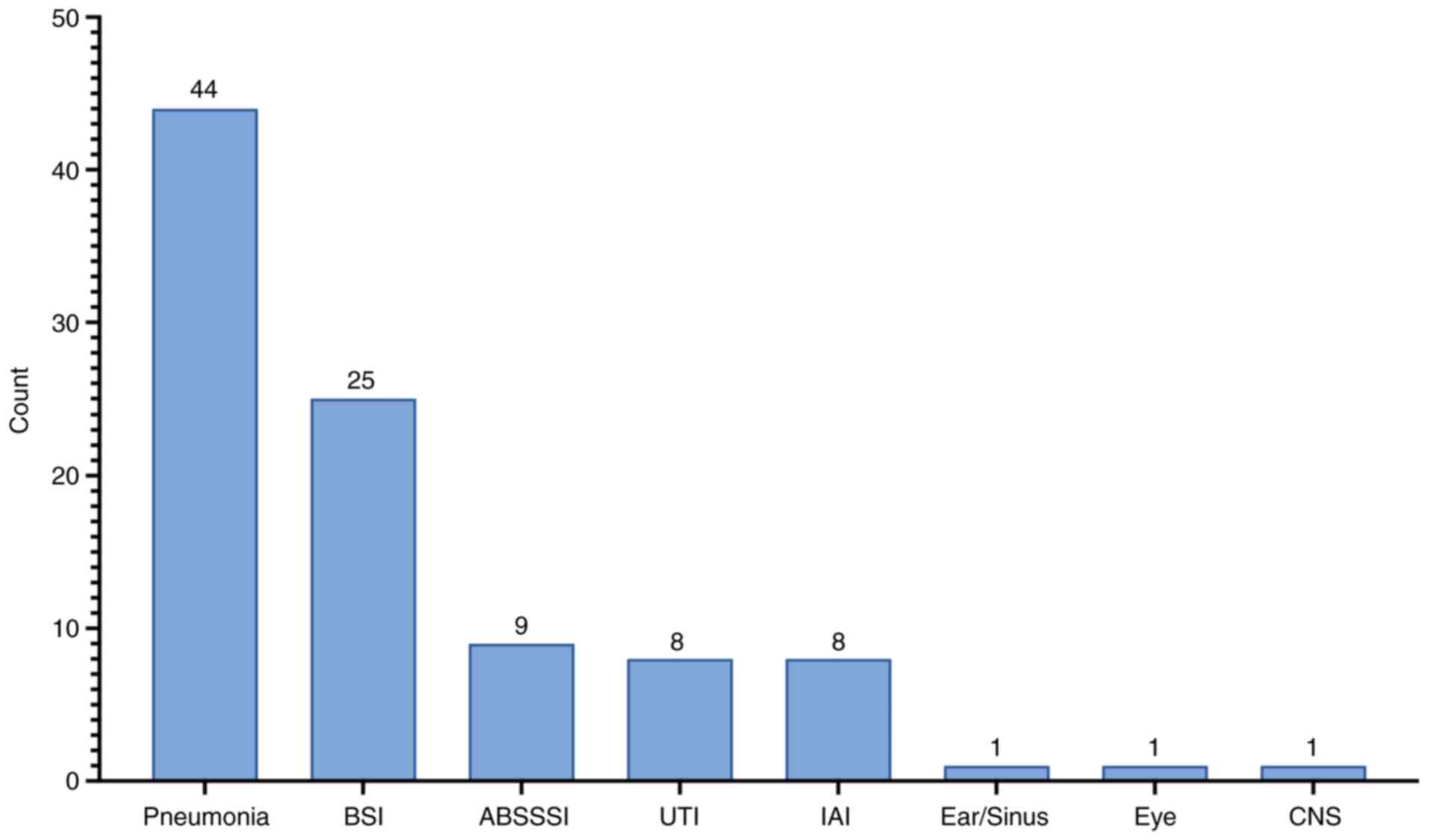
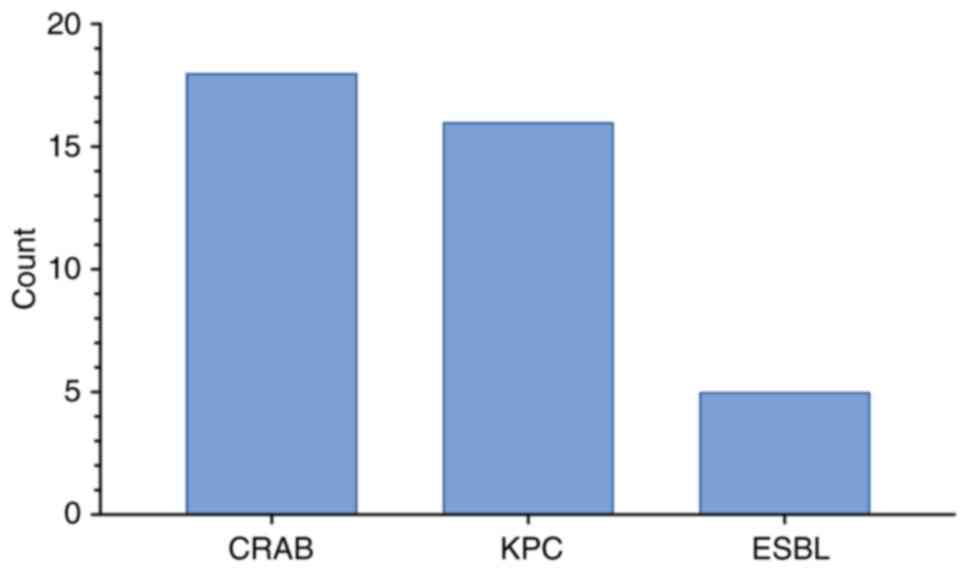
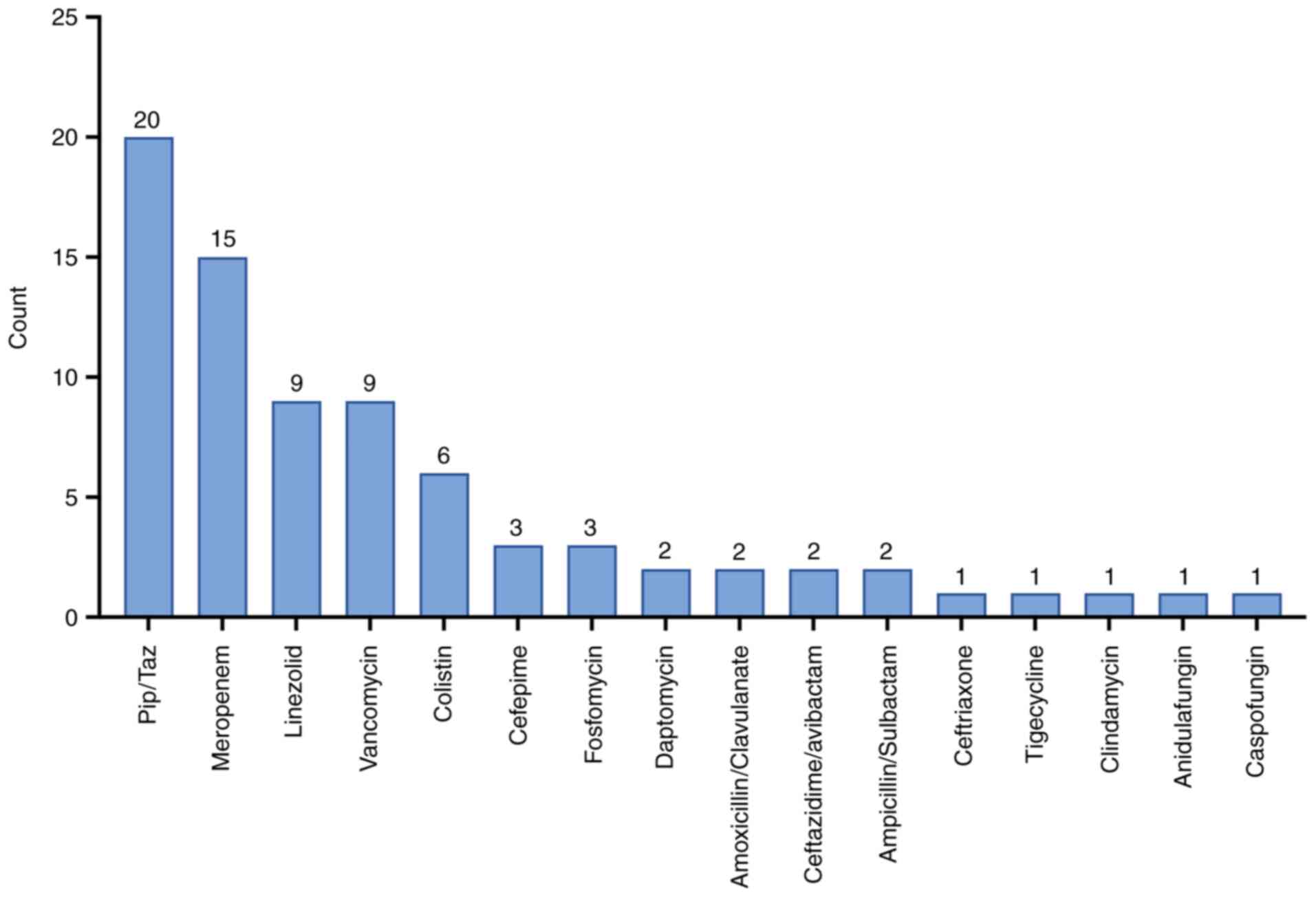
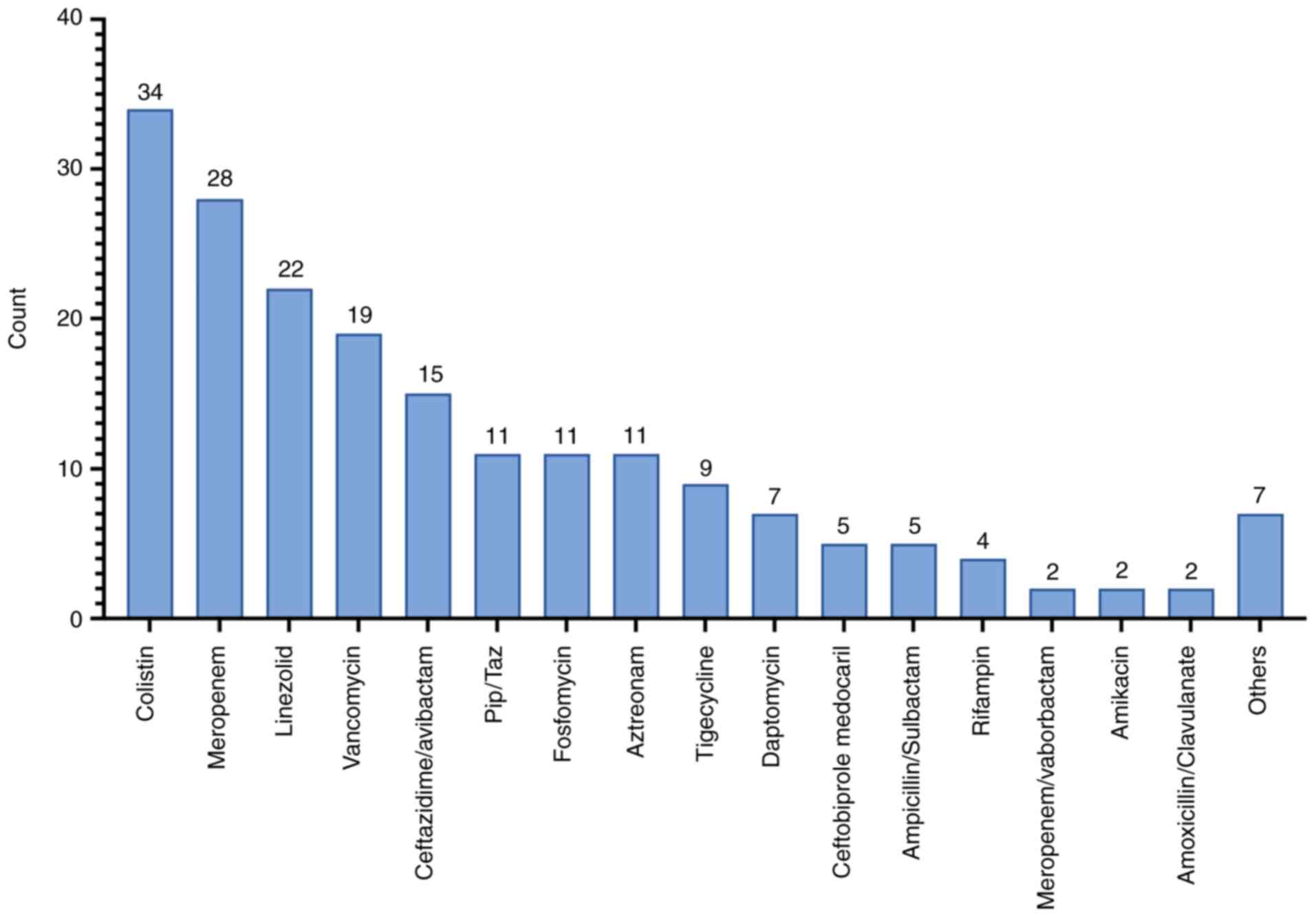
|
1
|
Marino A, Maniaci A, Lentini M, Ronsivalle
S, Nunnari G, Cocuzza S, Parisi FM, Cacopardo B, Lavalle S and La
Via L: The Global burden of multidrug-resistant bacteria.
Epidemiologia (Basel). 6(21)2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tiseo G, Galfo V, Carbonara S, Marino A,
Di Caprio G, Carretta A, Mularoni A, Mariani MF, Maraolo AE, Scotto
R, et al: Bacteremic nosocomial pneumonia caused by Gram-negative
bacilli: Results from the nationwide ALARICO study in Italy.
Infection. 53:1041–1050. 2025.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Giacobbe DR, Labate L, Russo Artimagnella
C, Marelli C, Signori A, Di Pilato V, Aldieri C, Bandera A, Briano
F, Cacopardo B, et al: Use of cefiderocol in adult patients:
Descriptive analysis from a prospective, multicenter, cohort study.
Infect Dis Ther. 13:1929–1948. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Antimicrobial Resistance Collaborators.
Global burden of bacterial antimicrobial resistance in 2019: A
systematic analysis. Lancet. 399:629–655. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stracquadanio S, Nicolosi A, Marino A,
Calvo M and Stefani S: Issues with cefiderocol testing: Comparing
commercial methods to broth microdilution in iron-depleted
medium-analyses of the performances, ATU, and trailing effect
according to EUCAST initial and revised interpretation criteria.
Diagnostics (Basel). 14(2318)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stracquadanio S, Nicolosi A, Privitera GF,
Massimino M, Marino A, Bongiorno D and Stefani S: Role of
transcriptomic and genomic analyses in improving the comprehension
of cefiderocol activity in Acinetobacter baumannii. mSphere.
9(e0061723)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bassetti M, Echols R, Matsunaga Y, Ariyasu
M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, et al:
Efficacy and safety of cefiderocol or best available therapy for
the treatment of serious infections caused by carbapenem-resistant
Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label,
multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet
Infect Dis. 21:226–240. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wunderink RG, Matsunaga Y, Ariyasu M,
Clevenbergh P, Echols R, Kaye KS, Kollef M, Menon A, Pogue JM,
Shorr AF, et al: Cefiderocol versus high-dose, extended-infusion
meropenem for the treatment of Gram-negative nosocomial pneumonia
(APEKS-NP): a randomised, double-blind, phase 3, non-inferiority
trial. Lancet Infect Dis. 21:213–225. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Portsmouth S, van Veenhuyzen D, Echols R,
Machida M, Ferreira JCA, Ariyasu M, Tenke P and Den Nagata T:
Cefiderocol versus imipenem-cilastatin for the treatment of
complicated urinary tract infections caused by Gram-negative
uropathogens: a phase 2, randomised, double-blind, non-inferiority
trial. Lancet Infect Dis. 18:1319–1328. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Magiorakos AP, Srinivasan A, Carey RB,
Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter
G, Olsson-Liljequist B, et al: Multidrug-resistant, extensively
drug-resistant and pandrug-resistant bacteria: An international
expert proposal for interim standard definitions for acquired
resistance. Clin Microbiol Infect. 18:268–281. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Falcone M, Tiseo G, Nicastro M, Leonildi
A, Vecchione A, Casella C, Forfori F, Malacarne P, Guarracino F,
Barnini S and Menichetti F: Cefiderocol as Rescue Therapy for
Acinetobacter baumannii and Other Carbapenem-resistant
Gram-negative Infections in Intensive Care Unit Patients. Clin
Infect Dis. 72:2021–2024. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu PY, Ko WC, Lee WS, Lu PL, Chen YH,
Cheng SH, Lu MC, Lin CY, Wu TS, Yen MY, et al: In vitro activity of
cefiderocol, cefepime/enmetazobactam, cefepime/zidebactam,
eravacycline, omadacycline, and other comparative agents against
carbapenem-non-susceptible Pseudomonas aeruginosa and
Acinetobacter baumannii isolates associated from bloodstream
infection in Taiwan between 2018-2020. J Microbiol Immunol Infect.
55:888–895. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bianco G, Boattini M, Comini S, Iannaccone
M, Casale R, Allizond V, Babrbui AM, Banche G, Cavallo R and Costa
C: Activity of ceftolozane-tazobactam, ceftazidime-avibactam,
meropenem-vaborbactam, cefiderocol and comparators against
Gram-negative organisms causing bloodstream infections in Northern
Italy (2019–2021): emergence of complex resistance phenotypes. J
Chemother. 34:302–310. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jabbour JF, Sharara SL and Kanj SS:
Treatment of multidrug-resistant Gram-negative skin and soft tissue
infections. Curr Opin Infect Dis. 33:146–154. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Palermo G, Medaglia AA, Pipitò L, Rubino
R, Costantini M, Accomando S, Giammanco GM and Cascio A:
Cefiderocol efficacy in a real-life setting: Single-centre
retrospective study. Antibiotics (Basel). 12(746)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Clancy CJ, Cornely OA, Slover CM, Nguyen
ST, Kung FH, Verardi S, Longshaw CM, Marcella S and Cai B: P-1475.
Real-world effectiveness and safety of cefiderocol in the treatment
of patients with serious Gram-negative bacterial infections:
Results of the PROVE chart review study. Open Forum Infect Dis. 12
(Suppl 1)(ofae631.1645)2025.
|
|
17
|
Meschiari M, Volpi S, Faltoni M, Dolci G,
Orlando G, Franceschini E, Menozzi M, Sarti M, Del Fabro G,
Fumarola B, et al: Real-life experience with compassionate use of
cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa
(DTR-P) infections. JAC Antimicrobial Resist.
3(dlab188)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bauer M, Gerlach H, Vogelmann T, Preissing
F, Stiefel J and Adam D: Mortality in sepsis and septic shock in
Europe, North America and Australia between 2009 and 2019- results
from a systematic review and meta-analysis. Crit Care.
24(239)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Azanza Perea JR and Sádaba Díaz de Rada B:
Pharmacokinetics/pharmacodynamics and tolerability of cefiderocol
in the clinical setting. Rev Esp Quimioter. 35 (Suppl 2):S28–S34.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hsueh SC, Chao CM, Wang CY, Lai CC and
Chen CH: Clinical efficacy and safety of cefiderocol in the
treatment of acute bacterial infections: A systematic review and
meta-analysis of randomised controlled trials. J Glob Antimicrob
Resist. 24:376–382. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Theriault N, Tillotson G and Sandrock CE:
Global travel and Gram-negative bacterial resistance; implications
on clinical management. Expert Rev Anti Infect Ther. 19:181–196.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Paterson DL, Kinoshita M, Baba T, Echols R
and Portsmouth S: Outcomes with cefiderocol treatment in patients
with Bacteraemia enrolled into prospective phase 2 and phase 3
randomised clinical studies. Infect Dis Ther. 11:853–870.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wright H, Harris PNA, Chatfield MD, Lye D,
Henderson A, Harris-Brown T, Donaldson A and Paterson DL:
Investigator-driven randomised controlled trial of cefiderocol
versus standard therapy for healthcare-associated and
hospital-acquired Gram-negative bloodstream infection: Study
protocol (the GAME CHANGER trial): Study protocol for an
open-label, randomised controlled trial. Trials.
22(889)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schmid H, Brown LK, Indrakumar B,
McGarrity O, Hatcher J and Bamford A: Use of cefiderocol in the
management of children with infection or colonization with
multi-drug resistant Gram-negative bacteria: A retrospective,
single-center case series. Pediatr Infect Dis J. 43:772–776.
2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Marino A, Augello E, Stracquadanio S,
Bellanca CM, Cosentino F, Spampinato S, Cantarella G, Bernardini R,
Stefani S, Cacopardo B and Nunnari G: Unveiling the secrets of
Acinetobacter baumannii: Resistance, current treatments, and
future innovations. Int J Mol Sci. 25(6814)2024.PubMed/NCBI View Article : Google Scholar
|