|
1
|
Taylor HS, Kotlyar AM and Flores VA:
Endometriosis is a chronic systemic disease: Clinical challenges
and novel innovations. Lancet. 397:839–852. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peiris AN, Chaljub E and Medlock D:
Endometriosis. JAMA. 320(2608)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Greene R, Stratton P, Cleary SD, Ballweg
ML and Sinaii N: Diagnostic experience among 4,334 women reporting
surgically diagnosed endometriosis. Fertil Steril. 91:32–39.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sanfilippo JS, Wakim NG, Schikler KN and
Yussman MA: Endometriosis in association with uterine anomaly. Am J
Obstet Gynecol. 154:39–43. 1986.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Imperiale L, Nisolle M, Noël JC and
Fastrez M: Three types of endometriosis: Pathogenesis, diagnosis
and treatment. state of the art. J Clin Med. 12(994)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
As-Sanie S, Shafrir AL, Halvorson L,
Chawla R, Hughes R and Merz M: The Burden of pelvic pain associated
with endometriosis among women in selected European countries and
the United States: A restricted systematic review. J Minim Invasive
Gynecol. 31:653–666.e5. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Katon JG, Plowden TC and Marsh EE: Racial
disparities in uterine fibroids and endometriosis: A systematic
review and application of social, structural, and political
context. Fertil Steril. 119:355–363. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Koninckx PR, Ussia A, Adamyan L, Wattiez
A, Gomel V and Martin DC: Pathogenesis of endometriosis: The
genetic/epigenetic theory. Fertil Steril. 111:327–340.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van Haaps AP, Wijbers JV, Schreurs AMF,
Vlek S, Tuynman J, De Bie B, de Vogel AL, van Wely M and Mijatovic
V: The effect of dietary interventions on pain and quality of life
in women diagnosed with endometriosis: A prospective study with
control group. Hum Reprod. 38:2433–2446. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Burney RO and Giudice LC: Pathogenesis and
pathophysiology of endometriosis. Fertil Steril. 98:511–519.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jensen JR and Coddington CC III: Evolving
spectrum: The pathogenesis of endometriosis. Clin Obstet Gynecol.
53:379–388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Maignien C, Santulli P, Chouzenoux S,
Gonzalez-Foruria I, Marcellin L, Doridot L, Jeljeli M, Grange P,
Reis FM, Chapron C and Batteux F: Reduced α-2,6 sialylation
regulates cell migration in endometriosis. Hum Reprod. 34:479–490.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hapangama DK, Raju RS, Valentijn AJ,
Barraclough D, Hart A, Turner MA, Platt-Higgins A, Barraclough R
and Rudland PS: Aberrant expression of metastasis-inducing proteins
in ectopic and matched eutopic endometrium of women with
endometriosis: Implications for the pathogenesis of endometriosis.
Hum Reprod. 27:394–407. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin LL, Makwana S, Chen M, Wang CM,
Gillette LH, Huang TH, Burney RO, Nicholson BJ and Kirma NB:
Cellular junction and mesenchymal factors delineate an
endometriosis-specific response of endometrial stromal cells to the
mesothelium. Mol Cell Endocrinol. 539(111481)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen CW, Chavez JB, Kumar R, Go VA, Pant
A, Jain A, Polusani SR, Hart MJ, Robinson RD, Gaczynska M, et al:
Hypersensitive intercellular responses of endometrial stromal cells
drive invasion in endometriosis. Elife. 13(e94778)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang X, Zheng Q, Sun M, Liu L, Zhang H and
Ying W: Signatures of necroptosis-related genes as diagnostic
markers of endometriosis and their correlation with immune
infiltration. BMC Womens Health. 23(535)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dai F, Li J and Liu Y: Phosphatase and
tensin homolog deficiency induces M2 macrophage polarization by
promoting glycolytic activity in endometrial stromal cells. Biol
Reprod. 112:640–650. 2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cordeiro MR, Carvalhos CA and
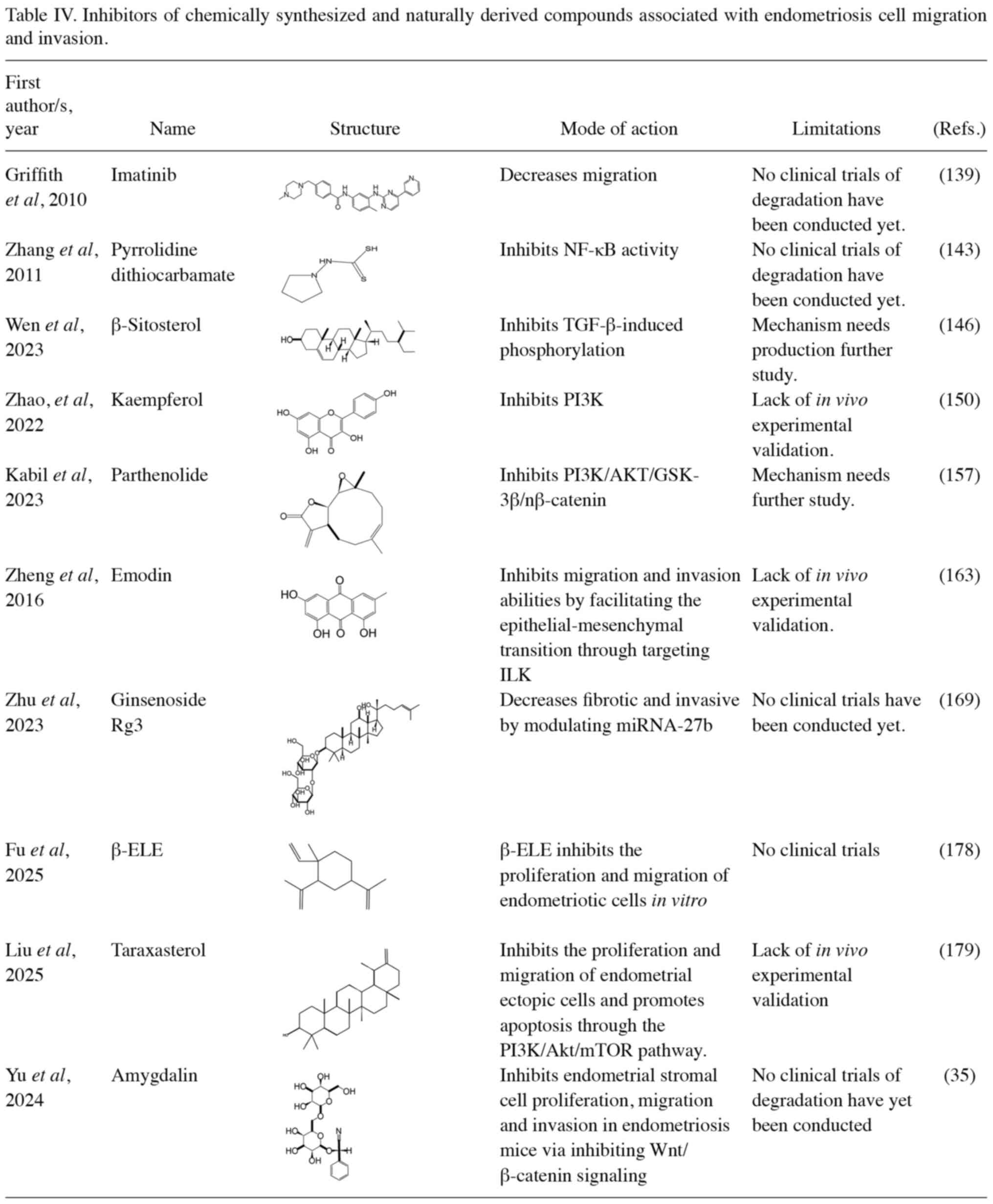
Figueiredo-Dias M: The emerging role of menstrual-blood-derived
stem cells in endometriosis. Biomedicines. 11(39)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hou S, Xu H, Lei S and Zhao D:
Overexpressed nicotinamide N-methyltransferase in endometrial
stromal cells induced by macrophages and estradiol contributes to
cell proliferation in endometriosis. Cell Death Discov.
10(463)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu M and Zhang Y: MiR-182 inhibits
proliferation, migration, invasion and inflammation of endometrial
stromal cells through deactivation of NF-κB signaling pathway in
endometriosis. Mol Cell Biochem. 476:1575–1588. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sarsenova M, Stepanjuk A, Saare M,
Kasvandik S, Soplepmann P, Mikeltadze I, Götte M, Salumets A and
Peters M: Carboxypeptidase inhibitor LXN expression in endometrial
tissue is menstrual cycle phase-dependent and is upregulated in
endometriotic lesions. Genes (Basel). 15(1086)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Peng Y, Xiong W, He H, Liu H, Fu T, Long
X, Li X, Dai X, Xu Y, Zhang L and Liu Y: Estradiol promotes
endometriosis progression via the eRβ/QKI/circSMAD2 Axis. Curr
Pharm Biotechnol: Feb 20, 2025 (Epub ahead of print).
|
|
23
|
Begum Y, Pandit A, Shukla D, Gupta R,
DasMahapatra P, Srivastava AK and Swarnakar S: Suppression of
endometriosis by miRNA-34a via inhibition of matrix
metalloproteinase-2: An alternative pathway to impede invasion.
Noncoding RNA Res. 12:92–101. 2025.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tang X, Li Q, Li L and Jiang J: Expression
of Talin-1 in endometriosis and its possible role in pathogenesis.
Reprod Biol Endocrinol. 19(42)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
D'Amico F, Skarmoutsou E, Quaderno G,
Malaponte G, La Corte C, Scibilia G, D'Agate G, Scollo P, Fraggetta
F, Spandidos DA and Mazzarino MC: Expression and localisation of
osteopontin and prominin-1 (CD133) in patients with endometriosis.
Int J Mol Med. 31:1011–1016. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xiong W, Zhang L, Liu H, Li N, Du Y, He H,
Zhang Z and Liu Y: E(2) -mediated EMT by activation of
β-catenin/Snail signalling during the development of ovarian
endometriosis. J Cell Mol Med. 23:8035–8045. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sesti F, Pietropolli A, Capozzolo T,
Broccoli P, Pierangeli S, Bollea MR and Piccione E: Hormonal
suppression treatment or dietary therapy versus placebo in the
control of painful symptoms after conservative surgery for
endometriosis stage III-IV. A randomized comparative trial. Fertil
Steril. 88:1541–1547. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qin Z, Dong Z, Liu J, Zhong A, Bao M, Wang
H, Yu H, Zhang S, Zhang W, Shen L, et al: A preliminary study on
the effects of black cohosh preparations on bone metabolism of rat
models with GnRH-a-induced peri-menopausal symptoms. Front
Endocrinol (Lausanne). 13(854345)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Houshdaran S, Oke AB, Fung JC, Vo KC,
Nezhat C and Giudice LC: Steroid hormones regulate genome-wide
epigenetic programming and gene transcription in human endometrial
cells with marked aberrancies in endometriosis. PLoS Genet.
16(e1008601)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moustafa S, Burn M, Mamillapalli R,
Nematian S, Flores V and Taylor HS: Accurate diagnosis of
endometriosis using serum microRNAs. Am J Obstet Gynecol.
223:557.e1–557.e11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Frisendahl C, Tang Y, Boggavarapu NR,
Peters M, Lalitkumar PG, Piltonen TT, Arffman RK, Salumets A, Götte
M, Korsching E and Gemzell-Danielsson K: miR-193b-5p and
miR-374b-5p are aberrantly expressed in endometriosis and suppress
endometrial cell migration in vitro. Biomolecules.
14(1400)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rodríguez E, Aburjania N, Priedigkeit NM,
DiFeo A and Martignetti JA: Nucleo-cytoplasmic localization domains
regulate Krüppel-like factor 6 (KLF6) protein stability and tumor
suppressor function. PLoS One. 5(e12639)2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shi J, Jing W, He Y and Huang Y: Decreased
expression of KLF6 in ectopic endometrial stromal cells contributes
to endometriosis progression by targeting CTNNB1. Cell Signal.
120(111230)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Meng F, Li J, Dong K, Bai R, Liu Q, Lu S,
Liu Y, Wu D, Jiang C and Li W: Juan-tong-yin potentially impacts
endometriosis pathophysiology by enhancing autophagy of endometrial
stromal cells via unfolded protein reaction-triggered endoplasmic
reticulum stress. J Ethnopharmacol. 325(117859)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yu M, Yang L, Pei Y and Xu M: Amygdalin
inhibits endometrial stromal cell proliferation, migration, and
invasion in endometriosis mice via inhibiting Wnt/β-catenin
signaling. J Mol Histol. 56(11)2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kao AP, Wang KH, Chang CC, Lee JN, Long
CY, Chen HS, Tsai CF, Hsieh TH and Tsai EM: Comparative study of
human eutopic and ectopic endometrial mesenchymal stem cells and
the development of an in vivo endometriotic invasion model. Fertil
Steril. 95:1308–1315.e1. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mao Z, Sang MM, Chen C, Zhu WT, Gong YS
and Pei DS: CSN6 promotes the migration and invasion of cervical
cancer cells by inhibiting autophagic degradation of cathepsin L.
Int J Biol Sci. 15:1310–1324. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gao S, Bian T, Su M, Liu Y and Zhang Y:
miR-26a inhibits ovarian cancer cell proliferation, migration and
invasion by targeting TCF12. Oncol Rep. 43:368–374. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Geng A, Luo L, Ren F, Zhang L, Zhou H and
Gao X: miR-29a-3p inhibits endometrial cancer cell proliferation,
migration and invasion by targeting VEGFA/CD C42/PAK1. BMC Cancer.
21(843)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cong Y, Cui Y, Zhu S, Cao J, Zou H, Martin
TA, Qiao G, Jiang W and Yu Z: Tim-3 promotes cell aggressiveness
and paclitaxel resistance through NF-κB/STAT3 signalling pathway in
breast cancer cells. Chin J Cancer Res. 32:564–579. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Altayyeb A, Othman E, Khashbah M, Esmaeel
A, El-Mokhtar M, Lambalk C, Mijatovic V and Abdelgawad M:
Characterization of mechanical signature of eutopic endometrial
stromal cells of endometriosis patients. Reprod Sci. 27:364–374.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Maurya VK, Szwarc MM, Fernandez-Valdivia
R, Lonard DM, Yong S, Joshi N, Fazleabas AT and Lydon JP: Early
growth response 1 transcription factor is essential for the
pathogenic properties of human endometriotic epithelial cells.
Reproduction. 164:41–54. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen SM, Liu YK, Ma XQ, Wei CY, Li MQ and
Zhu XY: Creatine promotes endometriosis progression by inducing M2
polarization of peritoneal macrophages. Reproduction.
169(e240278)2025.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bacci M, Capobianco A, Monno A, Cottone L,
Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S,
et al: Macrophages are alternatively activated in patients with
endometriosis and required for growth and vascularization of
lesions in a mouse model of disease. Am J Pathol. 175:547–556.
2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yu JJ, Sun HT, Zhang ZF, Shi RX, Liu LB,
Shang WQ, Wei CY, Chang KK, Shao J, Wang MY and Li MQ: IL15
promotes growth and invasion of endometrial stromal cells and
inhibits killing activity of NK cells in endometriosis.
Reproduction. 152:151–160. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vallvé-Juanico J, Houshdaran S and Giudice
LC: The endometrial immune environment of women with endometriosis.
Hum Reprod Update. 25:564–591. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Makoui MH, Fekri S, Makoui RH, Ansari N
and Esmaeilzadeh A: The role of mast cells in the development and
advancement of endometriosis. Am J Reprod Immunol.
93(e70019)2025.PubMed/NCBI View Article : Google Scholar
|
|
48
|
McCallion A, Nasirzadeh Y, Lingegowda H,
Miller JE, Khalaj K, Ahn S, Monsanto SP, Bidarimath M, Sisnett DJ,
Craig AW, et al: Estrogen mediates inflammatory role of mast cells
in endometriosis pathophysiology. Front Immunol.
13(961599)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tian J, Hoffmann V, Ibrahim MG, Hansen U,
Schüring AN, Velho RV, Mechsner S and Götte M: Characterization of
E-Cadherin, SSEA-1, MSI-1, and SOX-2 expression and their
association with pale cells in adenomyosis. Biomolecules.
14(1355)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Guo J, Gao J, Yu X, Luo H, Xiong X and
Huang O: Expression of DJ-1 and mTOR in eutopic and ectopic
endometria of patients with endometriosis and adenomyosis. Gynecol
Obstet Invest. 79:195–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gentilini D, Busacca M, Di Francesco S,
Vignali M, Viganò P and Di Blasio AM: PI3K/Akt and ERK1/2
signalling pathways are involved in endometrial cell migration
induced by 17beta-estradiol and growth factors. Mol Hum Reprod.
13:317–322. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guzeloglu Kayisli O, Kayisli UA, Luleci G
and Arici A: In vivo and in vitro regulation of Akt activation in
human endometrial cells is estrogen dependent. Biol Reprod.
71:714–721. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zheng T and Yang J: Differential
expression of EWI-2 in endometriosis, its functional role and
underlying molecular mechanisms. J Obstet Gynaecol Res.
43:1180–1188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chen J, Chang H, Peng X, Gu Y, Yi L, Zhang
Q, Zhu J and Mi M: 3,6-dihydroxyflavone suppresses the
epithelial-mesenchymal transition in breast cancer cells by
inhibiting the Notch signaling pathway. Sci Rep.
6(28858)2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yamaguchi M, Murata T, Shoji M and
Weitzmann MN: The flavonoid p-hydroxycinnamic acid mediates
anticancer effects on MDA-MB-231 human breast cancer cells in
vitro: Implications for suppression of bone metastases. Int J
Oncol. 47:1563–1571. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yu MM and Zhou QM: 3,6-dihydroxyflavone
suppresses the epithelial-mesenchymal transition, migration and
invasion in endometrial stromal cells by inhibiting the Notch
signaling pathway. Eur Rev Med Pharmacol Sci. 22:4009–4017.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Du X, Yang H, Kang X, Fu C and Yang T:
Blocking GATA6 alleviates pyroptosis and inhibits abdominal wall
endometriosis lesion growth through inactivating the PI3K/AKT
pathway. Cell Biochem Biophys. 83:1757–1770. 2025.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Qin R, Zheng F, Qin W, Wang J, Ma N, Tian
W, Li J, Liao M and Qin A: Progranulin promotes proliferation,
migration and invasion via the PI3K/Akt signalling pathway in a
model of endometriosis. Reprod Biomed Online. 46:425–435.
2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chen JJ, Xiao ZJ, Meng X, Wang Y, Yu MK,
Huang WQ, Sun X, Chen H, Duan YG, Jiang X, et al: MRP4 sustains
Wnt/β-catenin signaling for pregnancy, endometriosis and
endometrial cancer. Theranostics. 9:5049–5064. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lu Q, Huang Y, Wu J, Guan Y, Du M, Wang F,
Liu Z, Zhu Y, Gong G, Hou H, et al: T-cadherin inhibits invasion
and migration of endometrial stromal cells in endometriosis. Hum
Reprod. 35:145–156. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Matsuzaki S, Botchorishvili R, Pouly JL
and Canis M: Targeting the Wnt/β-catenin pathway in endometriosis:
A potentially effective approach for treatment and prevention. Mol
Cell Ther. 2(36)2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lepourcelet M, Chen YN, France DS, Wang H,
Crews P, Petersen F, Bruseo C, Wood AW and Shivdasani RA:
Small-molecule antagonists of the oncogenic Tcf/beta-catenin
protein complex. Cancer Cell. 5:91–102. 2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li Y, Wang X, Wang X, Wan L, Liu Y, Shi Y,
Zhang L, Fang Z and Wei Z: PDCD4 suppresses proliferation,
migration, and invasion of endometrial cells by inhibiting
autophagy and NF-κB/MMP2/MMP9 signal pathway. Biol Reprod.
99:360–372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Jung S, Jeong H and Yu SW: Autophagy as a
decisive process for cell death. Exp Mol Med. 52:921–930.
2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Doherty J and Baehrecke EH: Life, death
and autophagy. Nat Cell Biol. 20:1110–1117. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Cooper KF: Till death do us part: The
marriage of autophagy and apoptosis. Oxid Med Cell Longev.
2018(4701275)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Liu H, Du Y, Zhang Z, Lv L, Xiong W, Zhang
L, Li N, He H, Li Q and Liu Y: Autophagy contributes to
hypoxia-induced epithelial to mesenchymal transition of endometrial
epithelial cells in endometriosis. Biol Reprod. 99:968–981.
2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Pang C, Wu Z, Xu X, Yang W, Wang X and Qi
Y: Paeonol alleviates migration and invasion of endometrial stromal
cells by reducing HIF-1α-regulated autophagy in endometriosis.
Front Biosci (Landmark Ed). 26:485–495. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
70
|
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y,
Li N, He H, Du Y and Liu Y: Hypoxia-inducible factor-1α promotes
endometrial stromal cells migration and invasion by upregulating
autophagy in endometriosis. Reproduction. 153:809–820.
2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wu L, Lin S, Hu Y, Jing S, Sun B, Chen X,
Jia J, Zeng C and Pei F: Potential mechanism of Luoshi Neiyi
prescription in endometriosis based on serum pharmacochemistry and
network pharmacology. Front Pharmacol. 15(1395160)2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Foster WG: Hypoxia-induced autophagy,
epithelial to mesenchymal transition, and invasion in the
pathophysiology of endometriosis: A perspective. Biol Reprod.
99:905–906. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
McDonald PC, Swayampakula M and Dedhar S:
Coordinated regulation of metabolic transporters and
migration/invasion by carbonic anhydrase IX. Metabolites.
8(20)2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Quan Q, Wu J, Yu M and Tang J: Immune
micro-environment and drug analysis of peritoneal endometriosis
based on epithelial-mesenchymal transition classification. Front
Endocrinol (Lausanne). 13(1035158)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Acloque H, Thiery JP and Nieto MA: The
physiology and pathology of the EMT. Meeting on the
epithelial-mesenchymal transition. EMBO Rep. 9:322–326.
2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ang HL, Mohan CD, Shanmugam MK, Leong HC,
Makvandi P, Rangappa KS, Bishayee A, Kumar AP and Sethi G:
Mechanism of epithelial-mesenchymal transition in cancer and its
regulation by natural compounds. Med Res Rev. 43:1141–1200.
2023.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Matsuzaki S and Darcha C: Epithelial to
mesenchymal transition-like and mesenchymal to epithelial
transition-like processes might be involved in the pathogenesis of
pelvic endometriosis. Hum Reprod. 27:712–721. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Das V, Bhattacharya S, Chikkaputtaiah C,
Hazra S and Pal M: The basics of epithelial-mesenchymal transition
(EMT): A study from a structure, dynamics, and functional
perspective. J Cell Physiol. 234:14535–14555. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Yan L, Song Z, Yi L, Tian C, Zhang R, Qin
X, Wang X, Ren S, Ma X, Wang X, et al: TMEM176B inhibits ovarian
cancer progression by regulating EMT via the Wnt/β-catenin
signaling pathway. J Transl Med. 23(350)2025.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Yao Y, Niu Y, Zhou H and Yong M: KAT2B
inhibits proliferation and invasion via inactivating TGF-β/Smad3
pathway-medicated autophagy and EMT in epithelial ovarian cancer.
Sci Rep. 15(3417)2025.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Huang J, Sun X, Diao G, Li R, Guo J and
Han J: KIF15 knockdown inhibits the development of endometrial
cancer by suppressing epithelial-mesenchymal transition and
stemness through Wnt/β-catenin signaling. Environ Toxicol.
38:1824–1834. 2023.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Cassier PA, Navaridas R, Bellina M, Rama
N, Ducarouge B, Hernandez-Vargas H, Delord JP, Lengrand J, Paradisi
A, Fattet L, et al: Netrin-1 blockade inhibits tumour growth and
EMT features in endometrial cancer. Nature. 620:409–416.
2023.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Li N, Ji GX and Yang ZY: EFEMP2 increases
the invasion ability of cervical cancer cells by promoting EMT via
the Raf/MEK/ERK signaling pathway. Neoplasma. 69:1185–1197.
2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Zhu Y, Yin WF, Yu P, Zhang C, Sun MH, Kong
LY and Yang L: Meso-Hannokinol inhibits breast cancer bone
metastasis via the ROS/JNK/ZEB1 axis. Phytother Res. 37:2262–2279.
2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Wojtowicz-Praga SM, Dickson RB and Hawkins
MJ: Matrix metalloproteinase inhibitors. Invest New Drugs.
15:61–75. 1997.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Sharpe-Timms KL, Keisler LW, McIntush EW
and Keisler DH: Tissue inhibitor of metalloproteinase-1
concentrations are attenuated in peritoneal fluid and sera of women
with endometriosis and restored in sera by gonadotropin-releasing
hormone agonist therapy. Fertil Steril. 69:1128–1134.
1998.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wenzl RJ and Heinzl H: Localization of
matrix metalloproteinase-2 in uterine endometrium and ectopic
implants. Gynecol Obstet Invest. 45:253–257. 1998.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Cox KE, Piva M and Sharpe-Timms KL:
Differential regulation of matrix metalloproteinase-3 gene
expression in endometriotic lesions compared with endometrium. Biol
Reprod. 65:1297–1303. 2001.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lv X, Chen P and Liu W: Down regulation of
MiR-93 contributes to endometriosis through targeting MMP3 and
VEGFA. Am J Cancer Res. 5:1706–1717. 2015.PubMed/NCBI
|
|
90
|
Chung HW, Lee JY, Moon HS, Hur SE, Park
MH, Wen Y and Polan ML: Matrix metalloproteinase-2, membranous type
1 matrix metalloproteinase, and tissue inhibitor of
metalloproteinase-2 expression in ectopic and eutopic endometrium.
Fertil Steril. 78:787–795. 2002.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Collette T, Maheux R, Mailloux J and Akoum
A: Increased expression of matrix metalloproteinase-9 in the
eutopic endometrial tissue of women with endometriosis. Hum Reprod.
21:3059–3067. 2006.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Lin TC, Wang KH, Chuang KH, Kao AP and Kuo
TC: Interleukin-33 promotes invasiveness of human ovarian
endometriotic stromal cells through the ST2/MAPK/MMP-9 pathway
activated by 17β-estradiol. Taiwan J Obstet Gynecol. 60:658–664.
2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Gaetje R, Holtrich U, Engels K, Kourtis K,
Cikrit E, Kissler S, Rody A, Karn T and Kaufmann M: Expression of
membrane-type 5 matrix metalloproteinase in human endometrium and
endometriosis. Gynecol Endocrinol. 23:567–573. 2007.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Rodgers WH, Osteen KG, Matrisian LM, Navre
M, Giudice LC and Gorstein F: Expression and localization of
matrilysin, a matrix metalloproteinase, in human endometrium during
the reproductive cycle. Am J Obstet Gynecol. 168 (1 Pt 1):253–260.
1993.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Pearce CL, Templeman C, Rossing MA, Lee A,
Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund
KG, et al: Association between endometriosis and risk of
histological subtypes of ovarian cancer: A pooled analysis of
case-control studies. Lancet Oncol. 13:385–394. 2012.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Barnard ME, Farland LV, Yan B, Wang J,
Trabert B, Doherty JA, Meeks HD, Madsen M, Guinto E, Collin LJ, et
al: Endometriosis typology and ovarian cancer risk. JAMA.
332:482–489. 2024.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Yousefi M, Dehghani S, Nosrati R, Ghanei
M, Salmaninejad A, Rajaie S, Hasanzadeh M and Pasdar A: Current
insights into the metastasis of epithelial ovarian cancer-hopes and
hurdles. Cell Oncol (Dordr). 43:515–538. 2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Schröpfer A, Kammerer U, Kapp M, Dietl J,
Feix S and Anacker J: Expression pattern of matrix
metalloproteinases in human gynecological cancer cell lines. BMC
Cancer. 10(553)2010.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Cho-Clark M, Larco DO, Zahn BR, Mani SK
and Wu TJ: GnRH-(1-5) activates matrix metallopeptidase-9 to
release epidermal growth factor and promote cellular invasion. Mol
Cell Endocrinol. 415:114–125. 2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Kicman A, Gacuta E, Kulesza M, Będkowska
EG, Marecki R, Klank-Sokołowska E, Knapp P, Niczyporuk M and
Ławicki S: Diagnostic utility of selected matrix metalloproteinases
(MMP-2, MMP-3, MMP-11, MMP-26), HE4, CA125 and ROMA algorithm in
diagnosis of ovarian cancer. Int J Mol Sci. 25(6265)2024.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Shi L, Zhang Q, Zhu S, Tang Q, Chen X, Lan
R, Wang N and Zhu Y: Pharmacological inhibition of EZH2 using a
covalent inhibitor suppresses human ovarian cancer cell migration
and invasion. Mol Cell Biochem. 479:831–841. 2024.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Ünüvar S, Melekoğlu R, Yüce H, Çelik NZ,
Okumuş EB, Toprak S, Tanbek K, Yaşar Ş, Doğan A, Türkmen NB, et al:
Diagnostic utility of lipocalin 2 and metalloproteinase 9 levels in
early-stage endometrial cancer. Cancer Biomark.
41(18758592241290951)2024.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Shukla S, Qureshi S, Singh U and Khattri
S: A study of matrix metalloproteinase-2 and interleukin-18 in
preinvasive and invasive lesions of cancer cervix. J Midlife
Health. 11:236–239. 2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Chauhan R, Malhotra L, Gupta A, Dagar G,
Mendiratta M, Masoodi T, Hashem S, Al Marzooqi S, Das D, Uddin S,
et al: Bergenin inhibits growth of human cervical cancer cells by
decreasing Galectin-3 and MMP-9 expression. Sci Rep.
14(15287)2024.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kochumon S, Al-Sayyar A, Jacob T, Bahman
F, Akhter N, Wilson A, Sindhu S, Hannun YA, Ahmad R and Al-Mulla F:
TGF-β and TNF-α interaction promotes the expression of MMP-9
through H3K36 dimethylation: Implications in breast cancer
metastasis. Front Immunol. 15(1430187)2024.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Wan Y, Huang J, Song Y, Gu C, Kong J, Zuo
L and Chen J: hsa-miR-340-5p inhibits epithelial-mesenchymal
transition in endometriosis by targeting MAP3K2 and inactivating
MAPK/ERK signaling. Open Med (Wars). 17:566–576. 2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Zhang M, Wang X, Xia X, Fang X, Zhang T
and Huang F: Endometrial epithelial cells-derived exosomes deliver
microRNA-30c to block the BCL9/Wnt/CD44 signaling and inhibit cell
invasion and migration in ovarian endometriosis. Cell Death Discov.
8(151)2022.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Ntzeros K, Voros C, Mavrogianni D,
Kathopoulis N, Kypriotis K, Varthaliti A, Darlas M, Douligeris A
and Protopapas A: Expression of E-CADHERIN and miR-200b in
Different Forms of Endometriosis. Biomedicines.
13(524)2025.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Hagh YN, Ahmadifard M, Esmaelzadeh S,
Abbaszadeh S and Shokrzadeh N: Decreased expression of miR-200a and
miR-223-3p in endometriosis during the secretory phase of menstrual
cycle: Insights from a case-control study on molecular biomarkers
and disease-related infertility. Int J Reprod Biomed. 22:1003–1014.
2025.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Wang J, Li J, Han H, Wang C, Shi T and
Yang X: miR-375-3p predicts the severity of endometriosis and
regulates cellular progression by targeting NOX4. Mol Cell Probes.
79(101999)2025.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhang Z, Qin Y, Huang J, Wang Y, Zeng L,
Wang Y, Zhuyun F and Wang L: Oestrogen promotes the progression of
adenomyosis by inhibiting CITED2 through miR-145. Reprod Biomed
Online. 49(104108)2024.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Cui X, Zhou S and Lin Y: Long non-coding
RNA DHRS4 antisense RNA 1 inhibits ectopic endometrial cell
proliferation, migration, and invasion in endometriosis by
regulating microRNA-139-5p expression. Bioengineered. 13:9792–9804.
2022.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Bao Q, Zheng Q, Wang S, Tang W and Zhang
B: LncRNA HOTAIR regulates cell invasion and migration in
endometriosis through miR-519b-3p/PRRG4 pathway. Front Oncol.
12(953055)2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Liu L, Wang L, Hao N, Du N, Li Y and Kang
S: miRNA-1229-5p promotes migration and invasion and suppresses
apoptosis of endometrial cells via the STMN1/p38 MAPK axis in
endometriosis. Gene. 950(149385)2025.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Hudson QJ, Proestling K, Perricos A,
Kuessel L, Husslein H, Wenzl R and Yotova I: The role of long
non-coding RNAs in endometriosis. Int J Mol Sci.
22(11425)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Arendt W, Kleszczyński K, Gagat M and
Izdebska M: Endometriosis and cytoskeletal remodeling: The
functional role of actin-binding proteins. Cells.
14(360)2025.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Ma J and Jiang J: ATG8 inhibited
endometriosis formation by regulating Treg cells differentiation
via integrin α4β1 and Talin-1 interaction. Reprod Biomed Online.
48(103646)2024.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Knez J, Kovačič B and Goropevšek A: The
role of regulatory T-cells in the development of endometriosis. Hum
Reprod: May 19, 2024 (Epub ahead of print).
|
|
120
|
Sun H, Lagarrigue F and Ginsberg MH: The
connection between Rap1 and Talin1 in the activation of integrins
in blood cells. Front Cell Dev Biol. 10(908622)2022.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Rahmawati E, Yang WV, Lei YP, Maurya PK,
Chen HW and Tzeng CR: Gonadotropin-releasing hormone agonist
induces downregulation of tensin 1 in women with endometriosis.
Acta Obstet Gynecol Scand. 98:222–231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Zhang J, Wang L, Li C, Zhang H, Li R and
Li M: Letrozole promotes the expression of integrin αvβ3 and HOXA10
in endometrium of endometriosis. Syst Biol Reprod Med. 68:121–128.
2022.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Duan R, Wang Y, Lin A, Lian L, Cao H, Gu
W, Li T and Sun Q: Expression of nm23-H1, p53, and integrin β1 in
endometriosis and their clinical significance. Int J Clin Exp
Pathol. 13:1024–1029. 2020.PubMed/NCBI
|
|
124
|
Gao X, Shao W, Wang J, Gao H, Zhang X, Xia
C, Li M and Liu S: Integrin β3 enhances glycolysis and increases
lactate production in endometriosis. J Reprod Immunol.
165(104312)2024.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Rosa-E-Silva ACJS, Mamillapalli R,
Rosa-E-Silva JC, Ucar A, Schwartz J and Taylor HS: Uterine
administration of C-X-C motif chemokine ligand 12 increases the
pregnancy rates in mice with induced endometriosis. F S Sci.
4:65–73. 2023.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Harden S, Tan TY, Ku CW, Zhou J, Chen Q,
Chan JKY, Brosens J and Lee YH: Peritoneal autoantibody profiling
identifies p53 as an autoantibody target in endometriosis. Fertil
Steril. 120:176–187. 2023.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Raghuwanshi S and Gartel AL:
Small-molecule inhibitors targeting FOXM1: Current challenges and
future perspectives in cancer treatments. Biochim Biophys Acta Rev
Cancer. 1878(189015)2023.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Yu C, Chen L, Yie L, Wei L, Wen T, Liu Y
and Chen H: Targeting FoxM1 inhibits proliferation, invasion and
migration of nasopharyngeal carcinoma through the
epithelial-to-mesenchymal transition pathway. Oncol Rep.
33:2402–2410. 2015.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Yu M, Yang Y, Zhao H, Li M, Chen J, Wang
B, Xiao T, Huang C, Zhao H, Zhou W and Zhang JV: Targeting the
chemerin/CMKLR1 axis by small molecule antagonist α-NETA mitigates
endometriosis progression. Front Pharmacol.
13(985618)2022.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Lin Y, Kojima S, Ishikawa A, Matsushita H,
Takeuchi Y, Mori Y, Ma J, Takeuchi K, Umezawa K and Wakatsuki A:
Inhibition of MLCK-mediated migration and invasion in human
endometriosis stromal cells by NF-κB inhibitor DHMEQ. Mol Med Rep.
28(141)2023.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Xu X, Zheng Q, Zhang Z, Zhang X, Liu R and
Liu P: Periostin enhances migration, invasion, and adhesion of
human endometrial stromal cells through integrin-linked kinase
1/Akt signaling pathway. Reprod Sci. 22:1098–1106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Zheng QM, Lu JJ, Zhao J, Wei X, Wang L and
Liu PS: Periostin facilitates the epithelial-mesenchymal transition
of endometrial epithelial cells through ILK-Akt signaling pathway.
Biomed Res Int. 2016(9842619)2016.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Li YH, Geng YY, Liu L, Chen CY and Gao Y:
Lipoxin A4 inhibits the invasion and migration of endometrial
stromal cells by down-regulating NF-κB signaling-mediated
autophagy. Zhonghua Fu Chan Ke Za Zhi. 53:547–553. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
134
|
Marquardt RM, Kim TH, Shin JH and Jeong
JW: Progesterone and Estrogen Signaling in the Endometrium: What
Goes Wrong in Endometriosis? Int J Mol Sci. 20(3822)2019.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Wakatsuki A, Lin Y, Kojima S, Matsushita
H, Takeuchi K and Umezawa K: Inhibitory effects of estetrol on the
invasion and migration of immortalized human endometrial stromal
cells. Endocr J. 71:199–206. 2024.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Guo B, Zhu H, Xiao C, Zhang J, Liu X, Fang
Y, Wei B, Zhang J, Cao Y and Zhan L: NLRC5 exerts
anti-endometriosis effects through inhibiting ERβ-mediated
inflammatory response. BMC Med. 22(351)2024.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Wang S, Long S, Deng Z and Wu W: Positive
role of Chinese herbal medicine in cancer immune regulation. Am J
Chin Med. 48:1577–1592. 2020.PubMed/NCBI View Article : Google Scholar
|
|
138
|
He Q, Wan S, Jiang M, Li W, Zhang Y, Zhang
L, Wu M, Lin J, Zou L and Hu Y: Exploring the therapeutic potential
of tonic Chinese herbal medicine for gynecological disorders: An
updated review. J Ethnopharmacol. 329(118144)2024.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Griffith JS, Binkley PA, Kirma NB,
Schenken RS, Witz CA and Tekmal RR: Imatinib decreases endometrial
stromal cell transmesothial migration and proliferation in the
extracellular matrix of modeled peritoneum. Fertil Steril.
94:2531–2535. 2010.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Yao Z, Zhang J, Zhang B, Liang G, Chen X,
Yao F, Xu X, Wu H, He Q, Ding L and Yang B: Imatinib prevents lung
cancer metastasis by inhibiting M2-like polarization of
macrophages. Pharmacol Res. 133:121–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Ferraz CR, Calixto-Campos C, Manchope MF,
Casagrande R, Clissa PB, Baldo C and Verri WA Jr:
Jararhagin-induced mechanical hyperalgesia depends on TNF-α, IL-1β
and NFκB in mice. Toxicon. 103:119–128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Pinho-Ribeiro FA, Fattori V, Zarpelon AC,
Borghi SM, Staurengo-Ferrari L, Carvalho TT, Alves-Filho JC, Cunha
FQ, Cunha TM, Casagrande R and Verri WA Jr: Pyrrolidine
dithiocarbamate inhibits superoxide anion-induced pain and
inflammation in the paw skin and spinal cord by targeting NF-κB and
oxidative stress. Inflammopharmacology. 24:97–107. 2016.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ,
Wang XF and Li C: Pyrrolidine dithiocarbamate inhibits nuclear
factor-κB pathway activation, and regulates adhesion, migration,
invasion and apoptosis of endometriotic stromal cells. Mol Hum
Reprod. 17:175–181. 2011.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Babu S and Jayaraman S: An update on
β-sitosterol: A potential herbal nutraceutical for diabetic
management. Biomed Pharmacother. 131(110702)2020.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Khan Z, Nath N, Rauf A, Emran TB, Mitra S,
Islam F, Chandran D, Barua J, Khandaker MU, Idris AM, et al:
Multifunctional roles and pharmacological potential of
β-sitosterol: Emerging evidence toward clinical applications. Chem
Biol Interact. 365(110117)2022.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Wen Y, Pang L, Fan L, Zhou Y, Li R, Zhao T
and Zhang M: β-Sitosterol inhibits the proliferation of endometrial
cells via regulating Smad7-Mediated TGF-β/Smads signaling pathway.
Cell J. 25:554–563. 2023.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Periferakis A, Periferakis K, Badarau IA,
Petran EM, Popa DC, Caruntu A, Costache RS, Scheau C, Caruntu C and
Costache DO: Kaempferol: Antimicrobial properties, sources,
clinical, and traditional applications. Int J Mol Sci.
23(15054)2022.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Imran M, Salehi B, Sharifi-Rad J, Aslam
Gondal T, Saeed F, Imran A, Shahbaz M, Tsouh Fokou PV, Umair Arshad
M, Khan H, et al: Kaempferol: A key emphasis to its anticancer
potential. Molecules. 24(2277)2019.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Jantas D, Malarz J, Le TN and Stojakowska
A: Neuroprotective properties of kempferol derivatives from maesa
membranacea against oxidative stress-induced cell damage: An
association with cathepsin D inhibition and PI3K/Akt activation.
Int J Mol Sci. 22(10363)2021.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Zhao J, Wang J, Liu J, Li S, Liu P and
Zhang X: Effect and mechanisms of kaempferol against endometriosis
based on network pharmacology and in vitro experiments. BMC
Complement Med Ther. 22(254)2022.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Ren Y, Li Y, Lv J, Guo X, Zhang J, Zhou D,
Zhang Z, Xue Z, Yang G, Xi Q, et al: Parthenolide regulates
oxidative stress-induced mitophagy and suppresses apoptosis through
p53 signaling pathway in C2C12 myoblasts. J Cell Biochem.
120:15695–15708. 2019.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Freund RRA, Gobrecht P, Fischer D and
Arndt HD: Advances in chemistry and bioactivity of parthenolide.
Nat Prod Rep. 37:541–565. 2020.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Jafari N, Nazeri S and Enferadi ST:
Parthenolide reduces metastasis by inhibition of vimentin
expression and induces apoptosis by suppression elongation factor α
- 1 expression. Phytomedicine. 41:67–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Mathema VB, Koh YS, Thakuri BC and
Sillanpää M: Parthenolide, a sesquiterpene lactone, expresses
multiple anti-cancer and anti-inflammatory activities.
Inflammation. 35:560–565. 2012.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Wang M and Li Q: Parthenolide could become
a promising and stable drug with anti-inflammatory effects. Nat
Prod Res. 29:1092–1101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Huang L, Liu F, Liu X, Niu L, Sun L, Fang
F, Ma K and Hu P: Parthenolide inhibits the proliferation and
migration of cervical cancer cells via FAK/GSK3β pathway. Cancer
Chemother Pharmacol. 93:203–213. 2024.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Kabil SL, Rashed HE, Mohamed NM and Elwany
NE: Parthenolide repressed endometriosis induced surgically in
rats: Role of PTEN/PI3Kinase/AKT/GSK-3β/β-catenin signaling in
inhibition of epithelial mesenchymal transition. Life Sci.
331(122037)2023.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Cheng J, Li C, Ying Y, Lv J, Qu X, McGowan
E, Lin Y and Zhu X: Metformin alleviates endometriosis and
potentiates endometrial receptivity via decreasing VEGF and MMP9
and increasing leukemia inhibitor factor and HOXA10. Front
Pharmacol. 13(750208)2022.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Neto AC, Botelho M, Rodrigues AR, Lamas S,
Araújo B, Guimarães JT, Gouveia AM, Almeida H and Neves D:
Metformin reverses infertility in a mouse model of endometriosis:
Unveiling disease pathways and implications for future clinical
approaches. Reprod Biomed Online. 50(104474)2025.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Semwal RB, Semwal DK, Combrinck S and
Viljoen A: Emodin-A natural anthraquinone derivative with diverse
pharmacological activities. Phytochemistry.
190(112854)2021.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Demirezer LO, Kuruüzüm-Uz A, Bergere I,
Schiewe HJ and Zeeck A: The structures of antioxidant and cytotoxic
agents from natural source: Anthraquinones and tannins from roots
of Rumex patientia. Phytochemistry. 58:1213–1217. 2001.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Zhang Q, Chen WW, Sun X, Qian D, Tang DD,
Zhang LL, Li MY, Wang LY, Wu CJ and Peng W: The versatile emodin: A
natural easily acquired anthraquinone possesses promising
anticancer properties against a variety of cancers. Int J Biol Sci.
18:3498–3527. 2022.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Zheng Q, Xu Y, Lu J, Zhao J, Wei X and Liu
P: Emodin inhibits migration and invasion of human endometrial
stromal cells by facilitating the mesenchymal-epithelial transition
through targeting ILK. Reprod Sci. 23:1526–1535. 2016.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Cui Y, Chen LJ, Huang T, Ying JQ and Li J:
The pharmacology, toxicology and therapeutic potential of
anthraquinone derivative emodin. Chin J Nat Med. 18:425–435.
2020.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Liu X, Mi X, Wang Z, Zhang M, Hou J, Jiang
S, Wang Y, Chen C and Li W: Ginsenoside Rg3 promotes regression
from hepatic fibrosis through reducing inflammation-mediated
autophagy signaling pathway. Cell Death Dis. 11(454)2020.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Nakhjavani M, Smith E, Townsend AR, Price
TJ and Hardingham JE: Anti-angiogenic properties of ginsenoside
Rg3. Molecules. 25(4905)2020.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Ren B, Feng J, Yang N, Guo Y, Chen C and
Qin Q: Ginsenoside Rg3 attenuates angiotensin II-induced myocardial
hypertrophy through repressing NLRP3 inflammasome and oxidative
stress via modulating SIRT1/NF-κB pathway. Int Immunopharmacol.
98(107841)2021.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Zhu Y, Wang A, Zhang S, Kim J, Xia J,
Zhang F, Wang D, Wang Q and Wang J: Paclitaxel-loaded ginsenoside
Rg3 liposomes for drug-resistant cancer therapy by dual targeting
of the tumor microenvironment and cancer cells. J Adv Res.
49:159–173. 2023.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Kim MK, Lee SK, Park JH, Lee JH, Yun BH,
Park JH, Seo SK, Cho S and Choi YS: Ginsenoside Rg3 decreases
fibrotic and invasive nature of endometriosis by modulating
miRNA-27b: In vitro and in vivo studies. Sci Rep.
7(17670)2017.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Vlavcheski F, O'Neill EJ, Gagacev F and
Tsiani E: Effects of berberine against pancreatitis and pancreatic
cancer. Molecules. 27(8630)2022.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Habtemariam S: Berberine pharmacology and
the gut microbiota: A hidden therapeutic link. Pharmacol Res.
155(104722)2020.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Feng X, Sureda A, Jafari S, Memariani Z,
Tewari D, Annunziata G, Barrea L, Hassan STS, Šmejkal K, Malaník M,
et al: Berberine in cardiovascular and metabolic diseases: From
mechanisms to therapeutics. Theranostics. 9:1923–1951.
2019.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Wang K, Feng X, Chai L, Cao S and Qiu F:
The metabolism of berberine and its contribution to the
pharmacological effects. Drug Metab Rev. 49:139–157.
2017.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Chen Y, Li K, Zhao H, Hao Z, Yang Y, Gao M
and Zhao D: Integrated lipidomics and network pharmacology analysis
to reveal the mechanisms of berberine in the treatment of
hyperlipidemia. J Transl Med. 20(412)2022.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Hu S, Wang J, Liu E, Zhang X, Xiang J, Li
W, Wei P, Zeng J, Zhang Y and Ma X: Protective effect of berberine
in diabetic nephropathy: A systematic review and meta-analysis
revealing the mechanism of action. Pharmacol Res.
185(106481)2022.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Braicu OL, Budisan L, Buiga R, Jurj A,
Achimas-Cadariu P, Pop LA, Braicu C, Irimie A and Berindan-Neagoe
I: miRNA expression profiling in formalin-fixed paraffin-embedded
endometriosis and ovarian cancer samples. Onco Targets Ther.
10:4225–4238. 2017.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Gu Y and Zhou Z: Berberine inhibits the
proliferation, invasion and migration of endometrial stromal cells
by downregulating miR-429. Mol Med Rep. 23(416)2021.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Fu Z, Liu H, Kuang Y, Yang J, Luo M, Cao L
and Zheng W: β-elemene, a sesquiterpene constituent from Curcuma
phaeocaulis inhibits the development of endometriosis by inducing
ferroptosis via the MAPK and STAT3 signaling pathways. J
Ethnopharmacol. 341(119344)2025.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Liu Y, Cao H, Zheng S and Zhuang Y:
Unveiling the therapeutic mechanisms of taraxasterol from dandelion
in endometriosis: Network pharmacology and cellular insights.
Biochem Biophys Res Commun. 742(151079)2025.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Wu RF, Yang HM, Zhou WD, Zhang LR, Bai JB,
Lin DC, Ng TW, Dai SJ, Chen QH and Chen QX: Effect of
interleukin-1β and lipoxin A(4) in human endometriotic stromal
cells: Proteomic analysis. J Obstet Gynaecol Res. 43:308–319.
2017.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Matsuzaki S and Darcha C: In vitro effects
of a small-molecule antagonist of the Tcf/ß-catenin complex on
endometrial and endometriotic cells of patients with endometriosis.
PLoS One. 8(e61690)2013.PubMed/NCBI View Article : Google Scholar
|
|
182
|
van Winden AW, van den Broek I, Gast MC,
Engwegen JY, Sparidans RW, van Dulken EJ, Depla AC, Cats A,
Schellens JH, Peeters PH, et al: Serum degradome markers for the
detection of breast cancer. J Proteome Res. 9:3781–3788.
2010.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Chen Y, Li H, Cheng HY, Rui-Qiong M, Ye X,
Cui H, Hong-Lan Z and Chang XH: Fibrinogen alpha chain is
up-regulated and affects the pathogenesis of endometriosis. Reprod
Biomed Online. 39:893–904. 2019.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Li H, Ma RQ, Cheng HY, Ye X, Zhu HL and
Chang XH: Fibrinogen alpha chain promotes the migration and
invasion of human endometrial stromal cells in endometriosis
through focal adhesion kinase/protein kinase B/matrix
metallopeptidase 2 pathway†. Biol Reprod. 103:779–790.
2020.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Akbarzadeh M, Movassaghpour AA, Ghanbari
H, Kheirandish M, Fathi Maroufi N, Rahbarghazi R, Nouri M and
Samadi N: The potential therapeutic effect of melatonin on human
ovarian cancer by inhibition of invasion and migration of cancer
stem cells. Sci Rep. 7(17062)2017.PubMed/NCBI View Article : Google Scholar
|
|
186
|
Bu S, Wang Q, Sun J, Li X, Gu T and Lai D:
Melatonin suppresses chronic restraint stress-mediated metastasis
of epithelial ovarian cancer via NE/AKT/beta-catenin/SLUG axis.
Cell Death Dis. 11(644)2020.PubMed/NCBI View Article : Google Scholar
|
|
187
|
El-Sokkary GH, Ismail IA and Saber SH:
Melatonin inhibits breast cancer cell invasion through modulating
DJ-1/KLF17/ID-1 signaling pathway. J Cell Biochem. 120:3945–3957.
2019.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Qi S, Yan L, Liu Z, Mu YL, Li M, Zhao X,
Chen ZJ and Zhang H: Melatonin inhibits 17β-estradiol-induced
migration, invasion and epithelial-mesenchymal transition in normal
and endometriotic endometrial epithelial cells. Reprod Biol
Endocrinol. 16(62)2018.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Bhattacharya S, Patel KK, Dehari D,
Agrawal AK and Singh S: Melatonin and its ubiquitous anticancer
effects. Mol Cell Biochem. 462:133–155. 2019.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Reiter RJ, Rosales-Corral SA, Tan DX,
Acuna-Castroviejo D, Qin L, Yang SF and Xu K: Melatonin, a full
service anti-cancer agent: Inhibition of initiation, progression
and metastasis. Int J Mol Sci. 18(843)2017.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Cheng J, Yang HL, Gu CJ, Liu YK, Shao J,
Zhu R, He YY, Zhu XY and Li MQ: Melatonin restricts the viability
and angiogenesis of vascular endothelial cells by suppressing
HIF-1α/ROS/VEGF. Int J Mol Med. 43:945–955. 2019.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Kwon MR, Park JS, Ko EJ, Park J, Ju EJ,
Shin SH, Son GW, Lee HW, Park YY, Kang MH, et al: Ibulocydine
inhibits migration and invasion of TNBC cells via MMP-9 regulation.
Int J Mol Sci. 25(6123)2024.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Liang RN, Li PS, Zou Y, Liu YL, Jiang Z,
Liu Z, Fan P, Xu L, Peng JH and Sun XY: Ping-Chong-Jiang-Ni formula
induces apoptosis and inhibits proliferation of human ectopic
endometrial stromal cells in endometriosis via the activation of
JNK signaling pathway. Evid Based Complement Alternat Med.
2017(6489427)2017.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Huang J, Zhang X, Wang J, Gu C, Zhang Y,
Hu G and Chen J: Mechanism of Yushenhuoxue prescription in treating
endometriosis based on network pharmacology and the effect on the
TNF pathway. Heliyon. 9(e20283)2023.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Xu Y, Li Y, Zhang J and Cai P: Hua Yu Xiao
Zheng decoction induces ectopic endometrial stromal cell senescence
via inhibiting the PI3K/AKT signaling. Tissue Cell.
93(102763)2025.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Miller MA, Meyer AS, Beste MT, Lasisi Z,
Reddy S, Jeng KW, Chen CH, Han J, Isaacson K, Griffith LG and
Lauffenburger DA: ADAM-10 and -17 regulate endometriotic cell
migration via concerted ligand and receptor shedding feedback on
kinase signaling. Proc Natl Acad Sci USA. 110:E2074–E2083.
2013.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Zanon P, Terraciano PB, Quandt L, Palma
Kuhl C, Pandolfi Passos E and Berger M: Angiotensin II-AT1 receptor
signalling regulates the plasminogen-plasmin system in human
stromal endometrial cells increasing extracellular matrix
degradation, cell migration and inducing a proinflammatory profile.
Biochem Pharmacol. 225(116280)2024.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Chen J, Shen L, Wu T and Yang Y:
Unraveling the significance of AGPAT4 for the pathogenesis of
endometriosis via a multi-omics approach. Hum Genet. 143:1163–1174.
2024.PubMed/NCBI View Article : Google Scholar
|
|
199
|
Zheng R, Liu Y, Lei Y and Yue Y:
Upregulated microRNA-429 confers endometrial stromal cell
dysfunction by targeting HIF1AN and regulating the HIF1A/VEGF
pathway. Open Med (Wars). 18(20230775)2023.PubMed/NCBI View Article : Google Scholar
|
|
200
|
Zhang Y and Yang H: Silencing of FZD7
inhibits endometriotic cell viability, migration, and angiogenesis
by promoting ferroptosis. Cell Biochem Biophys. 83:2471–2480.
2025.PubMed/NCBI View Article : Google Scholar
|
|
201
|
Lv X and Li F: METTL14 promotes
proliferation, migration, and invasion in endometriotic stromal
cell growth by activating the ZEB1/MEK/ERK pathway. Gynecol Obstet
Invest. 90:42–54. 2025.PubMed/NCBI View Article : Google Scholar
|
|
202
|
Tang Y, Lu X and Lin K, Li J, Yuan M and
Lin K: m6A methylation of RNF43 inhibits the progression of
endometriosis through regulating oxidative phosphorylation via
NDUFS1. J Cell Physiol. 239(e31367)2024.PubMed/NCBI View Article : Google Scholar
|
|
203
|
Vu TH, Nakamura K, Shigeyasu K, Kashino C,
Okamoto K, Kubo K, Kamada Y and Masuyama H: Apolipoprotein-B
mRNA-editing complex 3B could be a new potential therapeutic target
in endometriosis. Sci Rep. 14(24968)2024.PubMed/NCBI View Article : Google Scholar
|
|
204
|
Practice bulletin no. 114. Management of
endometriosis. Obstet Gynecol. 116:223–236. 2010.
|
|
205
|
Vlahos N, Vlachos A, Triantafyllidou O,
Vitoratos N and Creatsas G: Continuous versus cyclic use of oral
contraceptives after surgery for symptomatic endometriosis: A
prospective cohort study. Fertil Steril. 100:1337–1342.
2013.PubMed/NCBI View Article : Google Scholar
|
|
206
|
Jewson M, Purohit P and Lumsden MA:
Progesterone and abnormal uterine bleeding/menstrual disorders.
Best Pract Res Clin Obstet Gynaecol. 69:62–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
207
|
Rafique S and Decherney AH: Medical
management of endometriosis. Clin Obstet Gynecol. 60:485–496.
2017.PubMed/NCBI View Article : Google Scholar
|
|
208
|
Resta C, Moustogiannis A, Chatzinikita E,
Malligiannis Ntalianis D, Malligiannis Ntalianis K, Philippou A,
Koutsilieris M and Vlahos N: Gonadotropin-releasing hormone
(GnRH)/GnRH receptors and their role in the treatment of
endometriosis. Cureus. 15(e38136)2023.PubMed/NCBI View Article : Google Scholar
|
|
209
|
Surrey ES, Katz-Jaffe M, Kondapalli LV,
Gustofson RL and Schoolcraft WB: GnRH agonist administration prior
to embryo transfer in freeze-all cycles of patients with
endometriosis or aberrant endometrial integrin expression. Reprod
Biomed Online. 35:145–151. 2017.PubMed/NCBI View Article : Google Scholar
|
|
210
|
Leconet W, Chentouf M, du Manoir S,
Chevalier C, Sirvent A, Aït-Arsa I, Busson M, Jarlier M,
Radosevic-Robin N, Theillet C, et al: Therapeutic activity of
Anti-AXL antibody against triple-negative breast cancer
patient-derived xenografts and metastasis. Clin Cancer Res.
23:2806–2816. 2017.PubMed/NCBI View Article : Google Scholar
|
|
211
|
Duan Y, Luo L, Qiao C, Li X, Wang J, Liu
H, Zhou T, Shen B, Lv M and Feng J: A novel human anti-AXL
monoclonal antibody attenuates tumour cell migration. Scand J
Immunol. 90(e12777)2019.PubMed/NCBI View Article : Google Scholar
|
|
212
|
Colavito SA: AXL as a target in breast
cancer therapy. J Oncol. 2020(5291952)2020.PubMed/NCBI View Article : Google Scholar
|
|
213
|
Davis JD, Bravo Padros M, Conrado DJ,
Ganguly S, Guan X, Hassan HE, Hazra A, Irvin SC, Jayachandran P,
Kosloski MP, et al: Subcutaneous administration of monoclonal
antibodies: Pharmacology, delivery, immunogenicity, and learnings
from applications to clinical development. Clin Pharmacol Ther.
115:422–439. 2024.PubMed/NCBI View Article : Google Scholar
|
|
214
|
Ye X, Li Y, Stawicki S, Couto S,
Eastham-Anderson J, Kallop D, Weimer R, Wu Y and Pei L: An anti-Axl
monoclonal antibody attenuates xenograft tumor growth and enhances
the effect of multiple anticancer therapies. Oncogene.
29:5254–5264. 2010.PubMed/NCBI View Article : Google Scholar
|
|
215
|
Netcharoensirisuk P, Abrahamian C, Tang R,
Chen CC, Rosato AS, Beyers W, Chao YK, Filippini A, Di Pietro S,
Bartel K, et al: Flavonoids increase melanin production and reduce
proliferation, migration and invasion of melanoma cells by blocking
endolysosomal/melanosomal TPC2. Sci Rep. 11(8515)2021.PubMed/NCBI View Article : Google Scholar
|
|
216
|
Li M, Guo T, Lin J, Huang X, Ke Q, Wu Y,
Fang C and Hu C: Curcumin inhibits the invasion and metastasis of
triple negative breast cancer via Hedgehog/Gli1 signaling pathway.
J Ethnopharmacol. 283(114689)2022.PubMed/NCBI View Article : Google Scholar
|
|
217
|
Yoo JY, Kim TH, Shin JH, Marquardt RM,
Müller U, Fazleabas AT, Young SL, Lessey BA, Yoon HG and Jeong JW:
Loss of MIG-6 results in endometrial progesterone resistance via
ERBB2. Nat Commun. 13(1101)2022.PubMed/NCBI View Article : Google Scholar
|
|
218
|
Jiang Y, Palomares AR, Munoz P, Nalvarte
I, Acharya G, Inzunza J, Varshney M and Rodriguez-Wallberg KA:
Proof-of-concept for long-term human endometrial epithelial
organoids in modeling menstrual cycle responses. Cells.
13(1811)2024.PubMed/NCBI View Article : Google Scholar
|
|
219
|
Abdolmaleki A, Jalili C, Mansouri K and
Bakhtiari M: New rat to mouse xenograft transplantation of
endometrium as a model of human endometriosis. Animal Model Exp
Med. 4:268–277. 2021.PubMed/NCBI View Article : Google Scholar
|
|
220
|
Ma J, Liao Z, Li J, Li X, Guo H, Zhong Q,
Huang J, Shuai X and Chen S: A cRGD-modified liposome for targeted
delivery of artesunate to inhibit angiogenesis in endometriosis.
Biomater Sci. 13:1045–1058. 2025.PubMed/NCBI View Article : Google Scholar
|
|
221
|
Abhang A and Burgess DJ: Recent
advancements and future applications of intrauterine drug delivery
systems. Expert Opin Drug Deliv. 22:841–856. 2025.PubMed/NCBI View Article : Google Scholar
|