Introduction
Bronchial asthma, a long-standing ailment, is a
prevalent respiratory disease that affects individuals of all ages
globally (1). Patients with chronic
persistent bronchial asthma frequently experience recurrent
episodes, significantly disrupting their daily life and work
(2). It is estimated that bronchial
asthma affects ~300 million people worldwide, a number projected to
rise to 400 million by 2025(3).
Bronchial asthma is responsible for ~500,000 hospitalizations
annually, with ~250,000 deaths attributed to the disease each year
(4). Bronchial asthma is
distinguished by varying degrees of persistent inflammation and
structural modifications in the airway (5). The most notable abnormalities include
epithelial denudation, goblet cell metaplasia, subepithelial
thickening, augmented airway smooth muscle mass, bronchial gland
enlargement, angiogenesis, and modifications in extracellular
matrix components, involving both large and small airways (6).
Alpha coronaviruses, including human coronavirus
229E (HCoV-229E) and HCoV-NL63, induce inflammation in the upper
respiratory tract and have the potential to exacerbate asthma
(7). Conversely,
bate-coronaviruses, such as severe acute respiratory syndrome-CoV-2
(SARS-CoV-2), SARS-CoV, and Middle East respiratory syndrome-CoV
(MERS-CoV), specifically target epithelial cells in both the upper
and lower airways. SARS-CoV-2 serves as the causative agent for the
disease known as coronavirus disease 2019 (COVID-19) (8). The COVID-19 pandemic originated in
Wuhan, China, and rapidly spread worldwide. The COVID-19 pandemic,
caused by the SARS-CoV-2, has emerged as a remarkable global health
concern (9). COVID-19 manifests in
distinct stages following a median incubation period of 5 days,
involving mild cases (80-90% of patients) characterized by upper
and lower airway responses, while severe cases (10-20%) progress to
bilateral pneumonia (10). A subset
of patients with severe COVID-19 may develop acute respiratory
distress syndrome, necessitating mechanical ventilation in an
intensive care setting (11). With
an estimated 300 million people globally affected by asthma
(12), understanding the clinical
characteristics of COVID-19-induced bronchial asthma is vital.
Notably, patients with pre-existing comorbidities, such as diabetes
and chronic obstructive pulmonary disease, are more prone to
experience severe manifestations of COVID-19(13). While there exists a potential
heightened risk of severe COVID-19 in asthmatic patients, it is
uncertain whether COVID-19 infection-induced bronchial asthma has
its own unique clinical characteristics. The present study examined
the medical history and clinical features of patients with COVID-19
infection-induced bronchial asthma, as well as those with typical
bronchial asthma who were diagnosed and treated in our hospital. A
comparative analysis was conducted to identify differences between
these two groups of asthmatic patients.
Materials and methods
Patients
The clinical data of 116 patients with COVID-19
infection-induced bronchial asthma, who were diagnosed and treated
in the outpatient department of Beijing Tsinghua Changgung Hospital
(Beijing, China) after the COVID-19 pandemic (post-March 2023),
were retrospectively analyzed. Additionally, data from 113 patients
with typical bronchial asthma (no history of COVID-19 infection),
who were diagnosed and treated before the pandemic (January 2022 to
November 2022), were included for comparable analysis.
Inclusion and exclusion criteria
Inclusion criteria were as follows: patients who
meet the GINA 2023 diagnostic criteria (https://ginasthma.org/2023-gina-main-report/) for
bronchial asthma and were initially diagnosed with bronchial
asthma; patients who aged between 18 and 75 years old; patients
with no obvious abnormalities on chest X-ray or computed tomography
(CT) examination.
Exclusion criteria were as follows: Patients with
other respiratory diseases, such as lung infection and
bronchiectasis; patients with severe diseases of other systems,
including autoimmune diseases, myocardial infarction, heart
failure, and malignant tumors; pregnant or lactating women.
Methods
The present study collected the medical history and
clinical characteristics of patients with bronchial asthma induced
by COVID-19 infection and patients with typical bronchial asthma
who were diagnosed and treated in our hospital. The time from the
onset of clinical manifestations of asthma to its diagnosis was
recorded. Additionally, routine blood test results, serum total IgE
levels, specific IgE levels, lung function, airway provocation test
results, fractional exhaled nitric oxide (FeNO) levels, and other
relevant information were collected. The differences between the
two groups of asthmatic patients were then compared and analyzed.
While the study concentrated on comparing clinical characteristics
and pulmonary function, the treatment methods for both outpatient
care and acute asthma control were standardized according to the
Global Initiative for Asthma (GINA) 2023 guidelines. All
patients received treatment based on these guidelines, ensuring
consistency in managing asthma symptoms. However, individualized
adjustments in therapy might occur, especially in outpatient
settings, which could introduce variability in the results. Future
studies should provide more detailed descriptions of the specific
treatment regimens used to assess their potential influence on
clinical outcomes and pulmonary function, thereby improving the
consistency and reproducibility of findings.
Pulmonary function tests
Once the acute phase of asthma attack was
controlled, pulmonary function tests were completed in both groups,
including pulmonary ventilation, pulmonary reserve function, small
airway function, diffusion function, carbon monoxide dispersion to
alveolar volume ratio, and residual volume/total lung capacity
(RV/TLC). Pulmonary ventilation tests included forced expiratory
volume in 1 sec as a percentage of the predicted value (FEV1%),
forced expiratory volume in 1 sec to forced vital capacity ratio
(FEV1/FVC), and peak expiratory flow (PEF). Maximal voluntary
ventilation (MVV) was assessed to evaluate pulmonary reserve
function. Small airway function tests included the maximal
expiratory flow rates at 75, 50 and 25% of lung capacity (MEF75,
MEF50 and MEF25) and the maximal mid-expiratory flow rate
(MMEF75/25). Diffusion function tests included the single-breath
diffusion capacity of carbon monoxide (DLCO SB) and DLCO normalized
for alveolar volume (DLCO/VA).
Statistical analysis
SPSS 20.0 (IBM Corp.) and GraphPad 9.0 (GraphPad
Software Inc.; Dotmatics) software were used to statistically
analyze the data. The measurement data of normal distribution were
expressed as the mean ± standard deviation (X ± s), and analyzed
using independent sample t-test. The measurement data of skewed
distribution were expressed as the median (quartile), and analyzed
via Mann-Whitney U test. The rates were compared using the χ² test.
The Pearson correlation analysis was conducted to identify the
factors associated with poor pulmonary function in the COVID-19
group. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients' clinical
characteristics
The range of age in the COVID-19 infection-induced
bronchial asthma group was 21.9-74.7 years old, with an average age
of 46.9±14.0 years. Among them, there were 69 women and 44 men,
with a female-to-male ratio of 1.57. In the general bronchial
asthma group, 7 (6.0%) cases had a history of allergy, including
inhalation, food, drugs, 51 (44.0%) cases had allergic rhinitis, 12
(10.3%) cases had eczema, and 7 (6.0%) cases had chronic Hives. The
range of age in the general bronchial asthma group was 21-77 years
old, with an average age of 44.5±14.7 years old. Among them, there
were 64 women and 30 men, with a female-to-male ratio of 2.13. In
the COVID-19 infection-induced bronchial asthma group, there were 7
(7.4%) cases with allergic history, 37 (39.4%) cases with allergic
rhinitis, 9 (9.6%) cases with Nasal polyp, 10 (10.6%) cases with
eczema, and 3 (3.2%) cases with chronic Hives (Table I).
 | Table IGeneral data of the two groups. |
Table I
General data of the two groups.
| | COVID-19 asthma
(116) | Asthma (113) | P-value |
|---|
| Age, years | 46.9±1.8 | 42.3±1.6 | >0.05 |
| Sex, female (%) | 75 (64.7) | 69 (61.1) | >0.05 |
| History of allergy
(%) | 7(6) | 4 (3.5) | >0.05 |
| Allergic Rhinitis
(%) | 51(44) | 52(46) | >0.05 |
| Nasal polyps (%) | 0 (0) | 0 (0.0) | >0.05 |
| Eczema (%) | 12 (10.3) | 17(15) | >0.05 |
| Urticaria (%) | 7(6) | 4 (3.5) | >0.05 |
The duration of disease was 3.371±0.11 months in the
COVID-19 infection-induced bronchial asthma group and 3.327±0.17
months in the general bronchial asthma group, and there was no
significant difference between the two groups (P>0.05).
Patients' main clinical characteristics were cough, sputum, chest
tightness, dyspnea and wheezing. There was no significant
difference in patients' main clinical characteristics between the
two groups (P>0.05) (Table
II).
 | Table IIClinical symptoms of the two
groups. |
Table II
Clinical symptoms of the two
groups.
| | COVID-19 asthma
(116) | Asthma (113) | P-value |
|---|
| Duration, months | 3.371±0.11 | 3.327±0.17 | >0.05 |
| Cough (%) | 85 (73.3) | 80 (70.8) | >0.05 |
| Sputum (%) | 13 (11.2) | 12 (10.6) | >0.05 |
| Chest tightness
(%) | 35 (27.6) | 43 (38.1) | >0.05 |
| Dyspnea (%) | 35 (30.2) | 28 (24.8) | >0.05 |
| Wheezing (%) | 3 (2.6) | 5 (4.4) | >0.05 |
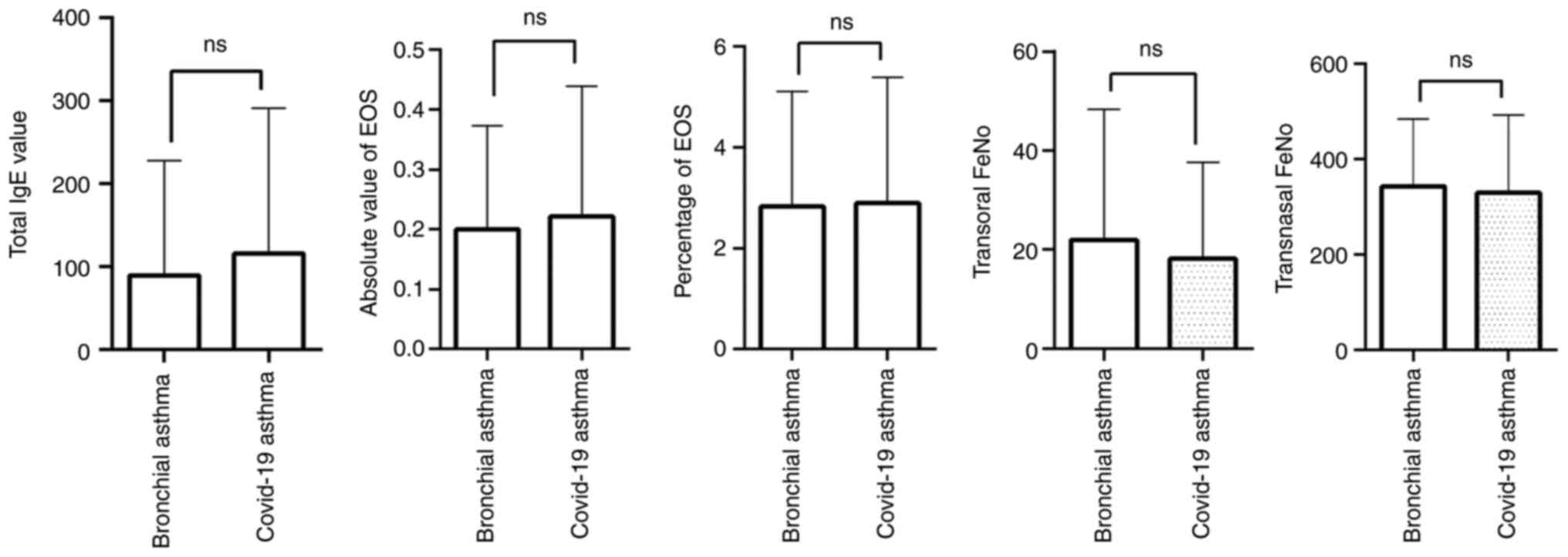
Comparison of inflammatory reaction
between the two groups
Patients with COVID-19 infection-induced bronchial
asthma and patients with common bronchial asthma all received
routine peripheral blood tests. The results indicated no
significant differences in total IgE levels, the absolute value and
percentage of eosinophils (EOS), transoral FeNO, or transnasal FeNO
in the peripheral blood between the COVID-19 infection-induced
bronchial asthma group and the normal bronchial asthma group
(P>0.05) (Table III, Fig. 1).
 | Table IIIComparison of pulmonary function
indexes between COVID-19 asthma and asthma groups. |
Table III
Comparison of pulmonary function
indexes between COVID-19 asthma and asthma groups.
| Indicator | Asthma (113) | COVID-19 asthma
(116) | P-value |
|---|
| Vcmax | 96.7±1.40 | 100.1±1.17 | 0.068 |
| FVC | 98.1±1.40 | 101.9±1.23 | 0.071 |
| FEV1% | 93.7±1.37 | 97.6±1.10 | 0.026 |
| FEV1/FVC | 81.4±0.67 | 80.36±0.77 | 0.293 |
| PEF | 99.0±1.58 | 92.1±1.67 | 0.003 |
| MEF75 | 97.4±1.98 | 89.7±2.14 | 0.009 |
| MEF50 | 81.5±2.13 | 76.4±2.48 | 0.121 |
| MEF25 | 71.0±5.54 | 62.8±2.80 | 0.180 |
| MMEF75/25 | 75.2±2.07 | 71.1±2.45 | 0.196 |
| MVV | 83.1±1.48 | 77.8±1.65 | 0.018 |
| FEV1*30 | 78.9±0.93 | 74.8±1.33 | 0.012 |
| DLCO SB | 85.4±1.39 | 87.3±1.59 | 0.372 |
| DLCO/VA | 91.5±1.65 | 92.9±1.85 | 0.571 |
| VA | 97.4±1.53 | 96.5±1.71 | 0.691 |
| R tot | 130.9±5.23 | 132.6±4.06 | 0.794 |
| RV | 105.4±2.57 | 117.2±4.42 | 0.026 |
| TLC | 96.4±1.12 | 96.9±2.02 | 0.842 |
| RV/TLC | 38.5±0.83 | 42.5±1.45 | 0.008 |
Comparison of pulmonary function
between the two groups
Pulmonary ventilation, pulmonary reserve function,
small airway function and diffusion function were evaluated between
the COVID-19 infection-induced bronchial asthma and typical
bronchial asthma groups. The pulmonary ventilation test indices,
such as FEV1%, FEV1*30, and PEF were significantly lower in the
COVID-19 infection-induced bronchial asthma group compared with the
typical bronchial asthma group (P<0.05). The MVV index was also
lower in the COVID-19 infection-induced bronchial asthma group
(P<0.05). Among the small airway function test indices, MEF75
was reduced in the COVID-19 infection-induced bronchial asthma
group (P<0.05). Additionally, RV and RV/TLC values were lower in
the COVID-19 infection-induced bronchial asthma group (P<0.05,
Table III, Fig. 2). However, no significant
differences were identified in the overall rates of impairment in
ventilation function, reserve function, small airway function,
diffusion function, airway resistance, or the ratio of residual
volume to total lung capacity (Table
IV). These findings suggested that patients with COVID-19
infection-induced bronchial asthma exhibited comparatively reduced
pulmonary function compared with those with bronchial asthma.
However, this difference was not considered clinically
significant.
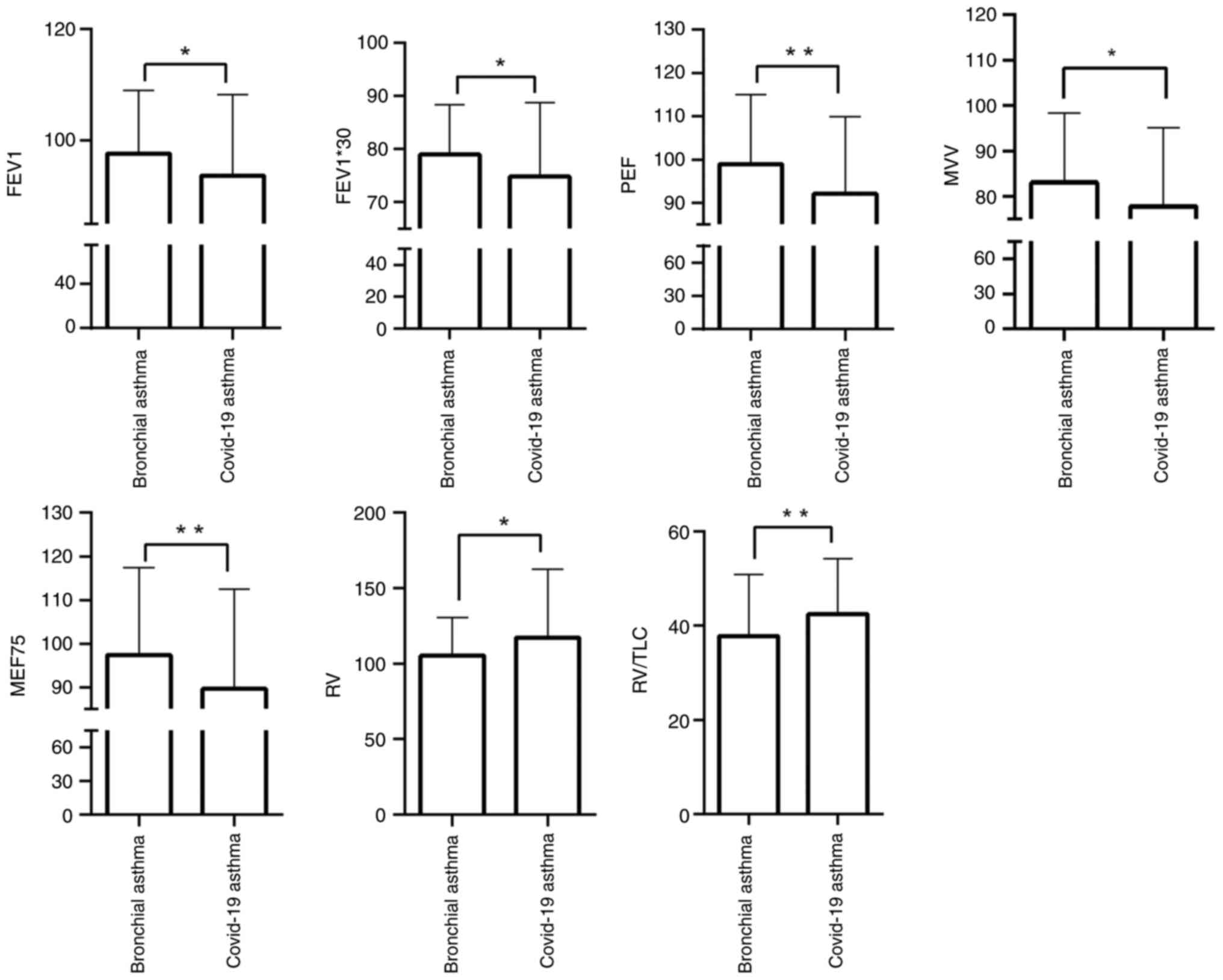 | Figure 2Comparison of pulmonary function
between patients with COVID-19 infection-induced bronchial asthma
and patients with typical bronchial asthma. FEV1 (FEV1/pred),
FEV1*30 (l/min), PEF (PEF/pred), MEF75 (MEF75/pred) and MVV
(MVV/pred) were higher in the COVID-19 infection-induced bronchial
asthma group than the typical bronchial asthma group, while RV
(RV-pred) and RV/TLC (%) were lower in the COVID-19
infection-induced bronchial asthma group than the typical bronchial
asthma group. *P<0.05 and **P<0.01.
FEV1%, forced expiratory volume in 1 sec as a percentage of the
predicted value; MEF, maximal expiratory flow rate; RV, residual
volume; MVV, maximal voluntary ventilation; TLC, total lung
capacity; PEF, peak expiratory flow. |
 | Table IVComparison of pulmonary function
between COVID-19 asthma and asthma groups. |
Table IV
Comparison of pulmonary function
between COVID-19 asthma and asthma groups.
| Indicator | Asthma (113)
(%) | COVID-19 asthma
(116) (%) | P-value |
|---|
| Impairment of
ventilatory function | 27 (23.9) | 34 (29.3) | 0.41 |
| Impairment of
reserve function | | | 0.37 |
| Mildly
impaired | 36 (31.9) | 36 (31.0) | |
| Moderately
impaired | 8 (7.1) | 17 (14.7) | |
| Impairment of small
airway function | | | 0.50 |
| Mildly
impaired | 37 (32.7) | 27(23.3) | |
| Moderately/severely
impaired | 8 (7.1) | 25(21.6) | |
| Impairment of
diffusion function | 42 (37.2) | 37 (31.9) | 0.49 |
| Increased airway
resistance | 42 (37.2) | 48(41.4) | 0.57 |
| Increased ratio of
residual volume to total lung capacity | 51 (45.1) | 54 (46.6) | 0.83 |
The factors related to poor pulmonary
function in the COVID-19 group
The Pearson correlation analysis was performed to
detect the factors related to poor pulmonary function in the
COVID-19 group. Patients were divided into two groups according to
the median age of 45 years old. Pearson correlation analysis
indicated that age was negatively correlated with MVV and FEV1*30
in the COVID-19 group (P<0.05), suggesting that the decline in
pulmonary function was associated with advancing age. Moreover, sex
was correlated with pulmonary function indices, including FEV1%,
MEF75, MVV, FEV1*30, and RV/TLC in the COVID-19 group (P<0.05),
indicating that male patients' pulmonary function is comparatively
inferior to that of female patients. However, the other indices,
such as allergic rhinitis, cough, expectoration, chest, dyspnea and
stridor, exhibited no correlation with pulmonary function in the
COVID-19 group (P>0.05) (Table
V).
 | Table VFactors related to poor lug functions
in COVID-19 group. |
Table V
Factors related to poor lug functions
in COVID-19 group.
| Index | FEV1% | PEF | MEF75 | MVV | FEV1*30 | RV | RV/TLC | |
|---|
| Age (median age, 45
years) | | | | | | | | |
| | | | | -0.255 | -0.301 | | | Correlation
coefficient |
| | 0.827 | 0.670 | 0.710 | 0.007a | 0.001a | 0.944 |
<0.001a | P-value |
| Sex (Male 41,
Female 75) | | | | | | | | |
| | -0.241 | | -0.263 | -0.197 | | | -0.265 | Correlation
coefficient |
| | 0.010a | 0.300 | 0.005a | 0.040a | 0.697 | 0.422 | 0.006a | P-value |
| Allergic
Rhinitis | 0.892 | 0.272 | 0.356 | 0.889 | 0.656 | 0.402 | 0.917 | P-value |
| Cough | 0.904 | 0.172 | 0.345 | 0.972 | 0.810 | 0.278 | 0.501 | P-value |
| Sputum | 0.934 | 0.266 | 0.305 | 0.509 | 0.470 | 0.339 | 0.822 | P-value |
| Chest
tightness | 0.986 | 0.986 | 0.771 | 0.462 | 0.756 | 0.188 | 0.507 | P-value |
| Dyspnea | 0.200 | 0.397 | 0.238 | 0.411 | 0.981 | 0.903 | 0.178 | P-value |
| Wheezing | 0.660 | 0.280 | 0.302 | 0.676 | 0.857 | 0.624 | 0.459 | P-value |
Discussion
The present study retrospectively analyzed 116 cases
of COVID-19 infection-induced bronchial asthma and 113 cases of
typical bronchial asthma. There was no significant difference
between the two groups in terms of age, sex ratio, history of
allergy, history of allergic rhinitis, history of nasal polyp,
history of eczema, and history of chronic Hives. There was no
significant difference in IgE, EOS, EOS percentage, oral FeNO, and
nasal FeNO between the two groups. The pulmonary function of
patients with COVID-19 infection-induced bronchial asthma appeared
worse than that of patients with typical bronchial asthma.
The pathophysiological changes in bronchial asthma
are mainly driven by airway smooth muscle spasms resulting from
inflammation of the airway walls. This leads to recurrent symptoms,
such as wheezing, shortness of breath, chest tightness and coughing
(14,15). In late 2019, COVID-19 outbreak,
caused by SARS-CoV-2, rapidly evolved into a global pandemic,
resulting in millions of fatalities (16). Patients diagnosed with bronchial
asthma are subjected to additional stressors due to the potential
development of COVID-19 and the impact of the COVID-19 pandemic on
societal and health-related services. Although clinical trials for
safe and efficacious antiviral agents are ongoing and vaccine
development programs have been accelerated, the long-term effects
of SARS-CoV-2 infection are becoming increasingly recognized
(17). The COVID-19 pandemic has
imposed remarkable challenges to the routine management and
diagnosis of bronchial asthma, including reduced face-to-face
consultations, limitations in conducting spirometry, and
restrictions on traditional pulmonary rehabilitation and home care
programs (18).
Eosinophil inflammation is a significant factor in
the pathogenesis of asthma. The parameters used to assess
eosinophilic inflammation include the absolute count and percentage
of EOS, total IgE level in the bloodstream, transoral FeNO, and
trans-nasal FeNO. These parameters are essential diagnostic
indicators for asthma and are also valuable for evaluating the
degree of inflammation control in asthmatic patients (19-21).
The present study found no significant differences in the absolute
count and percentage of EOS, IgE level, transoral FeNO, and
trans-nasal FeNO between the two groups, suggesting comparable
levels of eosinophilic inflammation. In allergic asthma,
eosinophilic inflammation is generally positively correlated with
the degree of pulmonary dysfunction (22,23),
demonstrating that higher levels of eosinophilic inflammation are
mainly associated with more severe pulmonary function deficits.
Pulmonary function is a fundamental measure for
evaluating asthma (24,25). The small airway, typically defined
as having a diameter of ≤2 mm, is a critical site for the early
development of several pulmonary diseases (26). In asthma, inflammation of the small
airway wall can lead to significant airway smooth muscle
contraction, resulting in recurrent symptoms, such as wheezing,
dyspnea, chest tightness and cough (27-29).
Despite the substantial cumulative cross-sectional area of small
airways, their resistance accounts for <20% of total airway
resistance (30). Traditional
pulmonary function tests mainly fail to detect abnormalities in
small airways, whereas specialized small airway function tests are
more sensitive in identifying early pathological changes (31). The emergence of COVID-19 has
presented unique challenges for patients with bronchial asthma, and
studies suggested potential interactions between the virus and
asthma pathophysiology, including small airway function and
systemic inflammation (16-18).
However, direct comparisons between COVID-19-induced bronchial
asthma and typical asthma remain scarce, particularly concerning
pulmonary dysfunction. Previous studies have highlighted the
importance of assessing small airway function as an early marker
for pulmonary diseases (26,31).
Previous research (32) has found
no significant impact of COVID-19 on asthma in children, while
studies on adult populations suggest variable outcomes, including
exacerbations and prolonged symptoms (33,34).
These findings underscore the need for further exploration of
pulmonary function in adult patients with COVID-19-induced
bronchial asthma. Eosinophilic inflammation, a hallmark of asthma
pathogenesis, has been extensively studied, and parameters, such as
total IgE, FeNO and eosinophil count served as diagnostic and
prognostic markers (19-21).
Although the findings indicated no significant differences in these
markers between the two groups, scholars (22,23)
suggested that variations in eosinophilic inflammation may
influence asthma severity, warranting further investigation in the
context of COVID-19. The COVID-19 pandemic has disrupted
traditional asthma management practices, including diagnostic
procedures and treatment adherence, potentially altering disease
trajectories (17,18).
In a study by Tosca et al (32), COVID-19 was found to have no
significant impact on pulmonary function or asthma control in
children. By contrast, isolated reports in adults have described
asthma exacerbations associated with COVID-19, although such events
are not prevalent in the general population (33,34).
The current analysis suggested a potential link between COVID-19
and asthma, particularly concerning pulmonary function (35). The present study identified
significant differences in key pulmonary function parameters,
comprising FEV1, RV/TLC, PEF, MEF75, MVV, FEV1*30 and RV, between
the two groups. These results indicated that patients with COVID-19
infection-induced bronchial asthma exhibited reduced pulmonary
function compared with those with typical bronchial asthma. While
no statistically significant differences were identified in the
overall rates of ventilation function impairment, reserve function
impairment, or small airway function impairment, the findings
suggest that patients with COVID-19-induced bronchial asthma may
have subclinical lesions contributing to distinct clinical features
compared with those with non-COVID-19-induced bronchial asthma. It
is noteworthy that the present study included only non-critically
ill patients receiving outpatient treatment. As a result,
variations in the completion of diagnostic tests among patients led
to inconsistencies in the statistical analysis of certain
indicators. The Pearson correlation analysis revealed that both age
and sex were significantly associated with poorer pulmonary
function in the COVID-19 infection-induced bronchial asthma group.
However, these findings require further investigation to determine
how these factors interact with COVID-19 infection and whether they
have a more notable impact on pulmonary function over time.
Although the present study identified differences in specific
pulmonary function tests, such as FEV1%, MEF75, and MVV, between
the two groups, the lack of statistical significance across all
parameters suggests that the clinical implications of COVID-19's
impact on asthma may be more remarkable. These differences may not
indicate a substantial deviation in overall pulmonary function,
particularly in non-critically ill patients. Further exploration of
the pathophysiological mechanisms contributing to these differences
in pulmonary function is essential. Specifically, the role of
COVID-19 in exacerbating asthma symptoms may involve complex
inflammatory processes that affect airway smooth muscle function,
while the exact mechanisms remain to be fully elucidated.
Although the present study found poorer pulmonary
function in patients with COVID-19 infection-induced bronchial
asthma, the potential mechanisms underlying these differences were
not thoroughly explored. Future studies should investigate how
COVID-19-induced alterations in airway function, such as changes in
airway smooth muscle tone or inflammation beyond
eosinophil-mediated pathways, may contribute to these findings.
Although the observed differences in pulmonary function were not
statistically significant in terms of impairment rates across
functions, the poorer pulmonary function in patients with COVID-19
infection-induced bronchial asthma highlights the potential need
for adjusted management strategies. These may include more frequent
monitoring, early intervention, and personalized treatment plans to
better address the unique needs of this patient population.
The findings suggested that while the pulmonary
function in patients with COVID-19 infection-induced bronchial
asthma was slightly poorer than that of patients with typical
asthma, these differences might not necessitate entirely distinct
treatment protocols, while indicated the need for enhanced
monitoring and individualized management strategies, particularly
for older and male patients who exhibited poorer pulmonary
function. Given the observed reduction in key pulmonary function
indices, such as FEV1% and MEF75, in COVID-19-induced asthma,
additional interventions concentrating on maintaining airway
patency and preventing further pulmonary decline might be
especially beneficial for this subgroup of patients. Management
strategies for COVID-19-induced asthma can benefit from
incorporating early detection and treatment of airway inflammation,
as well as an elevated concentration on small airway function given
the potential subclinical changes observed in these patients.
Future studies should investigate whether the pathophysiological
mechanisms unique to COVID-19-induced asthma, such as possible
non-eosinophilic inflammatory pathways, require specific
therapeutic modifications beyond standard asthma treatments,
particularly in terms of long-term control medications and
pulmonary rehabilitation. While current treatment guidelines, such
as GINA, provide a robust framework for asthma management, the
subtle pulmonary function differences in COVID-19-induced asthma
suggest that treatment intensification or modifications may be
warranted to address specific functional impairments.
The present study has several limitations. Firstly,
the differing inclusion periods for the two groups, one group
diagnosed and treated prior to the COVID-19 pandemic and the other
afterward, might introduce variability stemming from temporal
variations in environmental factors, clinical practices, or
healthcare accessibility. Secondly, the retrospective nature and
single-center setting of the study could limit the generalizability
of the findings and result in incomplete or inconsistent data for
certain variables. Thirdly, although the present study analyzed
routine inflammatory markers, such as total IgE, eosinophil count,
eosinophil percentage, and FeNO level, the retrospective design and
concentration on clinical outpatient data limited the inclusion of
more advanced biomarkers. Consequently, these parameters might not
fully capture the complexity of the inflammatory and immunological
responses in patients with COVID-19 infection-induced bronchial
asthma. Future investigations will benefit from the inclusion of
advanced inflammatory and immunological markers, such as cytokines
[for examle, interleukin-6 (IL-6), IL-13, and tumor necrosis
factor-α (TNF-α)] and chemokines, to provide a more comprehensive
evaluation of the inflammatory profile in this patient population.
Lastly, while the sample size was adequate for preliminary
analysis, it might not provide sufficient statistical power to
detect more significant differences between the two groups. Future
prospective, multicenter studies are essential with standardized
methodologies to confirm and expand the findings. To enhance the
understanding of these findings' clinical significance, future
research should concentrate on conducting prospective studies with
larger, multi-center cohorts. Such studies should investigate the
long-term effects of COVID-19 on asthmatic patients, emphasizing
both the underlying pathophysiological mechanisms and their
implications for clinical practice. Particular attention should be
given to develop personalized therapeutic strategies for patients
with COVID-19-induced bronchial asthma.
In conclusion, although no significant difference
was found between the two groups in the rates of impairment in
ventilation function, reserve function, and small airway function,
significant differences were identified in various indicators,
including FEV1, RV/TLC, PEF, MEF75, MVV, FEV*30 and RV between the
two groups. The findings indicated that pulmonary function of
patients with COVID-19 infection-induced bronchial asthma was worse
than that of patients with typical asthma.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81900021), the Special
‘Sailing’ Program for Clinical Medical Development (grant no.
ZLRK202323), and the Beijing Clinical Key Specialty (grant no.
XKB2022B1002).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MQZ, JQZ and XDM contributed substantially to the
conception and design of the study and acquisition of data, as well
as analysis and interpretation of the data. MQZ, LNZ and PZ were
involved in interpretation of the data, and in drafting the
manuscript. MQZ, JQZ and XDM have participated sufficiently in the
study to take public responsibility for appropriate portions of the
content and agree to be accountable for all aspects of the study in
ensuring that questions related to the accuracy or integrity of any
part of the study are appropriately investigated and resolved. MQZ
and JQZ confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alizadeh Z, Mortaz E, Adcock I and Moin M:
Role of epigenetics in the pathogenesis of asthma. Iran J Allergy
Asthma Immunol. 16:82–91. 2017.PubMed/NCBI
|
|
2
|
Barbi E and Longo G: Chronic and recurrent
cough, sinusitis and asthma. Much ado about nothing. Pediatr
Allergy Immunol. 18 (Suppl 18):S22–S24. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Akinbami LJ, Moorman JE and Liu X: Asthma
prevalence, health care use, and mortality: United States,
2005-2009. Natl Health Stat Report: Jan 12, 1-14, 2011.
|
|
4
|
Vora AC: Bronchial asthma. J Assoc
Physicians India. 62 (3 Suppl):5–6. 2014.PubMed/NCBI
|
|
5
|
Sugita M, Kuribayashi K, Nakagomi T,
Miyata S, Matsuyama T and Kitada O: Allergic bronchial asthma:
Airway inflammation and hyperresponsiveness. Intern Med. 42:636–43.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Banno A, Reddy AT, Lakshmi SP and Reddy
RC: Bidirectional interaction of airway epithelial remodeling and
inflammation in asthma. Clin Sci (Lond). 134:1063–1079.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu D, Chen C, Chen D, Zhu A, Li F, Zhuang
Z, Mok CKP, Dai J, Li X, Jin Y, et al: Mouse models susceptible to
HCoV-229E and HCoV-NL63 and cross protection from challenge with
SARS-CoV-2. Proc Natl Acad Sci USA. 120(e2202820120)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel Coronavirus-infected
pneumonia in wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mason RJ: Pathogenesis of COVID-19 from a
cell biology perspective. Eur Respir J. 55(2000607)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu Z and McGoogan JM: Characteristics of
and important lessons from the coronavirus disease 2019 (COVID-19)
outbreak in China: Summary of a report of 72 314 cases from the
Chinese center for disease control and prevention. JAMA.
323:1239–1242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Peters MC, Sajuthi S, Deford P,
Christenson S, Rios CL, Montgomery MT, Woodruff PG, Mauger DT,
Erzurum SC, Johansson MW, et al: COVID-19-related genes in sputum
cells in asthma relationship to demographic features and
corticosteroids. Am J Respir Crit Care Med. 202:83–90.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu Z, Hasegawa K, Ma B, Fujiogi M,
Camargo CA Jr and Liang L: Association of asthma and its genetic
predisposition with the risk of severe COVID-19. J Allergy Clin
Immunol. 146:327–329.e4. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
To T, Stanojevic S, Moores G, Gershon AS,
Bateman ED, Cruz AA and Boule LP: Global asthma prevalence in
adults: Findings from the cross-sectional world health survey. BMC
Public Health. 12(204)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liccardi G, Salzillo A, Sofia M, D'Amato M
and D'Amato G: Bronchial asthma. Curr Opin Anaesthesiol. 25:30–37.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kannan S, Shaik Syed Ali P, Sheeza A and
Hemalatha K: COVID-19 (Novel Coronavirus 2019)-recent trends. Eur
Rev Med Pharmacol Sci. 24:2006–2011. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang F, Kream RM and Stefano GB: An
evidence based perspective on mRNA-SARS-CoV-2 vaccine development.
Med Sci Monit. 26(e924700)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mahase E: Covid-19: Increased demand for
steroid inhalers causes ‘distressing’ shortages. BMJ.
369(m1393)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Badar A, Salem AM, Bamosa AO, Qutub HO,
Gupta RK and Siddiqui IA: Association between FeNO, total blood
IgE, peripheral blood eosinophil and inflammatory cytokines in
partly controlled asthma. J Asthma Allergy. 13:533–543.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hoshino Y, Soma T, Uchida Y, Shiko Y,
Nakagome K and Nagata M: Treatment resistance in severe asthma
patients with a combination of high fraction of exhaled nitric
oxide and low blood eosinophil counts. Front Pharmacol.
13(836635)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin SS, Liu H and Gao X: Elevated
fractional exhaled nitric oxide (FeNO) is a clinical indicator of
uncontrolled asthma in children receiving inhaled corticosteroids.
Int J Clin Pharmacol Ther. 55:66–77. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Bafadhel M, Rabe KF, Martinez FJ, Singh D,
Darken P, Jenkins M, Aurivillius M, Patel M and Dorinsky P:
Benefits of Budesonide/Glycopyrronium/Formoterol fumarate dihydrate
on COPD exacerbations, lung function, symptoms, and quality of life
across blood eosinophil ranges: A Post-hoc analysis of data from
ETHOS. Int J Chron Obstruct Pulmon Dis. 17:3061–3073.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pham DD, Lee JH, Kim JY, An J, Song WJ,
Kwon HS, Cho YS and Kim TB: Different impacts of blood and sputum
eosinophil counts on lung function and clinical outcomes in asthma:
Findings from the COREA cohort. Lung. 200:697–706. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaminsky DA and Irvin CG: What long-term
changes in lung function can tell us about asthma control. Curr
Allergy Asthma Rep. 15(505)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Koefoed HJL, Zwitserloot AM, Vonk JM and
Koppelman GH: Asthma, bronchial hyperresponsiveness, allergy and
lung function development until early adulthood: A systematic
literature review. Pediatr Allergy Immunol. 32:1238–1254.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
van der Wiel E, ten Hacken NH, Postma DS
and van den Berge M: Small-airways dysfunction associates with
respiratory symptoms and clinical features of asthma: A systematic
review. J Allergy Clin Immunol. 131:646–657. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tyler SR and Bunyavanich S:
Leveraging-omics for asthma endotyping. J Allergy Clin Immunol.
144:13–23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Milanese M, Miraglia Del Giudice E and
Peroni DG: Asthma, exercise and metabolic dysregulation in
paediatrics. Allergol Immunopathol (Madr). 47:289–294.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mitchell PD and O'Byrne PM: Biologics and
the lung: TSLP and other epithelial cell-derived cytokines in
asthma. Pharmacol Ther. 169:104–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Du K, Zheng M, Zhao Y, Xu W, Hao Y, Wang
Y, Zhao J, Zhang N, Wang X, Zhang L and Bachert C: Impaired small
airway function in non-asthmatic chronic rhinosinusitis with nasal
polyps. Clin Exp Allergy. 50:1362–1371. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yuan H, Liu X, Li L, Wang G, Liu C, Zeng
Y, Mao R, Du C and Chen Z: Clinical and pulmonary function changes
in cough variant asthma with small airway disease. Allergy Asthma
Clin Immunol. 15(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tosca MA, Crocco M, Girosi D, Olcese R,
Schiavetti I and Ciprandi G: Unaffected asthma control in children
with mild asthma after COVID-19. Pediatr Pulmonol. 56:3068–3070.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ono Y, Obayashi S, Horio Y, Niimi K,
Hayama N, Ito Y, Oguma T and Asano K: Asthma exacerbation
associated with COVID-19 pneumonia. Allergol Int. 70:129–130.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Codispoti CD, Bandi S, Patel P and
Mahdavinia M: Clinical course of asthma in 4 cases of coronavirus
disease 2019 infection. Ann Allergy Asthma Immunol. 125:208–210.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Garcia-Pachon E, Ruiz-Alcaraz S,
Baeza-Martinez C, Zamora-Molina L, Soler-Sempere MJ, Padilla-Navas
I and Grau-Delgado J: Symptoms in patients with asthma infected by
SARS-CoV-2. Respir Med. 185(106495)2021.PubMed/NCBI View Article : Google Scholar
|