Introduction
Antimicrobial resistance (AMR) poses a significant
global health threat, driving morbidity and mortality worldwide
(1,2). The emergence and spread of
multidrug-resistant (MDR) Gram-negative bacteria, particularly
carbapenem-resistant Enterobacterales, Acinetobacter
baumannii (A. baumannii) and Pseudomonas
aeruginosa (P. aeruginosa), are of critical concern,
severely limiting therapeutic options for serious infections
(1-4).
The overuse of broad-spectrum antibiotics, including carbapenems,
has contributed significantly to this challenge.
In response to this urgent need, new antimicrobial
agents have been developed. Cefiderocol is a novel siderophore
cephalosporin designed to overcome several common resistance
mechanisms in Gram-negative bacteria (3). Its unique mechanism of action,
utilizing bacterial iron transport systems for cell entry, allows
it activity against numerous difficult-to-treat resistant (DTR)
pathogens, including those producing carbapenemases (5,6).
While pivotal clinical trials such as CREDIBLE-CR
(7), APEKS-NP (8) and APEKS-cUTI (9) have provided essential data on
cefiderocol's efficacy and safety in specific patient populations,
understanding its performance in routine clinical practice is
crucial. Real-world settings often involve patients with complex
comorbidities, polymicrobial infections, and prior treatment
failures who may not have been fully represented in trial cohorts
(3).
Therefore, the primary aim of the present study was
to evaluate the real-life efficacy and safety of cefiderocol in
treating patients hospitalized at the University Hospital ‘Gaetano
Martino’ in Messina, Italy. The present study specifically focused
on patients with proven mono- or poly-microbial infections caused
by MDR/DTR Gram-negative bacteria, for whom cefiderocol was
prescribed due to the failure of previous therapeutic lines or the
absence of other suitable antibiotic options. The present
retrospective observational study examines clinical outcomes and
tolerability in this challenging patient population.
Materials and methods
Study design and setting
A monocentric, retrospective observational study was
conducted at the University Hospital ‘Gaetano Martino’ in Messina,
Italy, from July 2021 to April 2022. The present study was approved
by the Provincial Ethics Committee of Catania (approval no.
101/CECT2; Catania, Italy), with authorization from the local
institution. All participants provided written informed consent to
participate in the study.
Study objectives
The primary objectives were assessment of
in-hospital mortality and evaluating clinical and/or laboratory
resolution of infection following cefiderocol treatment. The
secondary objectives included: Evaluation of adverse drug reactions
related to cefiderocol, description of hospital epidemiology of MDR
organisms, and analysis of associations between MDR colonization at
admission and subsequent development of healthcare-associated
infections.
Inclusion and exclusion criteria
Adult patients (≥18 years) who: i) Were hospitalized
at the study center during the observation period; ii) received
cefiderocol for ≥48 h for any suspected or microbiologically
confirmed infection; and iii) had complete medical records and
microbiological data available. Pregnant women, patients who
received cefiderocol for <48 h and patients in whom cefiderocol
was prescribed but not administered were excluded.
Antimicrobial stewardship and
indication for cefiderocol
Cefiderocol use was restricted to the Infectious
Diseases Unit as part of the hospital's antimicrobial stewardship
protocol. It was prescribed in accordance with the drug's technical
sheet and local stewardship guidelines in the following cases:
Confirmed infection by carbapenem-resistant Gram-negative organisms
based on antibiogram; and severe infection with strong clinical
suspicion of carbapenem resistance, in the presence of: i)
documented failure of prior carbapenem-based treatment; ii) known
rectal colonization by carbapenem-resistant organisms; and iii)
known endemicity of carbapenem-resistant Gram-negative bacteria in
the treating ward.
Data collection and definitions
Patient data (including demographics, comorbidities,
microbiological results, treatment regimens and outcomes) were
extracted from electronic medical records and Infectious Diseases
consultation logs using a structured Excel database.
Microbiological diagnoses were established via FilmArray PCR panels
and/or standard cultures of relevant biological specimens (for
example, blood, urine and bronchoalveolar lavage). Organisms were
classified as MDR, extensively drug-resistant (XDR), or
pan-drug-resistant (PDR) based on the criteria of Magiorakos et
al (10).
Cefiderocol was administered at 2 g every 8 h
(adjusted in renal impairment), in accordance with the drug's
prescribing information. Clinical success was defined as the
complete resolution or significant improvement of signs, symptoms
and laboratory parameters of infection (for example, normalization
of white blood cell count and C-reactive protein) at the end of
cefiderocol therapy. Clinical failure was defined as death
attributable to the infection, persistence or worsening of
infection-related signs and symptoms, or the need to discontinue
cefiderocol due to a lack of efficacy.
Statistical analysis
All statistical analyses were performed using IBM
SPSS Statistics for Windows, Version 29.0 (IBM Corp.). Categorical
variables were summarized as absolute frequencies and percentages,
while continuous variables were assessed for normality using the
Shapiro-Wilk test. Normally distributed variables were expressed as
the mean ± standard deviation (SD), and non-normally distributed
variables were presented as median and interquartile range (IQR).
Group comparisons were conducted using the Chi-square test or
Fisher's exact test for categorical variables, and the unpaired
Student's t-test or Mann-Whitney U test for continuous variables,
as appropriate.
To explore factors independently associated with
in-hospital mortality, a multivariate logistic regression analysis
was performed, including variables that showed a P<0.10 in
univariate analysis. Results were reported as odds ratios (ORs)
with 95% confidence intervals (CIs). Missing data were handled
through case-wise deletion. A two-tailed P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
During the study period, cefiderocol was prescribed
to 65 patients. A total of 10 patients were excluded: 8 received
treatment for <48 h, and 2 were not administered the drug.
Therefore, 55 patients were included in the final analysis. A total
of 2 patients received cefiderocol twice (one in separate hospital
admissions, the other during a single admission).
The median age was 65.0 years (IQR 49.0-73.5), and
67.3% were male (n=37). Most patients had severe illness: 43
(78.2%) required intensive care unit (ICU) admission-15 (34.9%)
directly and 28 (65.1%) following transfer from other hospital
wards. Mechanical ventilation was needed in 32 patients (58.2%): 8
(14.6%) received non-invasive and 24 (43.6%) invasive
ventilation.
Infection types
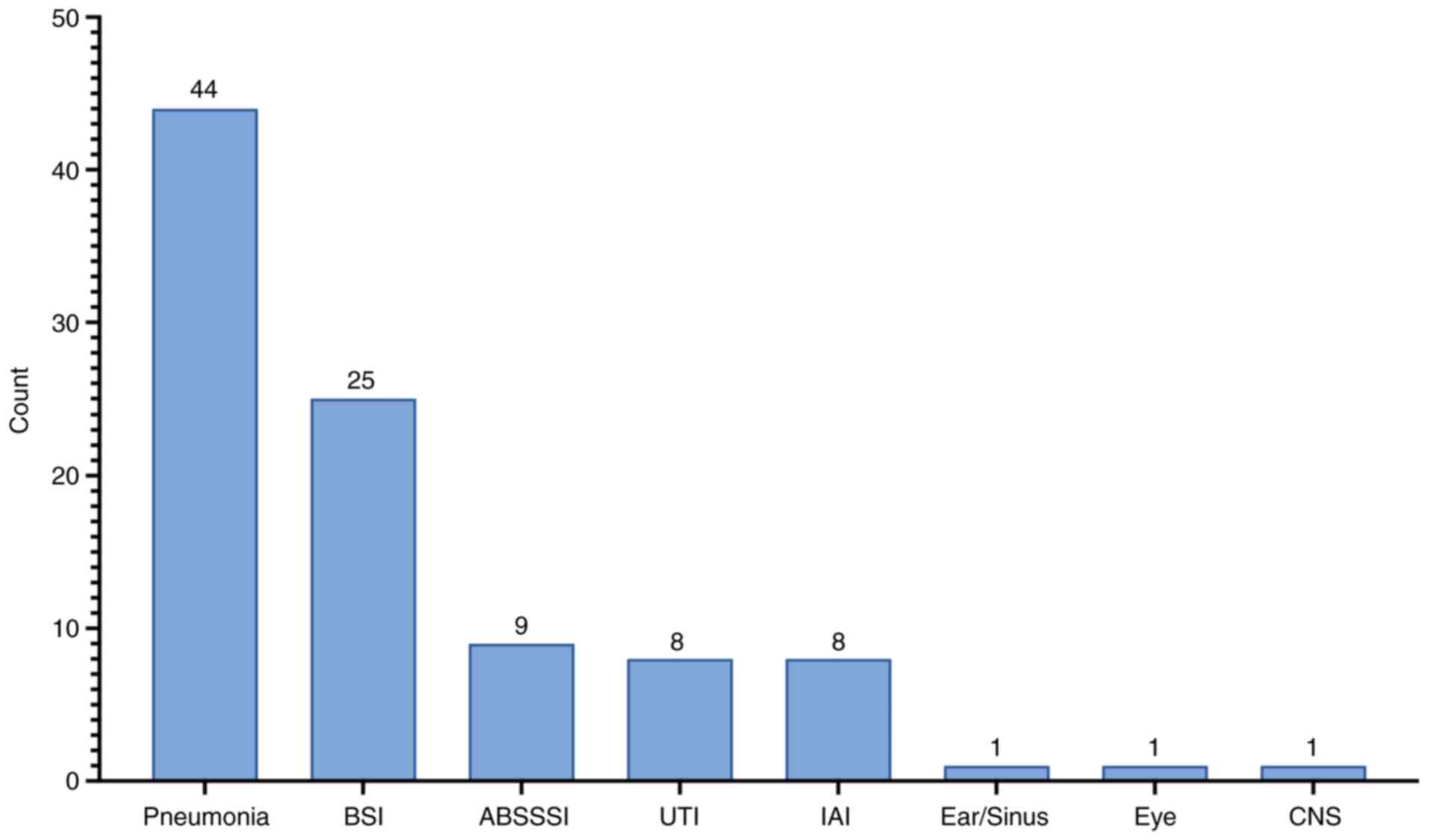
The most common infection treated was pneumonia
(80.0%, n=44), followed by bloodstream infections (BSI; 45.5%,
n=25), acute bacterial skin and soft tissue infections (16.4%,
n=9), intra-abdominal infections (IAI; 14.5%, n=8) and urinary
tract infections (UTI; 14.5%, n=8) (Fig. 1). One patient experienced cystic
fibrosis reactivation.
Microbiology and resistance
mechanisms
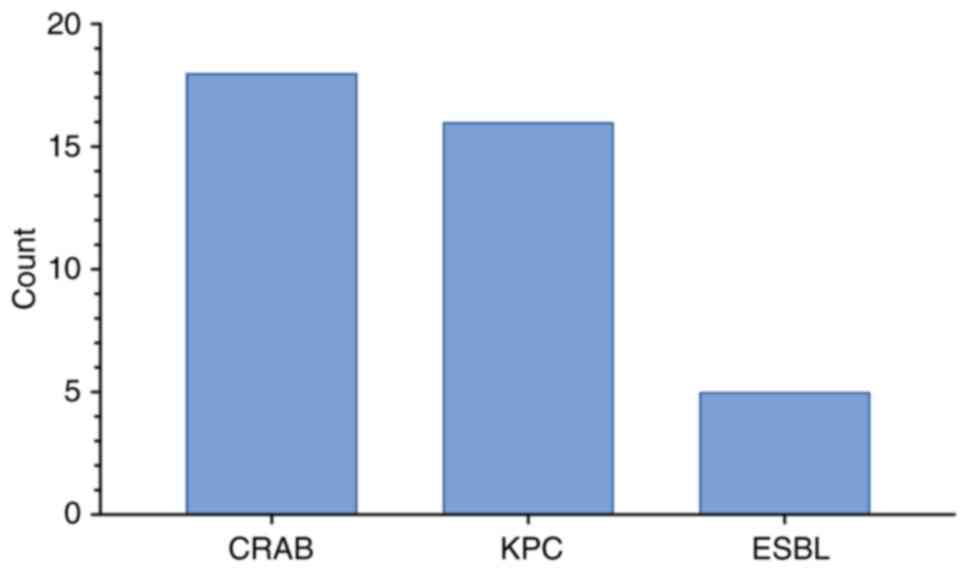
Among the 55 patients, 48 (87.3%) had confirmed
A. baumannii infection, 73.7% of which were XDR.
Additionally, 5 (10.5%) isolates were possibly PDR, 1 (2.1%) was
confirmed PDR, and 2 (4.2%) were not MDR. A total of 5 isolates
were identified via FilmArray only and not cultured.
Co-infections were frequent: 36 of the A.
baumannii cases involved at least one additional pathogen,
including P. aeruginosa, Klebsiella pneumoniae (K.
pneumoniae), Escherichia coli and Staphylococcus
aureus. The most frequently observed resistance profiles were
carbapenem-resistant A. baumannii, K. pneumoniae
carbapenemase-producing K. pneumoniae and extended-spectrum
beta-lactamase-producing Enterobacterales (Fig. 2).
Colonization and prior
antibiotics
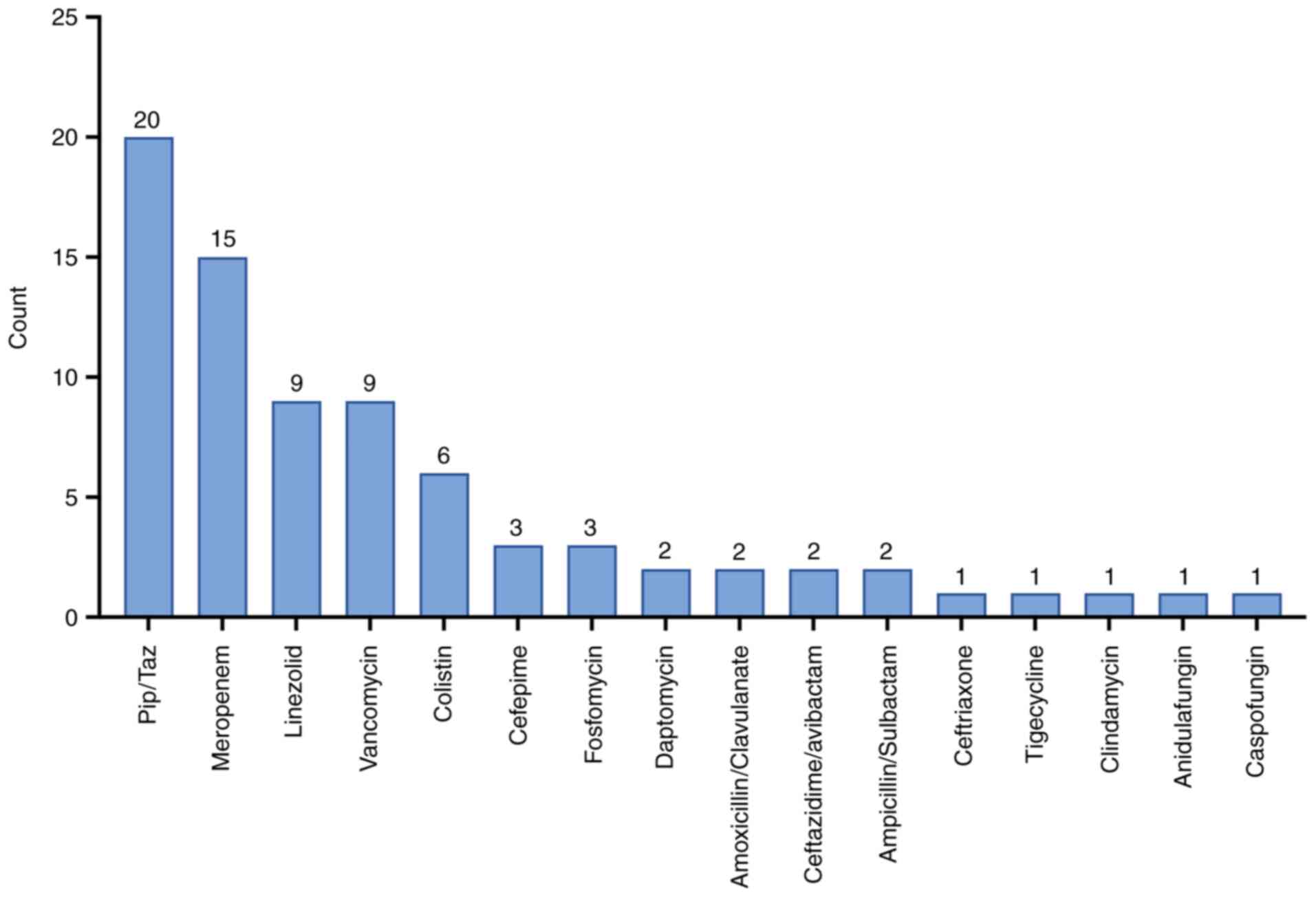
A total of 31 patients (57.4%) were colonized with
MDR organisms on admission, based on positive rectal swabs.
Colonization was significantly associated with increased mortality
(P=0.028). Additionally, 40 patients (72.7%) received empiric
in-hospital antibiotics prior to cefiderocol (Fig. 3).
Comorbidities and risk factors
A total of 30 patients (54.5%) had ≥2 comorbidities;
this was not significantly associated with mortality (P=0.126). The
most common comorbidities were cardiovascular disease (67.3%),
COVID-19 (52.7%), lymphopenia (47.3%), thrombocytopenia (34.5%) and
chronic kidney disease (29.1%). The median Charlson Comorbidity
Index was 4, indicating an estimated 10-year survival of 53%
(Table I).
 | Table IComorbidities in the population
studied. |
Table I
Comorbidities in the population
studied.
| Comorbidities | n | Percentage % |
|---|
| Cardiovascular
disorder | 37 | 67.3 |
| COVID-19 | 29 | 52.7 |
| Lymphopenia | 26 | 47.3 |
| Thrombocytopenia | 19 | 34.5 |
| Hemodialysis | 17 | 30.9 |
| Chronic kidney
failure | 16 | 29.1 |
| Diabetes | 15 | 27.3 |
| Cancer | 5 | 9.1 |
| Chronic obstructive
pulmonary disease | 5 | 9.1 |
| Neutropenia | 4 | 7.3 |
| Hematologic
cancer | 3 | 5.5 |
| Chronic liver
failure | 2 | 3.6 |
| HIV infection | 1 | 1.8 |
Clinical outcomes
The overall in-hospital mortality was 47.3% (n=26).
Mortality was significantly associated with septic shock (P=0.001)
and MDR colonization (P=0.028), but not with age (P=0.054),
COVID-19 status (P=0.215), or number of comorbidities (P=0.126).
Septic shock occurred in 30 patients (54.5%) with a median duration
of 5 days (IQR 0-15).
Median hospital length of stay was 40 days (IQR
28.5-60.0), significantly shorter in non-survivors (31.5 vs. 57.0
days; P=0.004), likely reflecting early death. Treatment success
(defined as clinical/laboratory improvement) varied by infection
site: ventilator-associated pneumonia (VAP), 40%; BSI, 66.7%; UTI,
50%; skin and soft tissue infections (SSTI), 100% and IAI, 50%.
Treatment details and adverse
events
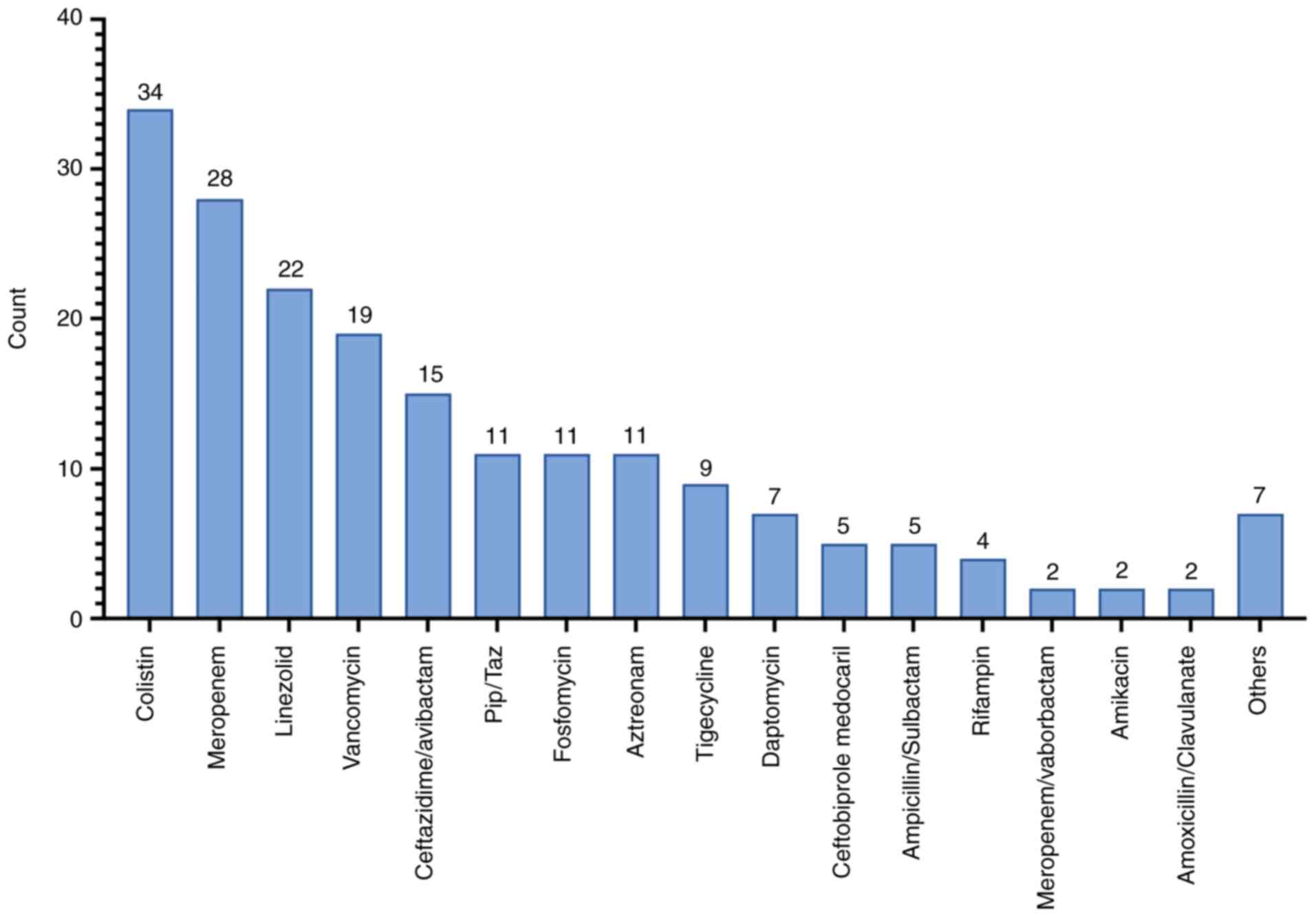
Cefiderocol was administered for a median of 14 days
(IQR 8.1-16.7). Only 2 patients (3.6%) received cefiderocol in
monotherapy. The majority received combination therapy, with
colistin used in 34 cases (61.8%) (Fig.
4). There was no significant difference in mortality between
patients treated with or without colistin (P=0.104). The median
number of antibiotics (excluding cefiderocol) used per patient was
3 (IQR 2-5). No serious cefiderocol-related adverse events were
reported. Minor adverse effects-such as rash, thrombocytopenia,
joint pain and sore throat-occurred in a few patients but did not
lead to drug discontinuation.
Discussion
The present retrospective observational study
evaluated the real-life use of cefiderocol in a critically ill
population infected with MDR Gram-negative bacteria, predominantly
A. baumannii. The current findings suggest that cefiderocol
may be a valuable therapeutic option in patients with limited
treatment alternatives, particularly for bloodstream and soft
tissue infections.
Clinical success was achieved in 66.7% of patients
with BSI, closely aligning with findings from the CREDIBLE-CR trial
(65.5%) (7) and the cohort study by
Falcone et al (11).
Similarly, a 100% success rate in skin and soft tissue infections
(SSTI) mirrors the strong in vitro activity of cefiderocol
against carbapenem-resistant pathogens documented in multiple
studies (12,13). Conversely, the lower efficacy
observed in VAP (40% success) reflects the ongoing challenges in
treating this condition; this high mortality rate highlights an
urgent need for optimized therapeutic strategies for VAP caused by
MDR pathogens. Future studies should investigate whether
alternative dosing regimens, such as prolonged or continuous
infusion of cefiderocol, could improve
pharmacokinetic/pharmacodynamic target attainment in the lung
parenchyma and lead to improved clinical outcomes (14).
A key finding was that cefiderocol was predominantly
used as part of a combination regimen (96.4%), with colistin being
the most frequent partner antibiotic. This reflects a common
clinical practice in treating severe infections caused by XDR
pathogens such as A. baumannii, where combination therapy is
often employed to achieve potential synergistic effects and
mitigate the risk of resistance development (15,16).
While the addition of colistin did not show a statistically
significant mortality benefit in our analysis (P=0.104), the choice
is often guided by the severity of illness and local resistance
patterns, despite ongoing debates regarding colistin's efficacy and
potential toxicity, particularly its limited pulmonary
penetration.
The microbiological landscape in our cohort was
dominated by A. baumannii (87.3%), a significantly higher
prevalence than in previous trials such as CREDIBLE-CR (46%)
(7), APEKS-NP (16%) (8) and Meschiari et al (5.9%)
(17). The high rate of ICU
admission and mechanical ventilation suggest these infections were
primarily nosocomial, with VAP being a major source.
Notably, over 70% of the isolates were classified as
XDR, with several cases potentially PDR. These findings emphasize
the severity of infections managed in our setting and underscore
the urgent need for effective antimicrobial options.
Septic shock was observed in 54.5% of patients and
was significantly associated with mortality (P=0.001), consistent
with global data estimating sepsis-related mortality at 30-40%
(18). Colonization with MDR
organisms at admission also associated with worse outcomes
(P=0.028), highlighting the prognostic value of routine rectal
swabs and active surveillance cultures in ICU settings.
The present study also sheds light on the
positioning of cefiderocol in clinical practice. The indications
for its use included both microbiologically confirmed infections
and cases of strong clinical suspicion based on risk factors such
as known colonization. This suggests a role for cefiderocol not
only as a targeted therapy for confirmed DTR pathogens but also as
a highly selective empiric option in critically ill patients with a
high pre-test probability of having such an infection, particularly
when prior broad-spectrum antibiotics have failed.
Cefiderocol demonstrated favorable tolerability in
our cohort, with no serious adverse effects leading to
discontinuation. Minor side effects (for example, rash,
thrombocytopenia and myalgia) were infrequent and self-limiting.
This safety profile aligns with published studies from randomized
trials and pharmacokinetic-pharmacodynamic studies (7,19). Our
findings are broadly consistent with prior clinical trials and
real-world studies (20-24).
Despite these encouraging observations, the present
study has important limitations. Its retrospective, single-center
nature limits external validity and introduces selection bias. The
absence of detailed molecular resistance testing (such as16S rRNA
sequencing) and minimum inhibitory concentration (MIC) data
restricts our ability to correlate outcomes with specific
resistance mechanisms and precise susceptibility profiles.
Additionally, the lack of a comparator arm precludes evaluation of
cefiderocol's relative efficacy. Lastly, the small sample size,
particularly within infection subgroups, limits the statistical
power of some analyses.
Furthermore, due to the retrospective data
collection, the precise timing of cefiderocol initiation relative
to hospital admission or infection onset could not be consistently
determined, although it was generally used after failure of prior
therapies. Lastly, the small sample size, particularly within
infection subgroups, limits the statistical power of some analyses.
Nevertheless, our study adds valuable real-life evidence to the
increasing body of literature supporting cefiderocol use in
critically ill patients with few alternative treatment options. In
particular, its observed efficacy in BSI and SSTI, combined with
its tolerability, support its consideration in the management of
infections caused by XDR A. baumannii and other MDR
Gram-negative bacteria (25).
Future prospective, multicenter studies are needed to further
define optimal patient selection, combination therapy strategies,
and the potential for resistance emergence. Moreover, long-term
strategies to combat AMR will require a multifaceted approach,
including robust antimicrobial stewardship, infection control, and
the exploration of novel therapeutic paradigms beyond conventional
antibiotics.
In conclusion, in the present real-life,
retrospective study involving critically ill patients with
infections caused by MDR Gram-negative bacteria-primarily A.
baumannii-cefiderocol demonstrated promising efficacy and
favorable tolerability, particularly in bloodstream and soft tissue
infections. Despite the complexity of the cohort, which included a
high prevalence of septic shock, ICU admission and XDR pathogens,
clinical outcomes were comparable to those observed in controlled
trials and recent observational data. Colonization with MDR
organisms and septic shock were independently associated with
higher mortality, emphasizing the importance of early
identification and targeted antimicrobial therapy in high-risk
settings. Cefiderocol was well tolerated, with no serious adverse
events reported, further supporting its use in fragile and
treatment-limited populations. While the retrospective,
single-center design and lack of MIC data limit the
generalizability of the present findings, our results contribute to
the increasing body of real-world evidence supporting the role of
cefiderocol as a valuable treatment option in severe infections
caused by DTR Gram-negative bacteria. Future prospective,
multicenter studies are needed to define optimal therapeutic
strategies, including the role of combination therapy and
resistance prevention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AM, EVR and GFP conceived the study. AM, EVR, CI and
GN designed the study. MV, CM, MC and AEC collected the data. EVR
and YR analyzed data. AM and EVR wrote the manuscript. GN and GFP
reviewed the manuscript. GFP and CI supervised the study. All
authors read and approved the final version of the manuscript. AM
and GN confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All participants provided written informed consent
to participate in the study. The present study was conducted in
accordance with the Declaration of Helsinki and was approved by the
Provincial Ethics Committee of Catania (approval no. 101/CECT2;
Catania, Italy).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marino A, Maniaci A, Lentini M, Ronsivalle
S, Nunnari G, Cocuzza S, Parisi FM, Cacopardo B, Lavalle S and La
Via L: The Global burden of multidrug-resistant bacteria.
Epidemiologia (Basel). 6(21)2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tiseo G, Galfo V, Carbonara S, Marino A,
Di Caprio G, Carretta A, Mularoni A, Mariani MF, Maraolo AE, Scotto
R, et al: Bacteremic nosocomial pneumonia caused by Gram-negative
bacilli: Results from the nationwide ALARICO study in Italy.
Infection. 53:1041–1050. 2025.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Giacobbe DR, Labate L, Russo Artimagnella
C, Marelli C, Signori A, Di Pilato V, Aldieri C, Bandera A, Briano
F, Cacopardo B, et al: Use of cefiderocol in adult patients:
Descriptive analysis from a prospective, multicenter, cohort study.
Infect Dis Ther. 13:1929–1948. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Antimicrobial Resistance Collaborators.
Global burden of bacterial antimicrobial resistance in 2019: A
systematic analysis. Lancet. 399:629–655. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stracquadanio S, Nicolosi A, Marino A,
Calvo M and Stefani S: Issues with cefiderocol testing: Comparing
commercial methods to broth microdilution in iron-depleted
medium-analyses of the performances, ATU, and trailing effect
according to EUCAST initial and revised interpretation criteria.
Diagnostics (Basel). 14(2318)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stracquadanio S, Nicolosi A, Privitera GF,
Massimino M, Marino A, Bongiorno D and Stefani S: Role of
transcriptomic and genomic analyses in improving the comprehension
of cefiderocol activity in Acinetobacter baumannii. mSphere.
9(e0061723)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bassetti M, Echols R, Matsunaga Y, Ariyasu
M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, et al:
Efficacy and safety of cefiderocol or best available therapy for
the treatment of serious infections caused by carbapenem-resistant
Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label,
multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet
Infect Dis. 21:226–240. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wunderink RG, Matsunaga Y, Ariyasu M,
Clevenbergh P, Echols R, Kaye KS, Kollef M, Menon A, Pogue JM,
Shorr AF, et al: Cefiderocol versus high-dose, extended-infusion
meropenem for the treatment of Gram-negative nosocomial pneumonia
(APEKS-NP): a randomised, double-blind, phase 3, non-inferiority
trial. Lancet Infect Dis. 21:213–225. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Portsmouth S, van Veenhuyzen D, Echols R,
Machida M, Ferreira JCA, Ariyasu M, Tenke P and Den Nagata T:
Cefiderocol versus imipenem-cilastatin for the treatment of
complicated urinary tract infections caused by Gram-negative
uropathogens: a phase 2, randomised, double-blind, non-inferiority
trial. Lancet Infect Dis. 18:1319–1328. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Magiorakos AP, Srinivasan A, Carey RB,
Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter
G, Olsson-Liljequist B, et al: Multidrug-resistant, extensively
drug-resistant and pandrug-resistant bacteria: An international
expert proposal for interim standard definitions for acquired
resistance. Clin Microbiol Infect. 18:268–281. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Falcone M, Tiseo G, Nicastro M, Leonildi
A, Vecchione A, Casella C, Forfori F, Malacarne P, Guarracino F,
Barnini S and Menichetti F: Cefiderocol as Rescue Therapy for
Acinetobacter baumannii and Other Carbapenem-resistant
Gram-negative Infections in Intensive Care Unit Patients. Clin
Infect Dis. 72:2021–2024. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu PY, Ko WC, Lee WS, Lu PL, Chen YH,
Cheng SH, Lu MC, Lin CY, Wu TS, Yen MY, et al: In vitro activity of
cefiderocol, cefepime/enmetazobactam, cefepime/zidebactam,
eravacycline, omadacycline, and other comparative agents against
carbapenem-non-susceptible Pseudomonas aeruginosa and
Acinetobacter baumannii isolates associated from bloodstream
infection in Taiwan between 2018-2020. J Microbiol Immunol Infect.
55:888–895. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bianco G, Boattini M, Comini S, Iannaccone
M, Casale R, Allizond V, Babrbui AM, Banche G, Cavallo R and Costa
C: Activity of ceftolozane-tazobactam, ceftazidime-avibactam,
meropenem-vaborbactam, cefiderocol and comparators against
Gram-negative organisms causing bloodstream infections in Northern
Italy (2019–2021): emergence of complex resistance phenotypes. J
Chemother. 34:302–310. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jabbour JF, Sharara SL and Kanj SS:
Treatment of multidrug-resistant Gram-negative skin and soft tissue
infections. Curr Opin Infect Dis. 33:146–154. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Palermo G, Medaglia AA, Pipitò L, Rubino
R, Costantini M, Accomando S, Giammanco GM and Cascio A:
Cefiderocol efficacy in a real-life setting: Single-centre
retrospective study. Antibiotics (Basel). 12(746)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Clancy CJ, Cornely OA, Slover CM, Nguyen
ST, Kung FH, Verardi S, Longshaw CM, Marcella S and Cai B: P-1475.
Real-world effectiveness and safety of cefiderocol in the treatment
of patients with serious Gram-negative bacterial infections:
Results of the PROVE chart review study. Open Forum Infect Dis. 12
(Suppl 1)(ofae631.1645)2025.
|
|
17
|
Meschiari M, Volpi S, Faltoni M, Dolci G,
Orlando G, Franceschini E, Menozzi M, Sarti M, Del Fabro G,
Fumarola B, et al: Real-life experience with compassionate use of
cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa
(DTR-P) infections. JAC Antimicrobial Resist.
3(dlab188)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bauer M, Gerlach H, Vogelmann T, Preissing
F, Stiefel J and Adam D: Mortality in sepsis and septic shock in
Europe, North America and Australia between 2009 and 2019- results
from a systematic review and meta-analysis. Crit Care.
24(239)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Azanza Perea JR and Sádaba Díaz de Rada B:
Pharmacokinetics/pharmacodynamics and tolerability of cefiderocol
in the clinical setting. Rev Esp Quimioter. 35 (Suppl 2):S28–S34.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hsueh SC, Chao CM, Wang CY, Lai CC and
Chen CH: Clinical efficacy and safety of cefiderocol in the
treatment of acute bacterial infections: A systematic review and
meta-analysis of randomised controlled trials. J Glob Antimicrob
Resist. 24:376–382. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Theriault N, Tillotson G and Sandrock CE:
Global travel and Gram-negative bacterial resistance; implications
on clinical management. Expert Rev Anti Infect Ther. 19:181–196.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Paterson DL, Kinoshita M, Baba T, Echols R
and Portsmouth S: Outcomes with cefiderocol treatment in patients
with Bacteraemia enrolled into prospective phase 2 and phase 3
randomised clinical studies. Infect Dis Ther. 11:853–870.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wright H, Harris PNA, Chatfield MD, Lye D,
Henderson A, Harris-Brown T, Donaldson A and Paterson DL:
Investigator-driven randomised controlled trial of cefiderocol
versus standard therapy for healthcare-associated and
hospital-acquired Gram-negative bloodstream infection: Study
protocol (the GAME CHANGER trial): Study protocol for an
open-label, randomised controlled trial. Trials.
22(889)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schmid H, Brown LK, Indrakumar B,
McGarrity O, Hatcher J and Bamford A: Use of cefiderocol in the
management of children with infection or colonization with
multi-drug resistant Gram-negative bacteria: A retrospective,
single-center case series. Pediatr Infect Dis J. 43:772–776.
2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Marino A, Augello E, Stracquadanio S,
Bellanca CM, Cosentino F, Spampinato S, Cantarella G, Bernardini R,
Stefani S, Cacopardo B and Nunnari G: Unveiling the secrets of
Acinetobacter baumannii: Resistance, current treatments, and
future innovations. Int J Mol Sci. 25(6814)2024.PubMed/NCBI View Article : Google Scholar
|