Introduction
Coronary artery disease (CAD) is a common condition,
affecting an estimated 315 million individuals worldwide, with a
prevalence of 3,605 cases per 100,000 population in 2022(1). In the USA, the prevalence rate of CAD
in 2022 was 4.9% (2). Coronary
artery bypass grafting (CABG) is a treatment aimed at
revascularizing the coronary arteries. CABG is recommended in
several situations, including left main disease with >50%
stenosis (3). In a previous study
the mortality rates of CABG at 30 days and 1 year were reported to
be 3.1 and 7.6%, respectively (4).
Notably, the mortality rate of CABG may vary among hospitals.
Currently, stem cell therapy has been shown to be
effective in CAD. Of note, two meta-analysis studies found that
stem cell therapy significantly improved left ventricular ejection
fraction (LVEF) compared with the control (5,6). The
LVEF in the stem cell therapy group improved by 3.89-4.8% compared
with the control (P=0.003 and P=0.001, respectively). However, the
cardiac-related mortality was not significantly different between
both groups; risk ratio of 0.78 (95% CI of 0.17, 3.56). Heart
failure is a condition that may decrease LVEF or diastolic
dysfunction. A previous meta-analysis revealed that stem cell
therapy significantly improved LVEF by 6.23% (P<0.0001) in
patients with CAD who underwent CABG with heart failure (7). As stem cell therapy may improve LVEF
by 5%, patients with CAD treated with CABG may receive benefits
from stem cell therapy (8). To the
best of our knowledge, there is no previous systematic review
evaluating the effects of stem cell therapy in this setting. The
aim of this study was to evaluate whether stem cell therapy is
effective in patients with CAD who underwent CABG and had no heart
failure.
Materials and methods
Purpose
The present research was a systematic review to
study whether there was any improvement in cardiac function or
mortality in patients with CAD who underwent CABG. This study was
exempted from ethical approval by the Ethics Committee of Khon Kaen
University (Khon Kaen, Thailand).
Eligibility criteria. Population
The inclusion criteria were studies conducted on
whether patients with CAD who underwent CABG, using stem cell
therapy from bone marrow, peripheral blood, or muscle and had no
heart failure either preserved or reduced LVEF. All studies,
regardless of the types of CAD and the types of CABG reported, were
included. Any research papers of the following categories:
Systematic review, case reports, and case series were excluded.
Intervention and control groups
The intervention group was defined by the use of
stem cells; which could be performed before or after the CABG.
There was no specification as to what type of stem cell therapy or
how the stem cells were introduced to the heart, as well as dosage
or numbers of injection. The control group was defined as receiving
no stem cells due to sham needles, placebo substance injections, or
nothing at all.
Outcomes
The outcomes of interest were cardiac function
represented by LVEF and mortality.
Study types
The only study types included in this study were
randomized controlled trials (RCTs). Non-randomized trials,
observation studies, systematic reviews, and meta-analyses were
excluded.
Search strategy
In total, four databases were used for systematic
searching; these were PubMed (https://pubmed.ncbi.nlm.nih.gov/), CENTRAL database
(https://www.cochranelibrary.com/central/), Scopus
(https://www.scopus.com/), and CINAHL Plus
(https://www.ebsco.com/). Search terms included
‘coronary artery disease’, ‘coronary artery bypass’, and ‘stem
cells’. The full list of search terms is shown in the Appendix. The final search was conducted
on October 22, 2022 (9,10).
Selection process
After duplicate removal, initial screening was
conducted for irrelevant articles. The initial screening process
was performed independently by two authors (TJ and SK). Studies
selected by the reviewer were compared and entered into the
full-text review process, then full-text reviews and data
extraction were performed independently by the two authors (TJ and
SK). In the event a final agreement could not be reached, consensus
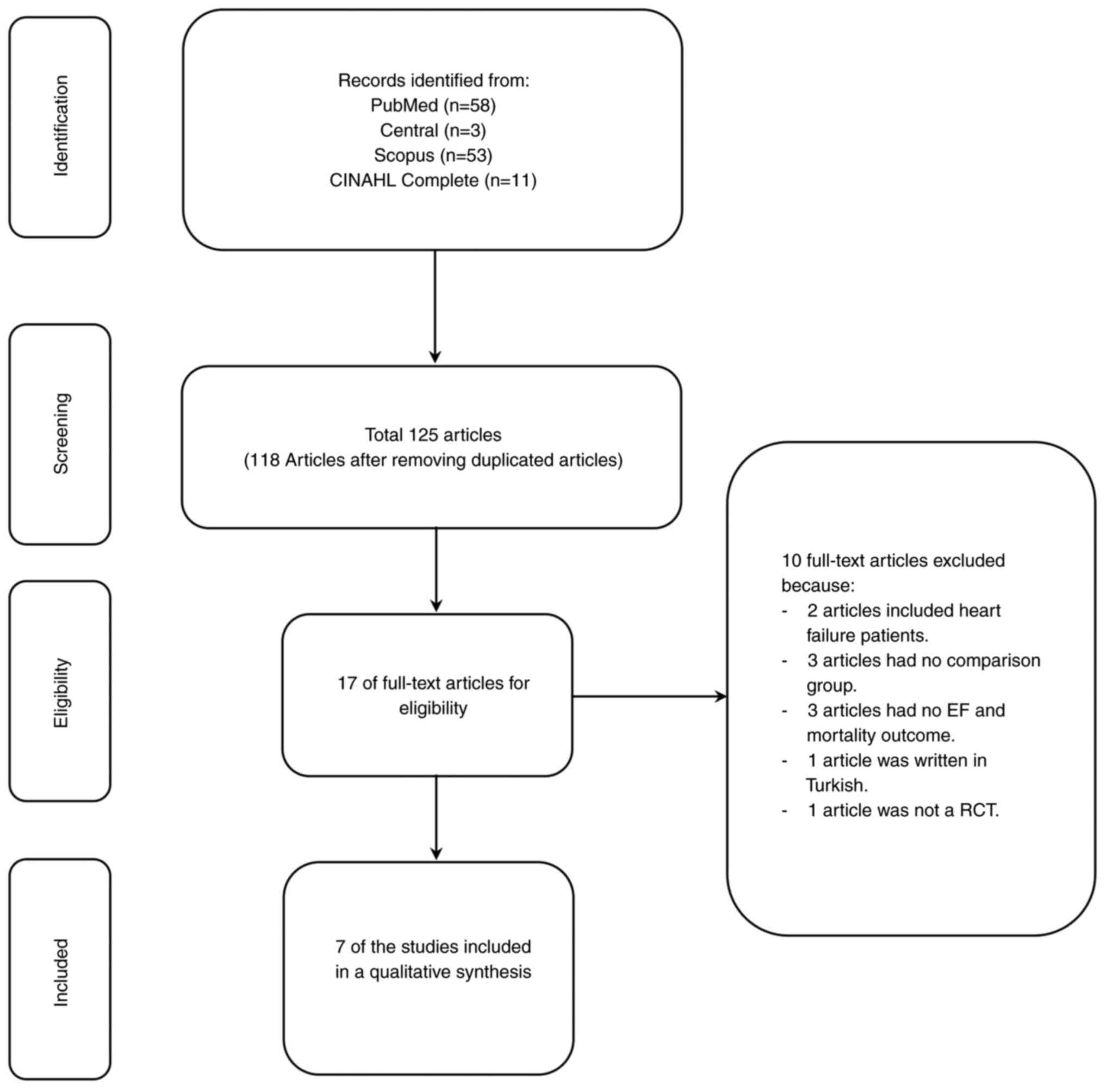
would be made by the third reviewer (KS). A PRISMA flowchart of
articles searching and data collection is illustrated in Fig. 1.
Data collection
Data collection included publication
characteristics, study characteristics, and outcome
characteristics. The publication characteristics contained the
first author, year of publication, and the country of origin. The
study characteristics were comprised of research design, research
duration, inclusion criteria, exclusion criteria, stem cell type,
site of injection, stem-cell dosage, and other outcomes of
interest. The outcome characteristics were methods of ejection
fraction measurement.
Data analysis
There were two groups assessed in the present study:
A stem cell therapy group and a control group. Cardiac function was
calculated by the standardized mean difference (SMD) of the LVEF
from both groups with a 95% confidence interval (CI). Mortality was
calculated by the risk ratio of death from both groups with a 95%
CI. I2 was used as a formal test of heterogeneity among
the results of the included trials. The following guide was used:
0-40% might not be important; 30-60% may represent moderate
heterogeneity; 50-90% may represent substantial heterogeneity; and
75-100% may represent considerable heterogeneity (11). A random-effect model was used to
perform a meta-analysis. A forest plot was created to show
differences between both groups. The analyses were performed by
Review Manager 5.4 (The Nordic Cochrane Centre, The Cochrane
Collaboration).
Risk of bias
The study quality of RCTs was evaluated using a
revised Cochrane risk-of-bias tool for randomized trials Version 2
(RoB 2) (12). The RoB 2 tool was
structured into five different domains of bias: Randomization
process, deviations from the intended interventions, missing
outcome data, measurement of the outcome, and selection of reported
results. Judgment of bias can be made from low to some concerns to
high. A risk-of-bias analysis was independently performed by two
authors (TJ and SK), and discrepancies were verified using the
Excel tool to implement RoB 2; in the event an agreement could not
be made, the final decision would be made by the third author (KS).
This evaluation was separately performed on both outcomes (namely
ejection fraction and mortality).
Results
There were 125 studies from a search in four
databases related to stem cell therapy in patients with CAD who had
undergone CABG treatment, and 118 remained after the removal of
duplicated studies. Of these, 50 of those were eligible for a
full-text review, and 10 were excluded due to the inclusion of
patients with heart failure (two), having no comparison group
(three), having no ejection fraction and mortality outcome (three),
written in Turkish (one), and not being an RCT (one). Thus, a total
of seven studies remained for evaluation (13-19).
These were published from 2007-2021 and were mostly conducted in
Europe (five studies), two studies were multicentered, and two
studies were conducted in Indonesia (Table I). The longest duration for the
research was 5 years, with an average duration of 3.29±1.25 years
(Table I). Most studies had as
inclusion criteria an LVEF of <35% (five out of seven studies)
and exhibiting akinetic left ventricular myocardium (five out of
seven studies) (Table I). Notably,
all included studies used autologous stem cell therapy.
 | Table IStudy characteristics of included
studies on stem cell therapy in patients with coronary artery
disease without heart failure who underwent CABG. |
Table I
Study characteristics of included
studies on stem cell therapy in patients with coronary artery
disease without heart failure who underwent CABG.
| First author,
year | Country | Research design | Setting | EF | Research
duration | Comparison | Mean age stem cell
group | Male/total (%): stem
cell group | Mean age control
group | Male/total (%)
Control group | (Refs.) |
|---|
| Soetisna et
al, 2020 | Indonesia | Single-blind
randomized clinical trial | Age, <70 years;
triple vessel disease | <35% (cardiac
MRI) | 2 years | Stem cell vs.
CABG | 54.61 (8.07) | 12/13 (92.30) | 57.46 (6.33) | 12/13 (92.30) | (17) |
| Soetisna, 2021 | Indonesia | Randomized trial | Triple vessel
disease | <35% | 2 years | Stem cell vs.
CABG | 54.61 (8.07) | 12/13 (92.30) | 57.46 (6.33) | 12/13 (92.30) | (18) |
| Nasseri et al,
2014 | Germany | Randomized,
placebo-controlled, and double-blinded trial | NA | <35% (Echo) | 4 years | Stem cell vs.
Placebo | 61.9 (7.3) | 28/30 (93.33) | 62.7 (10.6) | 29/30 (96.67) | (19) |
| Ang et al,
2008 | United Kingdom | Blinded randomized
controlled trial (physician and data analyzer blinded) | Post-myocardial
infarction (at least one vessel) | NA | 5 years | Stem cell vs.
CABG | IM, 64.7 (8.7); IC,
62.1 (8.7) | IM, 15/21 (71.4);
IC, 19/21 (90.5) | 61.3 (8.3) | 18/20 (90.0) | (14) |
| Stamm et al,
2007 | Germany | Single-blind
randomized clinical trial | Post-myocardial
infarction | NA | 4 years | Stem cell vs.
CABG | 62 (10.2) | 15/20(75) | 63.5 (8.4) | 16/20(80) | (13) |
| Menasché et
al, 2008 | France, Germany,
Belgium, United Kingdom, and Italy | Randomized,
placebo-controlled, 3-arm, double-blind trial | Age, 18-80 years;
NYHA class I-III | 15-35% (Echo) | 4 years | Stem cell vs.
Placebo | High dose, 59
(53-67) Low dose, 61 (54-70) | High dose;
28/30(93); Low dose, 33/33(100) | 61 (55-72) | 32/34(94) | (15) |
| Brickwedel et
al, 2014 | France, Germany,
Belgium, United Kingdom, and Italy | Randomized,
placebo-controlled, 3-arm, double-blind trial | Age, 18-80 years;
NYHA class I-III; post-myocardial infarction | 15-35% (Echo) | 2 years | Stem cell vs.
Placebo | 56.5 (5.1) | 4/4(100) | 62.0 (6.6) | 3/3(100) | (16) |
The most commonly used stem cell therapy was
autologous CD133+ cells from the bone marrow (five out
of seven studies) (Table II).
Additionally, the average injection volume was 14.86±14.32 ml.
Cardiac MRI and transthoracic echocardiography were both used for
the evaluation of the ejection fraction; the former was used
slightly more often (Table II).
Almost all studies (six out of seven studies) had both ejection
fraction and mortality as an outcome measurement, with the
exception of the study by Soetisna (18), which did not include mortality
(Table II).
 | Table IIIntervention and outcome measurements
of included studies on stem cell therapy in patients with coronary
artery disease without heart failure who underwent coronary artery
bypass graft. |
Table II
Intervention and outcome measurements
of included studies on stem cell therapy in patients with coronary
artery disease without heart failure who underwent coronary artery
bypass graft.
| First author,
year | Stem cells
type | Site of
injection | Stem cell
dosage | No. of
injections | EF measurement
method | (Refs.) |
|---|
| Soetisna et
al, 2020 | Bone marrow CD
133+ cells from the posterior iliac crest | (1) Transepicardial
segments (2) Transeptal segments (3) Hypokinetic/hypoperfused
segments | 0.5 ml of CD
133+ cells | 60 | Philips
Achieva® 1.5T MRI | (17) |
| Soetisna, 2021 | Autologous
CD133+ marrow cells | Hypoperfusion
area | 1 ml | 40 | 1.5 T cardiac
MRI | (18) |
| Nasseri et
al, 2014 | Autologous
CD133+ marrow cells |
Intramyocardial | 0.5 ml | 20 | Cardiac MRI | (19) |
| Ang et al,
2008 | Autologous
CD133+ bone marrow cells | Intramuscular,
intracoronary | 500 μl (IM) | 20 (IM) | Cardiac MRI | (14) |
| Stamm et al,
2007 | Autologous
CD133+ marrow cells |
Intramyocardial | 0.2 ml | 10 | Cardiac
transthoracic ultrasonography validated with cardiac MRI | (13) |
| Menasché et
al, 2008 | Autologous CD
56+ skeletal myoblasts |
Intramyocardial | 1 ml | 30 | Transthoracic
echocardiography | (15) |
| Brickwedel et
al, 2014 | Autologous CD
56+ skeletal myoblasts |
Intramyocardial | 1 ml | 30 | Transthoracic
echocardiography | (16) |
For the included studies without meta-analysis
calculations, a total of seven trials for the LVEF and seven trials
for the mortality outcomes are presented in Tables III and IV, respectively. Despite the
discrepancies in some characteristics in the research by Soetisna
et al (17) and Soetisna
(18) (Tables I and II), all the numerical data with regard to
baseline values, the number of participants, and the outcomes were
similar between studies (Tables
III and IV). As
aforementioned, since the study by Soetisna (18) did not analyze mortality as an
outcome, the dataset from this study was therefore excluded from
the meta-analysis. As a result, six trials were included in the
evaluation of cardiac function and mortality. Some trials such as
Ang et al (14), Menasché
et al (15), and Brickwedel
et al (16) were a three-arm
study; all intervention arms from these trials were compared
against their control in the meta-analysis as well (Fig. 2 and Fig.
3).
 | Table IIILVEF of included studies on stem cell
therapy in patients with coronary artery disease without heart
failure who underwent underwent coronary artery bypass graft. |
Table III
LVEF of included studies on stem cell
therapy in patients with coronary artery disease without heart
failure who underwent underwent coronary artery bypass graft.
| | Stem cell therapy
group | Control group | |
|---|
| First author,
year | Baseline LVEF
(%) | Total (n) | LVEF after
treatment (%) | Total (n) | Baseline LVEF
(%) | Total (n) | LVEF after
treatment (%) | Total (n) | (Refs.) |
|---|
| Soetisna et
al, 2020 | 25.88±5.66 | 13 | Δ 8.69±9.49 | 13 | 30.18±3.85 | 13 | Δ 1.43±7.87 | 13 | (17) |
| Soetisna, 2021 | 25.88±5.66 | 13 | 34.58±11.32 | 13 | 30.18±3.85 | 13 | 31.62±7.89 | 13 | (18) |
| Nasseri et
al, 2014 | 27±6 | 30 | 31±7 | 26 | 26±6 | 30 | 33±8 | 22 | (19) |
| Ang et al,
2008 | 25.4±8.1 (IM);
28.5±6.5 (IC) | 10 (IM) 8 (IC) | 29.7±9.1 (IM);
27.3±7.7 (IC) | 10 (IM); 8
(IC) | 20.9±8.9 | 7 | 22.3±5.8 | 7 | (14) |
| Stamm et al,
2007 | 37.4±8.4 | 20 | 47.1±8.3 | 20 | 37.9±10.3 | 20 | 41.3±9.1 | 20 | (13) |
| Menasché et
al, 2008 | 27.08±7.25 (LD);
28.83±7.64 (HD) | 28 (LD) 26
(HD) | 32.54±9.37 (LD);
33.66±8.92 (HD) | 28 (LD); 26
(HD) | 29.67±4.49 | 32 | 33.50±7.10 | 32 | (15) |
| Brickwedel et
al, 2014 | 33±1.41 (LD);
29±5.66 (HD) | 2 (LD) 2 (HD) | 35±8.49 (LD);
32±7.07 (HD) | 2 (LD); 2 (HD) | 31±4.62 | 3 | 25±13.43 | 3 | (16) |
 | Table IVMortality of included studies of stem
cell therapy in patients with coronary artery disease without heart
failure who underwent underwent coronary artery bypass graft. |
Table IV
Mortality of included studies of stem
cell therapy in patients with coronary artery disease without heart
failure who underwent underwent coronary artery bypass graft.
| | Stem cell therapy
group | Control group | |
|---|
| First author,
year | Deceased (n) | Total (n) | Deceased (n) | Total (n) | (Refs.) |
|---|
| Soetisna et
al, 2020 | 2 | 15 | 1 | 14 | (17) |
| Soetisna, 2021 | Not stated | Not stated | Not stated | Not stated | (18) |
| Nasseri et
al, 2014 | 0 | 28 | 2 | 28 | (19) |
| Ang et al,
2008 | 2 (IC group); 0 (IM
group) | 21 (IC group); 21
(IM group) | 1 | 20 | (14) |
| Stamm et al,
2007 | 0 | 20 | 0 | 20 | (13) |
| Menasché et
al, 2008 | Low dose group, 5;
High dose group, 4 | Low dose group, 33;
High dose group, 30 | 2 | 34 | (15) |
| Brickwedel et
al, 2014 | Low dose group, 0;
High dose group, 0 | Low dose group, 2;
High dose group, 2 | 0 | 3 | (16) |
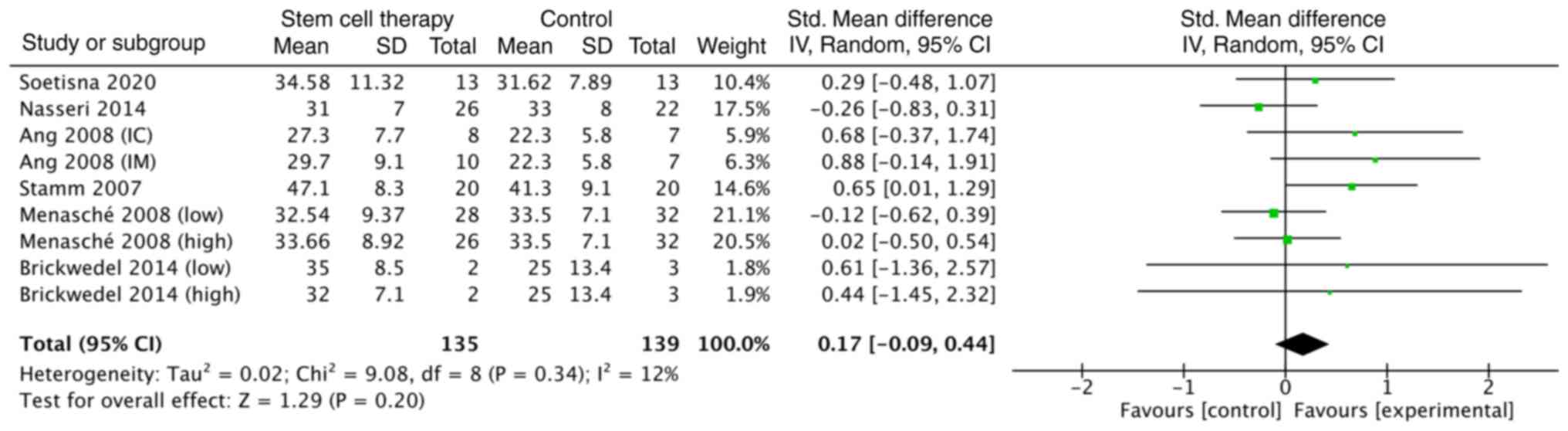
Regarding the outcome of cardiac function, which is
measured by LVEF, the SMD between the experimental and the control
groups was not statistically significant [SMD, 0.17; CI (-0.09,
0.44)], as shown in Fig. 2. The
formal test for heterogeneity showed a degree of freedom of 8
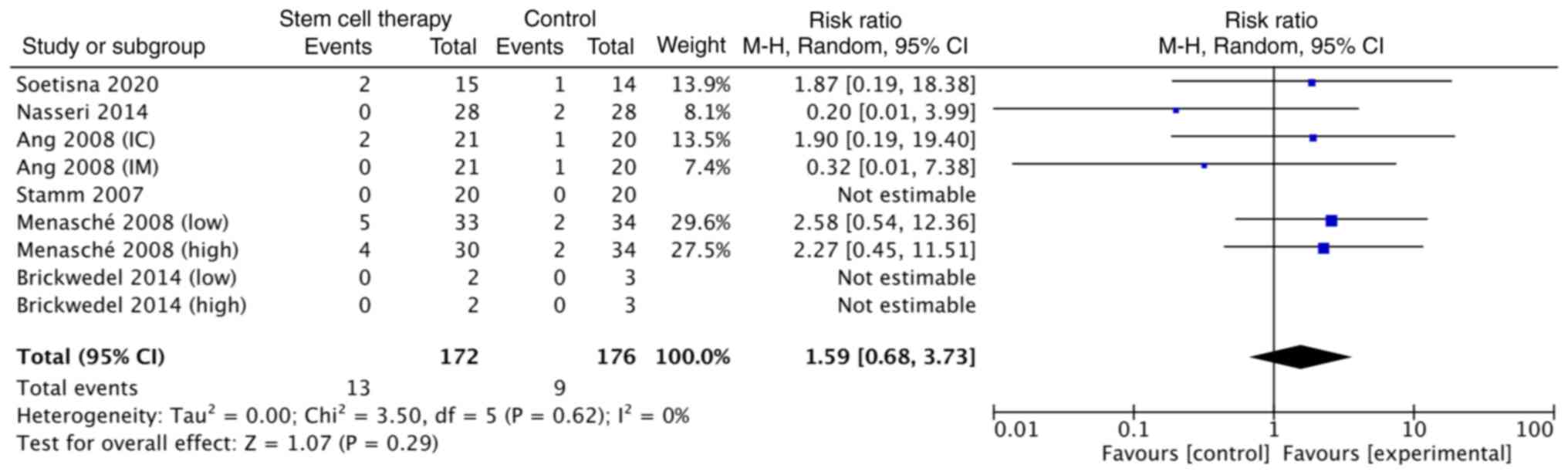
(P=0.34) and an I2 of 12% (Fig. 2). The risk ratio of mortality was
also not statistically significant [1.59; CI (0.68, 3.73)]
(Fig. 3). The formal test for
heterogeneity revealed a degree of freedom of 5 (P=0.62) and
an I2 of 0% (Fig. 3).
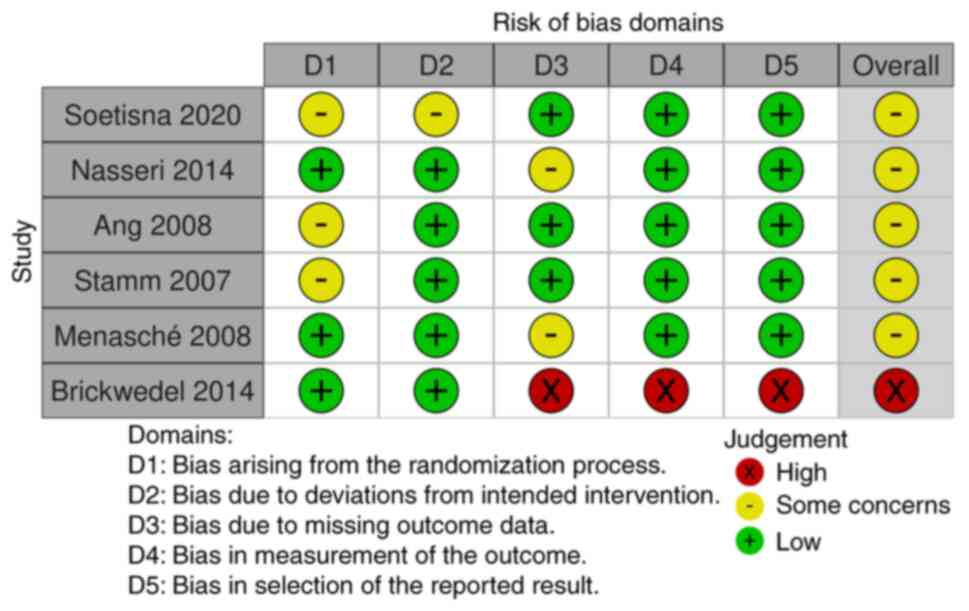
Since there was no significant heterogeneity observed, further
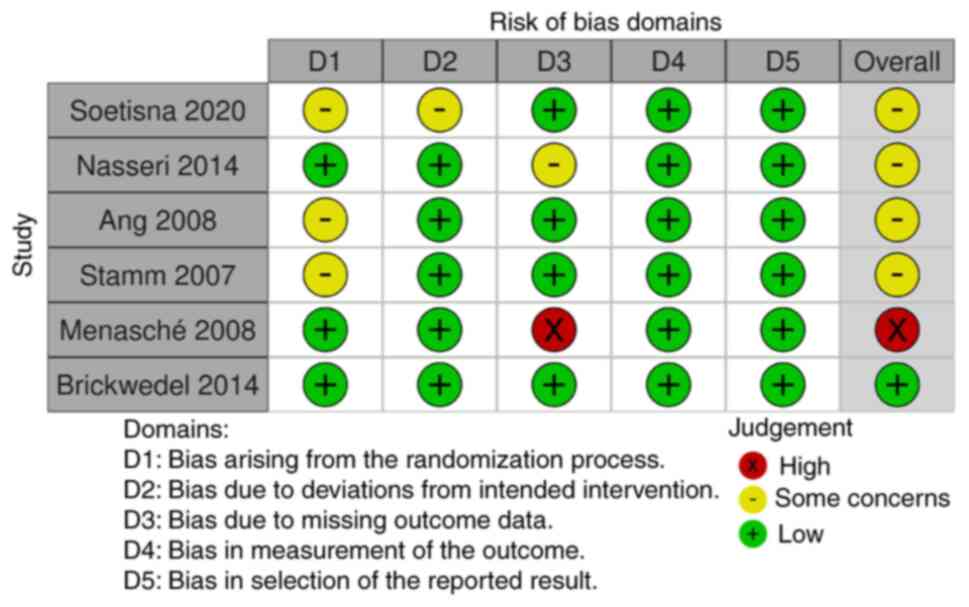
subgroup analyses were not indicated. In total, five out of six
studies had some concerns regarding the overall risk of bias for
the LVEF, while one study had a high risk of overall bias for LVEF
(Figs. 4 and 5) according to The Cochrane
Collaboration's tool for assessing risk of bias in randomized
trials (12). In addition, one
study in the overall bias evaluation for the mortality outcome had
low risk, while the majority (four out of six studies) had some
concerns, as shown in Fig. 5.
Discussion
The present meta-analysis determined that stem cell
therapy did not significantly improve LVEF and mortality compared
with the control treatment (Figs. 2
and 3). These results imply that in
patients with CAD who underwent CABG, this therapy may not be
beneficial in the patients without heart failure.
Stem cell therapy significantly improved LVEF by
several mechanisms, primarily related to cytokines. Stem cells may
differentiate into cardiomyocytes and improve cardiomyocyte
regeneration, suppress myocardial fibrosis or hypertrophy, and
improve angiogenesis of cardiac tissue (6). However, the findings of the present
study did not identify significant improvement of LVEF, although
the LVEF in the stem cell therapy group nearly reached a
significant increase of 0.17%, compared with the control group
(Fig. 2). These findings may be due
to the small sample size and the low average LVEF (Fig. 2); these two factors may require
larger differences of LVEF to be statistically significant.
As previously reported (6), stem cell therapy did not significantly
improve mortality compared with the control group (4.76% vs. 5.88%;
risk ratio, 0.78; 95% CI, 0.17, 3.56). By contrast, the present
study had a higher mortality rate in the stem-cell therapy group
than the control with a risk ratio of 1.59 and a 95% CI of
0.68-3.73, as shown in Fig. 3.
These differences may be attributed to two factors: First, the
mortality of patients undergoing CABG may vary depending on the
hospital and the expertise of the surgeon (4); second, several factors, such as age or
comorbidities, may also influence the mortality of patients with
CAD who undergo CABG (20-22).
Overall, the studies that were included had limited
and small sample sizes, which may have reduced the power to detect
significant differences between the stem cell therapy and control
treatment. Although stem cell therapy may be beneficial, the sample
size in each study was generally small probably due to the high
cost and difficult preparation of stem cells, particularly those
from bone marrow (23). Previous
systematic reviews revealed that most included studies had a sample
size between 20 and 40 patients per study (5-7).
There are several potential reasons for the lack of
significant improvement in LVEF and mortality in the present study,
other than the small sample size. As there are varied stem cell
therapy regimens in terms of timing, dosage, or type of stem cell
therapy, these factors may have affected both study outcomes. Only
one study (13) showed significant
improvement in LVEF (Fig. 2), while
none of the studies included reported favorable outcomes concerning
mortality (Fig. 3). Of note the
study by Stamm et al (13)
used 0.2 ml of stem cells with 10 injections in intramyocardial
areas (13). Additionally, patients
with CAD who did not have heart failure may survive longer than
those with heart failure. Previous research revealed that patients
with CAD and heart failure had a 48.7% survival rate at 720 days
(24,25). To achieve statistical significance
in patients without heart failure, a long follow-up duration and a
large sample size may be required. Similarly, left ventricular
function in patients without heart failure may not reveal marked
improvement.
There were some limitations in the present study.
First, broad inclusion criteria were used to reduce selection bias,
resulting in variations in stem cell therapy, including dosage,
number of injections, sites of injection, and type of stem cell
therapy employed. Therefore, the results of the present study may
not be specific to any particular stem cell therapy. Currently,
factors such as dosage and the source of stem cells for CAD
treatment remain uncertain (26).
Second, the included studies were limited and had a small sample
size. Additionally, as five studies were conducted in Europe and
two studies were conducted in Indonesia, evaluating racial
differences in this systematic review was challenging; future
additional RCTs in diverse settings are required to update the
meta-analysis for both outcomes. Finally, personal factors such as
hypertension and obstructive sleep apnea were not evaluated
(27-29),
nor was any other intervention implemented (30,31).
In conclusion, stem cell therapy did not exert
significant improvement of LVEF or the mortality rate compared with
the control in patients with CAD who underwent CABG and did not
have heart failure. Further studies are required to confirm the
findings of the present study and to provide greater insights into
the potential benefits of stem cell therapy by investigating other
patient subgroups or different stem cell therapy protocols.
Supplementary Material
Searching method for PubMed, Central,
Scopus and CINAHL (on October 22, 2022).
Acknowledgements
The authors would like to thank Dr Chetta Ngamjarus,
Khon Kaen University (Khon Kaen, Thailand) for assistance in the
literature search.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TJ, SK, and KS designed the study, collected and
analyzed the data, and wrote the manuscript. TJ and SK confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stark B, Johnson C and Roth GA: Global
prevalence of coronary artery disease: An update from the global
burden of disease study. J Am Coll Cardiol. 83 (13
Suppl)(S2320)2024.
|
|
2
|
Khalid N, Haider S, Abdullah M, Asghar S,
Laghari MA and Rajeswaran Y: Trends and disparities in coronary
artery disease prevalence among U.S. adults from 2019 to 2022. Curr
Probl Cardiol. 49(102645)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hillis LD, Smith PK, Anderson JL, Bittl
JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF,
Hutter AM Jr, et al: 2011 ACCF/AHA guideline for coronary artery
bypass graft surgery: A report of the American college of
cardiology foundation/American heart association task force on
practice guidelines. Circulation. 124:e652–e735. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brovman EY, James ME, Alexander B, Rao N
and Cobey FC: The association between institutional mortality after
coronary artery bypass grafting at one year and mortality rates at
30 days. J Cardiothorac Vasc Anesth. 36:86–90. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ayyat KS, Argawi A, Mende M, Steinhoff G,
Borger MA, Deebis AM, McCurry KR and Garbade J: Combined coronary
artery bypass surgery with bone marrow stem cell transplantation:
Are we there yet? Ann Thorac Surg. 108:1913–1921. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Soetisna TW, Thamrin AMH, Permadijana D,
Ramadhani ANE, Sugisman null, Santoso A and Mansyur M:
Intramyocardial stem cell transplantation during coronary artery
bypass surgery safely improves cardiac function: Meta-analysis of
20 randomized clinical trials. J Clin Med. 12(4430)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang Y, Yang Z, Shao L, Shen H, Teng X,
Chen Y, Ding Y, Fan J, Yu Y and Shen Z: Clinical outcomes by
consolidation of bone marrow stem cell therapy and coronary artery
bypass graft in patients with heart failure with reduced ejection
fraction: A meta-analysis. Cell Transplant.
32(9636897231152381)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Banerjee MN, Bolli R and Hare JM: Clinical
studies of cell therapy in cardiovascular medicine: Recent
developments and future directions. Circ Res. 123:266–287.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sawunyavisuth B, Ngamjarus C and
Sawanyawisuth K: Adherence to continuous positive airway pressure
therapy in pediatric patients with obstructive sleep apnea: A
meta-analysis. Ther Clin Risk Manag. 19:143–162. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Namwaing P, Ngamjarus C, Sakaew W,
Sawunyavisuth B, Sawanyawisuth K, Khamsai S and Srichaphan T: Chest
physical therapy and outcomes in primary spontaneous pneumothorax:
A systematic review. J Med Assoc Tha. 104 (Suppl 4):S165–S168.
2021.
|
|
11
|
Higgins J, Thompson S, Deeks J and Altman
D: Statistical heterogeneity in systematic reviews of clinical
trials: A critical appraisal of guidelines and practice. J Health
Serv Res Policy. 7:51–61. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stamm C, Kleine HD, Choi YH, Dunkelmann S,
Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski
D, et al: Intramyocardial delivery of CD133+ bone marrow cells and
coronary artery bypass grafting for chronic ischemic heart disease:
Safety and efficacy studies. J Thorac Cardiovasc Surg. 133:717–725.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ang KL, Chin D, Leyva F, Foley P, Kubal C,
Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT and
Galiñanes M: Randomized, controlled trial of intramuscular or
intracoronary injection of autologous bone marrow cells into
scarred myocardium during CABG versus CABG alone. Nat Clin Pract
Cardiovasc Med. 5:663–670. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Menasché P, Alfieri O, Janssens S, McKenna
W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour
B, Larghero J, et al: The myoblast autologous grafting in ischemic
cardiomyopathy (MAGIC) trial: First randomized placebo-controlled
study of myoblast transplantation. Circulation. 117:1189–1200.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brickwedel J, Gulbins H and Reichenspurner
H: Long-term follow-up after autologous skeletal myoblast
transplantation in ischaemic heart disease. Interact Cardiovasc
Thorac Surg. 18:61–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Soetisna TW, Sukmawan R, Setianto B,
Mansyur M, Murni TW, Listiyaningsih E and Santoso A: Combined
transepicardial and transseptal implantation of autologous CD 133+
bone marrow cells during bypass grafting improves cardiac function
in patients with low ejection fraction. J Card Surg. 35:740–746.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Soetisna TW: A new hope of CD133+ bone
marrow stem cell for functional exercise capacity improvement in
low ejection fraction coronary artery bypass graft patients: A
clinical trial. Bali Med J. 10:229–233. 2021.
|
|
19
|
Nasseri BA, Ebell W, Dandel M, Kukucka M,
Gebker R, Doltra A, Knosalla C, Choi YH, Hetzer R and Stamm C:
Autologous CD133+ bone marrow cells and bypass grafting for
regeneration of ischaemic myocardium: the Cardio133 trial. Eur
Heart J. 35:1263–1274. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu C, Camacho FT, Wechsler AS, Lahey S,
Culliford AT, Jordan D, Gold JP, Higgins RS, Smith CR and Hannan
EL: Risk score for predicting long-term mortality after coronary
artery bypass graft surgery. Circulation. 125:2423–2430.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goncharov M, Mejia OAV, Arthur CPDS,
Orlandi BMM, Sousa A, Oliveira MAP, Atik FA, Segalote RC, Tiveron
MG, de Barros E Silva PGM, et al: Mortality risk prediction in
high-risk patients undergoing coronary artery bypass grafting: Are
traditional risk scores accurate? PLoS One.
16(e0255662)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khamsai S, Mahawarakorn P, Limpawattana P,
Chindaprasirt J, Sukeepaisarnjaroen W, Silaruks S, Senthong V,
Sawunyavisuth B and Sawanyawisuth K: Prevalence and factors
correlated with hypertension secondary from obstructive sleep
apnea. Multidiscip Respir Med. 16(777)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chang D, Fan T, Gao S, Jin Y, Zhang M and
Ono M: Application of mesenchymal stem cell sheet to treatment of
ischemic heart disease. Stem Cell Res Ther. 12(384)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vicent L, Álvarez-García J, Vazquez-Garcia
R, González-Juanatey JR, Rivera M, Segovia J, Pascual-Figal D,
Bover R, Worner F, Fernández-Avilés F, et al: Coronary artery
disease and prognosis of heart failure with reduced ejection
fraction. J Clin Med. 12(3028)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Purek L, Laule-Kilian K, Christ A, Klima
T, Pfisterer ME, Perruchoud AP and Mueller C: Coronary artery
disease and outcome in acute congestive heart failure. Heart.
92:598–602. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu C, Han D, Liang P, Li Y and Cao F: The
current dilemma and breakthrough of stem cell therapy in ischemic
heart disease. Front Cell Dev Biol. 9(636136)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Khamsai S, Kachenchart S, Sawunyavisuth B,
Limpawattana P, Chindaprasirt J, Senthong V, Chotmongkol V,
Pongkulkiat P and Sawanyawisuth K: Prevalence and risk factors of
obstructive sleep apnea in hypertensive emergency. J Emerg Trauma
Shock. 14:104–107. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jeerasuwannakul B, Sawunyavisuth B,
Khamsai S and Sawanyawisuth K: Prevalence and risk factors of
proteinuria in patients with type 2 diabetes mellitus. Asia Pac J
Sci Technol. 26:APST–26. 2021.
|
|
29
|
Manasirisuk P, Chainirun N, Tiamkao S,
Lertsinudom S, Phunikhom K, Sawunyavisuth B and Sawanyawisuth K:
Efficacy of generic atorvastatin in a real-world setting. Clin
Pharmacol. 13:45–51. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sawunyavisuth B, Ngamjarus C and
Sawanyawisuth K: A meta-analysis to identify factors associated
with CPAP machine purchasing in patients with obstructive sleep
apnea. Biomed Rep. 16(45)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sawunyavisuth B, Ngamjarus C and
Sawanyawisuth K: Any effective intervention to improve CPAP
adherence in children with obstructive sleep apnea: A systematic
review. Glob Pediatr Health. 8(2333794X211019884)2021.PubMed/NCBI View Article : Google Scholar
|