Introduction
The neutrophil-enhancing effect of
granulocyte-colony stimulating factor (G-CSF) can directly
influence treatment effectiveness by maintaining the dosage and
intensity of chemotherapy and avoiding delays in treatment
intervals (1). Typical adverse
events of G-CSF include fever, back pain, headache, bone pain and
muscle aching. More serious adverse events include acute lung
injury, acute coronary syndrome, and acute aortitis (2). Aortitis induced by G-CSF treatment
typically presents with systemic symptoms including fever and
elevated C-reactive protein (CRP) levels. As these symptoms
resemble those of an infection, they are often mistaken for an
infection associated with chemotherapy-induced febrile neutropenia
(FN), which can lead to a delay in appropriate treatment for
aortitis. A computed tomography (CT) scan is an essential
diagnostic tool for confirming the diagnosis (3).
According to the Japanese Adverse Drug Event Report
database (JADER), of 3,409 patients with malignant tumors treated
with G-CSF, 16 (0.47%) developed aortitis. The cases included four
patients with breast and ovarian cancer, three with malignant
lymphoma, two with uterine cancer, and one with esophageal and
prostate cancer (4). In the present
study, a rare case of aortitis induced by G-CSF administration in a
patient undergoing chemotherapy for liver metastases from sacral
chordoma is presented.
Case report
A 59-year-old-man was referred to the Kobe
University hospital in Kobe City on October 2018 for the treatment
of multiple metastatic chordomas of the liver. He had already
initiated treatment with carbon ion radiotherapy [70.4 Gy
(RBE)/32fr] for sacral chordoma. A total of 22 months after
postradiotherapy, multiple metastases appeared in the liver, and
the patient received eight cycles of trabectedin (dose: 1.2
mg/m2, 24-h continuous infusion intravenously on day one
as palliative chemotherapy for metastases at the previous hospital.
As number of metastatic lesions gradually increased despite the
administration of initiation chemotherapy, the patient received
eribulin (dose: 1.4 mg/m2, intravenously on days one and
eight) in our hospital. As there is no established standard
treatment for chordoma, the next line of therapy was selected based
on treatment strategies for soft tissue sarcomas. The blood tests
conducted before the administration of eribulin did not reveal
neutropenia, renal or liver dysfunction, which were considered risk
factors for FN according to the NCCN guidelines (5).
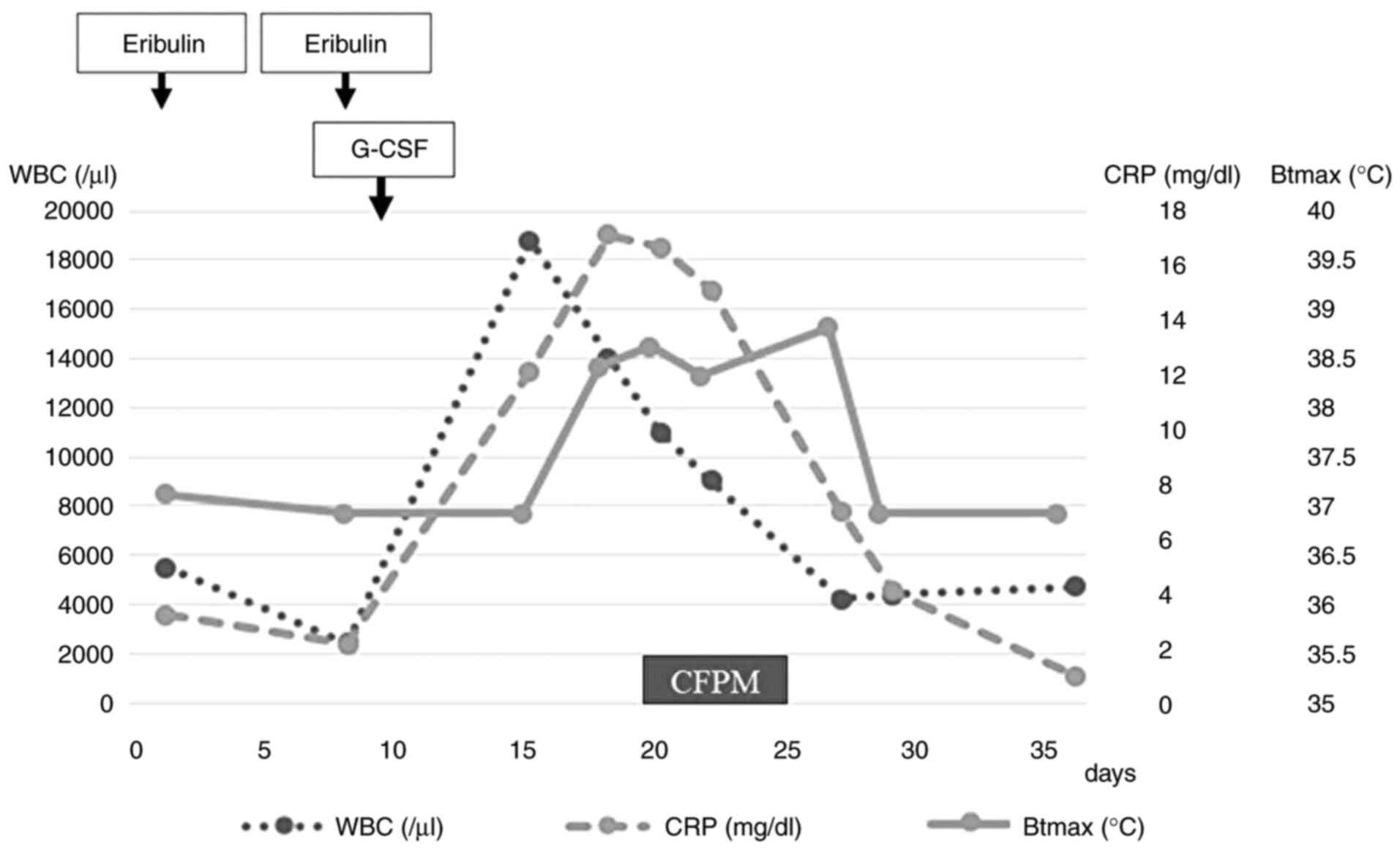
On day eight of the fifth course of eribulin, blood
tests showed a white blood cell (WBC) count of 2,400/µl and a
neutrophil count of 860/µl, which was classified as grade 3
neutropenia according to the Common Terminology Criteria for
Adverse Events Criteria (CTCAE) (6). Pegfilgrastim was administered as G-CSF
on day nine to prevent FN. A total of 3 days after G-CSF
administration, the patient had a fever (38˚C, grade 1 on CTCAE
criteria) (6) at home, which
continued for 6 days. The patient complained of a persistent fever
and abdominal pain on day 18 (day nine of G-CSF administration) of
the fifth cycle. Laboratory tests revealed a high WBC count
(14,000/µl), CRP level (17.11 mg/dl) and procalcitonin level (0.65
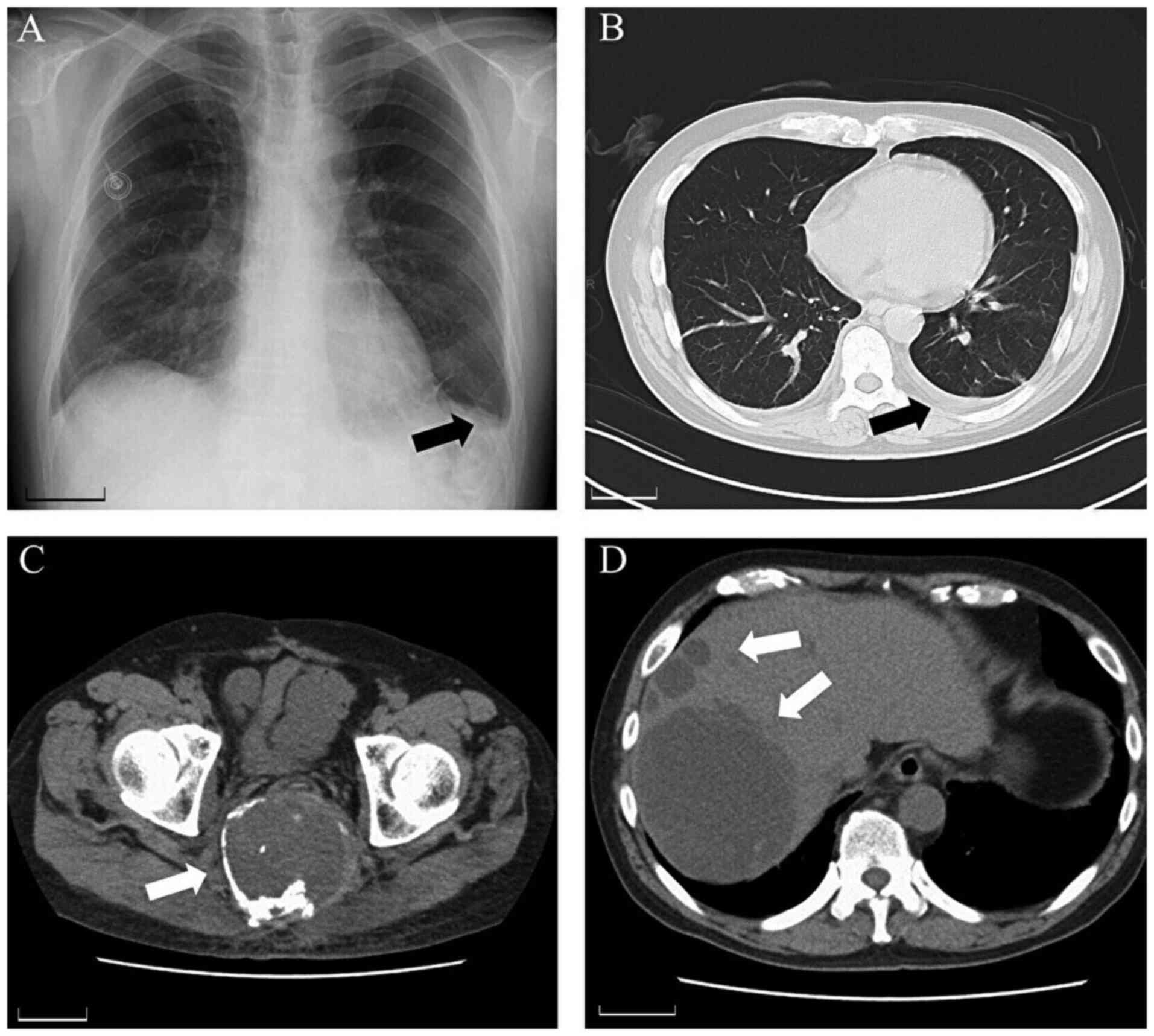
ng/ml). Blood, urine and sputum cultures were negative. Chest X-ray
revealed a blunt left costophrenic angle (Fig. 1A). Thoracic CT showed left pleural
effusion with no pneumonia (Fig.
1B). No obvious changes were observed at the primary site
(sacral) or metastatic lesion (liver) on abdominal CT (Fig. 1C and D).
FN was suspected and antibiotic treatment (cefepime,
4 g/day) was initiated on day 19. Neutrophil count before
administrating antibiotics was 15,600/µl (83% of WBC counts).
However, the patient did not achieve defervescence after six days
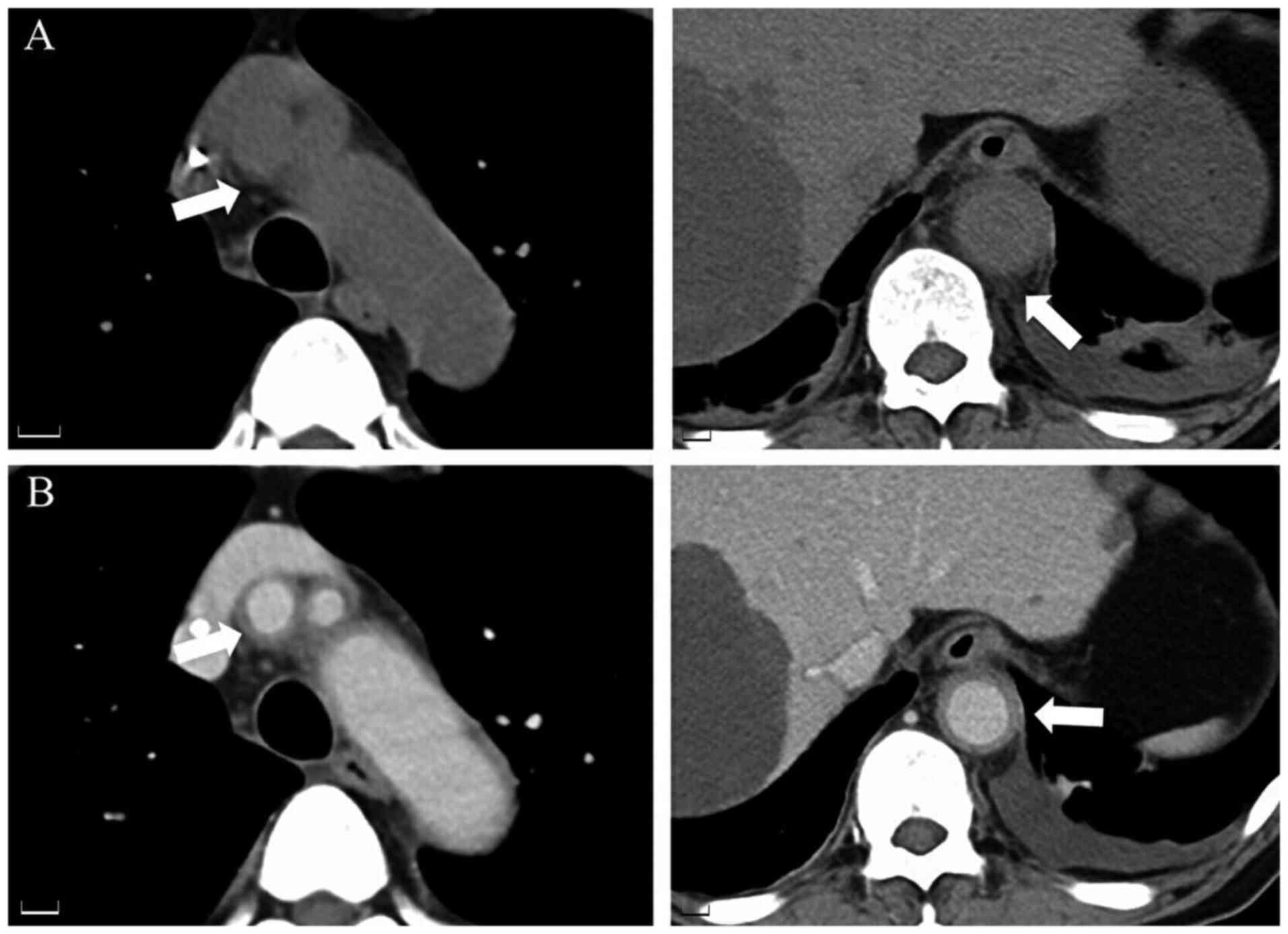
of antibiotic treatment. Therefore, contrast-enhanced CT was
performed, which revealed periaortic soft tissue inflammation
(Fig. 2A) and thickened walls of
the brachiocephalic artery and abdominal aorta (Fig. 2B). Based on the CT images,
G-CSF-induced aortitis was suspected, and antibiotic treatment was
discontinued. The patient's general condition improved naturally,
with the WBC count and CRP level decreasing to 4,400/µl and 4.08
mg/dl, respectively, on day 29.
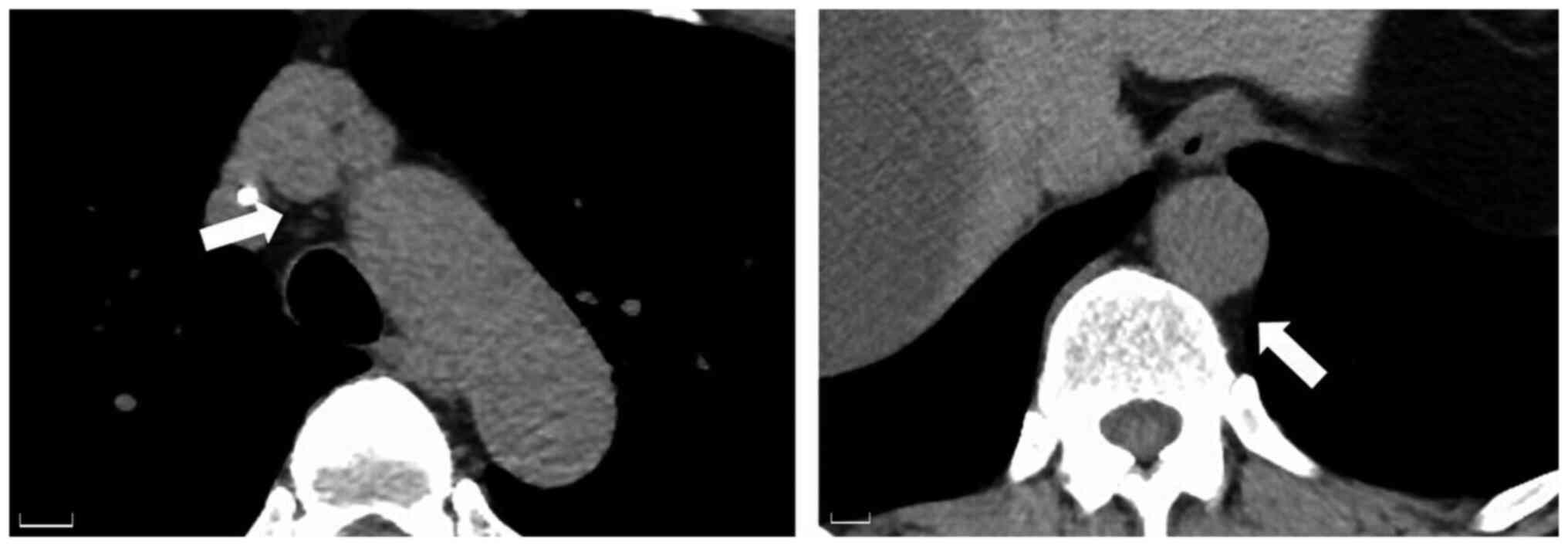
Follow-up CT revealed the disappearance of the
periaortic soft tissue inflammation (Fig. 3). Chemotherapy was continued without
any further complaints of fever. The clinical course and treatment
history of the patients are shown in Fig. 4.
Discussion
G-CSF attaches to receptors on neutrophil
progenitors in the bone marrow, stimulating their differentiation
into neutrophils and thereby boosting neutrophil counts in the
peripheral blood. It is widely used in chemotherapy to induce
myelosuppression (7). Various types
of G-CSF agents are available in clinical settings. Filgrastim and
lenograstim are short-acting G-CSF agents with biological
structures and activities closely resembling those of endogenous
human G-CSF (8). Pegfilgrastim, a
modified form of G-CSF, has a polyethylene glycol molecule attached
to its N-terminus, which extends its biological half-life in
peripheral blood (7).
Aortitis may develop as a result of an enhanced
pro-inflammatory response and neutrophil-induced tissue damage
(9); however, the exact mechanism
by which G-CSF administration induces aortitis remains unclear. In
a study using the JADER database, Oshima et al (4) reported that G-CSF treatment was linked
to a potentially increased risk of aortitis in patients with
malignant tumors, and the incidence of aortitis associated with
G-CSF was 0.47%. Their report included cases of four patients with
breast and ovarian cancer, three with malignant lymphoma, two with
uterine cancer, and one with esophageal and prostate cancer;
however, no case of patients with chordomas was included in their
report.
Regarding the characteristics of G-CSF-induced
aortitis, fever was the most common symptom, with additional
complaints of back, chest, abdominal and neck pain. The onset of
aortitis has been reported within 10 days after the initiation of
G-CSF in >50% of the patients (61.2%). CT was used as the
diagnostic imaging modality in most cases (89.8%) (10). In our case, the patient got fever 3
days after G-CSF administration and felt abdominal pain 9 days
after G-CSF administration, which was similar to the aforementioned
study (10). Regarding the utility
of contrast-enhanced CT, Takamatsu et al (11) reported that contrast-enhanced CT
enables differentiation between G-CSF-induced aortitis and
intramural hematoma based on their distinguishing imaging features
(11). With respect to the
differential diagnosis, Takayasu arteritis, giant cell arteritis
and IgG4-related disease were considered to be listed (11). In our case, internist determined
that the possibility of autoimmune diseases that cause aortitis was
low, therefore no additional tests were performed. The limitation
of the present case report is lack of the evaluation of
antineutrophil cytoplasmic antibody, rheumatoid factor, and serum
levels of IgG4 subclass to rule out these diseases.
The treatment of aortitis associated with G-CSF
remains controversial. The first and most common approach for
managing aortitis associated with G-CSF is to discontinue G-CSF
administration (12). Takahashi
et al (13) reported a case
successfully treated with intravenous administration of steroids.
In a systematic literature review on the treatment of aortic
arteritis, Hoshina and Takei (10)
reported that steroids were administered to 29 patients (59.2%),
whereas 19 patients (38.8%) healed without the use of steroids
(10). In the present case, the
patient became afebrile and showed improvement in inflammatory
markers in blood tests 20 days after G-CSF administration without
steroid treatment. Steroid administration may need to be considered
in more severe cases or in cases where aortitis persists even after
discontinuation of G-CSF (14).
The acceptability of re-administering G-CSF to
patients with a history of G-CSF-induced aortitis remains
controversial. Takamatsu et al (11) reported that among 11 asymptomatic
patients with clinically undiagnosed pegfilgrastim-induced
aortitis, eight patients received additional pegfilgrastim
treatment, and none of them showed recurrence of aortitis on CT.
They also stated that the results did not indicate the safety of
pegfilgrastim re-administration and that the risk of recurrence
after re-administration remained unclear (11).
Although G-CSF-induced aortitis is uncommon,
clinicians should exercise caution when treating patients with
malignant tumors. If a patient presents with a persistent fever of
unknown origin after receiving G-CSF, G-CSF-induced aortitis should
be considered as a potential cause during diagnostic workup. The
treatment for G-CSF-induced aortitis has not yet been established.
However, the discontinuation of G-CSF and, in some cases, steroid
administration should be considered depending on the severity of
the condition. Therefore, clinicians should closely monitor the
clinical course of patients to guide appropriate treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SF and HH designed the experiments and wrote the
initial draft of the manuscript. SF and HH confirm the authenticity
of all the raw data. SF, HH, NF, RS, TT, TM and YN provided medical
care for the patients and collected the data. RK and TA were
responsible for the design and interpretation of the study as well
as revisions and approval of the final draft of the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient was informed that data from the case
would be submitted for publication and provided consent for the
academic use of clinical information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lyman GH, Dale DC, Culakova E,
Poniewierski MS, Wolff DA, Kuderer NM, Huang M and Crawford J: The
impact of the granulocyte colony-stimulating factor on chemotherapy
dose intensity and cancer survival: A systematic review and
meta-analysis of randomized controlled trials. Ann Oncol.
24:2475–2484. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
D'Souza A, Jaiyesimi I, Trainor L and
Venuturumili P: Granulocyte colony-stimulating factor
administration: Adverse events. Transfus Med Rev. 22:280–290.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao T and Xu H: Literature review
analysis of aortitis induced by granulocyte-colony stimulating
factor. Front Pharmacol. 15(1487501)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oshima Y, Takahashi S, Tani K and Tojo A:
Granulocyte colony-stimulating factor-associated aortitis in the
Japanese adverse drug event report database. Cytokine. 119:47–51.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
National Comprehensive Cancer Network
(NCCN): NCCN Clinical Practice Guidelines in Oncology:
Hematopoietic Growth Factors. Version 1.2025. NCCN, Plymouth
Meeting, PA, 2025. https://www.nccn.org. Accessed May 7, 2025.
|
|
6
|
National Cancer Institute (NCI): Common
Terminol Criteria Adverse Events (ctcae) Version 5.0. NCI,
Bethesda, MD, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
Accessed March 23, 2025.
|
|
7
|
Takahashi M, Kondoh C and Takano T: The
history of granulocyte-colony stimulating factor. Drug Deliv Syst.
32:134–142. 2017.
|
|
8
|
Lee SY, Kim EK, Kim JY, Park TK, Choi SH,
Im YH, Kim MY, Park YH and Kim DK: The incidence and clinical
features of PEGylated filgrastim-induced acute aortitis in patients
with breast cancer. Sci Rep. 10(18647)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cuchacovich R: Immunopathogenesis of
vasculitis. Curr Rheumatol Rep. 4:9–17. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hoshina H and Takei H: Granulocyte-colony
stimulating factor-associated aortitis in cancer: A systematic
literature review. Cancer Treat Res Commun.
29(100454)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takamatsu A, Yoshida K, Toshima F, Kozaka
K, Yamamoto N, Sai Y and Gabata T: Single-center analysis of
pegfilgrastim-induced aortitis using a drug prescription database
and CT findings. Radiology. 305:729–740. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ito M, Amari M, Sato A and Hikichi M:
Granulocyte-colony stimulating factor (G-CSF)-induced aortitis: A
case report. Cureus. 16(e54845)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Takahashi T, Yamamoto K, Yamaguchi T,
Miura Y and Kizawa R: Granulocyte colony-stimulating
factor-associated aortitis. Lancet Oncol. 23(e155)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harada M, Motoki H, Sakai T and Kuwahara
K: Granulocyte colony stimulating factor-associated aortitis
evaluated via multiple imaging modalities including vascular
echography: A case report. Eur Heart J Case Rep.
5(ytaa503)2020.PubMed/NCBI View Article : Google Scholar
|