Introduction
Early diagnosis of oral head and neck squamous cell
carcinoma (HNSCC) is key to improving the probability of survival
(1,2). However, only 28% of HNSCCs are
diagnosed in the local stages of the disease, making treatment more
challenging (3). Early diagnosis of
oral carcinoma improves the 5-year survival rate to ~85% compared
with 40-60% associated with more advanced stages of the disease
(4).
Common precursors to oral HNSCC are leukoplakia or
erythroplakia, which are white or red lesions of the oral cavity,
collectively known as oral dysplasias (ODs) (5) that represent the most common oral
potentially malignant disorders (6). Currently, the only method to predict
malignant transformation (MT) of ODs is histological grading to
categorise lesions as ‘high’ or ‘low’ risk (7). A limitation of this method is its
subjectivity, which provides the potential for misdiagnosis. Other
methods have been researched, including DNA ploidy analysis
(8) and the use of biomarkers
(9,10); however, these methods are not
currently employed in clinical practice and are potentially
invasive due to requiring biopsy. In addition, the only approach to
prevent MT is surgical excision, which is invasive and often leads
to long-term morbidity, whilst not necessarily excluding recurrence
due to its significant association with MT (P<0.001) (11,12).
A notable cause of the late diagnosis of oral
carcinoma is the inaccurate prediction and prevention of MT of
potentially malignant ODs (7).
Moreover, ODs often remain asymptomatic (13). Thus, lesions are left undetected or
misdiagnosed and measures are not undertaken to halt development
into cancer. There is an unmet clinical need to improve OD
management. Chemoprevention is a primary research focus in HNSCC
for patients with high-risk OD to slow, stop or regress development
of neoplastic disease (14).
There are 18 known histone deacetylases (HDACs),
which are divided into four classes based on their sequence
structure (15,16). Class IIa represents a set of
classical HDACs (HDAC4, HDAC5, HDAC7 and HDAC9), which are
dependent on zinc for their enzymatic activity (17).
HDACs are complex enzymes that are yet to be fully
understood. HDACs may undergo post-translational modifications,
including methylation and ubiquitination, which can alter enzymatic
activity (16). This creates
functional variability between HDACs and causes distinct
subcellular localisation associated with different functions.
HDACs regulate expression of multiple proteins
implicated in tumorigenesis, including those involved in processes
such as the cell cycle (such as HDAC5-mediated Six1 upregulation),
apoptosis (for example, class IIa HDAC inhibition enhances
endoplasmic reticulum stress), angiogenesis (such as HDAC9-mediated
proangiogenic protein expression) and DNA damage (such as
HDAC4-mediated 53BP1 reduction) (18,19).
Consequently, aberrant expression of classical HDACs is associated
with numerous types of solid (20)
and haematological malignancies (21). Upregulation of HDACs is often
associated with poor prognosis (22-24).
Altered expression of HDACs is dependent on the cancer type and
location (25,26). For example, upregulation of HDAC9
has been found in oral cancer (27,28).
HDACs serve as promising targets for cancer therapy.
Inhibition of HDACs has notable antitumour effects (29-33).
So far, four HDAC inhibitors (HDACis) have been approved by the
Food and Drug Administration for clinical use and there are more
undergoing clinical trials (34).
Valproic acid (VPA) is a short-chain fatty acid that
has been under investigation for head and neck cancer
chemoprevention (35). VPA is a
commonly used therapy for epilepsy, as well as other neurological
disorders (36). VPA has been
established as a Class IIa HDACi (37), which impacts its neurological
indications but also suggests VPA as a potential agent for cancer
treatment. Studies have found promising in vitro and in
vivo anticancer effects of VPA, including anti-proliferative
effects and decreased cell viability (38,39).
Clinical trials are ongoing to determine its use as monotherapy or
combination therapy in multiple types of solid (40) and haematological malignancy
(41). To date, results indicate an
encouraging patient response, particularly when used as an adjuvant
therapy to cytotoxic chemotherapy (42).
A study has investigated the association between
long-term VPA treatment and the incidence of different types of
cancer in US Army veterans with psychiatric conditions (43). VPA prescription was associated with
risk reduction of HNSCC (HR, 0.66; 95% CI, 0.48-0.92), while no
significant differences in cancer incidence were identified for
malignancies of the lung, colon, prostate and bladder. These
results indicate that VPA may serve as a chemo-preventative agent
in HNSCC. In addition, certain HDACs such as HDAC1 and HDAC2 are
upregulated in potentially malignant ODs (27,44),
further supporting the use of HDACis in chemoprevention.
The ongoing SAVER trial conducted by the University
of Liverpool (Liverpool, UK) is investigating VPA as a
chemo-preventative epigenetic agent in patients with high-risk OD
(45). Moreover, a mechanistic
study to elucidate molecular changes that occur in response to VPA
exposure is being conducted in parallel. To the best of our
knowledge, how VPA influences its target genes, including if HDAC
expression is altered, remains unclear (46). Previous work investigating HDACis
has demonstrated that HDAC expression may be modified following
inhibition (47); however, this
research is limited to VPA and HNSCC. Mechanisms of other inhibitor
drugs include feedback loops (negative/positive), which adjust the
expression of respective targets in response to exposure (48). VPA may cause this same response,
resulting in altered expression of its target HDAC genes in
malignant and premalignant cell lines (46). Therefore, the present study aimed to
determine the effect of VPA on Class IIa HDAC expression in oral
cancer and premalignant cells.
Materials and methods
Cell lines and culture
Cell lines were authenticated (GenePrint 10 System;
Promega Corporation) and mycoplasma tested (Myco™ plus Mycoplasma
PCR Detection kit; Intron Biotechnology, Inc.). All cancer cell
lines were maintained in DMEM/Ham's Nutrient Mixture F-12 (1:1)
containing 5% FBS (Sigma-Aldrich; Merck KGaA). Human immortalised
bronchial epithelial cells (HBEC-3-KT; cat. no. CRL-4051; American
Type Culture Collection) were cultured in keratinocyte-serum-free
medium (Thermo Fisher Scientific, Inc.), supplemented with 50 mg/ml
bovine pituitary extract and 5 ng/ml human recombinant epidermal
growth factor (Thermo Fisher Scientific, Inc.). All cell lines were
maintained at 37˚C with 5% CO2.
Human oral cancer cell lines obtained from the
Liverpool University Biobank by the Liverpool Head and Neck group
(Liverpool, UK) were as follows: Squamous cell carcinoma of larynx
(UM-SCC-10A, UM-SCC-11B, UM-SCC-12 and UM-SCC-17A), tongue (HN5,
PE/CA-PJ15 and UM-SCC-1), oral cavity (BHY and PE/CA-PJ41) and
tonsil (UM-SCC-81B) cell lines (originally from American Type
Culture Collection or European Collection of Authenticated Cell
Cultures), in addition to three premalignant dysplastic oral mucosa
cell lines (D19, D20 and D35) derived at The Beatson Institute for
Cancer Research (Glasgow, Scotland) (49). The cells were cultured in
75-cm2 flasks and incubated in DMEM supplemented with
10% FBS at 37˚C with 5% CO2. At 50% confluence, cells
were treated, under the same aforementioned culture conditions,
with 1 mM VPA (Sigma-Aldrich; Merck KGaA), which is almost 2-fold
lower than the estimated IC50 for oral cancer cells
(50). Untreated cells were used as
the control. After 6, 24 or 48 h of incubation, the cells were
washed with phosphate buffer, lysed using 800 ml TRIzol™ (Thermo
Fisher Scientific, Inc.) and frozen at -80˚C for RNA
extraction.
Cell lines (BHY, D20 and UM-SCC-10A) of most
interest (at least ~2-fold differences in HDAC expression following
VPA treatment) were selected for further investigation. Each cell
line was cultured in 6-well plates and incubated in DMEM
supplemented with 10% FBS at 37.5˚C with 5% CO2. After
reaching 50% confluence, cells were treated with 1 mM VPA for 6 or
48 h, under the same aforementioned growth conditions. The cells
were lysed using 300 ml TRIzol and subsequently frozen for RNA
extraction. For 48 h time-point VPA exposure, the medium of 6-well
plates was changed after 24 h and fresh VPA (1 mM) was added.
Afterwards, the cells were lysed for RNA extraction as
aforementioned.
RNA extraction and reverse
transcription (RT)
Total RNA was extracted from cells using a
Direct-zol™ RNA Miniprep kit (Zymo Research Corp.) according to the
manufacturer's protocol. To quantify and qualify the extracted RNA,
a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.)
was used. The High-Capacity cDNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc.) was used for RT of 300 ng RNA according to
the manufacturer's instructions.
RT-quantitative (q)PCR
Primers and probes for genes of Class IIa HDACs and
the endogenous calibrator β-actin (ACTB) were designed using the
OLIGO 7 primer analysis software (Molecular Biology Insights, Inc.)
as follows: HDAC4 forward, 5'-TGGGAAACGAGCTTGATCCT-3', reverse,
5'-TGTGGAGGTTGTGCGCTG-3' and probe,
5'-VIC-AGGCAGCGCCAGTACTTGCTGTGG-NFQ-3'; HDAC5 forward,
5'-ATCCAGAGTGCGTGAGGAC-3', reverse, 5'-CATGGCGCTCACAGTCTC-3' and
probe, 5'-VIC-CCTCCTCGGTCTCACCTGCTTG-NFQ-3'; HDAC7 forward,
5'-AACCTCAATGCCATCCGCT-3', reverse, 5'-GGTCACTGCCTCCACTTCTTCT-3'
and probe, 5'-VIC-TCT GGAGGCCGTGATCCGGGT-NFQ-3'; HDAC9 forward
5'-AACTTGACACGGCAGCAC-3', reverse, 5'-CGATGCCTCTCTACTTCCTGT-3' and
probe, 5'-VIC-CTCAGCTTCAGGAGCATATCAAGGAACTT-NFQ-3'; and ACTB
forward, 5'-GGCACCCAGCACAATGAAG-3', reverse,
5'-CATACTCCTGCTTGCTGATCCA-3' and probe,
5'-VIC-CTCCTCCTGAGCGCAAGTACTCCGTG-NFQ-3'.
mRNA expression profiles of the tested Class IIa
HDAC genes were measured using RT-qPCR with a predesigned TaqMan
gene expression assay (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were 95˚C for 10 min (initial
denaturation), followed by 45 cycles of 95˚C for 15 sec
(denaturation) and 60˚C for 1 min (annealing and extension), on a
StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Inc.).
For normalisation, ROX dye was added, which reduces the influence
of well-to-well variability such as uneven illumination, slight
variation in optics and differences in the amount of condensation.
All qPCR assays were performed as four technical replicates.
Relative quantification values were calculated to
estimate mRNA expression levels according to the 2-ΔΔCq
method (51) using StepOne software
(version 1.2; Thermo Fisher Scientific, Inc.) and normalised to
ACTB. The mean of the 6-h control DCq values for each cell line was
used as the exogenous control for further normalisation (ΔΔCq=ΔCq
sample-ΔCq 6-h control).
Statistical analysis
To determine differences in Class IIa HDAC mRNA
expression, three biological replicates were used and estimated as
the mean of their values with their respective 95% confidence
intervals. The one-sample Kolmogorov-Smirnov test was used to test
the normality of the data. All data were confirmed as non-normally
distributed data, and the non-parametric Mann-Whitney U test was
used to determine the statistical significance of expression
levels. P<0.05 was considered to indicate a statistically
significant difference. To investigate the differential expression
of HDACs across both treatment and incubation time, Kruskal-Wallis
test with post-hoc Dunn test (with Bonferroni correction for
multiple testing) was performed for each HDAC in each cell line.
All statistical analyses were conducted using SPSS (version 27; IBM
Corp.).
Results
Class IIa HDAC expression in the 13
tested cell lines
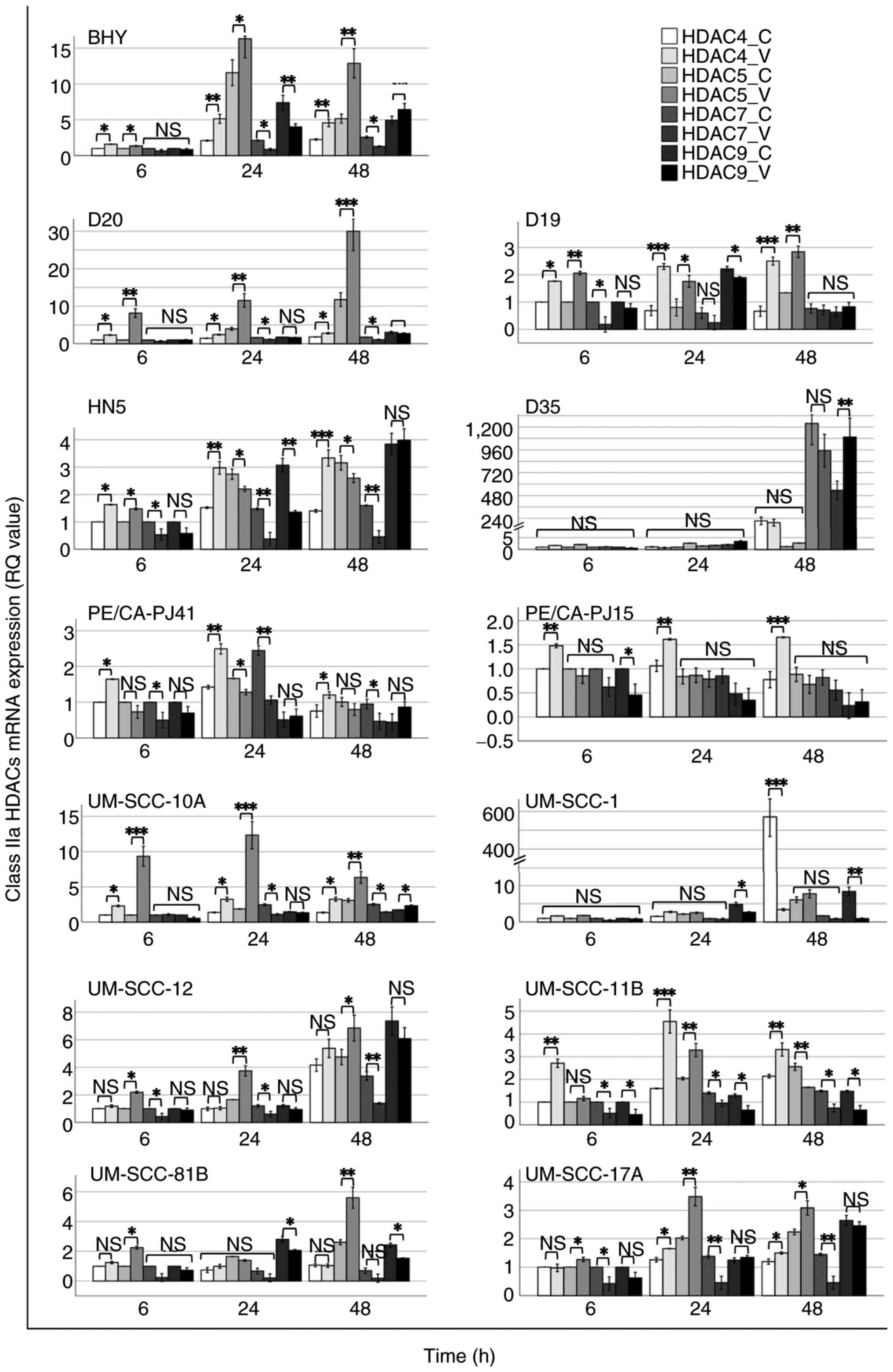
Preliminary results regarding the mRNA expression
levels of Class IIa HDAC genes indicated variable changes in HDAC
transcript levels following VPA exposure. According to the
comparisons of HDAC transcript levels independently performed for
each of the cell lines tested at each time point [VPA-treated vs.
untreated groups (controls)] (Fig.
1), the significance (Mann Whitney test; P<0.05) was
inferred to select three cell lines (D20, BHY and UM-SCC-10A) that
showed clear and consistent changes in expression (based on 95% CI)
across the multiple HDACs, and thus, were chosen for further
investigation.
Increased HDAC4 and HDAC5 and decreased HDAC7 mRNA
expression was observed across the cell lines, while no consistent,
except borderline significant, changes were caused to HDAC9 mRNA
expression following VPA treatment compared with controls (Fig. 1).
Following VPA treatment, BHY cells exhibited notable
alterations in HDAC expression for HDAC4, HDAC5 and HDAC7,
including a 2-fold increase and decrease in HDAC4 and HDAC7
expression, respectively. UM-SCC-10A cells displayed a 2-fold
increase in HDAC4 expression for all examined time points, while
2-fold and an 8-10-fold increases in HDAC5 expression for 48 and
6-24 h, respectively, were observed following VPA treatment
compared with controls (Fig. 1). A
number of the other cell lines also showed changes of interest in
the preliminary data. Increased HDAC4 and HDAC5 and decreased HDAC7
mRNA expression trends similar to those in BHY cells were observed
in D19 cells for all the tested time points except that at 24 h for
HDAC7 expression, in HN5 cells for all the examined time points
except at 24 and 48 h for HDAC5 expression, in PE/CA-PJ41 cells for
all the investigated time points except for HDAC5 expression, in
UM-SCC-12 cells for all the studied time points except for HDAC4
expression, in UM-SCC-11B cells for all the examined time points
except at 6 and 48 h for HDAC5 expression, and in UM-SCC-17A cells
for all the tested time points except at 6 h for HDAC4
expression.
Class IIa HDAC mRNA expression in BHY,
D20 and UM-SCC-10A cells
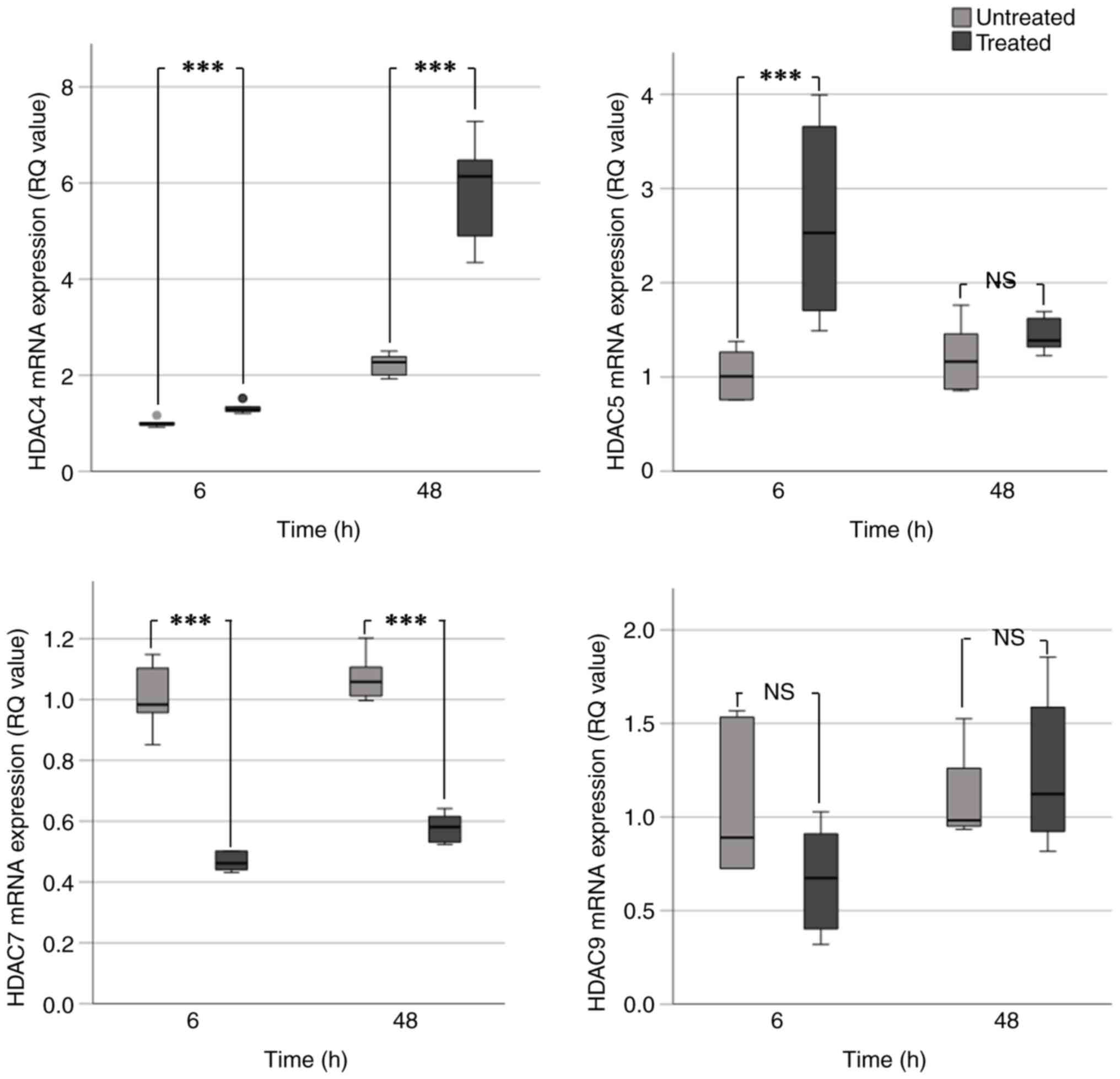
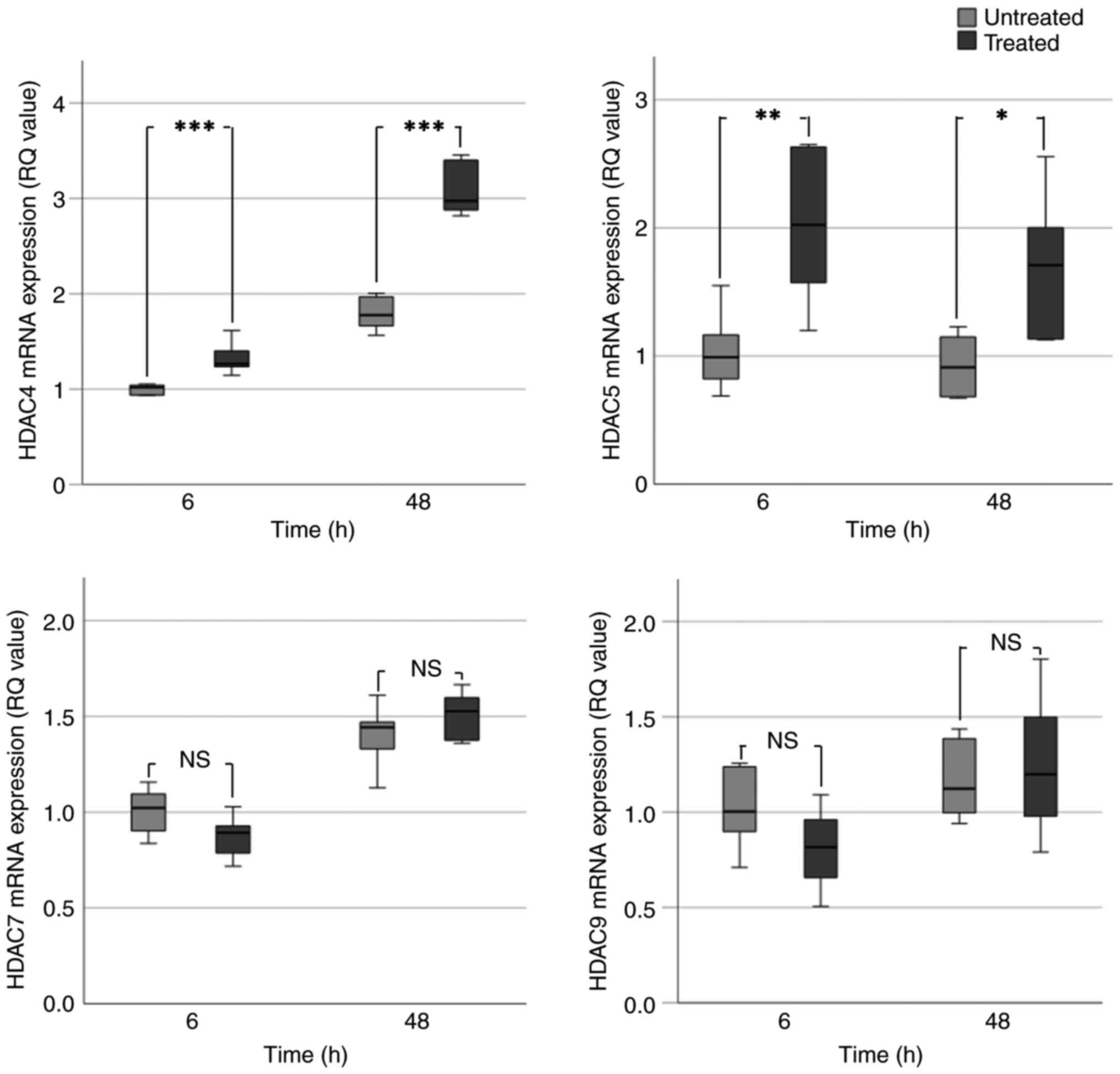
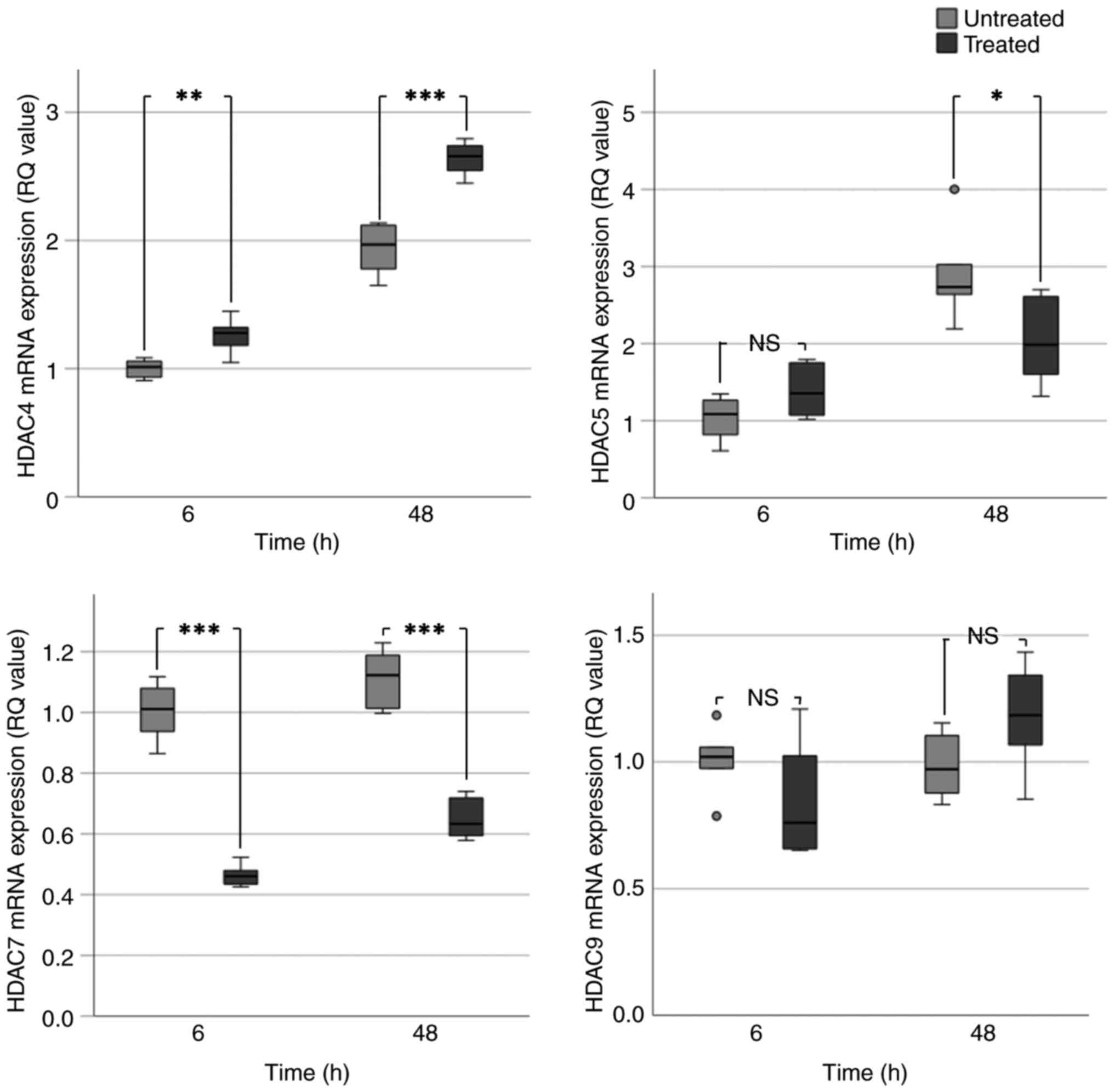
VPA treatment caused a significant increase in HDAC4
mRNA expression. This was particularly evident at 48 h in BHY
(Fig. 2) and UM-SCC-10A cells
(Fig. 3), which exhibited an
~2-fold increase in HDAC4 expression. In UM-SCC-10A cells, a
significant 2-fold increase in HDAC5 expression occurred following
VPA treatment for 6 and 48 h. An increase in HDAC5 expression was
observed in BHY cells; however, this was only significant at 6 h
(~2.5-fold increase). HDAC5 expression in D20 cells was increased
at 6 h but significantly decreased at 48 h (Fig. 4). This result did not correspond
with the evident increases in HDAC5 expression seen at all the time
points in the preliminary data (Fig.
1). BHY and D20 cells exhibited significant 1.5-2.0-fold
decreases in HDAC7 expression at 6 and 48 h. Consistent with the
aforementioned data, UM-SCC-10A cells exhibited no significant
change in HDAC7 expression following VPA treatment (Table IC). Significant decreases were also
observed for HDAC7 in treated BHY and D20 cells (Table IC).
 | Table IMedian RQ values representing mRNA
expression of Class IIa HDACs following VPA treatment. |
Table I
Median RQ values representing mRNA
expression of Class IIa HDACs following VPA treatment.
| A, HDAC4 |
|---|
| | BHY | D20 | UM-SCC-10A |
|---|
| Group | Median RQ | P-value | Median RQ | P-value | Median RQ | P-value |
|---|
| C6 | 0.97 | <0.01 | 1.01 | 0.01 | 1.02 | <0.01 |
| V6 | 1.29 | | 1.28 | | 1.26 | |
| C48 | 2.27 | <0.01 | 1.97 | <0.01 | 1.80 | <0.01 |
| V48 | 6.14 | | 2.66 | | 3.00 | |
| B, HDAC5 |
| | BHY | D20 | UM-SCC-10A |
| Group | Median RQ | P-value | Median RQ | P-value | Median RQ | P-value |
| C6 | 1.00 | <0.01 | 1.09 | 0.08 | 0.99 | <0.01 |
| V6 | 2.53 | | 1.36 | | 2.03 | |
| C48 | 1.16 | 0.52 | 2.73 | 0.02 | 0.91 | 0.03 |
| V48 | 1.39 | | 1.99 | | 1.71 | |
| C, HDAC7 |
| | BHY | D20 | UM-SCC-10A |
| Group | Median RQ | P-value | Median RQ | P-value | Median RQ | P-value |
| C6 | 0.98 | <0.01 | 1.01 | <0.01 | 1.02 | 0.11 |
| V6 | 0.46 | | 0.46 | | 0.89 | |
| C48 | 1.06 | <0.01 | 1.12 | <0.01 | 1.44 | 0.26 |
| V48 | 0.58 | | 0.63 | | 1.52 | |
| D, HDAC9 |
| | BHY | D20 | UM-SCC-10A |
| Group | Median RQ | P-value | Median RQ | P-value | Median RQ | P-value |
| C6 | 0.89 | 0.15 | 1.02 | 0.20 | 1.00 | 0.13 |
| V6 | 0.67 | | 0.76 | | 0.82 | |
| C48 | 0.98 | 0.75 | 0.97 | 0.15 | 1.12 | 0.88 |
| V48 | 1.12 | | 1.19 | | 1.20 | |
Both VPA treatment and incubation time had an effect
on HDAC4 levels (Kruskal-Wallis; P<0.001). In pairwise post hoc
analysis (Dunn test), HDAC4 expression significantly increased
between 6 and 48 h in both control and treatment groups across all
cell lines (P<0.027) (Table
SIA). The increase at 48 h was greater for VPA-treated cells
(2.66-6.14-fold) than control cells (1.80-2.27-fold) (Table IA), indicating that the combined
effect of time and VPA treatment increased HDAC4 expression.
However, pairwise post hoc analysis (Dunn test) indicated that the
incubation time had a more significant association with the HDAC4
increase than VPA treatment (Table
SIA). For HDAC7, the significant decrease seen was also more
closely associated with incubation time than VPA exposure; pairwise
post hoc tests were significant only for time in all cell lines.
Only for HDAC9 was there no association with incubation time.
Discussion
Epigenetic targeted therapy is an approach to
chemotherapy and chemoprevention (52). Epigenetic alterations, including
aberrant recruitment of HDACs, are observed in potentially
malignant lesions and may serve as an indicator of cancer
development. Therefore, methods to modify or reverse these
alterations, such as HDAC inhibition, may be an approach for
effective chemoprevention in high-risk patients (53).
VPA is a potent Class IIa HDACi under investigation
for cancer treatment (43),
indicating its potential use in HNSCC chemoprevention. Class IIa
HDACs are upregulated in HNSCC tissue and potentially malignant ODs
(27). To the best of our
knowledge, little research has been conducted on molecular changes
that occur in response to VPA treatment (47), particularly in oral cancer. It is
necessary to determine how VPA alters the expression of its targets
to understand its mechanism of action and provide potential
predictive markers of the response to VPA.
The present study aimed to expand on a previous
feasibility study to determine HDAC expression changes in oral
cancer cells in response to 1 mM VPA, a concentration 2-fold lower
than the estimated IC50 for oral cancer cells (50), and the lowest dose causing
VPA-mediated cytotoxicity and cell arrest (54). In addition, VPA at 1 mM is a
clinically meaningful dose (55).
The RT-qPCR analyses further confirmed the preliminary significant
changes in Class IIa HDAC mRNA expression in BHY, D20 and
UM-SCC-10A cells, where the HDAC4 and HDAC5 expression increased
and HDAC7 expression decreased (except in UM-SCC-10A cells)
following VPA treatment at the tested time points (6 and 48 h). No
significant changes were observed for HDAC9 in BHY, D20 and
UM-SCC-10A cells after VPA exposure at the examined time points (6
and 48 h). This may suggest that VPA may not target HDAC9 in
HNSCC.
The differential expression of HDACs may be due to
variation in the protein complexes associated with each HDAC or the
genes they target. The high variability between HDACs may result in
varied mechanisms of VPA and differences in response dependent on
the HDAC and its associated proteins (56).
VPA directly inhibits HDAC proteins. The present
results may indicate that the inhibition of HDAC triggered an
auto-regulatory feedback loop, resulting in altered RNA expression
to compensate for inhibition. This may be a transcriptional signal
to modify the number of transcripts or it may be a signal to adjust
RNA degradation (for example, by altering microRNA expression).
This auto-regulatory system has been suggested for HDACis,
including VPA (57,58).
Similarly, the opposing changes in HDAC expression
in the present study (the increased HDAC4 and HDAC5 expression and
decreased HDAC 7 expression) may also be attributed to a reciprocal
regulatory mechanism between HDACs. To compensate for inhibition,
the expression of one HDAC may be downregulated to allow the
upregulation of another (59,60). A
significant decrease in HDAC7 expression was observed in BHY and
D20 cells, while there was a significant increase in HDAC4
expression. The aforementioned regulatory mechanisms indicate that
VPA may alter the de novo synthesis of HDACs and contribute
to epigenetic reprogramming to counteract the loss of function
caused by protein inhibition.
HDAC expression in response to HDACis has been
investigated in other cancer types such as pancreatic, breast,
prostate and lung cancer (19),
revealing fluctuations similar to those found in the present study.
In addition, there is evidence that expression may change over
time. In a previous study, human leukaemia cell line MOLT4 was
treated with VPA and HDAC expression was analysed over 5 days;
expression of HDAC2, HDAC5, HDAC6, HDAC8 and HDAC9 increased until
day 3, then declined (61).
Furthermore, a study investigating Class II HDACs in breast cancer
found downregulation of HDAC3, HDAC7, HDAC8 and HDAC10 but
upregulation of HDAC2 following treatment with Trichostatin-A, a
non-selective HDACi (62). These
findings suggest that there may be compensatory mechanisms of HDAC
expression following inhibition, which differ depending on the
cancer type and inhibitor used.
In addition to changes in response to VPA exposure,
differences in HDAC expression were observed among different time
points. HDAC4 expression significantly increased between 6 and 48 h
for control and treated cells across all cell lines, HDAC7
expression decreased in UM-SCC-10A cells and HDAC5 expression
increased in D20 cells. The reason for selecting the aforementioned
time-points (6 and 48 h) for VPA exposure was to explore the VPA
epigenetic effect on HDACs expression compared with the early and
late log phase of cellular growth of controls (the growing and
surviving cells at 6 and 48 h time-points, respectively). It can be
hypothesized that the cell confluence may influence HDAC
regulation, as a part of a dynamic epigenetic reprogramming of the
cell population. However, this needs to be demonstrated in future
experiments to investigate the effect of cell confluence on
differential HDAC expression. As growth media with fresh VPA were
added at the midpoint (24 h) of the 48 h, the VPA activity was
maintained, thus differences are unlikely to be due to VPA
degradation; previously it has been shown that VPA does not affect
cell viability after 24 h (63). In
any case, differential expression of some HDACs in untreated cells
at 48 h indicated that cell confluence might also serve a role in
differential expression. Incubation time was the most significant
factor for changes in HDAC expression, when comparing across all
conditions. The present study lacked functional analysis, which is
required in the future.
The changes in HDAC expression induced by VPA varied
between cell lines. Genotypes and genomic mutation load differ
between HNSCC cell lines (64).
Therefore, the effect of VPA on HDAC expression may be dependent on
the expression and function of numerous gene effectors.
Overall, the present results indicated that
VPA-dependent epigenetic reprogramming was associated with
transcriptional alterations of Class IIa HDACs in HNSCC and
premalignant cells. The role of these alterations in the clinical
management of HNSCC must be determined to develop potential
epigenetic therapeutic regimens.
Supplementary Material
Kruskal-Wallis test pairwise post hoc
analysis (Dunn test) for comparisons of HDAC mRNA expression among
the groups of tested cell lines.
Acknowledgements
The authors would like to thank Dr Triantafillos
Liloglou (University of Liverpool, Liverpool, UK) for providing the
cancer cell lines. The authors would also like to thank Dr Caroline
McCarthy and Dr Keith Hunter (University of Liverpool) for gifting
the dysplastic cell lines.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ASKAK conceived and designed the study, analysed
data and wrote the manuscript. YBQ, LMW and HHA interpreted data
and revised the manuscript. LMW analysed data. ASKAK, YBQ, HHA and
LMW confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ho MW, Field EA, Field JK, Risk JM,
Rajlawat BP, Rogers SN, Steele JC, Triantafyllou A, Woolgar JA,
Lowe D and Shaw RJ: Outcomes of oral squamous cell carcinoma
arising from oral epithelial dysplasia: Rationale for monitoring
premalignant oral lesions in a multidisciplinary clinic. Br J Oral
Maxillofac Surg. 51:594–599. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sciubba JJ: Oral Cancer. The importance of
early diagnosis and treatment. Am J Clin Dermatol. 2:239–251.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
National Cancer Institute: Cancer Stat
Facts: Oral Cavity and Pharynx Cancer. https://seer.cancer.gov/statfacts/html/oralcav.html.
|
|
4
|
González-Moles MÁ, Aguilar-Ruiz M and
Ramos-García P: Challenges in the early diagnosis of oral cancer,
evidence gaps and strategies for improvement: A scoping review of
systematic reviews. Cancers (Basel). 14(4967)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van der Waal I: Potentially malignant
disorders of the oral and oropharyngeal mucosa; terminology,
classification and present concepts of management. Oral Oncol.
45:317–323. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Warnakulasuriya S: Oral potentially
malignant disorders: A comprehensive review on clinical aspects and
management. Oral Oncol. 102(104550)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Field EA, McCarthy CE, Ho MW, Rajlawat BP,
Holt D, Rogers SN, Triantafyllou A, Field JK and Shaw RJ: The
management of oral epithelial dysplasia: The Liverpool algorithm.
Oral Oncol. 51:883–887. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zaini ZM, McParland H, Møller H, Husband K
and Odell EW: Predicting malignant progression in clinically
high-risk lesions by DNA ploidy analysis and dysplasia grading. Sci
Rep. 8(15874)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Khafaji AS, Pantazi P, Acha-Sagredo A,
Schache A, Risk JM, Shaw RJ and Liloglou T: Overexpression of HURP
mRNA in head and neck carcinoma and association with in vitro
response to vinorelbine. Oncol Lett. 19:2502–2507. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rivera C, Gallegos R and Figueroa C:
Biomarkers of progression to oral cancer in patients with
dysplasia: A systematic review. Mol Clin Oncol.
13(42)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Balasundaram I, Payne KF, Al-Hadad I,
Alibhai M, Thomas S and Bhandari R: Is there any benefit in surgery
for potentially malignant disorders of the oral cavity? J Oral
Pathol Med. 43:239–244. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sundberg J, Korytowska M, Holmberg E,
Bratel J, Wallström M, Kjellström E, Blomgren J, Kovács A, Öhman J,
Sand L, et al: Recurrence rates after surgical removal of oral
leukoplakia-A prospective longitudinal multi-centre study. PLoS
One. 14(e0225682)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ranganathan K and Kavitha L: Oral
epithelial dysplasia: Classifications and clinical relevance in
risk assessment of oral potentially malignant disorders. J Oral
Maxillofac Pathol. 23:19–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Siemianowicz K, Likus W, Dorecka M, Wilk
R, Dziubdziela W and Markowski J: Chemoprevention of head and neck
cancers: Does it have only one face? Biomed Res Int.
2018(9051854)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park SY and Kim JS: A short guide to
histone deacetylases including recent progress on class II enzymes.
Exp Mol Med. 52:204–212. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Seto E and Yoshida M: Erasers of histone
acetylation: The histone deacetylase enzymes. Cold Spring Harb
Perspect Biol. 6(a018713)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Audia JE and Campbell RM: Histone
modifications and cancer. Cold Spring Harb Perspect Biol.
8(a019521)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Glozak MA and Seto E: Histone deacetylases
and cancer. Oncogene. 26:5420–5432. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y and Seto E: HDACs and HDAC inhibitors
in cancer development and therapy. Cold Spring Harb Perspect Med.
6(a026831)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zheng YC, Kang HQ, Wang B, Zhu YZ, Mamun
MAA, Zhao LF, Nie HQ, Liu Y, Zhao LJ, Zhang XN, et al: Curriculum
vitae of HDAC6 in solid tumors. Int J Biol Macromol.
230(123219)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang P, Wang Z and Liu J: Role of HDACs in
normal and malignant hematopoiesis. Mol Cancer.
19(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Osada H, Tatematsu Y, Saito H, Yatabe Y,
Mitsudomi T and Takahashi T: Reduced expression of class II histone
deacetylase genes is associated with poor prognosis in lung cancer
patients. Int J Cancer. 112:26–32. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cao LL, Song X, Pei L, Liu L, Wang H and
Jia M: Histone deacetylase HDAC1 expression correlates with the
progression and prognosis of lung cancer: A meta-analysis. Medicine
(Baltimore). 96(e7663)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Müller BM, Jana L, Kasajima A, Lehmann A,
Prinzler J, Budczies J, Winzer KJ, Dietel M, Weichert W and Denkert
C: Differential expression of histone deacetylases HDAC1, 2 and 3
in human breast cancer-overexpression of HDAC2 and HDAC3 is
associated with clinicopathological indicators of disease
progression. BMC Cancer. 13(215)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qaddoori YB, Al-Khafaji ASK, Khashman BM
and Abdulghafour KH: The study of histone deacetylases
immunoexpression in relation to regulating vascular endothelial
growth factor (VEGF) implicated in malignant progression of
colorectal cancer. Iraqi J Sci. 66:1861–1875. 2025.
|
|
26
|
Qaddoori YB, Al-Khafaji ASK, Khashman BM
and Abdulghafour KH: The potential role of HDAC1 and HDAC3
Immunoexpression in P53 downregulation and tumor aggressiveness of
colon and rectum carcinomas patients. Exp Oncol. 46:393–401.
2025.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rastogi B, Raut SK, Panda NK, Rattan V,
Radotra BD and Khullar M: Overexpression of HDAC9 promotes oral
squamous cell carcinoma growth, regulates cell cycle progression,
and inhibits apoptosis. Mol Cell Biochem. 415:183–196.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chang HH, Chiang CP, Hung HC, Lin CY, Deng
YT and Kuo MY: Histone deacetylase 2 expression predicts poorer
prognosis in oral cancer patients. Oral Oncol. 45:610–614.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kelly WK, Richon VM, O'Connor O, Curley T,
MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S,
Rosa E, et al: Phase I clinical trial of histone deacetylase
inhibitor: Suberoylanilide hydroxamic acid administered
intravenously. Clin Cancer Res. 9 (10 Pt 1):3578–3588.
2003.PubMed/NCBI
|
|
30
|
Duvic M, Talpur R, Ni X, Zhang C, Hazarika
P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM and Frankel
SR: Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic
acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood.
109:31–39. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
O'Connor OA, Horwitz S, Masszi T, Van Hoof
A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S,
et al: Belinostat in patients with relapsed or refractory
peripheral T-Cell lymphoma: Results of the pivotal phase II BELIEF
(CLN-19) study. J Clin Oncol. 33:2492–2499. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Piekarz RL, Frye R, Turner M, Wright JJ,
Allen SL, Kirschbaum MH, Zain J, Prince HM, Leonard JP, Geskin LJ,
et al: Phase II multi-institutional trial of the histone
deacetylase inhibitor romidepsin as monotherapy for patients with
cutaneous T-cell lymphoma. J Clin Oncol. 27:5410–5417.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
San-Miguel JF, Hungria VT, Yoon SS, Beksac
M, Dimopoulos MA, Elghandour A, Jedrzejczak WW, Günther A, Nakorn
TN, Siritanaratkul N, et al: Panobinostat plus bortezomib and
dexamethasone versus placebo plus bortezomib and dexamethasone in
patients with relapsed or relapsed and refractory multiple myeloma:
A multicentre, randomised, double-blind phase 3 trial. Lancet
Oncol. 15:1195–1206. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Eckschlager T, Plch J, Stiborova M and
Hrabeta J: Histone deacetylase inhibitors as anticancer drugs. Int
J Mol Sci. 18(1414)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
National Library of Medicine (NIH):
Chemoprevention of Head and Neck Squamous Cell Carcinoma (HNSCC)
With Valproic Acid (GAMA). NIH, Bethesda, MD, 2018. https://clinicaltrials.gov/study/NCT02608736. Accessed
March 16, 2025.
|
|
36
|
Romoli M, Mazzocchetti P, D'Alonzo R,
Siliquini S, Rinaldi VE, Verrotti A, Calabresi P and Costa C:
Valproic acid and epilepsy: From molecular mechanisms to clinical
evidences. Curr Neuropharmacol. 17:926–946. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gurvich N, Tsygankova OM, Meinkoth JL and
Klein PS: Histone deacetylase is a target of valproic acid-mediated
cellular differentiation. Cancer Res. 64:1079–1086. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Duenas-Gonzalez A, Candelaria M,
Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E and
Herrera LA: Valproic acid as epigenetic cancer drug: Preclinical,
clinical and transcriptional effects on solid tumors. Cancer Treat
Rev. 34:206–222. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sang Z, Sun Y, Ruan H, Cheng Y, Ding X and
Yu Y: Anticancer effects of valproic acid on oral squamous cell
carcinoma via SUMOylation in vivo and in vitro. Exp Ther Med.
12:3979–3987. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
National Library of Medicine (NIH):
MAGE-C2 TCR T Cell Trial to Treat Melanoma and Head and Neck Cancer
(MC2TCR). NIH, Bethesda, MD, 2023. https://clinicaltrials.gov/study/NCT04729543?cond=(head%20OR%20neck)%20AND%20cancer&term=(head%20OR%20neck)%20AND%20cancer&intr=Valproic%20Acid&page=1&rank=5).
Accessed March 16, 2025.
|
|
41
|
National Library of Medicine (NIH):
Repurposed Drugs to Improve Haematological Responses in
Myelodysplastic Syndromes (REPAIR-MDS). NIH, Bethesda, MD, 2023.
https://clinicaltrials.gov/study/NCT04997811?cond=Haematological%20Malignancy&term=Haematological%20Malignancy&intr=Valproic%20Acid&rank=1.
Accessed March 16, 2025.
|
|
42
|
Brodie SA and Brandes JC: Could valproic
acid be an effective anticancer agent? The evidence so far. Expert
Rev Anticancer Ther. 14:1097–1100. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kang H, Gillespie TW, Goodman M, Brodie
SA, Brandes M, Ribeiro M, Ramalingam SS, Shin DM, Khuri FR and
Brandes JC: Long-term use of valproic acid in US veterans is
associated with a reduced risk of smoking-related cases of head and
neck cancer. Cancer. 120:1394–1400. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Theocharis S, Klijanienko J, Giaginis C,
Rodriguez J, Jouffroy T, Girod A, Alexandrou P and Sastre-Garau X:
Histone deacetylase-1 and -2 expression in mobile tongue squamous
cell carcinoma: Associations with clinicopathological parameters
and patients survival. J Oral Pathol Med. 40:706–714.
2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
McCarthy C, Sacco J, Fedele S, Ho M,
Porter S, Liloglou T, Greenhalf B, Robinson M, Young B, Cicconi S,
et al: SAVER: Sodium valproate for the epigenetic reprogramming of
high-risk oral epithelial dysplasia-a phase II randomised control
trial study protocol. Trials. 22(428)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Al-Khafaji ASK, Wang LM, Alabdei HH and
Liloglou T: Effect of valproic acid on histone deacetylase
expression in oral cancer (Review). Oncol Lett.
27(197)2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dokmanovic M, Clarke C and Marks PA:
Histone deacetylase inhibitors: Overview and perspectives. Mol
Cancer Res. 5:981–989. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Iskar M, Campillos M, Kuhn M, Jensen LJ,
van Noort V and Bork P: Drug-induced regulation of target
expression. PLoS Comput Biol. 6(e1000925)2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
McGregor F, Muntoni A, Fleming J, Brown J,
Felix DH, MacDonald DG, Parkinson EK and Harrison PR: Molecular
changes associated with oral dysplasia progression and acquisition
of immortality: Potential for its reversal by 5-azacytidine. Cancer
Res. 62:4757–4766. 2002.PubMed/NCBI
|
|
50
|
Al-Khafaji ASK, Alnefaie G and Al-Shammari
AM: Valproic acid enhances the paclitaxel activity in respiratory
tract cancer cells. J Contemp Med Sci. 4:208–213. 2019.
|
|
51
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: Past, present and future. Nat Rev Drug Discov. 5:37–50.
2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gu M, Ren B, Fang Y, Ren J, Liu X, Wang X,
Zhou F, Xiao R, Luo X, You L and Zhao Y: Epigenetic regulation in
cancer. MedComm (2020). 5(e495)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Karagiannis TC, Kn H and El-Osta A: The
epigenetic modifier, valproic acid, enhances radiation sensitivity.
Epigenetics. 1:131–137. 2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Avallone A, Piccirillo MC, Di Gennaro E,
Romano C, Calabrese F, Roca MS, Tatangelo F, Granata V, Cassata A,
Cavalcanti E, et al: Randomized phase II study of valproic acid in
combination with bevacizumab and oxaliplatin/fluoropyrimidine
regimens in patients with RAS-mutated metastatic colorectal cancer:
The REVOLUTION study protocol. Ther Adv Med Oncol.
12(1758835920929589)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ukey S, Ramteke A, Choudhury C, Purohit P
and Sharma P: Differential expression of zinc-dependent HDAC
subtypes and their involvement in unique pathways associated with
carcinogenesis. Asian Pac J Cancer Prev. 23:877–883.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Castro LM, Gallant M and Niles LP: Novel
targets for valproic acid: Up-regulation of melatonin receptors and
neurotrophic factors in C6 glioma cells. J Neurochem. 95:1227–1236.
2005.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ajamian F, Salminen A and Reeben M:
Selective regulation of class I and class II histone deacetylases
expression by inhibitors of histone deacetylases in cultured mouse
neural cells. Neurosci Lett. 365:64–68. 2004.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jurkin J, Zupkovitz G, Lagger S,
Grausenburger R, Hagelkruys A, Kenner L and Seiser C: Distinct and
redundant functions of histone deacetylases HDAC1 and HDAC2 in
proliferation and tumorigenesis. Cell Cycle. 10:406–412.
2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pinkerneil M, Hoffmann MJ, Deenen R,
Köhrer K, Arent T, Schulz WA and Niegisch G: Inhibition of Class I
Histone Deacetylases 1 and 2 promotes urothelial carcinoma cell
death by various mechanisms. Mol Cancer Ther. 15:299–312.
2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yang H, Maddipoti S, Quesada A, Bohannan
Z, Cabrero Calvo M, Colla S, Wei Y, Estecio M, Wierda W,
Bueso-Ramos C and Garcia-Manero G: Analysis of class I and II
histone deacetylase gene expression in human leukemia. Leuk
Lymphoma. 56:3426–3433. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Duong V, Bret C, Altucci L, Mai A,
Duraffourd C, Loubersac J, Harmand PO, Bonnet S, Valente S,
Maudelonde T, et al: Specific activity of class II histone
deacetylases in human breast cancer cells. Mol Cancer Res.
6:1908–1919. 2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kwon HY, Kang NY, Dae HM, Kim KS, Kim CH,
Do SI and Lee Y: Valproic acid-mediated transcriptional regulation
of human GM3 synthase (hST3Gal V) in SK-N-BE(2)-C human
neuroblastoma cells. Acta Pharmacol Sin. 29:999–1005.
2008.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Li H, Wawrose JS, Gooding WE, Garraway LA,
Lui VWY, Peyser ND and Grandis JR: Genomic analysis of head and
neck squamous cell carcinoma cell lines and human tumors: A
rational approach to preclinical model selection. Mol Cancer Res.
12:571–582. 2014.PubMed/NCBI View Article : Google Scholar
|