Introduction
In 2023, the term used to replace non-alcoholic
fatty liver disease was metabolic dysfunction-associated steatotic
liver disease (MASLD) (1). A new
category, outside pure MASLD, termed metabolic and alcohol
related/associated liver disease (MetALD), was selected to describe
those with metabolic dysfunction-associated steatotic liver
disease. This included patients who consume greater amounts of
alcohol per week (140-350 g/week and 210-420 g/week for women and
men, respectively). Within the MetALD group, there is a continuum
across which the contribution of MASLD and alcohol-associated
(alcohol-related) liver disease (ALD) varies. Part of the renaming
of steatotic liver disease (SLD) is intended to define MetALD as a
specific disease; however, there are no drugs for the treatment of
MetALD as a separate disease (2).
Furthermore, metabolic risk factors and alcohol often coexist in
the same individual, and a synergistic effect of the interaction
markedly increasing the risk of liver disease has been elucidated
in recent years (3). Subsequent
studies investigated the prevalence of MetALD, which ranges from
1.7-17% in the total cohort (3). A
few cohort studies have also assessed the prognosis of this patient
population, with preliminary data suggesting that MetALD has an
intermediate risk of liver fibrosis, decompensation and mortality
among the SLD subtypes (3). MASLD
and alcohol consumption are independent risk factors for recurrence
after endoscopic treatment of esophageal squamous cell carcinoma
(4). The therapeutic significance
of diagnosing MetALD separately from MASLD and ALD remains
unclear.
In patients with MASLD, 7 to 10% weight loss occurs
at the beginning of the lifestyle improvement algorithm (5). The next step in the algorithm is to
increase physical activity; at least 150 min/week of
moderate-intensity physical activity or 75 min/week of
vigorous-intensity physical activity is recommended (5). Alcohol consumption is discouraged, or
abstinence is recommended in cases of advanced fibrosis or
cirrhosis (5). MetALD is an
overlapping disease of MASLD and ALD, and lifestyle changes are
required (2,3). However, the relationship among reduced
alcohol consumption, additional exercise, and weight loss in
patients with MetALD in terms of treatment efficacy remains
unclear.
In adults with MASLD, non-invasive scores based on
combinations of blood tests or combinations of blood tests with
imaging techniques measuring mechanical properties and/or hepatic
fat content should be used for the detection of fibrosis since their
diagnostic accuracy is higher than standard liver enzyme testing
[alanine (ALT) and aspartate aminotransferase (AST)] (5). With the vibration-controlled transient
elastography, liver stiffness (LS) measurement and controlled
attenuation parameter (CAP) values are determined, which allow for
a relatively reliable estimation of the degree of fibrosis and
steatosis, respectively (5). Mac-2
binding protein glycosylation isomer (M2BPGi) has favorable
diagnostic performance for significant and extensive fibrosis in
patients with non-alcoholic fatty liver disease and is an
effective, non-invasive and convenient marker (6). In the present study, lifestyle changes
(weight loss, additional exercise and abstinence from alcohol) were
introduced in patients with MASLD and MetALD, and changes in liver
fibrosis were assessed after 6 months using M2BPGi and LS as
noninvasive tests (NIT).
Materials and methods
Patients
The present study included 100 patients with MASLD
and MetALD who had visited liver disease outpatient clinic in
Nagasaki Harber Medical center between June 2017 and January 2024
for the first time (Table IA). Of
the 100 patients, 70 were women and 30 men. The median age of the
patients was 63 years (range: 83-18 years). These patients did not
include any cases that were anti-HCV antibody positive or HBs
antigen positive. Diagnostic criteria for MASLD and MetALD were
established as previously described (1). A total of 67 patients with MASLD and
33 patients with MetALD were retrospectively evaluated after six
months of lifestyle changes (weight loss, abstinence, and exercise)
(Table IB). The medical records of
100 patients were retrospectively reviewed. All laboratory
measurements were obtained from medical records. Hypertensive
patients (HT) were defined as those who had been taking
antihypertensive medication for at least six months prior to their
visit to our department. Patients with hyperlipidemia (HL) were
defined as those with a fasting low-density lipoprotein (LDL)
cholesterol level of 140 mg/dl or higher or a TG level of 150 mg/dl
or higher. Patients receiving statin therapy were included in the
HL group; however, those receiving fibrate therapy were excluded.
The diabetes group (diabetes mellitus; DM) consisted of patients
with fasting serum glucose ≥100 mg/dl or HbA1c ≥5.7%, or with a
diagnosis of type 2 diabetes or being treated for type 2 diabetes.
The DM group did not include patients treated with sodium-glucose
cotransporter 2 inhibitors, incretin preparations containing
glucagon-like peptide 1 agonists, or metformin.
 | Table IClinical characteristics. |
Table I
Clinical characteristics.
| A, Base line |
|---|
| | MASLD | MetALD | P-value |
|---|
| Sex | | | |
|
Female | 55 (82.1) | 15 (45.5) | 0.00017 |
|
Male | 12 (17.9) | 18 (54.5) | |
| Age, years | | | |
|
N | 67 | 33 | 0.38905 |
|
Me
(Q1~Q3) | 62.8
(51.6~71.6) | 65.1
(55.3~70.9) | |
| ALDG | | | |
|
0 | 62 (92.5) | 0 (0.0) | <0.00001 |
|
Alcoholic
consumption | 5 (7.5) | 33 (100.0) | |
| BMIG | | | |
|
Normal | 3 (4.5) | 5 (15.2) | 0.11092 |
|
Obesity | 64 (95.5) | 28 (84.8) | |
| BMI | | | |
|
N | 67 | 33 | 0.22226 |
|
Me (Q1,
Q3) | 27.2 (25.8,
30.1) | 27.3 (24.1,
29.8) | |
| AST | | | |
|
N | 67 | 33 | 0.68677 |
|
Me (Q1,
Q3) | 56.0 (36.0,
84.5) | 56.0 (30.0,
78.5) | |
| ALT | | | |
|
N | 67 | 33 | 0.13190 |
|
Me (Q1,
Q3) | 71.0 (43.5,
112.8) | 61.0 (38.5,
81.0) | |
| PLT | | | |
|
N | 67 | 33 | 0.03602 |
|
Me (Q1,
Q3) | 22.0 (19.1,
25.38) | 18.0 (14.58,
24.30) | |
| FIB-4 | | | |
|
N | 67 | 33 | 0.02465 |
|
Me (Q1,
Q3) | 1.658 (1.20,
2.639) | 2.279 (1.807,
3.459) | |
| LS | | | |
|
N | 66 | 32 | 0.02615 |
|
Me (Q1,
Q3) | 6.50 (5.00,
8.70) | 7.90 (6.35,
12.55) | |
| CAP | | | |
|
N | 66 | 32 | 0.06396 |
|
Me (Q1,
Q3) | 318.5 (290,
341) | 288.5 (270,
340) | |
| M2BPGi | | | |
|
N | 66 | 33 | 0.01600 |
|
Me (Q1,
Q3) | 0.880 (0.620,
1.210) | 1.110 (0.835,
2.538) | |
| HT | | | |
|
HT | 24 (35.8) | 17 (51.5) | 0.13350 |
|
none | 43 (64.2) | 16 (48.5) | |
| HT | | | |
|
HL | 25 (37.3) | 10 (30.3) | 0.48950 |
|
None | 42 (62.7) | 23 (69.7) | |
| DM | | | |
|
None | 50 (74.6) | 24 (72.7) | 0.83864 |
|
DM | 17 (25.4) | 9 (27.3) | |
| B, Change of
lifestyle |
| | MASLD | MetALD | P-value |
| Exercise | | | |
|
N | 67 | 33 | 0.77342 |
|
Me (Q1,
Q3) | 0.5 (0.0, 1.0) | 0.5 (0.0, 1.0) | |
| Exercise G | | | |
|
Ex | 47 (70.1) | 23 (69.7) | 0.96299 |
|
None | 20 (29.9) | 10 (30.3) | |
| Nutritional
Guidance | | | |
|
Done | 38 (56.7) | 10 (30.3) | 0.01292 |
| Abstinence | | | |
|
Abstinence | 1 (20.0) | 11 (33.3) | 1.00000 |
|
None | 4 (80.0) | 22 (66.7) | |
| dBW% | | | |
|
N | 67 | 33 | 0.18334 |
|
Me (Q1,
Q3) | -3.52 (-6.24,
-1.20) | -2.32 (-5.98,
0.70) | |
| dBW% G1 | | | |
|
G1 | 17 (25.4) | 9 (27.3) | 0.83864 |
|
G2-4 | 50 (74.6) | 24 (72.7) | |
| dBW% G12/34 | | | |
|
G1, 2 | 36 (53.7) | 14 (42.4) | 0.28762 |
|
G3, 4 | 31 (46.3) | 19 (57.6) | |
| dBW% G | | | |
|
G1 | 17 (25.4) | 8 (24.2) | 0.51125 |
|
G2 | 19 (28.4) | 6 (18.2) | |
|
G3 | 17 (25.4) | 8 (24.2) | |
|
G4 | 14 (20.9) | 33.3 | |
| C, Change of
clinical factors |
| | MASLD | MetALD | P-value |
| dAST | | | |
|
N | 67 | 33 | 0.82019 |
|
Me (Q1,
Q3) | -15.0 (-36.8,
0.0) | -13.0 (-37.0,
-2.0) | |
| dALT | | | |
|
N | 67 | 33 | 0.57236 |
|
Me (Q1,
Q3) | -24.0 (-51.8,
-4.3) | -17.0 (-43.5,
-3.0) | |
| dPLT | | | |
|
N | 67 | 33 | 0.39098 |
|
Me (Q1,
Q3) | -0.7 (-2.8,
1.0) | -0.3 (-1.7,
1.7) | |
| dLS | | | |
|
N | 66 | 32 | 0.66030 |
|
Me (Q1,
Q3) | -0.7 (-2.2,
0.7) | -0.8 (-2.5,
0.6) | |
| dLSG | | | |
|
Decrease | 44 (66.7) | 21 (65.6) | 0.91850 |
|
Increase | 22 (33.3) | 11 (34.4) | |
| dCAP | | | |
|
N | 66 | 32 | 0.77922 |
|
Me (Q1,
Q3) | -21.5 (-61.0,
11.0) | -23.0 (-60.0,
-2.0) | |
| dM2BPGi | | | |
|
N | 61 | 30 | 0.40795 |
|
Me (Q1,
Q3) | 0.0 (-0.2,
0.1) | -0.1 (-0.4,
0.2) | |
| dM2BG | | | |
|
Decrease | 27 (44.3) | 16 (53.3) | 0.41519 |
|
Increase | 34 (55.7) | 14 (46.7) | |
| dFIB-4 | | | |
|
N | 67 | 33 | 0.34621 |
|
Me (Q1,
Q3) | -0.1 (-0.4,
0.1) | -0.3 (-0.7,
0.3) | |
| dFIBG | | | |
|
Decrease | 41 (61.2) | 21 (63.6) | 0.81297 |
|
Increase | 26 (38.8) | 12 (36.4) | |
For lifestyle modification, the patients were
instructed to lose weight, abstain from alcohol, and increase
exercise (Table IB). All patients
with MASLD and MetALD were advised to add at least 30 min of
walking daily and were instructed to increase their walking speed
as they became accustomed to walking. Exercise was defined as 100%
of 30 min of walking; if less, the self-reported walking time was
divided by 30 min and expressed as a percentage. The exercise +
(Ex+) group was able to add any degree of exercise, while the None
group did not add any exercise. Nutritional guidance was
recommended to all patients except those who did not request it.
Nutritional guidance was provided by a dietitian with the goal of a
10% reduction in weight at the time of intervention to 25 calories
per target weight (kg). All patients were instructed to abstain
from alcohol; those who stopped drinking were defined as abstinent,
and those who drank less were defined as the reduced drinking
group. The abstinence period is six months, as is the observation
period. Abstinence was recommended at the first visit, total or
partial abstinence was confirmed at two weeks later, and alcohol
consumption was checked again at the final assessment six months
later. The AbEx group comprised of an Ex+ group and an
abstinence group. Patients were weighed at least once daily,
snacking and soft drinks were stopped, and had a goal of losing 10%
of their body weight in one year. Decreased body weight (dBW%) is
calculated as follows: (BW at entry-BW at 0.5 year after)/BW at
entry. BW decrease rate groups (dBWG) were divided by quartiles of
dBW% as follows: G1, -6.1%>; G2, -6.1 to -3.1; G3, -3.1 to
-0.77; G4, -0.77< (Fig.
S1A).
Informed consent was obtained from each patient
included in the study and they were guaranteed the right to leave
the study if desired. The study protocol conformed to the 1975
Declaration of Helsinki guidelines (7) and was approved by the Human Research
Ethics Committee of Nagasaki Harbor Medical Center (approval no.
H30-031; Nagasaki, China).
Laboratory measurements
At the start of change of lifestyle and 0.5 years
later, platelet [(PLT): M, 13.1-26.2; F, 13-36.9 X
104/µl], AST (10-40 U/l), ALT (5-40 U/l) and M2BPGi
[1> cut off index (C.O.I.)] levels were evaluated. Fibrosis-4
(FIB-4) levels were calculated based on age and AST, ALT and PLT
levels (8). Overall, 98 patients
were evaluated using FibroScan. LS (kPa) was evaluated using
vibration-controlled transient elastography, and liver fat content
(dB/m) was evaluated using the controlled attenuation parameter
(CAP). ‘d’ is the difference between baseline and endpoint
(Table IC). A decrease in liver
fibrosis (a decrease in dLSM2B) was defined as a reduction in LS
(dLS) using FibroScan and M2BPGi (dM2B) during the observation
period.
Statistical analysis
Data were analyzed using StatFlex (version 6.0;
Artech Co., Ltd.) and presented as median and 95% confidence
intervals (CI). Laboratory variables were compared using
Mann-Whitney U tests (for differences between the two groups) and
χ2 tests. The detection level was analyzed using
receiver operating characteristic (ROC) curves. P<0.05 was
considered to indicate a statistically significant difference.
Correlations were evaluated based on Pearson's correlation
coefficient (R). A multivariate analysis was performed using
logistic regression.
Results
Difference between MASLD and
MetALD
Patients with MetALD were more likely to be men and
had higher FIB-4, LS, and M2BPGi scores than those with MASLD
(Table IA). There were no
significant differences in age, body mass index (BMI), HT, HL, or
DM. Nutritional guidance interventions were more common in patients
with MASLD; however, exercise and dBW% did not differ significantly
(Table IB). The rate of abstinence
due to MetALD was 33.3%. No differences were found between the
MASLD and MetALD groups with respect to changes in clinical factors
(Table IC).
Weight loss is associated with a
decrease in liver fibrosis in patients with MASLD, but no
association can be observed in patients with MetALD
The correlation between dM2BPGi and dLS was R=0.5896
(P<0.00001) in all cases (N=87). When only one of LS and M2BPGi
was measured, fibrosis reduction was defined as a decrease in the
measured item. The rates of decrease in dLSM2B levels were 35.8 and
39.4% in MASLD and MetALD (no significance difference (Fig. S1B). Among patients with MASLD, a
decrease in the dLSM2B group was associated with weight loss
(Table II). Patients with MASLD
had greater dBW% in the decreased dLSM2B group; however, patients
with MetALD showed no significant differences (Fig. 1A). The distribution of the weight
loss rate was associated with a decrease in dLSM2B in patients with
MASLD, but not in those with MetALD (Figs. 1B and S1C).
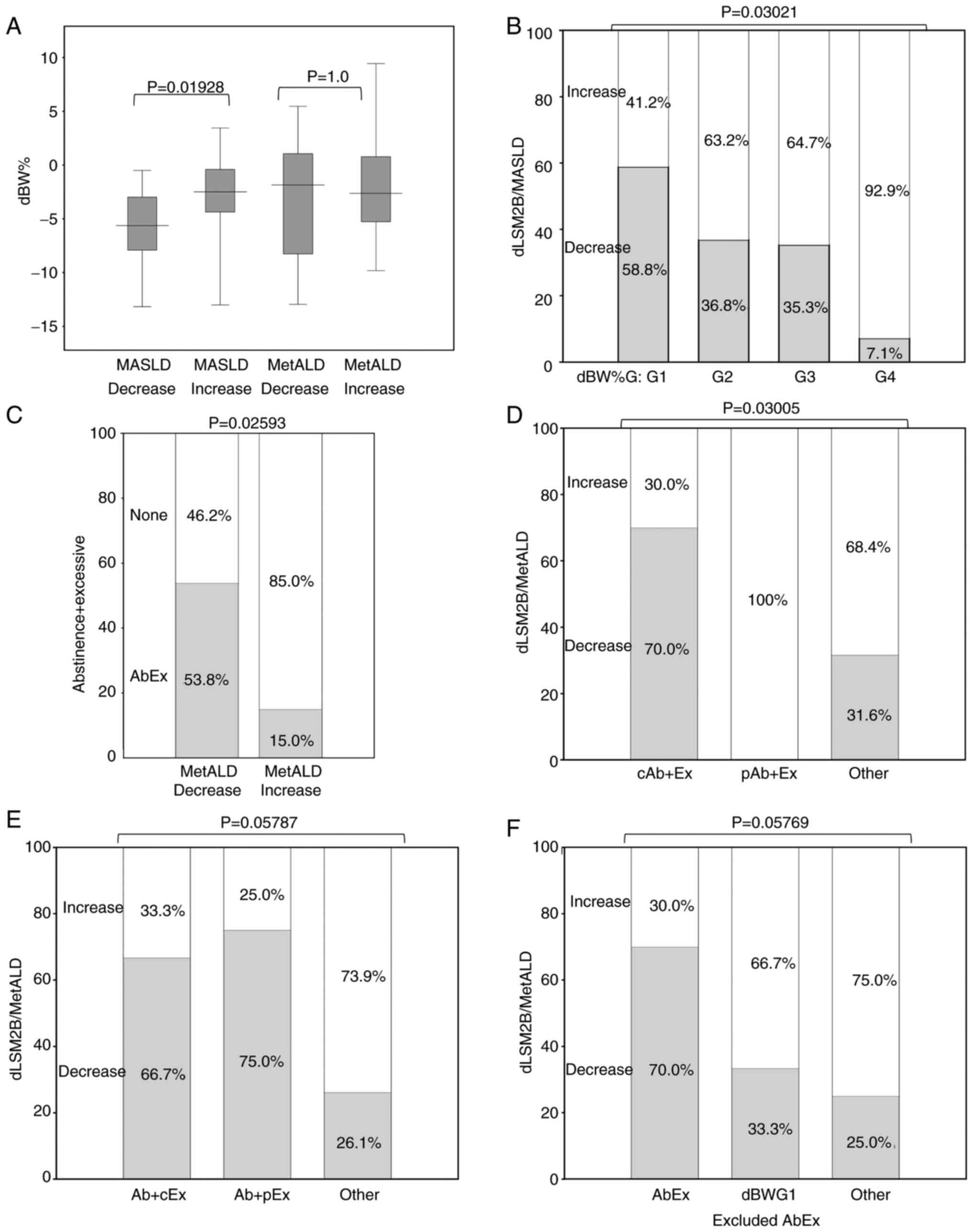 | Figure 1Relationship between with dLSM2B
decrease and change of lifestyle. The dLSM2B decreased group is the
group with no increase in LS (dLS) and no increase in M2BPI (dM2B),
and the dLSM2B increased group is the group other than the
decreased group. Change of LS and M2B were difference form entry to
0.5 year after. Exercise (Ex) was defined as 100% of 30 min of
walking; if less, self-reported walking time was divided by 30 min
and expressed as a percentage. The Ex+ group is the
group that was able to add any degree of exercise, and the None
group is the group that did not add any exercise at all. The
abstinence group was the group that completely stopped drinking
alcohol by self-report. The AbEx group is an Ex+ and
abstinence group. Decreased body weight (dBW%) is calculated as
follows: (BW at entry-BW at 0.5 year after)/BW at entry. BW
decrease rate groups (dBWG) were divided by quartiles of dBW% as
follows: G1: -6.1%>; G2, -6.1 to -3.1; G3, -3.1 to -0.77; G4,
-0.77<. (A) Differences in the relationship between weight loss
and liver fibrosis improvement in MASLD and MetALD. dBW% was
compered between dLSM2B decrease and increase groups in MASLD and
MetALD. Y axis is dBW%. (B) Association between weight loss and
liver fibrosis improvement in MASLD. In MASLD, dBWG and dLSM2B
decrease group. G1, 17 patients; G2, 19 patients; G3, 17 patients;
G4, 14 patients. Y axis is dLSM2B decrease group percentile. (C)
Association between abstinence + exercise and improvement of liver
fibrosis in MetALD. Rate of AbEx in groups related to dLSM2B
decrease group. Y axis is AbEx percentile. (D) Relationship between
complete abstinence and liver fibrosis improvement in MetALD. In
MetALD, the relation between degree of abstinence and dLSM2B
decrease group. cAb is complete abstinence and pAb is partial
abstinence included decrease drink. Ex is the group that was able
to add any degree of exercise. cAb + Ex group, 10 patients; pAb +
Ex group, 4 patients; other group, 19 patients. Y axis is dLSM2B
decrease group percentile. (E) Relationship between achievement of
exercise goal and improvement of liver fibrosis in MetALD. In
MetALD, the relation between degree of exercise and dLSM2B decrease
group. cEx is 30 min of more of walking and pEx is less than 30 min
of walking. Ab is stop of drinking. Ab + cEx group, 6 patients; Ab
+ pEx, 4 patients; other, 23 patients. (F) Comparison of liver
fibrosis improvement between alcohol abstinence exercise group and
weight loss group in MetALD. In MetALD, the relation between degree
of BW and dLSM2B decrease group. AbEx is an Ex+ group
and an abstinence group. dBWG1 excluded AbEx group is dBWG1 and
non-AbEx group. AbEx group, 10 patients; dBWG1 excluded AbEx, 3
patients; other, 20 patients. MASLD, metabolic
dysfunction-associated steatotic liver disease; MetALD, metabolic
dysfunction-associated steatotic liver disease and
alcoholic-related liver disease. |
 | Table IIThe relation with dLSM2B and clinical
factors. |
Table II
The relation with dLSM2B and clinical
factors.
| | MASLD | MetALD |
|---|
| | Decrease | Increase | P-value | Decrease | Increase | P-value |
|---|
| dLSM2B | | | | | | |
| dBW% | | | | | | |
|
n | 24 | 43 | 0.00152 | 13 | 20 | 0.58050 |
|
Me
(Q1~Q3) | -5.63
(-7.92~-2.98) | -2.50
(-4.37~-0.40) | | -1.84
(-8.28~1.04) | -2.63
(-5.28~0.77) | |
| dBW%G1 | | | | | | |
|
G1 | 10 (41.7) | 7 (16.3) | 0.02204 | 5 (38.5) | 3 (15.0) | 0.21338 |
|
G2-4 | 14 (58.3) | 36 (83.7) | | 8 (61.5) | 17 (85.0) | |
| Ex | | | | | | |
|
n | 24 | 43 | 0.94625 | 13 | 20 | 0.31540 |
|
Me (Q1,
Q3) | 0.5 (0.0, 0.9) | 0.5 (0.0, 1.0) | | 0.8 (0.2, 1.0) | 0.3 (0.0, 1.0) | |
| Ex G | | | | | | |
|
Ex+ | 17 (70.8) | 30 (69.8) | 0.92716 | 10 (76.9) | 13 (65.0) | 0.70059 |
|
None | 7 (29.2) | 13 (30.2) | | 3 (23.1) | 7 (35.0) | |
| Nutritional
guidance | | | | | | |
|
Done | 16 (66.7) | 22 (51.2) | 0.21942 | 5 (38.5) | 5 (25.0) | 0.46107 |
|
None | 8 (33.3) | 21 (48.8) | | 8 (61.5) | 15 (75.0) | |
| Abstinence | | | | | | |
|
abstinence | 0 (0.0) | 1 (2.3) | 1.00000 | 7 (53.8) | 4 (20.0) | 0.06455 |
|
None | 24 (100.0) | 42 (97.7) | | 6 (46.2) | 16 (80.0) | |
| abEx | | | | | | |
|
none | 24 (100.0) | 43 (100.0) | Insufficient
number | 6 (46.2) | 17 (85.0) | 0.02593 |
|
abEx | 0 (0.0) | 0 (0.0) | | 7 (53.8) | 3 (15.0) | |
In patients with MetALD, complete
abstinence from alcohol and the addition of exercise were
associated with a decrease in liver fibrosis
In patients with MetALD, alcohol abstinence and
additional exercise, but not weight loss, were associated with a
decrease in dLSM2B (Table II and
Fig. 1C). Patients with MetALD were
more likely to show a decrease in the dLSM2B group for complete
abstinence from alcohol (with exercise) than for those with partial
abstinence (with exercise) or others (Fig. 1D). There was no significant
difference in the decrease in dLSM2B between achieving exercise
goals (with abstinence), partial exercise goals (with abstinence),
and other goals in patients with MetALD (Fig. 1E). The percentage of patients with
MetALD with reduced dLSM2B levels was 23% in the abstinence alone
or exercise alone group, 30% in other (the non-abstinence and
non-exercise) groups, and 70% in the AbEx group, with no
significant difference between the three groups; however, a trend
toward a difference was observed (Fig.
S1D). Patients with MetALD treated with AbEx did not differ
from non-AbEx patients with dBW%G1 or others in terms of the rate
of dLSM2B reduction (Fig. 1F).
Although the difference was not significant, the rate of reduction
in dLSM2B was 80% with AbEx and G1 weight loss, 60% with AbEx and
no G1 weight loss, and 33% with G1 weight loss alone (Fig. S1E). In MetALD cases, there was no
significant difference in dLSM2B between the three groups of
complete Ab, partial Ab, and no Ab (Fig. S1F), and similarly no significant
difference between the three groups of complete Ex, partial Ex, and
no Ex (Fig. S1G).
Decrease in dLSM2B by lifestyle
modification for patients with MASLD is predicted by a decrease in
FIB-4 and weight loss, but not for patients with MetALD
Predictive factors that could improve liver fibrosis
in patients with MASLD were searched. In patients with MASLD, no
baseline factors differed significantly between the decreased and
increased dLSM2B groups (Table
SI). However, among the factors that changed over the
observation period, there were significant differences in the AST,
ALT, CAP and FIB-4 levels between the two groups (Table SII). Using ROC analysis, the cutoff
values for the dLSM2B reduced group were calculated for five
factors (these four factors plus dBW%). No significant differences
were observed in the area under the curve (AUC) of the five factors
(Fig. S2A). Since AST and ALT were
included in FIB-4, and dBW and dCAP were significantly correlated
(Table SIII), dFIB-4 and dBW% were
selected as predictors. dBW% (-3.8% over) and dFIB-4 (-0.201 over)
were used as predictors of a decrease in the dLSM2B group. The odds
ratios (ORs) of the dBW% (-3.8% or more) and dFIB-4 (-0.201 or
more) groups contributing to a decrease in dLSM2B were assessed
using logistic analysis. The results indicated that these two
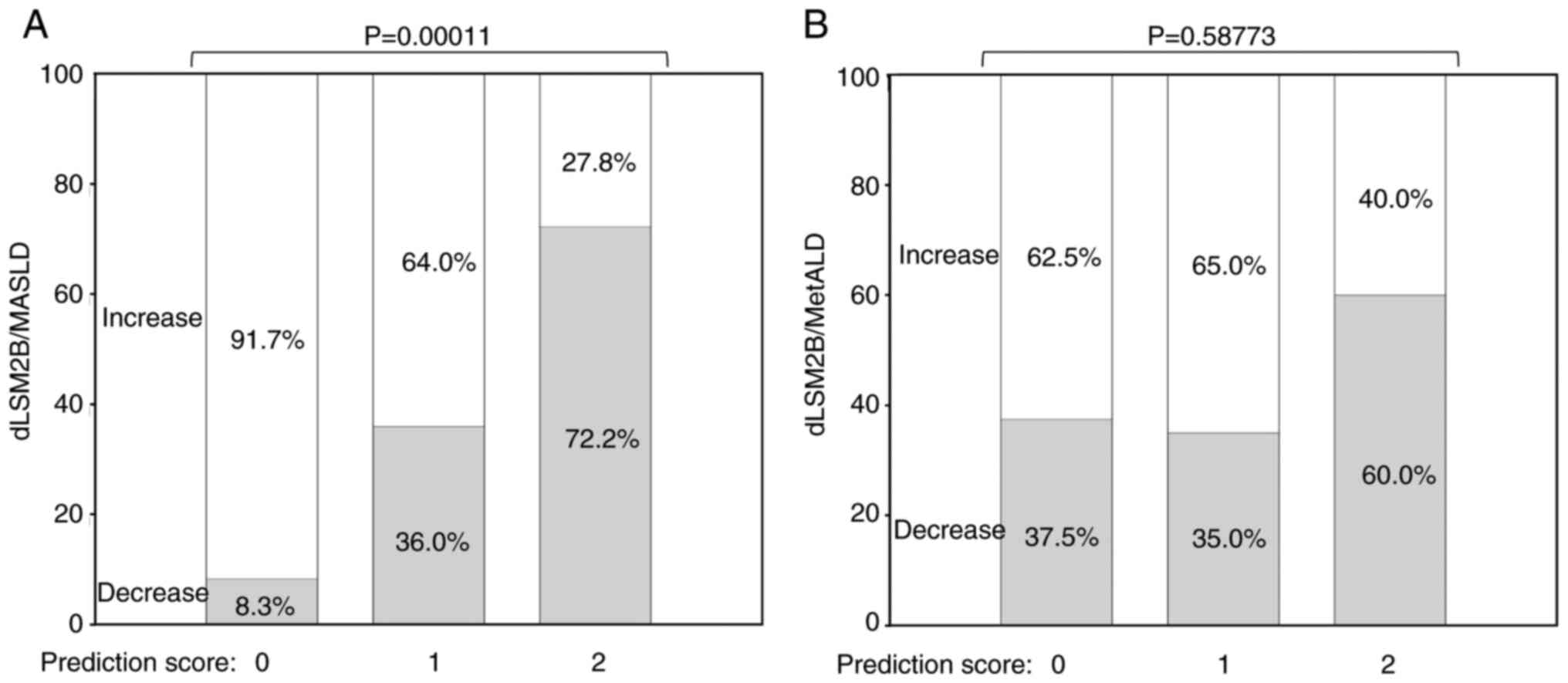
predictors contributed independently (Fig. S2B). A prediction score of 0, 1 or 2
points was developed to predict the decrease in dLSM2B, with 1
point awarded for a dBW% reduction of ≥3.8 and 1 point for a FIB-4
reduction of ≥0.201. In patients with MASLD, there was a
significant difference in the decrease in dLSM2B by 8.3% for a
prediction score of 0, 36% for 1, and 72.2% for 2 (Fig. 2A), whereas there was no significant
difference in the improvement rate for 0-2 points (Fig. 2B). In patients with MASLD, a
prediction score contributed to a decrease in dLSM2B in univariate
logistic analysis (OR, 5.224; 95% CI lower-upper: 2.231-12.232;
P=0.00014), but not in the MetALD group (OR, 1.482; 95% CI
lower-upper: 0.472-4.650; P=0.50048). In patients with MASLD,
multiple logistic analysis of the effects of a prediction score and
dBW% (-3.8% or more) on dLSM2B decrease showed that only a
prediction score was significant [Fig.
S2C; variance inflation factors (VIF); 2.667], as was the
comparison of a prediction score and dFIB-4 (-0.201 or more)
(Fig. S2D; VIF; 2.652).
Influence of alcohol consumption on
lifestyle modification in MetALD patients
The effects of alcohol consumption in patients with
MetALD were examined. The amount of alcohol consumed (ALD score)
was defined as grade 1 for 30-45 g of alcohol for men and 20-35 g
for women, and grade 2 for 45-60 g for men and 35-50 g for women.
Differences in sex and LS were observed for MetALD grade 1
(MetALD1) and MetALD2, but not significant for other pretreatment
factors (Table SIV). Particular
sex differences were observed in MASLD, MetALD1 and MetALD2
(Fig. S3A). In a comparison among
the three groups (MASLD, MetALD1, and MetALD2), differences in LS
were found between MASLD and MetALD2, whereas MASLD and MetALD1
were similar (Fig. S3B).
Considering lifestyle modification, weight loss was less frequent
in the MetALD2 group, and there were no significant differences in
abstinence from alcohol, physical activity, or frequency of
nutritional guidance (Table III).
The distribution of dBW% (dBW% G1-4) showed differences among
MASLD, MetALD1 and MetALD2, which were characterized by more G1 in
MetALD1 and G4 in MetALD2 (Fig.
S3C). The dBW% was also not significantly different between the
MASLD and MetALD1 groups; only the MetALD2 group showed a lower
rate than the MASLD and MetALD1 groups (Fig. S3D). There were no significant
differences in AST, ALT, PLT, LS, CAP, M2BPGi and FIB-4 levels
between the two groups over the treatment period (Table SV).
 | Table IIILifestyle change in MetALDG1/2. |
Table III
Lifestyle change in MetALDG1/2.
| MetALD Grade
1/2 | Grade1 | 2 | P-value |
|---|
| dBW% | | | |
|
Me (Q1,
Q3) | -4.086 (-6.98,
-1.596) | 1.423 (-1.331,
3.910) | 0.00243 |
| dBW%G | | | |
|
1 | 7 (33.3) | 1 (8.3) | 0.02123 |
|
2 | 5 (23.8) | 1 (8.3) | |
|
3 | 6 (28.6) | 2 (16.7) | |
|
4 | 3 (14.3) | 8 (66.7) | |
| dBW%G4 | | | |
|
4 | 3 (14.3) | 7 (58.3) | 0.01636 |
|
1-3 | 18 (85.7) | 5 (41.7) | |
| Abstinence/no | | | |
|
Abstinence | 7 (33.3) | 4 (33.3) | 1.00000 |
|
None | 14 (66.7) | 8 (66.7) | |
| abEx | | | |
|
None | 14 (66.7) | 9 (75.0) | 0.70981 |
|
abEx | 7 (33.3) | 3 (25.0) | |
| Ex G | | | |
|
Ex | 17 (81.0) | 6 (50.0) | 0.11422 |
|
None | 4 (19.0) | 6 (50.0) | |
| Nutritional
guidance | | | |
|
Done | 7 (33.3) | 3 (25.0) | 0.70981 |
|
None | 14 (66.7) | 9 (75.0) | |
Discussion
In the present study, 67 patients with MASLD and 33
patients with MetALD were instructed to improve their lifestyle,
and changes in liver fibrosis were assessed after six months. Prior
to lifestyle modifications, the evaluation revealed more men with
advanced liver fibrosis and MetALD. As a result of lifestyle
modifications, weight loss was associated with improved liver
fibrosis in patients with MASLD, but not in those with MetALD. By
contrast, complete abstinence from alcohol and exercise was
associated with improved liver fibrosis in patients with MetALD.
The predictive score, consisting of a decrease in FIB-4 index and
body weight, was associated with improved liver fibrosis in
patients with MASLD, but not in those with MetALD. Patients with
MetALD (MetALD1) who drank less alcohol had a similar degree of
weight loss to those with MASLD, and their liver fibrosis before
lifestyle modification was milder than that of patients (MetALD2)
patients who drank more. These results indicate a difference in the
effects of weight loss between MASLD and MetALD. Complete
abstinence from alcohol and exercise are considered effective in
improving the lifestyle of patients with MetALD. Thus, MetALD1 may
be similar to MASLD and MetALD2 in ALD.
In the present study, patients with MetALD had more
worsening liver fibrosis (FIB-4, M2BPGi and LS) and a male-dominant
sex difference than those with MALSD, but age and BMI did not
differ between the groups. Although one study did not find a sex
difference (9), most studies
indicate that MetALD is more common in males than in females as
compared with MASLD (10-12).
Even after adjusting for sex differences, MetALD has a worse
prognosis for liver and cardiovascular diseases than ALD (12-14).
The prevalence of MetALD in these studies ranged from 1.7-17.0% in
a resident-based cohort (3). When
the proportion of patients with MetALD was 1.7% in the overall
cohort, it was 2.5% in the SLD group, and when it was 17% in the
overall cohort, it was 24% in the SLD group (3). In the present study, 33 patients with
MetALD represented 33% of patients with SLD. When the target SLD is
narrowed down to cases with advanced fibrosis, MASLD and MetALD are
reported to represent 2.4 and 1.5% of all cases, respectively
(9), and it is estimated that the
number of MASLD and MetALD cases will converge as fibrosis worsens.
The large number of MetALD cases in the present study was
presumably because this was a hospital-based study with more cases
of advanced liver damage than studies in the general
population.
In the present study, differences were found between
the MASLD and MetALD groups in the relationship between weight loss
and decreased fibrosis. In MASLD, 7-10% weight loss is required to
improve MASH and liver fibrosis (5,15,16).
The FIB-4 is insufficient as a primary screening tool for liver
fibrosis in the general population (17). However, in MASLD, a decrease or
normalization of ALT levels is an indicator of improvement in the
MASH score, whereas changes in FIB-4 (14,15),
is an indicator of liver fibrosis (18). FIB-4 is a first-line test in MASLD
practice (5,15) and a cost-effective screening test
for high-risk MASLD (19).
Decreased FIB-4 and weight were independent ameliorating factors
for liver fibrosis in patients with MASLD (Fig. S2B). The combination of these two
parameters is a predictor of improvement of liver fibrosis is a new
finding. However, weight loss was not associated with improved
liver fibrosis in patients with MetALD, and the predictors created
by the MASLD results were not valid for MetALD. Because MetALD is a
condition that includes MASLD and ALD, MASLD and ALD should be
treated separately (2,3). However, because MetALD is a new
condition, no studies have examined the effect of weight loss;
therefore, it is necessary to develop a lifestyle intervention for
MetALD in the future.
Weight loss was not clearly associated with liver
fibrosis in MetALD, but complete abstinence from alcohol and the
addition of exercise were associated with liver fibrosis in MetALD.
Furthermore, complete abstinence from alcohol was more effective
than partial abstinence, and there was no difference in
effectiveness between performing daily exercise as directed and
performing it less than directed but as little as possible.
Education regarding lifestyle modifications for patients with ALD
is likely to be optimized by improving physical activity (20). Patients with alcohol use disorder
(AUD) who were able to add exercise were less associated with the
development of alcoholic liver disease (ALD) than those who did not
exercise (21). Patients with MASLD
and ALD understand the importance of exercise, but lack of
confidence in exercising and fear of falling are associated with
difficulties in exercising, which can be modified with education
and are goals of exercise acceptance (22). Based on these reports, exercise
education is very important for patients with MetALD, and if
exercise is enabled, improvement in liver fibrosis can be expected.
Complete abstinence from alcohol is recommended in cases of
cirrhosis, whereas alcohol reduction is recommended in SLD other
than advanced fibrosis (13),
although some studies have indicated that abstinence from alcohol
is also required for MetALD (2,15,23).
In the present study, complete alcohol abstinence was necessary to
improve liver fibrosis, and exercise may have been effective. Even
in patients with MASLD and low alcohol intake, there is a
relationship between alcohol consumption and risk of liver fibrosis
(24). Alcohol consumption and
mortality risk both increased, and the amount of alcohol consumed
that minimized health losses was zero (25). When metabolic dysfunction and
alcohol consumption coexist, pathogenic pathways leading to liver
injury are likely to be additive and synergistic (3,26).
These results indicate that patients with MetALD but without
cirrhosis should also be educated about complete abstinence from
alcohol. Alcohol consumption is the most important factor affecting
the severity of alcohol-related SLDs (MetALD + ALD) (27). Although the success rate of drinking
in patients with MetALD has not been reported, 21% of patients who
attended Alcoholics Anonymous (AA) with alcohol-related problems
successfully abstained from alcohol for one year if they wanted to
abstain, and 10% at one year if they did not want to (28). The effectiveness of AA meetings,
cognitive-behavioral therapy, and exercise in improving abstinence
rates is expected to be effective (29). All the patients in the present study
were referred by their general practitioners, and at the time of
their first visit to our department, they had already received an
explanation of the effectiveness and necessity of abstinence from
alcohol. Therefore, it is assumed that numerous came to our clinic
to abstain from alcohol. Additionally, the benefits of abstinence
from alcohol were explained to patients with MetALD, and have
sought their understanding before promoting physical exercise,
which it is considered that has led to the current abstinence rate
(33%) in our hospital. Therefore, alcohol abstinence may eliminate
aggravating factors and improve liver fibrosis in some patients,
independent of weight loss.
Our exercise instructions for patients with MASLD
and MetALD followed Western guidelines (4,14),
with instructions for 150 min of moderate exercise per week or 75
min of vigorous exercise per week. Specifically, they were
encouraged to exercise daily (30),
told them to work hard for an average of 30 min per day (31), and checked the amount of time they
could achieve. It has been reported that among people undergoing
health checkups, those aged 65 or older who have no exercise habits
are associated with liver fibrosis (32). Although the effect of exercise was
not related to changes in liver fibrosis in patients with MASLD,
the median value was 50%, and it is likely that the amount of
exercise was low. In the future, patients should be taught to
exercise at a minimal level (30 min of walking daily). Conversely,
excessive alcohol consumption is associated with increased dietary
intake and is related to obesity (33) and metabolic factor appearance
(27), and it is also known that
decreasing alcohol consumption results in improvement of obesity
(34). Weight loss had no clear
effect on liver fibrosis in patients with MetALD but should be
reexamined in more cases in the future. A comparison between
MetALD1 and MetALD2 showed that MetALD1 was closer to the MASLD (LS
and dBW%). In the future, classification of patients with MetALD in
terms of alcohol consumption may be necessary to predict the effect
of lifestyle modifications on liver fibrosis.
The limitation of the present study is that it was a
small, single-hospital, and retrospective study. In addition,
alcohol consumption and physical activity were patient-reported and
not quantifiable. Given the limited observation period of six
months in MetALD, it is possible that other factors may contribute
to long-term outcomes. Further follow-up and ongoing research are
therefore needed. However, the present study found that weight loss
in MASLD, alcohol abstinence, and additional exercise in MetALD
were associated with an improvement in liver fibrosis. When
teaching lifestyle modifications in SLD, it is important to first
teach weight loss for MASLD and complete abstinence and additional
exercises for MetALD. A combination of FIB-4 index reduction and
weight loss may be effective in predicting decreased liver fibrosis
in patients with MASLD. The effectiveness of weight loss in MetALD
needs to be further investigated in more cases, and the content of
exercise instruction also needs to be examined in the MASLD.
Screening for liver disease has been reported to increase
abstinence rates (35), and the
active involvement of hepatologists in MetALD (probably all
alcohol-related SLD) may increase abstinence rates. While a
multidisciplinary approach is necessary for patients with SLD
(5,15), education by hepatologists regarding
abstinence, weight loss, and exercise is also considered
necessary.
Supplementary Material
Distribution of decreased body
weights. Decreased body weight (dBW%) is calculated as follows: (BW
at entry-BW at 0.5 year after)/BW at entry. N is number. The y-axis
represents the number and the x-axis represents dBW%. Q1 and Q3
represent the first and third quartiles, respectively. (B) dLSM2B
ration in MASLD and MetALD. The dLSM2B decreased group had no
increase in LS or M2BPI, while the dLSM2B increased group was the
group other than the decreased group. The y-axis represents the
percentiles of the dLMM2B group in MASLD and MetALD. (C)
Distribution of the dBWG. Groups were divided by quartiles of dBW%
as follows: G1, -6.1%>; G2, -6.1 to -3.1; G3, -3.1 to -0.77; G4,
-0.77<. The y-axis represents the percentile of the dBWG. (D)
Relationship between the dLSM2B score group and
abstinence/exercise. The AbEx group discontinued drinking and
exercising. The Ab and Ex groups stopped drinking or exercising to
some degree. AbEx, 10 patients; Ab or Ex: 13 patients; other, 10
patients. The axis represents dLSM2B decrease percentile. (E)
Relationship between the dLSM2B decree group and the AbEx/dBWG1.
AbEx + dBWG1, 5 patients; AbEx-dBWG1, 5 patients; dBWG1-AbEx, 3
patients; other, 20 patients. (F) Relationship between the dLSM2B
decrease group and the Ab group. Complete Ab (cAb): 11 patients;
partial Ab (pAb): 10 patients; no Ab, 12 patients. (G) Relationship
between the dLSM2B decrease group and the Ex group. Complete Ex
(cEx): 13 patients; partial Ex (pEx): 10 patients; no Ex: 10
patients. MASLD, metabolic dysfunction-associated steatotic liver
disease; MetALD, metabolic dysfunction-associated steatotic liver
disease and alcoholic-related liver disease.
(A) Receiver Operating characteristic
curve for the dLSM2B decrease group. The cut-off value was set to
be equal to the sensitivity and 1-specificity. P-values are for the
comparison area under the curve of factors 1 and 2. (B) Logistic
multi-factor analysis of the dLSM2B decrease group in the MASLD.
The dBW%-3.8 and dFIB-4-0.201 groups were selected based on the
results shown in Fig. S2A and
Table SIV. (C) Logistic
multi-factors analysis of the effect of a prediction score and dBW%
(-3.8% or more) on dLSM2B decrease. One point was given for a
decrease of 0.201 or more in FIB-4 and one point for a decrease of
3.8% or more in BW, and the total score was used as the predictor.
(D) Logistic multi-factors analysis of the effect of a prediction
score and dFIB-4 (-0.201 or more). AUC, area under the curve;
MASLD, metabolic dysfunction-associated steatotic liver disease;
CI, confidence interval; BW, body weight; Fib-4, Fibrosis-4; AST,
aspartate aminotransferase; ALT, alanine aminotransferase; CAP,
CAP, controlled attenuation parameter.
(A) Amount of alcohol consumed (ALD
score) was defined as grade 1 for 30-45 g of alcohol for men and
20-35 g for women, and grade 2 for 45-60 g for men and 35-50 g for
women. Percentage of sex in MASLD, MetALD grade1 (MetALD1) and
MetALD grade 2 (MetALD2). Y axis is percentage. (B) Liver stiffness
at the first visit was compared with MASLD, MetALD1 and MetALD2.
The y-axis represents the LS (kPa). (C) Distribution of dBW%G
compared with that of MASLD, MetALD1 and MetALD2. The y-axis
represents the percentage of grade 1-4. (D) dBW% compared with
MASLD, MetALD1, and MetALD2. Y axis is dBW%. MASLD, metabolic
dysfunction-associated steatotic liver disease; MetALD, metabolic
dysfunction-associated steatotic liver disease and
alcoholic-related liver disease.
In the MASLD, the relationship between
the factors at entry and the dLSM2B group.
In MASLD, the relationship between
changes in clinical factors and the dLSM2B group.
In MASLD, the relation among
difference of clinical factors.
In MetALD, the relationship between
the factors at entry and alcohol grade.
Relationship between changes in
clinical factors and alcohol grade in patients with MetALD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to containing information that could
compromise the privacy of research participants, but may be
requested from the corresponding author.
Authors' contributions
TIc wrote the manuscript, analyzed the data and
designed the study. TIc, SM, MYa, SY, MK, YN, HY, OM, TIk, TO, NK,
MYo and HM collected the data. TIc and SM confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The protocol for this research project was approved
(approval no. H30-031) by the Ethics Committee of of Nagasaki
Harbor Medical Center (Nagasaki, Japan). Informed consent was
obtained from each patient included in the study and they were
guaranteed the right to leave the study if desired.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rinella ME, Lazarus JV, Ratziu V, Francque
SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab
JP, et al: A multisociety Delphi consensus statement on new fatty
liver disease nomenclature. J Hepatol. 79:1542–1556.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marek GW and Malhi H: MetALD: Does it
require a different therapeutic option? Hepatology. 80:1424–1440.
2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gratacós-Ginès J, Ariño S, Sancho-Bru P,
Bataller R and Pose E: MetALD: Clinical aspects, pathophysiology
and treatment. JHEP Rep. 7(101250)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fukunaga S, Mukasa M, Nakane T, Nakano D,
Tsutsumi T, Chou T, Tanaka H, Hayashi D, Minami S, Ohuchi A, et al:
Impact of non-obese metabolic dysfunction-associated fatty liver
disease on risk factors for the recurrence of esophageal squamous
cell carcinoma treated with endoscopic submucosal dissection: A
multicenter study. Hepatol Res. 54:201–212. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
European Association for the Study of the
Liver (EASL). Tacke F, Horn P, Wai-Sun Wong V, Ratziu V, Bugianesi
E, Francque S, Zelber-Sagi S, Valenti L, Roden M, et al:
EASL-EASD-EASO clinical practice guidelines on the management of
metabolic dysfunction-associated steatotic liver disease (MASLD). J
Hepatol. 81:492–542. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu X, Zhang W, Ma B, Lv C, Sun M and
Shang Q: The value of serum Mac-2 binding protein glycosylation
isomer in the diagnosis of liver fibrosis: A systematic review and
meta-analysis. Front Physiol. 15(1382293)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shephard DA: The 1975 declaration of
Helsinki and consent. Can Med Assoc J. 115:1191–1192.
1976.PubMed/NCBI
|
|
8
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oh JH, Ahn SB, Cho S, Nah EH, Yoon EL and
Jun DW: Diagnostic performance of non-invasive tests in patients
with MetALD in a health check-up cohort. J Hepatol. 81:772–780.
2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Männistö V, Salomaa V, Jula A, Lundqvist
A, Männistö S, Perola M and Åberg F: ALT levels, alcohol use, and
metabolic risk factors have prognostic relevance for liver-related
outcomes in the general population. JHEP Rep.
6(101172)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Díaz LA, Lazarus JV, Fuentes-López E,
Idalsoaga F, Ayares G, Desaleng H, Danpanichkul P, Cotter TG, Dunn
W, Barrera F, et al: Disparities in steatosis prevalence in the
United States by Race or Ethnicity according to the 2023 criteria.
Commun Med (Lond). 4(219)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Choe HJ, Moon JH, Kim W, Koo BK and Cho
NH: Steatotic liver disease predicts cardiovascular disease and
advanced liver fibrosis: A community-dwelling cohort study with
20-year follow-up. Metabolism. 153(155800)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Israelsen M, Francque S, Tsochatzis EA and
Krag A: Steatotic liver disease. Lancet. 404:1761–1778.
2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moon JH, Jeong S, Jang H, Koo BK and Kim
W: Metabolic dysfunction-associated steatotic liver disease
increases the risk of incident cardiovascular disease: A nationwide
cohort study. EClinicalMedicine. 65(102292)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rinella ME, Neuschwander-Tetri BA,
Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE and
Loomba R: AASLD practice guidance on the clinical assessment and
management of nonalcoholic fatty liver disease. Hepatology.
77:1797–1835. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huttasch M, Roden M and Kahl S: Obesity
and MASLD: Is weight loss the (only) key to treat metabolic liver
disease? Metabolism 157:. 155937(155937)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ogawa Y, Tomeno W, Imamura Y, Baba M, Ueno
T, Kobayashi T, Iwaki M, Nogami A, Kessoku T, Honda Y, et al:
Distribution of Fibrosis-4 index and vibration-controlled transient
elastography-derived liver stiffness measurement for patients with
metabolic dysfunction-associated steatotic liver disease in health
check-up. Hepatol Res: Oct 4, 2024 (Epub ahead of print).
|
|
18
|
Kaplan DE, Teerlink CC, Schwantes-An TH,
Norden-Krichmar TM, DuVall SL, Morgan TR, Tsao PS, Voight BF, Lynch
JA, Vujković M and Chang KM: Clinical and genetic risk factors for
progressive fibrosis in metabolic dysfunction-associated steatotic
liver disease. Hepatol Commun. 8(e0487)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Younossi ZM, Paik JM, Henry L, Stepanova M
and Nader F: Pharmaco-economic assessment of screening strategies
for high-risk MASLD in primary care. Liver Int.
45(e16119)2025.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Patel S, Kim RG, Shui AM, Magee C, Lu M,
Chen J, Tana M, Huang CY and Khalili M: Fatty liver education
promotes physical activity in vulnerable groups, including those
with unhealthy alcohol use. Gastro Hep Adv. 3:84–94.
2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shay JES, Vannier A, Tsai S, Mahle R, Diaz
PML, Przybyszewski E, Challa PK, Patel SJ, Suzuki J, Schaefer E, et
al: Moderate-high intensity exercise associates with reduced
incident alcohol-associated liver disease in high-risk patients.
Alcohol Alcohol. 58:472–477. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Frith J, Day CP, Robinson L, Elliott C,
Jones DEJ and Newton JL: Potential strategies to improve uptake of
exercise interventions in non-alcoholic fatty liver disease. J
Hepatol. 52:112–116. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Beygi M, Ahi S, Zolghadri S and Stanek A:
Management of metabolic-associated fatty liver disease/metabolic
dysfunction-associated steatotic liver disease: From medication
therapy to nutritional interventions. Nutrients.
16(2220)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marti-Aguado D, Calleja JL, Vilar-Gomez E,
Iruzubieta P, Rodríguez-Duque JC, Del Barrio M, Puchades L,
Rivera-Esteban J, Perelló C, Puente A, et al: Low-to-moderate
alcohol consumption is associated with increased fibrosis in
individuals with metabolic dysfunction-associated steatotic liver
disease. J Hepatol. 81:930–940. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Griswold MG, Fullman N, Hawley C, Arian N,
Zimsen SRM, Tymeson HD, Venkateswaran V, Tapp AD, Forouzanfar MH,
Salama JS and Abate KH: Alcohol use and burden for 195 countries
and territories, 1990-2016: A systematic analysis for the global
burden of disease study 2016. Lancet. 392:1015–1035.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Díaz LA, Arab JP, Louvet A, Bataller R and
Arrese M: The intersection between alcohol-related liver disease
and nonalcoholic fatty liver disease. Nat Rev Gastroenterol
Hepatol. 20:764–783. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arab JP, Díaz LA, Rehm J, Im G, Arrese M,
Kamath PS, Lucey MR, Mellinger J, Thiele M, Thursz M, et al:
Metabolic dysfunction and alcohol-related liver disease (MetALD):
Position statement by an expert panel on alcohol-related liver
disease. J Hepatol. 82:744–756. 2025.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Adamson SJ, Heather N, Morton V and
Raistrick D: UKATT Research Team. Initial preference for drinking
goal in the treatment of Alcohol problems: II. Treatment outcomes.
Alcohol Alcohol. 45:136–142. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Haber PS: Identification and treatment of
alcohol use disorder. N Engl J Med. 392:258–266. 2025.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu M, Ye Z, Zhang Y, He P, Zhou C, Yang
S, Zhang Y, Gan X and Qin X: Accelerometer-derived
moderate-to-vigorous physical activity and incident nonalcoholic
fatty liver disease. BMC Med. 22(398)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Stine JG, Schreibman IR, Faust AJ, Dahmus
J, Stern B, Soriano C, Rivas G, Hummer B, Kimball SR, Geyer NR, et
al: NASHFit: A randomized controlled trial of an exercise training
program to reduce clotting risk in patients with NASH. Hepatology.
76:172–185. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nakano M, Kawaguchi M, Kawaguchi T and
Yoshiji H: Profiles associated with significant hepatic fibrosis
consisting of alanine aminotransferase >30 U/l, exercise habits,
and metabolic dysfunction-associated steatotic liver disease.
Hepatol Res. 54:655–666. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim SY and Kim HJ: Obesity risk was
associated with alcohol intake and sleep duration among Korean men:
The 2016-2020 Korea national health and nutrition examination
survey. Nutrients. 16(3950)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Miller-Matero LR, Yeh HH, Ma L, Jones RA,
Nadolsky S, Medcalf A, Foster GD and Cardel MI: Alcohol use and
antiobesity medication treatment. JAMA Netw Open.
7(e2447644)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Avitabile E, Gratacós-Ginès J,
Pérez-Guasch M, Belén Rubio A, Herms Q, Cervera M, Nadal R, Carol
M, Fabrellas N, Bruguera P, et al: Liver fibrosis screening
increases alcohol abstinence. JHEP Rep. 6(101165)2024.PubMed/NCBI View Article : Google Scholar
|