1. Introduction
For numerous years, diagnostic tests have been the mainstay of research and monitoring the course of respiratory diseases. However, in the realm of diagnosing specific diseases, tests have often been either too insensitive, invasive, or lacking in specificity. This has led to the development of several exciting new diagnostic tools. Diagnostic tools continue to develop, and it is anticipated that in the next decade further advances in imaging techniques combined with developments in molecular biology translating into a more accurate and earlier diagnosis in the spectrum of respiratory diseases are expected (1,2). The advent of computed tomography (CT) almost three decades ago was the catalyst for the rapid progression in imaging technology that has led to the recent development of multi-slice scanners and magnetic resonance imaging. All of these advances have had a profound effect on the management of chest diseases. However, of a new era remains at the beginning with increasing precision in diagnosing diseases of the airways and lung parenchyma (3). The introduction of high-resolution CT (HRCT), positron emission tomography (PET), and single-photon emission CT (SPECT) has greatly expanded our ability to visualize and analyze the structure and function of the respiratory system. These imaging techniques provide detailed information about the lungs, airways, blood vessels and surrounding tissues (4,5). In addition, functional tests such as spirometry, diffusing capacity measurement, and exercise testing offer valuable insights into the physiological performance of the respiratory system (6,7).
Over the years, there have been significant advancements in the field of molecular biology, which have revolutionized the way of understanding and diagnosing respiratory diseases. The discovery of specific genes and biomarkers associated with various respiratory conditions has opened new possibilities for early detection and personalized treatment (8,9). Furthermore, advances in molecular imaging techniques, such as fluorescence imaging and molecular probes, have allowed for the visualization and tracking of disease processes at a cellular level (10).
Further innovations in diagnostic tools and imaging techniques for respiratory diseases are anticipated in the future. These may include the development of novel imaging agents, such as targeted nanoparticles or contrast agents, which can provide even more precise and specific information about the underlying pathology. Additionally, integrating artificial intelligence (AI) and machine learning algorithms into diagnostic systems holds great promise for improving accuracy and efficiency in the diagnosis and management of respiratory conditions (11).
By harnessing the power of advanced imaging and functional diagnostic tools, combined with the insights gained from molecular biology, a future where respiratory diseases are detected and treated at their earliest stages is possible, ultimately improving patient outcomes and quality of life. The present review discusses the current state and future of imaging and functional diagnostic tools for major respiratory diseases.
2. Imaging techniques
Magnetic resonance markers, despite their limited availability and higher cost, have led to increased use of magnetic resonance imaging (MRI) in lung disease assessment and pathology analysis (12). High-resolution imaging of pulmonary blood vessels plays a critical role in diagnosing pulmonary nodules, mediastinal masses and vascular disorders, including pulmonary hypertension. Magnetic resonance (MR) angiography serves as a valuable alternative imaging modality for suspected pulmonary embolism. Additionally, MRI enables the assessment of pulmonary perfusion, ventilation and lung mechanics, which are essential for evaluating disease severity and treatment response. Although early MRI techniques faced challenges due to the magnetic environment, recent advances in MR-compatible devices have expanded its utility, potentially offering a non-invasive alternative to lung biopsy (13).
Currently, chest X-rays are primarily employed to rule out other pathologies and establish baseline lung function. Radiographically, chest X-rays can reveal a variety of findings (14). Lung ultrasonography is beneficial for assessing lung consolidation, detecting pleural effusions, and aiding in pleural procedures. It is also useful for diagnosing pneumothorax (15). Sonoelastography, a novel technique, examines the structural elastic properties of tissues (16). HRCT is widely regarded as one of the most valuable tools in diagnosing respiratory diseases, often allowing radiological diagnosis and reducing the need for invasive tests or procedures. This can lead to more targeted and effective therapy for various lung diseases (17). Advances in multidetector technology have made CT scanning more accessible and affordable, resulting in increased use of CT pulmonary angiography for diagnosing pulmonary embolism (18).
Chest X-ray
Chest X-rays are the most frequently used imaging technique in respiratory practice due to their affordability, low radiation dosage, non-invasive nature, and ability to provide detailed structural information (19). They are particularly effective in examining the lungs to identify areas of increased or decreased density or abnormal masses. However, chest X-rays provide limited physiological information, such as malignancy of a mass (20). As most patients attending a chest clinic have a known abnormality, chest X-rays are now predominantly used for patient follow-up or to identify acute abnormalities, such as pleural fluid, air in the chest, failure of lung collapse, or acute heart failure. Chest X-rays remain the first-line investigation for suspected tuberculosis and can also aid in diagnosing pneumonia by identifying lung consolidation (21). Fluoroscopy, a variation of chest X-ray, provides functional information through continuous imaging in real-time, though its use has declined due to advancements in other imaging modalities (22).
CT
The primary limitation of CT is its use of ionizing radiation, particularly concerning for chest examinations given the radiosensitivity of the lungs. The cumulative radiation dose from serial CT scans to evaluate disease progression poses a concern, necessitating a risk-benefit analysis for the patient (23). The three-dimensional nature of CT datasets allows for endoscopic view simulation to detect lesions on the airway's luminal surface. However, studies indicate that virtual bronchoscopy's lesion detection rate is still inferior to fiberoptic bronchoscopy (24). CT, guided by multiple detectors and rapid computation, offers high-resolution imaging and is optimal for evaluating lung cavities, endobronchial lesions and vascular structures (25).
HRCT, using thin-section techniques, provides detailed analysis of lung parenchyma, offering a patient-friendly, cost-effective bedside assessment for critically ill patients. HRCT is essential for evaluating interstitial lung disorders (26). The relationship between HRCT and histopathology aids in determining lung parenchymal abnormalities, such as ground-glass opacities and reticular or nodular patterns. HRCT's ‘triple rule out’ capability enables simultaneous evaluation of acute aortic syndrome, pulmonary embolism and aortic dissection. CT-guided transbronchial aspiration needle (TBNA) and transbronchial needle biopsy (TTNB) offer higher diagnostic yield and are safer for patients with lung masses or lesions, minimizing pneumothorax risk (27).
Magnetic resonance imaging (MRI)
MRI is increasingly utilized for chest disease evaluation due to its ability to characterize tissues without exposing patients to ionizing radiation. Recent technological advances have significantly improved image quality. However, MRI remains underutilized for lung disease assessment, particularly for interstitial lung diseases and lung cancer (28). This underutilization is partly due to the established tradition of chest radiology and the relative lack of MRI capabilities for lung pathology assessment. High-resolution MRI provides detailed anatomical information about the thoracic viscera, chest wall and diaphragm. Advances in gradient echo and fast spin-echo techniques have enhanced lung image quality, aiding in the assessment of both focal and diffuse lung diseases (29).
Dynamic imaging techniques can evaluate diaphragm and chest wall movement, invaluable for assessing thoracic abnormalities in children and young adults. Flow void techniques reliably assess mediastinal and chest wall vascular anomalies. Current MR techniques lack sensitivity for detecting sclerotic and fibrotic changes in some interstitial lung diseases, but flexibility in image acquisition allows for rapid adoption of new techniques, increasing MRI's role in lung disease assessment (30).
Positron emission tomography (PET)
PET holds substantial potential to significantly impact patient care in respiratory medicine. Initially flourishing as an oncology diagnostic technique, PET's role in lung cancer management is now well established. As a cross-sectional imaging technique, PET produces tomographic images of tracer distribution in the body, using various labeled compounds to characterize disease processes at a molecular level (31). The PET scanner detects gamma rays emitted from positron decay produced by radiotracers, typically bound to biologically active molecules. PET excels in detecting functional and metabolic changes in diseases before anatomical changes are apparent, making it particularly effective in early lung cancer detection, distinguishing benign from malignant lesions, and staging and radiotherapeutic planning for patients with non-small cell lung carcinoma (NSCLC) (32).
PET demonstrates high sensitivity and specificity in diagnosing NSCLC, outperforming CT in some cases, potentially reducing unnecessary operations for benign disease and detecting secondary tumors and distant metastases missed by conventional staging techniques (33). However, PET's cost-effectiveness remains debated, with studies such as the pulmonary nodule project indicating lower diagnostic costs per patient but randomized controlled trials suggesting higher costs with only moderate clinical outcome improvements (34,35). The high false-positive rate in lymph node assessment primarily drives these costs. As PET technology advances and newer tracers are developed, its cost-effectiveness and role in NSCLC management may become more defined (36).
3. Pulmonary function tests
Spirometry is the most frequently performed lung function test, utilizing a spirometer to measure the volume of air inhaled and exhaled, as well as the airflow velocity. The test records the exhaled air volume over time, producing a spirogram that traces the inhaled and exhaled air volumes. These results are instrumental in assessing lung function, diagnosing the causes of shortness of breath, evaluating surgical readiness, and identifying restrictive lung diseases. Spirometry is also valuable for monitoring lung disease development due to exposure to occupational hazards including dust, gases, or fumes. Certain conditions, such as recent heart attacks, collapsed lungs, hernias and recent eye surgeries, can affect spirometry results, as can high air pollution levels and large meals before the test (37). The advancements in respiratory imaging modalities are summarized in Fig. 1.
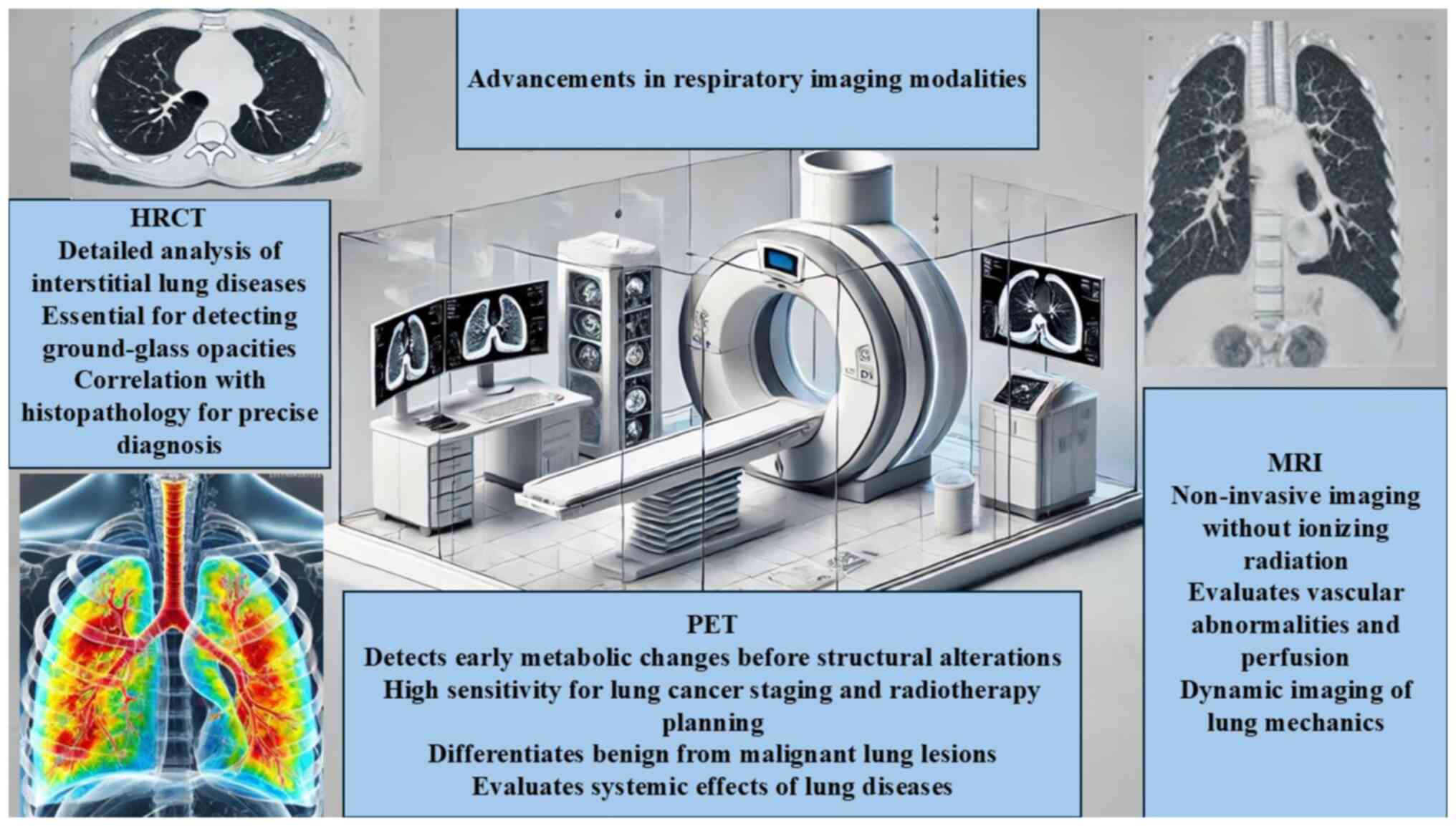 |
Figure 1
Advancements in respiratory imaging modalities. HRCT, high-resolution computed tomography; MRI, magnetic imaging resonance; PET, positron emission tomography.
|
Lung volume measurements, including body plethysmography, nitrogen washout, and helium dilution tests, further assess lung function. Static lung volume tests (body plethysmography) measure the lung air volume after deep inhalation and exhalation. Dynamic lung volume tests measure the speed of air intake and output, crucial for diagnosing restrictive and chronic obstructive pulmonary disease (COPD). These tests determine the air volume a patient can inhale and exhale and the residual lung air volume, comparing these measurements with expected volumes for healthy individuals of similar age, height and sex to determine disease severity (38).
Spirometry
In patients with obstructive lung diseases such as asthma or COPD, spirometry typically reveals a reduced forced expiratory volume at 1 second (FEV1) and an FEV1/forced vital capacity (FVC) ratio of <0.7, indicating airflow limitation. A significant improvement in FEV1 following bronchodilator administration suggests reversible airflow obstruction. Conversely, restrictive disorders result in a reduced FVC with a relatively normal FEV1/FVC ratio due to maintained airway function, allowing for general differential diagnosis (38).
The Global Lung Initiative recently published reference values for healthy non-smoking adults aged 20 to 79 years, considering height, weight, age, and occasionally ethnicity. Predicted values are given as z-scores, with a range of -1.64 to +1.64 considered normal, encompassing 90% of the reference population (39). Spirometry is the most performed lung function test, involving maximal inspiration followed by forced expiration from total lung capacity. Key parameters measured include FVC, FEV1 and the FEV1/FVC ratio (40).
Lung volume measurements
Alternative methods, such as helium or helium-oxygen mixtures, measure gas dilution to infer dynamic lung volumes from timed measurements. This method is applicable for forced vital capacity (FVC) and forced expiratory volume in a specific period (FEV), allowing for direct determination of lung gas concentrations and partial pressures. These measurements indirectly assess hemoglobin concentrations and oxygen transport (38).
Spirometry and lung volume measurements are fundamental in clinical assessments, providing critical information on ventilatory impairment. Lung volumes can be measured using body plethysmography or gas dilution techniques. The body plethysmograph method, based on Boyle's law (P1V1=P2V2), involves the subject sitting in a sealed box and breathing through a mouthpiece or facemask. Static lung volumes are inferred from pressure and volume changes at the mouth during panting maneuvers to determine functional residual capacity (FRC) or during expiratory and inspiratory valve closures to measure thoracic gas volume (VTG). The accuracy of this method depends on the box's tightness and the subject's cooperation (38).
Lung volume measurements provide an integrated assessment of lung air volume and ventilation distribution, subdivided into tidal volume (TV), inspiratory reserve volume (IRV), expiratory reserve volume (ERV), and residual volume (RV) (38).
Diffusion capacity testing
Diffusion capacity testing measures gas transfer from alveoli to erythrocytes, typically using carbon monoxide (CO) due to its high hemoglobin affinity. The test involves a small gas quantity over ~10 seconds, during which the subject breathes normally, inhales the test gas, holds their breath for 10 seconds, and then exhales the residual gas. CO concentration is measured at the test's beginning and end to calculate the absorbed gas volume, usually ~7 ml, with absorption quantities typically between 0.3-0.8 ml. The subject repeats the test 4-7 times for consistent results (41).
The test can indicate restrictions or limitations in gas transfer in refractory or inflammatory lung diseases. CO or smoke inhalation can damage lung architecture, increasing transfer rates and absorbed gas quantities, potentially fatally. However, the test has limitations and errors due to lung complexity. The test gas can be toxic, particularly for anemic subjects, as CO bonds with hemoglobin more readily than oxygen. Hemoglobin concentration is not constant, especially in heavy smokers or those with chronic hypoxic lung disease.
The test measures both diffusion and capillarization, making differentiation impossible. Breath-holding volume may vary due to respiratory muscle disorders or cardiac conditions. The effort-dependent nature of the test means restricted, or anxious subjects may not fully cooperate (41).
4. Bronchoscopy and endobronchial ultrasound (EBUS)
EBUS operates on principles akin to conventional sonography, wherein an ultrasound endoscope is inserted into the bronchoscope's working channel. This proximity enhances the imaging quality compared with conventional CT-guided fine needle aspiration (FNA). The ultrasound transducer, located at the endoscope's tip, generates images displayed on the EBUS processor, which can be recorded as still images or videos for documentation (42,43).
EBUS is particularly effective for imaging structures near the airways, such as lymph nodes, due to the attenuation of ultrasound beam intensity through these structures (44). Bronchoscopy remains the ‘gold standard’ for evaluating the respiratory tract from the trachea to the segmental bronchi. The advent of EBUS technology has enabled imaging and real-time FNA of hilar and mediastinal structures. EBUS is utilized for sampling mediastinal lymph nodes and lung masses, staging patients with lung cancer, re-staging previously treated intrathoracic malignancies, and diagnosing unexplained mediastinal or pulmonary pathology (45).
5. Sleep studies
Comprehensive overnight polysomnography is primarily indicated for patients with suspected sleep-disordered breathing, nocturnal hypoxemia, hypersomnia, parasomnia, or unexplained nocturnal sudden death. This test monitors and scores various physiological variables throughout the night, correlating them with sleep stages and arousals. Despite broad confidence intervals due to high night-to-night variability, polysomnography has significantly contributed to the understanding of the pathophysiology and clinical impact of numerous sleep-related respiratory and neurocognitive disorders (46).
Originating from electroencephalogram sleep studies in the 1950s and 60s, polysomnography faced technical challenges due to the numerous electrodes required. Advances in technology during the 70 and 80s led to the development of portable recorders and simplified systems for studying respiratory variables, highlighting the high prevalence of restrictive lung diseases and neuromuscular disorders, which can exacerbate sleep-related breathing disorders and hypoxemia (46).
Consequently, sleep studies have become a distinct discipline, linking respiratory abnormalities during sleep to various health outcomes and quality of life indicators. The increasing use of sleep studies for evaluating respiratory symptoms is evident, with substantial data indicating that a significant percentage of patients with isolated respiratory complaints may have sleep-disordered breathing or gas exchange abnormalities during sleep. Therefore, it is crucial for internists interested in respiratory diseases to understand these tests and their indications to select appropriate patients for study (47).
Polysomnography
Interest in sleep-disordered breathing has surged in recent years, driven by expanding knowledge of its previously underdiagnosed and underappreciated consequences. The morbidity and mortality associated with sleep-disordered breathing concern both physicians and the public. Over the past decade, substantial advancements have been achieved in sleep medicine and sleep-disordered breathing, leading to the development of new diagnostic technologies for evaluating the severity and consequences of these disorders and guiding appropriate therapy.
While home oximetry is a useful tool, it cannot identify specific sleep stages during desaturation events or detect sleep-related ventilation variations, making sleep studies the preferred diagnostic modality (46,47). Polysomnography remains the gold standard for diagnosing sleep-disordered breathing, involving the measurement of multiple physiological variables during sleep, typically conducted in a sleep laboratory. The recorded data helps classify abnormal respiratory events and determine their severity (47).
Previously, concerns over healthcare costs and increasing demands on hospital resources have prompted the development of limited channel sleep studies, which may play a larger role in diagnosing sleep-disordered breathing in the future (48).
Multiple sleep latency test (MSLT)
The MSLT is the standard measure of sleepiness, extensively used to diagnose conditions such as narcolepsy and idiopathic hypersomnia (49). The test involves a series of nap opportunities at 2-h intervals throughout the day, with each nap trial lasting 20 min (50). MSLT is based on the principle that the sleepier individuals are, the quicker they will fall asleep, while less sleepy individuals will take longer (51). The test also provides valuable information on rapid eye movement (REM) latency. If REM sleep is not observed during the MSLT trials, data should be collected from at least four nap opportunities to ensure accuracy.
Typically, MSLT is conducted following nocturnal polysomnography (PSG), which establishes a baseline for the patient's sleep quality (52). The MSLT consists of four to five naps, each lasting 20 min, with the first nap occurring 1.5 to 3 h after awakening from the nocturnal PSG. This test provides crucial information on sleep onset and REM periods within 8-24 h following PSG (52).
6. Molecular testing
PCR is a cornerstone molecular diagnostic tool that amplifies a few copies of nucleotides to detectable levels, thereby producing more nucleic blueprint than initially present in the sample (53). An advancement of this technology, real-time PCR assays, can simultaneously detect and quantify genes, making them widely utilized in both research and clinical practice (54). PCR tests are essential in clinical settings, such as confirming Pneumocystis pneumonia diagnoses, significantly influencing prophylactic and treatment strategies for vulnerable patients. This test is especially valuable for diagnosing severe and disseminated diseases in non-human immunodeficiency virus immunocompromised patients, where clinical and radiological presentations may not be specific (55).
Molecular diagnostics have become integral to managing patients with suspected respiratory conditions. Nucleic acid-based tests, sequencing technologies, and gene expression assays have revolutionized the identification of lower respiratory tract infections, aiding in defining the etiology of infections and airway diseases (56). These tests primarily focus on detecting nucleic acids derived from pathogens, though gene expression tests can also detect RNA transcribed from host or pathogen genes. For example, cytokeratins, markers of squamous epithelial cells, have been identified in asthmatic patients with increased cell shedding (57).
PCR
There are modified PCR techniques, such as nested or semi-nested PCR and touch-down PCR. The success of PCR has spurred the development of other molecular analysis methods, including DNA microarrays. However, PCR's high sensitivity often leads to contamination, amplifying unintended DNA samples (58). To mitigate this, various techniques are employed, such as using PCR cabinets, aerosol-resistant pipette tips, and UV light sterilization of PCR tubes (59).
There are several PCR techniques, including reverse transcription-PCR, which amplifies RNA to produce proteins (60), and real-time PCR, which quantifies DNA produced by PCR. Other techniques include immuno-PCR, hot start PCR and multiplex PCR, among others. PCR amplifies DNA primers rapidly in a sample, producing millions or billions of DNA strands. Typically, PCR is used to amplify a specific region or gene from a small DNA amount, enabling cloning, sequencing, or analysis (61).
Next-generation sequencing (NGS)
NGS data analyses have enhanced the understanding of genetic mutations in various diseases, ultimately leading to tailored therapies for different respiratory diseases (62). For instance, knowledge of numerous genetic mutations affecting the cystic fibrosis (CF) transmembrane conductance regulator gene in CF has led to mutation-specific medications that can alter the disease course (62). NGS has also identified shared genetic susceptibilities between different diseases. In respiratory disease, genetic variations in the immune system have been identified in both lung cancer and COPD, potentially allowing lung cancer medications to treat COPD if shared genetic mutations affect the phenotypes of both diseases (63).
NGS advances the analysis of microRNAs by enabling the study of the microRNAome rather than individual microRNAs. MicroRNAs play key roles in post-transcriptional gene regulation and numerous diseases (64). Identifying microRNA markers of disease in the blood suggests future blood-based tests for diagnosing respiratory diseases (65).
Gene expression profiling
In COPD, gene expression profiling in lung tissue and bronchial epithelial cells has identified several genes whose expression correlates with lung function and other disease severity measures. Identifying distinct molecular phenotypes within broader disease categories raises the possibility of more targeted treatments. However, translating these discoveries into improved respiratory health outcomes requires further study to identify druggable gene products and develop tests applicable in routine clinical practice (66).
Gene expression profiling, which measures the activity of thousands of genes simultaneously, has successfully distinguished diseases and identified new disease subtypes. For example, microarray analysis of bronchial biopsies revealed differing gene expression patterns in mild, moderate and severe asthma. Previously, specific gene expression signatures in peripheral blood cells have distinguished eosinophilic and non-eosinophilic asthma and Th2 high and Th2 low asthma, important as these phenotypes are likely to respond differently to new targeted therapies for severe asthma (67).
Genomic investigations of respiratory diseases have provided new insights into disease pathogenesis. Although much of this molecular information has not yet translated into improved patient outcomes, the volume of data produced offers hope that future genomic applications will significantly advance therapies (68).
7. Biomarkers in respiratory medicine
One biomarker meeting these criteria is the fraction of exhaled nitric oxide (FeNO), extensively studied in asthma. Nitric oxide (NO) impacts airway inflammation, sensory nerve activation and mucus production. It is easily detectable in breath using a simple, non-invasive method. FeNO correlates with sputum eosinophils and is elevated in steroid-responsive asthma, making it an ideal marker for guiding treatment decisions (69). Due to its simplicity and reliability, FeNO analysis has become widespread, with portable analyzers available for both primary care and hospital settings. This method exemplifies potential future directions for biomarker research in respiratory medicine.
Exhaled breath contains volatile organic compounds (VOCs), by-products of metabolic processes that may be altered by disease. Certain VOCs can change acidity or alkalinity in the presence of specific enzymes, relating to airway inflammation and damage. Aldehydes and malondialdehyde, markers of oxidative stress, are found in increased amounts in both asthma and COPD. Advances in analytical techniques have expanded the potential applications for VOC markers. These compounds, present in parts per billion (ppb), need to be easily detectable, specific to the condition, and unaffected by diet or environmental factors to serve as ideal markers (70).
Biomarker research has surged, recognizing that clinical evaluations can be invasive and expensive. Blood, sputum and exhaled breath markers are non-invasive, easy to obtain, and provide valuable information from genetic profiles to individual health status. Despite advances, numerous blood and sputum markers have not yet been fully integrated into respiratory medicine, with some exceptions. B-type natriuretic peptide, a blood peptide, guides heart failure diagnosis and treatment and identifies cor pulmonale, also indicating mortality in acute COPD exacerbations (71). Eosinophilic cationic protein and specific eosinophil markers differentiate between asthma and COPD in both blood and sputum. The most progress in respiratory biomarker research has been made in exhaled breath condensate analysis, significantly advancing over the past decade.
Blood biomarkers
High-throughput analytics have sparked interest in identifying clinical phenotype markers, particularly in COPD. Blood, easily obtained and analyzed, offers a snapshot of systemic processes. Blood-based biomarkers provide insights into disease pathogenesis. Elevated levels of inflammatory markers such as fibrinogen, C-reactive protein (CRP), leukocytes and tumor necrosis factor-alpha are linked to increased COPD incidence, clinical phenotypes and accelerated lung function decline (72).
Recent studies focus on markers specific to COPD and its phenotypes, such as genetic variants and their expression products, and molecules associated with disease pathways, including matrix metalloproteinases linked to emphysema. The ECLIPSE study aims to identify biomarkers predicting disease progression and defining clinically meaningful COPD phenotypes. Findings have highlighted blood-based biomarkers that differentiate COPD from normal aging and its phenotypes, such as chronic bronchitis and frequent exacerbations, including white cell count, leukocyte subtypes, plasma fibrinogen, and cytokines such as interleukin-6. These markers may identify active disease states and progression, leading to tailored therapeutic approaches (73). Future research could develop blood-based tests for identifying these biomarkers, aiding COPD management.
Sputum biomarkers
The analysis of breath and induced sputum has become a valuable, non-invasive method for sampling the lower respiratory tract (74,75). Induced sputum is attractive because it can be repeated without radiation exposure. Although sputum expectoration is simple and safe, some patients struggle to produce sufficient samples (76). The success of induction varies with agents and patient groups. Induced samples can differ from spontaneous ones, and repeatability over short periods is not established (77).
Sputum, a mix of bronchial and salivary secretions, can be contaminated by upper respiratory secretions or pollutants. Measuring sputum cell counts and differentials can assess this complexity, but the cellular response induction may confound inflammation markers (74). Sputum color subjectively indicates bacterial infection, and increased sputum purulence predicts chronic bronchitis exacerbations (78). Increased airway wall permeability allows plasma proteins to leak into the airways, but protein measurements in induced sputum have been inconsistent (79).
Exhaled breath biomarkers
NO, associated with inflammation, is produced from L-arginine via NO synthase (80). NO can be measured in exhaled breath due to its high blood solubility, diffusing from bronchial circulation into the airways and being released (81). Elevated NO levels are found in asthma and other inflammatory conditions (82). NO measurement is non-irritating, quick, and reproducible (83), using a NO analyzer to analyze expired air, with results given immediately in ppb (84).
Exhaled breath collection is non-invasive, suitable for all ages, and useful for daily patient monitoring (85). Higher levels of certain hydrocarbons have been identified in breath of patients with lung cancer compared with healthy individuals (86). Research has continued to analyze hydrocarbons and VOCs in the breath of patients with lung cancer and other respiratory diseases (87).
8. Telemedicine and remote monitoring
Remote monitoring is integral to telemedicine and essential for managing chronic diseases. Respiratory diseases, including COPD and asthma, impose a substantial burden on healthcare systems, significantly straining hospital resources (88,89). These diseases are characterized by acute exacerbations, often leading to unscheduled physician visits and inpatient care, especially during winter months (90,91). Evidence suggests that home telemonitoring can reduce hospital admissions for chronic respiratory diseases (92). Monitoring ranges from simple pulse oximetry systems, which transmit oxygen levels to a hospital physician during exacerbations, to complex health informatics systems (93,94). Providing preventative information on exacerbation prediction could allow for early pharmacological intervention, helping patients avoid acute deteriorations (95).
Telemedicine involves using electronic communication to provide remote healthcare, including medical conferences, consultations and knowledge exchange (96). This is facilitated through various media devices such as telephones, the internet, and home healthcare systems (97). The concept began in 1925 with radio technology, evolving over 90 years into today's sophisticated telemedicine platform (98). Over the last decade, telemedicine has gained feasibility and public acceptance (99). By 2007, 20 million Americans had received remote medical consultations, a figure expected to rise with increasing demand for remote care (100,101).
As technology integrates more into daily life, remote healthcare becomes more accessible, with smartphones, tablets, and other handheld devices enabling easy access to medical advice through internet connections (102).
9. AI in respiratory medicine
Research and innovation in AI are leading to the development of predictive models for various aspects of respiratory medicine, improving patient outcomes in numerous conditions (103,104). In 2014, Dutch researchers developed and validated a prediction model for COPD exacerbations using a support vector machine, demonstrating the utility of machine learning in enhancing respiratory healthcare (89,105). Another machine learning-based prediction model for lung cancer incidence in Ontario showed favorable accuracy in predicting lung cancer rates (106). Machine learning algorithms learn from data to make predictions or classifications, optimizing the internal model for accurate outcomes (107). The trained model is then applied to new, unseen data to make predictions (108).
10. Point-of-care testing (POCT)
Respiratory diseases vary greatly among individuals, often consuming significant healthcare resources due to their unpredictability. POCT has the potential to improve patient care in this context. A study by Bramley et al (109) demonstrated that POCT for CRP testing, compared with inpatient testing, reduced resource use, increased diagnostic clarity, and enhanced nurse confidence in treatment appropriateness. Another report by Daniels et al (110) indicated that POCT could differentiate bacterial from viral etiology in COPD exacerbations, reducing antibiotic exposure and associated costs through semi-quantitative Procalcitonin testing.
POCT's role in the early diagnosis and management of respiratory exacerbations, along with technical developments for direct patient diagnostic testing, is increasingly being explored. POCT can provide rapid, informed therapeutic decisions, such as detecting infections or airway inflammation. The potential for monitoring conditions such as decompensated respiratory failure with POCT tools also holds promise for palliative and end-of-life care. Implementing new technologies in healthcare must be cost-effective efficient, and economic modeling and modern healthcare structures are essential in evaluating POCT's future utilization for respiratory diseases (111,112).
11. Non-invasive ventilation (NIV)
NIV is crucial in managing ventilatory failure due to various acute and chronic conditions, including COPD exacerbations, acute-on-chronic heart failure, and chronic restrictive lung disorders and neuromuscular conditions (113). Traditionally, NIV is delivered using intermittent positive pressure ventilation via a nasal or oronasal interface. While effective in normalizing nocturnal hypoventilation in patients with restrictive lung disorders or scoliosis, its efficacy in acute-on-chronic type 2 respiratory failure in COPD is limited by patient tolerance and the high likelihood of requiring endotracheal intubation (114). Previous interest has focused on applying alternative pressure support modes in NIV, assessed using both standard and innovative monitoring techniques (115). These advancements aim to improve patient comfort and tolerance, reduce the need for invasive mechanical ventilation, and enhance the overall management of respiratory failure.
12. Lung biopsy techniques
The success of lung and heart-lung transplantation has driven the development of lung biopsy techniques for immunosuppressed patients with diffuse lung disease, who face a high risk of pneumothorax with traditional transbronchial biopsy. Consequently, various forms of open lung biopsy under local anesthesia have been developed to obtain larger lung tissue samples for comprehensive diagnosis (116,117).
Techniques such as video-assisted thoracoscopy and transbronchial biopsy using fluoroscopic or CT guidance, offer minimally invasive approaches, reducing complications and hospital stays (118). Fluoroscopy-guided bronchoscopic biopsy of solitary peripheral nodules yields ~70% diagnostic accuracy, though only 50% of small nodules can be localized by fluoroscopy, with higher yields in lesions close to the hilum (119,120).
Novel bronchoscopic techniques, such as transbronchial histology needle biopsies from a wedged position or peripheral lung marking with dye, have been developed to improve small peripheral nodule biopsy but face limitations due to nodule size and nature, often leading to pneumothorax (121,122). Alternative percutaneous methods, including fine needle aspiration and cutting needle biopsy under fluoroscopic and CT guidance, offer higher diagnostic yields up to 90%, though with higher complication rates compared with traditional transbronchial biopsy (91,123).
13. Lung cancer screening
A promising trial integrates serum biomarkers with CT scanning to determine if detected nodules are malignant (123). High false-positive rates often lead to more invasive procedures and surgeries, especially in individuals with a history of lung disease. These individuals face higher complication risks from unnecessary surgical interventions, increasing screening costs. Current research aims to enhance the efficacy and cost-effectiveness of this screening method (124).
CT screening aims to detect early-stage lung cancer in high-risk individuals but is unsuitable for diagnosing lung cancer in non-high-risk populations with symptoms such as hemoptysis or weight loss (125). CT scans effectively detect small pulmonary nodules, often precursors to lung cancer in high-risk individuals (124). Low-dose CT scanning has shown promise in lung cancer screening, with the National Lung Cancer Screening Trial in the U.S. indicating improved patient survival. This technique is under consideration for national screening programs in numerous countries (124,126).
14. Pulmonary rehabilitation
Pulmonary rehabilitation is crucial in managing chronic respiratory diseases, such as asthma, COPD, chronic bronchitis and emphysema (127). These diseases often lead to gradual deterioration in respiratory function and quality of life (128). Patients may become increasingly dependent due to lifestyle changes, breathlessness and reduced physical activity (129). Dependency often results in social isolation, exacerbating the problem. Pulmonary rehabilitation combines education, exercise, nutritional and psychological support to break this cycle, improving quality of life, exercise tolerance, and reducing breathlessness (130).
Measuring the effects of pulmonary rehabilitation on health is vital. Increased exercise tolerance and quality of life are positive outcomes, often assessed using pulmonary function and exercise tests. However, defining pulmonary rehabilitation's exact impact on various respiratory diseases is challenging (131). The complexity of patients and multidimensional health status changes require patient-centered outcomes reflecting mental and physical dimensions (128).
Health status represents the effects of a medical condition and its treatment as perceived by the patient, encompassing physical, psychological and social function (131). High health status is associated with decreased morbidity and mortality and improved quality of life (128). Health status questionnaires, simple yet comprehensive tools, effectively detect and quantify changes in health status over time, providing patient-centered outcomes reflecting the patient's health status (130).
15. Future perspectives and challenges
A prominent example of future advancements in pulmonary diagnostics is the evaluation and monitoring of pulmonary hypertension through the assessment of right ventricular function and hemodynamics using echocardiography. This method aims to replace the need for repetitive invasive measurements and right heart catheterization. The trend towards more quantitative and objective diagnostic techniques is expected to continue, with the ultimate goal of early disease detection and prevention, thereby reducing the overall burden of lung diseases on both individuals and the population as a whole.
The increasing demand for personalized healthcare, which involves tailored treatments for individuals, will undoubtedly influence diagnostic strategies aimed at differentiating specific disease phenotypes and endotypes. Future diagnostic tools may require less reliance on bronchoscopy or biopsy, as diseases will be characterized in greater detail through less invasive methods.
One of the recent advancements in genetic-based diagnostics is the measurement of telomere length in peripheral blood leukocytes. Telomere shortness has been associated with severe COPD and an accelerated decline in lung function. In the future, specific genetic mutations known to cause diseases are likely to be detected reliably, with potential screening for targeted genetic diseases using in vivo animal models.
The future of diagnostic tools in pulmonary medicine appears promising, with numerous advances achieved over the past decade. This progress is largely attributed to breakthroughs within the biomedical sciences. Enhanced knowledge of the human genome has led to an improved understanding of the genetic basis and mechanisms behind numerous lung diseases. As these diseases become better understood, the development of diagnostic tools to detect them will become simpler and more effective. The advancements in diagnostic tools in respiratory medicine are summarized in Table I.
 |
Table I
Summary of advancements in diagnostic tools in respiratory medicine.
|
Table I
Summary of advancements in diagnostic tools in respiratory medicine.
| Category |
Advancement |
Impact |
| Imaging techniques |
High-resolution CT, MRI, PET, SPECT |
Enhanced structural and functional assessment |
| Pulmonary function tests |
Advanced spirometry, body plethysmography |
More precise lung function analysis |
| Bronchoscopy and EBUS |
EBUS |
Real-time imaging and guided biopsy |
| Sleep studies |
Polysomnography, multiple sleep latency test |
Improved diagnosis of sleep-disordered breathing |
| Molecular testing |
PCR, NGS |
Rapid and accurate pathogen/genetic identification |
| Biomarkers |
FeNO, VOCs, blood/sputum biomarkers |
Non-invasive disease monitoring and early detection |
| Telemedicine and remote monitoring |
Home-based oximetry, wearable devices |
Reduced hospital admissions, improved disease management |
| AI |
Machine learning in diagnostics |
Enhanced predictive capabilities and automation |
| POCT |
CRP testing, procalcitonin analysis |
Faster, on-site decision-making |
| Non-invasive ventilation |
Advanced NIV devices |
Improved patient outcomes in ventilatory failure |
| Lung biopsy Techniques |
CT-guided and video-assisted techniques |
Less invasive, higher diagnostic yield |
| Lung cancer screening |
Low-dose CT scans, biomarker integration |
Earlier detection and reduced false positives |
| Pulmonary rehabilitation |
Tailored exercise programs |
Enhanced quality of life and function |
16. Limitations
While the present review provides a comprehensive overview of advancements in diagnostic tools in respiratory medicine, several limitations must be acknowledged. First, the field is rapidly evolving, with newer diagnostic modalities still in clinical trials. For instance, laryngeal ultrasonography, a promising non-invasive imaging technique, has shown potential in evaluating laryngeal pathology, predicting difficult intubation, and guiding percutaneous tracheostomy procedures. Despite its advantages, its clinical application remains limited due to the lack of standardized protocols and widespread expertise. As highlighted by Cergan et al (132), ultrasonography of the larynx has gained renewed attention during the SARS-CoV-2 pandemic, offering an alternative to aerosol-generating procedures such as laryngoscopy. However, further validation through large-scale clinical trials is necessary to establish its role in routine respiratory diagnostics.
Additionally, some of the emerging AI-driven diagnostic tools discussed in the present review still require extensive real-world validation before widespread adoption. Their clinical utility depends on robust datasets, ethical considerations regarding patient data, and seamless integration into existing healthcare infrastructures.
Finally, the current review is a narrative synthesis rather than a systematic review, meaning there is a potential for selection bias in the included literature. Future systematic reviews and meta-analyses should aim to quantify the diagnostic accuracy and clinical benefits of emerging technologies.
17. Conclusions
The present review detailed the various innovative diagnostic tools that are currently available and those that are the subject of ongoing research. These tools range from novel imaging modalities, such as confocal laser endomicroscopy and electrical impedance tomography, to the use of biomarkers in exhaled breath condensate and recently developed or improved functional tests, such as cardiopulmonary exercise testing and field walking tests. The diagnostic tools were discussed in the context of the wide array of respiratory conditions, from the more common conditions such as asthma and COPD, to the less prevalent conditions such as bronchiolitis obliterans.
In the last few years, there has been an increasing trend in the global prevalence of respiratory diseases, and this has led to an increasing need for accurate, sensitive and non-invasive diagnostic tools. This is important to detect the conditions in the early stages and to monitor disease progression and response to treatment. The invasive nature and relative insensitivity of the current ‘gold standard’ diagnostic tests for numerous respiratory conditions, such as lung biopsy for interstitial lung diseases and sputum microbiology for respiratory infections, mean that they are often impractical or of limited use. Diagnostic tools are also required that can identify the condition in a cost-effective manner in low-resource areas for diseases such as tuberculosis and childhood pneumonia.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AC and VEG conceptualized the study. AC, VEG and DAS made a substantial contribution to data interpretation and analysis and wrote and prepared the draft of the manuscript. AC and VEG analyzed the data and provided critical revisions. All authors contributed to manuscript revision, read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, artificial intelligence tool Chat GPT was used to improve the readability and language of the manuscript or to generate images, and subsequently, the authors revised and edited the content produced by the artificial intelligence tool as necessary, taking full responsibility for the ultimate content of the present manuscript.
References
|
1
|
Pircalabioru GG, Iliescu FS, Mihaescu G, Cucu AI, Ionescu ON, Popescu M, Simion M, Burlibasa L, Tica M, Chifiriuc MC and Iliescu C: Advances in the rapid diagnostic of viral respiratory tract infections. Front Cell Infect Microbiol. 12(807253)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vitazkova D, Foltan E, Kosnacova H, Micjan M, Donoval M, Kuzma A, Kopani M and Vavrinsky E: Advances in respiratory monitoring: A comprehensive review of wearable and remote technologies. Biosensors (Basel). 14(90)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hsieh J and Flohr T: Computed tomography recent history and future perspectives. J Med Imaging (Bellingham). 8(052109)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Müller NL: Computed tomography and magnetic resonance imaging: Past, present and future. Eur Respir J Suppl. 35:3s–12s. 2002.PubMed/NCBI
|
|
5
|
Beyer T, Freudenberg LS, Townsend DW and Czernin J: The future of hybrid imaging-part 1: Hybrid imaging technologies and SPECT/CT. Insights Imaging. 2:161–169. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ruppel GL and Enright PL: Pulmonary function testing. Respir Care. 57:165–175. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Georgakopoulou VE, Tarantinos K, Papalexis P, Spandidos DA, Damaskos C, Gkoufa A, Chlapoutakis S, Sklapani P, Trakas N and Mermigkis D: Role of pulmonary function testing in inflammatory bowel diseases (Review). Med Int (Lond). 2(25)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Obeidat M and Hall IP: Genetics of complex respiratory diseases: Implications for pathophysiology and pharmacology studies. Br J Pharmacol. 163:96–105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Georgakopoulou VE, Lempesis IG, Sklapani P, Trakas N and Spandidos DA: Precision medicine for respiratory diseases: A current viewpoint. Med Int (Lond). 4(31)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL and Remy J: Fleischner society: Glossary of terms for thoracic imaging. Radiology. 246:697–722. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, et al: International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 38:577–591. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sigrist RMS, Liau J, Kaffas AE, Chammas MC and Willmann JK: Ultrasound elastography: Review of techniques and clinical applications. Theranostics. 7:1303–1329. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Austin JH, Müller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, Webb WR and Zerhouni EA: Glossary of terms for CT of the lungs: Recommendations of the Nomenclature Committee of the Fleischner society. Radiology. 200:327–331. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brenner DJ and Hall EJ: Computed tomography-an increasing source of radiation exposure. N Engl J Med. 357:2277–2284. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mettler FA Jr, Huda W, Yoshizumi TT and Mahesh M: Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology. 248:254–263. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stapley S, Sharp D and Hamilton W: Negative chest X-rays in primary care patients with lung cancer. Br J Gen Pract. 56:570–573. 2006.PubMed/NCBI
|
|
17
|
Pysz MA, Gambhir SS and Willmann JK: Molecular imaging: Current status and emerging strategies. Clin Radiol. 65:500–516. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Krishnan G, Singh S, Pathania M, Gosavi S, Abhishek S, Parchani A and Dhar M: Artificial intelligence in clinical medicine: Catalyzing a sustainable global healthcare paradigm. Front Artif Intell. 6(1227091)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Campbell-Washburn AE: 2019 American Thoracic Society BEAR cage winning proposal: Lung imaging using high-performance low-field magnetic resonance imaging. Am J Respir Crit Care Med. 201:1333–1336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsuchiya N, van Beek EJ, Ohno Y, Hatabu H, Kauczor HU, Swift A, Vogel-Claussen J, Biederer J, Wild J, Wielpütz MO and Schiebler ML: Magnetic resonance angiography for the primary diagnosis of pulmonary embolism: A review from the international workshop for pulmonary functional imaging. World J Radiol. 10:52–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rea G, Sperandeo M, Lieto R, Bocchino M, Quarato CMI, Feragalli B, Valente T, Scioscia G, Giuffreda E, Barbaro MP and Lacedonia D: Chest imaging in the diagnosis and management of pulmonary tuberculosis: The complementary role of thoracic ultrasound. Front Med (Lausanne). 8(753821)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK and Smith JP: Early lung cancer action project: Overall design and findings from baseline screening. Lancet. 354:99–105. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Power SP, Moloney F, Twomey M, James K, O'Connor OJ and Maher MM: Computed tomography and patient risk: Facts, perceptions and uncertainties. World J Radiol. 8:902–915. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Calloway HE, Kimbell JS, Davis SD, Retsch-Bogart GZ, Pitkin EA, Abode K, Superfine R and Zdanski CJ: Comparison of endoscopic versus 3D CT derived airway measurements. Laryngoscope. 123:2136–2141. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ghaye B, Ghuysen A, Bruyere PJ, D'Orio V and Dondelinger RF: Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 26:23–39. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, Raghu G, King TE Jr, Bradford WZ, Schwartz DA, et al: High-resolution computed tomography in idiopathic pulmonary fibrosis: Diagnosis and prognosis. Am J Respir Crit Care Med. 172:488–493. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
DiBardino DM, Yarmus LB and Semaan RW: Transthoracic needle biopsy of the lung. J Thorac Dis. 7 (Suppl 4):S304–S316. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Biederer J, Mirsadraee S, Beer M, Molinari F, Hintze C, Bauman G, Both M, Van Beek EJ, Wild J and Puderbach M: MRI of the lung (3/3)-current applications and future perspectives. Insights Imaging. 3:373–386. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Foo CT, Langton D, Thompson BR and Thien F: Functional lung imaging using novel and emerging MRI techniques. Front Med (Lausanne). 10(1060940)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schrevens L, Lorent N, Dooms C and Vansteenkiste J: The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist. 9:633–643. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Herder GJ, Kramer H, Hoekstra OS, Smit EF, Pruim J, van Tinteren H, Comans EF, Verboom P, Uyl-de Groot CA, Welling A, et al: Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: A Dutch cooperative randomized study. J Clin Oncol. 24:1800–1806. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Miettinen OS and Henschke CI: CT screening for lung cancer: Coping with nihilistic recommendations. Radiology. 221:592–596. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gould MK, Maclean CC, Kuschner WG, Rydzak CE and Owens DK: Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: A meta-analysis. JAMA. 285:914–924. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Viney RC, Boyer MJ, King MT, Kenny PM, Pollicino CA, McLean JM, McCaughan BC and Fulham MJ: Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol. 22:2357–2362. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bradley JD, Bae K, Graham MV, Byhardt R, Govindan R, Fowler J, Purdy JA, Michalski JM, Gore E and Choy H: Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 28:2475–2480. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pulumati A, Pulumati A, Dwarakanath BS, Verma A and Papineni RVL: Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Rep (Hoboken). 6(e1764)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al: Standardisation of spirometry. Eur Respir J. 26:319–338. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al: Interpretative strategies for lung function tests. Eur Respir J. 26:948–968. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al: Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur Respir J. 40:1324–1343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al: Standardisation of spirometry. Eur Respir J. 26:319–338. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al: Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 26:720–735. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Balamugesh T and Herth FJ: Endobronchial ultrasound: A new innovation in bronchoscopy. Lung India. 26:17–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Steward M and Dickson C: Sonography Endobronchial Assessment, Protocols, and Interpretation. In: StatPearls. StatPearls Publishing, Treasure Island, FL, 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK570578/.
|
|
44
|
Jalil BA, Yasufuku K and Khan AM: Uses, limitations, and complications of endobronchial ultrasound. Proc (Bayl Univ Med Cent). 28:325–330. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chandrika S and Yarmus L: Recent developments in advanced diagnostic bronchoscopy. Eur Respir Rev. 29(190184)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, et al: Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep. 28:499–521. 2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Medical Advisory Secretariat. Polysomnography in patients with obstructive sleep apnea: An evidence-based analysis. Ont Health Technol Assess Ser. 6:1–38. 2006.PubMed/NCBI
|
|
48
|
Rundo JV and Downey R III: Polysomnography. Handb Clin Neurol. 160:381–392. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Huang YS, Guilleminault C, Lin CH, Chen CH, Chin WC and Chen TS: Multiple sleep latency test in narcolepsy type 1 and narcolepsy type 2: A 5-year follow-up study. J Sleep Res. 27(e12700)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kwon Y, Kazaglis L, Cho Y, Howell MJ and Mahowald MW: Test-retest reliability of two consecutive mean sleep latency tests in patients with hypersomnia. Sleep Biol Rhythms. 15:337–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dauvilliers Y, Arnulf I and Mignot E: Narcolepsy with cataplexy. Lancet. 369:499–511. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu H, Wang D, Li Y, Li Z, Zhang Y, Lei F, Du L and Tang X: Examination of daytime sleepiness and cognitive performance testing in patients with primary insomnia. PLoS One. 9(e100965)2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mojebi A, Wu P, Keeping S, Hale B, Chase JG and Beaubrun A: Clinical impact of rapid molecular diagnostic tests in patients presenting with viral respiratory symptoms: A systematic literature review. PLoS One. 19(e0303560)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhu N and Wong PK: Advances in viral diagnostic technologies for combating COVID-19 and future pandemics. SLAS Technol. 25:513–521. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Mühlethaler K, Bögli-Stuber K, Wasmer S, von Garnier C, Dumont P, Rauch A, Mühlemann K and Garzoni C: Quantitative PCR to diagnose pneumocystis pneumonia in immunocompromised non-HIV patients. Eur Respir J. 39:971–978. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Blumenfeld NR, Bolene MAE, Jaspan M, Ayers AG, Zarrandikoetxea S, Freudman J, Shah N, Tolwani AM, Hu Y, Chern TL, et al: Multiplexed reverse-transcriptase quantitative polymerase chain reaction using plasmonic nanoparticles for point-of-care COVID-19 diagnosis. Nat Nanotechnol. 17:984–992. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Choi JH, Nahm DH, Kim SH, Kim YS, Suh CH, Park HS and Ahn SW: Increased levels of IgG to cytokeratin 19 in sera of patients with toluene diisocyanate-induced asthma. Ann Allergy Asthma Immunol. 93:293–298. 2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Gill P and Ghaemi A: Nucleic acid isothermal amplification technologies: A review. Nucleosides Nucleotides Nucleic Acids. 27:224–243. 2008.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB and Erlich HA: Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 239:487–491. 1988.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bartlett JM and Stirling D: A short history of the polymerase chain reaction. Methods Mol Biol. 226:3–6. 2003.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mullis KB and Faloona FA: Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 155:335–350. 1987.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Cutting GR: Cystic fibrosis genetics: From molecular understanding to clinical application. Nat Rev Genet. 16:45–56. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Metzker ML: Sequencing technologies-the next generation. Nat Rev Genet. 11:31–46. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mardis ER: Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 9:387–402. 2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Inagaki M, Uchiyama M, Yoshikawa-Kawabe K, Ito M, Murakami H, Gunji M, Minoshima M, Kohnoh T, Ito R, Kodama Y, et al: Comprehensive circulating microRNA profile as a supersensitive biomarker for early-stage lung cancer screening. J Cancer Res Clin Oncol. 149:8297–8305. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, Suzuki M, Gosselink JV, Liu G, Alekseyev YO, et al: A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med. 4(67)2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR and Fahy JV: T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 180:388–395. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hersh CP: Pharmacogenetics of chronic obstructive pulmonary disease: Challenges and opportunities. Pharmacogenomics. 11:237–247. 2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL and Taylor DR: American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 184:602–615. 2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Fens N, van der Schee MP, Brinkman P and Sterk PJ: Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin Exp Allergy. 43:705–715. 2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sin DD, McAlister FA, Man SF and Anthonisen NR: Contemporary management of chronic obstructive pulmonary disease. JAMA. 290:2301–2312. 2003.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Gan WQ, Man SF, Senthilselvan A and Sin DD: Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax. 59:574–580. 2004.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E, et al: Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS One. 7(e37483)2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE and Dolovich J: Measurement of inflammatory indices in induced sputum: Effects of selection of sputum to minimize salivary contamination. Eur Respir J. 9:1174–1180. 1996.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Corradi M, Pignatti P, Manini P, Andreoli R, Goldoni M, Poppa M, Moscato G, Balbi B and Mutti A: Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur Respir J. 24:1011–1017. 2004.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Djukanović R, Sterk PJ, Fahy JV and Hargreave FE: Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 37:1s–2s. 2002.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Brightling CE, Monterio W, Green RH, Parker D, Morgan MD, Wardlaw AJ and Pavord D: Induced sputum and other outcome measures in chronic obstructive pulmonary disease: Safety and repeatability. Respir Med. 95:999–1002. 2001.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Stockley RA, O'Brien C, Pye A and Hill SL: Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 117:1638–1645. 2000.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Efthimiadis A, Spanevello A, Hamid Q, Kelly MM, Linden M, Louis R, Pizzichini MM, Pizzichini E, Ronchi C, Van Overvel F and Djukanović R: Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J Suppl. 37:19s–23s. 2002.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Gustafsson LE, Leone AM, Persson MG, Wiklund NP and Moncada S: Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 181:852–857. 1991.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Alving K, Weitzberg E and Lundberg JM: Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 6:1368–1370. 1993.PubMed/NCBI
|
|
82
|
Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA and Barnes PJ: Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 343:133–135. 1994.PubMed/NCBI View Article : Google Scholar
|
|
83
|
American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 171:912–930. 2005.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kharitonov SA, Yates D and Barnes PJ: Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur Respir J. 8:295–297. 1995.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Połomska J, Bar K and Sozańska B: Exhaled breath condensate-A non-invasive approach for diagnostic methods in asthma. J Clin Med. 10(2697)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Gordon SM, Szidon JP, Krotoszynski BK, Gibbons RD and O'Neill HJ: Volatile organic compounds in exhaled air from patients with lung cancer. Clin Chem. 31:1278–1282. 1985.PubMed/NCBI
|
|
87
|
Ratiu IA, Ligor T, Bocos-Bintintan V, Mayhew CA and Buszewski B: Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma, and COPD. J Clin Med. 10(32)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Pépin JL, Degano B, Tamisier R and Viglino D: Remote monitoring for prediction and management of acute exacerbations in chronic obstructive pulmonary disease (AECOPD). Life (Basel). 12(499)2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Almasi S, Shahbodaghi A and Asadi F: Efficacy of telemedicine for the management of asthma: a systematic review. Tanaffos. 21:132–145. 2022.PubMed/NCBI
|
|
90
|
Gaveikaite V, Fischer C, Schonenberg H, Pauws S, Kitsiou S, Chouvarda I, Chouvarda I, Maglaveras N and Roca J: Telehealth for patients with chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis protocol. BMJ Open. 8(e021865)2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Cruz-Correia R, Fonseca J, Lima L, Araújo L, Delgado L, Castel-Branco MG and Costa-Pereira A: Web-based or paper-based self-management tools for asthma-patients' opinions and quality of data in a randomized crossover study. Stud Health Technol Inform. 127:178–189. 2007.PubMed/NCBI
|
|
92
|
McLean S, Protti D and Sheikh A: Telehealthcare for long term conditions. BMJ. 342(d120)2011.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Snoswell CL, Taylor ML, Comans TA, Smith AC, Gray LC and Caffery LJ: Determining if telehealth can reduce health system costs: scoping review. J Med Internet Res. 22(e17298)2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Seto E: Cost comparison between telemonitoring and usual care of heart failure: A systematic review. Telemed J E Health. 14:679–686. 2008.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Stoddart A, van der Pol M, Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A and McKinstry B: Telemonitoring for chronic obstructive pulmonary disease: A cost and cost-utility analysis of a randomised controlled trial. J Telemed Telecare. 21:108–118. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Hilty DM, Ferrer DC, Parish MB, Johnston B, Callahan EJ and Yellowlees PM: The effectiveness of telemental health: A 2013 review. Telemed J E Health. 19:444–454. 2013.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Wootton R: Twenty years of telemedicine in chronic disease management-an evidence synthesis. J Telemed Telecare. 18:211–220. 2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Craig J and Patterson V: Introduction to the practice of telemedicine. J Telemed Telecare. 11:3–9. 2005.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Wade VA, Eliott JA and Hiller JE: Clinician acceptance is the key factor for sustainable telehealth services. Qual Health Res. 24:682–694. 2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Darkins A, Ryan P, Kobb R, Foster L, Edmonson E, Wakefield B and Lancaster AE: Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 14:1118–1126. 2008.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Bashshur RL, Shannon GW, Smith BR, Alverson DC, Antoniotti N, Barsan WG, Bashshur N, Brown EM, Coye MJ, Doarn CR, et al: The empirical foundations of telemedicine interventions for chronic disease management. Telemed J E Health. 20:769–800. 2014.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Haleem A, Javaid M, Singh RP and Suman R: Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens Int. 2(100117)2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Topol EJ: High-performance medicine: The convergence of human and artificial intelligence. Nat Med. 25:44–56. 2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Georgakopoulou VE: The role of artificial intelligence in combatting respiratory tract infections. Cureus. 16(e63635)2024.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Pascoe S, Locantore N, Dransfield MT, Barnes NC and Pavord ID: Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 3:435–442. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Chandran U, Reps J, Yang R, Vachani A, Maldonado F and Kalsekar I: Machine learning and real-world data to predict lung cancer risk in routine care. Cancer Epidemiol Biomarkers Prev. 32:337–343. 2023.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Beam AL and Kohane IS: Big data and machine learning in health care. JAMA. 319:1317–1318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Chen JH and Asch SM: Machine learning and prediction in medicine-beyond the peak of inflated expectations. N Engl J Med. 376:2507–2509. 2017.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Bramley TJ, Lerner D and Sames M: Productivity losses related to the common cold. J Occup Environ Med. 44:822–829. 2002.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Daniels JM, Schoorl M, Snijders D, Knol DL, Lutter R, Jansen HM and Boersma WG: Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 138:1108–1115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Bossuyt PM, Reitsma JB, Linnet K and Moons KG: Beyond diagnostic accuracy: The clinical utility of diagnostic tests. Clin Chem. 58:1636–1643. 2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, Hunt JF, Kita H, Liu AH, Panettieri RA Jr, et al: Asthma outcomes: Biomarkers. J Allergy Clin Immunol. 129 (Suppl 3):S9–S23. 2012.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Ambrosino N and Vagheggini G: Non-invasive ventilation in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2:471–476. 2007.PubMed/NCBI
|
|
114
|
Murphy PB, Rehal S, Arbane G, Bourke S, Calverley PMA, Crook AM, Dowson L, Duffy N, Gibson GJ, Hughes PD, et al: Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: A randomized clinical trial. JAMA. 317:2177–2186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Plant PK, Owen JL and Elliott MW: Early use of non-invasive ventilation for acute exacerbations of COPD on general respiratory wards: A multicentre randomised controlled trial. Lancet. 355:1931–1935. 2000.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Poletti V, Chilosi M and Olivieri D: Diagnostic invasive procedures in diffuse infiltrative lung diseases. Respiration. 71:107–119. 2004.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Cooper WA, Lam DC, O'Toole SA and Minna JD: Molecular biology of lung cancer. J Thorac Dis. 5 (Suppl 5):S479–S490. 2013.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Kebbe J and Abdo T: Interstitial lung disease: The diagnostic role of bronchoscopy. J Thorac Dis. 9 (Suppl 10):S996–S1010. 2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Rivera MP, Mehta AC and Wahidi MM: Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 143 (Suppl 5):e142S–e165S. 2013.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Chen LC, Yang SM, Malwade S, Chang HC, Chang LK, Chung WY, Ko JC and Yu CJ: Cone-beam computed-tomography-derived augmented fluoroscopy-guided biopsy for peripheral pulmonary nodules in a hybrid operating room: A case series. Diagnostics (Basel). 13(1055)2023.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Bae S, Lim S, Ahn JJ, Jegal Y, Seo KW, Ra SW, Kang BJ, Kim JH, Park SE, Han I, et al: Diagnosing peripheral lung lesions using endobronchial ultrasonography with guide sheath: A prospective registry study to assess the effect of virtual bronchoscopic navigation using a computed tomography workstation. Medicine (Baltimore). 99(e19870)2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Aravena C, Almeida FA, Culver DA and Neto ML: The utility of endobronchial ultrasound-transbronchial needle aspiration in patients with suspected extra-pulmonary sarcoidosis without thoracic lymphadenopathy. Respir Med. 171(106074)2020.PubMed/NCBI View Article : Google Scholar
|
|
123
|
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C, Yousaf-Khan U, Horeweg N, et al: Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 382:503–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM and Sicks JD: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 365:395–409. 2011.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Mazzone PJ, Obuchowski N, Fu AZ, Phillips M and Meziane M: Quality of life and healthcare use in a randomized controlled lung cancer screening study. Ann Am Thorac Soc. 10:324–329. 2013.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Guerreiro T, Aguiar P and Araújo A: Current evidence for a lung cancer screening program. Port J Public Health. 42:133–158. 2024.PubMed/NCBI View Article : Google Scholar
|
|
127
|
McCarthy B, Casey D, Devane D, Murphy K, Murphy E and Lacasse Y: Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(CD003793)2015.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al: An official American Thoracic Society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 188:e13–e64. 2013.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Man WD, Polkey MI, Donaldson N, Gray BJ and Moxham J: Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ. 329(1209)2004.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Puhan MA, Gimeno-Santos E, Cates CJ and Troosters T: Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 12(CD005305)2016.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Jones PW, Harding G, Berry P, Wiklund I, Chen WH and Leidy NK: Development and first validation of the COPD assessment test. Eur Respir J. 34:648–654. 2009.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Cergan R, Dumitru M, Vrinceanu D, Neagos A, Jeican II and Ciuluvica RC: Ultrasonography of the larynx: Novel use during the SARS-CoV-2 pandemic (Review). Exp Ther Med. 21(273)2021.PubMed/NCBI View Article : Google Scholar
|















