Introduction
Recent statistics highlighted the ongoing global
burden of cancer. In the United States alone, cancer continues to
pose a significant threat to human health, with ~2 million new
cancer cases and over 600,000 cancer-related deaths projected in
2024, emphasizing the critical need for improved diagnostic and
therapeutic strategies (1). The
incidence rates of several cancers, including breast cancer, have
steadily increased in recent years. Specifically, the number of
breast cancer cases rose by 0.6-1% annually between 2015 and 2019,
reflecting its increasing prevalence and the urgent need for
improved prevention and treatment strategies (1). Moreover, in 2024, lung cancer, along
with prostate and colorectal cancers, accounted for 48% of cases in
men, whereas in women, lung, breast, and colorectal cancers
comprised 51% of diagnoses (1).
Cancer therapy has advanced from conventional methods such as
chemotherapy to personalized treatments such as chimeric antigen
receptor T-cell therapy, antibody-drug conjugates and bispecific
T-cell engagers; however, improved treatments are continually
pursued (2).
The capacity of stem cells to self-renew and develop
into other types of cells makes them a potential source for
regenerative medicine (3). Stem
cells are classified into totipotent, pluripotent, multipotent and
unipotent stem cells based on their differentiation potential
(4). Mesenchymal stem cells (MSCs)
are obtained from various organs and tissues, including the brain,
liver, kidney, lung, muscle, thymus, pancreas, skin, adipose
tissue, fetal tissue, umbilical cord, Wharton's jelly (WJ) and
placenta (5). Bone marrow stromal
MSCs (BMSCs) are a subset of multipotent adult stem cells that are
derived primarily from the bone marrow and play a crucial role in
osteogenesis by differentiating into osteoblasts, which are
responsible for bone formation (6).
BMSCs are the most common type of MSCs used in cell therapy and
tissue repair (7). MSCs must meet
the following criteria as outlined by the International Society for
Cellular Therapy (ISCT): i) MSCs must express specific surface
markers including, CD105, CD90, and CD73; ii) they must not express
other surface markers such as CD45, CD34, CD14 or CD11b, CD79α or
CD19, and HLA-DR (8); iii) they
must adhere to plastic in culture conditions; and iv) they be able
to differentiate into adipocytes, chondrocytes, and osteoblasts
in vitro (8).
MSCs are considered promising sources for cell
therapy in regenerative medicine (7). However, several significant challenges
limit their potential for therapeutic applications, including their
limited ability to expand efficiently (9). Furthermore, MSCs are inherently
heterogeneous and display diverse biological properties that
contribute to inconsistent outcomes in clinical trials (10). This heterogeneity is attributed to
differences in donor characteristics, tissue sources, cell surface
markers and variations in microenvironmental and culture conditions
(10). As a result, an increasing
demand for alternative cell sources that can address these
limitations and enhance the effectiveness of cell therapy using
MSCs has been noted.
Adult somatic cells can be effectively converted
into undifferentiated cells, known as induced pluripotent stem
cells (iPSCs) (11,12). These iPSCs share similar
characteristics with embryonic stem cells (ESCs) (13). Notably, iPSCs have a high capacity
for self-renewal and pluripotent differentiation, allowing them to
develop into a variety of cell types, such as MSCs, neurons, and
cardiomyocytes (14). Compared with
conventional MSCs, iPSC-derived MSCs (iPSC-MSCs) have been shown to
exhibit superior cellular viability, including enhanced survival,
proliferation, and differentiation abilities. These properties are
due to a rejuvenation process that occurs during reprogramming
(15,16). The rejuvenation of iPSCs may also
reduce the heterogeneity typically associated with MSCs, which is
often influenced by the tissue source and donor age, thereby
providing a more consistent and reliable cell source for
therapeutic applications (16).
One of the primary challenges facing cell therapy is
the effective delivery of cells to damaged tissues (5). Although studies have revealed that
labeled MSCs delivered in vivo can migrate to injured
tissues, such as brain lesions or cardiac infarcts, only a small
number of MSCs engraft at the injury sites (17-19).
However, the paradigm has shifted toward the hypothesis that MSCs
influence host cells primarily through their paracrine factors
(20,21). The culture medium conditioned by
MSCs, known as conditioned medium (MSC-CM), contains a multitude of
bioactive soluble factors secreted by MSCs, including growth
factors, cytokines, chemokines, enzymes, and extracellular vesicles
(EVs) (22,23). MSC-CM is considered a preferred
option for use in cell therapy due to several key advantages over
cell-based applications: i) It avoids the risk of host immune
reactions; ii) it can be stored for relatively long periods without
the use of toxic cryopreservatives such as DMSO; iii) it is
cost-effective; and iv) the process of evaluating the safety and
efficacy of MSC-CM is considerably simpler than that of
conventional pharmaceutical agents or MSCs (21). MSC-derived extracellular vesicles
(MSC-EVs) have garnered significant attention due to their primary
role in mediating cellular communication (24). MSC-EVs also modify the activity of
target cells by transferring mRNAs, microRNAs (miRNAs or miRs),
lipids, and proteins (25).
The therapeutic importance of EVs, and their ability
to modulate target cells and enhance tissue repair, has been
previously demonstrated (26).
Traditionally, EVs are classified into three subtypes based on
their size and biogenesis, namely, exosomes, microvesicles (MVs),
and apoptotic bodies (27-29).
However, some studies have categorized apoptotic bodies, exosomes
and exosome-like vesicles as distinct types of EVs, whereas other
studies have focused only on microvesicles and exosomes due to a
lack of sufficient evidence supporting the classification of other
types of EVs (30,31).
EVs are heterogeneous membranous structures that
originate from the endosomal system or are shed from the plasma
membrane and are secreted by cells through different mechanisms
(32). EVs are composed of
different subtypes, primarily exosomes and microvesicles, which
vary in size, origin, and content (33,34).
Exosomes are nanosized vesicles ranging from 30 to 200 nm in
diameter that contain proteins, mRNAs, and miRNAs (35,36).
They play key roles in various cancer-related biological processes,
such as angiogenesis, metastasis, and immune system regulation, and
influence the tumor microenvironment (37). Moreover, MSC-derived exosomes have
been utilized in engineered systems for targeted delivery,
enhancing therapeutic efficacy in various diseases and tissue
regeneration applications (38-40).
MSC-exosomes (Exos) including BMSC-Exos have been
studied for their potential in cancer therapy, particularly as a
delivery system for therapeutic molecules, with numerous studies
focusing on their effects on tumor progression and the treatment
response. For example, Zhou et al (41) demonstrated that BM-MSC-derived
exosomes loaded with paclitaxel and gemcitabine monophosphate
improved drug delivery and efficacy in PDAC, addressing
chemoresistance and ECM abnormalities. Lin et al (42) reviewed the role of MSC-Exos in
shaping the tumor microenvironment and driving therapy resistance,
providing insights into their molecular mechanisms and clinical
applications. In breast cancer, MSC-EVs modulated stemness markers
(OCT4 and ALDH1) and proliferation in a concentration-dependent
manner in MCF7 cells (43). In lung
cancer, BMSC-Exos promoted invasion, migration, and metastasis by
delivering miR-425 which suppressed cytoplasmic polyadenylation
binding protein 1 (CPEB1) expression (44). Together, these findings highlight
the therapeutic potential of MSC-Exos across various cancer
models.
Moreover, Zhao et al (45) described the therapeutic potential of
induced pluripotent stem cell-derived MSC (iMSC)-Exos in enhancing
treatment responses in metastatic prostate cancer, highlighting the
potential role of iMSC-Exos as anticancer agents. However, in
contrast to that of BMSC-Exos, the role of iMSC-Exos in cancer
therapy remains less explored. The present study focused on
comparing the effects of BMSC-Exos and iMSC-Exos on MCF7 and A549
cancer models. The MCF7 cell line, a widely recognized model of
human breast cancer, has been used extensively in advancing
therapeutic strategies and exploring cancer biology (46,47).
Similarly, the A549 cell line, a model of non-small cell lung
cancer, has been pivotal for exploring cancer treatments and their
underlying biology (48-50).
The present study explored the effects of exosomes derived from
BMSCs and iMSCs on lung and breast cancer, specifically the A549
lung cancer and MCF7 breast cancer cell lines.
Material and methods
Cell culture
In the present study, BMSCs were previously isolated
from 2 male and 2 female non-smoking patients (25-45 years old),
who were referred to the Orthopedic Department of Jordan University
Hospital (JUH)/University of Jordan (Amman, Jordan) after
sustaining orthopedic fractures from road traffic accidents
(51). Institutional Review Board
(IRB) approval (approval no. IRB/7/2019) for obtaining BMSCs was
issued by the IRB Committee of the Cell Therapy Center
(CTC)/University of Jordan. Informed consent was also obtained from
all patients to donate their tissues, as specified in The
Declaration of Helsinki, prior to participation.
Bone marrow samples were collected by iliac crest
aspiration. The inclusion criteria for patient selection were as
follows: i) Males or females, 25-45 years old; ii) those with
orthopedic fractures or trauma resulting from traffic accidents
requiring orthopedic intervention, in which iliac crest bone marrow
aspiration did not pose any additional risk; and iii)
hemodynamically stable (normal vital signs or stabilized
post-resuscitation). While the exclusion criteria were as follows:
i) History of, or evidence of, hematologic disorders, including
leukemia, lymphoma, or aplastic anemia; ii) major mental health
disorders that preclude participation in the present study; iii)
type I or type II diabetes mellitus, chronic hypertension, or
receiving antidiabetic medications; iv) severe anemia (hemoglobin
<8 g/dl); v) active systemic infection (such as sepsis and
osteomyelitis) that may contaminate the bone marrow; vi) a
clinically active autoimmune condition; vii) history of
chemotherapy or radiation therapy, which may alter bone marrow cell
composition; and viii) positive serological evidence of HIV I and
II, HBV, HCV, and VDRL.
All participants met the inclusion criteria and had
none of the exclusion conditions, confirming their suitability for
the present study.
Bone marrow sample collection began on December 1,
2019, and continued for 1 year until January 1, 2021. Bone marrow
samples were aseptically collected in 12-16 EDTA tubes.
Subsequently, the buffy coat was isolated by centrifugation [300 x
g, 6 min, at room temperature (RT)], suspended in 1.5 ml
phosphate-buffered saline (PBS), and used for culture. Then, 5 ml
of the separated buffy coat was layered onto an equal volume of
Ficoll (Cytiva) and centrifuged (500 x g, 30 min, at RT with the
centrifuge brake turned to off).
For the present study, all the experimental
procedures were approved (approval no. IRB-7-2019-8) by the IRB
Committee at the CTC, University of Jordan. BMSCs were cultured at
passage 3 in minimum essential medium Eagle-alpha modification
(αMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 15%
fetal bovine serum (FBS; Cytiva), 1% of 100X Glutamax (Gibco;
Thermo Fisher Scientific, Inc.), and 1% of 100X
antibiotic-antimycotic mixture (Gibco; Thermo Fisher Scientific,
Inc.). Breast cancer (MCF7, ATCC® HTB-22™)
and alveolar basal epithelial adenocarcinoma (A549,
ATCC® CCL-185™) were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS, 1% of 100X Glutamax, and 1% of 100X
antibiotic-antimycotic. Moreover, human dermal fibroblast cells
used were previously prepared (49,50)
and cultured in Advanced DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS, 1% of 100X Glutamax, and 1% of
100X antibiotic-antimycotic mixture. All cells were maintained and
incubated at 37˚C, 21% O2, and 5% CO2, with
media changes every 2 days.
Generation of embryoid body-derived
iMSCs (EB-iMSCs)
The four iPSC lines used in the present study are as
follows: JUCTCi010-A, derived from skin dermal fibroblasts of a
healthy 27-year-old Jordanian female (49); JUCTCi010-B, derived from MSCs
obtained from skin dermal fibroblasts of a 27-year-old Jordanian
female (49); JUCTCi011-A and
JUCTCi011-B, derived from skin dermal fibroblasts of a 34-year-old
Jordanian male (50). The
generation of these lines was approved (approval no. IRB/07/2017)
by the Cell therapy Center (CTC), University of Jordan, Amman,
Jordan, which covered JUCTCi010-A and B as well as JUCTCi011-A and
B. This IRB approval has been documented in previous publications
by the authors on female (52) and
male (53) iPSC lines.
For coating plates used to culture iPSCs in mTeSR
media (STEMCELL Technologies), Matrigel was diluted 1:100 in
DMEM/F12 to achieve a final concentration of ~500 µg/ml. For a
6-well plate, 1 ml of the diluted Matrigel solution was added per
well and incubated for at least 1 h at 37˚C before use (51). For differentiation, the iPSC
monolayer cultures were detached using 0.5 M EDTA (Gibco; Thermo
Fisher Scientific, Inc.), and the resulting cell suspensions were
plated in MSC differentiation media on ultra-low attachment plates
to form EBs (54). The MSC
differentiation media consisted of αMEM, supplemented with 15% FBS,
1% 100X Glutamax, and 1% 100X antibiotic-antimycotic mixture
(54).
On days 2 and 4 of differentiation, the media were
replaced with fresh media containing 10 µM retinoic acid (RA; Merck
KGaA) and 0.1 µM RA, respectively. On day 6, the media were
switched to RA-free differentiation media and on day 7, the EBs
were plated on Matrigel-coated plates and maintained in MSC
differentiation media. The differentiation media were replaced
every two days. On day 12, 2.5 ng/ml basic fibroblast growth factor
(R&D Systems, Inc.) was added, with media changes occurring
every 2 days thereafter. iMSCs were passaged upon reaching 80-90%
confluency and were then cryopreserved in 1X freezing media
composed of 90% FBS and 10% DMSO and stored in liquid nitrogen.
Osteogenic differentiation
Cells were seeded in triplicate at a density of
200,000 cells per well in 6-well plates and cultured in complete
culture medium (CCM) until reaching 50% confluency. The medium was
then replaced with osteogenic differentiation medium consisting of
αMEM supplemented with 15% FBS, 1% 100X Glutamax, 1% 100X
antibiotic-antimycotic mixture, 10 mM dexamethasone (Merck KGaA),
50 µg/ml ascorbic acid 2-phosphate (Merck KGaA), and 10 mM
β-glycerophosphate (Carbosynth Ltd.) for 21-28 days, or until
calcium deposits appeared. Control cells remained in CCM with
medium changes every 2-3 days. Upon observation of mineral
deposits, one well from each sample was stained with Alizarin Red
(Merck KGaA) for 5 min at RT to visualize calcium deposits, which
were then imaged using the EVOS XL Core Imaging System (Thermo
Fisher Scientific, Inc.).
Adipogenic differentiation
EB-iMSCs and BMSCs were seeded in triplicate at a
density of 200,000 cells per well and cultured in CCM until
reaching 50% confluency. They were then switched to adipogenic
differentiation medium consisting of αMEM supplemented with 15%
FBS, 1% 100X Glutamax, 1% 100X antibiotic-antimycotic mixture, 10
mM dexamethasone, 500 µM 3-isobutyl-1-methylxanthine (IBMX), 0.2 mM
indomethacin, and 10 µg/ml insulin (all from Merck KGaA) and
maintained for 14-21 days, with medium changes every 2-3 days,
until fat vacuoles were visible. Control cells remained in CCM.
Upon differentiation, the cells were stained with Oil Red-O (Merck
KGaA) for 5 min at RT to visualize fat vacuoles, which were then
imaged using the EVOS XL Core Imaging System.
Flow cytometry of iMSC and human
(h)MSC surface markers
Cells were assessed by flow cytometry using BD Stem
Flow hMSC Analysis kit (cat. no. 562245, BD Biosciences) and
according to the manufacturer's instructions. iMSCs and BMSCs were
trypsinized at early passages (passage <8) and washed with 1X
PBS. The cells were then resuspended in 800 µl of 1% bovine serum
albumin (BSA) staining buffer. Next, the cells were incubated for
30 min with fluorescently conjugated antibodies targeting human MSC
surface markers (FITC anti-CD90, PerCP anti-CD105, APC anti-CD73,
and PE anti-CD44; all included in th aforementioned kit) or with
appropriate isotype controls (all from BD Biosciences) at a
concentration of 50 µg/ml for FITC anti-CD90, PerCP anti-CD105, and
APC anti-CD73, and 20 µg/ml for anti-CD44 and the isotype controls.
The absence of negative cocktail surface markers was also.
Following incubation, the cells were washed twice with 1X PBS to
remove any unbound antibodies and then resuspended in 200 µl of
PBS. The expression of surface markers was analyzed using a BD
FACSCanto II flow cytometer (BD Biosciences), and the data were
processed using BD FACSDiva software (BD Biosciences) (55).
Exosome preparation
After reaching 70-80% confluency, cells of iMSCs and
BMSCs at passage 3 were washed with PBS and then cultured in
serum-free α-MEM for 48 h. The conditioned media were then
collected and centrifuged at 300 x g for 10 min at 4˚C, followed by
centrifugation in 2,000 x g for 20 min at 4˚C. Following
centrifugation, the supernatants were filtered through 0.22-µm
filter units to remove any remaining cell debris, which could
contribute to exosome aggregation during the filtration process. To
ensure consistent exosome pelleting, the filtered supernatant was
then ultracentrifuged at 110,000 x g for 2 h at 4˚C using a
fixed-angle rotor. After purification, the exosome pellets
(iMSC-Exos and BMSC-Exos) were gently resuspended in 500 µl
filtered PBS to avoid disrupting the lipid bilayer, which could
promote aggregation. The concentration of the exosomes was measured
using the Micro BCA™ Protein Assay Kit (cat. no. 23235;
Thermo Fisher Scientific, Inc.), strictly following the
manufacturer's instructions. The measured concentration was
standardized to 1 mg/ml. The exosomes were then aliquoted,
resuspended in filtered PBS, and stored at -80˚C to preserve their
structural integrity and reduce aggregation over time for future
use (56).
iMSC-Exos and BMSC-Exos surface
markers analysis
A total of ~100 µg of isolated exosomes were
incubated with 3 µl of aldehyde/sulfate latex beads (4% w/v, 4 µm,
Invitrogen; Thermo Fisher Scientific, Inc.) (57) for 15 min and then incubated
overnight at RT with gentle shaking (270˚ shaking) after adding 10
µl of filtered 1X PBS. Subsequently, the exosome-bead binding was
blocked by adding 1M glycine for 15 min at RT, which was prepared
by dissolving 0.7507 g of 0.01 M glycine (Millipore, Sigma), 200 mg
of 2% BSA (Abcam), and 10 ml of 1X PBS, followed by filtration
using 0.22 µm filter units. The exosome-coated beads were then
incubated for 40 min at 37˚C with various antibodies (A5488
anti-CD9 (cat. no. FAB1880G), AF647 anti-CD81 (cat. no. FAB4615R),
and APC anti-CD63 (cat no. FAB5417A) or their respective isotype
controls (all from BioTechne; R&D Systems, Inc.) according to
the manufacturer's instructions. Finally, 150 µl of filtered 1X PBS
was added to the samples, which were then processed on a BD
FACSCanto II and analyzed using BD FACSDiva software.
Transmission electron microscopy
(TEM)
A total of 100 µg of purified exosomes were mixed
1:1 with 2% paraformaldehyde (PFA; MilliporeSigma), and applied to
Formvar-carbon-coated electron microscopy grids [Electron
Microscopy Sciences (EMS)] to promote membrane absorption for 20
min in a dry environment at RT. The grids were then rinsed with 1X
PBS and subsequently immersed in 1% glutaraldehyde for 5 min at RT
to eliminate any negative background. Following this, the grids
were washed seven times with distilled water, with each wash
lasting 2 min. To enhance contrast, the grids were treated with
uranyl oxalate for 5 min at RT and then placed on methyl
cellulose-UA for 10 min on ice. Finally, the grids were allowed to
air dry for 10 min at RT before being examined under TEM with a
magnification of 500 nm at an acceleration voltage of 30 kV, using
VERSA 3D (FEI; Thermo Fisher Scientific, Inc.).
Size distribution measurement of
exosomes
Size distribution was measured using a dynamic light
scattering (DLS) nanosizer, the ZetaView (serial no. MAL1137709)
(Malvern Nano ZS; Malvern Panalytical, Ltd.), which analyzes
particle sizes ranging from 0.3 nm to 10 µm. Data were processed
using Zetasizer software version 7.11 (Malvern Panalytical, Ltd.).
The temperature was maintained at 24˚C throughout the process. The
data acquisition settings were configured as follows: A measurement
angle of 173˚ backscatter, 10 runs per measurement, 60 sec per run,
3 total measurements, and a 10-sec delay between measurements. All
settings followed the manufacturer's recommendations for EV
analysis. Prior to measurement, samples were diluted with filtered
PBS.
Cellular uptake of exosomes
iMSC-Exos and BMSC-Exos were labeled with
1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate
(DiI-O; Thermo Fisher Scientific, Inc.) fluorescent dye according
to the manufacturer's instructions. Briefly, 200 µl of the isolated
exosomes were incubated with 5-8 µl/1 ml of the dye for 1 h at RT,
then added to 4 ml of filtered 1X PBS, ultracentrifuged at 110,000
x g for 1.5 h at 4˚C, and resuspended in 700 µl of filtered 1X PBS.
The medium of MCF7 or A549 or fibroblasts cells (all at
1.5x105 cells per well) was then replaced with
serum-free medium (SFM) containing 50 µg/ml of iMSC-Exos or
BMSC-Exos. After 16 h of treatment, the cells were washed with 1X
PBS and incubated for an additional 16 h. Subsequently, the cells
were incubated for 30 min at RT with 5-chloromethylfluorescein
diacetate dye (CMFDA; Invitrogen; Thermo Fisher Scientific, Inc.),
fixed with 4% PFA, incubated for 15 min at RT, washed with 1X PBS,
and stained for 5 min at RT with 4',6-diamidino-2-phenylindole
(DAPI; Invitrogen; Thermo Fisher Scientific, Inc.). Finally, the
cells were mounted with mounting medium (Abcam). The cellular
internalization of iMSC-Exos or BMSC-Exos was observed under a
time-lapse microscope (Carl Zeiss).
Cell proliferation assay
MCF7, A549 and fibroblast cells (8x103
cells per well) were cultured in 96-well plates with 100 µl of
RPMI-1640 medium for 24 h at 37˚C and 5% CO2. Following
this initial incubation, the medium was replaced with serum-free
RPMI-1640 containing 350 µl of either iMSC-Exos or BMSC-Exos (50
µg/ml). Cell proliferation was measured after 24, 48, and 72 h by
adding 10 µl of thiazolyl blue tetrazolium bromide reagent (MTT;
Promega) to each well, followed by a 3-h incubation at 37˚C.
Subsequently, 100 µl of solubilization stop solution (Promega
Corporation) was added, and the plates were incubated for an
additional 30 min at 37˚C. The absorbance of the cells was then
measured at 570 nm using a BioTek microplate reader (5), and the data were analyzed with BioTek
Gen 5 data analysis software (BioTek; Agilent Technologies,
Inc.).
Apoptosis analysis
Cell apoptosis was assessed using the
eBioscience™ Annexin V-FITC Apoptosis Detection Kit
(cat. no. 88-8005-74; Invitrogen; Thermo Fisher Scientific, Inc.)
and analyzed by flow cytometry. Briefly, A549 and MCF7 cell lines
were seeded at a density of 3x105 cells per well and
treated with or without 50 µg/ml of iMSC-Exos or BMSC-Exos for 48 h
after replacing the medium with serum-free RPMI-1640. Following
treatment, cells were collected and washed with 1X PBS. The cell
pellet was then resuspended in 100 µl of 1X Binding Buffer, mixed
with 5 µl of FITC-conjugated Annexin V and incubated in the dark at
RT for 15 min. Subsequently, 5 µl of propidium iodide (PI) was
added to each sample, followed by 100 µl of 1X Binding Buffer to
dilute the cell suspension. The samples were then analyzed using a
BD FACSCanto II flow cytometer and BD FACSDiva software.
Scratch wound assay
MCF7 or A549 cells were seeded at a density of
3x105 cells per well. Upon reaching 90% confluency, an
artificial wound was created by scratching the cell monolayer with
a 200-µl pipette tip. The medium was then changed to serum-free
RPMI-1640, and the cells were washed twice with 1X PBS before being
treated with 50 µg/ml of iMSC-Exos or BMSC-Exos. Images were
recorded using EVOS XL Core Imaging System (Thermo Fisher
Scientific, Inc.), at 0, 9, 20, and 47 h after treatment and the
distance of cell migration was measured using ImageJ software
(version 1.53; National Institutes of Health) (5).
Senescence assay
Senescence-associated β-galactosidase (SA-βGal)
staining was performed on treated cells using a senescence
detection kit (cat. no. ab65351; Abcam). Briefly, MCF7 or A549
cells were seeded at a density of 3x105 cells per well
in RPMI-1640 medium. The following day, the medium was replaced
with serum-free RPMI-1640 containing 50 µg/ml of either iMSC-Exos
or BMSC-Exos. After 48 h, the medium was aspirated, and the cells
were washed once with 1 ml of 1X PBS and fixed with 0.5 ml of
fixative solution provided in the kit for 15 min at RT.
Subsequently, 0.5 ml of staining solution, prepared by mixing 5 µl
of 100X staining supplement, and 25 µl of 20 mg/ml X-Gal in
dimethylsulfoxide with 470 µl of staining solution (all included in
the aforementioned kit), was added, and the cells were incubated at
37˚C for 12 h. The cells were then observed under a microscope
(EVOS XL Core Imaging System; Thermo Fisher Scientific, Inc.) to
investigate the development of blue color, which indicates
senescence, and were manually counted and compared to untreated
cells.
Statistical analysis
All data were analyzed using GraphPad Prism version
9.1.0 (Dotmatics). The statistical tests included an unpaired
Student's t-test or a two-way analysis of variance (ANOVA),
followed by a Bonferroni post-hoc test when indicated. Data are
presented as the mean ± standard error (SE), and a P≤0.05 was
considered to indicate a statistically significant difference. All
the experiments were conducted in three independent replicates
(n=3).
Results
Characterization of iMSC and BMSC
surface markers by flow cytometry
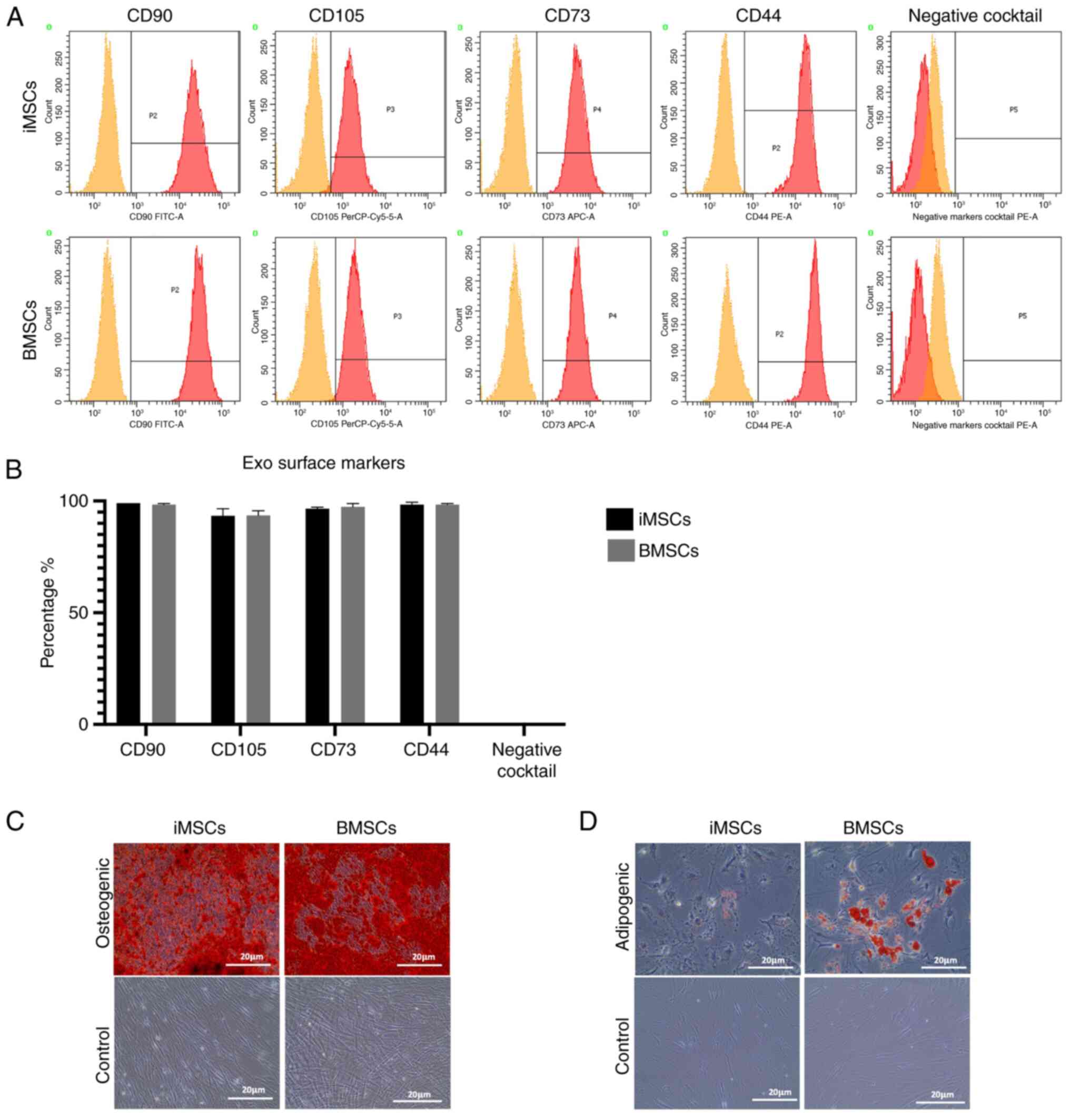
A flow cytometric analysis was initially performed
to detect the expression of stem cell surface markers on iMSCs and
BMSCs. The analysis clearly revealed the expression of CD105, CD90,
CD73, and CD44 on both iMSCs and BMSCs (Fig. 1A), with negative expression of CD34,
CD11b, CD19, CD45, and HLA-DR surface markers. The expression
percentages of the aforementioned markers in the BMSCs were as
follows: CD105 was expressed in 97% of the cells, CD90 was
expressed in 98.6%, CD73 was expressed in 99%, and CD44 was
expressed in 98.7% (Fig. 1B). In
comparison, the average expression percentages for iMSCs were 91%
for CD105, 98.3% for CD90, 99% for CD73, and 99% for CD44 (Fig. 1B). Additionally, both iMSCs and
BMSCs were tested for their ability to differentiate into
osteogenic (Fig. 1C) and adipogenic
(Fig. 1D) lineages. The results
obtained from the present study revealed the positive
differentiation potential of both cell types, with BMSCs showing
higher levels of adipogenic differentiation than iMSCs.
Characterization of isolated BMSC-Exos
and iMSC-Exos
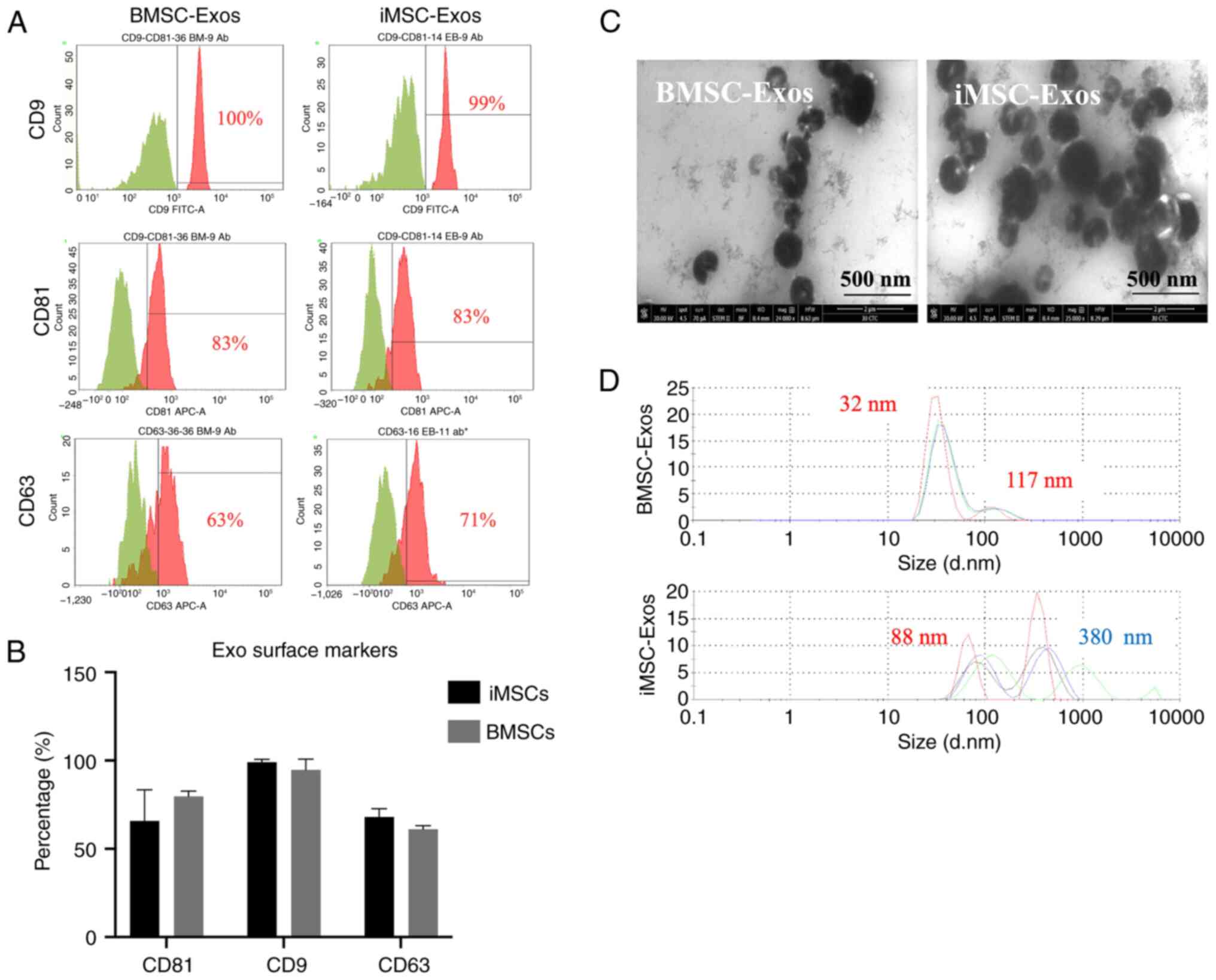
The exosomes were bound to sulfate-latex beads to
detect exosome surface markers using flow cytometry (CD81, CD9, and
CD63), as aforementioned. The analysis confirmed the successful
expression of these markers in both the iMSC-Exos and the
BMSC-Exos. Notably, both types of exosomes presented higher
expression levels of the CD9 tetraspanin protein than CD81 and
CD63. However, no significant differences in the expression of
exosome surface markers were observed between the two groups
(Fig. 2A and B). The average percentages of these
markers in iMSC-Exos were as follows: CD9 (99%), CD81 (79%), and
CD63 (64%). In comparison, BMSC-Exos exhibited average percentages
of CD9 of 95%, CD81 of 77%, and CD63 of 40% (Fig. 2A and B). The size distribution analysis revealed
that both iMSC- and BMSC-derived exosomes exhibited heterogeneous
size ranges of 88-220 nm and 32-117 nm, respectively, with
iMSC-Exos having a larger average size than BMSC-Exos (Fig. 2D). TEM revealed a typical cup-like
shape of both iMSC-Exos and BMSC-Exos (Fig. 2C). Additionally, the aggregates
observed for the iMSC-Exos were consistent with the DLS findings,
which indicated a larger average size for the iMSC-Exos than for
the BMSC-Exos. Overall, the characteristics of the isolated EVs
were consistent with those of the exosomes.
Internalization of iMSC- and
BMSC-derived exosomes
Subsequently, the uptake efficiency of exosomes by
two cancer cell lines was assessed (namely, MCF7 and A549, as well
as nonmalignant fibroblasts) by incubating them with either
iMSC-derived or BMSC-derived exosomes for 12 h. The exosomes were
prelabeled with DiI-O, a lipophilic red-orange, fluorescent dye.
The cells were also stained with CMFDA (green) to visualize the
cytoplasm, and DAPI (blue) was used for nuclear staining. After 12
h of incubation with DiI-O-labeled exosomes, the cells were fixed
and examined under a fluorescence microscope. Imaging data
confirmed the successful internalization of iMSC-Exos and BMSC-Exos
into MCF7 (Fig. 3A) and A549 cells
(Fig. 3B), as well as nonmalignant
fibroblasts (Fig. S1).
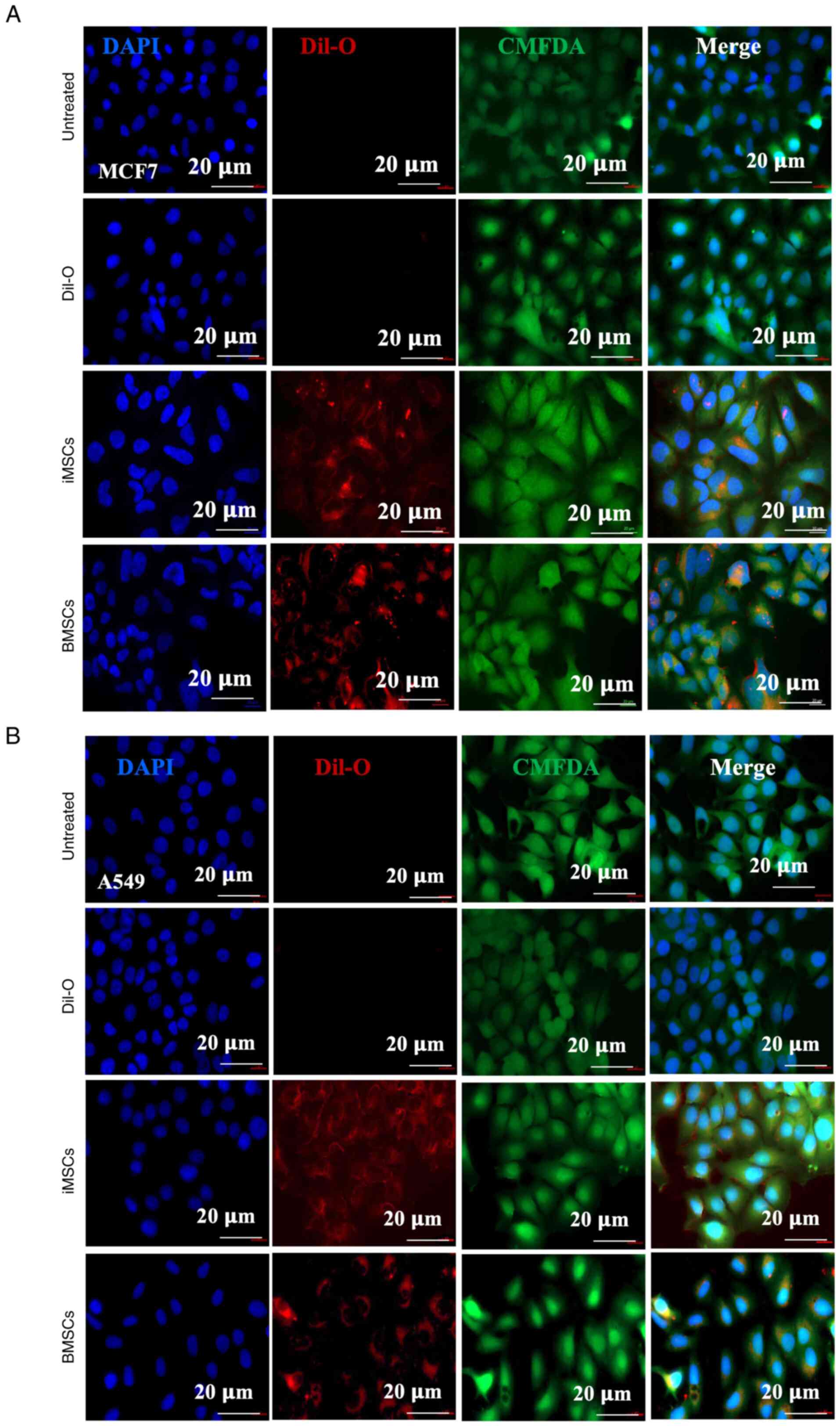 | Figure 3Assessment of the internalization of
iMSC-Exos and BMSC-Exos into cancer cells. (A) MCF7 and (B) A549
cells were either left untreated (first panel), incubated with
DiI-O dye only (second panel), or incubated with DiI-O-labeled
exosomes from iMSCs (third panel) and BMSCs (fourth panel). DAPI
staining was used to visualize the nuclei and CMFDA to stain the
cell bodies. Scale bar, 20 µm. (n=3). iMSCs, induced pluripotent
stem cell-derived mesenchymal stem cells; Exos, exosomes; BMSCs,
bone marrow stromal mesenchymal stem cells; DiI-O,
1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate;
CMFDA, 5-chloromethylfluorescein diacetate dye; DAPI,
4',6-diamidino-2-phenylindole. |
Transient suppression of the
proliferation of MCF7 cells is observed with both exosome types,
whereas the proliferation of A549 cells is suppressed by
iMSC-Exos
The effects of both exosome types on cancer cell
proliferation and survival were examined. Cancer cell proliferation
was assessed using an MTT assay to evaluate the effects of
iMSC-Exos and BMSC-Exos at 24 and 48 h of treatment. Compared to
untreated SFM cells, both MCF7 and A549 cells showed a significant
suppression of proliferation after 24 h of treatment with either
type of exosome (P≤0.0001 for both) (Fig. 4A and B). Moreover, BMSC-Exos had a greater
inhibitory effect than iMSC-Exos on MCF7 cells at 24 h after
treatment (P≤0.01).
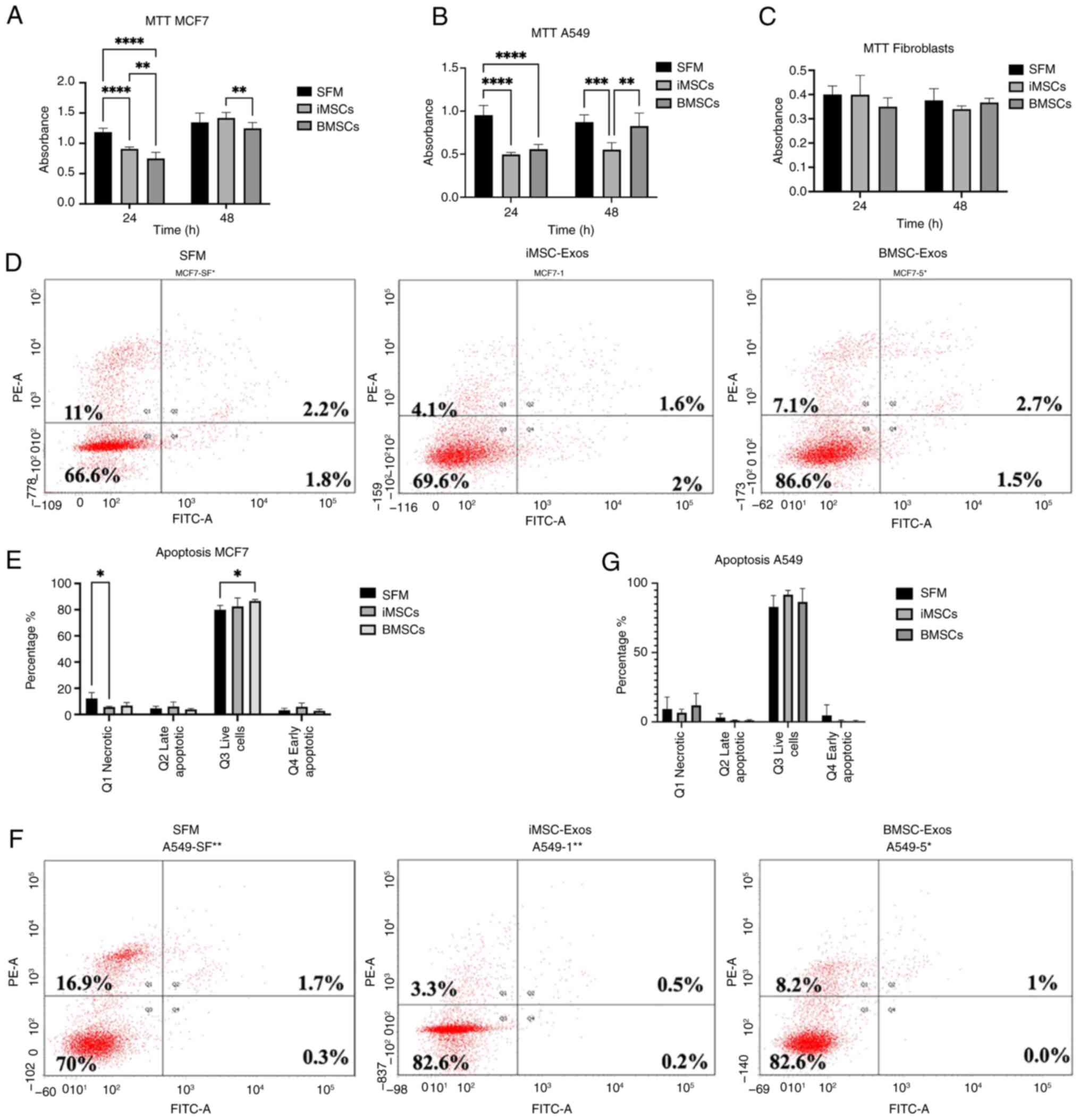 | Figure 4Assessment of proliferation and
apoptosis on treated MCF7 and A549 cells with iMSC-Exos or
BMSC-Exos. An MTT assay was used to assess the proliferation of (A)
MCF7, (B) A549, and (C) fibroblasts following 24 and 48 h of
incubation with iMSC-Exos, BMSC-Exos, or SFM. Representative FACS
plot images of (D) MCF7 and (F) A549 cells. (E) Apoptosis assay
results for treated MCF7 cells, showing Q1 (necrotic cells), Q2
(late apoptotic cells), Q3 (live cells), and Q4 (early apoptotic
cells). (G) Apoptosis assay results for treated A549 cells. Data
are presented as the mean ± SE; (n=3). *P≤0.05,
**P≤0.01 ***P≤0.001, ****P≤0.0001.
iMSCs, induced pluripotent stem cell-derived mesenchymal stem
cells; Exos, exosomes; BMSCs, bone marrow stromal mesenchymal stem
cells; SFM, serum-free medium; FACS, fluorescence-activated cell
sorting; SE, standard error. |
Notably, the significant antitumor effect observed
at 24 h compared with that of SFM did not persist at 48 h in MCF7
cells treated with both types of exosomes or in A549 cells treated
with BMSC-Exos, indicating a transient effect of BMSC-Exos on MCF7
and A549 cells and of iMSC-Exos on MCF7 cells. However, within 48 h
of treating the MCF7 cells with the BMSC-Exos, cell proliferation
decreased compared with that of the MCF7 cells treated with the
iMSC-Exos (P≤0.01). In A549 cells, the suppressive effect of
iMSC-Exos persisted over time, as evidenced by a continued
reduction in proliferation at 48 h compared with that of the SFM
control (P≤0.001) and BMSC-Exos (P≤0.01). Additionally, the
treatment of fibroblasts that were used as control cells with
either type of exosome had no significant effect on proliferation
(Fig. 4C). The findings of the
present study suggested that the antiproliferative effect may be
cancer-specific.
Next, the effect of the BMSC-Exos and iMSC-Exos on
the induction of apoptosis in the MCF7 and A549 cells was assessed
using flow cytometry of the Annexin V/PI-stained cells. Treatment
of MCF7 cells with either iMSC-Exos or BMSC-Exos for 48 h resulted
in no significant increase in the number of early apoptotic cells
(Annexin V+/PI−) or late apoptotic cells
(Annexin V+/PI+) (Fig. 4E). Similarly, no significant
differences in apoptosis were observed in A549 cells treated with
either BMSC-Exos or iMSC-Exos (Fig.
4G).
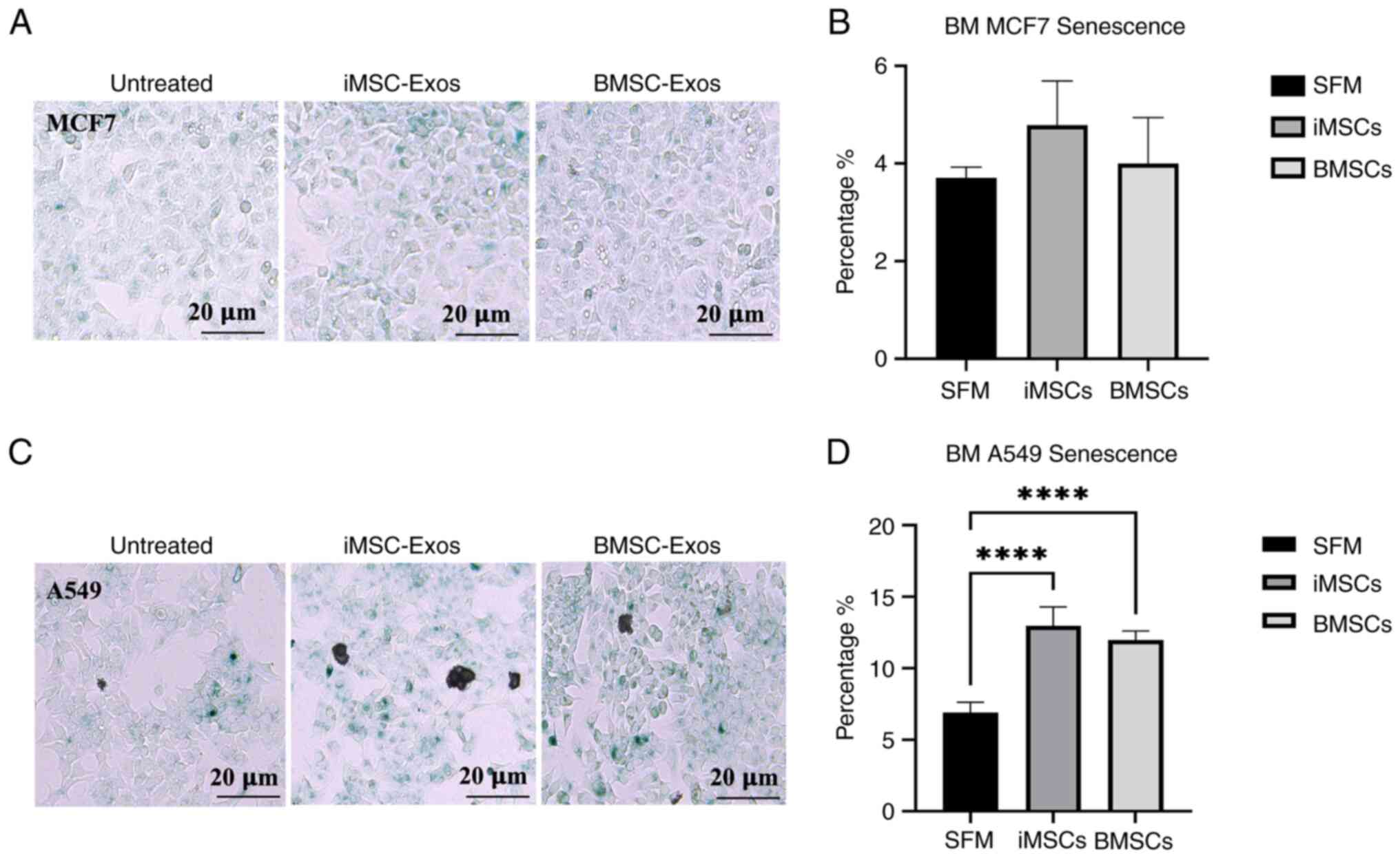
Significant increase in the number of
SA-βGal-positive A549 cells after treatment
The percentage of cells positive for SA-βGal
activity, which reflects cellular senescence, was determined by
counting the number of blue cells in the total population, as
described by Debacq-Chainiaux et al (58). In MCF7 cells treated with iMSC-Exos
or BMSC-Exos, the percentage of SA-βGal-positive cells was not
significantly different (Fig. 5A
and B). The findings of the present
study indicated that no significant induction of cellular
senescence occurred in MCF7 cells treated with either type of
exosome. By contrast, treatment of A549 cells with either BMSC-Exos
or iMSC-Exos resulted in a significant increase in senescence
compared with that of the control (both P<0.0001) (Fig. 5C and D). These results indicate that the effect
of exosome treatment on cellular senescence in tumor cells may be
minimal.
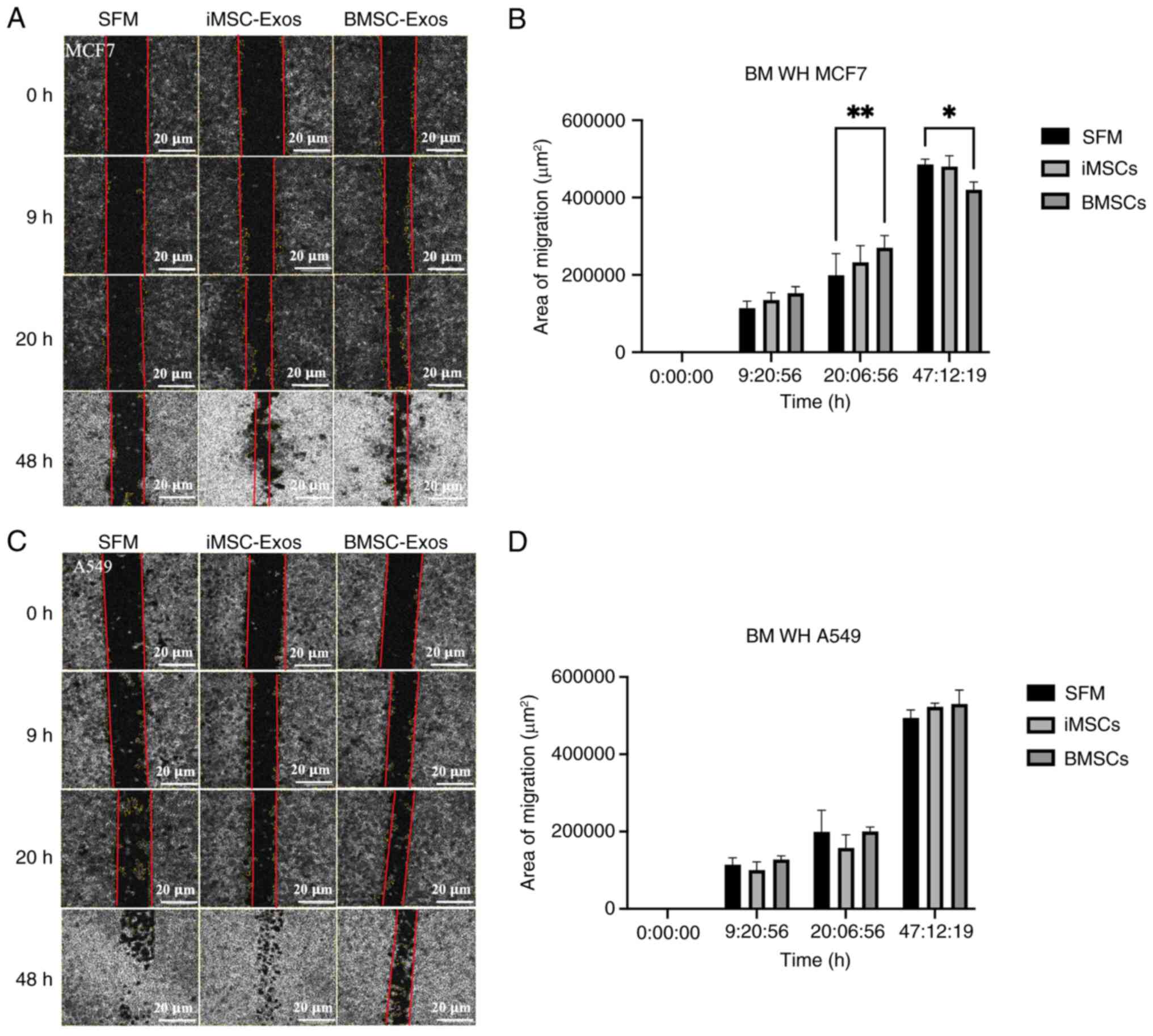
Time-dependent effects of iMSC- and
BMSC-derived exosomes on migration
A scratch wound healing assay was performed to
assess the effects of iMSC- and BMSC-derived exosomes on MCF7 and
A549 cancer cells. A scratch was made through the monolayers of
cancer cells, and microscopy images of the scratch wounds were
captured at ~9, 20, and 47 h after treatment with iMSC- or
BMSC-derived exosomes. In MCF7 cells treated with BMSC-derived
exosomes, a significant increase in migration was observed at ~20 h
(P≤0.01) (Fig. 6A and B). However, this effect was reversed at
~47 h (P≤0.05), indicating a time-dependent effect of the BMSC-Exos
on these cells. By contrast, no significant difference in migration
was observed in A549 cells treated with either type of exosomes
compared with the SFM control (Fig.
6C and D).
Discussion
iPSCs represent a promising alternative to
traditional MSC sources, potentially overcoming the challenges
associated with limited expandability and source variability
(16). BMSC-derived exosomes have
demonstrated significant therapeutic potential in various bone
diseases and are gaining recognition for their broader applications
in regenerative medicine, particularly in orthopedic conditions
(59). For example, they protect
against cartilage damage and alleviate knee pain in osteoarthritis
models (60) and promote
chondrocyte proliferation to mitigate osteoarthritis (61). The relationship between MSC-derived
exosomes and cancer remains ambiguous, as studies have reported
conflicting results regarding the effects of MSC-derived EVs on
cancer (62,63). These conflicting results may be
attributed to variations in the origin of MSCs, the methods used
for isolating MSCs (64), or the
specific types of tumor models employed in the studies (64,65).
However, the therapeutic potential of iPSC-derived MSCs and their
exosomes requires further investigation to fully understand their
impacts on cancer. The present study provided insights into the
comparative effects of exosomes derived from iMSCs and BMSCs on
cancer cells, contributing to ongoing research efforts aimed at
elucidating their roles in cancer biology.
In the present study, both iMSCs and BMSCs were
successfully characterized according to the ISCT criteria (66). These cells exhibited positive
expression of stem cell surface markers (CD90, CD105, CD73, and
CD44), a spindle-like morphology, adherence to plastic, and the
ability to differentiate into osteogenic and adipogenic lineages
in vitro. Previous research, such as work by Maleki et
al (67), has reported that the
expression of MSC surface markers can vary significantly depending
on the source of the MSCs. In their study, four types of MSCs
[spermatogonial stem cells (SSCs), hair follicle stem cells,
granulosa cells, and WJ-MSCs], were analyzed. SSCs showed the
highest expression of CD44, which is associated with maintaining
stemness, CD90, that is linked to growth and differentiation, and
CD105, which plays a role in osteogenesis. However, despite these
potential differences, both the BMSCs and iMSCs in the present
study exhibited high expression levels of classical hMSC markers
(>90%). Exosomes were isolated through the sequential
centrifugation and filtration of CM, followed by
ultracentrifugation. These exosomes were characterized using flow
cytometry to detect exosome surface markers from the tetraspanin
family, including CD9, CD81, and CD63(68). Both groups of exosomes expressed
these markers, with a higher percentage of CD9 expression observed
in each set. Notably, CD9 is associated with modulating cell
adhesion and migration in breast cancer-derived EVs, suggesting a
potential role in cancer metastasis (69).
Further analysis of the isolated exosomes revealed a
size distribution of 32-117 nm for the BMSC-Exos and 88-220 nm for
the iMSC-Exos. An additional reading with an average size of 380
nm, which appeared to have a lower intensity in the size
distribution measurement of iMSC-Exos, likely resulted from the
presence of iMSC-Exo aggregates. This aggregation was confirmed via
TEM when morphology was studied. Aggregation is a primary
limitation of collecting exosomes following sequential
ultracentrifugation; however, it remains an effective method for
collecting sufficient exosomes from large volumes of conditioned
media (70). To minimize
aggregation, several exosome isolation methods can be used. Exosome
precipitation kits that utilize reagents such as PEG to precipitate
exosomes from the sample are one option (71). Magnetic bead-based isolation, using
magnetic beads coated with antibodies targeting specific exosome
surface markers (for example CD9 and CD63), selectively captures
exosomes, providing high specificity and avoiding aggregation that
can occur with ultracentrifugation (72). Polymer-based precipitation (for
example ExoQuick) can also be used to precipitate exosomes from
biological fluids, offering a simpler and quicker alternative to
ultracentrifugation (73,74). While these alternative methods can
help reduce aggregation, ultracentrifugation remains a widely
employed, cost-effective, and scalable technique, making it
well-suited for the requirements the present study. However,
despite the heterogeneity in size, iMSC-Exos were generally larger.
Given that the typical size range of exosomes is 30-150 nm
(35,75), both sets of isolated exosomes
generally fell within this range.
The results of the proliferation assay using MCF7
and A549 cells revealed that both iMSC-Exos and BMSC-Exos
significantly decreased cell proliferation at 24 h, with no
significant decrease in MCF7 cell proliferation observed at 48 h
after treatment with iMSC-Exos. However, A549 cells were
significantly affected after 48 h of treatment with iMSC-Exos. In
fact, iMSC-Exos resulted in greater proliferation at 48 h than did
BMSC-Exos. The findings of the present study suggested that while
the exosomes initially inhibited MCF7 cell proliferation, their
effects may have diminished over time. By contrast, for A549 cells,
iMSC-Exos had a greater inhibitory effect on proliferation at 48 h
than did BMSC-Exos. The finding of the present study aligned with
that of previous research documenting the effects of human stem
cells on reducing the proliferation of the A549 cell line (76). However, the proliferation rate of
MCF7 cells was not significantly different from that of cells
cultured in SFM at 48 h after treatment with either iMSC-Exos or
BMSC-Exos, despite the initial antitumor effect observed at 24 h.
Additionally, a decrease in necrosis was observed in cells treated
with iMSC-Exos compared with those treated with SFM. The findings
of the present study suggested a potential suppressive effect of
BMSC-Exos on MCF7 cells at 48 h, which may have contributed to the
diminished antitumor effect observed at 24 h in the proliferation
assay, possibly due to the increased number of viable cells at the
later time points.
Overall, these observations are consistent with
previous studies that reported variability in the impact of
MSC-derived exosomes on cancer cell proliferation, which is often
influenced by the source of the MSCs (64) and the specific cancer model used
(64,74). Previous studies revealed that
BMSC-Exos can exert either pro- or anti-tumor effects depending on
their molecular content and target pathways. Wu et al
(77) reported that BMSC-Exos
carrying miR-30b-5p suppressed non-small cell lung cancer
proliferation by targeting EZH2 and other genes involved in the
PI3K/AKT signaling pathway, while Wang et al (44) found that BMSC-Exos promote lung
cancer progression, as shown in the A549 cell model through
miR-425-mediated suppression of CPEB1. These findings suggest that
the cargo composition of exosomes plays a crucial role in
determining their effects on proliferation. Similarly, in breast
cancer, Chen et al (78)
showed that BMSC-Exos promote breast cancer proliferation in the
MCF7 model by activating the Hedgehog signaling pathway, whereas Hu
et al (79) demonstrated
that BMSC-Exos carrying AlkB homolog 5 (ALKBH5) suppress
triple-negative breast cancer cell growth by regulating the
ALKBH5-dependent mechanism, involving the UBE2C gene and p53
regulation. Further studies are needed to determine the molecular
pathways responsible for the differential and time-dependent impact
of BMSC-Exos and iMSC-Exos on cancer cell proliferation. However,
although the MTT assay has been widely employed to measure cell
viability, it is worth emphasizing, according to Liu et al
(80), that utilizing alternative
assays, such as WST-based methods (for example CCK-8), could
address the limitations of MTT and enhance the reliability and
efficiency of viability analyses. The MTT assay relies on
mitochondrial enzymes to reduce MTT into insoluble formazan
crystals, but it has limitations including toxicity and the need
for solubilization. By contrast, WST assays, such as CCK-8, use
water-soluble tetrazolium salts reduced by dehydrogenases into
soluble formazan, offering advantages such as non-toxicity, no
solubilization, and improved enzymatic detection, which could make
them a better alternative to MTT, as discussed by Liu et al
(80).
Although no significant migration of A549 cells was
observed following exosome treatment, compared with SFM,
BMSC-derived exosomes increased the migration of MCF7 cells at 20
h. Notably, this effect was reversed at 47 h, with increased
migration observed in the serum-free condition. This behavior
suggests a dynamic interaction between exosomes and the cell
migration process over time or potentially due to the experimental
conditions. Considering the original hypothesis that increased
proliferation rates are associated with increased migration rates,
the findings of the present study indicated that the relationship
between exosome treatment and cell behavior may be more complex.
Further investigation is needed to understand the underlying
mechanisms driving these time-dependent changes in migration
(81,82). The findings of the present study
from MCF7 cells contradict the theory that increased proliferation
is associated with increased migration. Instead, they are
consistent with other studies suggesting that proliferation and
migration can be contrasting events (82-84).
Notably, both the proliferation and migration assays revealed a
time-dependent effect on MCF7 cells treated with BMSC-Exos. A
significant antitumor effect was observed in the proliferation
assay at 24 h, whereas the migration assay showed the opposite
effect at 47 h compared with 20 h, indicating complex and dynamic
cellular responses to exosome treatment over time.
The quantification of SA-βGal-positive cells via a
senescence assay revealed an increase in the senescence of both
A549 and MCF7 cells following treatment with iMSC-Exos and
BMSC-Exos, with only A549 cells showing a statistically significant
effect. Cellular senescence is characterized by irreversible cell
cycle arrest, leading to the inhibition of proliferation, thus
highlighting its role as a tumor-suppressive mechanism (85). This increase in senescence aligns
with the decreased proliferation of A549 cells observed in the MTT
assay, suggesting that the antitumor effects of iMSC-Exos and
BMSC-Exos on A549 cells are mediated, at least in part, by the
induction of senescence. The finding of the present study is
consistent with previous studies highlighting the tumor-suppressive
role of senescence in cancer (25,86,87).
By contrast, the absence of a significant effect on MCF7 cells may
reflect differences in cell type-specific responses to exosome
treatment, potentially due to variations in the molecular pathways
regulating proliferation and senescence. The increased senescence
observed in A549 cells treated with iMSC-Exos compared with
BMSC-Exos could be attributed to differences in their molecular
cargo, such as increased levels of senescence-inducing microRNAs,
proteins, or epigenetic regulators (85).
It is suggested that future studies use
patient-derived xenograft models and larger cohorts to confirm
these findings and ensure broader applicability. For example,
Yaghoubi et al (88)
reported that the overexpression of miR-145-5p in human umbilical
MSC-derived exosomes reduced xenograft tumor growth, highlighting
the potential of exosomes in cancer treatment. In conclusion, the
present study described the distinct effects of exosomes derived
from BMSCs and iMSCs on MCF7 and A549 cancer cells, highlighting
the variability in cellular responses to exosome treatment. Both
types of exosomes exerted potential antitumor effects. These
findings underscore the importance of further research to enhance
our understanding and optimize the therapeutic use of MSC-derived
exosomes in cancer treatment.
Supplementary Material
Assessment of the internalization of
BMSC- and iMSC-derived exosomes into HDF. Dil-O-labeled exosomes
were taken up by HDF cells, with DAPI staining used to visualize
the nuclei and CMFDA to stain the cell bodies. Scale bar, 200
μm (n=3). BMSCs, bone marrow stromal mesenchymal stem cells;
iMSCs, induced pluripotent stem cell-derived mesenchymal stem
cells; HDF, human dermal fibroblasts; DiI-O,
1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate;
CMFDA, 5-chloromethylfluorescein diacetate dye; DAPI,
4',6-diamidino-2-phenylindole.
Acknowledgements
The authors would like to thank Professor Hatem
Al-Kateib from the University of Jordan/Faculty of Pharmacy for
helping with the DLS measurement and Miss Rola Bqaien at the Cell
Therapy Center/University of Jordan for her assistance in TEM
imaging of purified EVs.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NAA performed the formal analysis, designed the
methodology, validated the data, wrote, reviewed, and edited the
manuscript as well as supervised the project. RA wrote the original
draft and contributed to data acquisition and analysis. SN designed
the methodology, performed the formal analysis, validated the data
and contributed to writing the original draft. MAI designed the
methodology and performed formal analysis. RB, SAH, AAl and FKA
validated the methodological procedures and contributed to data
acquisition. AHAH and TS wrote, reviewed and edited the manuscript
and contributed to data analysis and interpretation. AAw provided
the resources, and conceptually designed the project. NAA and AAw
confirm the authenticity of all the raw data generated as part of
this work. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved
(approval no. IRB/7/2019) by the Ethics Committee Institutional
Review Board of the Cell Therapy Center/University of Jordan
(Amman, Jordan). All participants provided written informed consent
to participate in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sonkin D, Thomas A and Teicher BA: Cancer
treatments: Past, present, and future. Cancer Genet. 286-287:18–24.
2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hoang DM, Pham PT, Bach TQ, Ngo ATL,
Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, et
al: Stem cell-based therapy for human diseases. Signal Transduct
Target Ther. 7(272)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fus-Kujawa A, Mendrek B, Trybus A,
Bajdak-Rusinek K, Stepien KL and Sieron AL: Potential of induced
pluripotent stem cells for use in gene therapy: History, molecular
bases, and medical perspectives. Biomolecules.
11(699)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shojaei S, Hashemi SM, Ghanbarian H,
Salehi M and Mohammadi-Yeganeh S: Effect of mesenchymal stem
cells-derived exosomes on tumor microenvironment: Tumor progression
versus tumor suppression. J Cell Physiol. 234:3394–3409.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li X, Peng B, Zhu X, Wang P, Xiong Y, Liu
H, Sun K, Wang H, Ou L, Wu Z, et al: Changes in related circular
RNAs following ERβ knockdown and the relationship to rBMSC
osteogenesis. Biochem Biophys Res Commun. 493:100–107.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song
AG: Mesenchymal stem cell migration and tissue repair. Cells.
8(784)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Olmedo-Moreno L, Aguilera Y,
Baliña-Sánchez C, Martín-Montalvo A and Capilla-González V:
Heterogeneity of in vitro expanded mesenchymal stromal cells and
strategies to improve their therapeutic actions. Pharmaceutics.
14(1112)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vizoso FJ, Eiro N, Cid S, Schneider J and
Perez-Fernandez R: Mesenchymal stem cell secretome: Toward
cell-free therapeutic strategies in regenerative medicine. Int J
Mol Sci. 18(1852)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang W and Han ZC: Heterogeneity of human
mesenchymal stromal/stem cells. Adv Exp Med Biol. 1123:165–177.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cerneckis J, Cai H and Shi Y: Induced
pluripotent stem cells (iPSCs): Molecular mechanisms of induction
and applications. Signal Transduct Target Ther.
9(112)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bindhya S, Sidhanth C, Shabna A,
Krishnapriya S, Garg M and Ganesan TS: Induced pluripotent stem
cells: A new strategy to model human cancer. Int J Biochem Cell
Biol. 107:62–68. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou X, Liu J, Wu F, Mao J, Wang Y, Zhu J,
Hong K, Xie H, Li B, Qiu X, et al: The application potential of
iMSCs and iMSC-EVs in diseases. Front Bioeng Biotechnol.
12(1434465)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sabapathy V and Kumar S: hiPSC-derived
iMSCs: NextGen MSCs as an advanced therapeutically active cell
resource for regenerative medicine. J Cell Mol Med. 20:1571–1588.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wruck W, Graffmann N, Spitzhorn LS and
Adjaye J: Human induced pluripotent stem cell-derived mesenchymal
stem cells acquire rejuvenation and reduced heterogeneity. Front
Cell Dev Biol. 9(717772)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kopen GC, Prockop DJ and Phinney DG:
Marrow stromal cells migrate throughout forebrain and cerebellum,
and they differentiate into astrocytes after injection into
neonatal mouse brains. Proc Natl Acad Sci USA. 96:10711–10716.
1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bittira B, Shum-Tim D, Al-Khaldi A and
Chiu RC: Mobilization and homing of bone marrow stromal cells in
myocardial infarction. Eur J Cardiothorac Surg. 24:393–398.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Cir Res.
95:9–20. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Phinney DG and Pittenger MF: Concise
review: MSC-derived exosomes for cell-free therapy. Stem Cells.
35:851–858. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin H, Chen H, Zhao X, Chen Z, Zhang P,
Tian Y, Wang Y, Ding T, Wang L and Shen Y: Advances in mesenchymal
stem cell conditioned medium-mediated periodontal tissue
regeneration. J Transl Med. 19(456)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kumar MA, Baba SK, Sadida HQ, Marzooqi SA,
Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra AB,
Kumar R, et al: Extracellular vesicles as tools and targets in
therapy for diseases. Signal Transduct Target Ther.
9(27)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bogatcheva NV and Coleman ME: Conditioned
medium of mesenchymal stromal cells: A new class of therapeutics.
Biochemistry (Mosc). 84:1375–1389. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cheshomi H and Matin MM: Exosomes and
their importance in metastasis, diagnosis, and therapy of
colorectal cancer. J Cell Biochem. 120:2671–2686. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fuloria S, Subramaniyan V, Dahiya R,
Dahiya S, Sudhakar K, Kumari U, Sathasivam K, Meenakshi DU, Wu YS,
Sekar M, et al: Mesenchymal stem cell-derived extracellular
vesicles: Regenerative potential and challenges. Biology (Basel).
10(172)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brossa A, Fonsato V, Grange C, Tritta S,
Tapparo M, Calvetti R, Cedrino M, Fallo S, Gontero P, Camussi G and
Bussolati B: Extracellular vesicles from human liver stem cells
inhibit renal cancer stem cell-derived tumor growth in vitro and in
vivo. Int J Cancer. 147:1694–1706. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Simeone P, Bologna G, Lanuti P,
Pierdomenico L, Guagnano MT, Pieragostino D, Del Boccio P, Vergara
D, Marchisio M, Miscia S and Mariani-Costantini R: Extracellular
vesicles as signaling mediators and disease biomarkers across
biological barriers. Int J Mol Sci. 21(2514)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abels ER and Breakefield XO: Introduction
to extracellular vesicles: Biogenesis, RNA cargo selection,
content, release, and uptake. Cell Mol Neurobiol. 36:301–312.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jin Y, Ma L, Zhang W, Yang W, Feng Q and
Wang H: Extracellular signals regulate the biogenesis of
extracellular vesicles. Biol Res. 55(35)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vinaiphat A and Sze SK: Advances in
extracellular vesicles analysis. Adv Clin Chem. 97:73–116.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Greening DW and Simpson RJ: Understanding
extracellular vesicle diversity-current status. Expert Rev
Proteomics. 15:887–910. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
van Niel G, D'Angelo G and Raposo G:
Shedding light on the cell biology of extracellular vesicles. Nat
Rev Mol Cell Biol. 19:213–228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells.
8(727)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Del Fattore A, Luciano R, Saracino R,
Battafarano G, Rizzo C, Pascucci L, Alessandri G, Pessina A,
Perrotta A, Fierabracci A and Muraca M: Differential effects of
extracellular vesicles secreted by mesenchymal stem cells from
different sources on glioblastoma cells. Expert Opin Biol Ther.
15:495–504. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Berumen Sánchez G, Bunn KE, Pua HH and
Rafat M: Extracellular vesicles: Mediators of intercellular
communication in tissue injury and disease. Cell Commun Signal.
19(104)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Skotland T, Sagini K, Sandvig K and
Llorente A: An emerging focus on lipids in extracellular vesicles.
Adv Drug Deliv Rev. 159:308–321. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Muralikumar M, Manoj Jain S, Ganesan H,
Duttaroy AK, Pathak S and Banerjee A: Current understanding of the
mesenchymal stem cell-derived exosomes in cancer and aging.
Biotechnol Rep (Amst). 31(e00658)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J,
Wang X, Jing Y, Chen X and Su J: Exosome-guided bone targeted
delivery of Antagomir-188 as an anabolic therapy for bone loss.
Bioact Mater. 6:2905–2913. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo H and Huang X: Engineered exosomes for
future gene-editing therapy. Biomater Transl. 3:240–242.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu H, Song P, Zhang H, Zhou F, Ji N, Wang
M, Zhou G, Han R, Liu X, Weng W, et al: Synthetic biology-based
bacterial extracellular vesicles displaying BMP-2 and CXCR4 to
ameliorate osteoporosis. J Extracell Vesicles.
13(e12429)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen
Q, Zhang Y, Lu Y, Ding X and Jiang C: Bone marrow mesenchymal stem
cells-derived exosomes for penetrating and targeted chemotherapy of
pancreatic cancer. Acta Pharm Sin B. 10:1563–1575. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lin Z, Wu Y, Xu Y, Li G, Li Z and Liu T:
Mesenchymal stem cell-derived exosomes in cancer therapy
resistance: Recent advances and therapeutic potential. Mol Cancer.
21(179)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zakiyah N, Wanandi SI, Antarianto RD,
Syahrani RA and Arumsari S: Mesenchymal stem cell-derived
extracellular vesicles increase human MCF7 breast cancer cell
proliferation associated with OCT4 expression and ALDH activity.
Asian Pac J Cancer Prev. 24:2781–2789. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang G, Ji X, Li P and Wang W: Human bone
marrow mesenchymal stem cell-derived exosomes containing
microRNA-425 promote migration, invasion and lung metastasis by
down-regulating CPEB1. Regen Ther. 20:107–116. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao Q, Hai B, Kelly J, Wu S and Liu F:
Extracellular vesicle mimics made from iPS cell-derived mesenchymal
stem cells improve the treatment of metastatic prostate cancer.
Stem Cell Res Ther. 12(29)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu H, Dilger JP and Lin J: Lidocaine
suppresses viability and migration of human breast cancer cells:
TRPM7 as a target for some breast cancer cell lines. Cancers
(Basel). 13(234)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li R, Xiao C, Liu H, Huang Y, Dilger JP
and Lin J: Effects of local anesthetics on breast cancer cell
viability and migration. BMC Cancer. 18(666)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Santucci KL, Snyder KK, Van Buskirk RG,
Baust JG and Baust JM: Investigation of lung cancer cell response
to cryoablation and adjunctive gemcitabine-based cryo-chemotherapy
using the A549 cell line. Biomedicines. 12(1239)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li J, Zhong X, Zhao Y, Shen J, Xiao Z and
Pilapong C: Acacetin inhibited non-small-cell lung cancer (NSCLC)
cell growth via upregulating miR-34a in vitro and in vivo. Sci Rep.
14(2348)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huang PH, Duan XB, Tang ZZ, Zou ZX, Song
WM, Gao G, Li D, Nie FQ, Yan X, Fu YX, et al: Betulinaldehyde
exhibits effective anti-tumor effects in A549 cells by regulating
intracellular autophagy. Sci Rep. 13(743)2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ababneh NA, Al-Kurdi B, Jamali F and Awidi
A: A comparative study of the capability of MSCs isolated from
different human tissue sources to differentiate into neuronal stem
cells and dopaminergic-like cells. PeerJ. 10(e13003)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ababneh NA, Al-Kurdi B, Ali D, Abuarqoub
D, Barham R, Salah B and Awidi A: Establishment of a human induced
pluripotent stem cell (iPSC) line (JUCTCi010-A) from a healthy
Jordanian female skin dermal fibroblasts. Stem Cell Res.
47(101891)2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
53
|
Ababneh NA, Al-Kurdi B, Ali D, Barham R,
Sharar N, Mrahleh MM, Salah B and Awidi A: Generation of a human
induced pluripotent stem cell (iPSC) line (JUCTCi011-A) from skin
fibroblasts of a healthy Jordanian male subject. Stem Cell Res.
48(101923)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Karam M and Abdelalim EM: Robust and
highly efficient protocol for differentiation of human pluripotent
stem cells into mesenchymal stem cells. Methods Mol Biol.
2454:257–271. 2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sober SA, Darmani H, Alhattab D and Awidi
A: Flow cytometric characterization of cell surface markers to
differentiate between fibroblasts and mesenchymal stem cells of
different origin. Arch Med Sci. 19:1487–1496. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang S, Hou Y, Li X, Song Z, Sun B, Li X
and Zhang H: Comparison of exosomes derived from induced
pluripotent stem cells and mesenchymal stem cells as therapeutic
nanoparticles for treatment of corneal epithelial defects. Aging
(Albany NY). 12:19546–19562. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Koliha N, Wiencek Y, Heider U, Jüngst C,
Kladt N, Krauthäuser S, Johnston ICD, Bosio A, Schauss A and Wild
S: A novel multiplex bead-based platform highlights the diversity
of extracellular vesicles. J Extracell Vesicles.
5(29975)2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Debacq-Chainiaux F, Erusalimsky JD,
Campisi J and Toussaint O: Protocols to detect
senescence-associated beta-galactosidase (SA-betagal) activity, a
biomarker of senescent cells in culture and in vivo. Nat Protoc.
4:1798–1806. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zeng ZL and Xie H: Mesenchymal stem
cell-derived extracellular vesicles: A possible therapeutic
strategy for orthopaedic diseases: A narrative review. Biomater
Transl. 3:175–187. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
He L, He T, Xing J, Zhou Q, Fan L, Liu C,
Chen Y, Wu D, Tian Z, Liu B and Rong L: Bone marrow mesenchymal
stem cell-derived exosomes protect cartilage damage and relieve
knee osteoarthritis pain in a rat model of osteoarthritis. Stem
Cell Res Ther. 11(276)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhou X, Liang H, Hu X, An J, Ding S, Yu S,
Liu C, Li F and Xu Y: BMSC-derived exosomes from congenital
polydactyly tissue alleviate osteoarthritis by promoting
chondrocyte proliferation. Cell Death Discov. 6(142)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang F, Guo J, Zhang Z, Qian Y, Wang G,
Duan M, Zhao H, Yang Z and Jiang X: Mesenchymal stem cell-derived
exosome: A tumor regulator and carrier for targeted tumor therapy.
Cancer Lett. 526:29–40. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jahangiri B, Khalaj-Kondori M, Asadollahi
E, Kian Saei A and Sadeghizadeh M: Dual impacts of mesenchymal stem
cell-derived exosomes on cancer cells: Unravelling complex
interactions. J Cell Commun Signal. 17:1229–1247. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Tan TT, Lai RC, Padmanabhan J, Sim WK,
Choo ABH and Lim SK: Assessment of tumorigenic potential in
mesenchymal-stem/stromal-cell-derived small extracellular vesicles
(MSC-sEV). Pharmaceuticals (Basel). 14(345)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Heidegger S, Stritzke F, Dahl S,
Daßler-Plenker J, Joachim L, Buschmann D, Fan K, Sauer CM, Ludwig
N, Winter C, et al: Targeting nucleic acid sensors in tumor cells
to reprogram biogenesis and RNA cargo of extracellular vesicles for
T cell-mediated cancer immunotherapy. Cell Rep Med.
4(101171)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Maleki M, Ghanbarvand F, Reza Behvarz M,
Ejtemaei M and Ghadirkhomi E: Comparison of mesenchymal stem cell
markers in multiple human adult stem cells. Int J Stem Cells.
7:118–126. 2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Willms E, Cabañas C, Mäger I, Wood MJA and
Vader P: Extracellular vesicle heterogeneity: Subpopulations,
isolation techniques, and diverse functions in cancer progression.
Front Immunol. 9(738)2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ekström K, Crescitelli R, Pétursson HI,
Johansson J, Lässer C and Olofsson Bagge R: Characterization of
surface markers on extracellular vesicles isolated from lymphatic
exudate from patients with breast cancer. BMC Cancer.
22(50)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Varderidou-Minasian S and Lorenowicz MJ:
Mesenchymal stromal/stem cell-derived extracellular vesicles in
tissue repair: Challenges and opportunities. Theranostics.
10:5979–5997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Yu J, Huang D, Liu H and Cai H: Optimizing
conditions of polyethylene glycol precipitation for exosomes
isolation from MSCs culture media for regenerative treatment.
Biotechnol J. 19(e202400374)2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Oksvold MP, Neurauter A and Pedersen KW:
Magnetic bead-based isolation of exosomes. Methods Mol Biol.
1218:465–481. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Niu Z, Pang RTK, Liu W, Li Q, Cheng R and
Yeung WSB: Polymer-based precipitation preserves biological
activities of extracellular vesicles from an endometrial cell line.
PLoS One. 12(e0186534)2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Simon L, Lapinte V and Morille M:
Exploring the role of polymers to overcome ongoing challenges in
the field of extracellular vesicles. J Extracell Vesicles.
12(e12386)2023.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Schulz-Siegmund M and Aigner A: Nucleic
acid delivery with extracellular vesicles. Adv Drug Deliv Rev.
173:89–111. 2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Li L, Tian H, Chen Z, Yue W, Li S and Li
W: Inhibition of lung cancer cell proliferation mediated by human
mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai).
43:143–148. 2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wu T, Tian Q, Liu R, Xu K, Shi S, Zhang X,
Gao L, Yin X, Xu S and Wang P: Inhibitory role of bone marrow
mesenchymal stem cells-derived exosome in non-small-cell lung
cancer: microRNA-30b-5p, EZH2 and PI3K/AKT pathway. J Cell Mol Med.
27:3526–3538. 2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Chen R, Liu X and Tan N: Bone marrow
mesenchymal stem cell (BMSC)-derived exosomes regulates growth of
breast cancer cells mediated by hedgehog signaling pathway. J
Biomater Tissue Eng. 13:157–161. 2023.
|
|
79
|
Hu Y, Liu H, Xiao X, Yu Q, Deng R, Hua L,
Wang J and Wang X: Bone marrow mesenchymal stem cell-derived
exosomes inhibit triple-negative breast cancer cell stemness and
metastasis via an ALKBH5-dependent mechanism. Cancers (Basel).
14(6059)2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Liu H, Dilger JP and Lin J: Effects of
local anesthetics on cancer cells. Pharmacol Ther.
212(107558)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Maretzky T, Evers A, Zhou W, Swendeman SL,
Wong PM, Rafii S, Reiss K and Blobel CP: Migration of growth
factor-stimulated epithelial and endothelial cells depends on EGFR
transactivation by ADAM17. Nat Commun. 2(229)2011.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yan M, Yang X, Shen R, Wu C, Wang H, Ye Q,
Yang P, Zhang L, Chen M, Wan B, et al: miR-146b promotes cell
proliferation and increases chemosensitivity, but attenuates cell
migration and invasion via FBXL10 in ovarian cancer. Cell Death
Dis. 9(1123)2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Svensson S, Nilsson K, Ringberg A and
Landberg G: Invade or proliferate? Two contrasting events in
malignant behavior governed by p16(INK4a) and an intact Rb pathway
illustrated by a model system of basal cell carcinoma. Cancer Res.
63:1737–1742. 2003.PubMed/NCBI
|
|
84
|
Evdokimova V, Tognon C, Ng T and Sorensen
PH: Reduced proliferation and enhanced migration: Two sides of the
same coin? Molecular mechanisms of metastatic progression by YB-1.
Cell Cycle. 8:2901–2906. 2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ou HL, Hoffmann R, González-López C,
Doherty GJ, Korkola JE and Muñoz-Espín D: Cellular senescence in
cancer: From mechanisms to detection. Mol Oncol. 15:2634–2671.
2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Calcinotto A, Kohli J, Zagato E,
Pellegrini L, Demaria M and Alimonti A: Cellular senescence: Aging,
cancer, and injury. Physiol Rev. 99:1047–1078. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Domen A, Deben C, Verswyvel J, Flieswasser
T, Prenen H, Peeters M, Lardon F and Wouters A: Cellular senescence
in cancer: Clinical detection and prognostic implications. J Exp
Clin Cancer Res. 41(360)2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Yaghoubi Y, Movassaghpour A, Zamani M,
Talebi M, Mehdizadeh A and Yousefi M: Human umbilical cord
mesenchymal stem cells derived-exosomes in diseases treatment. Life
Sci. 233(116733)2019.PubMed/NCBI View Article : Google Scholar
|