Introduction
Gastroesophageal reflux disease (GERD) is a notable
global health concern. As of 2024, the global prevalence of GERD is
~13.9%, affecting nearly 1.03 billion individuals worldwide.
However, prevalence rates exhibit notable regional variation: Rates
range from 8.7 to 33.1% in the Middle East, 8.8 to 25.9% in Europe
and 2.5 to 7.8% in East Asia (1).
This increasing trend in GERD prevalence is attributed to multiple
factors, including shifts in dietary and lifestyle habits,
declining rates of Helicobacter pylori infection, enhanced
diagnostic capability and evolving criteria in the endoscopic
evaluation of reflux-related pathology (2,3).
Reflux esophagitis (RE), the erosive form of GERD, is now estimated
to affect ~10% of the adult population (4,5). GERD
is characterized by the abnormal reflux of gastric content into the
esophagus, resulting in symptoms (primarily heartburn and
regurgitation) that can disrupt daily functioning and impair
quality of life (QOL). It may also present with mucosal injury or
both symptomatology and endoscopic abnormality (2,6).
Clinically, GERD is broadly categorized into two forms: RE, which
is defined by visible mucosal damage on endoscopy, and non-erosive
reflux disease, where patients report similar symptoms despite the
absence of endoscopic findings (7).
The primary goals of GERD management are sustained symptom relief,
improved QOL and preventing complications such as anemia,
esophageal strictures, Barrett's esophagus and esophageal
adenocarcinoma (8). Guidelines for
gastroesophageal reflux disease 2021 recommend an individualized
treatment approach based on disease severity (2). Proton pump inhibitors (PPIs) or
potassium-competitive acid blocker (P-CABs) are typically used as
initial therapy, with P-CABs often preferred in more severe or
refractory cases. In patients with severe RE, long-term maintenance
therapy is generally recommended to prevent recurrence and
complications. By contrast, individuals with mild RE may benefit
from an on-demand or intermittent treatment strategy. Previous
studies (9,10) have demonstrated that progression of
mild RE [particularly Los Angeles (LA) classification grades A and
B] is relatively infrequent. Mucosal deterioration is observed in
~10% of untreated cases over time, suggesting a generally stable
course in the absence of ongoing mucosal injury.
Vonoprazan, a P-CAB developed in Japan, represents a
class of acid-suppressing agents that reversibly inhibit the
H+/K+-ATPase enzyme in gastric parietal cells
by competitively binding the potassium site, thereby blocking the
final step of acid secretion (11-15).
By contrast with PPIs, vonoprazan does not require activation in an
acidic environment and is unaffected by Cytochrome P450 2C19
(CYP2C19) polymorphisms (12).
These pharmacological characteristics contribute to its more rapid,
consistent and sustained acid suppression. Its high acid
dissociation constant (9.06) facilitates selective accumulation in
the acidic canaliculi of parietal cells, supporting a prolonged
duration of action and effective acid control from the first dose
(11-15).
Clinical studies have demonstrated that vonoprazan provides more
potent and sustained acid suppression compared with conventional
PPIs (13-17).
Multiple clinical trials have confirmed the efficacy of vonoprazan
(20 mg) in patients with PPI-refractory RE, demonstrating faster
symptom relief and superior mucosal healing compared with
lansoprazole (13,18). At 8 weeks, vonoprazan (20 mg) has
shown healing rates that are 3.0-8.5% higher than those of
lansoprazole (30 mg), and up to 1.1-fold greater efficacy in cases
of severe erosive esophagitis. In PPI-refractory populations,
vonoprazan is associated with a 2-3-fold improvement in mucosal
healing compared with historical data on PPIs (19,20).
Studies have further supported the effectiveness of vonoprazan (20
mg) in most patients with PPI-refractory RE (21-23).
In addition to mucosal healing, vonoprazan has demonstrated more
rapid and sustained relief of persistent heartburn symptoms
compared with lansoprazole (18-20).
Our previous study assessed the efficacy of vonoprazan (10 mg)
administered once daily as maintenance therapy over a 48-week
period in patients with PPI-refractory RE who achieved mucosal
healing following vonoprazan (20 mg) induction therapy (24). The maintenance regimen demonstrated
high efficacy, with an endoscopic non-recurrence rate of 88.0% at
week 48, and symptom control was maintained in the majority of
patients. These results highlighted the potential of vonoprazan (10
mg) as a viable long-term maintenance strategy for individuals
unresponsive to standard PPI treatment (24). Despite its proven efficacy in
healing erosive esophagitis and its superiority in PPI-refractory
cases, data on vonoprazan long-term use in patients who experience
symptom or mucosal relapse following initial healing remain limited
(25). A previous study (26) have addressed outcomes in patients
who relapse following treatment discontinuation and require
retreatment or dose adjustment.
Therefore, the present study aimed to evaluate the
long-term effectiveness of vonoprazan in maintaining remission and
preventing recurrence in patients with erosive esophagitis,
particularly those who had previously achieved mucosal healing but
later relapsed. This represents a critical unmet need in GERD
management and may provide evidence to guide therapeutic strategies
for difficult-to-treat cases.
Materials and methods
Study design
This was an open-label, single-center follow-up
study extending from our previous prospective investigation
(24), conducted to evaluate the
efficacy of vonoprazan over a 96-week maintenance period in
patients with healed RE. The study was performed at Toyama City
Hospital (Toyama, Japan) between March 2016 and March 2019. The
present follow-up analysis included only patients who had
successfully completed the initial 48-week vonoprazan maintenance
therapy without discontinuation due to adverse events, mucosal
relapse or loss to follow-up. The study protocol was approved by
the Ethics Committee of Toyama City Hospital (approval no.
2014-21). Prior to enrollment, all participants received a
comprehensive explanation of the study purpose, procedures,
potential risks and anticipated benefits. Written informed consent
was obtained in accordance with the principles outlined in the
Declaration of Helsinki.
Patients and treatment
Inclusion criteria were as follows: Age ≥20 years;
endoscopically confirmed RE classified as LA grades A-D (27) prior to PPI therapy; diagnosis of
PPI-refractory RE, defined by a total score ≥8 on the Frequency
Scale for the Symptoms of GERD (FSSG) (28) despite receiving standard-dose PPI
therapy (rabeprazole 10 mg/day, omeprazole 20 mg/day, esomeprazole
20 mg/day or lansoprazole 30 mg/day) for ≥8 weeks and documented
endoscopic healing of erosive lesions following a 4-week course of
vonoprazan (20 mg/day).
Exclusion criteria were as follows: Notable organ
dysfunction; malignancy or coexisting gastrointestinal disorder,
including esophageal stricture, achalasia, eosinophilic
esophagitis, inflammatory bowel disease, primary esophageal
motility disorders, Zollinger-Ellison syndrome or malabsorption
syndromes.
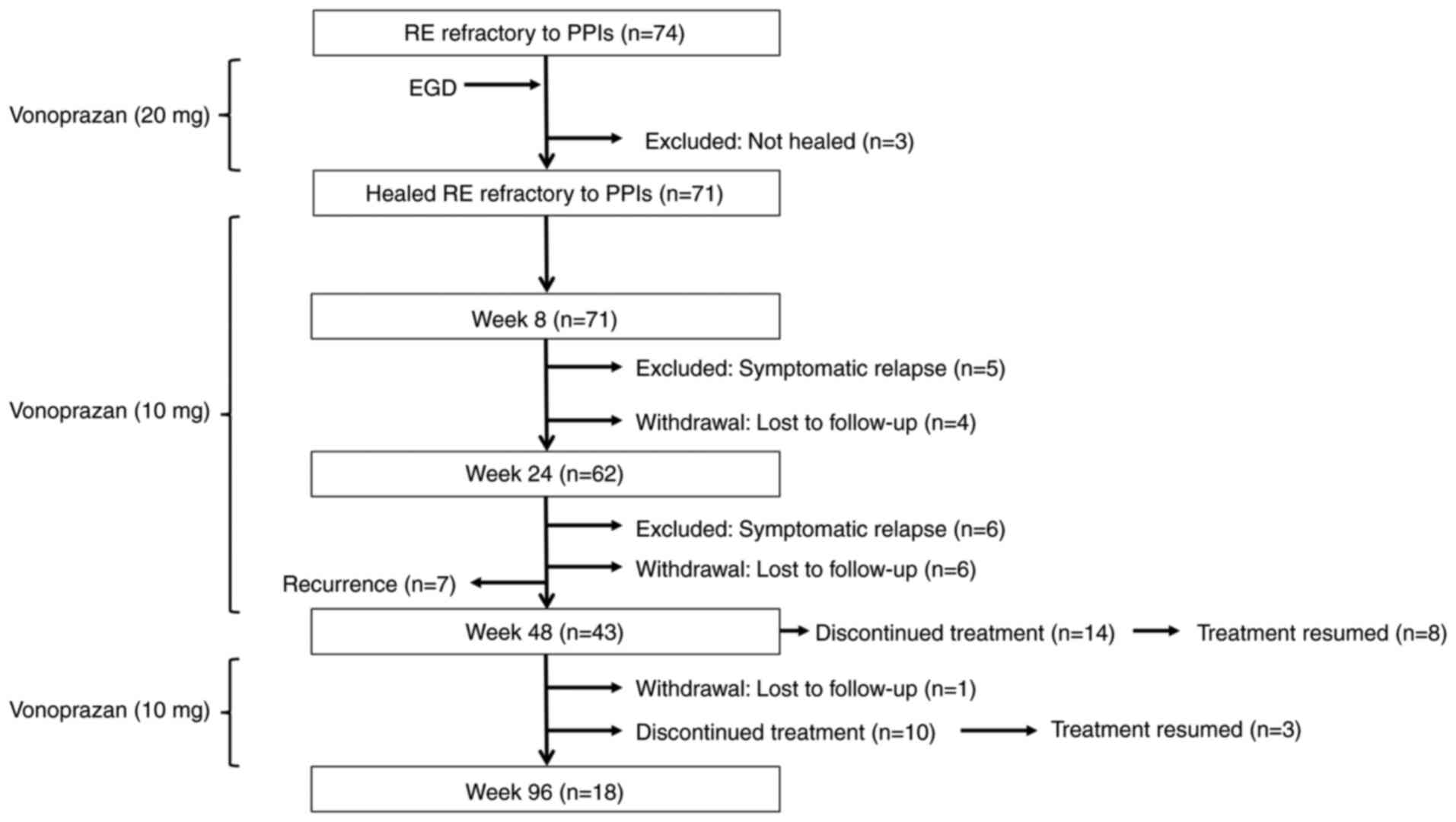
Initially, 71 patients (Age, 28-85 years,
male/female 34/37) with PPI-refractory RE who achieved mucosal
healing following 4-week induction therapy with vonoprazan (20
mg/day) were enrolled in a 48-week maintenance phase with
vonoprazan (10 mg/day; Fig. 1). Of
these, 50 patients successfully completed the full 48-week regimen,
however, seven patients were excluded from the final analysis due
to recurrence of erosive lesions: Four with LA grade A, one with
grade B and two with grade C. The remaining 43 patients were
enrolled in the 96-week maintenance phase. All patients received
vonoprazan (10 mg) once daily, with the option to temporarily
discontinue medication if symptoms resolved. Patients who elected
to pause treatment were monitored on an outpatient basis every 8-12
weeks for up to 96 weeks. In cases of symptomatic relapse or
endoscopic evidence of mucosal recurrence, therapy was reinitiated
with vonoprazan (20 mg) once daily. Morning fasting serum gastrin
levels were measured either at the end of the vonoprazan (10 mg)
maintenance phase or at the 96-week time point to monitor
hypergastrinemia, a known pharmacological effect associated with
prolonged potent acid suppression (29). These measurements also served to
assess the safety profile of long-term vonoprazan therapy and
explore potential associations between gastrin levels, symptom
recurrence and mucosal healing outcomes. H. pylori infection
was assessed using serum anti-H. pylori IgG antibody titers,
measured by the H. pylori-LATEX ‘SEIKEN’ assay (Denka Seiken
Co., Ltd.), according to the manufacturer's instructions. A cut-off
value of ≥10 U/ml was used to define H. pylori
seropositivity.
Endoscopic evaluation and study
endpoints
At the time of diagnosis and during follow-up, upper
gastrointestinal endoscopy was performed by four experienced
endoscopists to assess mucosal recurrence following maintenance
therapy. The grading of erosive esophagitis was independently
confirmed by two endoscopists to ensure diagnostic consistency. The
severity of esophagitis was evaluated using a modified LA
classification system (27). LA
classification system is based on the length and extent of mucosal
breaks observed during upper gastrointestinal endoscopy and offers
a reproducible framework for both clinical diagnosis and
therapeutic evaluation. The present study employed a modified LA
classification that included the standard LA grades A to D, as well
as two additional non-erosive categories: Grade M, indicating
minimal changes such as erythema or whitish turbidity and grade N,
indicating no visible mucosal abnormalities (30,31).
This expanded classification allowed more comprehensive assessment
of the full spectrum of mucosal findings. Healing of reflux
esophagitis was defined as an endoscopic assessment of grade N or
M, reflecting the absence of erosive lesions.
Hiatal hernia was diagnosed when retroflexed
endoscopic examination under gastric insufflation revealed a
widened esophageal hiatus with visualization of squamous epithelium
below the diaphragmatic impression (32).
The presence of atrophic gastritis was assessed
endoscopically using the Kimura-Takemoto classification system,
which categorizes the extent of gastric mucosal atrophy into six
stages: C-0 for no atrophy; C-1 and C-2 for mild atrophy; C-3 and
O-1 for moderate atrophy; and O-2 and O-3 for severe atrophy
(33). The staging of atrophic
gastritis was independently confirmed by two endoscopists to ensure
diagnostic consistency.
Upper gastrointestinal endoscopy was performed at
the end of the treatment or at 96 weeks to evaluate the presence or
absence of recurrent esophageal mucosal erosion. The primary
endpoint was the endoscopic remission rate, defined as the
proportion of patients maintaining mucosal healing (grade N or M)
throughout the 96-week follow-up period.
Symptom evaluation and study
endpoints
GERD symptoms were assessed using FSSG (28), a validated questionnaire comprising
12 items, each scored on a 5-point Likert scale: 0=never,
1=occasionally, 2=sometimes, 3=often and 4=always. The items are
grouped into two subscales: Acid reflux-related symptoms and
dysmotility-related symptoms. A cumulative score ≥8 is considered
indicative of GERD or RE (28).
Participants completed the FSSG questionnaire at the
end of the treatment period or at the 96-week follow-up. The
results were compared with baseline scores obtained prior to the
initiation of maintenance therapy. Symptomatic improvement was
defined as a total FSSG score equal to or lower than the baseline
value. The rates of symptom improvement were analyzed separately
for acid reflux-symptoms and dysmotility-related symptoms to assess
therapeutic response. Symptom recurrence was specifically evaluated
based on acid reflux-related complaints (2) and defined as the presence of moderate
to severe heartburn and acid regurgitation persisting for 3
consecutive days during the maintenance phase. In addition, symptom
recurrence was corroborated by an increase in the total FSSG score
above the clinical threshold of ≥8, consistent with validated GERD
symptom assessment tools (28). In
cases of symptomatic exacerbation or endoscopic recurrence of
mucosal lesions, vonoprazan (20 mg) once daily was reintroduced as
re-treatment. The secondary endpoint was to assess the efficacy of
vonoprazan in patients who experienced recurrent RE during the
follow-up period.
Statistical analysis
Efficacy parameters were analyzed in the
per-protocol (PP) population. Continuous variables are presented as
median and ranges, while categorical variables are expressed as
frequency and percentages. The Mann-Whitney U test was used to
compare continuous variables, and the χ2 or Fisher's
exact test was used for categorical variables, as appropriate.
Kaplan-Meier analysis was used to estimate the time to first
symptom relapse, based on FSSG subscale score during the
maintenance phase. Patients who were lost to follow-up or
discontinued therapy for reasons unrelated to relapse were censored
in the analysis. Changes in FSSG scores from baseline to follow-up
were analyzed using paired t-test, with a two-sided significance
level of 5%. Data are presented as the mean ± SD. All statistical
analyses were performed using IBM SPSS Statistics for Windows,
version 29.0 (IBM Corp.).
Results
Study profile
A total of 43 patients were enrolled in the 96-week
maintenance phase (Fig. 1). Of
these, 14 patients (32.6%) experienced symptoms resolution and
elected to discontinue treatment during the early phase of
follow-up. The remaining 29 patients continued vonoprazan (10 mg)
once daily as maintenance therapy. During the maintenance phase, an
additional 10 patients (23.3%) discontinued treatment following
symptom resolution, and one patient (2.3%) was lost to follow-up. A
total of 18 patients (41.9%) completed the full 96-week maintenance
regimen with vonoprazan.
Patient demographics and clinical
characteristics [intention-to-treat (ITT) population]
A total of 43 patients were included in the ITT
population. Of these, 17 were male, representing 39.5% of the
cohort (Table I). The mean age of
the ITT population was 66.3±11.9 years, and the mean BMI was
23.6±3.7 kg/m². Prior to initiation of vonoprazan (20 mg) therapy,
the distribution of LA classification was as follows: Grade A in
72.1% of patients, grade B in 11.6%, grade C in 9.3% and grade D in
7.0%. The mean disease duration was 19.3±10.8 months. H.
pylori infection, as determined by serological testing, was
present in 32.6% of patients. Gastrointestinal comorbidities were
observed in 51.2% of patients, with the most common being hiatal
hernia (32.6%) and atrophic gastritis (20.9%; Table I). In addition to baseline
comorbidities, the mean fasting serum gastrin levels at the end of
the maintenance phase were 1,029.8±751.7 pg/ml (Table I). On-demand therapy was used by 26
patients, representing 60.5% of the study population. Overall, the
study cohort was composed primarily of older adults (aged ≥65
years) with mild to moderate reflux esophagitis and a relatively
high prevalence of gastrointestinal comorbidity, reflecting a
representative clinical population for evaluating the long-term
efficacy and safety of vonoprazan (Table I).
 | Table IDemographic and clinical
characteristics of patients at baseline (ITT population; n=43). |
Table I
Demographic and clinical
characteristics of patients at baseline (ITT population; n=43).
| Characteristic | Value |
|---|
| Mean age,
years | 66.3±11.9 |
| Sex,
male/female | 17/26 |
| Mean BMI,
kg/m2 | 23.6±3.7 |
| BMI ≥25 | 14 (32.6) |
| Duration of
illness, months (mean ± SD) | 19.3±10.8 |
| PPI used before VPZ
20 mg, RPZ/OPZ/EPZ/LPZ | 13/6/19/5 |
| Baseline LA
classification (grade A/B/C/D) | 31/5/4/3 |
| Helicobacter
pylori infection (%) | 14 (32.6) |
| Gastrointestinal
comorbidities, n (%) | 22 (51.2) |
|
Atrophy of
gastric mucosa, n (%) | 9 (20.9) |
|
Esophageal
hiatal hernia, n (%) | 14 (32.6) |
| Mean serum gastrin,
pg/ml | 1,029.8±751.7 |
| On-demand therapy,
n (%) | 26 (60.5) |
Endoscopic outcomes of maintenance
therapy (PP population)
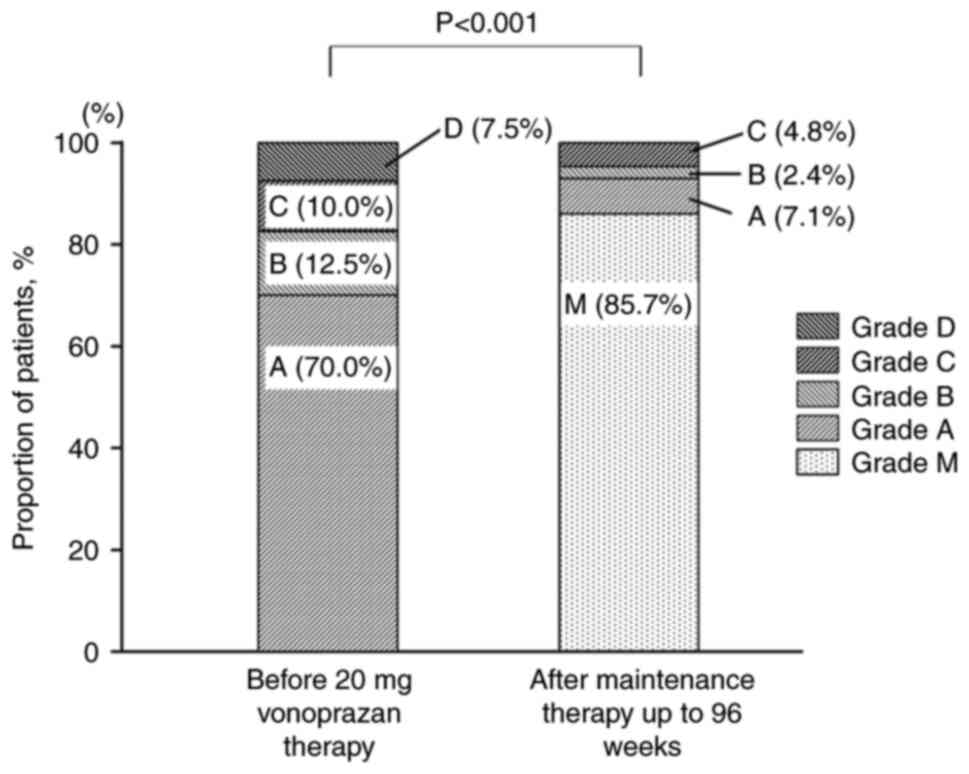
At the end of the 96-week maintenance period, 36/42
patients in the PP population were endoscopically classified as
grade M, corresponding to a mucosal non-recurrence rate of 85.7%
(Fig. 2). Of the six patients who
experienced recurrence of esophageal mucosal erosion, the severity
was classified as grade A in three cases, B in one case and C in
two cases. These findings suggest that long-term maintenance
therapy with vonoprazan (10 mg) once daily was effective in
sustaining mucosal healing in the majority of patients.
Efficacy of maintenance therapy: GERD
symptoms and gastrointestinal QoL (PP population)
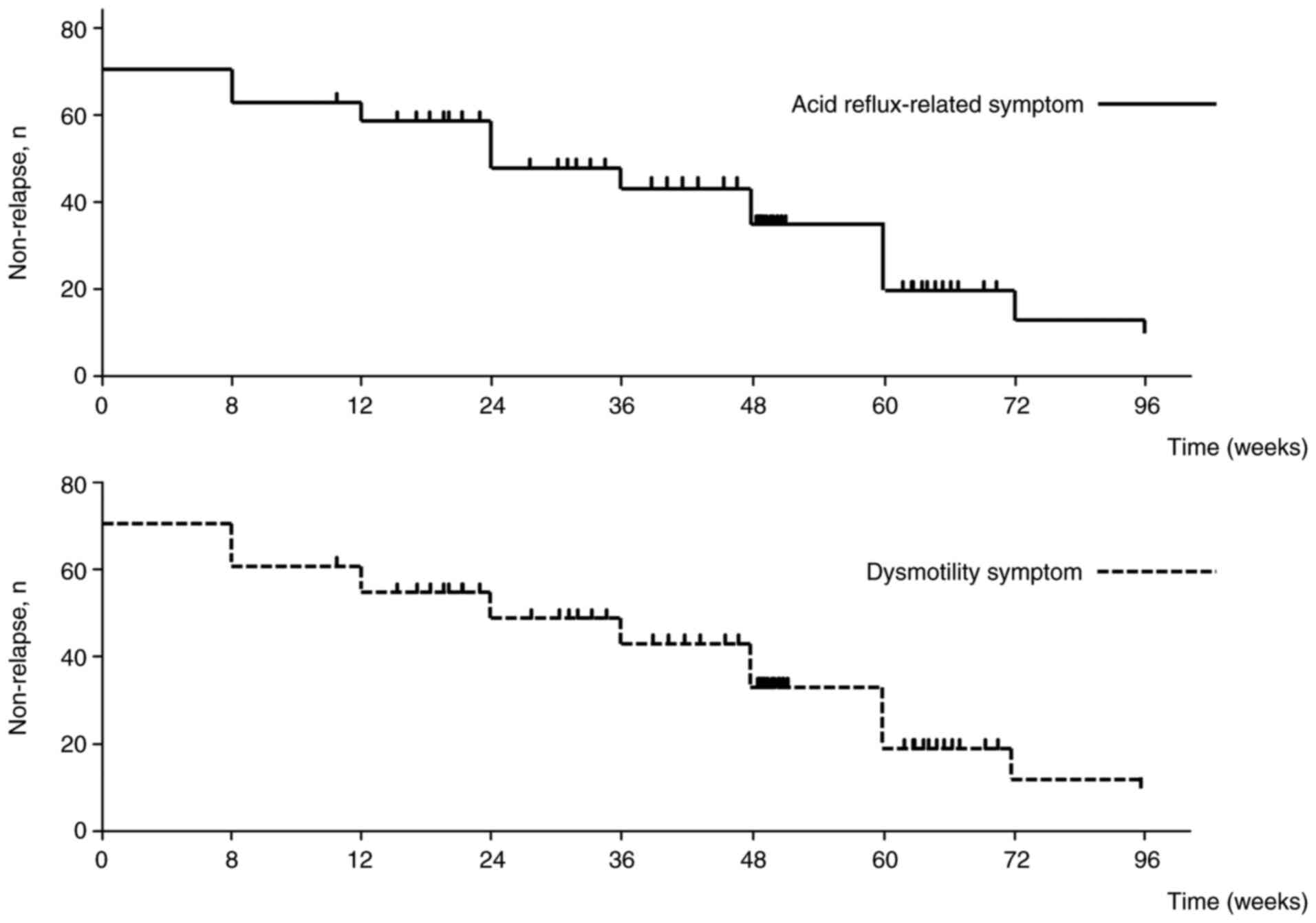
The symptom non-relapse rates for acid
reflux-related symptoms at weeks 8, 24, 48 and 96 were 88.4, 76.7,
69.8 and 55.8%, respectively (Fig.
3). For dysmotility-related symptoms, the corresponding rates
were 86.0, 79.1, 65.1 and 51.2%. These data indicated that
vonoprazan maintenance therapy offered strong initial symptomatic
control, however, its effectiveness in maintaining relief from GERD
symptoms gradually declines over time. This trend underscores the
importance of individualized, long-term management strategies for
optimizing patient outcomes.
Efficacy of vonoprazan in patients
with symptom relapse following completion of treatment (PP
population)
A total of 18 patients completed the full 96-week
course of vonoprazan maintenance therapy. Among the remaining
patients, 24 elected to discontinue treatment following symptom
resolution, with a mean treatment duration of 56.6±12.4 weeks
(range, 48-82 weeks). The mean fasting serum gastrin concentration
was 1,130.9±831.0 pg/ml in the continuous maintenance therapy group
and 864.7±748.5 pg/ml in the discontinuous maintenance therapy
group (Table II). Although serum
gastrin levels tended to be higher in patients who experienced
relapse, the difference was not statistically significant. These
findings suggest that fasting gastrin concentrations were not
strongly associated with either clinical or endoscopic recurrence
following discontinuation of vonoprazan therapy (Table II). Among the 11 patients who
experienced symptom recurrence following the discontinuation of
vonoprazan therapy, the mean duration of drug withdrawal prior to
relapse was 22.3±9.5 weeks (range, 8-38 weeks; Table III). The mean age of these
patients was 65.8±12.2 years, with a mean BMI of 22.8±3.6 kg/m².
H. pylori infection was present in 27.3% of cases.
Gastrointestinal comorbidities were reported in 45.5% of patients,
including atrophic gastritis in 9.1% and hiatal hernia in 36.4%.
Endoscopic recurrence of esophageal mucosal erosion was observed in
5/11 patients who experienced symptom relapse following
discontinuation of vonoprazan therapy. The severity of mucosal
relapse based on the LA classification was distributed as follows:
Grade A, n=2; B, n=1 and C, n=2. All 11 patients were re-treated
with vonoprazan (20 mg) once daily. Following re-initiation of
therapy, symptomatic improvement was achieved in all cases and
treatment was subsequently discontinued following a mean
retreatment duration of 21.5±9.6 weeks (range, 6-40 weeks; Table III). These findings suggest that
recurrence may occur even several months after initial drug
withdrawal, particularly in patients with anatomical or mucosal
risk factors such as hiatal hernia. Nevertheless, the high success
rate of retreatment highlighted the efficacy of vonoprazan in
managing recurrent GERD symptoms following treatment
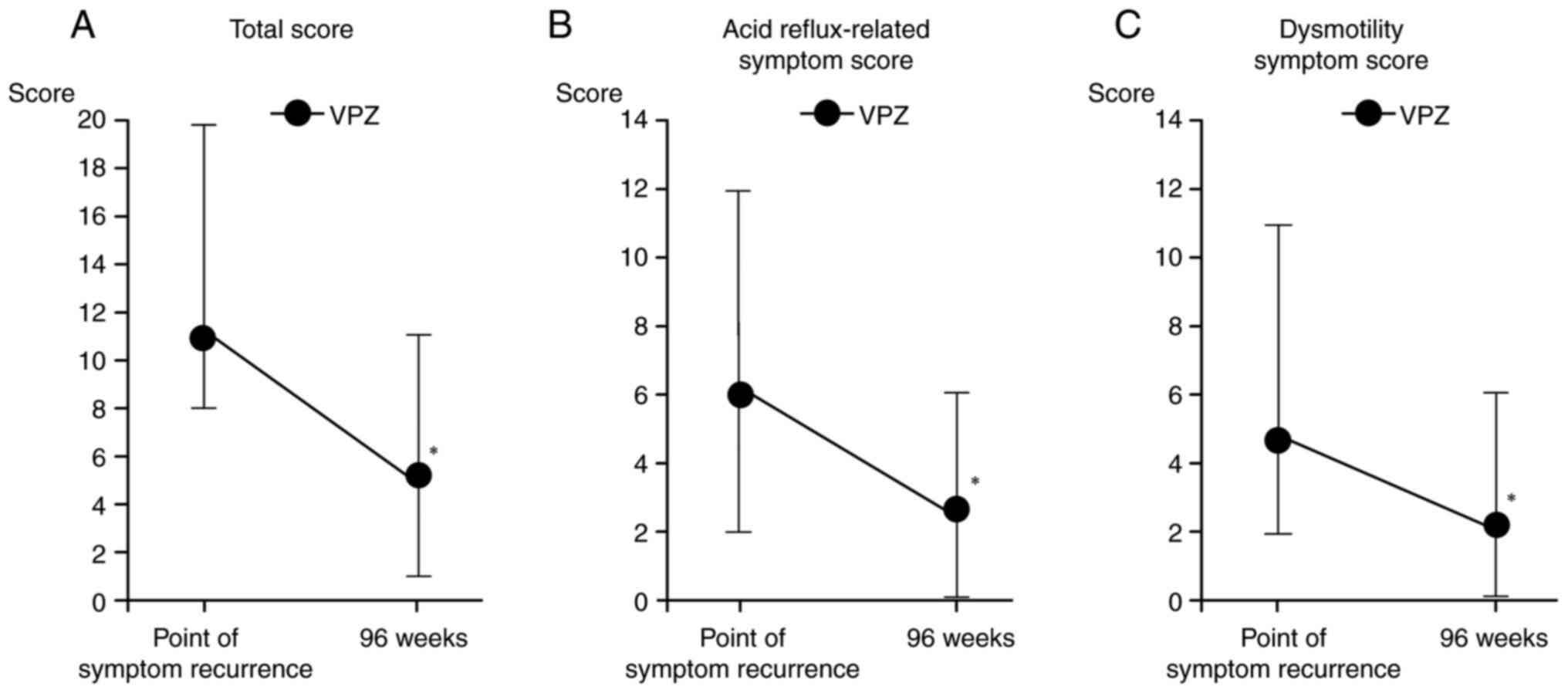
discontinuation. At the time of symptom recurrence, the mean total
FSSG score was 10.8±4.4, with acid reflux-related and dysmotility
symptom subscale scores of 6.0±3.1 and 4.8±2.9, respectively.
Following retreatment, these scores decreased significantly to
5.1±2.9 (total), 2.6±1.6 (acid reflux-related) and 2.5±1.9
(dysmotility-related; Fig. 4A-C).
The decreases in total, acid reflux- and dysmotility-related
symptom scores were significant (P=0.002, 0.004 and 0.037,
respectively), supporting the robust symptomatic efficacy of
vonoprazan following re-administration. These results reinforce the
use of vonoprazan not only as an effective long-term maintenance
therapy but also as a flexible and reliable rescue treatment
strategy for patients who relapse following therapy
discontinuation.
 | Table IIClinical characteristics between the
patients undergoing continuous and discontinuous maintenance
therapy. |
Table II
Clinical characteristics between the
patients undergoing continuous and discontinuous maintenance
therapy.
| Characteristic | Continuous
maintenance therapy (n=18) | Discontinuous
maintenance therapy (n=24) | P-value |
|---|
| Mean age,
years | 65.2±13.2 | 67.2±11.2 | 0.619 |
| Sex,
male/female | 5/13 | 11/13 | 0.192 |
| Mean BMI,
kg/m2 | 23.6±3.7 | 23.1±3.8 | 0.683 |
| Mean duration of
illness, months | 22.6±23.4 | 20.3±21.0 | 0.735 |
| Baseline LA
classification (grade A/B/C/D) | 14/2/0/2 | 16/3/4/1 | 0.351 |
| Helicobacter
pylori infection, positive/negative | 2/7 | 5/7 | 0.161 |
| Atrophy of gastric
mucosa, n (%) (closed type/open type) | 3 (25.0) 3/0 | 6 (21.2) 6/0 | 0.398 |
| Esophageal hiatal
hernia (%) | 6 (33.3) | 8 (33.3) | 0.627 |
| Mean serum gastrin,
pg/ml | 1,130.9±831.0 | 864.7±748.5 | 0.346 |
| On-demand therapy,
n (%) | 8 (44.4) | 17 (70.8) | 0.081 |
| Mean duration of
treatment, weeks | 96 | 56.6±12.4 | 0.001 |
 | Table IIIClinical characteristics of patients
with recurrence of symptoms after discontinuation of
vonoprazan. |
Table III
Clinical characteristics of patients
with recurrence of symptoms after discontinuation of
vonoprazan.
| Age, years | Sex | LA grade at
recurrence | Duration until
recurrence, weeks | Duration of
re-treatment of vonoprazan, weeks | BMI
kg/m2 | Helicobacter
pylori infection | Atrophy of gastric
mucosa | Esophageal hiatal
hernia | FSSG total score at
recurrence |
|---|
| 68 | Female | LA-C | 24 | 24 | 17.5 | Negative | No | No | 9 |
| 46 | Female | LA-M | 24 | 24 | 32.4 | Negative | No | No | 9 |
| 71 | Male | LA-M | 20 | 24 | 24.9 | Positive | No | Yes | 8 |
| 76 | Male | LA-M | 32 | 16 | 21.9 | Negative | No | No | 8 |
| 68 | Male | LA-B | 16 | 32 | 19.5 | Negative | No | Yes | 8 |
| 80 | Female | LA-C | 26 | 20 | 24.1 | Negative | No | Yes | 18 |
| 59 | Male | LA-M | 8 | 40 | 22.5 | Negative | No | No | 8 |
| 70 | Male | LA-A | 38 | 10 | 28.9 | Negative | No | No | 10 |
| 84 | Male | LA-A | 14 | 25 | 19.6 | Negative | Closed type | No | 11 |
| 53 | Female | LA-M | 11 | 6 | 18.3 | Positive | No | Yes | 21 |
| 49 | Female | LA-M | 32 | 16 | 21.3 | Positive | No | No | 9 |
Safety profile
Throughout the 96-week study period, no serious
adverse events were observed. Treatment-related adverse effects
associated with vonoprazan (10 mg) occurred in 3/43 patients
(7.0%), all of whom experienced mild constipation. These symptoms
resolved spontaneously during continued administration, and no
additional therapeutic intervention was required. Although gastrin
levels were elevated [a known pharmacodynamic effect of potent acid
suppression (34)], no cases of
hypergastrinemia-related complications, including carcinoid tumors,
were identified. These findings indicated that while vonoprazan
induces sustained elevations in serum gastrin during long-term use,
the increase remained clinically benign within the 96-week
observation period. This supported the safety of extended
vonoprazan maintenance therapy and efficacy in a real-world
clinical setting.
Discussion
To the best of our knowledge, the present study is
the first to evaluate the clinical effectiveness of vonoprazan
re-treatment in patients who experience symptom recurrence
following completion of long-term maintenance therapy, offering
novel insights into relapse management strategies in GERD. The
present findings demonstrated that on-demand or intermittent use of
vonoprazan (10 mg) once daily maintained endoscopic remission in
85.7% of patients over a 96-week period. Furthermore, re-initiation
of vonoprazan (20 mg) once daily in patients with symptomatic or
endoscopic relapse resulted in rapid and effective resolution of
both symptoms and mucosal erosion. These results highlighted the
dual therapeutic utility of vonoprazan as a potent and durable
long-term maintenance agent and as a reliable rescue therapy
following recurrence, even in patients previously exposed to
treatment. This flexibility may support individualized treatment
approaches, particularly in patients requiring intermittent acid
suppression tailored to fluctuating symptom severity or risk of
mucosal relapse. The 96-week observation period was selected to
capture long-term maintenance outcomes that extend beyond the
conventional 6-12-month follow-up commonly reported in GERD studies
(2,35). This extended duration was designed
to assess the durability of mucosal healing and the incidence of
symptomatic or endoscopic recurrence over time. The 96-week
framework also allowed for assessment of vonoprazan performance in
patients who temporarily discontinued therapy or required
re-treatment during the follow-up period.
GERD is a chronic condition with a high tendency to
recur, often necessitating long-term maintenance therapy to prevent
symptom relapse and mucosal deterioration. The principal
therapeutic goals in GERD management include sustained symptom
relief and continued healing of esophageal mucosa (36,37).
Previous studies have reported that >70% of patients experience
symptom recurrence within 6-12 months following discontinuation of
initial therapy, underscoring the persistent and relapsing nature
of the disease (38,39). The present study further confirmed
the therapeutic efficacy of vonoprazan in the management of
recurrent reflux esophagitis. The ability of vonoprazan to induce
symptom resolution and mucosal healing even after prior treatment
discontinuation supports its role as a reliable agent in the
long-term management of GERD.
The exploratory VISION study evaluated the long-term
safety profile of vonoprazan maintenance therapy over a 5-year
period in patients with healed erosive esophagitis, demonstrating
its tolerability and sustained efficacy (34). Emerging evidence from suggests that
long-term therapy with vonoprazan provides superior control of GERD
symptoms and mucosal healing compared with conventional PPIs
(34,40). By contrast with large-scale
investigations such as the VISION trial, which evaluated vonoprazan
in a broad population of patients with healed erosive esophagitis,
the present study focused on patients with PPI-refractory disease.
It further explored the clinical outcomes of a personalized
management approach, incorporating long-term maintenance therapy,
on-demand use and retreatment strategies. This tailored framework
offers practical insight into real-world therapeutic flexibility
and supports the use of vonoprazan in managing patients at higher
risk of relapse or requiring individualized dosing regimens.
The safety profiles of vonoprazan at both 10 and 20
mg are comparable with that of lansoprazole (15 mg) in patients
with healed erosive esophagitis (41,42).
In the present study, on-demand administration of vonoprazan (10
mg) was effective in maintaining endoscopic remission within a
96-week period, with a mucosal non-recurrence rate of 85.0%. These
findings support the feasibility of an individualized,
symptom-guided dosing strategy for select patients. The cohort
analyzed in the present study represented a treatment-responsive
and tolerant subgroup, as it includes only those patients who
completed the initial 48-week vonoprazan maintenance therapy
without significant adverse events or therapeutic failure. As such,
the findings primarily reflect the long-term efficacy and safety of
vonoprazan in responders, rather than the general population with
PPI-refractory reflux esophagitis. Furthermore, Oshima et al
(18) demonstrated that vonoprazan
(20 mg) once daily provided significantly faster and more complete
relief of heartburn during the first week of treatment compared
with lansoprazole (30 mg) once daily in patients with erosive
esophagitis. Together, these data underscore the potent acid
suppression, rapid onset of action and safety of vonoprazan, making
it a valuable therapeutic option across various GERD treatment
scenarios, including both maintenance and rescue settings.
In the present study, 11 patients required
re-treated with vonoprazan (20 mg) once daily following symptom
recurrence. Symptom resolution was achieved following a median
treatment duration of 25 weeks. A subset of patients with GERD
experience spontaneous symptom resolution or maintain symptom
control without continuous medication (9,43-46).
In the present study, 74.4% of patients who achieved symptom
resolution maintained good symptom control without ongoing
pharmacological therapy. A large cohort study reported that 24-26%
of patients with mild erosive esophagitis and 16-18% with severe
erosive esophagitis remained off medication during each year of a
4-year follow-up period (47). In
the present cohort, 11/24 patients (45.8%) who discontinued
vonoprazan experienced symptom recurrence. This recurrence rate is
considerably lower than that reported in a systematic review of 19
studies, which found a mean recurrence rate of 75% (95% CI: 68-82%)
in patients receiving placebo over follow-up periods ranging from 6
months to 5 years (48). The
relatively low recurrence rate in the present study is likely
attributable to the robust mucosal healing achieved during the
initial induction phase with vonoprazan (20 mg). This observation
aligns with findings from Hsu et al (49), who reported that longer and more
potent acid suppression during the initial treatment phase
significantly decreased the risk of recurrence during maintenance
therapy. Therefore, the strong initial activity of vonoprazan
serves an important role in both the initial and maintenance
therapy. During the initial treatment phase, its rapid and potent
acid suppression facilitates faster mucosal healing and symptom
relief, which is key for patients with PPI-refractory disease. In
the maintenance phase, vonoprazan sustains intragastric pH levels
>4 for extended durations, thereby minimizing acid exposure to
the esophageal mucosa and decreasing the risk of mucosal relapse.
This dual-phase pharmacological profile makes vonoprazan
particularly well-suited for comprehensive management of GERD,
serving effectively as both an aggressive induction agent and a
long-term maintenance option. Its consistent acid suppression is
especially advantageous in patients with fluctuating symptom
patterns or anatomical risk factors such as hiatal hernia, where
stable pH control is essential to maintaining clinical
remission.
During the course of long-term maintenance therapy,
some patients elected to use vonoprazan (20 mg) on an on-demand
basis. Among the 18 patients who completed the 96-week maintenance
period, eight adopted this strategy and achieved satisfactory
symptom control without requiring daily medication. Various
therapeutic strategies have been proposed for the long-term
management of GERD, including continuous therapy, where PPIs are
taken daily, and several non-continuous approaches. These include
on-demand therapy, in which PPIs are used only when symptoms arise
and discontinued upon relief; intermittent therapy, typically
consisting of short treatment courses (1-2 weeks) in response to
symptom recurrence, and threshold therapy, where the interval
between doses is progressively extended as long as symptoms remain
absent (38). The present findings
support the feasibility of a flexible, symptom-guided approach
using vonoprazan, particularly in patients with stable or mild
disease following mucosal healing, and underscore its potential use
within personalized GERD treatment paradigms. A key advantage of
on-demand therapy is its efficiency in decreasing medication
burden: Studies (38,50) have shown that total PPI consumption
in this group is approximately half that of patients receiving
continuous therapy. In a real-world survey evaluating long-term PPI
maintenance therapy for GERD, no significant differences were
observed among continuous, on-demand and intermittent therapy
groups with regard to patient-reported outcomes, including symptom
control, satisfaction or preference for the type of maintenance
regimen (39). Patients with a
longer duration of GERD were more likely to adopt non-continuous
strategies, including on-demand use (39). Moreover, patients in the
non-continuous therapy group demonstrated higher awareness of the
potential adverse effects associated with long-term PPI use
compared with those on continuous therapy (39). On-demand therapy with vonoprazan is
an effective alternative maintenance strategy for patients with
mild RE (51,52). These findings suggest that on-demand
therapy not only decreases medication exposure but may align better
with patient preferences and safety awareness, especially in
individuals with chronic GERD who seek to minimize long-term
pharmacological dependency. The present study implemented an
individualized on-demand approach by monitoring patient adherence
through the remaining amount of vonoprazan tablets at each
follow-up visit. A total of 26/43 patients (60.5%) elected to
discontinue regular therapy, indicating successful symptom control
without daily medication. This approach is cost-effective, as it
minimizes unnecessary prescriptions and decreases overall drug
consumption. The findings further support the utility of a
flexible, patient-centered dosing strategy (particularly on-demand
use), which can achieve sustained symptom control in a substantial
proportion of patients. Such strategies not only lessen medication
burden but also improve cost-efficiency without compromising
therapeutic efficacy, making them a viable option for long-term
GERD management in appropriately selected individuals.
Vonoprazan is distinguished by its potent and rapid
acid-suppressive properties, which offer notable therapeutic
advantages in the treatment of GERD. However, such notable acid
inhibition has raised concerns regarding long-term safety,
particularly the potential risk of carcinoid tumor development
associated with sustained hypergastrinemia (53). Despite these concerns, vonoprazan
has consistently demonstrated a favorable safety and tolerability
profile in multiple clinical studies (19,21-23,34,40).
Notably, a 5-year clinical trial found no histological evidence of
hypergastrinemia-associated changes in the gastric mucosa,
including neuroendocrine cell hyperplasia or carcinoid tumor
formation (34). These findings
reinforce the long-term safety of vonoprazan, even during extended
use, and support its suitability as a maintenance therapy for GERD.
In the present study, the mean morning fasting serum gastrin levels
measured following a treatment regimen consisting of vonoprazan (20
mg) for 4 weeks followed by 10 mg for 96 weeks was 1,029.8 pg/ml.
This elevation is consistent with the pharmacological profile of
vonoprazan, which is known to induce hypergastrinemia due to
sustained acid suppression (34).
Monitoring of serum gastrin levels is an integral component of the
safety evaluation and provided supporting evidence that long-term
vonoprazan therapy, including on-demand or retreatment use, did not
result in clinically notable hypergastrinemia-associated
complications in the present cohort (34,40).
This degree of hypergastrinemia is anticipated, given the potent
and prolonged acid suppression of vonoprazan, which leads to
compensatory gastrin secretion via negative feedback mechanisms
(54). Elevated gastrin levels
stimulate enterochromaffin-like cell proliferation, raising
concerns regarding the potential development of gastric
neuroendocrine tumors or mucosal hyperplasia (34). However, clinical evidence suggests
that this elevation does not translate into clinically meaningful
pathological changes within a 2-year observation period (25,52).
In the present cohort, no histological abnormality, carcinoid
tumors or serious gastric mucosal events were observed. These
results are consistent with findings from the 5-year VISION study,
which also reported no hypergastrinemia-related neoplasia despite
sustained gastrin elevation (34).
Clinically, this implies that while serum gastrin levels should be
monitored during long-term vonoprazan therapy (particularly in
high-risk individuals) the elevations observed in typical patients
with GERD are physiologically adaptive and not inherently harmful.
Nonetheless, ongoing surveillance in larger, more diverse
populations and extended follow-up durations is key to exclude any
potential late-emerging risks.
The present study had limitations. First, the
single-center design and small sample size may limit the
generalizability of the findings to broader GERD populations.
Second, the open-label nature of the study introduced potential
patient-associated bias in symptom reporting and treatment
adherence. Third, objective physiological assessment of acid
suppression, such as 24-h esophageal pH monitoring, was not
performed; inclusion of such measures would have strengthened the
association between pharmacological effect and clinical outcomes.
Fourth, due to the non-randomized and observational design, the
potential for selection bias and confounding cannot be excluded.
Additionally, a key limitation was the enrichment of the analyzed
population with patients who had already demonstrated clinical
benefit and tolerability during a preceding 48-week course of
vonoprazan maintenance therapy. As a result, the findings primarily
reflect outcomes in a responder subgroup and may not be fully
generalizable to all patients with PPI-refractory erosive
esophagitis. Despite these limitations, the present study provided
real-world insight into the management of a high-risk GERD
population, including patient-driven decisions regarding treatment
continuation, discontinuation and re-initiation. Future studies
should aim to include larger, more diverse patient cohorts and
incorporate randomized, controlled designs with comparator arms to
evaluate the long-term efficacy and safety of vonoprazan across the
full clinical spectrum of GERD.
In conclusion, long-term maintenance therapy with
vonoprazan, particularly when individualized through on-demand use,
is both safe and effective in preventing symptom recurrence and
mucosal relapse in patients with erosive esophagitis. The present
study highlighted the practical utility of vonoprazan as a flexible
and reliable treatment option in real-world GERD management. While
the results are encouraging, confirmation through larger,
multicenter, randomized controlled trials is warranted to validate
these findings and broaden their generalizability.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HM, KY and SK were involved in the study concept and
design and performed the experiments. HM and SI contributed to data
analysis and interpretation. HM drafted and revised the manuscript.
All authors have read and approved the final manuscript. KY and SK
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Helsinki Declaration of 1964 and later versions and approved by
the Ethics Committee of Toyama City Hospital (Toyama, Japan;
approval no. 2014-21). Written informed consent was obtained from
all the patients prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alqassab DF, Hasan MJ, AlSaadoon AM,
AlMuqahwi AJ, AlAwadhi FA, Bahram SA and Alsayyad AS: Prevalence
and risk factors of gastroesophageal reflux disease among adults
attending primary healthcare in Bahrain. J Family Med Prim Care.
13:5758–5765. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iwakiri K, Fujiwara Y, Manabe N, Ihara E,
Kuribayashi S, Akiyama J, Kondo T, Yamashita H, Ishimura N,
Kitasako Y, et al: Evidence-based clinical practice guidelines for
gastroesophageal reflux disease 2021. J Gastroenterol. 57:267–285.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
El-Serag HB: Time trends of
gastroesophageal reflux disease: A systematic review. Clin
Gastroenterol Hepatol. 5:17–26. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fujiwara Y and Arakawa T: Epidemiology and
clinical characteristics of GERD in the Japanese population. J
Gastroenterol. 44:518–534. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wickramasinghe N and Devanarayana NM:
Insight into global burden of gastroesophageal reflux disease:
Understanding its reach and impact. World J Gastrointest Pharmacol
Ther. 16(97918)2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vakil N, Sander ZV, Peter K, John D and
Roger J: The Montreal definition and classification of GERD. Am J
Gastroenterol. 101:1900–1920. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Katz PO, Gerson LB and Marcelo VF:
Guidelines for the diagnosis and management of GERD. Am J
Gastroenterol. 108:308–328. 2013.PubMed/NCBI
|
|
8
|
Shaheen NJ and Richter JE: Barrett's
esophagus. N Engl J Med. 360:925–935. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Manabe N, Yoshihara M, Sasaki A, Tanaka S,
Haruma K and Chayama K: Clinical characteristics and natural
history of patients with low grade reflux esophagitis. J
Gastroenterol Hepatol. 17:949–954. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Savarino E, Marabotto E, Bodini G,
Pellegatta G, Coppo C, Giambruno E, Brunacci M, Zentilin P and
Savarino V: Epidemiology and natural history of gastroesophageal
reflux disease. Minerva Gastroenterol Dietol. 63:175–183.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Andersson K and Carlsson E:
Potassium-competitive acid blockade: A new therapeutic strategy in
acid-related diseases. Pharmacol Ther. 108:294–307. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hori Y, Imanishi A, Matsukawa J, Tsukimi
Y, Nishida H, Arikawa Y, Hirase K, Kajino M and Inatomi N:
1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438), a novel and potent potassium-competitive
acid blocker for the treatment of acid-related diseases. J
Pharmacol Exp Ther. 335:231–238. 2010.
|
|
13
|
Ashida K, Sakurai Y, Nishimura A, Kudou K,
Hiramatsu N, Umegaki E, Iwakiri K and Chiba T: Randomised clinical
trial: A dose-ranging study of vonoprazan, a novel
potassium-competitive acid blocker, vs. lansoprazole for the
treatment of erosive oesophagitis. Aliment Pharmacol Ther.
42:685–695. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jenkins H, Sakurai Y, Nishimura A, Okamoto
H, Hibberd M, Jenkins R, Yoneyama T, Ashida K, Ogama Y and
Warrington S: Randomised clinical trial: Safety, tolerability,
pharmacokinetics and pharmacodynamics of repeated doses of
TAK-438(vonoprazan), a novel potassium-competitive acid blocker, in
healthy male subjects. Aliment Pharmacol Ther. 41:636–648.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Randomised clinical trial:
Acid-inhibitory effect of vonoprazan 20 mg compared with
esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male
subjects-a randomised open-label cross-over study. Aliment
Pharmacol Ther. 42:719–730. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oshima T and Miwa H: Potent
Potassium-competitive Acid Blockers: A new era for the treatment of
Acid-related diseases. J Neurogastroenterol Motil. 24:334–344.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Kinoshita Y, Ishimura N and Ishihara S:
Management of GERD: Are Potassium-Competitive acid blockers
superior to proton pump inhibitors? Am J Gastroenterol.
113:1417–1419. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Oshima T, Arai E, Taki M, Kondo T, Tomita
T, Fukui H, Watari J and Miwa H: Randomised Clinical trial:
Vonoprazan versus lansoprazole for the initial relief of heartburn
in patients with erosive oesophagitis. Aliment Pharmacol Ther.
49:140–106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ashida K, Sakurai Y, Hori T, Kudou K,
Nishimura A, Hiramatsu N, Umegaki E and Iwakiri K: Randomised
clinical trial: Vonoprazan, a novel potassium-competitive acid
blocker, vs. lansoprazole for the healing of erosive oesophagitis.
Aliment Pharmacol Ther. 43:240–251. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akazawa Y, Fukuda D and Fukuda Y:
Vonoprazan-based therapy for Helicobacter pylori
eradication: Experience and clinical evidence. Therap Adv
Gastroenterol. 9:845–852. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hoshino S, Kawami N, Takenouch N, Umezawa
M, Hanada Y, Hoshikawa Y, Kawagoe T, Sano H, Hoshihara Y, Nomura T
and Iwakiri K: Efficacy of Vonoprazan for proton pump
inhibitor-resistant reflux esophagitis. Digestion. 95:156–161.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iwakiri K, Sakurai Y, Shiino M, Okamoto H,
Kudou K, Nishimura A, Hiramatsu N, Umegaki E and Ashida K: A
randomized, double-blind study to evaluate the acid-inhibitory
effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump
inhibitor-resistant erosive esophagitis. Ther Adv Gastroenterol.
10:439–451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Okamura M, Nakahara K, Iwakura N, Hasegawa
T, Oyama M, Inoue A, Ishizu H, Satoh H and Fujiwara Y: Factors
associated with Patassium-competitive acid blocker Non-response in
patients with proton pump Inhibitor-refractory gastroesophageal
reflux disease. Digestion. 95:281–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mizuno H, Nishino M, Yamada K,
Kamiyamamoto S and Hinoue Y: Efficacy of vonoprazan for 48-week
maintenance therapy of patients with healed reflux esophagitis.
Digestion. 101:411–421. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tanabe T, Hoshino S, Kawami N, Hoshikawa
Y, Hanada Y, Takenouchi N, Goto O, Kaise M and Iwakir K: Efficacy
of long-term maintenance therapy with 10-mg vonoprazan for proton
pump inhibitor-resistant reflux esophagitis. Esophagus. 16:377–381.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shinozaki S, Sakamoto H, Osawa H, Yano T,
Lazaridis N and Yamamoto H: Timing and predictors for Vonoprazan
dose escalation in refractory gastroesophageal reflux disease: A
Long-term observational study. Digestion: 1-9 doi:
10.1159/000546992 (Epub ahead of print).
|
|
27
|
Armstrong D, Bennett JR, Blum AL, Dent J,
Dombal FTD, Galmiche JP, Lundell LR, Margulies M, Richter JE,
Spechler SJ, et al: The endoscopic assessment of esophagitis: A
progress report on observer agreement. Gastroenterology. 111:85–92.
1996.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kusano M, Shimoyama Y, Sugimoto S,
Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H,
Ino K, et al: Development and evaluation of FSSG: Frequency scale
for the symptoms of GERD. J Gastroenterol. 39:888–891.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shinozaki S, Osawa H, Miura Y, Hayashi Y,
Sakamoto H, Yano T, Lefor AK and Yamamoto H: Long-term changes in
serum gastrin levels during standard dose vonoprazan therapy. Scand
J Gastroenterol. 57:1412–1416. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hoshihara Y: Endoscopic findings of GERD.
Nihon Rinsho. 62:1459–1464. 2004.PubMed/NCBI(In Japanese).
|
|
31
|
Miwa H, Yokoyama T, Hori K, Sakagami T,
Oshima T, Tomita T, Fujiwara Y, Saita H, Itou T, Ogawa H, et al:
Interobserver agreement in endoscopic evaluation of reflux
esophagitis using a modified Los Angeles classification
incorporating grades N and M: A validation study in a cohort of
Japanese endoscopists. Dis Esophagus. 21:355–363. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kinoshita Y and Adachi K: Hiatal hernia
and gastroesophageal flap valve as diagnostic indicators in
patients with gastroesophageal reflux disease. J Gastroenterol.
41:720–721. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969.
|
|
34
|
Uemura N, Kinoshita Y, Haruma K, Kushima
R, Yao T, Akiyama J, Aoyama N, Baba Y, Suzuki C and Ishiguro K:
Vonoprazan as a Long-term maintenance treatment for erosive
esophagitis: VISION, a 5-Year, randomized, Open-label study. Clin
Gastroenterol Hepatol. 23:748–757.e5. 2025.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gyawali CP, Kahrilas PJ, Savarino E,
Zerbib F, Mion F, Smout AJPM, Vaezi M, Sifrim D, Fox MR, Vela MF,
et al: Modern diagnosis of GERD: The Lyon Consensus. Gut.
67:1351–1362. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dent J, Brun J, Fendrick A, Fendrick M,
Janssens J, Kahrilas P, Lauritsen K, Reynolds J, Shaw M and Tally
N: An evidence-based appraisal of reflux disease management-the
Genval Workshop Report. Gut. 44 (Suppl 2):S1–S16. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
DeVault KR and Castell DO: American
College of Gastroenterology. Updated guidelines for the diagnosis
and treatment of gastroesophageal reflux disease. Am J
Gastroenterol. 100:190–200. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim SY and Lee KJ: Potential risks
associated with Long-term use of proton pump inhibitors and the
maintenance treatment modality for patients with mild
gastroesophageal reflux disease. J Neurogastroenterol Motil.
30:407–20. 2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Huh CW, Son NH, Youn YH, Jung DH, Kim MK,
Gong EJ, Huh KC, Kim SY, Park MI, Lee JY, et al: Real-world
prescription patterns and patient satisfaction regarding
maintenance therapy of gastroesophageal reflux disease: An
observational, Cross-sectional, multicenter study. J
Neurogastroenterol Motil. 29:470–477. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Laine L, DeVault K, Katz P, Mitev S, Lowe
J, Hunt B and Spechler S: Vonoprazan versus lansoprazole for
healing and maintenance of healing of erosive esophagitis: A
randomized trial. Gastroenterology. 164:61–71. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ashida K, Iwakiri K, Hiramatsu N, Sakurai
Y, Hori T, Kudou K, Nishimura A and Umegaki E: Maintenance for
healed erosive esophagitis: Phase III comparison of vonoprazan with
lansoprazole. World J Gastroenterol. 24:1550–1561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiao Y, Qian J, Zhang S, Dai N, Chun HJ,
Chiu C, Chong CF, Funao N, Sakurai Y, Eisner JD, et al: Vonoprazan
10 mg or 20 mg vs. lansoprazole 15 mg as maintenance therapy in
Asian patients with healed erosive esophagitis: A randomized
controlled trial. Chin Med J (Engl). 137:962–971. 2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Malfertheiner P, Nocon M, Vieth M, Stolte
M, Jaspersen D, Koelz HR, Labenz J, Leodolter A, Lind T, Richter K
and Willich SN: Evolution of gastro-oesophageal reflux disease over
5 years under routine medical care-the ProGERD study. Aliment
Pharmacol Ther. 35:154–164. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ness-Jensen E, Lindam A, Lagergren J and
Hveem K: Changes in prevalence, incidence and spontaneous loss of
gastro-oesophageal reflux symptoms: A prospective Population-based
cohort study, the HUNT study. Gut. 61:1390–1397. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shah A, Shibli F, Kitayama Y and Fass R:
The natural course of gastroesophageal reflux disease: A critical
appraisal of the literature. J Clin Gastroenterol. 55:12–20.
2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shaqran TM, Ismaeel MM, Alnuaman AA, Al
Ahmad FA, Albalawi GA, Almubarak JN, AlHarbi RS, Alaqidi RS, ALALi
YA, Alfawaz KS, et al: Epidemiology, causes, and management of
Gastro-esophageal reflux disease: A systematic review. Cureus.
15(e47420)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nocon M, Labenz J, Jaspersen D,
Meyer-Sabellek W, Stolte M, Lind T, Malfertheiner P and Willich SN:
Long-term treatment of patients with gastro-oesophageal reflux
disease in routine care-results from the ProGERD study. Aliment
Pharmacol Ther. 25:715–722. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Armstrong D: Systematic review:
Persistence and severity in gastro-oesophageal reflux disease.
Aliment Pharmacol Ther. 28:841–853. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hsu PI, Lu CL, Wu DC, Kuo CH, Kao SS,
Chang CC, Tai WC, Lai KH, Chen WC, Wang HM, et al: Eight weeks of
esomeprazole therapy reduces symptom relapse, compared with 4
weeks, in patients with Los Angeles grade A or B erosive
esophagitis. Clin Gastroenterol Hepatol. 13:859–866.e1.
2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jung DH, Youn YH, Jung HK, Kim SY, Huh CW,
Shin CM, Oh JH, Huh KC, Park MI, Choi SC, et al: On-demand versus
continuous maintenance treatment with a proton pump inhibitor for
mild Gastroesophageal reflux disease: A prospective randomized
multicenter study. J Neurogastroenterol Motil. 29:460–469.
2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Umezawa M, Kawami N, Hoshino S, Hoshikawa
Y, Koizumi E, Takenouchi N, Hanada Y, Kaise M and Iwakiri K:
Efficacy of on-demand therapy using 20-mg vonoprazan for mild
reflux esophagitis. Digestion. 97:309–315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Okanobu H, Kohno T, Boda K, Ochi H and
Furukawa Y: Long-term clinical course after successful initial
treatment in patients with mild erosive esophagitis: A prospective
Follow-Up study. Digestion. 104:283–290. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
McCarthy DM: Proton pump inhibitor use,
Hypergastrinemia, and gastric carcinoids-what is the relationship?
Int J Mol Sci. 21(662)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fossmark R, Johnsen G, Johanessen E and
Helge W: Rebound acid hypersecretion after long-term inhibition of
gastric acid secretion. Aliment Pharmacol Ther. 21:149–154.
2005.PubMed/NCBI View Article : Google Scholar
|