Introduction
Hypogonadism, a condition characterized by reduced
or absent secretion of gonadal hormones, significantly impacts
multiple physiological systems, including cognition. Testosterone,
the primary androgen affected in hypogonadism, plays an essential
role in cognitive processes such as memory, attention and executive
function (1). Studies indicate that
men with hypogonadism exhibit reduced cognitive abilities compared
with age-matched healthy individuals, highlighting testosterone's
role in cognition (2). However,
hypogonadism's impact extends beyond testosterone deficiency,
implicating interactions with other physiological systems, such as
neuroinflammation, metabolic dysregulation and vascular health, all
of which can contribute to cognitive decline. Studies have shown
that low testosterone levels are often accompanied by higher
inflammatory markers and impaired cerebrovascular function, further
exacerbating cognitive impairment (3,4).
Hypogonadal men have shown specific impairments in memory tasks,
working memory, attention-switching and visuospatial processing
(5). Androgen replacement therapy
(ART) has been proposed as a potential intervention to mitigate
cognitive deficits in hypogonadal patients by restoring
testosterone levels to a normative range (6).
Research on ART and cognitive function in
hypogonadal men has increased, yet findings are inconclusive. Some
studies report improvements in cognitive performance, particularly
in areas such as spatial memory and verbal fluency, following ART
administration (7). For instance,
Cherrier et al (8) found
that testosterone supplementation improved verbal memory in older
hypogonadal men, suggesting testosterone's potential role in
enhancing specific cognitive domains. However, other studies find
no significant impact of ART on cognitive functions, attributing
these results to variability in study design, testosterone dosing
and cognitive assessment methods (9). These discrepancies raise questions
about the generalizability of ART effects across different
cognitive domains and patient populations. Several systematic
reviews and meta-analyses, such as Zhang et al (10) and Hong et al (11), have explored the relationship
between testosterone supplementation and cognitive function in
aging men. Zhang et al (10)
concluded that testosterone deficiency may increase the risk of
all-cause dementia but showed inconsistent results regarding the
efficacy of ART in improving cognitive outcomes. Similarly, Hong
et al (11) reported no
significant effect of ART on cognitive improvement across cognitive
domains such as memory and executive function. However, these
studies were based on some pooled data without differentiating
between younger and older hypogonadal patients or those with
cognitive impairment vs. cognitively healthy participants.
The proposed mechanisms by which testosterone may
influence cognitive function are multifaceted. Testosterone exerts
neuroprotective effects by modulating neurotransmitter levels,
enhancing neurogenesis, and reducing neuroinflammation (12). Additionally, androgen receptors
(ARs) are distributed in key brain regions associated with memory
and learning, such as the hippocampus and prefrontal cortex,
suggesting a direct link between androgen levels and cognitive
function (13). Testosterone also
acts as a precursor for estradiol, which has well-documented
effects on synaptic plasticity and cognitive function, adding
another layer to the complex interplay between androgens and
cognitive health (14).
While some studies have demonstrated that ART may
improve cognitive performance, the evidence remains mixed due to
variability in study designs and methodologies. Furthermore, prior
meta-analyses have not thoroughly explored the interplay between
testosterone dose, duration of therapy, and specific cognitive
outcomes, particularly in visuospatial skills and memory. Thus, a
comprehensive meta-analysis (CMA) incorporating recent findings is
crucial for delineating the specific domains most affected by
testosterone replacement. A systematic review and meta-analysis of
studies on ART and cognitive outcomes in hypogonadal patients is
warranted to clarify these effects and provide a synthesized
understanding. Prior systematic reviews in this area often exclude
significant sources of heterogeneity, such as the age of patients,
baseline testosterone levels and duration of therapy, which may
influence cognitive outcomes (15).
By conducting a systematic review and meta-analysis, it is possible
to quantitatively assess the overall impact of ART on cognitive
domains, identify any moderating factors, and provide a basis for
clinical recommendations in the management of hypogonadism-related
cognitive impairments (16).
The present study aims to systematically review and
quantitatively analyze the effects of ART on cognitive function in
subjects with hypogonadism, focusing on domains frequently impacted
by testosterone fluctuations, such as memory, attention and
executive function. Understanding the efficacy of ART in preserving
or enhancing cognitive abilities in hypogonadal individuals is
critical, as cognitive decline can significantly impair the quality
of life and daily functioning in this patient population (17). The present review also aims to
evaluate the methodological rigor of existing studies, with an
emphasis on exploring heterogeneity in outcomes related to
differences in study designs, dosages and cognitive assessment
techniques.
Materials and methods
The present systematic review and meta-analysis were
conducted following the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) guidelines, ensuring a
standardized and transparent approach to the study selection, data
extraction and analysis procedures (18).
Inclusion and exclusion criteria
The inclusion criteria for the present study were as
follows: studies that enrolled male participants diagnosed with
age-related hypogonadism, defined as total testosterone levels
below the clinical reference threshold of <300 ng/dl or as
reported by study authors, who underwent ART; studies where ART was
administered through testosterone or related androgen formulations
(including injections, gels and patches) at therapeutic doses;
studies assessing cognitive outcomes using validated
neuropsychological tests across cognitive domains such as memory
(for example, verbal recall tests), attention (for example, digit
span) and executive function (for example, verbal fluency tests);
and studies designed as randomized controlled trials (RCTs), cohort
studies, or case-control studies reporting quantitative data
sufficient to calculate standardized effect sizes (for example,
providing mean scores, standard deviations, confidence intervals,
or effect size estimates). Sufficient data were defined as studies
reporting pre- and post-intervention cognitive test scores with
variability measures, such as standard deviations or standard
errors, or studies that reported effect sizes with confidence
intervals. To account for key hormonal factors potentially
associated with cognitive function, studies that reported
bioavailable testosterone levels, sex hormone-binding globulin
(SHBG) levels, or other relevant biomarkers when available were
noted during data extraction to evaluate their influence on
cognitive outcomes.
Studies were excluded if they were non-primary
research articles, such as case reports, reviews, editorials, or
conference abstracts; if they lacked sufficient quantitative data
to compute effect sizes, such as those reporting only subjective
assessments or narrative descriptions; or if they were published in
languages other than English to ensure consistency in
interpretability and analysis. This approach aimed to ensure that
included studies met uniform methodological standards and provided
reliable quantitative measures of cognitive outcomes. Additionally,
studies that investigated the association between AR levels and
cognitive performance were considered, although none directly
measured AR levels.
Search strategy
A comprehensive literature search was conducted
across multiple databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/), Scopus (https://www.scopus.com/home.uri), Google Scholar
(https://scholar.google.com/) and the
Cochrane Library (https://www.cochranelibrary.com/). The following
search terms were used: [TITLE-ABS-KEY (‘cognitive function’ OR
‘memory’ OR ‘executive function’ OR ‘attention’ OR ‘verbal fluency’
OR ‘spatial memory’ OR ‘cognition’ AND TITLE-ABS-KEY (‘testosterone
replacement therapy’ OR ‘androgen therapy’ OR ‘hormone replacement
therapy’ OR ‘ART’ OR ‘TRT’)]. The reference lists of included
studies were manually screened to identify any additional relevant
studies. No language or publication date restrictions were applied
in the initial search. The final search was completed in October
2024.
Study selection and data
extraction
All retrieved articles were imported into reference
management software, and duplicates were removed. Two independent
reviewers screened the titles and abstracts of the remaining
studies for relevance. Full-text versions of potentially eligible
articles were then assessed to determine final inclusion. Any
discrepancies between reviewers were resolved through discussion or
by consulting a third reviewer. Data were extracted from each
eligible study, including author names and the year of publication,
sample size and patient demographics, such as age and baseline
testosterone levels, study design, including whether the study was
an RCT, cohort study, or case-control study, type, and dosage of
intervention, specifying the form of androgen used, the dose, and
the duration of therapy, cognitive outcome measures, detailing the
specific tests administered to assess domains including memory,
attention and executive function, and follow-up period and the
timing of cognitive assessments. This approach ensured
comprehensive data collection for a nuanced analysis of cognitive
outcomes across various studies.
Risk of bias assessment
Each study's risk of bias was independently
evaluated by two reviewers using the CONSORT checklist for RCTs.
The CONSORT checklist assessed adherence to key reporting standards
across 17 domains, including clarity of title and abstract,
descriptions of participant selection, randomization, allocation
concealment, blinding, statistical methods and outcome reporting.
Any discrepancies between reviewers were addressed through
discussion or, if needed, by consulting a third reviewer to reach a
consensus. To address potential biases in non-randomized studies,
the Risk of Bias in Non-randomized Studies-of Interventions
(ROBINS-I) tool was applied. This tool evaluates biases across
domains, such as confounding, selection bias and outcome
measurement bias. ROBINS-I allows for a more tailored assessment,
addressing potential limitations inherent in cohort studies or
pre-post designs. Each domain was scored as ‘low’, ‘moderate’, or
‘high’ risk of bias. Discrepancies in scoring were resolved by
discussion among reviewers until a consensus was reached.
Statistical analysis
Meta-analyses were performed using CMA (Version 3;
CMA; Biostat,) software Standardized mean differences (SMD) were
calculated to synthesize cognitive outcomes across studies with
different scales and measures. A random-effects model was applied
due to expected heterogeneity among studies, particularly in terms
of differences in ART dosages, durations, patient demographics and
cognitive tests used. In the present meta-analysis, the precision
interval approach was used to quantify heterogeneity instead of the
I² statistic due to several methodological benefits. Precision
intervals offer a more intuitive and clinically relevant measure of
uncertainty around the combined effect size, which helps in
understanding the practical range of ART's impact on cognitive
outcomes. Unlike I2, which quantifies the proportion of
variance attributable to between-study heterogeneity (19), precision intervals provide a direct
reflection of the confidence range around the pooled effect size.
This method aligns closely with random-effects models, which
account for variability both within and between studies, making it
particularly well-suited for our dataset that encompasses diverse
study designs, populations and cognitive measures (20). By contrast, I2 values can
be inflated in meta-analyses involving small or numerous studies,
potentially exaggerating heterogeneity. Additionally,
I2's sensitivity to sample size and clinical diversity
can complicate interpretation, especially in contexts with
significant methodological heterogeneity. Precision intervals, by
contrast, offer a clearer representation of how closely the true
effect sizes are clustered around the overall estimate. This
approach has been recommended in previous meta-analytic studies to
provide a more nuanced and interpretable measure of variability,
supporting our objective of accurately estimating the cognitive
impact of ART while accounting for study differences (20). A leave-one-out sensitivity analysis
was conducted to assess the influence of each study on the overall
effect size. This process involved sequentially removing each study
from the analysis to determine whether the exclusion of any single
study significantly altered the results. Potential publication bias
was assessed visually using funnel plots and statistically with
Egger's and Begg's tests. When asymmetry was detected, the
trim-and-fill method was employed to adjust for potential missing
studies and provide an adjusted effect size estimate. A
dose/duration-response analysis was conducted to explore the
relationship between the testosterone dose/duration and also
baseline testosterone levels and cognitive outcomes. The regression
of SMD on ART dosage was performed, allowing for an evaluation of
any potential dose/duration-dependent effects of testosterone on
cognitive functions. Statistical significance was set at P<0.05
for all analyses.
Results
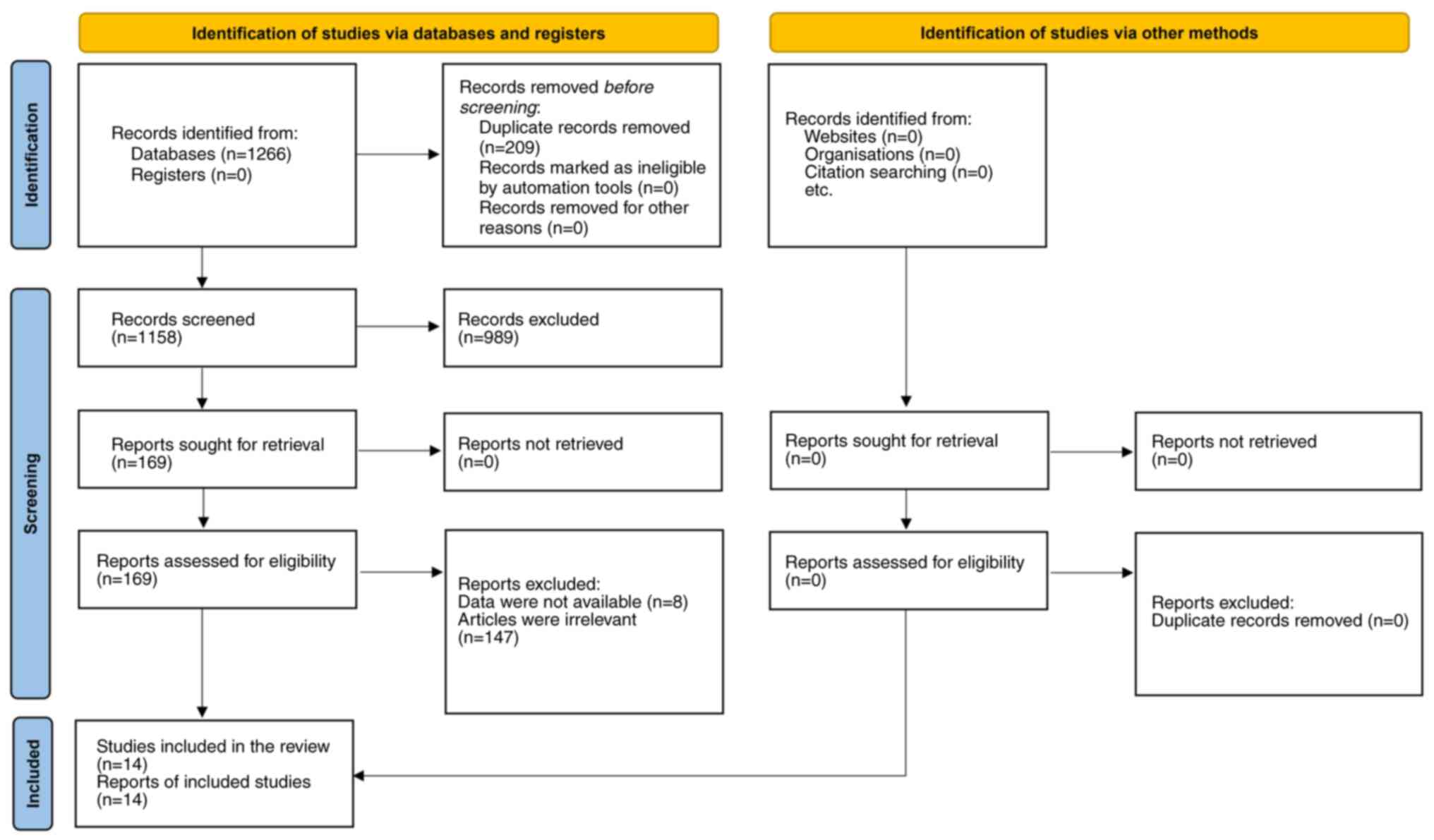
The study selection process is illustrated in the
PRISMA flow diagram (Fig. 1). The
initial database search yielded a total of 1,266 records, of which
209 duplicate records were removed, resulting in 1,158 records for
screening. After title and abstract screening, 989 studies were
excluded, and 169 studies remained for full-text review. Following
the full-text assessment, an additional 155 studies were excluded
for reasons such as irrelevance to the research question or
insufficient data for extraction (21-30).
Ultimately, a total of 14 studies were included in the
meta-analysis (5,7-9,31-40).
The characteristics of the included studies are
detailed in Table I. Each study
provided data on the effects of androgen supplementation on
cognitive function, with notable variations in sample size,
participant demographics, study design and intervention type.
Sample sizes ranged from 19 to 493 participants, with ages spanning
from 35 to 75 years. Most studies reported baseline testosterone
levels, which ranged from 215-350 ng/dl or specified hypogonadal
status. The designs of the included studies comprised RCTs,
crossover studies and cohort studies, with the majority being RCTs.
The interventions included various forms of androgen
supplementation, such as testosterone enanthate, testosterone
cypionate, testosterone undecanoate and transdermal testosterone
gel, administered at doses ranging from 5 mg daily to 250 mg per
week or biweekly. The duration of androgen therapy varied widely
across studies, from 5 days to 36 months. Cognitive outcomes were
assessed using a variety of neuropsychological tests targeting
specific cognitive domains, including memory (verbal memory tests,
paragraph recall), attention (Stroop interference test) and
executive function (Trail-Making Tests A and B). Follow-up periods
also varied, with assessments conducted immediately
post-intervention in some studies, while others included follow-up
intervals of up to 36 months to evaluate sustained effects.
 | Table ICharacteristics of included studies
on testosterone supplementation and cognitive function in older
men.a |
Table I
Characteristics of included studies
on testosterone supplementation and cognitive function in older
men.a
| First author/s,
year | Sample size | Age (mean ±
SD) | Baseline
testosterone level (ng/dl) | Study design | Intervention type
and dosage | Duration of
therapy | Cognitive outcome
measures | Follow-up
period | (Refs.) |
|---|
| Cherrier et
al, 2001 | 60 | 70±6 | 240 | RCT | Testosterone gel,
50 mg | 12 months | Verbal memory,
spatialcognition | 6 and 12
months | (8) |
| Cherrier et
al, 2005 | 36 | 65.2±5.3 | 215 | RCT | Testosterone
enanthate, 100 mg weekly | 6 months | Spatial memory,
verbal memory | 6 months | (31) |
| Emmelot-Vonk et
al, 2008 | 237 | 74.7±5.3 | 300 | RCT | Testosterone
undecanoate, 80 mg daily | 6 months | Trail-Making Test,
Stroop, memory recall tests | 6 months | (9) |
| Huang et al,
2016 | 44 | 72±5.5 | 220 | RCT | Testosterone
injection, 200 mg | 24 weeks | Complex figure
test, Stroop interference, verbal memory | 24 weeks | (35) |
| Janowsky et
al, 1994 | 25 | 68±4.1 | 275 | RCT | Testosterone patch,
6 mg/day | 3 months | Verbal memory,
spatial ability | 3 months | (32) |
| Janowsky et
al, 2000 | 30 | 70±5 | 230 | Crossover RCT | Testosterone
cypionate, 200 mg | 6 weeks | Memory tests,
executive function tests | 6 weeks | (7) |
| Kenny et al,
2002 | 20 | 67±3.8 | 245 | RCT | Testosterone
cypionate, 100 mg weekly | 6 months | Trail-Making Test,
Stroop, verbal fluency | 6 months | (33) |
| Kenny et al,
2004 | 32 | 70±7 | 250 | RCT | Testosterone gel,
75 mg daily | 1 year | MMSE, Digit Span,
memory recall | 1 year | (34) |
| Lašaitė et
al, 2017 | 19 | 35 | Low
(Hypogonadal) | Cohort Study | Testosterone
replacement therapy | 2 years | Digit Span,
Trail-Making Test A and B | 2 years | (5) |
| Maki et al,
2007 | 32 | 68±6 | 230 | Cross-over RCT | Transdermal
testosterone, 10 mg | 12 weeks | Trail-Making Test,
Paragraph Recall | 3 and 6 months | (36) |
| Resnick et
al, 2017 | 493 | 72.3±5.8 | <275 | RCT (Testosterone
Trials) | Testosterone gel,
5-10 mg daily | 1 year | Delayed paragraph
recall, Trail-Making Test B | 6 and 12
months | (40) |
| Vaughan et
al, 2007 | 69 | 70.8±4.2 | <350 | RCT | Testosterone
enanthate, 200 mg biweekly | 36 months | Digit span, visual
memory, verbal memory | 4 and 36
months | (37) |
| Wahjoepramono et
al, 2016 | 44 | 65±8 | 300 | Cross-over RCT | Testosterone cream,
50 mg daily | 6 months | MMSE, RAVLT,
depression scales | 6 months | (38) |
| Wolf et al,
2000 | 30 | 68.7±1.9 | 230 | RCT | Testosterone
injection, 250 mg | 5 days | Verbal fluency,
spatial memory | 5 days | (39) |
Among the included studies, only a few explicitly
excluded participants with conditions known to influence
testosterone levels and cognitive outcomes. Notably, studies by
Emmelot-Vonk et al (9) and
Huang et al (35) excluded
participants with diabetes and severe cardiovascular disease, while
others did not report comorbidity screening in detail. None of the
studies specifically examined populations with schizophrenia,
multiple sclerosis, or leukemia.
The CONSORT checklist assessment of 13 RCTs and 1
cohort study reveals variable adherence to reporting standards.
Most studies fulfilled the fundamental criteria, including clear
descriptions of the title, abstract, background, objectives,
participants, interventions and outcomes. However, specific
criteria, such as allocation concealment, blinding and participant
flow, demonstrated inconsistencies across studies. While the
majority of trials detailed statistical methods and participant
recruitment effectively, several provided only partial information
on ancillary analyses and harms, which are essential for
understanding treatment implications (Table II). The assessment of the risk of
bias using the ROBINS-I tool revealed that the majority of included
studies had a low to moderate risk of bias across most domains,
with some variability in areas such as confounding and selection
bias. A few studies exhibited concerns related to deviations from
the intended intervention, particularly when blinding was
inadequate or when participant adherence was unclear. Reporting and
measurement biases were generally low, as most studies used
validated cognitive measures. However, certain observational
studies had limitations in terms of selective reporting and missing
data. Overall, while the studies demonstrated acceptable
methodological rigor, a few exhibited methodological weaknesses
that should be considered when interpreting the findings (Table III).
 | Table IICONSORT checklist assessment for RCTs
on testosterone and cognition in older men.a |
Table II
CONSORT checklist assessment for RCTs
on testosterone and cognition in older men.a
| First author/s,
year | Title and
Abstract | Background and
objectives | Participants | Interventions | Outcomes | Sample size | Randomization:
Sequence Generation | Randomization:
Allocation Concealment | Blinding | Statistical
Methods | Participant
Flow | Recruitment | Baseline Data | Numbers
analyzed | Outcomes and
estimation | Ancillary
analyses | Harms | (Refs.) |
|---|
| Cherrier et
al, 2001 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Partial | (8) |
| Cherrier et
al, 2005 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | (31) |
| Emmelot-Vonk et
al, 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | (9) |
| Huang et al,
2016 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | (35) |
| Janowsky et
al, 2000 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | (32) |
| Janowsky et
al, 1994 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | No | (7) |
| Kenny et al,
2002 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | Yes | Yes | Partial | No | (33) |
| Kenny et al,
2004 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | Yes | Partial | Yes | No | (34) |
| Lašaitė et
al, 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | (5) |
| Maki et al,
2007 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Partial | Yes | No | (36) |
| Resnick et
al, 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | (40) |
| Vaughan et
al, 2007 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | (37) |
| Wahjoepramono et
al, 2016 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | (38) |
| Wolf et al,
2000 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | Yes | Partial | Yes | No | (39) |
 | Table IIIRisk of Bias Assessment of Included
Studies using ROBINS-I tool.a |
Table III
Risk of Bias Assessment of Included
Studies using ROBINS-I tool.a
| First author/s,
year | Bias due to
confounding | Bias in selection
of participants | Bias in
classification of interventions | Bias due to
deviations from intended interventions | Bias due to missing
data | Bias in measurement
of outcomes | Bias in selection
of reported results | Overall bias | (Refs.) |
|---|
| Cherrier et
al, 2001 | Low | Low | Low | Low | Low | Low | Low | Low | (8) |
| Cherrier et
al, 2005 | Low | Low | Low | Low | Low | Low | Low | Low | (31) |
| Emmelot-Vonk et
al, 2008 | Low | Low | Low | Low | Low | Low | Low | Low | (9) |
| Huang et al,
2016 | Low | Low | Low | Low | Low | Low | Low | Low | (35) |
| Janowsky et
al, 2000 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate | (7) |
| Janowsky et
al, 1994 | Moderate | Low | Low | Low | Low | Low | Low | Low | (32) |
| Kenny et al,
2002 | Low | Low | Low | Low | Low | Low | Low | Low | (33) |
| Kenny et al,
2004 | Low | Low | Low | Low | Low | Low | Low | Low | (34) |
| Wahjoepramono et
al, 2016 | Low | Low | Low | Low | Low | Low | Low | Low | (38) |
| Wolf et al,
2000 | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate | (39) |
| Lašaitė et
al, 2017 | Low | Low | Low | Low | Low | Low | Low | Low | (5) |
| Maki et al,
2007 | Low | Low | Low | Low | Low | Low | Low | Low | (36) |
| Resnick et
al, 2017 | Low | Low | Low | Low | Low | Low | Low | Low | (40) |
| Vaughan et
al, 2007 | Low | Low | Low | Low | Low | Low | Low | Low | (37) |
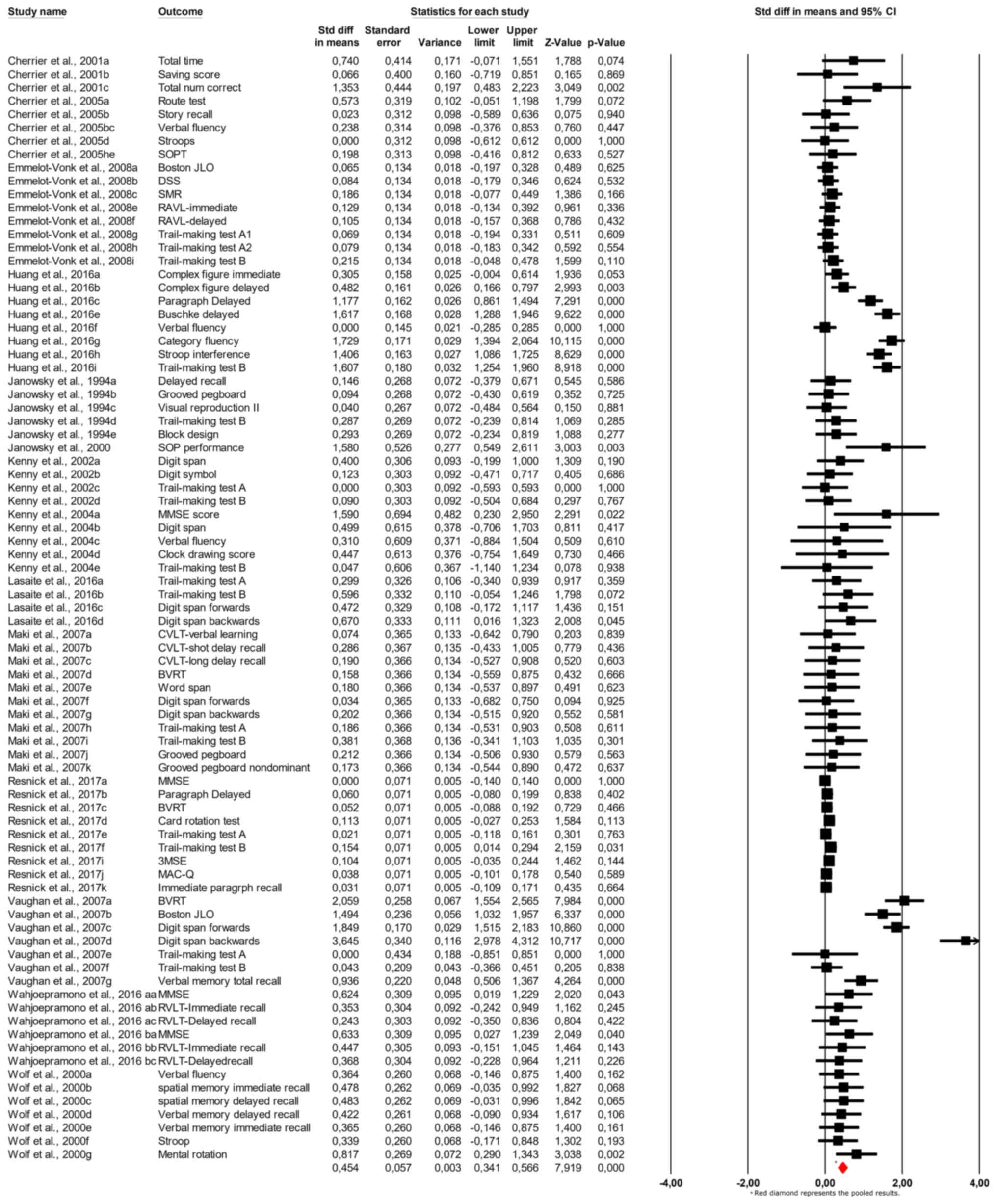
It was chosen not to combine multiple cognitive test
outcomes within the same population in the present analysis due to
several methodological and interpretative challenges. This approach
would require estimating correlations between outcomes, which are
often unreported in studies and difficult to accurately infer,
potentially introducing bias. Additionally, combining outcomes
could disproportionately emphasize certain cognitive domains, such
as memory, if they were assessed more frequently than others, thus
skewing the overall effect size. It was also aimed to preserve
domain-specific insights into ART's effects; combining results
would obscure differences between cognitive domains, such as memory
and executive function, which is crucial for understanding ART's
specific cognitive benefits. Moreover, cumulative variance
calculations in this method could lead to broader confidence
intervals, reducing precision and potentially masking statistically
significant results. Given these concerns, and to align with
standard practices in cognitive meta-analyses, each cognitive
outcome was analyzed separately to retain clarity, precision and
interpretability in our findings. The results of the meta-analysis,
shown in the forest plot (Fig. 2),
indicate a pooled SMD of 0.454 (95% CI: 0.341 to 0.566;
P<0.001), suggesting a small but statistically significant
effect of androgen therapy on cognitive function. The forest plot
displays each study's effect sizes and confidence intervals,
showing variability across the studies but with a consistent
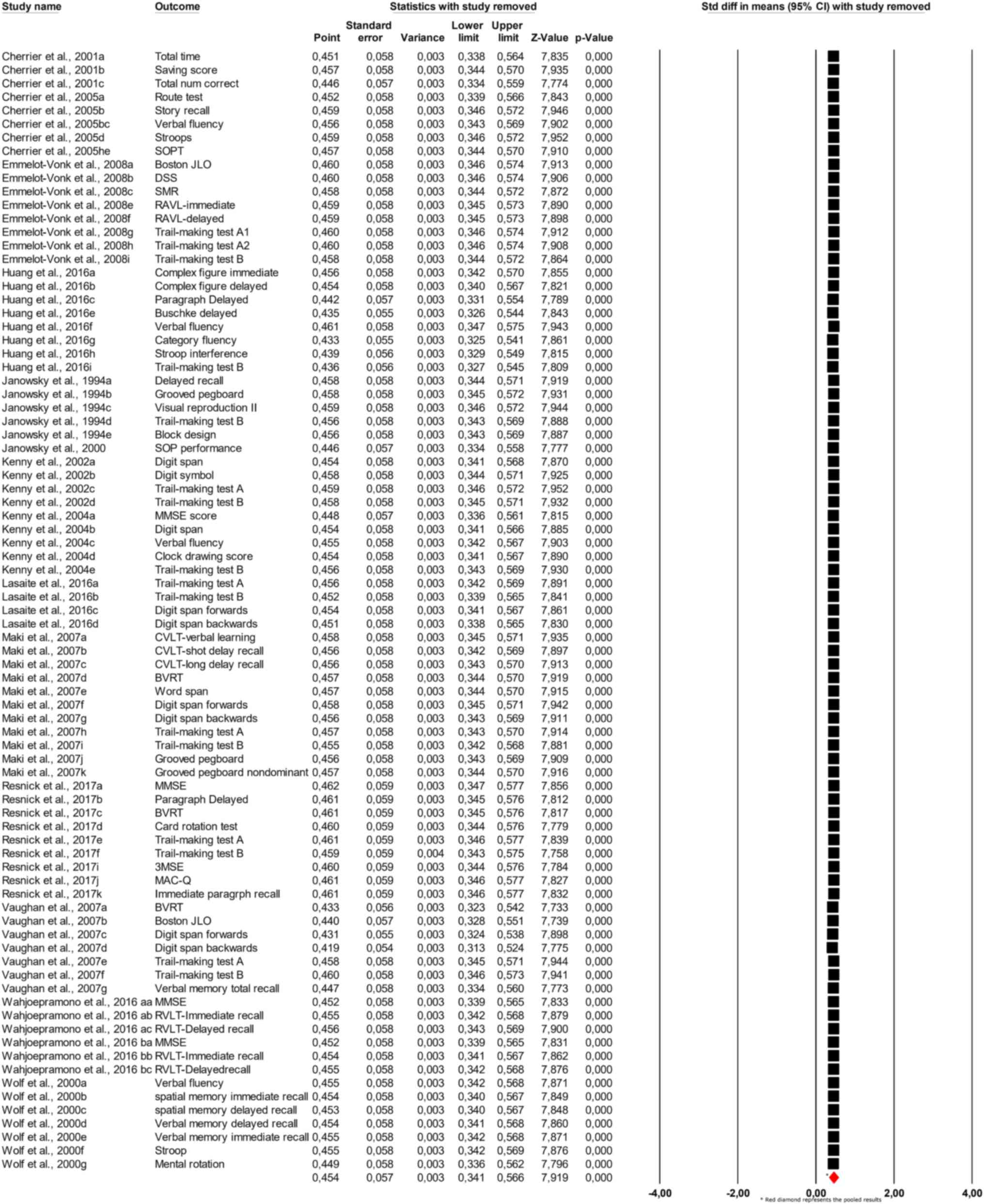
positive effect trend. Sensitivity analysis, depicted in Fig. 3, revealed that removing individual
studies did not substantially alter the pooled estimate, which
ranged from 0.451 to 0.457 across different iterations, confirming
the robustness of the findings.
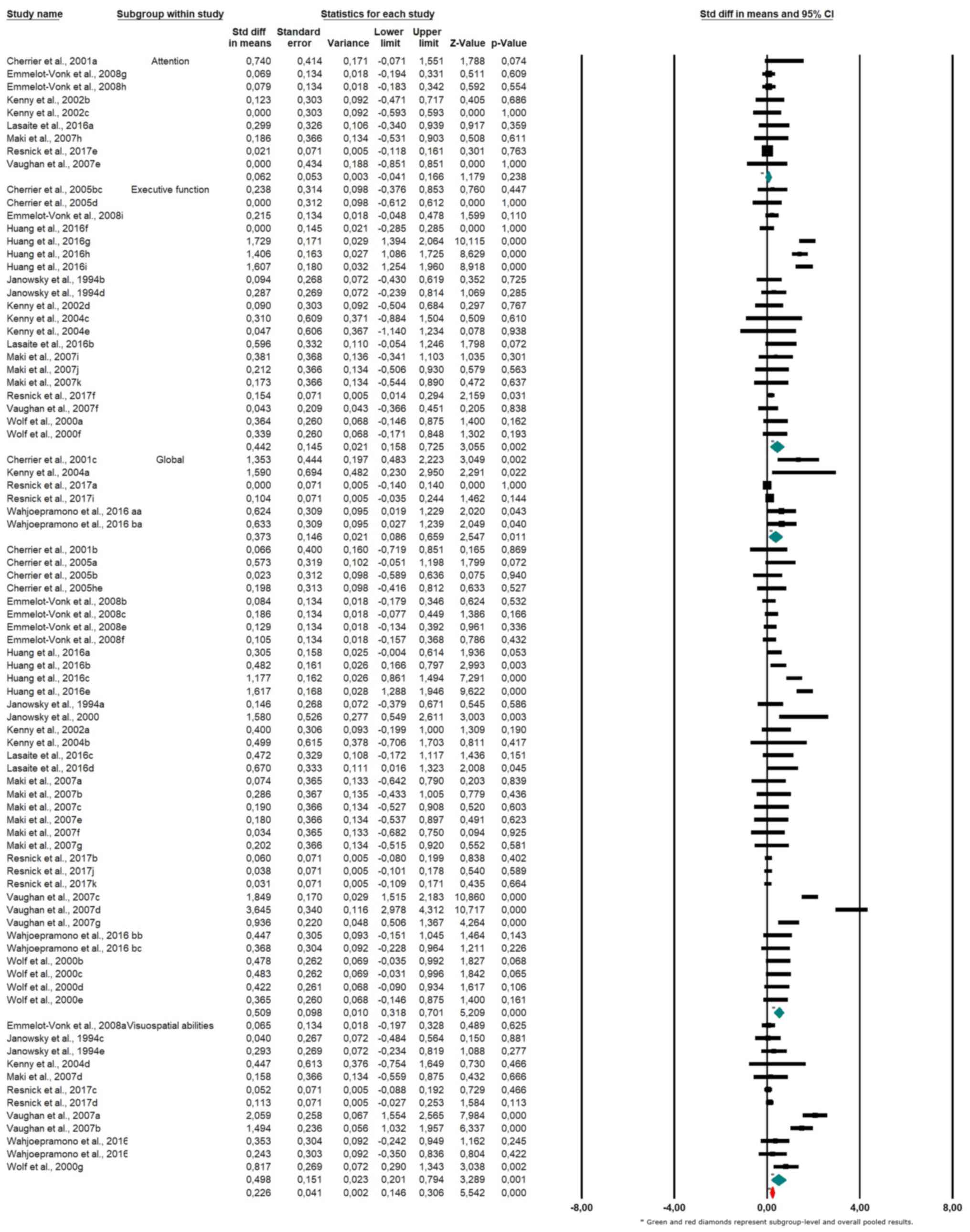
The results of the subgroup meta-analysis, shown in
the forest plot (Fig. 4), indicate
the following pooled SMDs across cognitive domains: for attention,
the SMD was 0.226 (95% CI: 0.041 to 0.411; P=0.016), suggesting a
small but statistically significant positive effect; for executive
function, the SMD was 0.226 (95% CI: 0.146 to 0.306; P<0.001),
indicating a small but robust positive effect; for global cognitive
function, the SMD was 0.488 (95% CI: 0.306 to 0.669; P<0.001),
representing a moderate and statistically significant improvement;
and for visuospatial abilities, the SMD was 0.226 (95% CI: -0.012
to 0.465; P=0.062), indicating a small and non-significant trend
toward improvement.
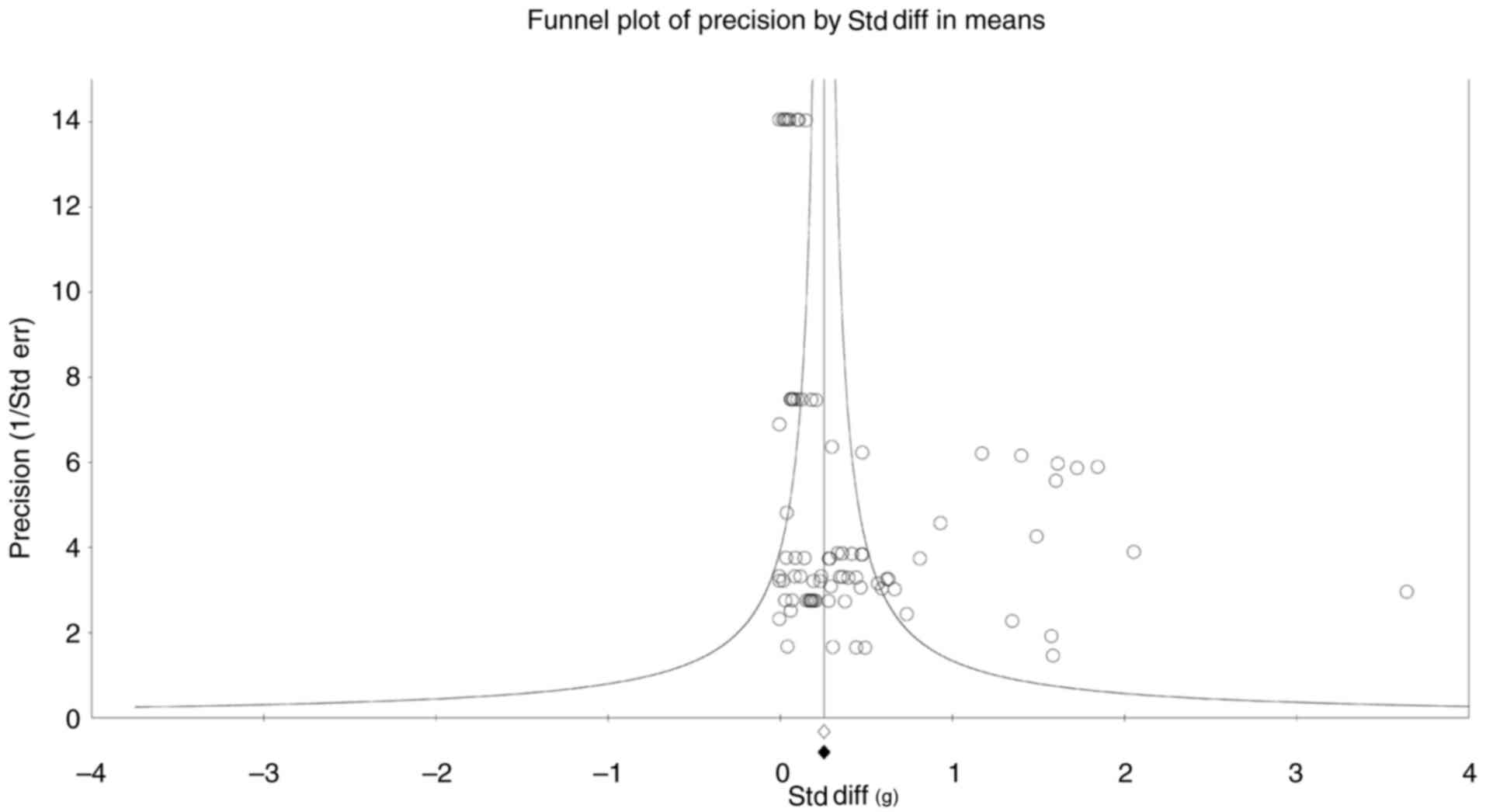
A funnel plot (Fig.
5) was constructed to evaluate publication bias, and asymmetry
was assessed with Begg and Mazumdar's rank correlation and Egger's
regression intercept. Begg's test yielded Kendall's tau of 0.265
with a P<0.001, and Egger's test showed an intercept of 1.92
(P<0.001), indicating significant publication bias. Furthermore,
the classic fail-safe N test indicated that 6,476 studies would be
required to nullify the observed effect, suggesting that the
results are highly resistant to the impact of unpublished null
findings. Orwin's fail-safe N supported these findings, setting a
trivial effect size criterion of 0.25 and yielding consistent
results. The Duval and Tweedie trim-and-fill method (Fig. 6) was applied to adjust for potential
publication bias. The adjusted pooled effect size under both fixed
and random effects models remained similar to the observed values
(SMD=0.454), reinforcing the robustness of the results despite the
detected asymmetry. Detecting significant publication bias
underscores the potential influence of unpublished studies with
null results on the pooled effect size. However, the fail-safe N
tests indicate a robust effect that is resistant to the inclusion
of additional null studies. While the Duval and Tweedie
trim-and-fill method suggests that the adjusted pooled effect size
remains consistent, indicating that the observed effect is not
solely attributable to bias, it is important to interpret these
findings with caution. The observed asymmetry may also reflect
differences in study quality, reporting practices, or sample sizes,
which can skew the distribution of reported effect sizes. Future
studies with rigorous methodology and transparent reporting are
crucial to validate these findings and mitigate potential
biases.
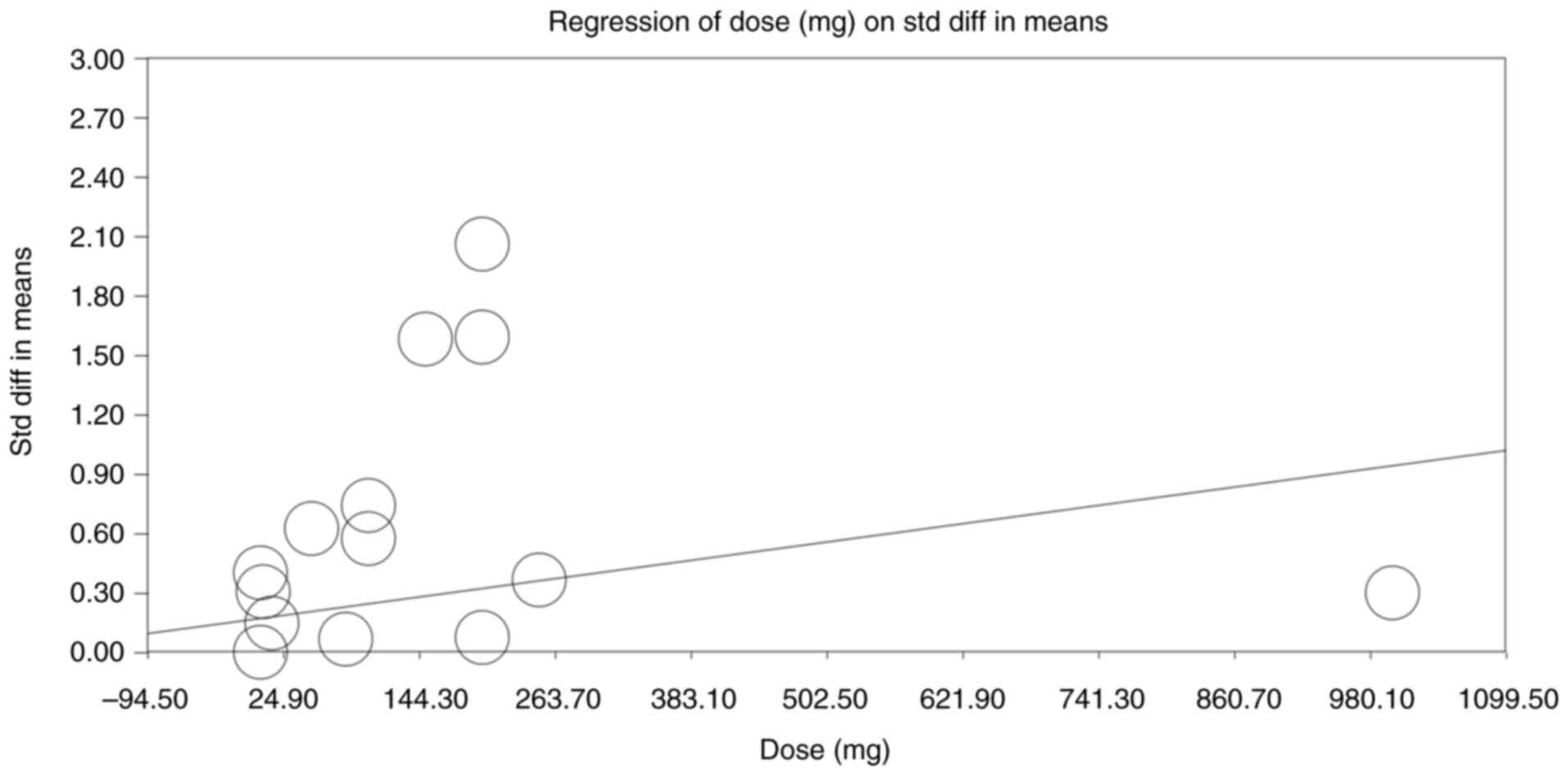
Additionally, a dose-response regression analysis,
examining the relationship between androgen dose and effect size,
is presented in Fig. 7. A slight
positive trend was observed and the association reached statistical
significance (slope P=0.01), suggesting that higher doses do yield
greater cognitive benefits. However, the baseline testosterone
level and duration of treatment showed no effect on the cognitive
outcomes (data not shown, P>0.05)
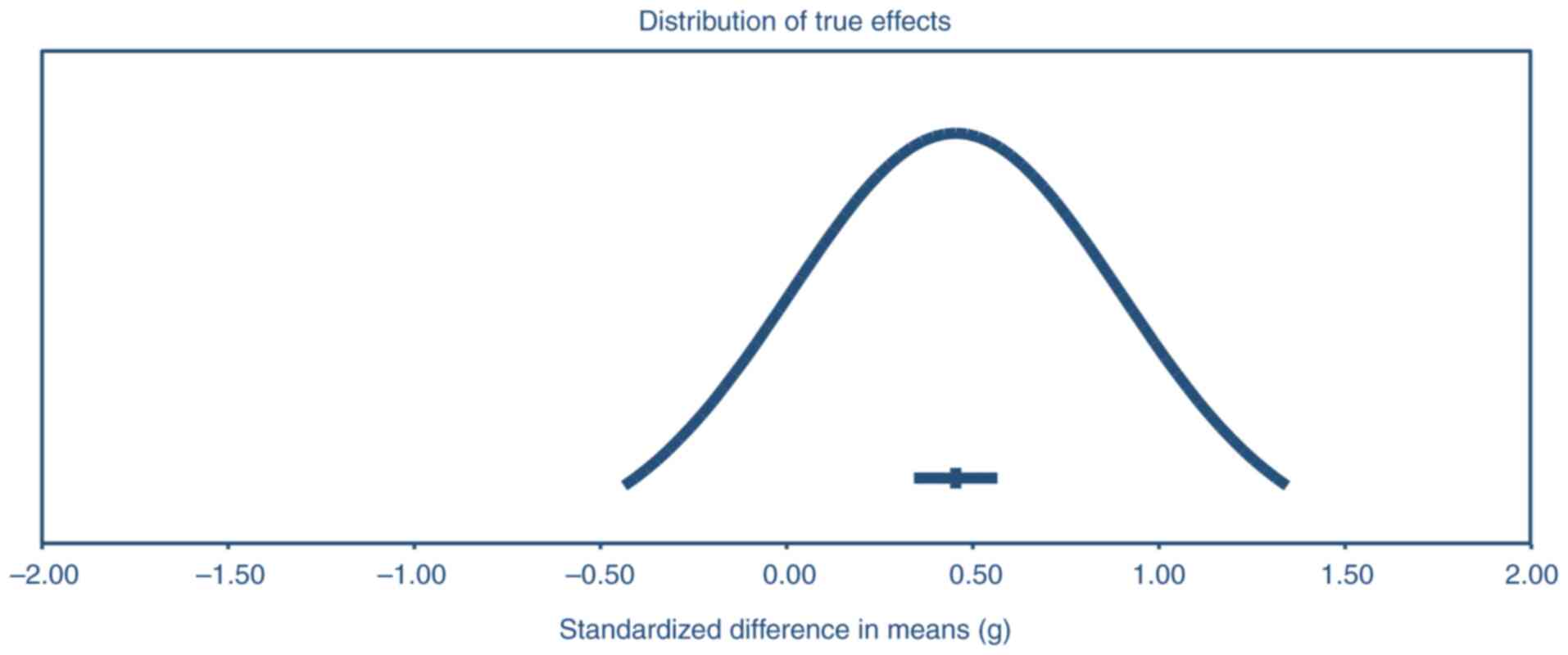
The distribution of true effects across studies
(Fig. 7) displays the results of
the precision analysis revealing a significant positive effect,
with a mean effect size of 0.45 and a 95% confidence interval
ranging from 0.34 to 0.57. This indicates a moderate and
statistically significant impact of the intervention or treatment
being studied. However, it is important to note the considerable
variability in the true effect sizes across different populations.
The analysis suggests that in 95% of comparable populations, the
true effect size could fall anywhere between-0.44 and 1.34. This
wide range encompasses both negative and positive effects,
suggesting that while the overall trend is positive, the
intervention's impact may vary substantially depending on the
specific population or context. This heterogeneity in effects
underscores the importance of considering individual differences
and contextual factors when interpreting and applying these
findings to real-world scenarios. A significant source of
heterogeneity was identified in participant demographics, such as
baseline cognitive status, age and baseline testosterone levels,
which ranged from 215 to 350 ng/dl. Variations in study design,
including the mode of testosterone administration (injections,
gels, patches), dosage and treatment duration, further contributed
to heterogeneity. Additionally, cognitive testing protocols varied
across studies, with some utilizing comprehensive
neuropsychological batteries while others focused on specific
domains (these sources of heterogeneity persisted even in the
subgroup analyses). The random-effects model was chosen to account
for this diversity, and subgroup analyses were conducted to explore
potential sources of variability.
Discussion
The present systematic review and meta-analysis
examined the impact of ART on cognitive functions in hypogonadal
men, synthesizing data across studies to assess outcomes in domains
such as memory, attention and executive function. The meta-analysis
yielded a pooled SMD of 0.454 (95% CI: 0.341 to 0.566; P<0.001),
indicating a statistically significant but small-to-moderate effect
size in favor of ART's role in enhancing cognitive performance in
hypogonadal individuals. The results showed consistency across
various studies despite differences in sample sizes, cognitive
assessment tools and ART administration protocols. Sensitivity
analyses further confirmed the robustness of these findings, as the
exclusion of any individual study did not significantly alter the
overall effect size.
The heterogeneity in ART regimens, including
differences in dosage, duration and administration methods, likely
influenced the overall findings of the present meta-analysis.
Studies with longer treatment durations (≥12 months) (37) and higher doses (200 mg biweekly)
(37) demonstrated more consistent
improvements in memory and executive function, possibly due to
sustained neuroprotective effects of stable testosterone levels
over time. Conversely, studies with shorter treatment durations
(for example, <4 weeks) (39)
often reported minimal cognitive improvements, potentially
reflecting an insufficient timeframe for neurogenesis or synaptic
remodeling to occur. Variations in the mode of administration also
played a role, as intramuscular injections tended to produce more
rapid and pronounced cognitive effects compared with transdermal
formulations, which provide more stable but slower-acting
testosterone levels. This variability may have contributed to the
observed discrepancies in domains such as visuospatial ability,
where ART appeared to have limited effects regardless of the
regimen. Additionally, the absence of ART-combination treatments,
such as concurrent cognitive training or physical exercise, may
have further constrained the potential for synergistic cognitive
gains. These findings underscore the importance of considering
treatment parameters and individual response variability when
interpreting the effects of ART on cognitive function.
Standardization in ART protocols across future studies,
particularly with respect to dose and duration, may help clarify
the true domain-specific cognitive benefits of testosterone
supplementation.
The cognitive benefits of ART observed in the
present study align with findings from previous research. Previous
studies, such as Cherrier et al (8), found significant improvements in
verbal memory following testosterone supplementation in older
hypogonadal men, which aligns with our meta-analysis results
showing positive effects in memory-related tasks (7). Similarly, Janowsky et al
(32) found that testosterone
administration improved spatial cognition in older men, suggesting
that spatial memory and navigation skills may be particularly
responsive to testosterone treatment (32). In line with that, a later study by
Janowsky et al (7) confirmed
these findings, showing that testosterone improved performance on
tasks requiring spatial and working memory, further indicating a
consistent impact of ART on specific memory-related cognitive
domains (7). Further evidence comes
from Kenny et al (33), who
conducted an RCT in which hypogonadal men received transdermal
testosterone over 6 months. The study found significant
improvements in attention and executive functioning, as assessed by
the Trail-Making Test and Stroop interference test, both sensitive
to attentional and cognitive control processes. These findings
suggest that ART may benefit memory and positively impact executive
functions, such as processing speed and cognitive flexibility. This
effect is particularly relevant for daily functioning, as cognitive
control is crucial for managing complex tasks (33). However, results across studies have
not been uniformly positive. For example, Emmelot-Vonk et al
(9) reported mixed findings, where
testosterone supplementation improved specific areas including
attention but did not significantly enhance overall cognitive
performance. In their large-scale RCT involving 237 older men,
cognitive benefits were observed primarily in attention, with
minimal effects on global cognitive scores. The lack of improvement
in global cognition might be attributed to the variability in
cognitive assessments used or individual differences in baseline
cognitive functioning. The authors suggested that the limited
impact on broader cognitive domains could be due to insufficient
dosage or duration of ART to elicit larger cognitive effects across
all domains (9). More recently,
Huang et al (35) examined
the effects of long-term testosterone administration on cognition
in older men with low to low-normal testosterone concentrations.
Their study, a secondary analysis of the TEAAM trial, found some
improvement in specific cognitive tasks, particularly in verbal
memory and complex figure tests, which assess visuospatial and
memory functions. However, similar to Emmelot-Vonk et al
(9), Huang et al (35) found that the effects of testosterone
were not pervasive across all cognitive domains, indicating that
ART may selectively enhance certain cognitive functions while
having little to no impact on others (35).
The findings of the present study are consistent
with this pattern, where improvements in memory, especially verbal
memory, were evident across studies. However, ART's effect on
broader cognitive domains remains less consistent. These variations
could result from differences in study design, such as testosterone
administration method (injections, gels, patches), dose, therapy
duration, and variations in cognitive testing protocols.
Additionally, factors such as baseline testosterone levels and the
age of participants likely influence the cognitive response to ART.
For instance, studies involving younger hypogonadal men [for
example, Lašaitė et al (5)]
have shown that younger age groups may exhibit more pronounced
cognitive benefits, possibly due to higher neuroplasticity in
younger individuals.
In line with this, the current meta-analysis found a
significant effect on executive function and global cognition,
indicating that ART may enhance higher-order cognitive processes
and overall cognitive performance. However, the effects on
attention and visuospatial abilities were less pronounced. These
findings underscore the complexity of ART's impact on cognition,
with domain-specific effects that may depend on both biological and
methodological factors. The observed improvements in executive
function, for example, may reflect the role of testosterone in
modulating brain regions such as the prefrontal cortex, which
governs cognitive control and working memory. Conversely, the lack
of significant improvements in attention and visuospatial tasks
suggests that these domains may be less sensitive to androgen
levels or require longer intervention periods to exhibit measurable
changes. Further research should aim to address these discrepancies
by standardizing study designs and exploring potential moderators,
such as participant age, baseline cognitive status, and genetic
predispositions, to improve understanding of the nuanced cognitive
effects of ART.
The administration method also plays a crucial role
in ART outcomes. Studies that used injectable testosterone, such as
testosterone enanthate or testosterone cypionate, tended to show
more immediate cognitive effects (7) compared with slower-releasing
transdermal gels and patches (31).
However, the long-term benefits of transdermal testosterone suggest
it may offer more stable cognitive gains over extended periods,
particularly for memory-related tasks (9,31).
The current findings align with and extend those of
previous meta-analyses by Zhang et al (10) and Hong et al (11) (the latter shares 75% of the eligible
studies with our meta-analysis), but with notable methodological
and interpretive differences. Zhang et al (10) conducted a CMA that included both
observational studies and RCTs, highlighting mild cognitive
improvements, particularly in verbal memory, but only in short-term
follow-ups (up to 6 months). Their analysis was limited by small
sample sizes in intervention studies and a lack of detailed
exploration of dose-response relationships. By contrast, Hong et
al (11) focused exclusively on
elderly populations across 15 RCTs and found no significant
improvements in cognitive speed, working memory, or
attention-related tasks, attributing these null findings to study
heterogeneity and potential publication bias. Unlike these studies,
the current meta-analysis included longer follow-up periods (up to
36 months) and incorporated diverse ART administration methods,
allowing us to capture potential long-term cognitive effects.
Precision intervals were also employed instead of relying solely on
I², providing a more nuanced assessment of heterogeneity and
identifying modest but statistically significant improvements in
executive function, which were absent in the findings of Hong et
al (11). Additionally, our
dose-response analysis revealed a significant positive association
between testosterone dosage and cognitive improvement (P=0.01),
suggesting that dosage optimization may play a critical role in ART
efficacy. These findings underscore the importance of personalized
ART regimens and suggest that future research should focus on
optimizing treatment protocols and conducting long-term RCTs
stratified by cognitive risk profiles.
In addition, the study by Ponce et al
(41) conducted a systematic review
of RCTs on testosterone replacement therapy (TRT) in hypogonadal
men, focusing on validated patient-important outcomes (PIOs)
including sexual function, mood and adverse events such as
erythrocytosis, with strict inclusion criteria (TT ≤300 ng/dl,
≥12-week duration). Their findings indicated modest improvements in
sexual desire (SMD: 0.17), erectile function (SMD: 0.16) and sexual
satisfaction (SMD: 0.16), but no significant effect on mood or
energy, alongside an increased risk of erythrocytosis (RR: 8.14).
By contrast, the present study incorporated broader testosterone
thresholds, longer follow-up durations and additional cognitive
assessments, allowing for the capturing of potential long-term
effects on cognition. Furthermore, dose-response and subgroup
analyses were performed, strengthening insights into treatment
variability, while Ponce et al's (41) focus remained on general PIOs without
stratification for cognitive or individual-level factors, making
the current findings more comprehensive for personalized TRT
strategies.
An important consideration in our meta-analysis is
the potential age-related bias in study selection. The majority of
the included studies focused on older men with age-related
hypogonadism (typically aged ≥50 years). This focus is clinically
relevant, as older adults represent the primary population for ART
due to the natural decline in testosterone levels with age and the
increased prevalence of cognitive complaints (42). However, this also limits the
generalizability of the present findings to younger hypogonadal
men, who may exhibit different cognitive responses to ART.
Age-related biological factors, such as reduced AR density and
decreased neuroplasticity, may attenuate the cognitive benefits of
ART in older individuals (43).
Moreover, the presence of age-related comorbidities, such as
cardiovascular disease and diabetes, may confound cognitive
outcomes, as these conditions can independently affect cognitive
function (44). The observed modest
effect sizes in the present analysis may reflect these age-related
influences. By contrast, younger individuals with non-age-related
hypogonadism may experience more pronounced cognitive benefits due
to greater baseline neuroplasticity, fewer comorbidities, and
potentially higher AR sensitivity (45).
A review of the 14 included studies revealed that
bioavailable testosterone levels were commonly reported alongside
total testosterone, with several studies, such as those by Kenny
et al (33,34), showing significant increases in
bioavailable testosterone in treatment groups following ART. By
contrast, no notable changes were observed in control groups.
Additionally, SHBG levels, which influence the proportion of free
and bioavailable testosterone, were consistently monitored in
studies such as Lašaitė et al (5) and Cherrier et al (8). While SHBG levels showed variability
across participants, they generally remained stable during ART.
However, none of the included studies directly measured AR levels,
though some studies, such as Lašaitė et al (5), discussed the potential role of AR
polymorphisms and receptor sensitivity in influencing cognitive
outcomes. To address this limitation, it is important to note that
AR-related factors were not quantitatively assessed but were
acknowledged as potential moderators in cognitive responses to ART.
Including bioavailable testosterone as a key outcome alongside
total testosterone aligns with common endocrinological protocols,
as it provides a more precise indicator of the active hormonal
fraction. Therefore, while hormonal measures such as bioavailable
testosterone and SHBG were routinely considered, the absence of
direct AR-level data highlights an area for further research.
The mechanisms underlying the cognitive effects of
ART are indeed complex and appear to involve multiple pathways,
both direct and indirect. One major pathway is the action of
testosterone through ARs, which are widely distributed in brain
regions that are crucial for cognitive functions, particularly
memory and learning. Key areas such as the hippocampus, amygdala
and prefrontal cortex have high densities of ARs, which suggests a
direct link between testosterone levels and cognitive performance
in these regions (13). Activation
of these receptors is considered to facilitate neuroprotective
effects, including the enhancement of synaptic connectivity,
modulation of neurotransmitter levels and protection against
neurodegenerative processes (14).
Additionally, testosterone undergoes aromatization in the brain to
form estradiol, a process that plays a significant role in synaptic
plasticity, neurogenesis and neuronal survival. Estradiol, the
primary estrogen in the brain, has well-documented effects on
synaptic density and cognitive function. It enhances dendritic
spine growth in the hippocampus and prefrontal cortex, which are
structures critical for memory formation and executive function
(46). By acting as a precursor to
estradiol, testosterone contributes indirectly to these cognitive
benefits, particularly in brain areas involved in verbal memory,
spatial navigation and attention (14,24).
The neuroprotective effects of testosterone may also be attributed
to its influence on neurotransmitter systems. For example,
testosterone has been shown to enhance dopamine release, a
neurotransmitter essential for motivation, reward processing and
executive functioning (47).
Dopaminergic pathways are particularly dense in the prefrontal
cortex, a brain area involved in planning, problem-solving and
impulse control. Furthermore, testosterone may positively affect
serotonin and GABAergic systems, contributing to mood regulation
and stress resilience, which indirectly supports cognitive
performance by reducing anxiety and depressive symptoms commonly
associated with cognitive decline (48).
Despite these plausible mechanisms, the effect sizes
observed in the current analysis were modest. Although testosterone
appears to have neuroprotective and cognitive-enhancing effects,
these benefits might be limited by several factors. First,
individual variability in AR density, which declines with age, may
reduce the brain's responsiveness to ART, particularly in older men
(32). Second, cognitive decline is
a multifactorial process that involves numerous interacting
elements beyond testosterone levels, such as genetic
predisposition, cardiovascular health, lifestyle factors and other
hormonal changes. Therefore, while ART may provide some cognitive
benefits, it may not fully counteract or reverse cognitive deficits
that result from these broader, complex mechanisms (15). Finally, while testosterone's
conversion to estradiol is beneficial for synaptic health,
excessive estradiol in men could potentially lead to adverse
effects, including mood instability and even cognitive impairments
if levels exceed the optimal range (25). This suggests a need for personalized
ART dosing to balance the benefits of testosterone, particularly
given the variability in individual responses to testosterone
supplementation.
One advantage of the present study is the rigorous
methodology, adhering to PRISMA guidelines, with thorough data
extraction and evaluation of each study's methodological quality
using the CONSORT and ROBINS-I tool checklist. The current analysis
also included a dose-response assessment, revealing a slight
positive correlation between testosterone dose and cognitive effect
size (slope P=0.01). This suggests a dose-dependent relationship,
though the absence of a significant duration-response effect
indicates that the duration of ART may not be as influential as
dosage in determining cognitive outcomes. Our findings underscore
the importance of dosage and duration in determining ART efficacy.
However, the variability in dosages (ranging from 5 mg/day to 250
mg/week) and therapy durations (5 days to 36 months) complicates
direct comparisons between studies. This highlights the need for
standardizing ART protocols and further clarifying the relationship
between these parameters and cognitive outcomes. Future research
should investigate the effects of varying regimens across different
populations to identify optimal ART combinations and dosing
schedules for specific cognitive domains. Nevertheless, there are
limitations to the present study. First, substantial heterogeneity
was present across studies, with variations in ART protocols,
cognitive tests and participant demographics, which may confound
results. Although a random-effects model and precision intervals
were used to mitigate this heterogeneity, these factors remain
potential sources of bias. The age-related focus of the included
studies may limit the generalizability of findings to younger men
with hypogonadism. Additionally, the presence of comorbid
conditions, such as cardiovascular disease and diabetes, and
comorbidities associated with decreased androgen levels
(schizophrenia, multiple sclerosis and acute lymphoblastic
leukemia) may have influenced cognitive outcomes independently,
which could have attenuated the observed effect sizes (49). Future studies should stratify
participants by age and comorbidity status to account for these
confounding factors. Furthermore, the presence of publication bias,
as indicated by the funnel plot and Begg's and Egger's tests,
suggests an overrepresentation of positive findings. The
trim-and-fill method adjusted for this bias, but the overall effect
size remained relatively unchanged, underscoring that ART's
cognitive effects, though consistent, may be modest. Additionally,
the generalizability of the current findings may be limited by the
characteristics of the included studies, which primarily involved
older men with age-related hypogonadism. Cognitive effects may
differ in younger populations or those with non-age-related
hypogonadism, necessitating caution in extrapolating our results
beyond the studied demographic.
The findings of the present study have potential
implications for the clinical management of cognitive deficits in
hypogonadal patients. Given the observed cognitive benefits, ART
could be considered as part of a broader therapeutic approach for
older men with hypogonadism and cognitive complaints. Clinicians
should temper expectations, as the modest effect sizes indicate
that ART alone is unlikely to yield substantial cognitive gains for
all patients. The decision to initiate ART should balance potential
cognitive benefits against associated risks, particularly
cardiovascular health and prostate complications. ART may be most
beneficial when combined with lifestyle interventions such as
exercise and cognitive training, which have independently
demonstrated positive effects on cognitive function.
Non-pharmacological interventions, such as cognitive training,
aerobic exercise and dietary supplementation, have also
demonstrated efficacy in improving cognitive outcomes in
hypogonadal men (50). For
instance, aerobic exercise has shown significant improvements in
executive function and memory, suggesting that combining ART with
lifestyle modifications may yield more robust cognitive benefits
(51). Currently, there is a lack
of studies directly comparing ART with non-pharmacological
interventions, such as cognitive training, aerobic exercise, or
dietary supplementation, in terms of cognitive benefits for
hypogonadal men. While ART has been shown to improve certain
cognitive functions, such as verbal and spatial memory, in
hypogonadal men (52), and
non-pharmacological interventions such as aerobic exercise have
demonstrated improvements in executive function and memory
(53), head-to-head comparative
studies are needed to determine the relative efficacy of these
treatments and to assess whether combining ART with lifestyle
modifications offers synergistic cognitive benefits.
Additionally, tailoring ART regimens to individual
cognitive risk profiles, baseline testosterone levels and
comorbidity status may optimize therapeutic outcomes while
minimizing adverse effects. In clinical practice, ART should be
integrated into a comprehensive cognitive health strategy that
includes lifestyle interventions such as exercise, nutrition and
cognitive training. Future research should focus on understanding
how ART interacts with these factors to optimize cognitive
resilience.
Several factors may contribute to the absence of new
RCTs or large-scale studies meeting our inclusion criteria after
2023. First, conducting RCTs in this domain is resource-intensive,
requiring significant time and funding, particularly given the need
for long-term follow-up to capture meaningful clinical outcomes and
adverse events. Second, ethical considerations surrounding placebo
use in symptomatic hypogonadal men could limit the feasibility of
new trials. Additionally, recent systematic reviews and
meta-analyses may have already synthesized existing evidence
comprehensively, potentially disincentivizing the initiation of new
large-scale studies. Finally, there may be an ongoing shift towards
real-world evidence and large observational studies, which some
researchers may prioritize over RCTs to capture broader patient
populations and real-world treatment effects.
In conclusion, the present systematic review and
meta-analysis provide evidence that ART has a modest but
statistically significant positive effect on cognitive function in
hypogonadal men, particularly in memory and executive function
domains. Domain-specific findings indicate that ART shows the
strongest effects in verbal memory and working memory, with smaller
but notable improvements in executive functions such as cognitive
flexibility and processing speed. However, the effects on attention
and visuospatial abilities were less consistent, indicating that
some cognitive domains may be more responsive to ART than others.
These findings suggest that ART may be most beneficial for
individuals with mild to moderate cognitive deficits and low
baseline testosterone levels, as observed in studies involving
younger cohorts, which reported more pronounced cognitive
improvements. Furthermore, differences in ART administration
methods, such as transdermal testosterone gels vs. intramuscular
injections, may influence outcomes, with transdermal forms
potentially offering imroved adherence and fewer adverse events.
Despite the observed benefits, the modest effect sizes suggest that
ART alone may not suffice as a comprehensive cognitive intervention
for hypogonadal men with significant cognitive impairments. The
variability in cognitive outcomes across studies underscores the
need to consider individual factors, including age, baseline
cognitive status, comorbid conditions and genetic predispositions,
when evaluating ART's potential benefits. Clinicians should
carefully balance the potential cognitive benefits of ART with its
associated risks, particularly in patients with cardiovascular risk
factors or a history of prostate disease. Future research should
prioritize long-term RCTs involving both younger and older
hypogonadal populations to improve understanding of the
differential cognitive effects across age groups. Additionally,
there is a need to examine the interaction of ART with comorbid
conditions and lifestyle factors to better delineate its cognitive
impact. Standardizing cognitive assessments and ART dosing
protocols across studies is essential to reduce methodological
variability and improve comparability. Mechanistic studies
investigating the role of ARs, neuroplasticity, and the influence
of estradiol conversion on synaptic connectivity will also provide
important insights into the pathways through which ART influences
cognitive function. These targeted research efforts will support
the development of more personalized and effective ART regimens to
address cognitive deficits in hypogonadal men.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Tianjin Health
Technology Project (grant no. ZC20185).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BW and XL developed the study protocol and were
responsible for data collection. WC and LL analyzed the data. BW
and XL confirm the authenticity of all the raw data. All authors
contributed to the writing of the manuscript, and read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shores MM, Sloan KL, Matsumoto AM, Moceri
VM, Felker B and Kivlahan DR: Increased incidence of diagnosed
depressive illness in hypogonadal older men. Arch Gen Psychiatry.
61:162–167. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Barrett-Connor E, Goodman-Gruen D and
Patay B: Endogenous sex hormones and cognitive function in older
men. J Clin Endocrinol Metab. 84:3681–3685. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jayaraman A, Lent-Schochet D and Pike CJ:
Diet-induced obesity and low testosterone increase
neuroinflammation and impair neural function. J Neuroinflammation.
11(162)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Munari EV, Amer M, Amodeo A, Bollino R,
Federici S, Goggi G, Giovanelli L, Persani L, Cangiano B and Bonomi
M: The complications of male hypogonadism: Is it just a matter of
low testosterone? Front Endocrinol (Lausanne).
14(1201313)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lašaitė L, Čeponis J, Preikša RT and
Žilaitienė B: Effects of two-year testosterone replacement therapy
on cognition, emotions and quality of life in young and middle-aged
hypogonadal men. Andrologia. 49:2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nieschlag E, Behre HM, Kliesch S and
Nieschlag S (eds): Andrology: Male Reproductive Health and
Dysfunction. 4th edition. Springer Nature, 2023. https://doi.org/10.1007/978-3-031-31574-9. Accessed
October 27, 2023.
|
|
7
|
Janowsky JS, Chavez B and Orwoll E: Sex
steroids modify working memory. J Cogn Neurosci. 12:407–414.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cherrier MM, Asthana S, Plymate S, Baker
L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W,
Petrova A, et al: Testosterone supplementation improves spatial and
verbal memory in healthy older men. Neurology. 57:80–88.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour
HR, Aleman A, Lock TM, Bosch JL, Grobbee DE and van der Schouw YT:
Effect of testosterone supplementation on functional mobility,
cognition, and other parameters in older men: A randomized
controlled trial. JAMA. 299:39–52. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Z, Kang D and Li H: Testosterone and
cognitive impairment or dementia in middle-aged or aging males:
Causation and intervention, a systematic review and meta-analysis.
J Geriatr Psychiatry Neurol. 34:405–417. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hong SW, Cho YJ, Lee JH, Suh YS and Kim
DH: Effect of testosterone supplementation on cognition in elderly
men: A systematic meta-analysis. Korean J Geriatr Gerontol.
24:24–33. 2023.
|
|
12
|
Farajdokht F, Farhoudi M, Majdi A, Zamanlu
M, Sadigh-Eteghad S, Vahedi S and Mahmoudi J: Testosterone may hold
therapeutic promise for the treatment of ischemic stroke in aging:
A closer look at laboratory findings. Adv Pharm Bull. 9:48–55.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Roselli CE, Abdelgadir SE, Rønnekleiv OK
and Klosterman SA: Anatomic distribution and regulation of
aromatase gene expression in the rat brain. Biol Reprod. 58:79–87.
1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Luine VN: Sex steroids and cognitive
function. J Neuroendocrinol. 20:866–872. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Holland J, Bandelow S and Hogervorst E:
Testosterone levels and cognition in elderly men: A review.
Maturitas. 69:322–337. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huo S, Scialli AR, McGarvey S, Hill E,
Tügertimur B, Hogenmiller A, Hirsch AI and Fugh-Berman A: Treatment
of men for ‘low testosterone’: A systematic review. PLoS One.
11(e0162480)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zitzmann M, Faber S and Nieschlag E:
Association of specific symptoms and metabolic risks with serum
testosterone in older men. J Clin Endocrinol Metab. 91:4335–4343.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Thorlund K, Imberger G, Johnston BC, Walsh
M, Awad T, Thabane L, Gluud C, Devereaux PJ and Wetterslev J:
Evolution of heterogeneity (I2) estimates and their 95% confidence
intervals in large meta-analyses. PLoS One.
7(e39471)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
IntHout J, Ioannidis JP, Rovers MM and
Goeman JJ: Plea for routinely presenting prediction intervals in
meta-analysis. BMJ Open. 6(e010247)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Asih PR, Wahjoepramono EJ, Aniwiyanti V,
Wijaya LK, de Ruyck K, Taddei K, Fuller SJ, Sohrabi H, Dhaliwal SS,
Verdile G, et al: Testosterone replacement therapy in older male
subjective memory complainers: Double-blind randomized crossover
placebo-controlled clinical trial of physiological assessment and
safety. CNS Neurol Disord Drug Targets. 14:576–586. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cherrier MM, Plymate S, Mohan S, Asthana
S, Matsumoto AM, Bremner W, Peskind E, Raskind M, Latendresse S,
Haley AP and Craft S: Relationship between testosterone
supplementation and insulin-like growth factor-I levels and
cognition in healthy older men. Psychoneuroendocrinology. 29:65–82.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cherrier MM, Matsumoto AM, Amory JK,
Johnson M, Craft S, Peskind ER and Raskind MA: Characterization of
verbal and spatial memory changes from moderate to
supraphysiological increases in serum testosterone in healthy older
men. Psychoneuroendocrinology. 32:72–79. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cherrier MM, Anderson K, Shofer J, Millard
S and Matsumoto AM: Testosterone treatment of men with mild
cognitive impairment and low testosterone levels. Am J Alzheimers
Dis Other Demen. 30:421–430. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gray PB, Singh AB, Woodhouse LJ, Storer
TW, Casaburi R, Dzekov J, Dzekov C, Sinha-Hikim I and Bhasin S:
Dose-dependent effects of testosterone on sexual function, mood,
and visuospatial cognition in older men. J Clin Endocrinol Metab.
90:3838–3846. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gregori G, Celli A, Barnouin Y, Paudyal A,
Armamento-Villareal R, Napoli N, Qualls C and Villareal DT:
Cognitive response to testosterone replacement added to intensive
lifestyle intervention in older men with obesity and hypogonadism:
Prespecified secondary analyses of a randomized clinical trial. Am
J Clin Nutr. 114:1590–1599. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Haren MT, Wittert GA, Chapman IM, Coates P
and Morley JE: Effect of oral testosterone undecanoate on
visuospatial cognition, mood and quality of life in elderly men
with low-normal gonadal status. Maturitas. 50:124–133.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Konaka H, Sugimoto K, Orikasa H, Iwamoto
T, Takamura T, Takeda Y, Shigehara K, Iijima M, Koh E and Namiki M:
EARTH study group. Effects of long-term androgen replacement
therapy on the physical and mental statuses of aging males with
late-onset hypogonadism: A multicenter randomized controlled trial
in Japan (EARTH study). Asian J Androl. 18:25–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ly LP, Jimenez M, Zhuang TN, Celermajer
DS, Conway AJ and Handelsman DJ: A double-blind,
placebo-controlled, randomized clinical trial of transdermal
dihydrotestosterone gel on muscular strength, mobility, and quality
of life in older men with partial androgen deficiency. J Clin
Endocrinol Metab. 86:4078–4088. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamaguchi K, Ishikawa T, Chiba K and
Fujisawa M: Assessment of possible effects for testosterone
replacement therapy in men with symptomatic late-onset
hypogonadism. Andrologia. 43:52–56. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cherrier MM, Matsumoto AM, Amory JK, Ahmed
S, Bremner W, Peskind ER, Raskind MA, Johnson M and Craft S: The
role of aromatization in testosterone supplementation: Effects on
cognition in older men. Neurology. 64:290–296. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Janowsky JS, Oviatt SK and Orwoll ES:
Testosterone influences spatial cognition in older men. Behav
Neurosci. 108:325–332. 1994.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kenny AM, Bellantonio S, Gruman CA, Acosta
RD and Prestwood KM: Effects of transdermal testosterone on
cognitive function and health perception in older men with low
bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci.
57:M321–M325. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kenny AM, Fabregas G, Song C, Biskup B and
Bellantonio S: Effects of testosterone on behavior, depression, and
cognitive function in older men with mild cognitive loss. J
Gerontol A Biol Sci Med Sci. 59:75–78. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang G, Wharton W, Bhasin S, Harman SM,
Pencina KM, Tsitouras P, Li Z, Hally KA, Asthana S, Storer TW and
Basaria S: Effects of long-term testosterone administration on
cognition in older men with low or low-to-normal testosterone
concentrations: A prespecified secondary analysis of data from the
randomised, double-blind, placebo-controlled TEAAM trial. Lancet
Diabetes Endocrinol. 4:657–665. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Maki PM, Ernst M, London ED, Mordecai KL,
Perschler P, Durso SC, Brandt J, Dobs A and Resnick SM:
Intramuscular testosterone treatment in elderly men: Evidence of
memory decline and altered brain function. J Clin Endocrinol Metab.
92:4107–4114. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vaughan C, Goldstein FC and Tenover JL:
Exogenous testosterone alone or with finasteride does not improve
measurements of cognition in healthy older men with low serum
testosterone. J Androl. 28:875–882. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wahjoepramono EJ, Asih PR, Aniwiyanti V,
Taddei K, Dhaliwal SS, Fuller SJ, Foster J, Carruthers M, Verdile
G, Sohrabi HR and Martins RN: The effects of testosterone
supplementation on cognitive functioning in older men. CNS Neurol
Disord Drug Targets. 15:337–343. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wolf OT, Preut R, Hellhammer DH, Kudielka
BM, Schürmeyer TH and Kirschbaum C: Testosterone and cognition in
elderly men: A single testosterone injection blocks the practice
effect in verbal fluency, but has no effect on spatial or verbal
memory. Biol Psychiatry. 47:650–654. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Resnick SM, Matsumoto AM, Stephens-Shields
AJ, Ellenberg SS, Gill TM, Shumaker SA, Pleasants DD,
Barrett-Connor E, Bhasin S, Cauley JA, et al: Testosterone
treatment and cognitive function in older men with low testosterone
and age-associated memory impairment. JAMA. 317:717–727.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ponce OJ, Spencer-Bonilla G,
Alvarez-Villalobos N, Serrano V, Singh-Ospina N,
Rodriguez-Gutierrez R, Salcido-Montenegro A, Benkhadra R, Prokop
LJ, Bhasin S and Brito JP: The efficacy and adverse events of
testosterone replacement therapy in hypogonadal men: A systematic
review and meta-analysis of randomized, placebo-controlled trials.
J Clin Endocrinol Metab: Mar 17, 2018, (Epub ahead of print).
|
|
42
|
Cai Z and Li H: An updated review:
Androgens and cognitive impairment in older men. Front Endocrinol
(Lausanne). 11(586909)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kuwahara N, Nicholson K, Isaacs L and
MacLusky NJ: Androgen effects on neural plasticity. Androg Clin Res
Ther. 2:216–230. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bidzan L, Jurek P, Olech M, Bidzan-Wiącek
M, Bidzan-Bluma I and Bidzan M: Somatic comorbidity and the
progression of cognitive impairment. Front Aging Neurosci.
15(1219449)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hua JT, Hildreth KL and Pelak VS: Effects
of testosterone therapy on cognitive function in aging: A
systematic review. Cogn Behav Neurol. 29:122–138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
McEwen BS and Woolley CS: Estradiol and
progesterone regulate neuronal structure and synaptic connectivity
in adult as well as developing brain. Exp Gerontol. 29:431–436.
1994.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Amin Z, Canli T and Epperson CN: Effect of
estrogen-serotonin interactions on mood and cognition. Behav Cogn
Neurosci Rev. 4:43–58. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Frye CA: Effects and mechanisms of
progestogens and androgens in ictal activity. Epilepsia. 51 (Suppl
3):S135–S140. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yeo S, Holl K, Peñaherrera N, Wissinger U,
Anstee K and Wyn R: Burden of male hypogonadism and major
comorbidities, and the clinical, economic, and humanistic benefits
of testosterone therapy: A narrative review. Clinicoecon Outcomes
Res. 13:31–38. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hoffmann CM, Petrov ME and Lee RE: Aerobic
physical activity to improve memory and executive function in
sedentary adults without cognitive impairment: A systematic review
and meta-analysis. Prev Med Rep. 23(101496)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kleinloog JPD, Mensink RP, Ivanov D, Adam
JJ, Uludağ K and Joris PJ: Aerobic exercise training improves
cerebral blood flow and executive function: A randomized,
controlled cross-over trial in sedentary older men. Front Aging
Neurosci. 11(333)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lisco G, Giagulli VA, De Tullio A, De
Pergola G, Guastamacchia E and Triggiani V: Age-related male
hypogonadism and cognitive impairment in the elderly: Focus on the
effects of testosterone replacement therapy on cognition.
Geriatrics (Basel). 5(76)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Čeponis J, Swerdloff RS and Wang C:
Androgen replacement therapy in hypogonadal men. In: Male
Hypogonadism: Basic, Clinical and Therapeutic Principles. Winters S
and Huhtaniemi I (eds). Humana Press, Cham, pp367-397, 2017.
https://link.springer.com/book/10.1007/978-3-319-53298-1.
Accessed April 26, 2017.
|