Introduction
Tooth extraction frequently results in notable
alveolar bone loss, with a significant portion of bone resorption
occurring within the first 3 to 6 months post-extraction, posing
challenges for subsequent implant placement and aesthetic outcomes
(1,2). This bone remodeling process is
inevitable, characterized by horizontal and vertical bone
reduction, particularly pronounced on the buccal side (3,4).
Consequently, alveolar ridge preservation (ARP) techniques have
become essential for minimizing bone loss and enhancing tissue
regeneration. Recent clinical interest has focused on using
high-concentration growth factors, such as concentrated growth
factors (CGF) and plasma rich in growth factors (PRGF), which are
derived from autologous blood and exhibit potent regenerative
properties (5-7).
High-concentration growth factors such as CGF are
advanced formulations of platelet concentrates designed to release
growth factors more sustainably over time, closely mimicking
natural healing mechanisms. These growth factors, including
platelet-derived growth factor (PDGF), transforming growth
factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and
insulin-like growth factor (IGF), play crucial roles in cell
proliferation, angiogenesis and osteogenesis (8,9). CGF,
in particular, is known for its dense fibrin matrix, which enhances
tissue repair by supporting soft and hard tissue healing
post-extraction. Fang et al (6) demonstrated that CGF can reduce
postoperative complications such as pain and dry socket formation
while promoting faster soft tissue regeneration. Growth factors
such as TGF-β and VEGF also play pivotal roles in bone metabolism
and regeneration. TGF-β is crucial in regulating the
differentiation of mesenchymal stem cells into osteoblasts,
promoting bone formation. However, its role can be complex, as it
may have positive and negative effects on osteogenic
differentiation depending on the context (8,10,11).
The clinical effectiveness of CGF in reducing
alveolar bone resorption has been observed in multiple trials. For
instance, Assadi et al (3)
noted that CGF reduced bone loss in alveolar sockets and enhanced
soft tissue preservation, facilitating improved outcomes in implant
therapy. Similarly, Liu et al (5) found that CGF membranes, when used to
seal extraction sockets, effectively maintained soft tissue healing
rates. However, results on long-term bone preservation were mixed
when compared with conventional collagen-based membranes. By
contrast, the application of PRGF has also been explored
extensively, with Farina et al (12) highlighting PRGF's role in early bone
formation through enhanced cell differentiation and matrix
formation. However, the benefits of the bone density and volume
preservation were modest (12).
Comparative studies indicate varying efficacy levels
between CGF and other biomaterials. For example, Stumbras et
al (2) observed that bone
substitutes, such as bovine-derived bone minerals combined with
collagen membranes, achieved minimal horizontal bone resorption. In
contrast, PRGF offered comparable benefits, particularly in
vertical bone preservation (2).
However, other trials have reported inconsistent results. Anitua
et al (1) noted that while
PRGF improved soft tissue healing and reduced inflammation
post-extraction, it demonstrated limited impact on overall bone
regeneration compared with control groups.
While using high-concentration growth factors in ARP
offers promise, there is no consensus on optimal protocols. Farina
et al (12) highlighted that
CGF applications could reduce vertical and horizontal bone loss and
enhance new bone formation in posterior tooth extractions. However,
they emphasized that additional research is needed to establish
consistent outcomes and explore the underlying mechanisms of action
(12). Elayah et al
(8) also advocated for CGF as a
cost-effective, efficient option for ARP, recommending further
studies to assess its full potential and applicability across
various clinical scenarios.
While previous studies have demonstrated the general
benefits of high-concentration growth factors, comprehensive
meta-analyses that explore the variability in clinical outcomes,
such as early bone formation, complication rates, and the influence
of protocol differences, are needed. The present study provides new
insights by employing precision interval analysis and cumulative
sensitivity assessments to assess the reliability and range of
outcomes associated with high-concentration growth factors.
Materials and methods
Study design
The present meta-analysis was conducted following
the Preferred Reporting Items for Systematic Reviews and
meta-analyses (PRISMA) guidelines, which require the studies to be
included no later than November 2024. This ensures a structured and
transparent approach to the systematic review and synthesis of data
(13).
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) studies that
assessed the effect of high-concentration growth factors (such as
CGF and PRGF) on alveolar bone preservation during tooth
extraction; ii) randomized controlled trials (RCTs) or controlled
clinical trials that reported quantitative outcomes related to
alveolar bone dimensions, including but not limited to alveolar
ridge width, bone volume and socket width; iii) studies that
provided sufficient statistical data to calculate standardized
means differences (such as mean values, standard deviations,
confidence intervals), and iv) studies published in English and
peer-reviewed journals.
The exclusion criteria were as follows: i) studies
that did not specifically focus on high-concentration growth
factors for alveolar bone preservation (such as studies on other
biomaterials or techniques), ii) observational studies, case
series, case reports, reviews and studies without a control group,
iii) studies lacking sufficient statistical data for meta-analysis,
and iv) studies with severe methodological flaws or low quality
based on the quality assessment criteria.
Data extraction process
In total, two reviewers independently conducted data
extraction. Extracted data included study characteristics (such as
author, year, sample size, population characteristics), details of
the intervention (type of growth factor and application method),
and primary outcomes such as changes in alveolar ridge width, bone
volume and socket width. Secondary outcomes, such as soft tissue
healing and probing depth, were also recorded when available. In
cases where discrepancies occurred between the reviewers, a third
reviewer was consulted to resolve disagreements. If consensus could
not be reached, the study was excluded from the analysis to ensure
consistency and reliability in the data extraction process.
Quality assessment
The quality of each included study was assessed
using the Jadad Scale (14), a
reliable tool specifically designed to evaluate the methodological
rigor of RCTs. The Jadad Scale, or the Oxford Quality Scoring
System, evaluates studies based on three key criteria: i)
Randomization: Assessment of whether the study explicitly describes
the randomization method and ensures its adequacy. Studies received
points if they described both the process and adequacy of
randomization, reducing selection bias. ii) Blinding: Evaluation of
the implementation and description of blinding within the study.
Studies that described an appropriate method of blinding received
higher scores, as blinding minimizes detection and performance
biases. iii) Withdrawals and dropouts: Consideration of whether the
study reported the number and reasons for withdrawals or dropouts,
providing transparency in handling incomplete outcome data and
ensuring that the analysis accounts for all participants.
Each study received a Jadad score out of a maximum
of 5 points, with higher scores reflecting better methodological
quality and a lower risk of bias. This assessment allowed the
authors to identify high-quality studies and contributed to the
interpretation of results by considering the rigor of each study's
design and implementation. The use of the Jadad Scale thus ensured
a consistent and structured evaluation of study quality, supporting
the overall robustness of the present meta-analysis.
Statistical analysis
Statistical analyses were performed using
Comprehensive Meta-Analysis software v.3 (Biostat). A
random-effects model was applied to estimate pooled effect sizes,
presented as standardized differences in means. These models
accounted for variations across study populations and
methodologies. Instead of the I² statistic, the precision interval
approach was used to evaluate heterogeneity, providing a range
where the actual effect size is expected to fall across similar
populations. This method was selected as it offers a more nuanced
understanding of variability, giving insights into the range of
actual effects rather than a single heterogeneity value (15). Publication bias was assessed using
Begg and Mazumdar's rank correlation test and Egger's regression
intercept. Both tests revealed no significant evidence of
publication bias (P>0.05), indicating that selective reporting
did not likely influence the results. Additionally, Duval and
Tweedie's trim-and-fill method was applied to adjust for
potentially missing studies, confirming that the pooled estimates
remained stable even with hypothetical missing data. A sensitivity
analysis was conducted by sequentially removing each study and
recalculating the pooled effect size to ensure the robustness of
the results. This analysis confirmed that no single study had an
undue influence on the overall findings, enhancing the reliability
of the conclusions of the present study. Classic and Orwin's
fail-safe N tests were used to determine the number of unpublished
studies required to negate the observed effects. These tests
indicated a high degree of stability in the findings, demonstrating
that a substantial number of studies would need to be missing to
reduce the P-value to non-significant levels. A moderator analysis
was conducted to explore potential effect modifiers, such as
participant age, which may influence the efficacy of
high-concentration growth factors on alveolar bone preservation.
This analysis provided insights into whether specific study
characteristics were associated with differences in effect sizes,
aiding in the interpretation of heterogeneity across studies.
Results
Study selection
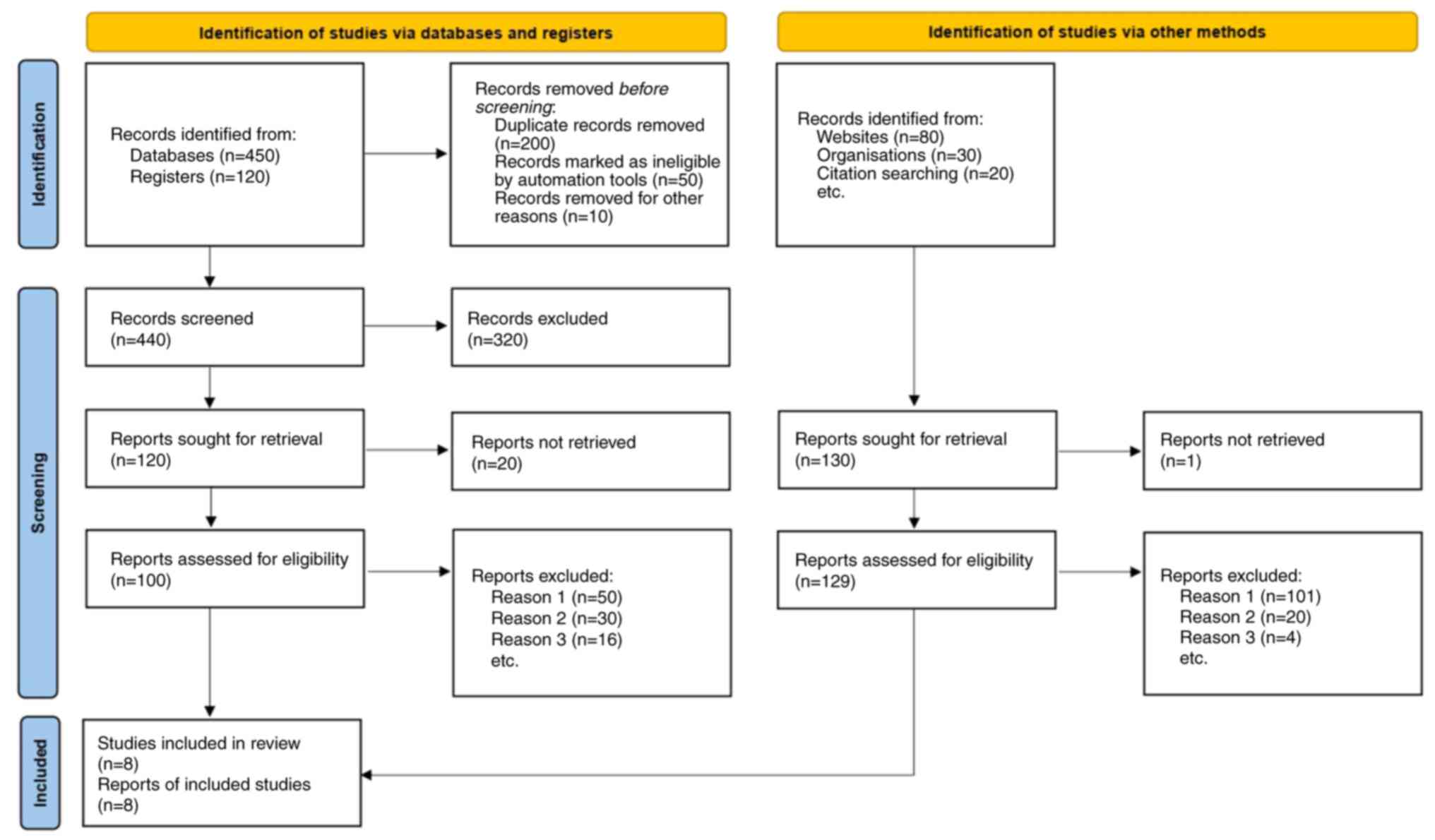
The study selection process was conducted according
to PRISMA guidelines. A comprehensive literature search initially
identified a total of 570 studies. After removing duplicates, 250
studies remained for the title and abstract screening. Of these,
229 studies were excluded for not meeting the inclusion criteria,
such as inappropriate study design, lack of relevant outcome
measures, or focus on interventions other than high-concentration
growth factors. A full-text review was then conducted on 21
studies, from which 13 were further excluded due to methodological
flaws or insufficient data. A total of 8 studies met the inclusion
criteria and were included in the meta-analysis (Fig. 1). These studies are summarized in
Table I, detailing sample size,
interventions, control groups, primary outcomes and follow-up
duration. The differences in outcomes presented in Table I reflect the variability in study
designs, follow-up durations, and primary endpoints. For instance,
while Assadi et al (3) and
Liu et al (5) emphasized
bone density and soft tissue healing, Farina et al (12) focused on early bone deposition.
These variations highlight the heterogeneity in clinical protocols
and intervention applications, which were accounted for using a
random-effects model in the meta-analysis.
 | Table IStudy characteristics and outcomes of
included trials on high-concentration growth factors in alveolar
bone preservation. Summary of the characteristics and main outcomes
of studies included in the meta-analysis and examination of the
effects of high-concentration growth factors, such as PRGF and CGF,
on alveolar bone preservation following tooth extraction. Study
details include sample size, intervention type, control group,
primary and secondary outcome measures, main results, and follow-up
duration. Each study's design and specific findings are provided to
facilitate comparison across trials. |
Table I
Study characteristics and outcomes of
included trials on high-concentration growth factors in alveolar
bone preservation. Summary of the characteristics and main outcomes
of studies included in the meta-analysis and examination of the
effects of high-concentration growth factors, such as PRGF and CGF,
on alveolar bone preservation following tooth extraction. Study
details include sample size, intervention type, control group,
primary and secondary outcome measures, main results, and follow-up
duration. Each study's design and specific findings are provided to
facilitate comparison across trials.
| First author,
year | Study design | Sample size | Intervention | Control group | Outcome measures | Main results | Follow-up
duration | (Refs.) |
|---|
| Anitua et al,
2015 | Randomized controlled
trial | 60 | PRGF application in
extraction socket | Blood clot | Bone regeneration,
soft tissue healing | Improved bone
regeneration and soft tissue healing with PRGF | 10-12 weeks | (1) |
| Assadi et al,
2023 | Randomized controlled
trial | 45 | CGF application in
alveolar socket post-extraction | Natural healing in
opposite socket | Alveolar bone width,
pain management | CGF reduced bone
resorption and pain; improved soft tissue healing | 2 months | (3) |
| Elayah et al,
2023 | Randomized controlled
trial | 30 | CGF in
post-extraction socket | Natural healing in
opposite socket | Bone height, bone
density, socket surface area | Higher bone height
and density with CGF | 3 months | (8) |
| Fang et al,
2021 | Randomized controlled
clinical study | 118 | CGF in mandibular
third molar extraction site | Serum in extraction
socket | Pain, swelling, bone
density, dry socket incidence | CGF reduced pain and
dry socket incidence; improved bone density | 24 weeks | (6) |
| Farina et al,
2012 | Controlled clinical
trial | 28 | PRGF in human
extraction sockets | Spontaneous
healing | Early bone
deposition, tissue mineral content | No significant
enhancement in early bone deposition with PRGF | 4-10 weeks | (12) |
| Liu et al,
2022 | Randomized controlled
trial | 22 | CGF membrane for
socket sealing in ARP | Bio-Gide collagen
membrane | Soft tissue healing
rate, bone resorption | CGF led to faster
soft tissue healing but no significant bone resorption
difference | 6 months | (5) |
| Ma et al,
2021 | Randomized controlled
clinical trial | 50 | CGF application for
alveolar ridge preservation | No intervention | Bone resorption, new
bone formation | CGF reduced vertical
and horizontal bone resorption | 3 months | (16) |
| Stumbras et
al, 2020 | Randomized
controlled clinical trial | 40 | PRGF in alveolar
ridge preservation post-extraction | Spontaneous
healing | Horizontal and
vertical bone changes | PRGF reduced bone
resorption, like bone substitute group | 3 months | (2) |
Meta-analysis outcomes
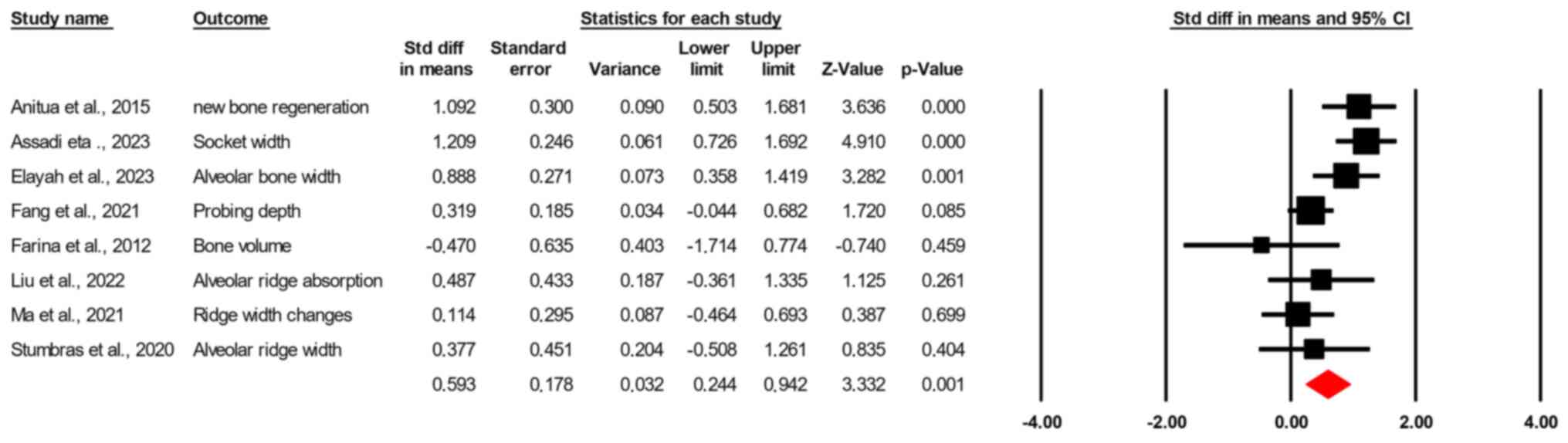
The random-effects model demonstrated a significant
pooled effect size, with a standardized mean difference of 0.593
(standard error=0.178; 95% CI, 0.2443-0.942; Z=3.332; P<0.001)
(Fig. 2). Heterogeneity was
assessed using the Q statistic, yielding a value of 18.084 with 7
degrees of freedom (P=0.012), and an I² value of 61.3%, suggesting
moderate to substantial heterogeneity among the included studies.
The observed heterogeneity (I²=61.3%) indicates moderate to
substantial variability, which is expected given the differences in
study populations and intervention protocols. The tau-squared value
was 0.143 (standard error=0.133), indicating variability in the
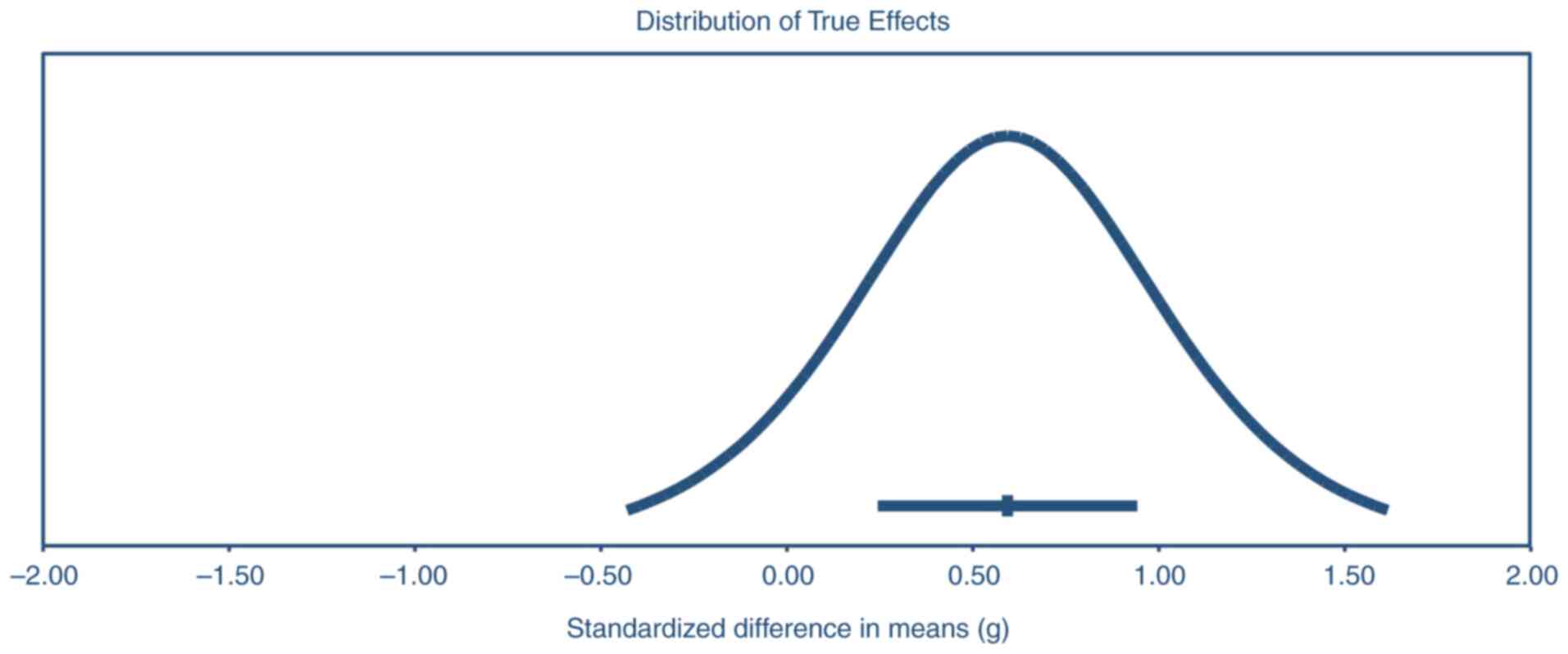
effect size estimates beyond chance. A precision interval approach
was used to assess heterogeneity further. The mean effect size was
estimated at 0.59 (95% CI, 0.24-0.94), with the actual effect size
in 95% of comparable populations expected to lie within -0.43 to
1.62. This broader range provides insights into variability across
similar populations and supports the consistency of the positive
effects of growth factor interventions on alveolar bone
preservation across different settings (Fig. 3).
Sensitivity analysis
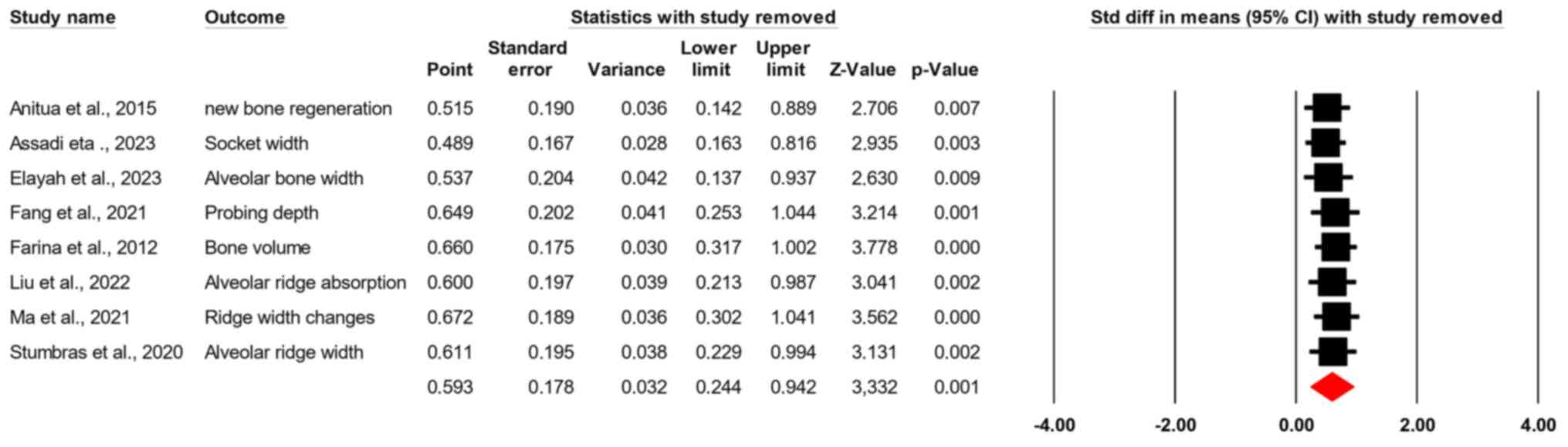
A sensitivity analysis was performed to evaluate the
influence of individual studies on the overall effect size. When
each study was sequentially removed, the standardized mean
differences remained consistent, ranging from 0.489 (95% CI,
0.163-0.816; Z=2.935; P=0.003) to 0.672 (95% CI, 0.302-1.041;
Z=3.562; P<0.001). This consistency indicates that no single
study unduly influenced the pooled effect size, supporting the
robustness of the findings (Fig.
4).
Both Classic and Orwin's fail-safe N tests were
performed to further ensure the robustness of the findings. The
Classic fail-safe N yielded a Z-value of 5.36805 with P<0.0001,
indicating that 52,000 additional studies with null results would
be required to bring the cumulative P-value above the alpha
threshold of 0.05. This high fail-safe N supports the stability and
reliability of the observed positive effect, confirming that
unpublished studies with null findings are unlikely to overturn the
results.
Methodological quality
The methodological quality of each included study
was assessed using the Jadad Scale (Table II). This scale evaluates three
critical components: i) Randomization, ii) blinding and iii)
handling of withdrawals/dropouts. Out of a maximum score of 5, most
studies scored 4, indicating moderate to high methodological
quality. All studies appropriately reported randomization and
withdrawals or dropouts, contributing to reliability in handling
incomplete data. However, blinding was only partially implemented
across studies, with all receiving a score of 1 in this category.
Overall, the Jadad Scale assessment suggested that the studies
included were of sufficient quality with minimal risk of bias.
 | Table IIAssessment of publication bias in
high-concentration growth factor studies for alveolar bone
preservation. This table presents The Jadad Scale assessment is
presented for each included study, evaluating methodological
quality based on three criteria: i) Randomization (0-2 points), ii)
blinding (0-2 points) and iii) withdrawals/dropouts (0-1 point).
Each study's overall quality is reflected by the total Jadad score
(0-5 points), with higher scores indicating lower risk of bias and
greater methodological rigor. |
Table II
Assessment of publication bias in
high-concentration growth factor studies for alveolar bone
preservation. This table presents The Jadad Scale assessment is
presented for each included study, evaluating methodological
quality based on three criteria: i) Randomization (0-2 points), ii)
blinding (0-2 points) and iii) withdrawals/dropouts (0-1 point).
Each study's overall quality is reflected by the total Jadad score
(0-5 points), with higher scores indicating lower risk of bias and
greater methodological rigor.
| First author,
year | Randomization
(0-2) | Blinding (0-2) |
Withdrawals/Dropouts (0-1) | Total Jadad score
(0-5) | (Refs.) |
|---|
| Anitua et
al, 2015 | 2 | 1 | 1 | 4 | (1) |
| Assadi et
al, 2023 | 2 | 1 | 1 | 4 | (3) |
| Elayah et
al, 2023 | 2 | 1 | 1 | 4 | (8) |
| Fang et al,
2021 | 2 | 1 | 1 | 4 | (6) |
| Farina et
al, 2012 | 1 | 1 | 1 | 3 | (12) |
| Liu et al,
2022 | 2 | 1 | 1 | 4 | (5) |
| Ma et al,
2021 | 2 | 1 | 1 | 4 | (16) |
| Stumbras et
al, 2020 | 2 | 1 | 1 | 4 | (2) |
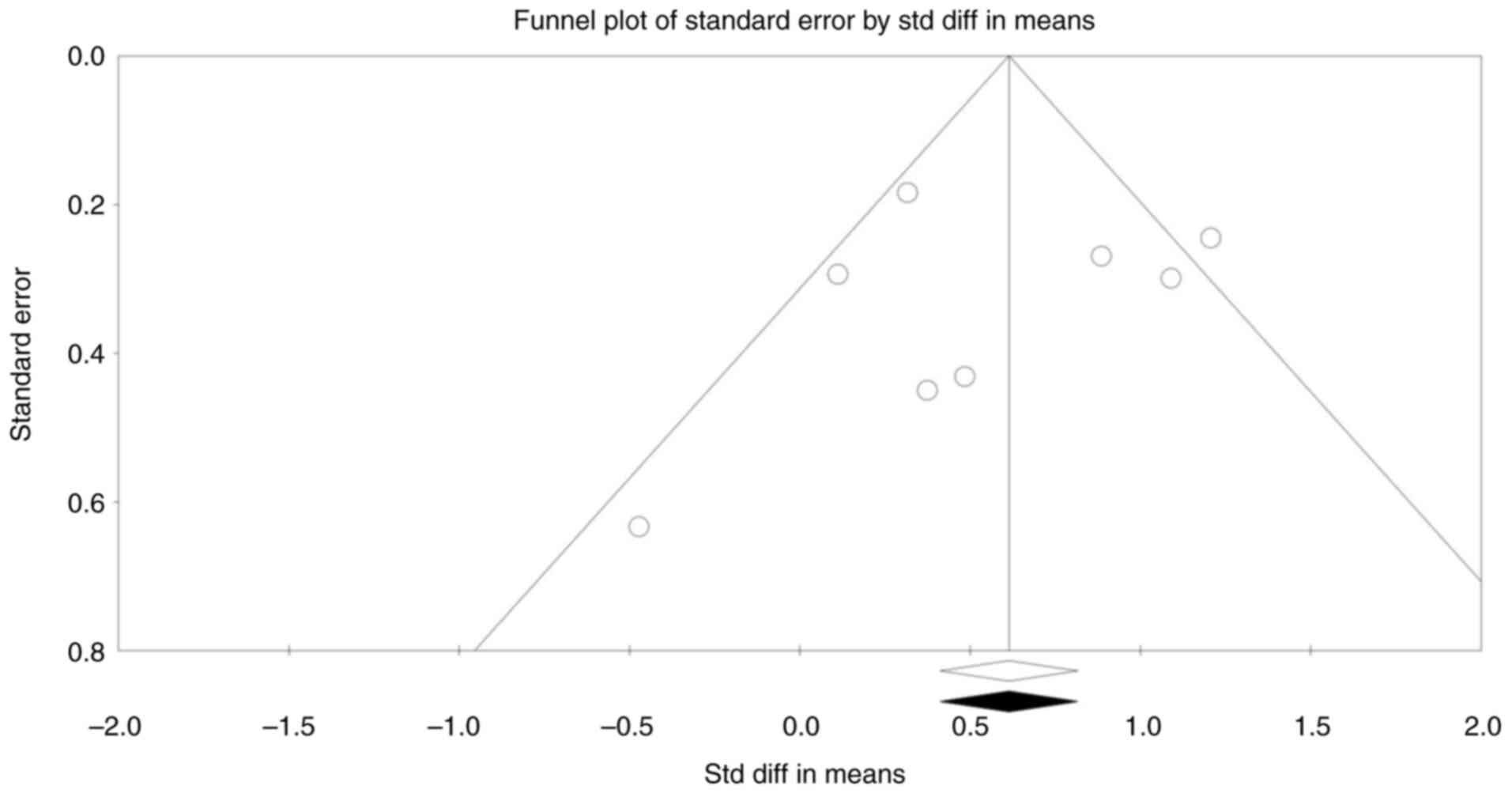
Publication bias
Publication bias was assessed through several
methods to ensure the robustness of the results. The funnel plot
appeared visually symmetrical, indicating no substantial
publication bias (Fig. 5). Begg and
Mazumdar's rank correlation test returned Kendall's tau values of
-0.1429 (P=0.3105) without continuity correction and -0.1071
(P=0.3552) with continuity correction, both of which were not
statistically significant. Egger's regression intercept was -0.9090
(95% CI, -5.3816-3.5636; P=0.6367), indicating no significant
funnel plot asymmetry. These results suggest that publication bias
is unlikely to have influenced the overall findings.
Furthermore, Duval and Tweedie's trim-and-fill
method was applied to adjust for any hypothetical missing studies.
This method did not trim any studies, as no missing studies were
estimated, and the observed effect sizes remained consistent with
the adjusted values. For the fixed-effects model, the point
estimate was 0.614 (95% CI, 0.4122-0.815), and for the
random-effects model, the point estimate was 0.593 (95% CI,
0.2443-0.942), with no adjustments needed. The Q value remained at
18.084, reinforcing the stability and reliability of the effect
estimates without any detected publication bias.
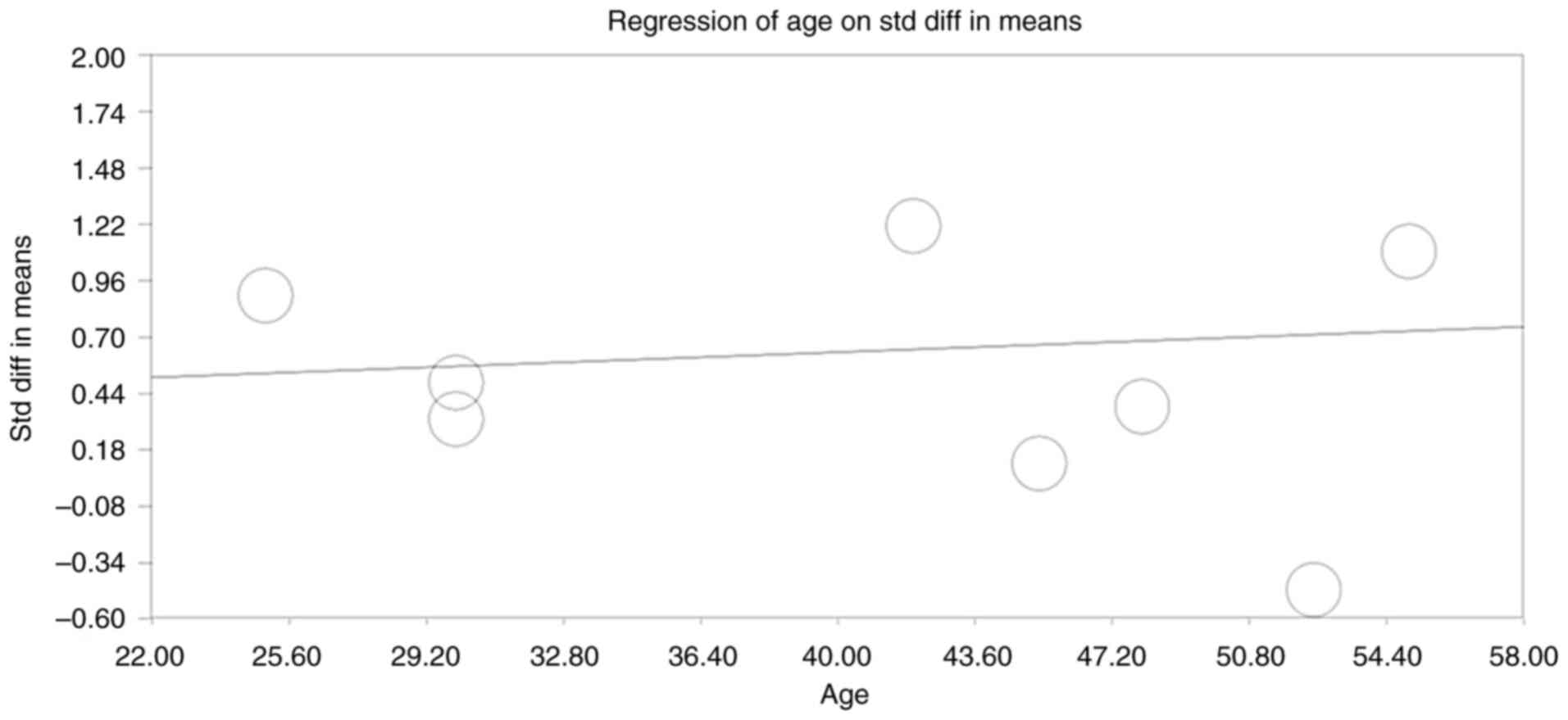
Moderator analysis
Moderator analysis evaluated whether participant age
influenced the standardized mean differences across studies. The
regression analysis indicated no significant effect modification by
age, suggesting that the positive effect of high-concentration
growth factors was consistent across various age groups, enhancing
the generalizability of the findings (Fig. 6).
Discussion
The present meta-analysis assessed the impact of
high-concentration growth factors, such as PRGF and CGF, on
alveolar bone preservation following tooth extraction. The pooled
results from eight studies, with a combined sample size of 393
participants, demonstrated a significant positive effect of these
growth factors in enhancing bone preservation. Sensitivity and
cumulative analyses indicated the robustness and stability of these
findings over time, with no single study unduly influencing the
pooled effect size. Furthermore, publication bias assessments
suggested minimal publication bias, including Begg's test, Egger's
test, and Duval and Tweedie's trim-and-fill method. These findings
support the beneficial impact of high-concentration growth factors
in promoting alveolar bone preservation. The initial hypothesis
anticipated consistent improvements in bone preservation and soft
tissue healing with PRGF and CGF application. While the pooled
effect size (SMD=0.593; P<0.001) confirmed this, certain
studies, such as Farina et al (2012) (12), reported minimal early bone formation
with PRGF. This discrepancy may be due to differences in the early
bioactivity of PRGF compared to CGF or the timing of follow-up
assessments. These findings underscore the importance of follow-up
timing in evaluating bone regeneration.
The results of this meta-analysis align with and add
to the growing body of literature supporting the use of PRGF and
CGF in dental applications, particularly in alveolar ridge
preservation. Previous individual studies, such as those by Assadi
et al (3) and Fang et
al (6), demonstrated that CGF
significantly reduces alveolar bone loss and improves soft tissue
healing after tooth extraction. Similarly, Anitua et al
(1) reported enhanced bone
regeneration and tissue healing with PRGF application, highlighting
the effectiveness of growth factors in facilitating faster and more
robust healing processes in extraction sites. These studies
underscore the biological mechanisms of PRGF and CGF, which are
rich in growth factors such as PDGF and transforming growth
factor-beta (TGF-β). These factors stimulate cellular
proliferation, angiogenesis, and osteogenesis, key bone and tissue
regeneration processes.
However, there have been some inconsistencies across
studies regarding the effectiveness of these interventions. For
instance, Farina et al (12)
found that PRGF did not significantly enhance bone deposition
early, indicating outcome variability based on application method
or patient characteristics. The present study provides quantitative
confirmation that the pooled effects of PRGF and CGF consistently
enhance bone preservation across varying follow-up durations,
indicating robustness regardless of study-level differences in
population characteristics.
CGFs have gained attention in recent years as a
biologically potent and minimally invasive treatment to enhance
tissue and bone regeneration in dental applications, including
alveolar ridge preservation after tooth extraction. CGFs are
derived from autologous blood through a specific centrifugation
process, resulting in a fibrin-rich matrix with a high
concentration of growth factors and cytokines. These biologically
active components play critical roles in cellular processes
essential for wound healing, angiogenesis and osteogenesis, thus
contributing to improved clinical outcomes in bone preservation.
The mechanisms by which CGFs exert their effects were explored to
support the findings of the present meta-analysis. The unique
centrifugation process was used to produce CGF, resulting in a
dense fibrin matrix enriched with various growth factors, including
PDGF. PDGF promotes cell migration, proliferation and
differentiation, particularly of osteoblast, which are essential
for new bone formation. It also stimulates the recruitment of
mesenchymal stem cells to the extraction site, enhancing bone
regeneration (8). Furthermore,
TGF-β plays a vital role in collagen synthesis and extracellular
matrix formation, crucial for stabilizing newly formed bone tissue.
It also promotes the differentiation of osteoblasts and other
bone-forming cells, which helps maintain alveolar ridge height and
density (16,17). Besides, VEGF is a key driver of
angiogenesis, forming new blood vessels, which improves blood flow
and nutrient supply to the healing site. Enhanced vascularization
supports cellular activities in bone regeneration, accelerating
wound healing and reducing post-extraction complications such as
dry sockets (1,6). In addition, the fibrin matrix in CGF
acts as a three-dimensional scaffold, providing structural support
and stability to the extraction socket. This matrix facilitates the
gradual and sustained release of growth factors over time, closely
mimicking the body's natural healing processes. The dense fibrin
structure anchors cells in the extraction site and serves as a
protective barrier that shields the wound from bacterial invasion,
thereby reducing the risk of infection. The scaffold's mechanical
properties also allow for the integration of osteoblasts and other
bone-forming cells, which aids in new bone deposition and
stabilizes the alveolar ridge (3).
Furthermore, the growth factors in CGF have been shown to enhance
the proliferation and differentiation of various cell types
involved in bone healing, including osteoblasts, fibroblasts and
endothelial cells. PDGF and TGF-β stimulate osteoblast activity,
leading to increased production of bone matrix proteins, such as
collagen, which forms the foundational structure of new bone.
Additionally, these factors promote the
differentiation of mesenchymal stem cells into osteogenic lineages,
further enhancing the regeneration of lost bone tissue. By
accelerating these cellular processes, CGFs contribute to faster
and more efficient alveolar ridge preservation (6). Moreover, CGFs contain
anti-inflammatory cytokines, such as interleukin-4 (IL-4) and
TGF-β, which help modulate the inflammatory response at the
extraction site. Excessive inflammation can lead to delayed healing
and increased bone resorption. The anti-inflammatory properties of
CGF minimize inflammation, creating an optimal environment for bone
and tissue regeneration. By reducing inflammation, CGFs also help
mitigate postoperative pain and swelling, improving patient comfort
and reducing complications (16).
Finally, angiogenesis, the formation of new blood vessels, is
critical for successful bone regeneration, as it ensures an
adequate supply of oxygen and nutrients to the healing site. VEGF,
a key component of CGF, stimulates angiogenesis within the
extraction socket, promoting vascularization of the newly forming
tissue. Enhanced blood vessel formation supports the survival and
activity of osteoblasts and other cells involved in bone repair,
thereby accelerating the healing process. Improved vascularization
also facilitates soft tissue healing, contributing to the
preservation of keratinized gingiva and the aesthetic outcome of
the treatment (1,6,18,19).
The findings of the present meta-analysis are
clinically relevant for several reasons. First, alveolar ridge
preservation is critical for optimizing the success of subsequent
implant placement and maintaining the aesthetic outcomes of dental
procedures. Bone resorption following tooth extraction can
complicate implant placement, requiring additional augmentation
procedures, which may be costly and carry risks. By demonstrating
that high-concentration growth factors can significantly reduce
alveolar bone loss, this meta-analysis supports integrating PRGF
and CGF as effective, minimally invasive options for ridge
preservation.
The Jadad Scale assessment revealed that most
studies scored 4 out of 5, indicating moderate to high
methodological quality. While randomization and reporting of
withdrawals were well-documented, blinding was consistently
underreported across studies. This limitation may introduce
performance and detection bias, especially in subjective outcomes
such as pain assessment. However, the cumulative and sensitivity
analyses confirmed that the overall findings remain robust despite
these methodological differences, indicating that lower blinding
scores do not disproportionately influence the positive effects of
high-concentration growth factors on alveolar bone preservation.
Despite variability in methodological rigor, the authors' precision
interval approach estimated a range of true effects that underscore
the robustness of high-concentration growth factor interventions
across studies of varying quality. This consistency suggests that
high-concentration growth factors improve bone preservation even
when study designs differ in methodological rigor. Nonetheless,
future studies could benefit from more rigorous implementation of
blinding protocols to enhance reliability.
While the present study has several strengths,
including rigorous adherence to PRISMA guidelines, comprehensive
publication bias assessment, and cumulative and sensitivity
analyses to evaluate the robustness of the findings, some
limitations should be acknowledged. Furthermore, unlike previous
studies, moderator analysis was applied to examine participant age
as a potential effect modifier and found no significant age-related
differences. This finding supports the generalizability of
high-concentration growth factor effects across diverse age groups,
reinforcing their clinical relevance for a broad patient
population. One limitation is the moderate heterogeneity observed
(I²=61.293%). This suggests some variability in study outcomes,
potentially due to differences in study populations, intervention
protocols, and follow-up durations. The precision interval analysis
offered a range (-0.43 to 1.62) where the actual effect size may
lie across comparable populations, providing a broader perspective
on potential heterogeneity sources. However, variations in growth
factor preparation methods, application techniques, and patient
characteristics could contribute to outcome differences. Future
studies may benefit from standardizing protocols to reduce
heterogeneity and enhance the comparability of results. Another
limitation is related to the Jadad Scale's focus on randomization
and blinding, which may not fully capture the methodological rigor
of these studies, mainly since blinding was inconsistently applied
across the studies. Although the quality assessment revealed that
most studies were of moderate to high quality, further refinement
in assessing study quality, such as using a tool specifically
tailored for non-pharmacological interventions, could provide
additional insights. Finally, while publication bias was assessed
using multiple methods, the relatively small number of studies
(n=8) included in the meta-analysis may limit the power of these
tests. Although Begg's test, Egger's test, and the trim-and-fill
method did not indicate significant publication bias, future
research with larger sample sizes may yield even more robust
results.
Additionally, the fail-safe N value of 52,000
supports the robustness of these findings, suggesting that a
substantial number of null-effect studies would be required to
nullify the observed effects. Finally, the variability in study
outcomes may also reflect differences between predicted and
observed effects due to variations in growth factor preparation
methods, application techniques, and follow-up periods.
Standardizing these protocols minimizes variability and ensures
comparable outcomes across future studies.
In conclusion, this meta-analysis proves that
high-concentration growth factors, such as PRGF and CGF, enhance
alveolar bone preservation following tooth extraction. The pooled
results from eight studies consistently indicate positive effects
on bone volume and width preservation, underscoring the potential
of these growth factors to support effective bone regeneration in
clinical settings. The findings were confirmed through cumulative
and sensitivity analyses, demonstrating the stability and
robustness of the pooled effect sizes despite some observed
heterogeneity.
Using high-concentration growth factors represents a
promising, minimally invasive strategy for promoting bone
preservation and improving outcomes in dental surgeries,
particularly for patients undergoing extractions and preparing for
future implant placement. The present study provides novel evidence
that confirms the robustness of interventions involving
high-concentration growth factors across diverse settings. The
findings of the present study also highlight the potential of
precision interval analysis to guide future research by identifying
ranges of clinical outcomes rather than single-point estimates.
Future research should aim to standardize application protocols and
evaluate the long-term impact of these growth factors on implant
stability, bone quality, and patient satisfaction. Further studies
are needed to explore their effectiveness across diverse patient
populations, investigate cost-effectiveness, and examine potential
synergistic effects with other biomaterials. These directions will
help refine clinical practices and broaden the integration of
growth factor therapies into routine dental and surgical care
(20).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The manuscript was written and drafted by XS, YY, LL
and WY. Additionally, they gathered and examined the data. XQ
provided general supervision, made intellectual content revisions,
and granted final publishing permission. QY acquired resources,
edited the language, and conducted data analysis. XQ and XS created
the study protocol and confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anitua E, Murias-Freijo A, Alkhraisat MH
and Orive G: Clinical, radiographical, and histological outcomes of
plasma rich in growth factors in extraction socket: A randomized
controlled clinical trial. Clin Oral Investig. 19:589–600.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stumbras A, Galindo-Moreno P, Januzis G
and Juodzbalys G: Three-dimensional analysis of dimensional changes
after alveolar ridge preservation with bone substitutes or plasma
rich in growth factors: Randomized and controlled clinical trial.
Clin Implant Dent Relat Res. 23:96–106. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Assadi H, Asadi M, Sadighi M, Faramarzi M
and Kouhsoltani M: Concentrated growth factor application in
alveolar ridge preservation on anterior teeth. A split-mouth,
randomized, controlled clinical trial. J Osseointegration.
15:249–255. 2023.
|
|
4
|
Weinreb M, Tsesis I, Rosen E, Taschieri S,
Del Fabbro M and Nemcovsky CE: Evolving new strategies for
periodontal, endodontic, and alveolar bone regeneration. In:
Evidence-Based Decision Making in Dentistry: Multidisciplinary
Management of the Natural Dentition. Rosen E, Nemcovsky C and
Tsesis I (eds). Springer, Cham, pp109-137, 2017.
|
|
5
|
Liu Y, Li X, Jiang C, Guo H, Luo G, Huang
Y and Yuan C: Clinical applications of concentrated growth factors
membrane for sealing the socket in alveolar ridge preservation: A
randomized controlled trial. Int J Implant Dent.
8(46)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fang D, Li D, Li C, Yang W, Xiao F and
Long Z: Efficacy and safety of concentrated growth factor fibrin on
the extraction of mandibular third molars: A prospective,
randomized, double-blind controlled clinical study. J Oral
Maxillofac Surg. 80:700–708. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baniasadi B and Evrard L: Alveolar ridge
preservation after tooth extraction with DFDBA and platelet
concentrates: A radiographic retrospective study. Open Dent J.
11:99–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Elayah SA, Younis H, Cui H, Liang X,
Sakran KA, Alkadasi B, Al-Moraissi EA, Albadani M, Al-Okad W, Tu J
and Na S: Alveolar ridge preservation in post-extraction sockets
using concentrated growth factors: A split-mouth, randomized,
controlled clinical trial. Front Endocrinol (Lausanne).
14(1163696)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ai-Aql ZS, Alagl AS, Graves DT,
Gerstenfeld LC and Einhorn TA: Molecular mechanisms controlling
bone formation during fracture healing and distraction
osteogenesis. J Dent Res. 87:107–118. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wei E, Hu M, Wu L, Pan X, Zhu Q, Liu H and
Liu Y: TGF-β signaling regulates differentiation of MSCs in bone
metabolism: Disputes among viewpoints. Stem Cell Res Ther.
15(156)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crane JL and Cao X: Bone marrow
mesenchymal stem cells and TGF-β signaling in bone remodeling. J
Clin Invest. 124:466–472. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Farina R, Bressan E, Taut A, Cucchi A and
Trombelli L: Plasma rich in growth factors in human extraction
sockets: A radiographic and histomorphometric study on early bone
deposition. Clin Oral Implants Res. 24:1360–1368. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Clark HD, Wells GA, Huët C, McAlister FA,
Salmi LR, Fergusson D and Laupacis A: Assessing the quality of
randomized trials: Reliability of the Jadad scale. Control Clin
Trials. 20:448–452. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
IntHout J, Ioannidis JP, Rovers MM and
Goeman JJ: Plea for routinely presenting prediction intervals in
meta-analysis. BMJ Open. 6(e010247)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma F, Lin Y, Sun F, Jiang X and Wei T: The
impact of autologous concentrated growth factors on the alveolar
ridge preservation after posterior tooth extraction: A prospective,
randomized controlled clinical trial. Clin Implant Dent Relat Res.
23:579–592. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mangano A, Mangano A, Lianos GD, Picone M
and Dionigi G: TGF-β superfamily, molecular signaling and
biomimetic features for bone regeneration: Historical perspectives
and future applications. Updates Surg. 67:321–323. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4(16009)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rachmawat D, Saskianti T, Ridwan RD,
Prasetyaningrum N and Kanawa M: VEGF as alveolar bone regeneration
key protein in SHED secretome, hydroxyapatite and collagen type 1
scaffold: An in-silico study. Res J Pharm Technol. 17:4975–4980.
2024.
|
|
20
|
Marian D, Toro G, D'Amico G, Trotta MC,
D'Amico M, Petre A, Lile I, Hermenean A and Fratila A: Challenges
and innovations in alveolar bone regeneration: A narrative review
on materials, techniques, clinical outcomes, and future directions.
Medicina (Kaunas). 61(20)2024.PubMed/NCBI View Article : Google Scholar
|