Introduction
Colorectal cancer (CRC) is the third most diagnosed
cancer worldwide, accounting for >10% of all cancer cases, and
is the second leading cause of cancer-related mortality globally.
Predictions indicate that the number of new CRC cases will rise by
20% by 2030 (1,2). In Thailand, CRC is a significant
health issue and is the third most common cancer in men and the
fourth in women. Unlike other cancer types, a steady increase in
CRC cases has been observed for both sexes in Thailand (3,4).
Studies have consistently highlighted the critical role of early
diagnosis and intervention in improving the treatment outcomes of
this disease (5,6). Therefore, the identification and
development of predictive biomarkers are essential for optimizing
treatment strategies for CRC.
The SWitch/sucrose non-fermentable (SWI/SNF)
chromatin remodeling complex serves a crucial role as a tumor
suppressor that regulates gene expression, transcription and DNA
repair (7,8). Within this complex, the AT-rich
interactive domain (ARID) proteins are key regulators of
transcription, cell cycle control, growth and differentiation
(9,10). Among the ARID family of proteins,
ARID1A, ARID1B and ARID2 have demonstrated a strong association
with cancer progression (11).
ARID1A is a well-known tumor suppressor gene that is
frequently mutated in several cancer types and its loss is
correlated with advanced cancer stages and metastasis (9,12,13).
ARID1B, which shares 66% sequence similarity with ARID1A, has also
been linked to cancer (9). Although
ARID1B expression in human cancer remains unclear, its
mutations have been identified and are considered to promote
tumorigenesis in various cancer types, including breast, ovarian,
pancreatic and bladder cancer (14-17).
ARID2, another important SWI/SNF subunit, is essential for
chromatin remodeling and tumor suppression (18). Loss of ARID2 has been
detected in several cancer types, including liver, melanoma, lung
and CRC (18-20).
Emerging evidence has highlighted the tumor-suppressive roles of
ARID1A, ARID1B and ARID2 in various cancer types, including CRC
(21-24).
ARID1A upregulation has been revealed to suppress invasion
and migration by modulating the expression of
epithelial-mesenchymal transition (EMT)-related markers (24) and ARID1A inhibition has been
shown to enhance metastatic potential (22,23).
Similarly, ARID1B knockdown was shown to disrupt DNA repair
and chromatin accessibility (25),
while ARID2 deficiency was demonstrated to promote cancer
cell proliferation and metastasis (18). Despite these insights, the
relationships among the ARID protein expression levels in CRC
remain largely unexplored. Furthermore, to the best of our
knowledge, no study has specifically investigated the expression
patterns and prognostic implications of ARID1A, ARID1B and ARID2 in
Thai patients with CRC. Given that genetic and environmental
factors influence CRC development differently across populations
(26), region-specific studies are
essential for identifying novel prognostic markers and potential
therapeutic targets.
The present study aimed to investigate the gene
mutations in ARID1A, ARID1B and ARID2 and
explore the correlations in their expression in CRC through
bioinformatics analysis. Additionally, the protein expression
levels of these three ARIDs in CRC tissues and their association
with the clinicopathological characteristics of Thai patients with
CRC were assessed to gain insights into their role and prognostic
value in CRC.
Materials and methods
Bioinformatics analysis of the ARID1A,
ARID1B and ARID2 gene mutations and expression correlations in
CRC
Genomic alterations of the ARID1A,
ARID1B and ARID2 genes were explored using The Cancer
Genome Atlas-colon adenocarcinoma (TCGA-COAD) dataset through the
Cancer Virtual Cohort Discovery Analysis Platform (CVCDAP)
(https://omics.bjcancer.org/cvcdap/)
(27). The expression correlations
among the ARID1A, ARID1B and ARID2 genes in
TCGA-COAD dataset were assessed using the Pearson correlation
coefficient in the Gene Expression Profiling Interactive Analysis 2
platform (28). The promoter
methylation levels of the ARID1A, ARID1B and
ARID2 genes in TCGA-COAD dataset were examined through The
University of ALabama at Birmingham CANcer data analysis portal
(https://ualcan.path.uab.edu/) (29,30).
The Kaplan-Meier (KM) plotter database (https://kmplot.com/analysis/) (31) was used to evaluate the association
of ARID1A (Affymetrix probe ID: 218917_s_at; n=1,061),
ARID1B (Affymetrix probe ID: 238043_at; n=814) and
ARID2 (Affymetrix probe ID: 225486_at; n=814) with overall
survival (OS) in patients with CRC. Low- and high-expression groups
were determined using the ‘Auto select best cut-off’ option, which
is based on the median expression values. The hazard ratio and
log-rank P-value were automatically computed by the database for
survival analysis. The KM plots for overall survival were generated
directly by the KM plotter tool without any additional statistical
analysis or modification. A late-stage crossover was observed in
the survival curves, as provided by the tool.
Patient samples
The present study was approved by the Human Research
Ethics Committee of Sawanpracharak Hospital (Nakhon Sawan,
Thailand; certificate of approval no. 53/2567) and the Naresuan
University Human Research Ethics Committee (Phitsanulok, Thailand;
approval no. P1-0107/2567; certificate of approval no. 139/2024),
and conducted in accordance with the principles of the Declaration
of Helsinki. Tissue biopsies from 63 patients diagnosed with CRC of
varying pathological differentiation were submitted to the
Pathology Unit at Sawanpracharak Hospital between 2017 and 2021.
This patient cohort included 27 males and 36 females, with a median
age of 66 years (range, 52-97 years). Formalin-fixed,
paraffin-embedded (FFPE) blocks containing the CRC tissues,
including both cancerous and adjacent non-cancerous areas, were
obtained for each patient. The clinicopathological data, including
age, sex, tumor location, tumor size, pathological differentiation,
American Joint Committee on Cancer (AJCC) staging 8th edition
(32), tumor invasion, metastasis,
lymphovascular invasion, comorbidities and follow-up period
post-operation, were comprehensively assessed by a clinical
pathologist. The exclusion criteria included patients with
incomplete data, patients diagnosed with hereditary CRC syndromes,
those with cancer of unknown primary origin and cases where a
pathologist or researcher was unable to clarify the findings of the
histological and/or immunohistochemical investigation. To ensure
anonymity, each FFPE block was labeled with a unique research code
and sensitive patient information was carefully protected.
Immunohistochemistry (IHC)
To assess the expression of the ARID proteins, IHC
was performed using the following specific antibodies: Anti-ARID1A
rabbit polyclonal antibody (1:400; cat. no. HPA005456;
MilliporeSigma), anti-ARID1B mouse monoclonal antibody (1:200; cat.
no. ab57461; Abcam) and anti-ARID2 rabbit polyclonal antibody
(1:250; cat. no. ab113283; Abcam). CRC tissue samples were
initially fixed in 10% neutral buffered formalin (NBF), and then
processed into FFPE blocks. These blocks were sectioned into
3-µm-thick slices, followed by deparaffinization in xylene and
rehydration using an ethanol gradient. Antigen retrieval was
achieved at 97˚C for 35 min using the heat-induced epitope
retrieval method in citrate buffer (pH 6.0). Endogenous peroxidase
activity was blocked with 3% hydrogen peroxide/sodium azide
(NaN3) for 25 min at room temperature (RT). After
washing the slides three times with PBS (5 min each), non-specific
protein binding was blocked with 0.1% NaN3 for 20 min at
RT. The slides were then incubated with the specified primary
antibodies at 4˚C overnight in a humidified chamber. As a negative
control, the primary antibody was replaced with PBS. Subsequent
steps involved incubation with biotinylated goat anti-rabbit IgG
(H+L) (from the Rabbit specific HRP/DAB Detection IHC Kit; cat. no.
ab64261; Abcam) or goat anti-mouse IgG (H+L) secondary antibody
[from the Mouse-specific HRP/DAB (ABC) Detection IHC Kit; cat. no.
ab64259; Abcam] for 15 min at RT, followed by three washes with PBS
(5 min each). The slides were then incubated with streptavidin
peroxidase for 15 min at RT. After three additional washes with
PBS, immunostaining was performed using the chromogen
3,3'-diaminobenzidine (DAB) substrate (1:50; cat. no. ab64238;
Abcam) for 4 min at RT to detect specific antigen-antibody
interactions, with the DAB reaction halted using distilled water.
The slides were counterstained with Mayer's hematoxylin (C.V.
Laboratories Co., Ltd.) by 3 dips at RT, washed in running tap
water for 5 min, dehydrated with increasing concentrations of
ethanol, cleared with xylene, mounted with mounting media
(Permount; Thermo Fisher Scientific, Inc.) and covered with a cover
slip.
Quantitative analysis of ARID1A,
ARID1B and ARID2 protein expression
In total, five independent areas per slide, covering
both cancerous and adjacent non-cancerous regions in the same CRC
tissues, were analyzed using the ZEN program (Rushmore Precision
Co., Ltd.) and an Axiocam 105 color ZEISS microscope (Carl Zeiss
AG) at high power fields with x40 magnification. ImageJ software
(version 1.53c; National Institutes of Health) was employed to
detect and analyze the ARID-positive cells. All images were
evaluated in a double-blind manner by both a pathologist and the
investigators. IHC scoring utilized the modified histoscore
(H-score), which combines staining intensity with the percentage of
positively stained cells to assess the abundance and distribution
of proteins in tissue samples (33). Staining intensity was classified as
follows: Negative staining (0), weak positivity (1), moderate positivity (2) and strong positivity (3) (34).
The H-score was calculated using the following formula: H-score=[(0
x % negative cells) + (1 x % weak positive cells) + (2 x % moderate
positive cells) + (3 x % strong positive cells)] (33). The H-score values ranged from 0 to
300. The ARID protein expression levels were categorized into two
groups based on the median value: Low (< median value) and high
(≥ median value). This classification approach aligns with the
previously established optimal cut-off for SWI/SNF component
expression (35).
Statistical analysis
Descriptive statistics are expressed as the mean ±
SD and the median. Quantitative data are presented as the mean ±
SEM. Comparisons between two groups were performed using the
Mann-Whitney U test. The correlation among the protein expression
levels of ARID1A, ARID1B and ARID2 were determined using Spearman's
rank correlation coefficient. Pearson's χ2 test was used
to analyze the association between the ARID1A, ARID1B and ARID2
protein expression levels and the clinicopathological
characteristics of patients with CRC when the expected value in
<20% of cells was <5. In cases where this condition was
violated in a 2x2 table, Fisher's exact probability test was
performed (36). The relationship
between ARID protein expression and progression-free survival (PFS)
was assessed using KM analysis and the log-rank test. Moreover, the
PFS univariate and multivariate analyses were performed using Cox
proportional hazards regression analysis. All statistical analyses
were performed using IBM SPSS statistical software (version 25; IBM
Corp.) and GraphPad Prism 9 (Dotmatics). P<0.05 was considered
to indicate a statistically significant difference.
Results
Gene mutations, expression
correlations and promoter methylation levels of ARID1A, ARID1B and
ARID2 in CRC
Gene mutations in ARID1A, ARID1B and
ARID2 in TCGA-COAD dataset were investigated using the
CVCDAP platform. The analysis revealed that ~20.75% of patients
with CRC had mutations in at least one of these ARID genes.
Among these genes, ARID1A was the most prevalent, accounting
for 13% of all samples. Mutations in ARID1B and ARID2
were each identified in 8% of the samples. Frameshift mutations
were the most common in ARID1A, while missense mutations
were the most frequently observed in both ARID1B and
ARID2 (Fig. 1A).
Additionally, significant positive correlations were found among
the expression of ARID1A, ARID1B and ARID2. A
strong correlation was found between ARID1A and
ARID1B expression (r=0.71, P<0.001), while moderate
correlations were observed between ARID1A and ARID2
(r=0.48, P<0.001) as well as between ARID1B and
ARID2 (r=0.43, P<0.001). Pearson correlation analysis
revealed linear relationships between gene expression pairs, as
visualized in the scatter plots with clear positive trend lines in
each panel (Fig. 1B-D).
Furthermore, promoter methylation analysis revealed that all three
ARID genes exhibited significantly higher methylation levels
in the COAD samples compared with the normal tissues (Fig. 1E-G). These findings suggest that
genetic mutations and epigenetic regulation may contribute to the
altered expression of ARID1A, ARID1B and ARID2 in CRC.
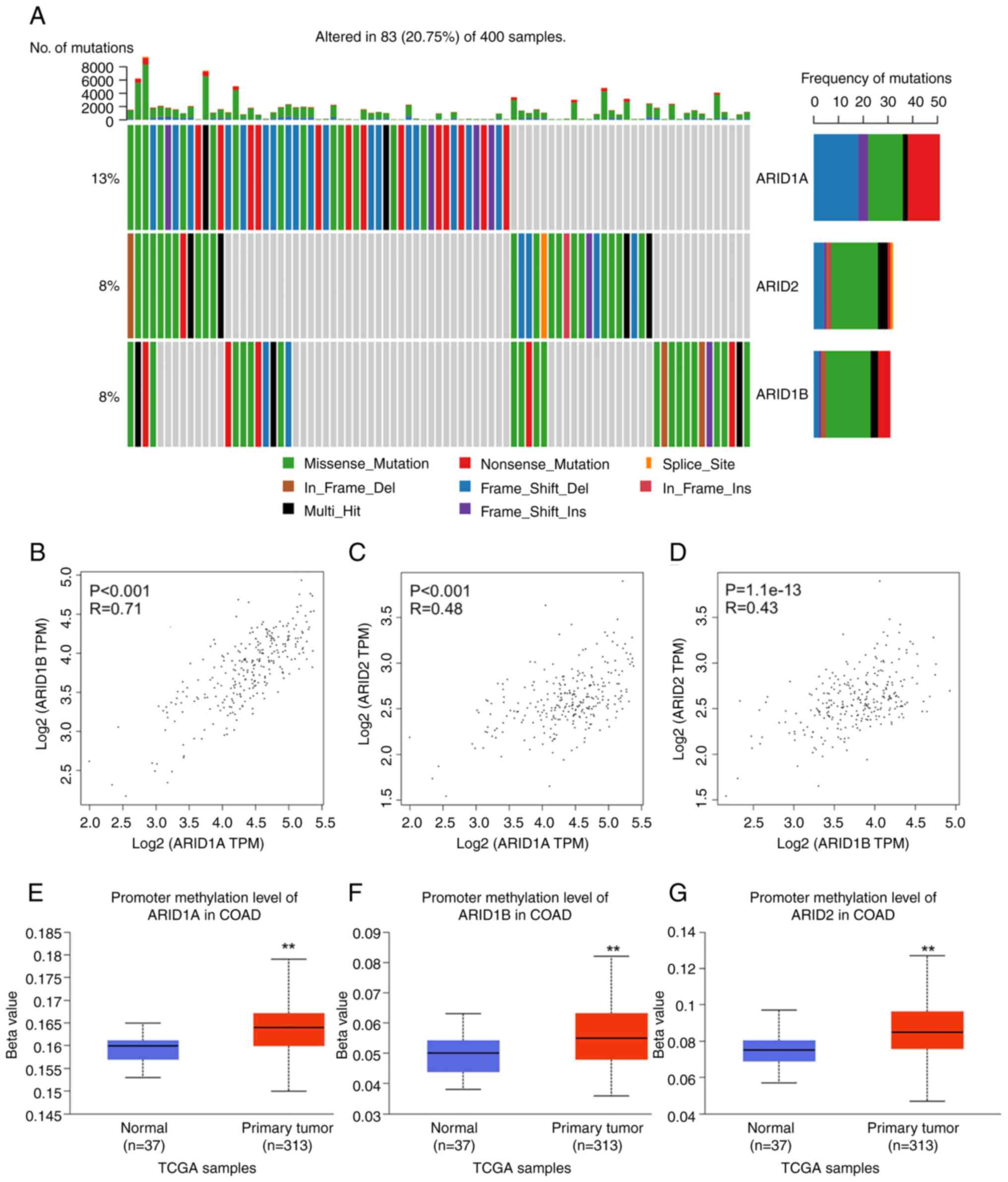 | Figure 1Gene mutations, expression
correlations and promoter methylation levels of ARID1A,
ARID1B and ARID2 in COAD. (A) Genetic alterations of
ARID1A, ARID1B and ARID2 in patients from
TCGA-COAD cohort, analyzed using the Cancer Virtual Cohort
Discovery Analysis Platform. Expression correlations between (B)
ARID1A and ARID1B, (C) ARID1A and ARID2
and (D) ARID1B and ARID2 in TCGA-COAD cohort,
analyzed via the Gene Expression Profiling Interactive Analysis 2
platform. The promoter methylation level of (E) ARID1A, (F)
ARID1B and (G) ARID2 in TCGA-COAD samples, analyzed
via The University of ALabama at Birmingham CANcer data analysis
portal. **P<0.01. ARID, AT-rich interactive domain;
COAD, colon adenocarcinoma; TCGA, The Cancer Genome Atlas. |
Demographic and clinical
characteristics of patients with CRC
A total of 63 patients diagnosed with CRC were
included in the present study, with ages ranging from 52 to 97
years, yielding a mean age of 67.17±8.83 years and a median age of
66 years. Among the cohort, 36 patients (57.14%) were women and 27
(42.86%) were men. The tumor location was predominantly in the
rectum/sigmoid colon (47.62%), followed by the right-sided colon
(38.09%) and the left-sided colon (14.29%). Tumor sizes varied from
2.00 to 12.50 cm, with a mean size of 5.52±2.07 cm and a median
size of 5.00 cm. Pathological differentiation examination indicated
that the majority of tumors were well-differentiated
adenocarcinomas, found in 42 patients (66.67%), followed by
moderately differentiated tumors in 15 patients (23.81%) and poorly
differentiated tumors in 6 patients (9.52%). According to the AJCC
staging, patients were classified as Stage I (12.17%), Stage II
(26.98%), Stage III (36.51%) and Stage IV (23.81%). The depth of
tumor invasion examination indicated that 80.95% of cases were
categorized as late-stage (pT3-pT4). Lymph node involvement (pN)
was absent in 58.73% of patients (pN0), while 41.27% had one or
more positive lymph node (pN1-pN2). Distant metastasis (pM1) was
identified in 23.81% of the cohort. Additionally, nearly half of
the patients (49.21%) exhibited lymphovascular invasion. Lymph node
metastasis was detected in 26 of the 63 patients (41.27%).
Furthermore, 73.02% of patients had comorbidities, including
diabetes mellitus, hypertension and dyslipidemia, highlighting the
complexity of the patient population. The clinicopathological
characteristics of the 63 patients with CRC are summarized in
Table I.
 | Table IClinicopathological characteristics
in 63 patient samples of CRC. |
Table I
Clinicopathological characteristics
in 63 patient samples of CRC.
| Clinicopathological
characteristics | Value |
|---|
| Age (years) | |
|
Age range
(mean ± SD) | 52-97
(67.17±8.83) |
|
Median of
age | 66 |
| Sex [n (%)] | |
|
Men | 27 (42.86) |
|
Women | 36 (57.14) |
| Location of tumor
[n (%)] | |
|
Left-sided
colon | 9 (14.29) |
|
Right-sided
colon | 24 (38.09) |
|
Rectum/sigmoid
colon | 30 (47.62) |
| Largest dimension
of tumor (cm) | |
|
Size range
(mean ± SD) | 2.0-12.5
(5.52±2.07) |
|
Median of
the largest dimension of tumor | 5.0 |
| Pathological
differentiation [n (%)] | |
|
Well | 42 (66.67) |
|
Moderate | 15 (23.81) |
|
Poor | 6 (9.52) |
| AJCC CRC staging [n
(%)] | |
|
Stage I | 8 (12.70) |
|
Stage
II | 17 (26.98) |
|
Stage
III | 23 (36.51) |
|
Stage
IV | 15 (23.81) |
| Depth of tumor
invasion (pT stage) [n (%)] | |
|
Early stage
(pT0-pT2) | 12 (19.05) |
|
Late stage
(pT3-pT4) | 51 (80.95) |
| No. of positive
lymph nodes (pN stage) [n (%)] | |
|
Not
identified (negative; pNX-pN0) | 37 (58.73) |
|
1 node or
>1 node (positive) (pN1-pN2) | 26 (41.27) |
| Distant metastasis
(pM stage) [(%)] | |
|
Not
identified (pM0) | 48 (76.19) |
|
Metastasized
other organs (pM1) | 15 (23.81) |
| Lymphovascular
invasion [n (%)] | |
|
Not
identified | 32 (50.79) |
|
Presence | 31 (49.21) |
| Lymph node
metastasis [n (%)] | |
|
Negative | 37 (58.73) |
|
Positive | 26 (41.27) |
| Comorbidity [n
(%)] | |
|
Absence | 17 (26.98) |
|
Presence | 46 (73.02) |
ARID1A, ARID1B and ARID2 protein
expression in adjacent non-cancerous vs. cancerous areas
The expression levels of ARID1A, ARID1B and ARID2 in
the cohort of 63 patients with CRC were analyzed using IHC. The
results indicated that nuclear ARID1A, ARID1B and ARID2 proteins
were predominantly found in the colonic epithelial cells that form
the intestinal glands. Strong nuclear expression of these ARIDs was
observed in the intestinal cells of adjacent non-cancerous areas,
while cancerous regions exhibited weaker staining. Additionally,
ARID2 also exhibited cytoplasmic localization (Fig. 2A-C).
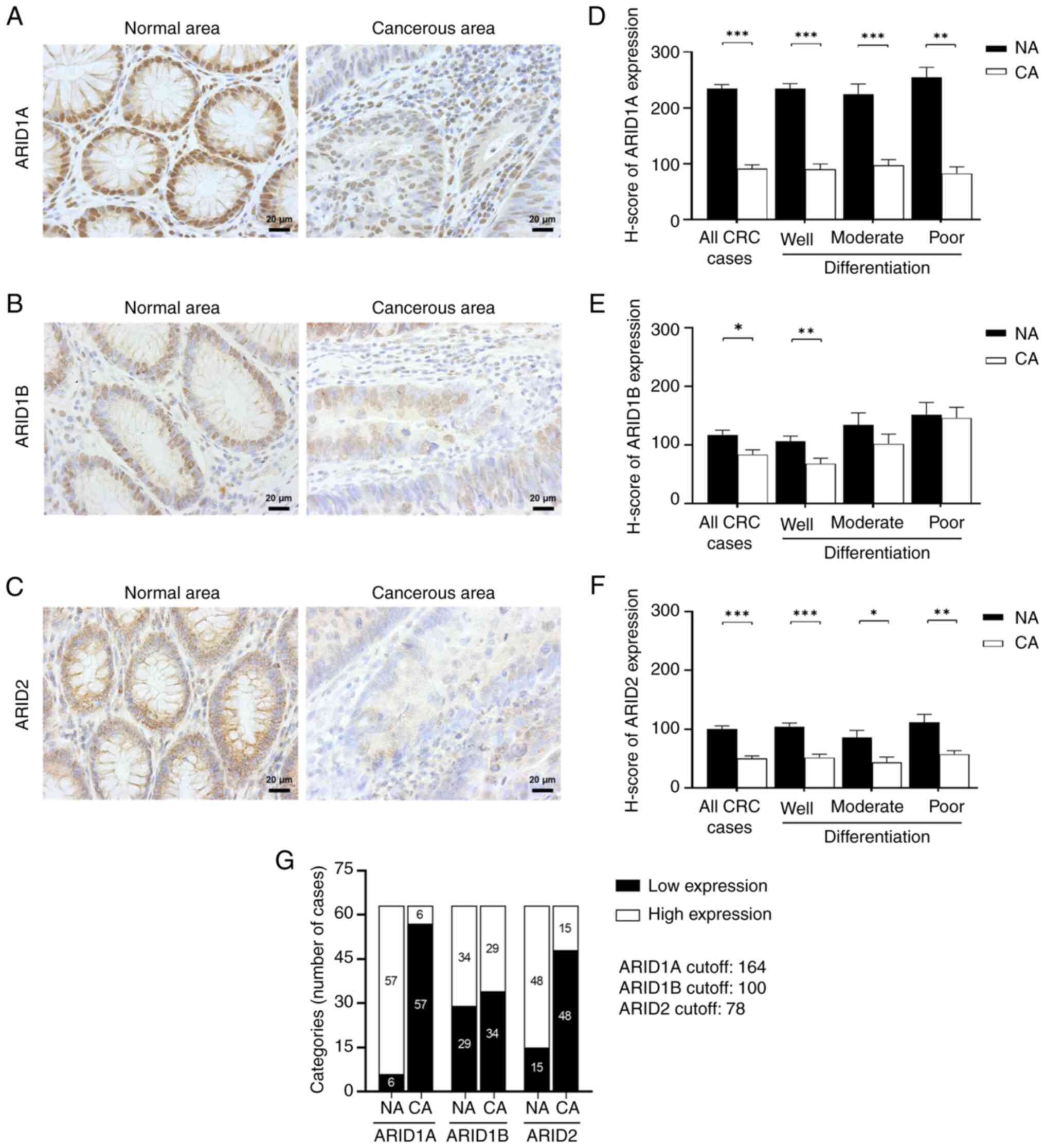 | Figure 2ARID1A, ARID1B and ARID2 protein
expression in colorectal cancer tissues. Immunohistochemistry of
(A) ARID1A, (B) ARID1B and (C) ARID2 proteins in the adjacent
non-cancerous area compared with the cancerous area. ARID protein
staining appears brown (magnification, x400; scale bar, 20 µm). The
H-score of (D) ARID1A, (E) ARID1B and (F) ARID2 protein expression
in the non-cancerous area (black bar) compared with the cancerous
area (white bar). The data are presented as the mean ± SEM and
analyzed by the Mann-Whitney U test. (G) Distribution of ARID1A,
ARID1B and ARID2 expression categorization in adjacent
non-cancerous and cancerous areas. Expression is classified as low
or high depending on the median H-score. ARID1A cut-off, 164;
ARID1B cut-off, 100; ARID2 cut-off, 78. *P<0.05,
**P<0.01 and ***P<0.001. ARID, AT-rich
interactive domain; H-score, histoscore; SEM, standard error of the
mean; NA, adjacent non-cancerous area; CA, cancerous area. |
Semi-quantitative analysis (Fig. 2D) revealed a significant decrease in
ARID1A protein expression in cancerous areas (90.89±6.67) compared
with adjacent non-cancerous areas (234.23±7.40) in all CRC cases
(P<0.001). In well-differentiated tumors, ARID1A protein
expression was significantly lower in cancerous areas (89.92±9.69)
compared with adjacent non-cancerous areas (234.59±8.84)
(P<0.001). Similarly, moderately differentiated cancerous areas
exhibited reduced ARID1A levels (96.94±10.40) compared with
adjacent non-cancerous areas (224.95±17.74) (P<0.001). Poorly
differentiated tissues also displayed decreased ARID1A protein
expression in cancerous areas (82.58±11.79) compared with adjacent
non-cancerous areas (254.88±17.69) (P<0.01).
ARID1B protein expression was significantly
decreased in cancerous areas (83.87±8.04) compared with adjacent
non-cancerous areas (117.34±8.13) in all CRC cases (P<0.05).
Well-differentiated cancerous areas had lower ARID1B levels
(68.46±9.20) than adjacent non-cancerous areas (106.25±9.08)
(P<0.01). However, no significant differences were observed in
tissues with moderate or poor differentiation grades, although
ARID1B expression tended to be lower in cancerous areas (P=0.351
and P=0.818, respectively) (Fig.
2E).
ARID2 expression was significantly lower in
cancerous areas (50.51±4.43) than in adjacent non-cancerous tissues
(114.26±14.45) in all CRC cases (P<0.001). In
well-differentiated tumors, ARID2 expression was significantly
lower in cancerous areas (51.83±5.80) compared with adjacent
non-cancerous areas (104.44±6.11) (P<0.001). Moderately
differentiated cancerous areas also had decreased ARID2 expression
(43.94±8.94) compared with adjacent non-cancerous areas
(85.66±12.53) (P<0.05). Similarly, ARID2 expression in poorly
differentiated cancerous areas was significantly lower (57.69±6.02)
than in adjacent non-cancerous areas (111.96±13.39) (P<0.01)
(Fig. 2F).
Next, the H-score for the cancerous areas was used
to categorize ARID expression as either low or high based on the
median cut-off value. For ARID1A, scores <164 were categorized
as ‘low expression’ and scores of ≥164 as ‘high expression.’ There
were 57 cases with low ARID1A expression (90.48%) and 6 cases with
high ARID1A expression (9.52%). The median H-score of ARID1B
expression was 100, with 34 cases showing low ARID1B expression
(53.97%) and 29 cases showing high ARID1B expression (46.03%). For
ARID2, the median H-score was 78, with 15 cases showing high ARID2
expression (23.81%) and 48 cases showing low ARID2 expression
(76.19%) (Fig. 2G).
Correlation between the ARID1A, ARID1B
and ARID2 protein expression levels in CRC tissues
The Spearman's correlation coefficient was used to
assess the linear correlation among the expression levels of
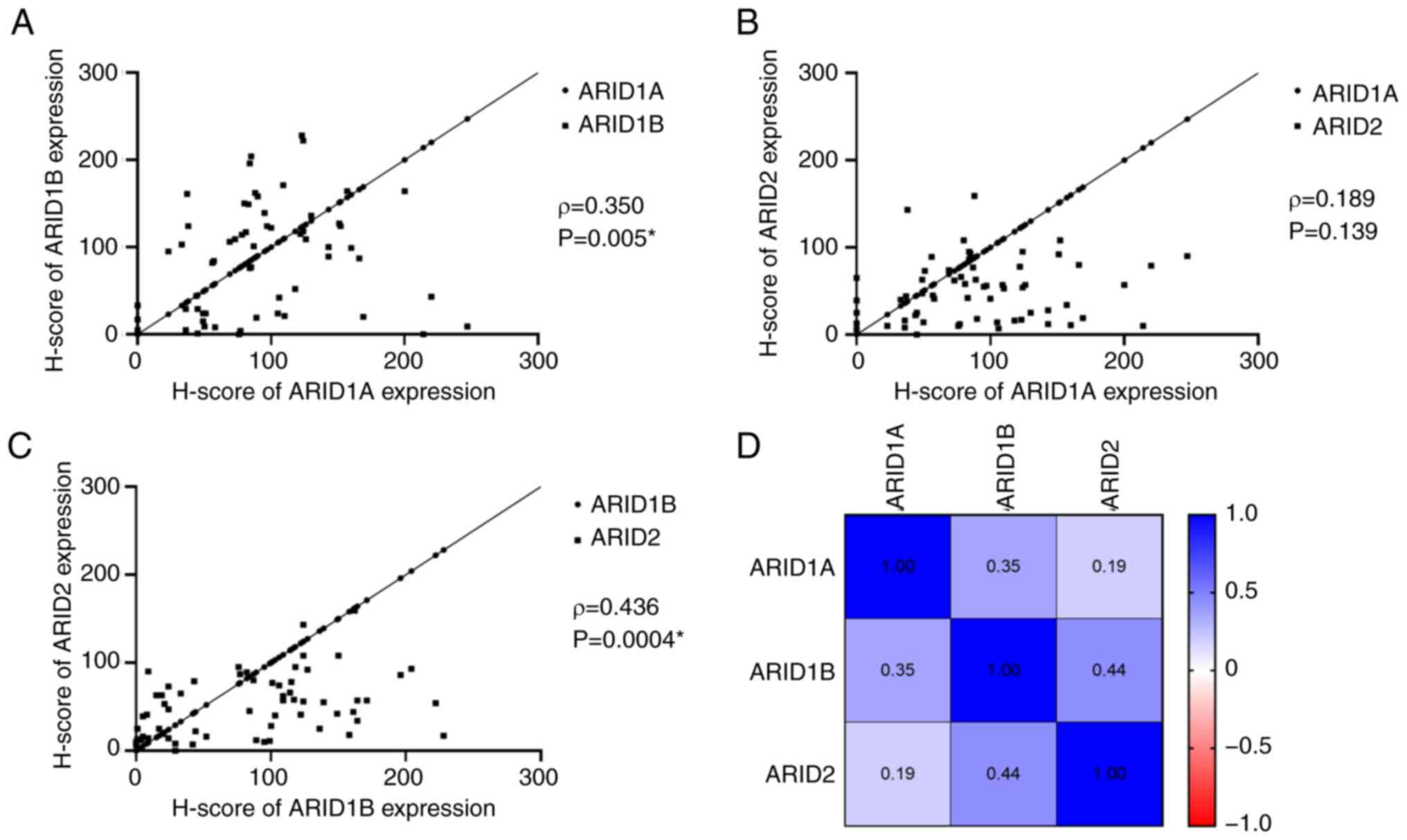
ARID1A, ARID1B and ARID2 in CRC tissues (Fig. 3). The results showed a moderate
correlation between the H-scores of ARID1A and ARID1B (ρ=0.350,
P=0.005), but no significant correlation between ARID1A and ARID2
(ρ=0.189, P=0.139) (Fig. 3A and
B). Additionally, ARID1B expression
was moderately correlated with ARID2 expression (ρ=0.436, P=0.0004;
Fig. 3C). The ρ values of the
correlation coefficients from the Spearman's correlation analysis
are presented in a heatmap (Fig.
3D).
Association between ARID expression
and the clinicopathological characteristics of patients with
CRC
Fisher's exact and χ2 analysis revealed
that low ARID1A expression in patients with CRC was significantly
associated with late-stage disease (P=0.049), a higher pN stage
(P=0.038), the pM stage (P=0.025) and LNM (P=0.038) compared with
those exhibiting high ARID1A expression. However, no significant
differences were observed in age, sex, tumor location, tumor size,
pathological differentiation, pT stage, lymphovascular invasion or
comorbidities between the two expression groups. Furthermore, low
ARID1B expression in patients with CRC was significantly associated
with pathological differentiation (P=0.004), late-stage disease
(P=0.038), pN stage (P=0.010) and LNM (P=0.010) compared with those
exhibiting high ARID1B expression. However, ARID2 expression did
not show a significant association with the clinicopathological
characteristics (Table II). After
applying the Bonferroni adjustment (adjusted P=0.0014) to all ARID
associations, none of these associations remained statistically
significant.
 | Table IIAssociation of ARID1A, ARID1B, and
ARID2 expression with clinicopathology of CRC patients (n=63). |
Table II
Association of ARID1A, ARID1B, and
ARID2 expression with clinicopathology of CRC patients (n=63).
| | ARID1A | ARID1B | ARID2 |
|---|
| Clinicopathological
characteristics | Low n (%) | High n (%) | χ2 | P-value | Low n (%) | High n (%) | χ2 | P-value | Low n (%) | High n (%) | χ2 | P-value |
|---|
| Age | | | 0.003 | 1.000a | | | 0.501 | 0.479b | | | 0.233 | 1.000a |
|
<60 years
old | 10 (15.87) | 1 (7.94) | | | 7 (11.11) | 4 (6.35) | | | 9 (14.29) | 2 (3.17) | | |
|
≥60 years
old | 47 (65.15) | 5 (16.67) | | | 27 (42.86) | 25 (39.68) | | | 39 (61.90) | 13 (20.63) | | |
| Sex | | | 0.138 | 1.000a | | | 0.048 | 0.827b | | | 0.729 | 0.393 |
|
Men | 24 (38.10) | 3 (4.76) | | | 15 (23.81) | 12 (19.05) | | | 22 (34.92) | 5 (7.94) | | |
|
Women | 33 (52.38) | 3 (4.76) | | | 19 (30.16) | 17 (26.98) | | | 26 (41.27) | 10 (15.87) | | |
| Location of
tumor | | | 4.716 | 0.095b | | | 0.518 | 0.772b | | | 1.260 | 0.533 |
|
Left-sided
colon | 7 (11.11) | 2 (3.17) | | | 4 (6.35) | 5 (7.94) | | | 6 (9.52) | 3 (4.76) | | |
|
Right-sided
colon | 24 (38.10) | 0 (0.00) | | | 14 (22.22) | 10 (15.87) | | | 20 (31.75) | 4 (6.35) | | |
|
Rectum/sigmoid
colon | 26 (41.27) | 4 (6.35) | | | 16 (25.40) | 14 (22.22) | | | 22 (34.92) | 8 (12.70) | | |
| Largest dimension
of tumor | | | 1.239 | 0.355a | | | 0.925 | 0.336b | | | 2.547 | 0.196a |
|
<4.50
cm | 16 (25.40) | 3 (4.76) | | | 12 (19.05) | 7 (11.11) | | | 12 (19.05) | 7 (11.11) | | |
|
≥4.50
cm | 41 (65.08) | 3 (4.76) | | | 22 (34.92) | 22 (34.92) | | | 36 (57.14) | 8 (12.70) | | |
| Pathological
differentiation | | | 3.316 | 0.191b | | | 10.939 | 0.004b,c | | | 2.074 | 0.355b |
|
Well | 36 (57.14) | 6 (9.52) | | | 28 (44.44) | 14 (22.22) | | | 31 (49.21) | 11 (17.46) | | |
|
Moderate | 15 (23.81) | 0 (0.00) | | | 6 (9.52) | 9 (14.29) | | | 11 (17.46) | 4 (6.35) | | |
|
Poor | 6 (9.52) | 0 (0.00) | | | 0 (0.00) | 6 (9.52) | | | 6 (9.52) | 0 (0.00) | | |
| AJCC CRC
staging | | | 7.881 | 0.049b,c | | | 8.415 | 0.038b,c | | | 1.763 | 0.623b |
|
Stage I | 7 (11.11) | 1 (1.59) | | | 5 (7.94) | 3 (4.76) | | | 5 (7.94) | 3 (4.76) | | |
|
Stage
II | 16 (25.40) | 1 (1.59) | | | 11 (17.46) | 6 (9.52) | | | 12 (19.05) | 5 (7.94) | | |
|
Stage
III | 23 (36.51) | 0 (0.00) | | | 7 (11.11) | 16 (25.40) | | | 19 (30.16) | 4 (6.35) | | |
|
Stage
IV | 11 (17.46) | 4 (6.35) | | | 11 (17.46) | 4 (6.35) | | | 12 (19.05) | 3 (4.76) | | |
| pT stage | | | 0.878 | 0.320a | | | 0.962 | 0.327b | | | 0.741 | 0.457a |
|
Early stage
(pT0-pT2) | 10 (15.87) | 2 (3.17) | | | 8 (12.70) | 4 (6.35) | | | 8 (12.70) | 4 (6.35) | | |
|
Late stage
(pT3-pT4) | 47 (74.60) | 4 (6.35) | | | 26 (41.27) | 25 (39.68) | | | 40 (63.49) | 11 (17.46) | | |
| pN stage | | | 4.660 | 0.038a,c | | | 6.674 | 0.010b,c | | | 1.732 | 0.188b |
|
pNX-pN0 | 31 (49.21) | 6 (9.52) | | | 25 (39.68) | 12 (19.05) | | | 26 (41.27) | 11 (17.46) | | |
|
pN1-pN2 | 26 (41.27) | 0 (0.00) | | | 9 (14.29) | 17 (26.98) | | | 22 (34.92) | 4 (6.35) | | |
| pM stage | | | 6.714 | 0.025a,c | | | 2.972 | 0.085b | | | 0.158 | 1.000a |
|
pM0 | 46 (73.02) | 2 (3.17) | | | 23 (36.51) | 25 (39.68) | | | 36 (57.14) | 12 (19.05) | | |
|
pM1 | 11 (17.46) | 4 (6.35) | | | 11 (17.46) | 4 (6.35) | | | 12 (19.05) | 3 (4.76) | | |
| Lymphovascular
invasion | | | 2.809 | 0.196a | | | 0.765 | 0.382b | | | 0.668 | 0.414b |
|
Not
identified | 27 (42.62) | 5 (7.94) | | | 19 (30.16) | 13 (20.63) | | | 23 (36.51) | 9 (14.29) | | |
|
Presence | 30 (47.62) | 1 (1.59) | | | 15 (23.81) | 16 (25.40) | | | 25 (39.68) | 6 (9.52) | | |
| Lymph node
metastasis | | | 4.660 | 0.038a,c | | | 6.674 | 0.010b,c | | | 1.732 | 0.188b |
|
Negative | 31 (49.21) | 6 (9.52) | | | 25 (39.68) | 12 (19.05) | | | 26 (41.27) | 11 (17.46) | | |
|
Positive | 26 (41.27) | 0 (0.00) | | | 9 (14.29) | 17 (26.98) | | | 22 (34.92) | 4 (6.35) | | |
| Comorbidity | | | 2.451 | 0.178a | | | 0.447 | 0.504b | | | 1.862 | 0.317a |
|
Absence | 17 (26.98) | 0 (0.00) | | | 8 (12.70) | 9 (14.29) | | | 15 (23.81) | 2 (3.17) | | |
|
Presence | 40 (63.49) | 6 (9.52) | | | 26 (41.27) | 20 (31.75) | | | 33 (52.38) | 13 (20.63) | | |
Association between the protein
expression levels of ARID1A, ARID1B and ARID2 with the survival
outcomes of patients with CRC
The associations between the 5-year PFS of patients
and the expression levels of ARID1A, ARID1B and ARID2 were analyzed
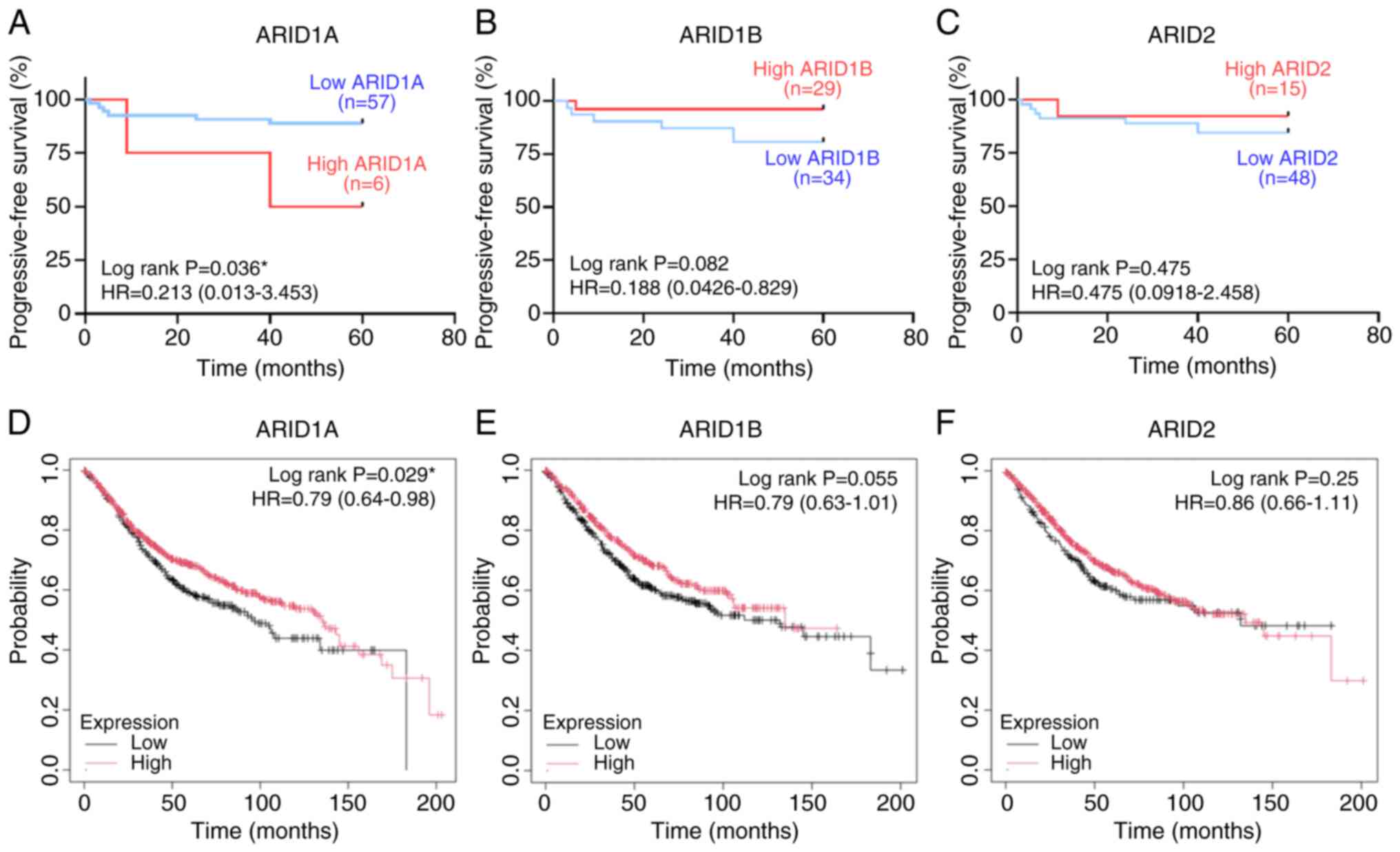
using KM curve and log-rank test analysis (Fig. 4A-C). The results revealed that
patients with CRC exhibiting high ARID1A expression had a
significantly shorter PFS time compared with those exhibiting low
ARID1A expression (P=0.036). Additionally, there was a trend
towards a shorter PFS time in patients with low ARID1B and ARID2
expression compared with those with high expression; however, the
differences were not statistically significant.
Cox proportional hazards regression analysis was
conducted to assess the significance of potential prognostic
factors in patients with CRC. Univariate analysis revealed that low
ARID1A expression (P=0.005) was significantly associated with PFS.
Furthermore, the multivariate analysis, which included ARID1A,
ARID1B and ARID2 expression as well as LNM status, indicated that
low ARID1A expression (P=0.015) was an independent prognostic
factor related to PFS (Table
III).
 | Table IIIUnivariate and multivariate analyses
of clinicopathological characteristics in 63 patients of CRC using
Cox hazard regression analysis. |
Table III
Univariate and multivariate analyses
of clinicopathological characteristics in 63 patients of CRC using
Cox hazard regression analysis.
| | Univariate | Multivariate |
|---|
| Variable | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valuea |
|---|
| ARID1A | | | | | | |
|
Low vs. high
ARID1A | 0.187 | 0.057-0.610 | 0.005b | 5.502 | 1.402-21.594 | 0.015b |
|
Low vs. high
ARID1B | 1.963 | 0.604-6.375 | 0.262 | 0.627 | 0.179-2.190 | 0.464 |
| ARID2 | | | | | | |
|
Low vs. high
ARID2 | 1.017 | 0.280-3.697 | 0.979 | 0.716 | 0.183-2.805 | 0.632 |
| Age | | | | | | |
|
≥60 vs.
<60 years | 0.828 | 0.184-3.738 | 0.806 | | | |
| Sex | | | | | | |
|
Men vs.
Women | 2.697 | 0.742-9.808 | 0.132 | | | |
| Tumor location | | | | | | |
|
Rectum/sigmoid
vs. right/left | 0.760 | 0.255-2.262 | 0.622 | | | |
| Tumor largest
dimension | | | | | | |
|
≥4.5 vs.
<4.5 cm | 1.015 | 0.313-3.298 | 0.980 | | | |
| Pathological
differentiation | | | | | | |
|
Poor/moderate
vs. well | 2.955 | 0.655-13.336 | 0.159 | | | |
| AJCC stage | | | | | | |
|
Late-stage
vs. early-stage | 0.019 | 0.000-1.770 | 0.087 | | | |
| Tumor invasion | | | | | | |
|
High (T3-T4)
vs. Low (T1-T2) | 0.721 | 0.160-3.256 | 0.671 | | | |
| Positive lymph
node | | | | | | |
|
Positive
(N1-N2) vs. Negative (N0) | 1.633 | 0.503-5.303 | 0.415 | | | |
| Distance
metastasis | | | | | | |
|
Presence
(M1) vs. absence (M0) | 0.000 | 0.000-194.851 | 0.232 | | | |
| Lymphovascular
invasion | | | | | | |
|
Presence vs.
absence | 2.244 | 0.691-7.288 | 0.179 | | | |
| Lymph node
metastasis | | | | | | |
|
Presence vs.
absence | 1.6333 | 0.503-5.303 | 0.415 | 0.922 | 0.233-3.656 | 0.908 |
To further assess the prognostic significance of
ARID1A, ARID1B and ARID2, additional analysis was conducted using
the KM plotter database. The results demonstrated that lower
expression of all three ARID genes showed a general trend
towards a shorter OS time. However, only ARID1A expression showed
prognostic significance, with patients with CRC exhibiting low
ARID1A expression having a significantly shorter OS time compared
with those exhibiting high expression (Fig. 4D-F).
Discussion
CRC is a prevalent cancer worldwide, with incidence
rates that are increasing, including in Thailand (4). The SWI/SNF chromatin remodeling
complex, particularly the ARID1A, ARID1B and ARID2 subunits, plays
a critical role in CRC pathogenesis, affecting transcription
regulation, cell differentiation, cell growth and cell progression
(9,10). In the present study, bioinformatics
analysis revealed frequent mutations in these ARID genes,
with ARID1A predominantly exhibiting frameshift mutations
and ARID1B and ARID2 primarily exhibiting missense
mutations. These findings align with a previous study reporting
that certain cancers with a high frequency of ARID1A
mutations also exhibit recurrent mutations in ARID1B and
ARID2 (37). ARID1A
predominantly exhibited frameshift mutations, particularly at
codons such as Gln456fs and Ser1315fs, which result in premature
stop codons and loss of function (38). ARID1B and ARID2
primarily exhibited missense mutations, including Arg1271Cys and
Gly1973Arg in ARID1B, and Phe105Leu in ARID2
(39,40). These mutations are known to disrupt
the function of the SWI/SNF chromatin remodeling complex, leading
to impaired transcriptional regulation and promoting oncogenesis
(41). Previous studies have
demonstrated that ARID1A loss-of-function mutations are
associated with microsatellite instability and are prevalent in CRC
(42). Similarly, missense
mutations in ARID1B and ARID2 can alter DNA
accessibility and impact tumor suppressor gene expression (43). Additionally, in the present study,
increased methylation levels were observed in the promoters of all
three ARID genes, suggesting that both genetic mutations and
epigenetic regulation contribute to their altered expression in
CRC. However, the direct impact of ARID mutations on CRC
development and progression requires further investigation. Future
studies should also include a comprehensive assessment of how
specific ARID mutations affect ARID protein function and
expression, to fully understand their role in CRC.
ARID proteins regulate gene expression and chromatin
remodeling through their DNA-binding domains (9). All ARID family members are involved in
tumorigenesis (44), with mutations
often leading to decreased protein expression (45,46) or
loss in various cancer types (7,11,14-16,19,47).
As tumor suppressors, the ARIDs regulate pathways involved in
cancer development and progression (21-24).
Upregulation of ARID1A expression in CRC cells was shown to
suppress cell invasion and migration (24), while downregulation enhanced these
processes (22,23). In lung cancer cells, ARID1B
knockdown increased DNA damage and impaired DNA repair mechanisms
(25), and ARID2 deficiency
promoted cancer growth and metastasis (18). However, the relationships between
the ARID protein expression levels in CRC remain largely
unexplored.
In the present study, immunohistochemical analysis
revealed decreased ARID1A, ARID1B and ARID2 protein levels in
tissues from Thai patients with CRC, with ARID1A exhibiting the
strongest staining. This supports the previous findings that ARID1A
is the most effective suppressor among the three ARIDs (11). In the present study, using the
H-score system for quantitative analysis, patients were classified
into the high and low expression groups according to the median
H-score (35,48). The H-score classification showed
that 90.48% of cancerous areas had low ARID1A expression,
consistent with previous reports indicating ARID1A loss in over
half of CRC cases (13,49). Similarly, 54 and 76% of cancerous
areas exhibited low ARID1B and ARID2 expression, respectively
(50). It was also observed that
the ARID proteins were localized to the nucleus and cytoplasm,
predominantly in the epithelial cells of the intestinal glands in
adjacent non-cancerous areas. ARID1A and ARID1B showed a nuclear
distribution, whereas ARID2 was largely present in the cytoplasm. A
previous study reported ARID2 localization in both the nucleus and
cytoplasm in colon tissue, with its downregulation linked to
altered cell proliferation, invasion, migration and EMT (51). While the role of cytoplasmic ARID2
remains unclear, it may regulate cytoplasmic signaling or other
processes; however, this requires further investigation.
In the present study, immunohistochemical analysis
revealed a significant association among the ARID1A, ARID1B and
ARID2 proteins in CRC, similar to the correlation observed at the
mRNA level and consistent with findings in gastric cancer (11). These results suggest a co-expression
at both the mRNA and protein levels; however, the underlying
regulatory mechanisms remain unclear. Future studies should include
a detailed assessment of mRNA levels to explore the regulatory
mechanisms of this co-expression. ARID family members may share
overlapping roles in transcriptional regulation and form complex
networks (52), with their positive
correlations indicating a potential cooperative role in CRC
pathogenesis. Additionally, tumor suppressor proteins such as p53,
MYC, retinoblastoma protein and BRCA1 interact with the SWI/SNF
subunits (53). In CRC,
ARID1A mutations may contribute to disease progression
through co-occurring mutations in other cancer-related genes (such
as TP53, KRAS, APC and PIK3CA) and
dysregulated pathways (such as WNT, Akt and MEK/ERK), affecting key
cellular processes including cell cycle regulation and chromatin
remodeling (54).
Loss of ARID expression is associated with various
clinicopathological characteristics in cancer (9,11,13,17,55).
In the present study, decreased ARID1A expression was found to be
linked to late-stage disease and LNM, while low ARID1B expression
was associated with poor differentiation, late-stage disease, pN
stage and LNM. No significant associations were found for ARID2
expression in CRC. These findings align with previous studies that
showed reduced ARID1A expression is correlated with advanced
disease and LNM in CRC (13,49,55),
and other cancer types (56,57).
ARID1B promotor methylation has been linked to tumor stage
and LNM in COAD (50), and its loss
was demonstrated to be associated with lymphatic infiltration and
LNM in gastric cancer (11). By
contrast, high ARID1B expression was linked to favorable outcomes
in breast cancer (58) and bladder
urothelial carcinoma (17). In
hepatocellular carcinoma and oral cancer, low ARID2 expression was
correlated with advanced clinicopathological factors (45,59).
Although the present study demonstrated an association between the
ARID1A and ARID1B expression levels and advanced
clinicopathological characteristics in CRC, the Bonferroni
correction result underscores the need for cautious interpretation.
Future studies with larger sample sizes or alternative correction
methods are warranted to validate these findings and clarify the
role of ARIDs in CRC.
Among the three ARIDs, only ARID1A expression was an
independent prognostic factor for patients with CRC in the present
study. In the Thai CRC cohort, KM survival analysis showed that
high ARID1A expression was associated with a significantly shorter
PFS time, contradicting previous studies that found low ARID1A
expression was correlated with poorer survival (13,55).
These discrepancies may be due to differences in methodologies,
such as antibodies, cut-off values and IHC scoring (54), as well as the small number of
patients with high ARID1A expression in the present study. Notably,
the present study found that 4 out of 6 patients with high ARID1A
expression had metastases and complications, such as hypertension
and dyslipidemia, which may influence survival. A tendency towards
a shorter PFS time was also noted in patients with low ARID1B and
ARID2 expression, consistent with a prior report on oral squamous
cell carcinoma (59). These
findings suggest ARID1A as a potential prognostic marker, while the
roles of ARID1B and ARID2 require further exploration. However, the
prognostic value of ARID1A in Thai patients with CRC also requires
further validation.
Of the poorly differentiated tissues collected in
the present study, all six samples exhibited low ARID1A expression
and high ARID1B expression, suggesting a possible compensatory
upregulation of ARID1B in response to ARID1A loss. This aligns with
previous studies that have shown ARID1B upregulation when ARID1A is
inactivated (60,61). Helming et al reported that at
least one ARID1B allele is retained in ARID1A-deficient cancer to
maintain SWI/SNF complex functionality, supporting cancer cell
survival. The synthetic lethality of targeting ARID1B in
ARID1A-deficient cells was further confirmed in a previous CRC
study (61). These findings
underscore the potential compensatory role of ARIDs in cancer
cells.
The present study has several limitations. First,
the present study was retrospective with a small sample size and
future prospective studies with larger cohorts are required.
Second, although positive correlations in protein expression among
ARID1A, ARID1B and ARID2 were observed in CRC, the regulatory
mechanisms underlying this co-expression remain unclear.
Investigating these pathways could reveal their potential
synergistic roles in cancer development. Future studies using
chromatin immunoprecipitation sequencing or reporter gene assays
could help identify specific binding sites and the regulatory
mechanisms of these proteins. Third, while the prognostic value of
each ARID protein was evaluated individually, exploring combined
expression patterns could lead to more accurate biomarkers for
predicting patient outcomes. Lastly, the specific functional roles
of ARID1A, ARID1B and ARID2 in CRC progression are not fully
understood and further in vitro and in vivo
experiments are required to clarify their contributions. Genetic
manipulation of ARIDs in CRC cells followed by functional assays
could provide a deeper insight into their roles and mechanisms in
CRC development; specifically, future directions should include
subcellular fractionation and siRNA knockdown experiments to
elucidate their precise functions and localization.
In conclusion, the present study demonstrated that
ARID1A, ARID1B and ARID2 are frequently mutated and exhibit reduced
expression in CRC tissues compared with adjacent non-cancerous
tissues. The expression levels of these ARIDs are correlated with
each other and are associated with advanced clinicopathological
features. These findings suggest that decreased ARID expression may
indicate cancer progression and prognosis in CRC, although further
research is needed to validate their clinical significance.
Acknowledgements
The authors are grateful to Mr. Olalekan Israel
Aiikulola, Faculty of Medical Science at Naresuan University
(Phitsanulok, Thailand), for proofreading the English writing of
this manuscript. Furthermore, we would like to thank the Pathology
Unit, Sawan Pracharak Hospital (Nakhon Sawan, Thailand) for kindly
providing the FFPE tissue blocks.
Funding
Funding: This research was supported by Naresuan University,
Thailand Science Research and Innovation (TSRI), and the National
Science Research and Innovation Fund (NSRF) (grant n.
R2567B027).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WM contributed to the study design, performed the
experiments, data analysis, and manuscript writing. KS contributed
to the pathologic analysis and interpretation of data. SA
contributed to data analysis and manuscript editing. PS performed
experiments and pathologic analysis. RS contributed to collecting
samples and patient data. NS and SWU contributed to the conception
and study design, supervision, funding acquisition, and manuscript
editing. WM and SWU confirm the authenticity of all the raw data.
All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Research
Ethics Committee of Sawanpracharak Hospital (Nakhon Sawan,
Thailand; certificate of approval no. 53/2567) and the Naresuan
University Human Research Ethics Committee (Phitsanulok, Thailand;
approval no. P1-0107/2567; certificate of approval no. 139/2024),
and conducted in accordance with the principles of the Declaration
of Helsinki. Written informed consent was obtained from all
subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no completing
interests.
References
|
1
|
Mattiuzzi C, Sanchis-Gomar F and Lippi G:
Concise update on colorectal cancer epidemiology. Ann Transl Med.
7(609)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rawla P, Sunkara T and Barsouk A:
Epidemiology of colorectal cancer: Incidence, mortality, survival,
and risk factors. Prz Gastroenterol. 14:89–103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lohsiriwat V, Chaisomboon N and
Pattana-Arun J: Current colorectal cancer in Thailand. Ann
Coloproctol. 36:78–82. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tiankanon K, Aniwan S and Rerknimitr R:
Current status of colorectal cancer and its public health Burden in
Thailand. Clin Endosc. 54:499–504. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Koncina E, Haan S, Rauh S and Letellier E:
Prognostic and predictive molecular biomarkers for colorectal
cancer: Updates and challenges. Cancers (Basel).
12(319)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sawicki T, Ruszkowska M, Danielewicz A,
Niedźwiedzka E, Arłukowicz T and Przybyłowicz KE: A review of
colorectal cancer in terms of epidemiology, risk factors,
development, symptoms and diagnosis. Cancers (Basel).
13(2025)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pagliaroli L and Trizzino M: The
evolutionary conserved SWI/SNF Subunits ARID1A and ARID1B are key
modulators of pluripotency and cell-fate determination. Front Cell
Dev Biol. 9(643361)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schaefer IM and Hornick JL: SWI/SNF
complex-deficient soft tissue neoplasms: An update. Semin Diagn
Pathol. 38:222–231. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin C, Song W, Bi X, Zhao J, Huang Z, Li
Z, Zhou J, Cai J and Zhao H: Recent advances in the ARID family:
Focusing on roles in human cancer. Onco Targets Ther. 7:315–324.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun J and Cheng NS: Comprehensive
landscape of ARID family members and their association with
prognosis and tumor microenvironment in hepatocellular carcinoma. J
Immunol Res. 2022(1688460)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aso T, Uozaki H, Morita S, Kumagai A and
Watanabe M: Loss of ARID1A, ARID1B, and ARID2 expression during
progression of gastric cancer. Anticancer Res. 35:6819–6827.
2015.PubMed/NCBI
|
|
12
|
Fantone S, Mazzucchelli R, Giannubilo SR,
Ciavattini A, Marzioni D and Tossetta G: AT-rich interactive domain
1A protein expression in normal and pathological pregnancies
complicated by preeclampsia. Histochem Cell Biol. 154:339–346.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wei XL, Wang DS, Xi SY, Wu WJ, Chen DL,
Zeng ZL, Wang RY, Huang YX, Jin Y, Wang F, et al: Clinicopathologic
and prognostic relevance of ARID1A protein loss in colorectal
cancer. World J Gastroenterol. 20:18404–18412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khursheed M, Kolla JN, Kotapalli V, Gupta
N, Gowrishankar S, Uppin SG, Sastry RA, Koganti S, Sundaram C,
Pollack JR and Bashyam MD: ARID1B, a member of the human SWI/SNF
chromatin remodeling complex, exhibits tumour-suppressor activities
in pancreatic cancer cell lines. Br J Cancer. 108:2056–2062.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li K, Wang B and Hu H: Research progress
of SWI/SNF complex in breast cancer. Epigenetics Chromatin.
17(4)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sato E, Nakayama K, Razia S, Nakamura K,
Ishikawa M, Minamoto T, Ishibashi T, Yamashita H, Iida K and Kyo S:
ARID1B as a potential therapeutic target for ARID1A-mutant ovarian
clear cell carcinoma. Int J Mol Sci. 19(1710)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang B, Xie H, Ma C, Zhang G, Gan H, Wang
Q, Liu X, Zhu Y, Zhu Y, Shi G, et al: Expression of ARID1B is
associated with poor outcomes and predicts the benefit from
adjuvant chemotherapy in bladder urothelial carcinoma. J Cancer.
8:3490–3497. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moreno T, Monterde B, González-Silva L,
Betancor-Fernández I, Revilla C, Agraz-Doblas A, Freire J, Isidro
P, Quevedo L, Blanco R, et al: ARID2 deficiency promotes tumor
progression and is associated with higher sensitivity to
chemotherapy in lung cancer. Oncogene. 40:2923–2935.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cajuso T, Hänninen UA, Kondelin J, Gylfe
AE, Tanskanen T, Katainen R, Pitkänen E, Ristolainen H, Kaasinen E,
Taipale M, et al: Exome sequencing reveals frequent inactivating
mutations in ARID1A, ARID1B, ARID2 and ARID4A in microsatellite
unstable colorectal cancer. Int J Cancer. 135:611–623.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Duan Y, Tian L, Gao Q, Liang L, Zhang W,
Yang Y, Zheng Y, Pan E, Li S and Tang N: Chromatin remodeling gene
ARID2 targets cyclin D1 and cyclin E1 to suppress hepatoma cell
progression. Oncotarget. 7:45863–45875. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aluksanasuwan S, Somsuan K, Wanna-Udom S,
Roytrakul S, Morchang A, Rongjumnong A and Sakulsak N: Proteomic
insights into the regulatory function of ARID1A in colon cancer
cells. Oncol Lett. 28(392)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Angelico G, Attanasio G, Colarossi L,
Colarossi C, Montalbano M, Aiello E, Di Vendra F, Mare M, Orsi N
and Memeo L: ARID1A mutations in gastric cancer: A review with
focus on clinicopathological features, molecular background and
diagnostic interpretation. Cancers. 16(2062)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jin M, Xu S, Li J, Li L and Tang C: Role
of ARID1A in the regulation of human trophoblast migration and
invasion. Reprod Sci. 29:2363–2373. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wanna-Udom S, Aluksanasuwan S, Somsuan K,
Mongkolwat W and Sakulsak N: ARID1A overexpression inhibits
colorectal cancer cell migration through the regulation of
epithelial-mesenchymal transition. Mol Med Rep.
30(201)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu G, Liu J, Li Y, Huang H, Chen C, Wu D,
Cao P, Su L, Wang Y, Zhang H, et al: ARID1B deficiency leads to
impaired DNA damage response and activated cGAS-STING pathway in
non-small cell lung cancer. J Cancer. 15:2601–2612. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shen Y, Chen W, Fu C, Liu X, Miao J, Li J,
Li N and Hang D: Polygenic risk score, healthy lifestyle score, and
colorectal cancer risk: A prospective cohort study. Cancer
Epidemiol Biomarkers Prev. 34:290–297. 2025.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guan X, Cai M, Du Y, Yang E, Ji J and Wu
J: CVCDAP: An integrated platform for molecular and clinical
analysis of cancer virtual cohorts. Nucleic Acids Res.
48:W463–W471. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47:W556–W560.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Győrffy B: Integrated analysis of public
datasets for the discovery and validation of survival-associated
genes in solid tumors. Innovation (Camb). 5(100625)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ushakov E, Naumov A, Fomberg V,
Vishnyakova P, Asaturova A, Badlaeva A, Tregubova A, Karpulevich E,
Sukhikh G and Fatkhudinov T: EndoNet: Model for automatic
calculation of H-score on histological slides. Informatics.
10(90)2023.
|
|
34
|
Bencze J, Szarka M, Kóti B, Seo W,
Hortobágyi TG, Bencs V, Módis LV and Hortobágyi T: Comparison of
semi-quantitative scoring and artificial intelligence aided digital
image analysis of chromogenic immunohistochemistry. Biomolecules.
12(19)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Numata M, Morinaga S, Watanabe T, Tamagawa
H, Yamamoto N, Shiozawa M, Nakamura Y, Kameda Y, Okawa S, Rino Y,
et al: The clinical significance of SWI/SNF complex in pancreatic
cancer. Int J Oncol. 42:403–410. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim HY: Statistical notes for clinical
researchers: Chi-squared test and Fisher's exact test. Restor Dent
Endod. 42:152–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu JN and Roberts CW: ARID1A mutations in
cancer: Another epigenetic tumor suppressor? Cancer Discov.
3:35–43. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jones S, Wang TL, Shih Ie M, Mao TL,
Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, et
al: Frequent mutations of chromatin remodeling gene ARID1A in
ovarian clear cell carcinoma. Science. 330:228–231. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fujimoto A, Furuta M, Totoki Y, Tsunoda T,
Kato M, Shiraishi Y, Tanaka H, Taniguchi H, Kawakami Y, Ueno M, et
al: Whole-genome mutational landscape and characterization of
noncoding and structural mutations in liver cancer. Nat Genet.
48:500–509. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mathur R, Alver BH, San Roman AK, Wilson
BG, Wang X, Agoston AT, Park PJ, Shivdasani RA and Roberts CW:
ARID1A loss impairs enhancer-mediated gene regulation and drives
colon cancer in mice. Nat Genet. 49:296–302. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kadoch C, Hargreaves DC, Hodges C, Elias
L, Ho L, Ranish J and Crabtree GR: Proteomic and bioinformatic
analysis of mammalian SWI/SNF complexes identifies extensive roles
in human malignancy. Nat Genet. 45:592–601. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cancer Genome Atlas Network. Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bitler BG, Aird KM, Garipov A, Li H,
Amatangelo M, Kossenkov AV, Schultz DC, Liu Q, Shih IeM,
Conejo-Garcia JR, et al: Synthetic lethality by targeting EZH2
methyltransferase activity in ARID1A-mutated cancers. Nat Med.
21:231–238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhu Y, Yan C, Wang X, Xu Z, Lv J, Xu X, Yu
W, Zhou M and Yue L: Pan-cancer analysis of ARID family members as
novel biomarkers for immune checkpoint inhibitor therapy. Cancer
Biol Ther. 23:104–111. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jiang H, Cao HJ, Ma N, Bao WD, Wang JJ,
Chen TW, Zhang EB, Yuan YM, Ni QZ, Zhang FK, et al: Chromatin
remodeling factor ARID2 suppresses hepatocellular carcinoma
metastasis via DNMT1-Snail axis. Proc Natl Acad Sci USA.
117:4770–4780. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Odnokoz O, Wavelet-Vermuse C, Hophan SL,
Bulun S and Wan Y: ARID1 proteins: From transcriptional and
post-translational regulation to carcinogenesis and potential
therapeutics. Epigenomics. 13:809–823. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xu S and Tang C: The Role of ARID1A in
tumors: Tumor initiation or tumor suppression? Front Oncol.
11(745187)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hu WH, Chen HH, Yen SL, Huang HY, Hsiao CC
and Chuang JH: Increased expression of interleukin-23 associated
with progression of colorectal cancer. J Surg Oncol. 115:208–212.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Erfani M, Hosseini SV, Mokhtari M, Zamani
M, Tahmasebi K, Alizadeh Naini M, Taghavi A, Carethers JM, Koi M,
Brim H, et al: Altered ARID1A expression in colorectal cancer. BMC
Cancer. 20(350)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Baldi S, He Y, Ivanov I, Khamgan H, Safi
M, Alradhi M, Shopit A, Al-Danakh A, Al-Nusaif M, Gao Y and Tian H:
Aberrantly hypermethylated ARID1B is a novel biomarker and
potential therapeutic target of colon adenocarcinoma. Front Genet.
13(914354)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhenzong T, Wang H and Peng Y: ARID2
suppresses cell proliferation, migration, and invasion of colon
cancer by the TGF-β1/Smad pathway. Journal of Biological Regulators
and Homeostatic Agents. 37:2095–2103. 2023.
|
|
52
|
Raab JR, Resnick S and Magnuson T:
Genome-Wide transcriptional regulation mediated by biochemically
distinct SWI/SNF complexes. PLoS Genet. 11(e1005748)2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Reddy D, Bhattacharya S and Workman JL:
(mis)-Targeting of SWI/SNF complex(es) in cancer. Cancer Metastasis
Rev. 42:455–470. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhao S, Wu W, Jiang Z, Tang F, Ding L, Xu
W and Ruan L: Roles of ARID1A variations in colorectal cancer: A
collaborative review. Mol Med. 28(42)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kishida Y, Oishi T, Sugino T, Shiomi A,
Urakami K, Kusuhara M, Yamaguchi K, Kitagawa Y and Ono H:
Associations between loss of ARID1A expression and
clinicopathologic and genetic variables in T1 early colorectal
cancer. Am J Clin Pathol. 152:463–470. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cho HD, Lee JE, Jung HY, Oh MH, Lee JH,
Jang SH, Kim KJ, Han SW, Kim SY, Kim HJ, et al: Loss of tumor
suppressor ARID1A protein expression correlates with poor prognosis
in patients with primary breast cancer. J Breast Cancer.
18:339–346. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Namjan A, Techasen A, Loilome W,
Sa-Ngaimwibool P and Jusakul A: ARID1A alterations and their
clinical significance in cholangiocarcinoma. PeerJ.
8(e10464)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cui Y, Bai X, Niu M, Qin Y, Zhang X and
Pang D: Upregulated expression of AT-rich interactive
domain-containing protein 1B predicts poor prognosis in patients
with triple-negative breast cancer. Oncol Lett. 17:3289–3295.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wu M, Duan Q, Liu X, Zhang P, Fu Y, Zhang
Z, Liu L, Cheng J and Jiang H: MiR-155-5p promotes oral cancer
progression by targeting chromatin remodeling gene ARID2. Biomed
Pharmacother. 122(109696)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Fukumoto T, Park PH, Wu S, Fatkhutdinov N,
Karakashev S, Nacarelli T, Kossenkov AV, Speicher DW, Jean S, Zhang
L, et al: Repurposing Pan-HDAC inhibitors for ARID1A-mutated
ovarian cancer. Cell Rep. 22:3393–3400. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Helming KC, Wang X, Wilson BG, Vazquez F,
Haswell JR, Manchester HE, Kim Y, Kryukov GV, Ghandi M, Aguirre AJ,
et al: ARID1B is a specific vulnerability in ARID1A-mutant cancers.
Nat Med. 20:251–254. 2014.PubMed/NCBI View Article : Google Scholar
|