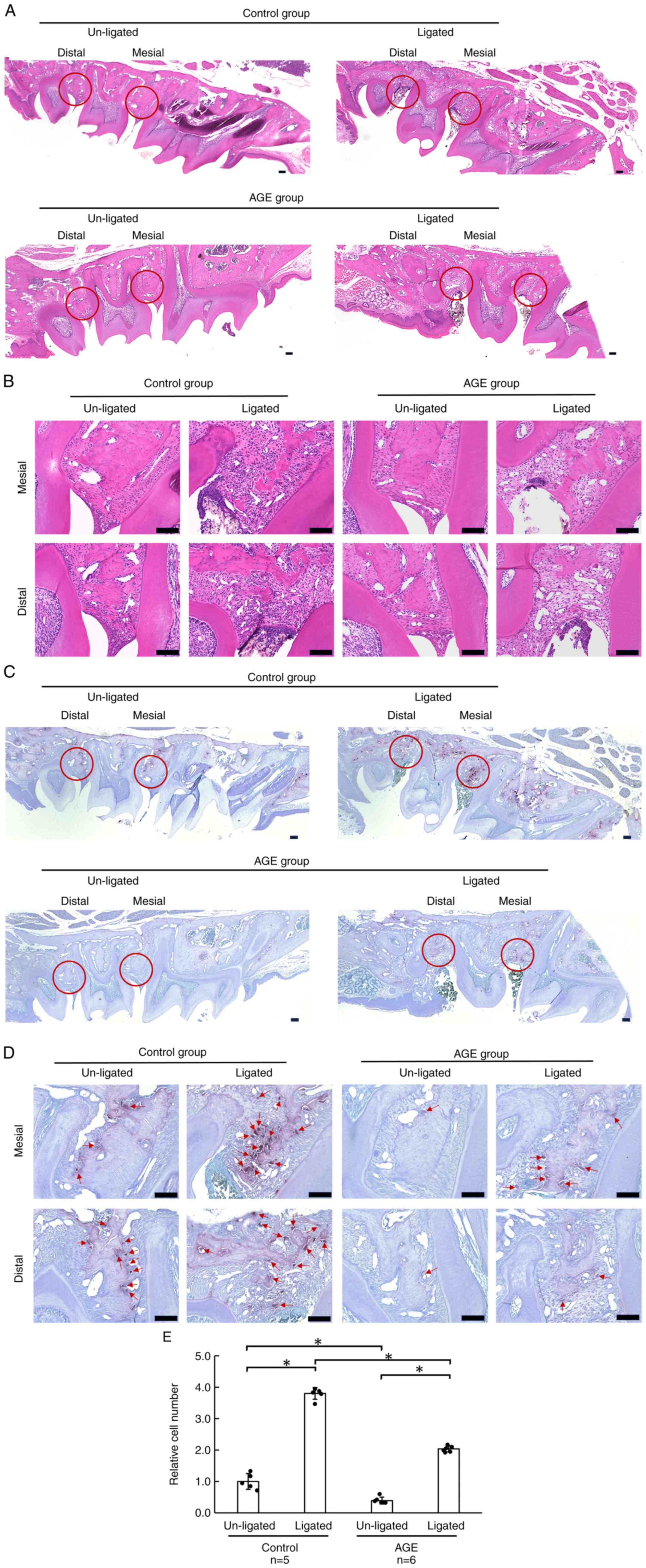
Reduced number of osteoclasts in
ligature-induced periodontitis tissue in AGE-fed mice
Around the periodontal tissue surrounding the
ligature, the interdental papillae and alveolar bone crests were
destroyed, and cells had infiltrated these areas (Fig. 3A). These phenomena were observed in
both the control and AGE groups (Fig.
3B). TRAP staining revealed more osteoclasts at the ligated
sites in the control group than in the AGE group (Fig. 3C and D). The number of TRAP-positive osteoclasts
increased on the ligature side in both control and AGE-fed mice.
However, under ligature-induced periodontitis conditions, it
decreased when the mice were fed AGE (Fig. 3E). Even under un-ligated conditions,
it also decreased with AGE feeding.
Discussion
AGE has been reported to show suppressive effects in
inflammatory periodontal diseases (14-16).
Therefore, in the present study, its effects were evaluated on
alveolar bone resorption. It was demonstrated that AGE inhibited
alveolar bone resorption in an experimental periodontitis model
(Fig. 1). In the inflamed
periodontal tissue, the expression of inflammatory cytokine genes
(especially TNF) was suppressed (Fig.
2). Furthermore, the number of osteoclasts in the inflamed
periodontal tissue was reduced when AGE was fed to experimental
mice (Fig. 3). These results
suggest that AGE suppresses inflammation and osteoclast
differentiation, leading to reduced alveolar bone resorption.
The anti-inflammatory effects of garlic have been
reported previously (12). In
particular, AGE has been shown to reduce interleukin (IL)-6
production in both clinical trials (34,35)
and in vitro cell culture models (24). Since IL-6 is known to be involved in
periodontitis (36,37), the regulation of IL-6 production by
AGE is a notable finding. Furthermore, AGE as well as garlic
extracts and derivatives may suppress numerous inflammatory
processes. Thiosulfinate-enriched garlic extract was shown to
inhibit the production of TNF-α, IL-1β and IL-6 in LPS-stimulated
monocytes obtained from healthy individuals (38).
Furthermore, allicin, one of the bioactive
components of garlic, has been shown to suppress the LPS-induced
increases in the levels of inflammatory cytokines, such as IL-1β,
IL-6, IL-8 and TNF-α in bovine epithelial cell culture experiments,
and to alter nuclear factor kappa-B (NF-κB) signaling pathway
(39). In a human gingival cell
line, the AGE components SAC and SAMC reduced TNF-α-induced
increments in IL-6 production by suppressing NF-κB. In addition,
S1PC inhibited the expression of intercellular adhesion molecule-1.
AGE may inhibit inflammatory reactions by preventing the migration
of inflammatory immune cells and interrupting the mitogen-activated
protein kinase signaling pathway (24). In a clinical application of garlic
extracts and derivatives for reducing inflammation, a randomized,
double-blind clinical trial in patients undergoing peritoneal
dialysis demonstrated that administering of 400 mg of garlic
extract twice daily for 8 weeks resulted in anti-inflammatory
effects on IL-6 and C-reactive protein levels, as well as on the
erythrocyte sedimentation rate (40). Unfortunately, no blood test was
conducted in the present study. Thus, the effect on blood factors
in mice remains unknown. However, thiosulfinate-enriched garlic
extract has been suggested to enhance the inflammatory response in
monocytes from patients with sepsis, manifesting as increased
expression of human leukocyte antigen-DR (38). Thus, the anti-inflammatory effects
of garlic extracts and their derivatives may require more careful
consideration since they may also have opposite effects. Therefore,
garlic extracts and their derivatives should be included in the
category of herbal medicines and supplements until their mechanism
of action is clarified.
Numerous types of cytokines are involved in
osteoclast differentiation (41).
Certain cytokines play key roles in the interaction between
receptor activator of NF-κB (RANK), its ligand RANKL, and
osteoprotegerin, which are crucial for osteoclast differentiation
(42). Other cytokines influence
the activities of factors such as nuclear factor of activated
T-cells and cytoplasmic 1, which promotes osteoclast activation and
inhibits osteogenic differentiation factors such as runt-related
transcription factor 2 and osterix (43). TNF-α is one of the major cytokines
involved in osteoimmunology and stimulates
osteoclastogenesis-related other factors such as macrophage
colony-stimulating factor (M-CSF) and RANKL, thereby causing
pathological bone resorption such as alveolar bone resorption in
periodontitis (44). The present
results demonstrated that AGE reduced alveolar bone resorption, the
expression level of TNF mRNA, and the number of osteoclasts in
periodontal tissue, suggesting that further investigation is needed
to clarify osteoclastogenesis, especially TNF-α secretion from
monocytes/macrophages, and effects on the expression of factors
related to osteoclastogenesis.
There are certain limitations to the present study.
The suppressive effect of AGE on alveolar bone resorption was not
as strong as the average bone level, which was evident in AGE-fed
mice (Fig. 1B; P=0.14 between
control and AGE groups at ligature sites). These effects were
observed when bone levels were compared in the individual mice
(Fig. 1C). This suggests that AGE
does not exert any apparent suppressive effects on bone resorption,
like certain bisphosphonate derivatives. This weak suppressive
effect may be related to the signaling pathways that induce
osteoclast differentiation. However, the present study evaluated
only three inflammatory cytokines. Further studies are needed to
assess inflammatory cytokine-related osteoclast differentiation
signaling pathways, such as those involving M-CSF and RANKL.
Further experiments on how AGE affects osteoclast differentiation
are conducted by the authors based on the results of the present
study. The involvement of reactive oxygen species is another topic
of further research (45). Aged
black garlic extract has been reported to have higher antioxidant
activity than fresh raw garlic extract (46). This represents another perspective
worth exploring in future studies. A series of studies on
AGE-induced improvement of osteoimmunology is needed to expand the
potential applications of AGE in healthy living (12).
In conclusion, AGE prevented alveolar bone loss by
suppressing the expression of inflammatory cytokines related to
osteoclast differentiation in periodontal tissues. Further studies
are needed to elucidate how AGE alters the secretion of
osteoclastogenesis-related cytokines in inflamed periodontal
tissues.
Supplementary Material
Body weight of the mice during the
experiment. Each mouse was weighed before daily oral
administration, and the results are represented as a line graph.
Individuals are numbered and shown as colored lines.
Blood biochemistry test at the end of
the experiment. Serum collected at the end of the experiment was
analyzed. Total protein and albumin concentrations were not
significantly different between the AGE and control groups. Data
are presented as the mean ± SD. Student's t-test was
performed.
Primer sequences used for reverse
transcription-quantitative PCR.
Acknowledgements
The authors would like to thank Dr Toshiaki
Matsutomo (Wakunaga Pharmaceutical Co., Ltd.) for his helpful
advice, encouragement, and critical evaluation of this manuscript.
The authors would also like to thank Drs Jun Sakurai and Yasuhiro
Fujii (Center for Innovative Clinical Medicine, Okayama University
Hospital, Japan) for organizing and supporting the collaborative
research.
Funding
Funding: The present study was supported by the Collaborative
Research Contract (grant no. 7262000084) between Okayama University
and Wakunaga Pharmaceutical Co., Ltd.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CK conceptualized the study, developed methodology,
performed formal analysis and investigation, and wrote the original
draft. AH conceptualized the study, developed methodology,
validated data, performed investigation and visualization, provided
resources and supervised the study. CKN developed methodology and
conducted investigation. HN and MO performed formal analysis, data
curation and validation. KO validated data, supervised the study,
wrote, reviewed and edited the manuscript. ST conceptualized the
study, validated data, reviewed and edited the manuscript,
performed project administration and acquired funding. CK, AH and
ST confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Animal care and experiments were performed in
accordance with Okayama University's Guidelines for the Care and
Use of Laboratory Animals, which were reviewed and approved
(approval no. OKU-2022934) by the Animal Care and Use Committee of
Okayama University (Okayama, Japan).
Patient consent for publication
Not applicable.
Competing interests
MO and HN are employed by Wakunaga Pharmaceutical
Co., Ltd., who provided the funding for the present study.
Specifically, the funder provided the AGE for the present
study.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Kassebaum NJ, Smith AGC, Bernabé E,
Fleming TD, Reynolds AE, Vos T, Murray CJL and Marcenes W: GBD 2015
Oral Health Collaborators. Global, regional, and national
prevalence, incidence, and disability-adjusted life years for oral
conditions for 195 countries, 1990-2015: A systematic analysis for
the global burden of diseases, injuries, and risk factors. J Dent
Res. 96:380–387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Periodontal diseases-level 4 cause. Global
Health Metrics. https://www.thelancet.com/pb-assets/Lancet/gbd/summaries/diseases/periodontal-diseases.pdf.
|
|
3
|
Socransky SS, Haffajee AD, Cugini MA,
Smith C and Kent RL Jr: Microbial complexes in subgingival plaque.
J Clin Periodontol. 25:134–144. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cochran DL: Inflammation and bone loss in
periodontal disease. J Periodontol. 79 (Suppl 8):S1569–S1576.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hajishengallis G and Chavakis T: Local and
systemic mechanisms linking periodontal disease and inflammatory
comorbidities. Nat Rev Immunol. 21:426–440. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Balta MG, Papathanasiou E, Blix IJ and Van
Dyke TE: Host modulation and treatment of periodontal disease. J
Dent Res. 100:798–809. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ito T, Yoshida Y, Shiota Y, Ito Y,
Yamamoto T and Takashiba S: Effects of Lectins on initial
attachment of cariogenic Streptococcus mutans. Glycoconj J.
35:41–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ito M, Ito T, Aoki H, Nishioka K, Shiokawa
T, Tada H, Takeuchi Y, Takeyasu N, Yamamoto T and Takashiba S:
Isolation and identification of the antimicrobial substance
included in tempeh using Rhizopus stolonifer NBRC 30816 for
fermentation. Int J Food Microbiol. 325(108645)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Siddiqui YD, Omori K, Ito T, Yamashiro K,
Nakamura S, Okamoto K, Ono M, Yamamoto T, Van Dyke TE and Takashiba
S: Resolvin D2 induces resolution of periapical inflammation and
promotes healing of periapical lesions in rat periapical
periodontitis. Front Immunol. 10(307)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang L, Liu Z, Wang J, Fu J, Jia Y, Ji L
and Wang T: Bioactivity and health effects of garlic essential oil:
A review. Food Sci Nutr. 11:2450–2470. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grant JK, Dangl M, Ndumele CE, Michos ED
and Martin SS: A historical, evidence-based, and narrative review
on commonly used dietary supplements in lipid-lowering. J Lipid
Res. 65(100493)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Verma T, Aggarwal A, Dey P, Chauhan AK,
Rashid S, Chen KT and Sharma R: Medicinal and therapeutic
properties of garlic, garlic essential oil, and garlic-based snack
food: An updated review. Front Nutr. 10(1120377)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sunanta P, Kontogiorgos V, Pankasemsuk T,
Jantanasakulwong K, Rachtanapun P, Seesuriyachan P and Sommano SR:
The nutritional value, bioactive availability and functional
properties of garlic and its related products during processing.
Front Nutr. 10(1142784)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zini A, Mann J, Mazor S and Vered Y: The
efficacy of aged garlic extract on gingivitis-a randomized clinical
trial. J Clin Dent. 29:52–56. 2018.PubMed/NCBI
|
|
15
|
Zini A, Mann J, Mazor S and Vered Y:
Beneficial effect of aged garlic extract on periodontitis: A
randomized controlled double-blind clinical study. J Clin Biochem
Nutr. 67:297–301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Takahashi K, Nango H, Ushijima M,
Takashima M, Nakamoto M, Matsutomo T, Jikihara H, Arakawa N, Maki
S, Yabuki A, et al: Therapeutic effect of aged garlic extract on
gingivitis in dogs. Front Vet Sci. 10(1277272)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Morihara N, Hayama M and Fujii H: Aged
garlic extract scavenges superoxide radicals. Plant Foods Hum Nutr.
66:17–21. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang X, Yang Y and Zhang M: The vivo
antioxidant activity of self-made aged garlic extract on the
d-galactose-induced mice and its mechanism research via gene chip
analysis. RSC Adv. 9:3669–3678. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ushijima M, Takashima M, Kunimura K,
Kodera Y, Morihara N and Tamura K: Effects of S-1-propenylcysteine,
a sulfur compound in aged garlic extract, on blood pressure and
peripheral circulation in spontaneously hypertensive rats. J Pharm
Pharmacol. 70:559–565. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim MJ, Yoo YC, Kim HJ, Shin SK, Sohn EJ,
Min AY, Sung NY and Kim MR: Aged black garlic exerts
anti-inflammatory effects by decreasing no and proinflammatory
cytokine production with less cytoxicity in LPS-stimulated raw
264.7 macrophages and LPS-induced septicemia mice. J Med Food.
17:1057–1063. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suzuki JI, Kodera Y, Miki S, Ushijima M,
Takashima M, Matsutomo T and Morihara N: Anti-inflammatory action
of cysteine derivative S-1-propenylcysteine by inducing MyD88
degradation. Sci Rep. 8(14148)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahmadabad HN, Hassan ZM, Safari E,
Bozorgmehr M, Ghazanfari T and Moazzeni SM: Evaluation of the
immunomodulatory effect of the 14 kDa protein isolated from aged
garlic extract on dendritic cells. Cell Immunol. 269:90–95.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr. 131
(Supple 3):1075S–1079S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ohtani M and Nishimura T:
Sulfur-containing amino acids in aged garlic extract inhibit
inflammation in human gingival epithelial cells by suppressing
intercellular adhesion molecule-1 expression and IL-6 secretion.
Biomed Rep. 12:99–108. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nango H and Ohtani M:
S-1-propenyl-L-cysteine suppresses lipopolysaccharide-induced
expression of matrix metalloproteinase-1 through inhibition of
tumor necrosis factor-α converting enzyme-epidermal growth factor
receptor axis in human gingival fibroblasts. PLoS One.
18(e0284713)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ohtani M and Nishimura T: The preventive
and therapeutic application of garlic and other plant ingredients
in the treatment of periodontal diseases. Exp Ther Med.
19:1507–1510. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ryu K, Ide N, Matsuura H and Itakura Y: N
alpha-(1-deoxy-D-fructos-1-yl)-L-arginine, an antioxidant compound
identified in aged garlic extract. J Nutr. 131 (Suppl 3):972S–976S.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Matsutomo T, Ushijima M, Kodera Y,
Nakamoto M, Takashima M, Morihara N and Tamura K: Metabolomic study
on the antihypertensive effect of S-1-propenylcysteine in
spontaneously hypertensive rats using liquid chromatography coupled
with quadrupole-Orbitrap mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1046:147–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ushijima M, Kunimura K and Suzuki JI:
S-1-propenylcysteine, a sulfur compound in aged garlic extract,
alleviates cold-induced reduction in peripheral blood flow in rat
via activation of the AMPK/eNOS/NO pathway. Exp Ther Med.
20:2815–2821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kunimura K, Nakamoto M and Ushijima M:
S-1-propenylcysteine enhances endurance capacity of mice by
stimulating fatty acid metabolism via muscle isoform of carnitine
acyltransferase-1. J Nutr. 154:2707–2716. 2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Ideguchi H, Aoyagi H, Yamashiro
K, Yamamoto T, Nishibori M and Takashiba S: Malnutrition delayed
wound healing after tooth extraction by HMGB1-related prolonged
inflammation. Int Immunopharmacol. 96(107772)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Abe T and Hajishengallis G: Optimization
of the ligature-induced periodontitis model in mice. J Immunol
Methods. 394:49–54. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gadidala SK, Johny E, Thomas C, Nadella M,
Undela K and Adela R: Effect of garlic extract on markers of lipid
metabolism and inflammation in coronary artery disease (CAD)
patients: A systematic review and meta-analysis. Phytother Res.
37:2242–2254. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wlosinska M, Nilsson AC, Hlebowicz J,
Hauggaard A, Kjellin M, Fakhro M and Lindstedt S: The effect of
aged garlic extract on the atherosclerotic process - a randomized
double-blind placebo-controlled trial. BMC Complement Med Ther.
20(132)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mazurek-Mochol M, Bonsmann T, Mochol M,
Poniewierska-Baran A and Pawlik A: The role of interleukin 6 in
periodontitis and its complications. Int J Mol Sci.
25(2146)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Takashiba S, Naruishi K and Murayama Y:
Perspective of cytokine regulation for periodontal treatment:
Fibroblast biology. J Periodontol. 74:103–110. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Avendaño-Ortiz J, Redondo-Calvo FJ,
Lozano-Rodríguez R, Terrón-Arcos V, Bergón-Gutiérrez M,
Rodríguez-Jiménez C, Rodríguez JF, Del Campo R, Gómez LA,
Bejarano-Ramírez N, et al: Thiosulfinate-enriched Allium
sativum extract exhibits differential effects between healthy
and sepsis patients: The implication of HIF-1α. Int J Mol Sci.
24(6234)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Che HY, Zhou CH, Lyu CC, Meng Y, He YT,
Wang HQ, Wu HY, Zhang JB and Yuan B: Allicin alleviated LPS-induced
mastitis via the TLR4/NF-κB signaling pathway in bovine mammary
epithelial cells. Int J Mol Sci. 24(3805)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zare E, Alirezaei A, Bakhtiyari M and
Mansouri A: Evaluating the effect of garlic extract on serum
inflammatory markers of peritoneal dialysis patients: A randomized
double-blind clinical trial study. BMC Nephrol.
20(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao Z, Du Y, Yan K, Zhang L and Guo Q:
Exercise and osteoimmunology in bone remodeling. FASEB J.
38(e23554)2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Boyce BF and Xing L: Biology of RANK,
RANKL, and osteoprotegerin. Arthritis Res Ther. 9 (Suppl
1)(S1)2007.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Takayanagi H: RANKL as the master
regulator of osteoclast differentiation. J Bone Miner Metab.
39:13–18. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Marahleh A, Kitaura H, Ohori F, Kishikawa
A, Ogawa S, Shen WR, Qi J, Noguchi T, Nara Y and Mizoguchi I: TNF-α
directly enhances osteocyte RANKL expression and promotes
osteoclast formation. Front Immunol. 10(2925)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sczepanik FSC, Grossi ML, Casati M,
Goldberg M, Glogauer M, Fine N and Tenenbaum HC: Periodontitis is
an inflammatory disease of oxidative stress: We should treat it
that way. Periodontol 2000. 84:45–68. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jeong YY, Ryu JH, Shin JH, Kang MJ, Kang
JR, Han J and Kang D: Comparison of anti-oxidant and
anti-inflammatory effects between fresh and aged black garlic
extracts. Molecules. 21(430)2016.PubMed/NCBI View Article : Google Scholar
|