Introduction
Chronic recurrent multifocal osteomyelitis (CRMO)
represents the most severe manifestation of chronic non-bacterial
osteomyelitis (CNO), constituting a rare autoinflammatory bone
disorder (1). CRMO predominantly
affects the metaphysis of long bones, pelvis, clavicle and spine
(2). This rare condition is part of
the spectrum of autoinflammatory rheumatological diseases together
with Majeed syndrome, IL-1 receptor antagonist deficiency (DIRA),
IL-36 receptor deficiency (DITRA) and Pyoderma gangrenosum
(PAPA). CRMO is also known by various names such as SAPHO
(synovitis, acne, pustulosis, hyperostosis and osteomyelitis) or
non-bacterial osteomyelitis (NBO), both referring to the same
disease entity (3). Described first
in 1972 by Giedion et al (4), CRMO predominantly affects female
children, with an average onset age of 10 years. The worldwide
prevalence is estimated at 1-9 cases per million, with ~480 cases
documented in the Eurofever registry (5). In total, ~50% patients experience
persistent chronic disease, and ~20% of patients with CRMO
experience recurrences. Because CRMO is a diagnosis of exclusion
and can mimic other inflammatory bone conditions, the condition is
considered to be underdiagnosed. Several cases occur associated
with other autoinflammatory and autoimmune diseases, such as skin
disorders, peripheral arthritis, inflammatory bowel disease, and
granulomatosis with polyangiitis. Association with several
autoinflammatory conditions can be detected in ~1/3 of patients
with CRMO (6).
CRMO presents with an insidious onset and
polymorphic clinical manifestations that can mimic infections or
malignancies. Bone pain is the most common initial symptom, often
accompanied by tenderness at the affected site, with or without
swelling. Nocturnal bone pain can be misinterpreted as growing
pains (7). Mild febrile conditions,
asthenia and fatigue occur in <5% of patients. Laboratory
analyses typically reveal elevated inflammatory markers, including
elevated erythrocyte sedimentation rate (ESR) in 59% of patients,
elevated C-reactive protein (CRP) in 49%, elevated leukocyte count
in 14%, and elevated serum amyloid A in 12%. No relevant increases
are observed in immunoglobulin levels. Additionally, HLA-B27 is
present in 7.9% of individuals, and 38% of tested patients have
elevated ANA titers (5).
CRMO diagnosis often involves a process of
exclusion, occasionally requiring a bone biopsy. Diagnostic
criteria, such as the Jansson and Bristol criteria, aid in
establishing a diagnosis. In the Jansson classification, meeting
two major criteria or one major and three minor criteria is
necessary. Major criteria include radiologically demonstrated
osteolytic/osteosclerotic bone lesions, multifocality of bone
lesions, palmoplantar dermatosis (psoriasis-pustulosis), and
sterile bone biopsy with signs of inflammation, fibrosis, or
sclerosis. Minor criteria consist of normal blood count and
favorable general health, increased CRP and ESR, symptom duration
of more than half a year, hyperostosis, and positive family history
with first or second-degree relatives diagnosed with any autoimmune
disease (8). Within the Bristol
system, diagnostic criteria encompass characteristic clinical
presentations (such as localized bone pain with or without
localized swelling, without signs of infection). Typical
radiological observations include plain X-ray findings (showing a
combination of lytic areas, sclerosis, and new bone formation), or
preferably STIR magnetic resonance imaging (MRI) findings, which
may include bone marrow edema, bone expansion, lytic areas, and
periosteal reaction (9)
Furthermore, diagnosis requires fulfillment of one of the
subsequent criteria: (i) Involvement of >1 bone (or clavicle
only) without significantly elevated C-reactive protein (CRP <30
g/l); and (ii) unifocal lesion (other than clavicle) or CRP >30
g/l, with bone biopsy demonstrating inflammatory changes (such as
plasma cells, osteoclasts, fibrosis, or sclerosis) without
bacterial growth while not under antibiotic therapy (8).
Subgroup classification of CRMO based on the
distribution of bone lesions has been proposed, including the
‘tibio-appendicular multifocal model’ and the ‘clavicular-spinal
pauci-focal model’, although these remain purely descriptive
(3).
Most patients with CRMO likely possess a genetic
predisposition that, on its own, may not be sufficiently strong
enough to induce the disease without the influence of additional
factors. One of the environmental factors that can influence or
determine this disease is Cutibacterium acnes (C.
acnes), previously known as Propionibacterium acnes or
Corynebacterium parvum. C. acnes, the primary
bacteria implicated in acne, naturally resides in the sebaceous
glands of all individuals, where it contributes to the balance of
the skin microbiome. As a saprophytic bacterium, it feeds on
decaying organic matter such as sebum.
Each person possesses a unique microbiome profile.
Typically, sebaceous skin is predominantly inhabited by
Cutibacteria spp. (formally known as
Propionibacteria), Staphylococci spp.,
β-Proteobacteria and Corynebacteria spp., while dry
skin is characterized by an abundance of β-Proteobacteria,
Corynebacteria spp., Flavobacteriales and Cutibacterium
spp. This diverse microbial community is vital for maintaining
skin health, as it helps establish and modulate skin immunity and
host defense by producing antimicrobial peptides (10). A decrease in the abundance of C.
acnes is often associated with various skin conditions,
including acne, atopic dermatitis, rosacea and psoriasis. C.
acnes and the diversity of its clonal population actively
contribute to the skin's normal physiological functions by
modulating lipid metabolism and mitigating oxidative stress
(11). In patients with psoriasis,
itching can cause skin wounds, allowing certain bacteria to
penetrate deep into the dermis or even enter the peripheral blood,
where they stimulate both innate and adaptive immune responses. A
decrease in Corynebacterium spp., Lactobacillus spp.,
Burkholderia spp. and Propionibacterium acnes were observed in
the skin of patients with psoriasis lesions compared with healthy
skin (12).
Case presentation
The patient is a 9-year-old female with a personal
history of psoriasis since the age of 5. Family history reveals two
sisters with psoriasis. In December 2023, she was hospitalized due
to a left shoulder injury sustained two months prior, resulting in
pain and limited mobility. Orthopedic consultation suggested a
possible clavicle fracture in the healing process, leading to
immobilization in a plaster cast. However, symptoms persisted after
cast removal, with added swelling, pain and functional impairment,
prompting admission to the ‘Louis Turcanu’ Children's Emergency
Clinical Hospital in Timisoara for further investigation.
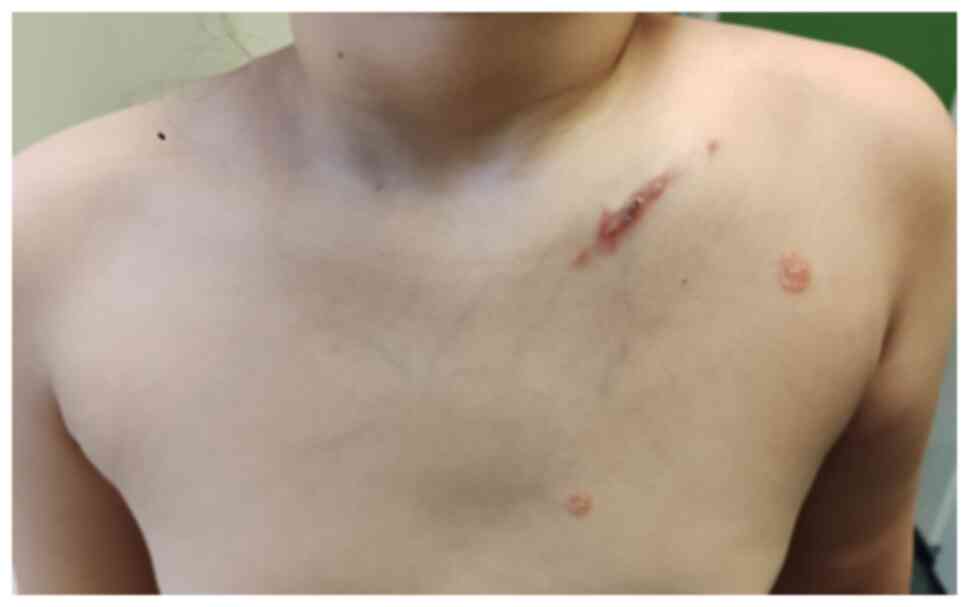
On admission, clinical examination revealed a
swollen, hardened area with intense pain on palpation and marked
functional impairment at the left clavicle (Figs. 1 and 2). Additionally, discomfort while walking
was noted, attributed to hip pain.
Laboratory investigations showed normal blood count,
liver and kidney function, with slightly increased inflammatory
markers: CRP=4.97 mg/l (normal values 0-5 mg/l) and ESR=32 mm/h
(normal values 0-13 mm/h). Procalcitonin, HLA-B27, and antinuclear
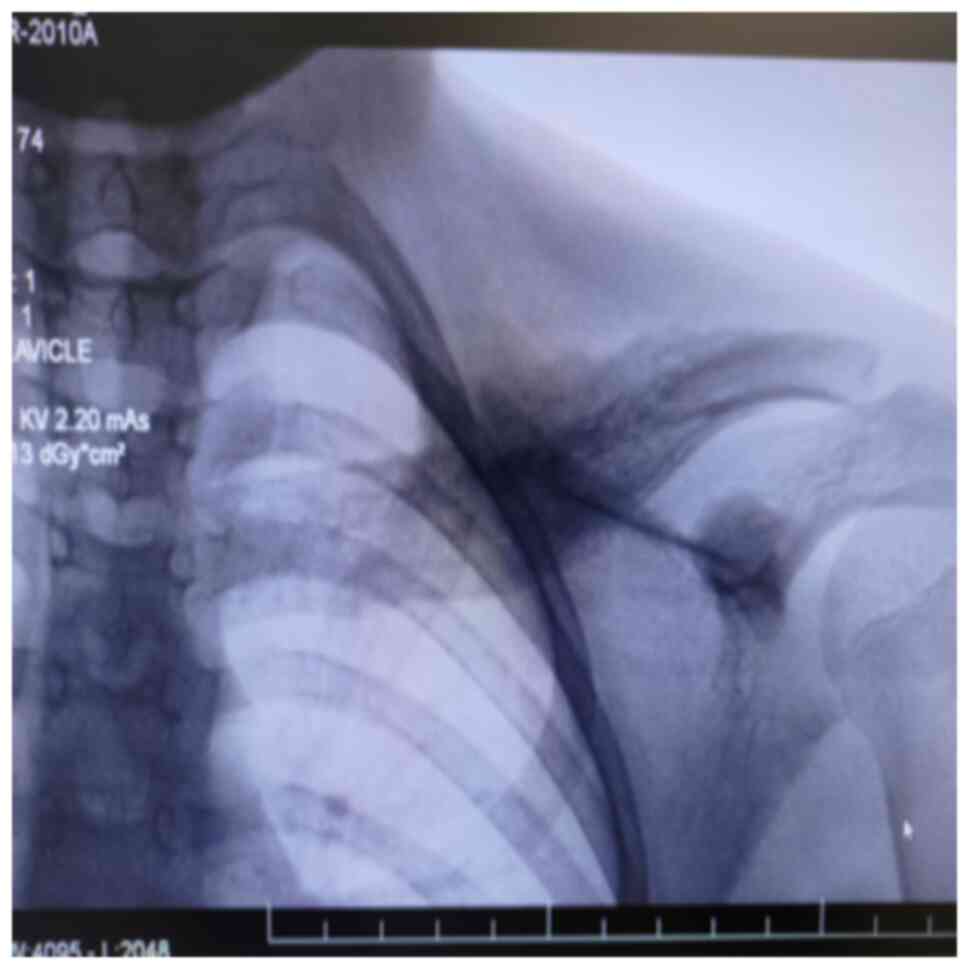
anti-nuclear antibodies (ANA) were negative. An X-ray of the left
clavicle revealed changes in its shape and bone structure, along
with an exaggerated periosteal reaction in the middle third.
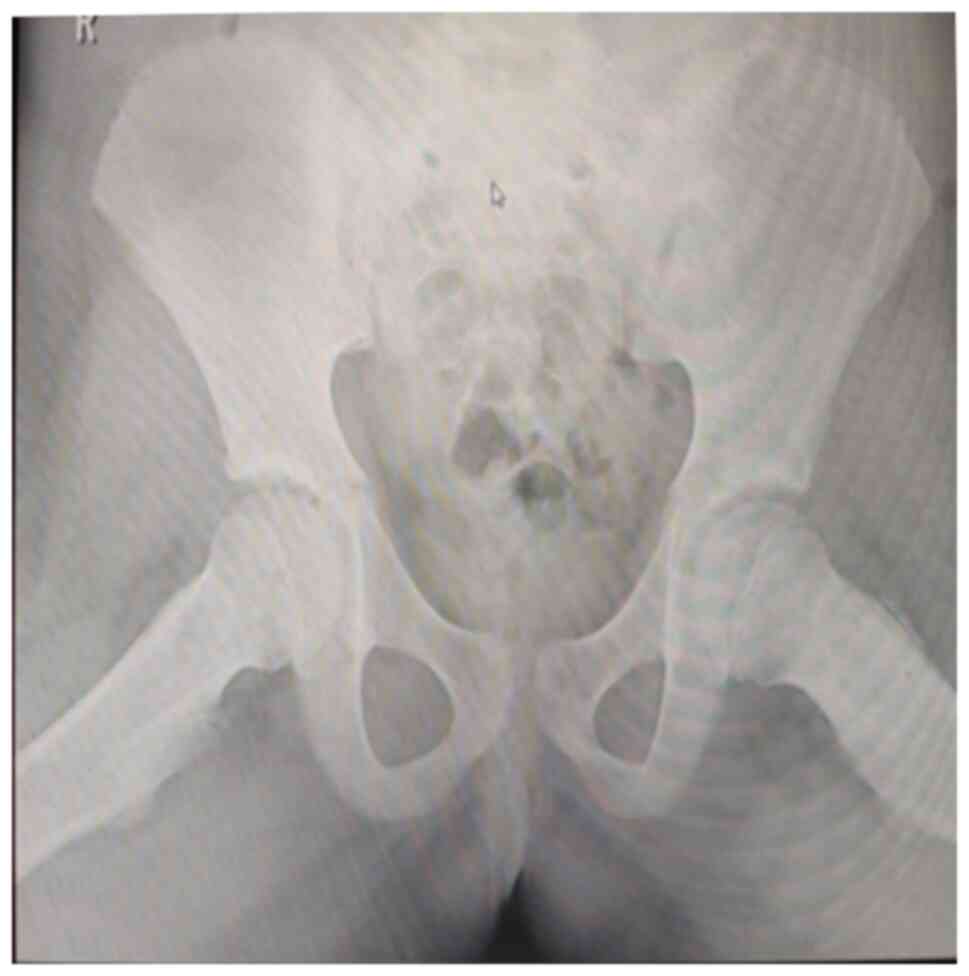
Additionally, the coxo-femoral X-ray of the left femur exhibited
morphological and structural changes in the intertrochanteric
region, characterized by widening of the femoral neck and the area
with a mixed osteolytic and osteosclerotic pattern, predominantly
osteosclerotic. The ‘frog-leg’ incidence highlights linear opacity
parallel to the femoral neck (Figs.
3 and 4).
Considering the absence of infectious history,
fever, or weight loss, along with the discrepancy in inflammatory
factors and multifocal radiological lesions, further imaging and
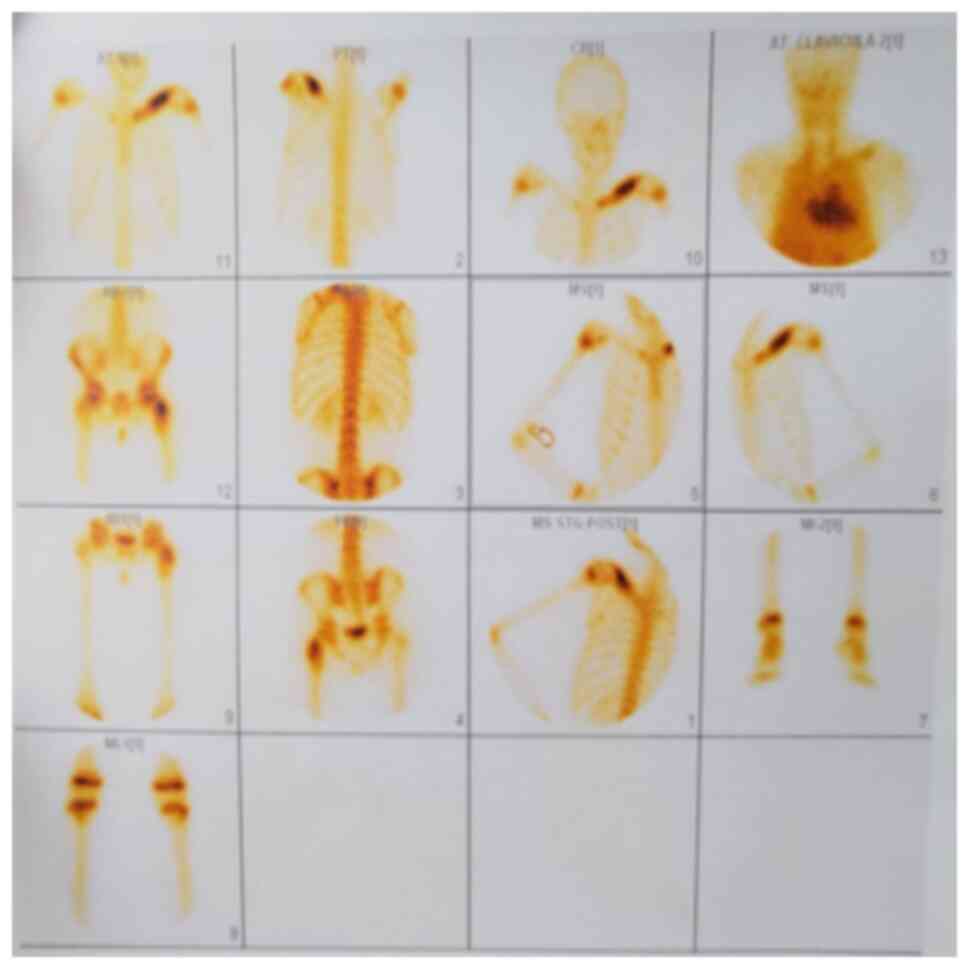
biopsy were pursued. Subsequently, a scintigraphic examination was
performed revealing intense hyper uptake of contrast substance at
the left clavicle (sternal extremity, body of the clavicle) with an
uneven distribution indicative of thickening of the bone outline.
Similarly, heightened contrast substance capture was observed at
the trochanteric region of the left femur, particularly at the
greater trochanter (Fig. 5).
The histopathological result of the sampled
formation describes spongy bone tissue with areas of necrosis and
edema, congested vascular structures, infiltration of lymphocytes
and plasma cells consistent with non-specific chronic inflammation,
medullary fibrosis and osteonecrosis.
Given the autoimmune and autoinflammatory nature of
the patient's condition, attention was directed towards
understanding the underlying pathophysiological mechanisms. Thus,
it was found out from the patient's history that she had been
receiving off-label immunostimulation therapy for psoriasis since
the age of 5. The treatment involved injection with C.
acnes, one weekly injection. A favorable evolution of skin
lesions was initially observed but with multiple relapses during
the years when it stops.
Non-steroidal anti-inflammatory therapy (Naproxen
7.5 mg/kg/day) was chosen as the first-line therapeutic approach,
resulting in an initial improvement, pain and swelling decreased,
arm mobility returned and hip discomfort resolved. From a
biological point of view, an initial decrease in ESR was observed.
After 3 months, the painful symptomatology accompanied by swelling
and functional impotence reappeared. The patient was clinically
reevaluated, against the background of active psoriasis and
reactivated autoinflammatory pathology; it was decided to initiate
treatment with subcutaneously injected Methotrexate 15
mg/m2/weekly. Subsequently, the evolution was slowly
favorable both clinically and biologically with the improvement of
painful symptoms, and the reduction of bone swelling. Psoriatic
lesions were substantially reduced. Biological investigations did
not detect inflammatory signs.
A total of ~2 months after initiation of
disease-modifying antirheumatic drugs (DMARDs) treatment, the
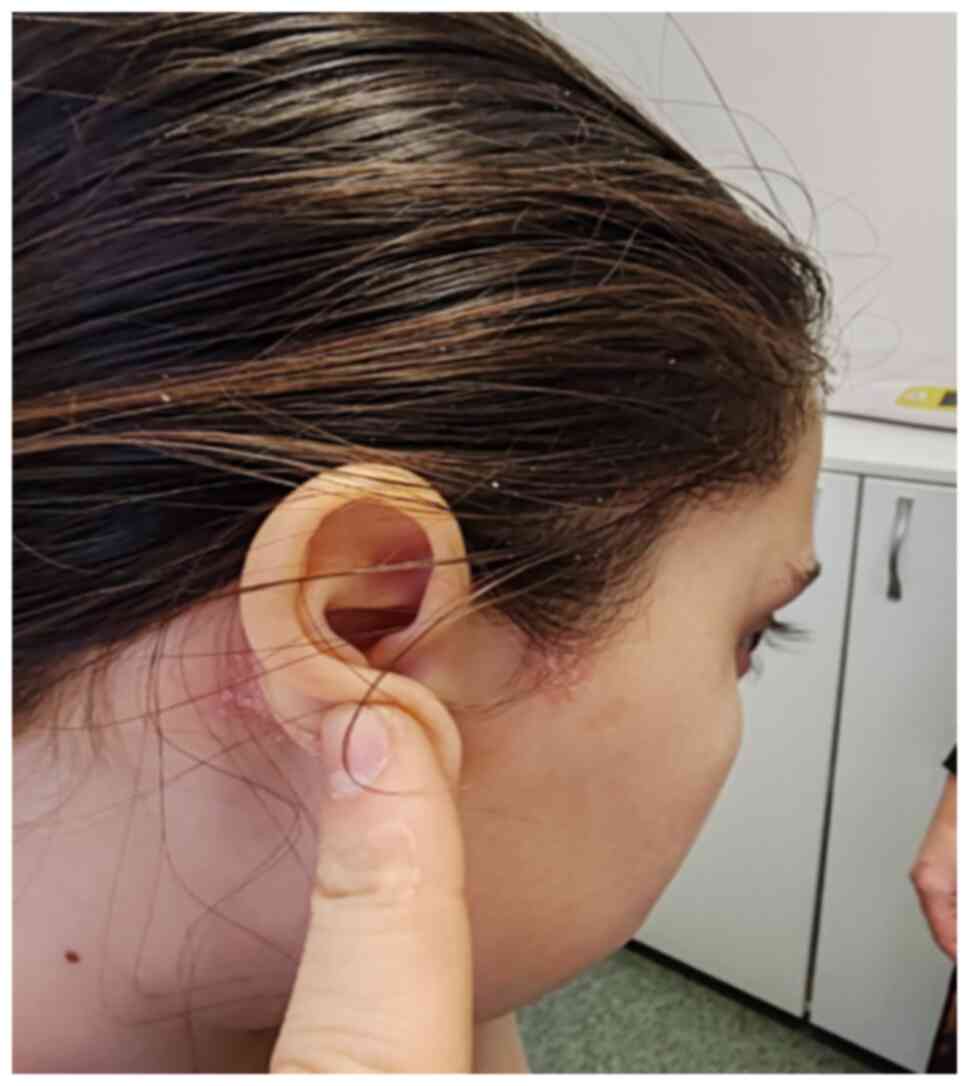
patient presented with fever, odynophagia and left otalgia. A
pharyngeal swab was performed, which tested positive for Group A
beta-hemolytic Streptococcus. An ENT evaluation was
conducted, diagnosing muco-purulent otitis, for which a 7-day
antibiotic treatment was initiated. For hearing disturbances, an
ENT consultation was performed after antibiotic treatment finished,
diagnosing chronic hypertrophic tonsillitis and hypertrophic
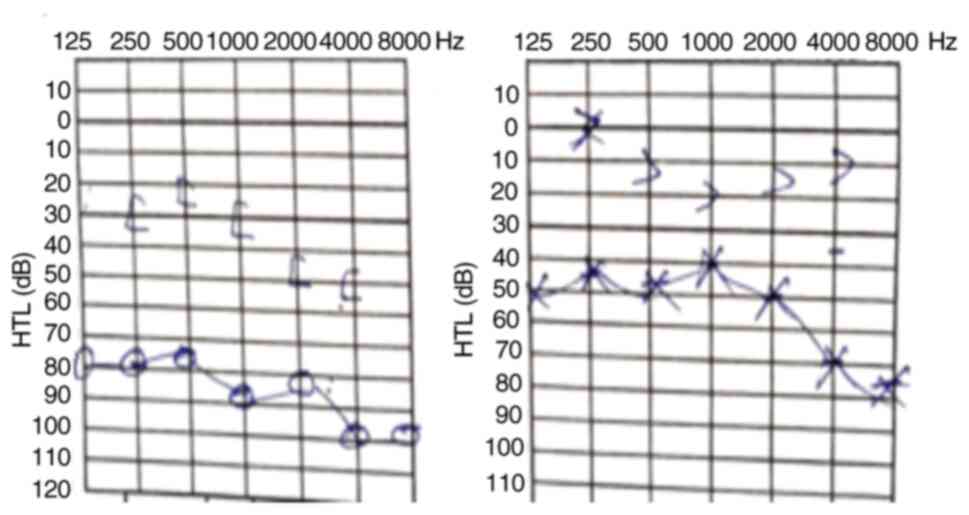
adenoid vegetation. An audiogram (Fig.
6) confirmed bilateral hypoacusis.
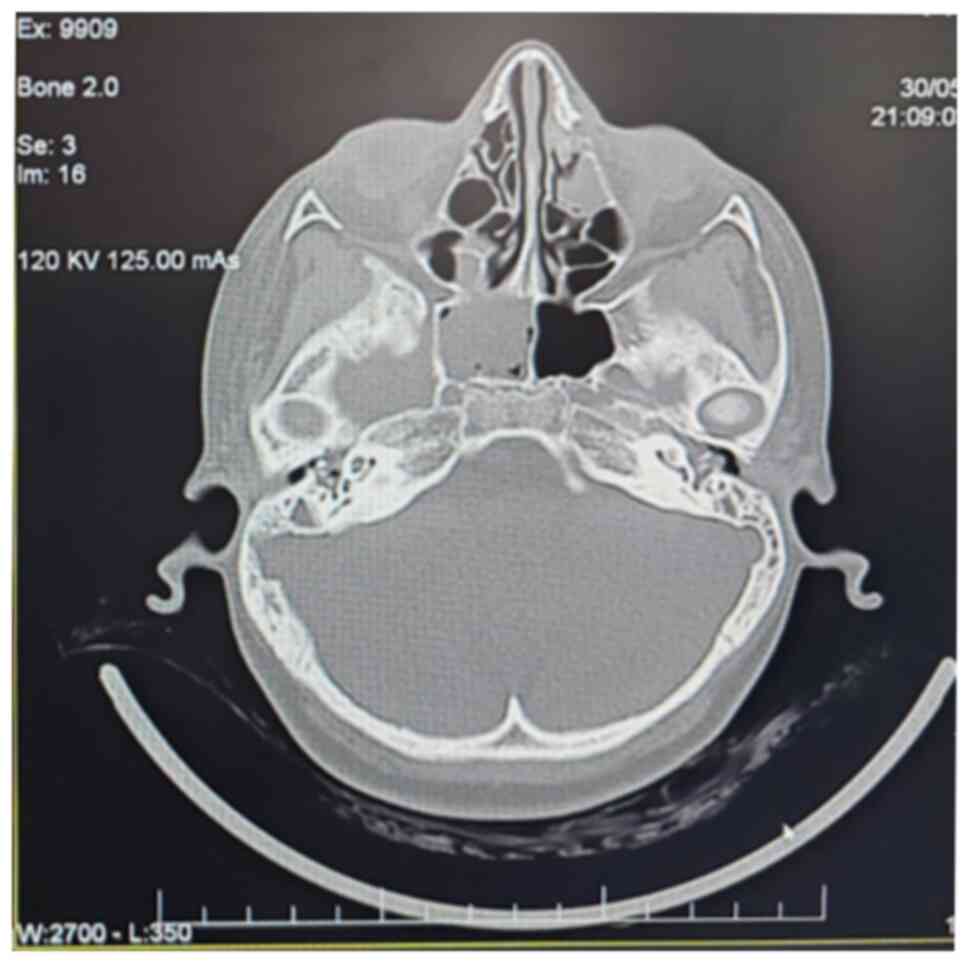
A subsequent computed tomography scan (Fig. 7) revealed complete bilateral
opacification of the mastoid cells, dense material in the left
middle ear, and marked hypertrophy of lymphoid tissue in the
nasopharynx, leading to lumen obstruction. The findings suggested
bilateral mastoiditis, left middle ear otitis with a possible
cholesteatoma, and proximal nasopharyngeal lumen obstruction due to
hypertrophic adenoid tissue. Additionally, chronic inflammatory
changes were observed in the right sphenoidal and ethmoid-maxillary
regions.
Discussion
Although the precise mechanism of CRMO remains
incompletely understood, it has been included in the family of
autoinflammatory diseases because, primarily due to the predominant
involvement of autoinflammatory bone. This condition is
characterized by an imbalance of cytokines, patients often
exhibiting decreased production of anti-inflammatory cytokines such
as interleukins (IL)-9, -10 and -18, and increased levels of
pro-inflammatory cytokines including IL-1β, IL-6 and tumor necrosis
factor-alpha (TNF-α) (9,13). Notably, monocytes from patients with
CRMO exhibit impaired immune regulatory IL-10 in response to
Toll-like receptor stimulation (14), which is partially attributed to
reduced activation of mitogen-activated protein kinases, ERK1 and
2, resulting in deficient expression of anti-inflammatory cytokines
(15). However, the disease-causing
mutations in one or more genes remain unknown (16).
There is controversy about whether
Cutibacterium plays a role in the etiology of CRMO. It has
been postulated that this bacterium could trigger chronic
inflammation in genetically predisposed patients. Dysregulation of
IL-1 is important in the pathogenesis of autoinflammatory bone
diseases. When healthy individuals are exposed to C. acnes
stimulation, there is an increase in caspase-1 activity in
neutrophils, which is associated with the production of IL-1β and
IL-18. Additionally, in vitro studies have demonstrated that
C. acnes stimulation results in elevated production of IL-8 and
TNF-α by monocytes, keratinocytes and dendritic cells (DCs)
(17). Surgical interventions,
particularly orthopedic device implantation, may inadvertently
promote infection (18). It is
worth mentioning that 2 years ago our patient underwent surgery to
remove a foreign body (needle) that had become lodged in the bone
at the level of the tibial plateau, an event that occurred as a
play accident. These types of infections are associated with
bacteremia, endocarditis, systemic inflammation, and even bone
destruction, underscoring the potential role of bacterial
infections in CRMO pathogenesis (19).
Further studies are needed to improve the
understanding of the role of skin microbiome in individuals
predisposed to autoimmune or autoinflammatory disease. Skin
dysbiosis may trigger conventional DCs to secrete IL-23,
stimulating Th17 cells to produce IL-17, which in turn promotes
keratinocyte hyperproliferation and leukocyte infiltration
(20,21). In addition, innate lymphoid type 3
cells (ILC3) can respond to stimulatory cytokines, including IL-1β,
IL-18 and IL-23, and secrete IL-17, IL-22 and IFN-γ. IL-17
stimulates keratinocytes to produce chemokines such as CXCL1,
CXCL2, CXL20, IL-6 and IL-8, resulting in leukocyte infiltration.
Infiltrating leukocytes can further produce IL-1β and IL-18 to
stimulate ILC3 cells to produce more IL-22 and IL-22, promoting
keratinocyte hyperproliferation (22,23).
To establish the diagnosis CRMO, several potential
differential diagnoses were eliminated through comprehensive
laboratory and imaging investigations. Infections, including
bacterial osteomyelitis and tuberculosis, were ruled out due to the
absence of recent infection history, lack of active infection
signs, negative Quantiferon test results, and normal findings on
chest X-ray without pneumonic foci. Immune deficiency was
considered and ultimately dismissed as the patient had no history
of severe or recurrent infections requiring hospitalization or
antibiotic treatment. Furthermore, immunoglobulin levels (IgM, IgG,
IgA) and serum protein electrophoresis were found to be within
normal limits. The possibility of malignant hematological tumors
such as leukemia or lymphoma was also investigated. Clinical
examination revealed no suspicious adenopathy, and biological
assessments showed no abnormalities in blood counts or blasts in
the peripheral blood smear. Additionally, chest X-ray and
ultrasound examinations of the cervical zone and abdominal regions
revealed no evidence of malignancy. Malignant bone tumors were
ruled out based on findings from scintigraphy examination and bone
biopsy results, which did not align with the characteristics of
such tumors. Metabolic bone disease was considered and excluded
based on laboratory tests that revealed no deficiencies in blood
microelements. Lastly, the potential for monogenic autoinflammatory
disorders with bone damage, including PAPA, DIRA and Majeed
syndromes, was explored (24).
However, the onset of pathology did not occur in infancy or early
childhood, and there were no accompanying features such as
dys-erythropoietic anemia, pustulosis, or joint swelling indicative
of these monogenic disorders.
The radiographic evaluation depends on the stage of
the disease. Decalcification or osteolysis can be observed in an
early stage, whereas advanced stages often manifest with
hyperostosis and sclerosis. The periosteal reaction can occur at
any stage. Tubular bone lesions are most often located at the
metaphysis of long bones but may extend to the diaphysis and
occasionally to the epiphysis. Initially, radiographs reveal
metaphyseal involvement, with eccentric lytic lesions adjacent to
the growth plate, a sclerotic rim separating it from the underlying
bone, and a limited periosteal reaction (25). Identification of the multifocal
configuration through two-phase bone scintigraphy is crucial for
accurate CRMO diagnosis (26). Both
bone scintigraphy and MRI have been shown to be useful tools for
detecting CRMO lesions. In the evaluation of the axial skeleton
(particularly the spine), MRI offers higher spatial resolution
compared with planar scintigraphy (27). Fluid-sensitive sequences can
visualize bone marrow edema, a typical feature of CRMO. Small bone
changes may be detected, and clinical symptoms may emerge as the
disease progresses (28). As a
modern method of investigation, the magnetic resonance of the whole
body has become the method of choice because it does not expose the
patient to radiation and offers superior evaluation capability. MRI
can also demonstrate marrow edema, periostitis, soft tissue
inflammation and joint involvement (29). In our case, scintigraphy was chosen
for its utility in confirming the diagnosis at low financial
costs.
The current hearing disorders need to be
investigated and possibly correlated with the underlying pathology,
not just in the context of infectious issues in the ENT sphere. The
term ‘autoinflammatory’ in autoinflammatory diseases (ADs) refers
to the seemingly spontaneous onset of inflammation, in the absence
of infectious triggers, autoreactive T lymphocytes and specific
autoantibodies. ADs result from dysregulated production of
pro-inflammatory cytokines, prominently IL-1, and a delayed
termination of the immune response (30). The inflammasomes are large
multimeric protein complexes that regulate the activation of
proinflammatory cytokines such as interleukin-1β and -18, and
inflammatory cell death known as pyroptosis. NLRP1, NLRP3, NLRC4,
AIM2 and pyrin can induce the formation of inflammasomes. Among
these, the NLRP3 inflammasome is the most well-characterized.
Previous studies have revealed that variants in the NLRP3 gene can
cause genetic diseases, including a systemic inflammatory syndrome
called cryopyrin-associated periodic syndrome and non-syndromic
neurosensory hearing loss DFNA34(31). Auto-inflammatory diseases can lead
to hearing loss, and evidence suggests that inflammation may play a
role in hearing loss associated with other conditions. The
inflammasome is a multi-molecular pro-inflammatory protein complex
formed in activated macrophages, which may contribute to hearing
loss (32). Macrophage-like cells
are distributed throughout all cochlear tissues, including the
auditory nerve, spiral ganglion, basilar membrane, stria vascularis
and spiral ligament. The p.Arg918Gln mutation in NLRP3 can lead to
non-syndromic sensorineural hearing loss (33).
Regarding treatment, non-steroidal
anti-inflammatories remain the first-line treatment option for most
patients. In cases of non-responsiveness to non-steroidal
anti-inflammatory drugs (NSAID), corticosteroids, DMARDs, TNF
inhibitors and bisphosphonates (such as Pamidronate) may be
considered. Since the NSAID action starts after at least 4 weeks of
therapy, it is important to maintain the treatment for at least 1
month before declaring therapeutic failure (34). If discovered and treated promptly,
CRMO can show a favorable evolution. In the present case, it was
recommended starting treatment with injectable Methotrexate after
surgery.
The uniqueness of the present case resides in the
fact that the pre-existing chronic autoimmune pathology (psoriasis)
was treated for a long period with an injectable immune stimulant
based on Propionibacterium Parvum, which is incriminated in
multiple studies as a trigger factor in patients with pre-existing
genetic susceptibility. Familial psoriasis (genetic substrate)
combined with environmental factors (skin dysmicrobism) may have
led to the development of psoriasis since early childhood. The
immune dysregulation prompted by psoriasis, along with the
immunostimulation with C. acnes, may have resulted in the
development of bone lesions specific to CRMO.
In conclusion, this case report presents a rare and
complex pathology from an etiopathogenic perspective, involving
both autoinflammatory and autoimmune elements. It is considered
that the genetic predisposition, in combination with bacterial
immunostimulation in the context of an autoimmune disease, may
contribute to the development of a concurrent autoinflammatory
disease. Investigating any potential specific pathogenic
relationship between these two conditions would necessitate
multicenter studies. Furthermore, there remains a lack of specific
biomarkers for CRMO, highlighting the need for further research to
identify characteristic patterns of CRMO and optimal methods for
monitoring disease progression.
Acknowledgements
Not applicable.
Funding
Funding: The authors would like to acknowledge ‘Victor Babes’
University of Medicine and Pharmacy Timisoara (Timisoara, Romania)
for their support in covering the costs of publication for this
research paper.
Availability of data and materials
The data generated in the present study are not
publicly available due to ethical restrictions but may be requested
from the corresponding author.
Authors' contributions
AIM conceptualized the study, provided resources,
wrote the original draft of the manuscript and developed
methodology. IJ and MAB validated data. AIM and DMN performed
formal analysis, conducted investigation and data curation. DMN and
MAB wrote, reviewed and edited the manuscript. OM and IJ confirm
the authenticity of all the raw data, supervised the study, and
edited language. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and was approved
(approval no. 3157/07.02.2024) by the Ethics Committee for Research
of ‘Louis Turcanu’, Children's Emergency Hospital (Timisoara,
Romania).
Patient consent for publication
Consent for publication of data and associated
images of the patient was provided by the legal guardian of the
minor.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Kaut S, Van den Wyngaert I, Christiaens D,
Wouters C, Noppe N, Herregods N, Dehoorne J and De Somer L: Chronic
nonbacterial osteomyelitis in children: A multicentre Belgian
cohort of 30 children. Pediatr Rheumatol Online J.
20(41)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Koné-Paut I, Mannes I and Dusser P:
Chronic recurrent multifocal osteomyelitis (CRMO) and juvenile
spondyloarthritis (JSpA): To what extent are they related? J Clin
Med. 12(453)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao DY, McCann L, Hahn G and Hedrich CM:
Chronic nonbacterial osteomyelitis (CNO) and chronic recurrent
multifocal osteomyelitis (CRMO). J Transl Autoimmun.
4(100095)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Giedion A, Holthusen W, Masel LF and
Vischer D: Subacute and chronic ‘symmetrical’ osteomyelitis. Ann
Radiol (Paris). 15(32942)1972.PubMed/NCBI(In Multiple languages).
|
|
5
|
Girschick H, Finetti M, Orlando F, Schalm
S, Insalaco A, Ganser G, Nielsen S, Herlin T, Koné-Paut I, Martino
S, et al: The multifaceted presentation of chronic recurrent
multifocal osteomyelitis: A series of 486 cases from the Eurofever
international registry. Rheumatology (Oxford).
57(1504)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Snipaitiene A, Sileikiene R, Klimaite J,
Jasinskiene E, Uktveris R and Jankauskaite L: Unusual case of
chronic recurrent multifocal osteomyelitis. Pediatr Rheumatol
Online J. 16(49)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Costi S, Germinario S, Pandolfi M, Pellico
MR, Amati A, Gattinara M, Chighizola CB, Caporali R and Marino A:
Chronic nonbacterial osteomyelitis and inflammatory bowel disease:
A literature review-based cohort. Children (Basel).
10(502)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roderick MR, Shah R, Rogers V, Finn A and
Ramanan AV: Chronic recurrent multifocal osteomyelitis
(CRMO)-advancing the diagnosis. Pediatr Rheumatol Online J.
14(47)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sergi CM, Miller E, Demellawy DE, Shen F
and Zhang M: Chronic recurrent multifocal osteomyelitis. A
narrative and pictorial review. Front Immunol.
13(959575)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schneider AM and Nelson AM: Skin
microbiota: Friend or foe in pediatric skin health and skin
disease. Pediatr Dermatol. 36:815–822. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rozas M, Hart de Ruijter A, Fabrega MJ,
Zorgani A, Guell M, Paetzold B and Brillet F: From dysbiosis to
healthy skin: Major contributions of cutibacterium acnes to skin
homeostasis. Microorganisms. 9(628)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chang HW, Yan D, Singh R, Liu J, Lu X,
Ucmak D, Lee K, Afifi L, Fadrosh D, Leech J, et al: Alteration of
the cutaneous microbiome in psoriasis and potential role in Th17
polarization. Microbiome. 6(154)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Swords M, Lakehomer H, McDonald M and
Patel J: Symposium-Hindfoot and Ankle Trauma. Indian J
Orthopaedics. 52:161–169. 2018.
|
|
14
|
Kostik MM, Makhova MA, Maletin AS,
Magomedova SM, Sorokina LS, Tsukasaki M, Okamoto K, Takayanagi H,
Vasiliev DS, Kozlova DI and Mushkin AY: Cytokine profile in
patients with chronic non-bacterial osteomyelitis, juvenile
idiopathic arthritis, and insulin-dependent diabetes mellitus.
Cytokine. 143(155521)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hofmann SR, Kapplusch F, Girschick HJ,
Morbach H, Pablik J, Ferguson PJ and Hedrich CM: Chronic recurrent
multifocal osteomyelitis (CRMO): Presentation, pathogenesis, and
treatment. Curr Osteoporos Rep. 15:542–554. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hedrich CM, Hofmann SR, Pablik J, Morbach
H and Girschick HJ: Autoinflammatory bone disorders with special
focus on chronic recurrent multifocal osteomyelitis (CRMO). Pediatr
Rheumatol. 11(47)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zimmermann P and Curtis N: The role of
Cutibacterium acnes in auto-inflammatory bone disorders. Eur J
Pediatr. 178:89–95. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zaid M, Chavez MR, Carrasco AE, Zimel MN,
Zhang AL, Horvai AE, Link TM and O'Donnell RJ: Cutibacterium
(formerly Propionibacterium) acnes clavicular infection. J Bone Jt
Infect. 4:40–49. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rausch P, Hartmann M, Baines JF and von
Bismarck P: Analysis of the fecal and oral microbiota in chronic
recurrent multifocal osteomyelitis. Arthritis Res Ther.
24(54)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jin Z, Song Y and He L: A review of skin
immune processes in acne. Front Immunol. 14(1324930)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Menter A, Krueger GG, Paek SY, Kivelevitch
D, Adamopoulos IE and Langley RG: Interleukin-17 and
Interleukin-23: A narrative review of mechanisms of action in
psoriasis and associated comorbidities. Dermatol Ther (Heidelb).
11:385–400. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hsu DK, Fung MA and Chen HL: Role of skin
and gut microbiota in the pathogenesis of psoriasis, an
inflammatory skin disease. Med Microecol. 4(100016)2020.
|
|
23
|
Nguyen CT, Sah SK, Zouboulis CC and Kim
TY: Inhibitory effects of superoxide dismutase 3 on
Propionibacterium acnes-induced skin inflammation. Sci Rep.
8(4024)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Singhal S, Landes C, Shukla R, McCann LJ
and Hedrich CM: Classification and management strategies for
paediatric chronic nonbacterial osteomyelitis and chronic recurrent
multifocal osteomyelitis. Expert Rev Clin Immunol. 19:1101–1116.
2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Quon JS, Dzus AK and Leswick DA: Case
study: Chronic recurrent multifocal osteomyelitis in the femoral
diaphysis of a young female. Case Rep Radiol.
2012(515761)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mandell GA, Contreras SJ, Conard K, Harcke
HT and Maas KW: Bone scintigraphy in the detection of chronic
recurrent multifocal osteomyelitis. J Nucl Med. 39:1778–1783.
1998.PubMed/NCBI
|
|
27
|
Villani MF, De Horatio LT, Garganese MC,
Casazza I, Savelli S, Pardeo M, Messia V, De Benedetti F and
Insalaco A: Whole-Body MRI versus bone scintigraphy: Which is the
best diagnostic tool in patients with chronic recurrent multifocal
osteomyelitis (CRMO)? Pediatr Rheumatol. 13 (Suppl 1)(P58)2015.
|
|
28
|
Schaal MC, Gendler L, Ammann B, Eberhardt
N, Janda A, Morbach H, Darge K, Girschick H and Beer M: Imaging in
non-bacterial osteomyelitis in children and adolescents: Diagnosis,
differential diagnosis and follow-up-an educational review based on
a literature survey and own clinical experiences. Insights Imaging.
12(113)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen HC, Wuerdeman MF, Chang JH and
Nieves-Robbins NM: The role of whole-body magnetic resonance
imaging in diagnosing chronic recurrent multifocal osteomyelitis.
Radiol Case Rep. 13:485–489. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sangiorgi E and Rigante D: The clinical
chameleon of autoinflammatory diseases in children. Cells.
11(2231)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nakanishi H, Yamada S, Kita J, Shinmura D,
Hosokawa K, Sahara S and Misawa K: Auditory and vestibular
characteristics of NLRP3 inflammasome related autoinflammatory
disorders: Monogenic hearing loss can be improved by
anti-interleukin-1 therapy. Front Neurol. 13(865763)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gregory GE, Munro KJ, Couper KN,
Pathmanaban ON and Brough D: The NLRP3 inflammasome as a target for
sensorineural hearing loss. Clin Immunol.
249(109287)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nakanishi H, Prakash P, Ito T, Kim HJ,
Brewer CC, Harrow D, Roux I, Hosokawa S and Griffith AJ: Genetic
hearing loss associated with autoinflammation. Front Neurol.
11(141)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Taddio A, Zennaro F, Pastore S and Cimaz
R: An update on the pathogenesis and treatment of chronic recurrent
multifocal osteomyelitis in children. Pediatr Drugs. 19:165–172.
2017.PubMed/NCBI View Article : Google Scholar
|