Introduction
Bladder cancer (BC) is one of the most prevalent
malignancies globally, with an estimated 613,791 new cases reported
in 2022 according to GLOBOCAN 2022(1). Clinically, BC is categorized into
three main subtypes, namely non-muscle-invasive (NMI), MIB and
metastatic BC (2). While NMIBC
accounts for ~80% of initial BC diagnoses and is associated with a
favorable 5-year survival rate >90% (3), it has a 1-year recurrence rate of
15-61%, and a 5-year recurrence rate of 31-78% (4). Its high recurrence rate imposes
substantial healthcare burdens (5-9).
By contrast, MIBC and metastatic BC, accounting for ~25% of all
diagnoses, are associated with aggressive progression and poorer
prognosis (10). Disparities in BC
outcomes are associated with ethnicity, sex and socioeconomic
factors (11-16).
Management strategies for NMIBC and MIBC include transurethral
resection combined with intravesical therapies, such as mitomycin C
and Bacillus Calmette-Guérin (17-21),
and platinum-based neoadjuvant chemotherapy followed by radical
cystectomy, respectively (10,22).
However, chemotherapy resistance and toxicity underscore the need
for more safe and effective therapeutic agents (22).
Pueraria spp. (Leguminosae), notably P.
lobata and P. thomsonii, are medicinal plants that are
widely distributed in East Asia. The 2020 edition of the Chinese
Pharmacopoeia recognizes the dried roots (radix) of Pueraria
as a source of bioactive compounds, particularly isoflavonoids such
as puerarin, which are the primary active components of
Puerariae radix flavones (PRF) (23-43).
Previous studies demonstrated that PRF exhibit multifaceted
pharmacological properties, such as anti-cancer effects, in colon
(HT-29 cells), cervical (HeLa cells), liver (SMMC-7721 cells) and
breast cancer (HS578T, MDA-MB-231 and MCF-7 cells), acute
promyelocytic leukemia (NB4 cells), neuroblastoma (SHSY5Y cells),
pancreatic carcinoma (BxPC-3 cells) and lung cancer (A549 cells)
(44-48).
Despite these findings, the anti-tumor activity of PRF against BC
remains unexplored.
T24 cells serve as the preferred model for research
on invasion, metastasis and drug resistance mechanisms in BC. Their
intrinsic cisplatin resistance (49) makes T24 cells a classical system for
exploring drug mechanisms of action (50) that are widely used in fundamental BC
research (51,52). Moreover, in natural compound
anticancer mechanism studies, most researchers employ single-cell
models to investigate mechanisms of action (47,50).
Therefore, the present study investigates the effects of PRF on
human bladder cancer T24 cells and its underlying molecular
mechanisms.
Materials and methods
Drug preparation and cell culture
PRF (Taobao; purity ≥80%) was dissolved in anhydrous
ethanol to prepare an 8,000 µg/ml stock solution, which was stored
at 4˚C. T24 cells (Procell Life Science & Technology Co., Ltd.)
were authenticated by STR profiling (Mayo Clinic Cytogenetics Core)
and confirmed to be free of mycoplasma contamination. Cells were
cultured in RPMI-1640 (Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (Shanghai VivaCell Biosciences, Ltd.)
at 37˚C in a humified atmosphere with 5% CO2. Cells were
maintained at a density of <1x106 cells/ml and used
within three passages.
MTT assay
Cells (5x10³/well) in 200 µl medium were treated
with 0, 25, 50, 100 and 200 µg/ml PRF at 37˚C for 24 or 48 h.
Following treatment with MTT reagent (Sigma-Aldrich, Merck KGaA),
cells were incubated at 37˚C for an additional 4 h. Finally, the
absorbance at 490 nm was measured using a microplate reader.
Acridine orange/ethidium bromide
(AO/EB) fluorescence staining
Following treatment with 0-200 µg/ml PRF at 37˚C for
24 or 48 h, T24 cells were stained with AO/EB (Sigma-Aldrich, Merck
KGaA) in the dark at room temperature for 5 min. Images were
captured under a fluorescence microscope (magnification, x200).
DNA ladder assay
Following incubation with PRF (0, 50, 100 and 200
µg/ml) at 37˚C for 24 or 48 h, 1x106 T24 cells were
collected. Subsequently, genomic DNA was extracted from untreated
and PRF-treated cells using the Apoptosis DNA Ladder Extraction kit
(cat. no. #C0007; Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. Finally, 5 µl DNA (containing 1 µg
DNA) was mixed with 6X Loading Buffer and loaded onto a 1% agarose
gel prepared in TBE buffer containing 0.5 µg/ml ethidium bromide
(EB; Thermo Fisher Scientific, Inc.). Electrophoresis was performed
at a constant voltage of 100 V for 30 min. DNA bands were
visualized under UV irradiation (302 nm) using a gel imaging
system. Caution: Ethidium bromide is a mutagenic agent. All
procedures were conducted with nitrile gloves, and waste was
disposed as hazardous material.
Reverse transcription-quantitative
(RT-q)PCR
Following the manufacturer's protocols, total RNA
was extracted from cells using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and reverse-transcribed into cDNA using
the Transcriptor First Strand cDNA synthesis kit (cat. no.
#4897030001; Roche Diagnostics). cDNA amplification was performed
in triplicate with the FastStart Universal SYBR Green Master (ROX)
kit (cat. no. #4913850001; Roche Diagnostics) on an ABI Prism 7500
system (Thermo Fisher Scientific, Inc.). The primer sequences for
BCL2, BAX, FAS receptor (FAS), FAS ligand (FASL), tumor necrosis
factor receptor 1 (TNFR1), tumor necrosis factor-α (TNF-α),
cysteinyl aspartate-specific protease-3 (CASP3), NF-κB and GAPDH
are listed in Table I. The primers
were designed and synthesized by Sangon Biotech Co., Ltd.
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence,
5'→3' | Product length,
bp |
|---|
| GAPDH |
F:GCATCCTGGGCTACACTG | 103 |
| |
R:TGGTCGTTGAGGGCAAT | |
| BAX |
F:CGGAACTGATCAGAACCATCA | 97 |
| |
R:AGGAGTCTCACCCAACCA | |
| BCL2 |
F:GTGGATGACTGAGTACCTGAAC | 125 |
| |
R:GAGACAGCCAGGAGAAATCAA | |
| CASP3 |
F:CTCCACAGCACCTGGTTATT | 106 |
| |
R:AAATTCAAGCTTGTCGGCATAC | |
| NF-kB |
F:GGTGCGGCTCATGTTTACAG | 85 |
| |
R:GATGGCGTCTGATACCACGG | |
| TNFR1 |
F:CTCCAAATGCCGAAAGGAAATG | 100 |
| |
R:ATAATGCCGGTACTGGTTCTTC | |
| TNFα |
F:CCAGGGACCTCTCTCTAATCA | 106 |
| |
R:TCAGCTTGAGGGTTTGCTAC | |
| FAS |
F:GTGATGAAGGACATGGCTTAGA | 115 |
| |
R:GGGTCACAGTGTTCACATACA | |
| FASL |
F:CATTTAACAGGCAAGTCCAACTC | 105 |
| |
R:CACAAGGCCACCCTTCTTAT | |
The thermocycling conditions were as follows:
Initial denaturation at 50˚C for 2 min and 95˚C for 10 min,
followed by 45 cycles of 95˚C for 15 sec and 60˚C for 1 min. The
relative mRNA expression levels were calculated using the
2-ΔΔCq method (53).
ELISA
Following incubation with PRF (0, 50, 100 and 200
µg/ml) at 37˚C for 24 or 48 h, cells and culture medium were
collected. The protein expression levels of FAS and TNF-α were
quantified in T24 cells and culture supernatant using the
corresponding ELISA kits (cat. nos. #JL14207 and #JL10208,
respectively; both Shanghai Future Industrial Co., Ltd.) according
to the manufacturer's instructions.
Statistical analysis
All data were analyzed using SPSS Statistics
(version 25; IBM Corporation) or GraphPad Prism (version 8.1;
GraphPad Software, Inc.; Dotmatics). Experiments were performed in
triplicate, and all data are presented as the mean ± SD. Data were
analyzed using two-way repeated-measures ANOVA followed by
Dunnett's post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
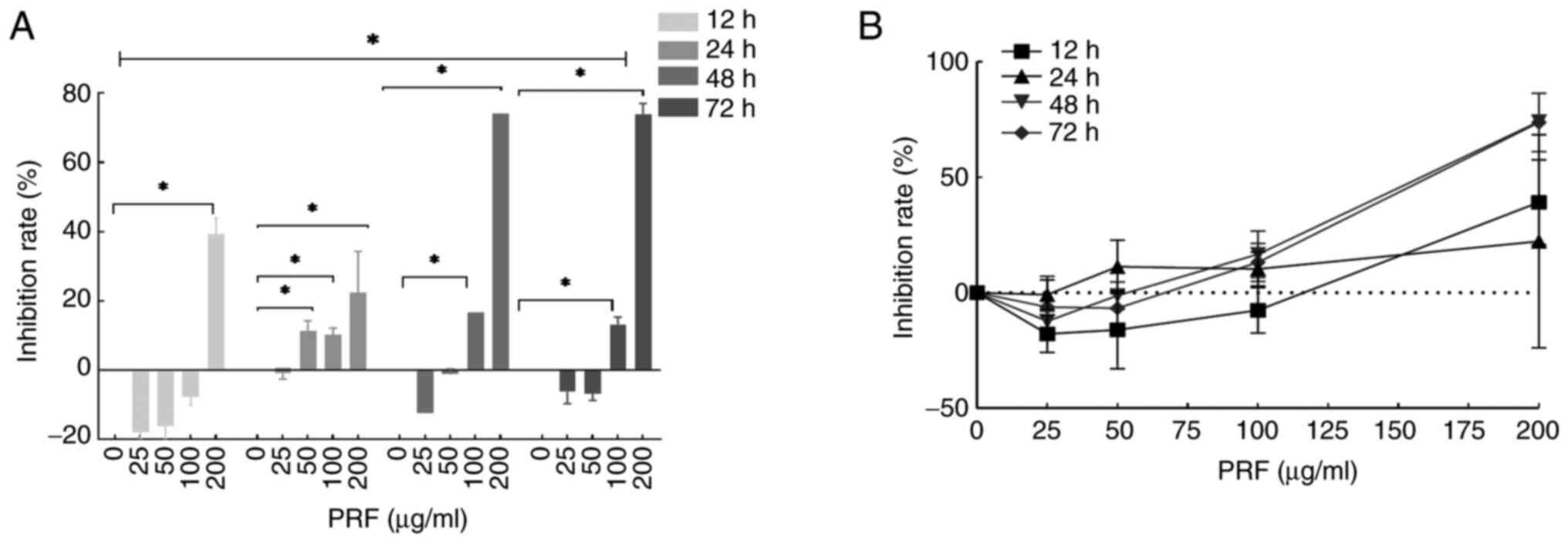
PRF inhibits the viability of T24
cells
MTT assay demonstrated that PRF could significantly
inhibit the viability of T24 cells in a time- and dose-dependent
manner (Fig. 1A). The inhibition
rate in cells treated with 200 µg/ml PRF for 12 h was significantly
higher compared with that in the control group (Fig. 1A). Additionally, the inhibition
rates of cells exposed to 50, 100 and 200 µg/ml PRF for 24 h were
all increased compared with the control group. Consistently, at 48
and 72 h, the inhibition rates in the 100 and 200 µg/ml PRF-treated
groups were significantly higher compared with the control group.
PRF exhibited a clear dose- and time-dependent inhibitory effect on
cell viability, with inhibition increasing with enhanced
concentration and exposure duration (Fig. 1B). Significant differences in
inhibition rates were observed between time points (12, 24, 48 and
72 h).
Morphological changes in T24 cells
treated with PRF
AO/EB staining revealed distinct apoptotic
progression in T24 cells (Fig. 2).
Increasing PRF concentrations induced a progressive fluorescence
shift from green to orange-red emission. Morphologically, cells
progressed from intact plasma membranes with uniform chromatin
distribution to display early apoptotic hallmarks (cellular
shrinkage, nuclear condensation, and apoptotic body formation) and
late-stage apoptosis typified by membrane rupture, chromatin
fragmentation, and nuclear dissolution. Control cells exhibited a
uniform green staining (Fig. 2A and
E). However, 50 µg/ml PRF induced
early apoptotic features, such cell shrinkage, condensed nuclei and
apoptotic bodies (Fig. 2B and
F). Additionally, higher PRF
concentrations (100-200 µg/ml) induced features of late apoptosis,
including enhanced membrane permeability, chromatin fragmentation
(orange/red staining) and nuclear disintegration (Fig. 2C, D,
G and H). Notably, 100-200 µg/ml PRF exposure for
24-48 h promoted concentration- and time-dependent morphological
alterations, characteristic of late-stage apoptosis.
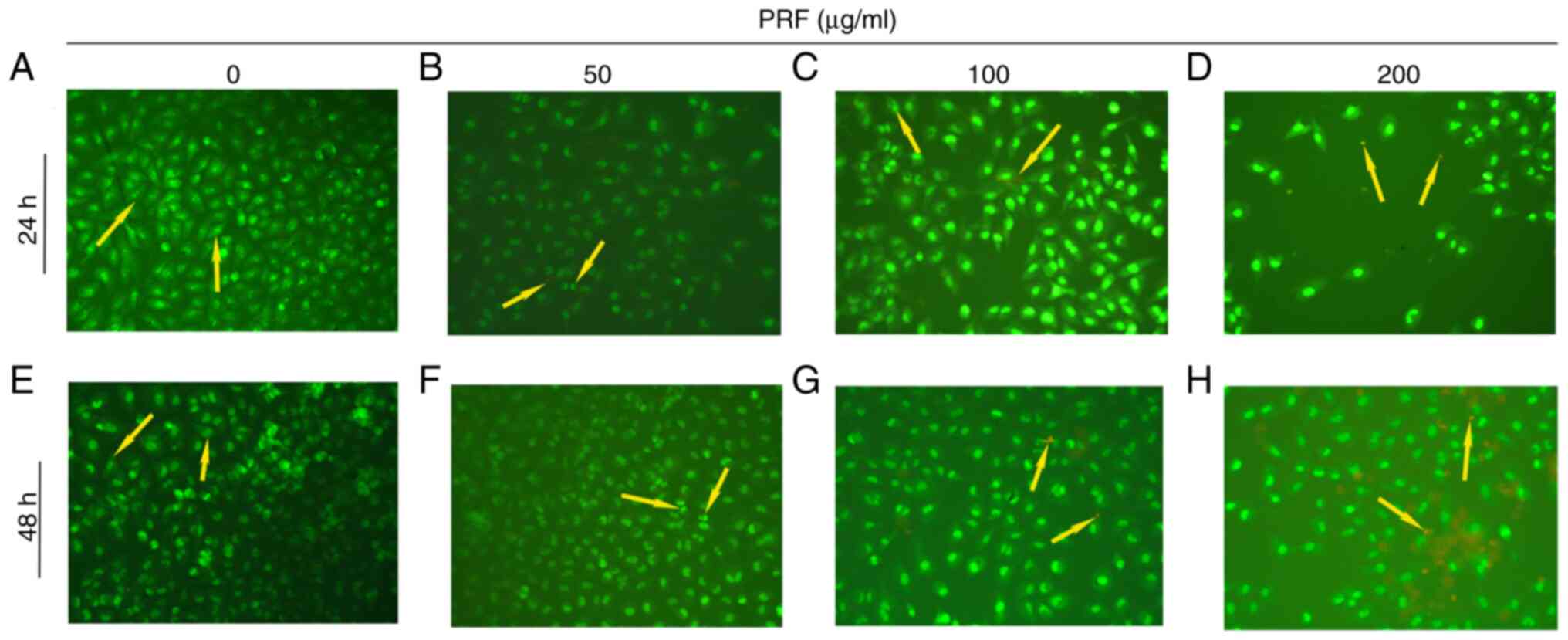 | Figure 2Acridine orange/ethidium bromide
staining of T24 cells exposed PRF. (A) Control for 24 h, the yellow
arrow indicates viable T24 cells exhibiting green fluorescence with
intact plasma membranes. (B) 50 µg/ml and 24 h, yellow arrow
indicates early apoptotic cells demonstrating green fluorescence
with punctate orange-red fluorescence, along with characteristic
morphological changes including cell shrinkage, nuclear
condensation, and apoptotic body formation. (C) 100 µg/ml and 24 h,
yellow arrow indicates apoptotic cells in early and late stages,
exhibiting green fluorescence accompanied by orange-red
fluorescence. Early-stage cells show apoptotic body formation,
while late-stage cells display plasma membrane disintegration,
chromatin fragmentation, and diffuse nuclear orange-red
fluorescence. (D) 200 µg/ml and 24 h, the yellow arrow indicates
late apoptotic cells exhibiting orange-red fluorescence,
characterized by chromatin fragmentation and nuclear
disintegration. (E) Control and 48 h, the yellow arrow indicates
viable T24 cells exhibiting green fluorescence with intact plasma
membranes. (F) 50 µg/ml and 48 h, yellow arrow indicates early
apoptotic cells demonstrating green fluorescence with punctate
orange-red fluorescence, along with characteristic morphological
changes including cell shrinkage, nuclear condensation, and
apoptotic body formation. (G) 100 µg/ml and 24 h, yellow arrow
indicates apoptotic cells in early and late stages, exhibiting
green fluorescence accompanied by orange-red fluorescence.
Early-stage cells show apoptotic body formation, while late-stage
cells display plasma membrane disintegration, chromatin
fragmentation and diffuse nuclear orange-red fluorescence. (H) 200
µg/ml and 48 h, the yellow arrow indicates late apoptotic cells
exhibiting orange-red fluorescence, characterized by chromatin
fragmentation and nuclear disintegration. Magnification, x200. PRF,
Puerariae radix flavone. |
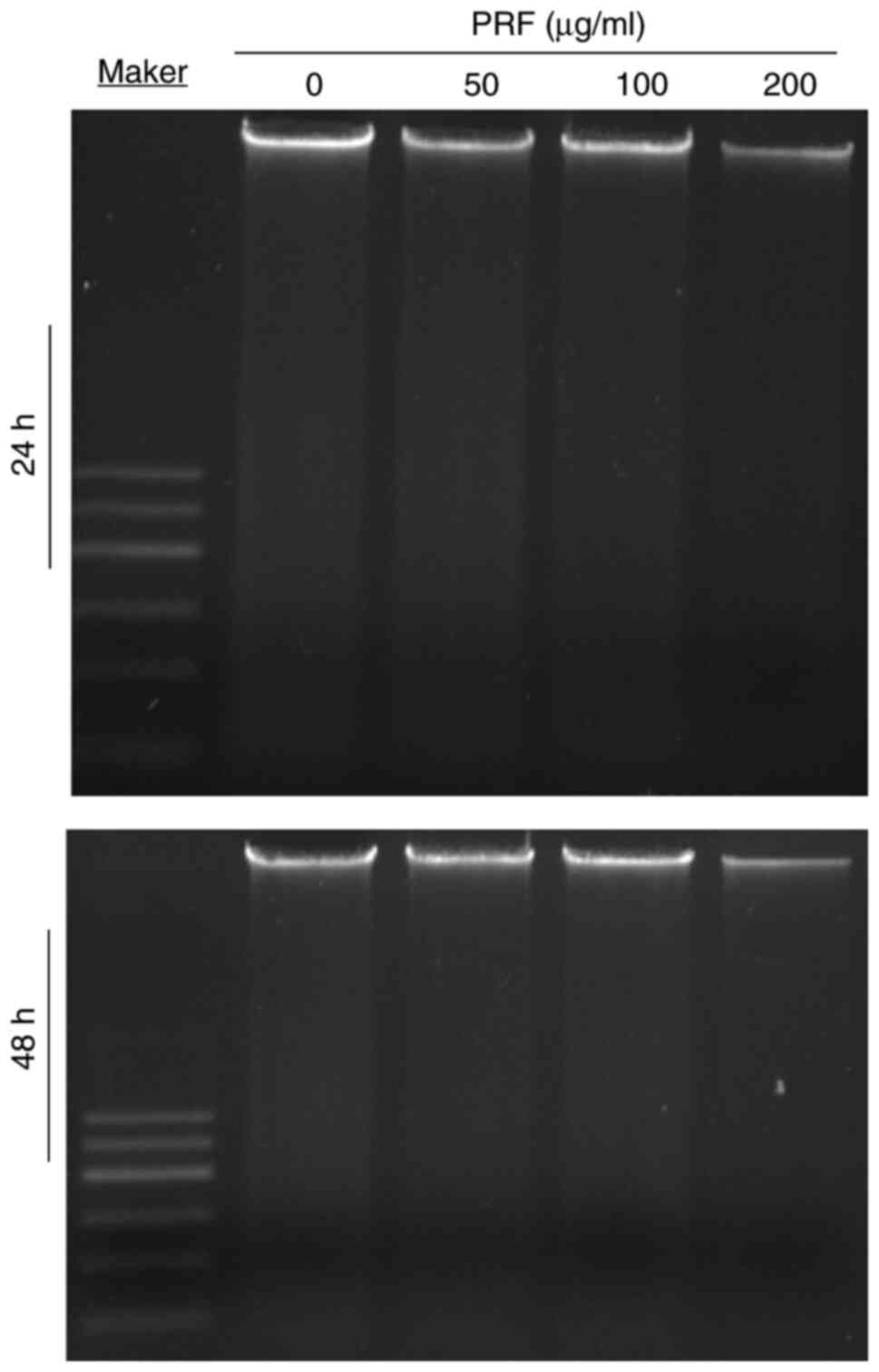
Assessment of DNA fragmentation in T24
cells following PRF treatment
The DNA ladder assay showed no apoptotic
fragmentation in T24 cells treated with PRF (0-200 µg/ml) for 24-48
h, thus suggesting that PRF-induced apoptosis was triggered via a
DNA fragmentation-independent pathway (Fig. 3).
PRF modulates the expression of
apoptosis-related genes
RT-qPCR demonstrated that treatment with 100 or 200
µg/ml PRF for 24 h upregulated FAS (2.0- and 2.3-fold,
respectively), FASL (48.9- and 15.2-fold, respectively), TNF-α
(1.4- and 2.2-fold, respectively) and CASP3 (2.1- and 1.8-fold,
respectively; Fig. 4A-C and
E). However, the expression of
TNFR1 only significantly increased in cells treated with 200 µg/ml
PRF (1.2-fold; Fig. 4D). No
significant changes were observed in the mRNA expression of NF-κB,
BCL-2 and BAX (Fig. 4F-H).
Additionally, treatment with PRF for 48 h slightly upregulated FAS
at 100 and 200 µg/ml (1.3- and 1.2-fold, respectively; Fig. 4A), and markedly upregulated TNF-α at
200 µg/ml (1.6-fold; Fig. 4C). By
contrast, FASL (0.5-fold at 100 µg/ml), TNFR1 (0.8-fold at 200
µg/ml), and NF-κB (0.8- and 0.6-fold at 100 and 200 µg/ml,
respectively) were significantly downregulated (Fig. 4B, D
and F). No significant alterations
were detected in the mRNA expression of CASP3, BCL-2 and BAX
(Fig. 4E, G and H).
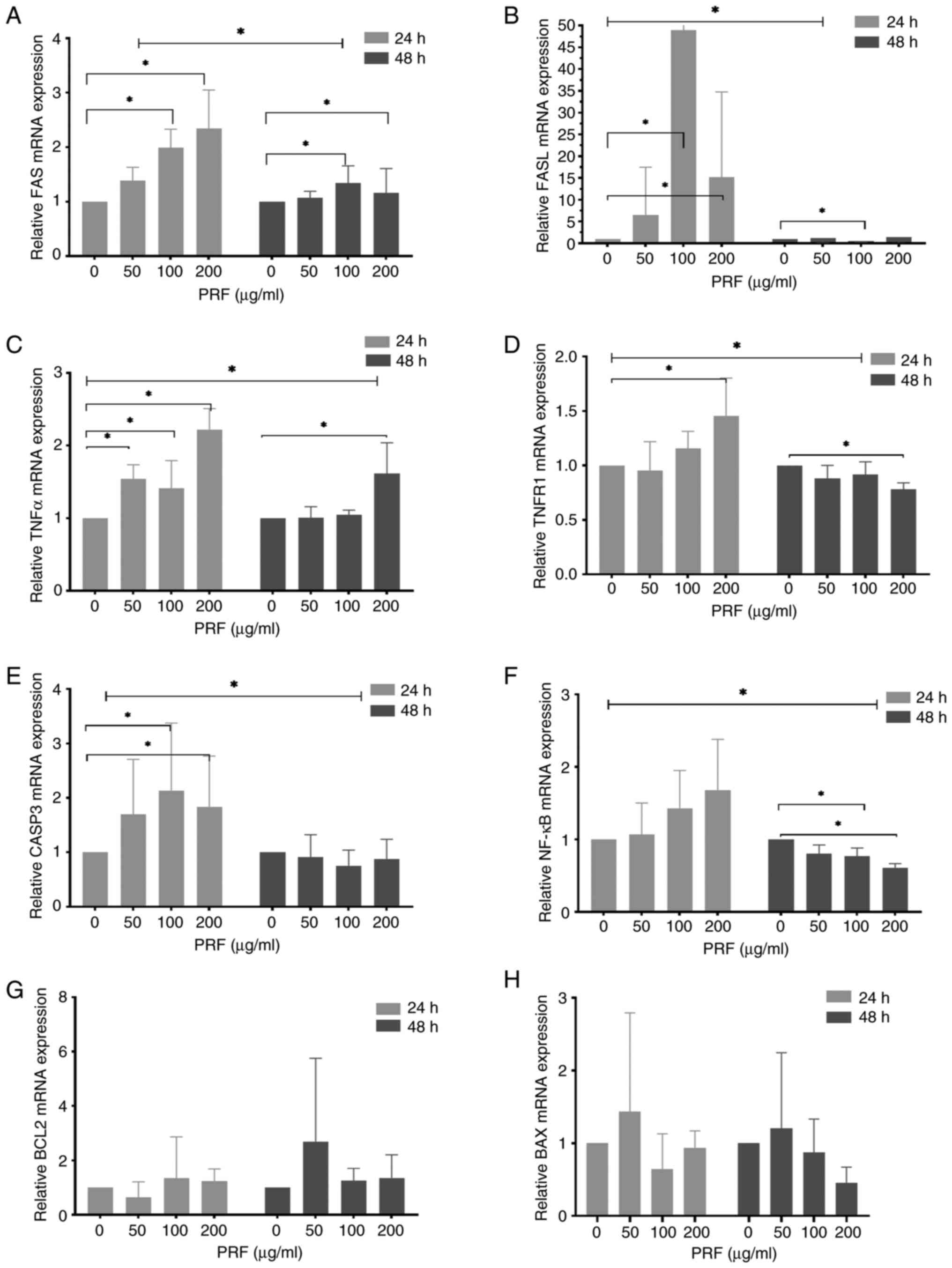 | Figure 4Effects of PRF on the expression of
apoptosis-related genes in T24 cells. T24 cells were treated with
PRF (0, 50, 100 and 200 µg/ml) for 24 or 48 h. The mRNA levels of
(A) FAS, (B) FASL, (C) TNFα, (D) TNFR1, (E) CASP3, (F) NF-κB, (G)
BCL2 and (H) BAX were assessed by quantitative PCR (normalized to
GAPDH). *P<0.05. PRF, Puerariae radix
flavones; CASP3, cysteinyl aspartate-specific protease-3. |
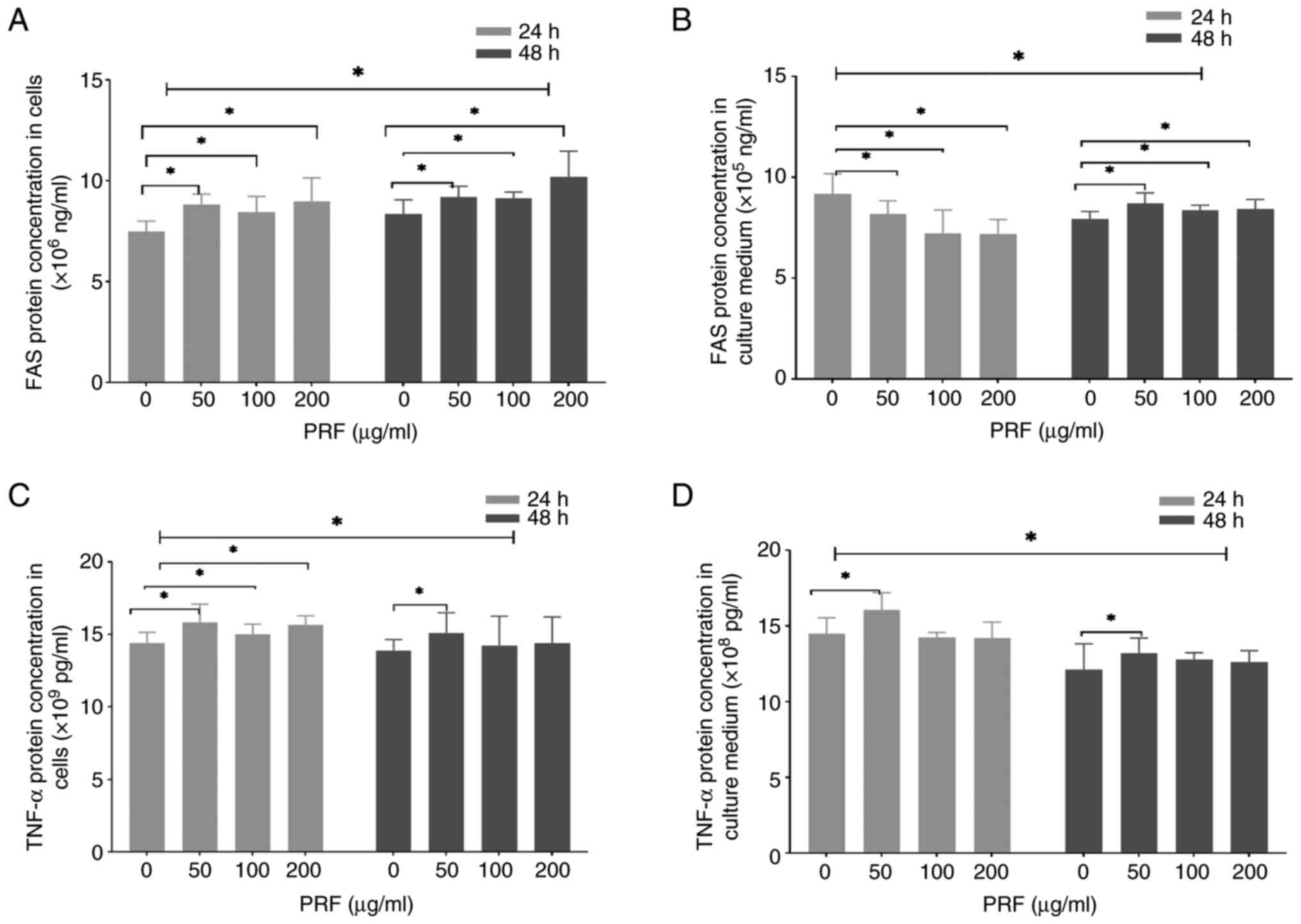
PRF modulates the protein expression
of FAS and TNF-α
ELISA showed that treatment with PRF for 24 h
resulted in a dose-dependent increase of intracellular FAS and
TNF-a protein levels (50-200 µg/ml; Fig. 5A and C). By contrast, the secreted levels of FAS
were reduced in the culture supernatant (50-200 µg/ml; Fig. 5B). In addition, the secreted levels
of TNF-α were elevated only at 50 µg/ml (Fig. 5D). Furthermore, T24 cell exposure to
PRF for 48 h dose-dependently increased the intracellular and
secreted levels of FAS (50-200 µg/ml; Fig. 5A and B). However, intracellular and secreted
TNF-α levels were only significantly elevated at 50 µg/ml (Fig. 5C and D).
Discussion
The present study demonstrated that treatment with
PRF for 24 and 48 h significantly inhibited T24 cell viability in a
dose- and time-dependent manner, with the most pronounced effects
observed at 100 and 200 µg/ml. The ability of PRF to promote T24
cell apoptosis was shown by flow cytometry in our previous study
(54). Here, morphological
examination revealed characteristic apoptotic features, including
early-stage membrane shrinkage and late-stage apoptotic body
formation. Notably, the absence of DNA laddering suggested that PRF
induced apoptosis via a non-canonical DNA fragmentation pathway.
Although no direct evidence currently demonstrates PRF-induced
apoptosis via this mechanism in other cancer cells, it is
hypothesized that negative DNA fragmentation results may contribute
to the underreporting of such findings. Consistent with this
observation, our team previously demonstrated flavonoid compounds
extracted from Galium verum L. likewise yield negative DNA
fragmentation results when inducing apoptosis in HepG2
hepatocellular carcinoma cells (unpublished data). The hypothesis
that PRF induces apoptosis through non-classical DNA fragmentation
pathways is based on the T24 cell model. To the best of our
knowledge, the present study is the first to report a non-canonical
DNA fragmentation route during PRF-induced apoptosis in T24
cells.
PRF treatment significantly upregulated FAS, FASL,
TNFR1, TNFα and CASP3 in T24 cells, while NF-κB was downregulated.
mRNA expression levels of both BCL2 and BAX remained unchanged,
indicating that PRF-mediated apoptosis in T24 cells may involve the
coordinated regulation of FAS, FASL, TNFR1, TNFα, CASP3 and NF-κB
expression dynamics (Fig. 6).
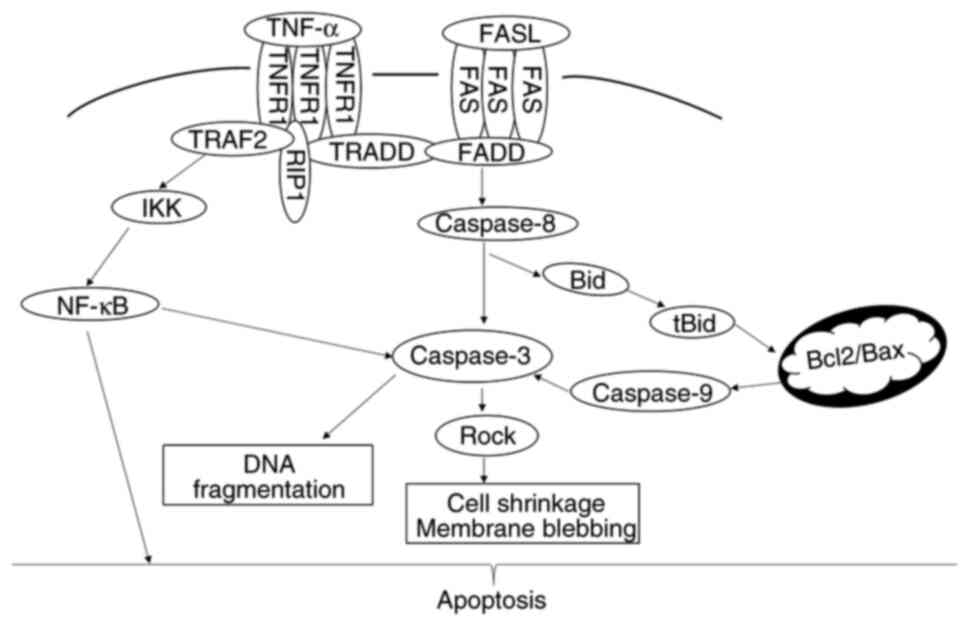 | Figure 6Mechanism of apoptosis. Apoptotic
signaling primarily occurs via two pathways: the extrinsic death
receptor pathway and the intrinsic mitochondrial pathway. In T24
cells, PRF induces apoptosis predominantly by activating FAS and
TNFR1, thereby initiating the death receptor pathway. This
ultimately leads to the activation of CASPASE3, resulting in
characteristic biochemical and morphological changes characteristic
of programmed cell death. Concurrently, NF-κB participates in the
regulation of this apoptotic pathway. TNFR, tumor necrosis factor
receptor; TRAF, TNFR-Associated Factor; TRADD, TNFR-associated
death domain; RIP, receptor-interacting protein; IKK, IκB kinase;
tBid, truncated Bid; PRF, Puerariae radix flavone. |
Binding of FASL to its receptor induces receptor
trimerization and activation (55,56).
Subsequently, activated FAS can recruit Fas-associated death domain
(FADD), which undergoes conformational changes that allow it to
bind and cleave procaspase-8, promoting the formation of the
death-inducing signaling complex (DISC). Within this complex,
activated caspase-8 propagates the apoptotic cascade via cleaving
and activating caspase-3 (55,56).
Here, the pronounced upregulation of FAS, FASL and CASP3,
accompanied by the increased intracellular FAS protein levels,
supported the activation of the extrinsic apoptotic pathway.
Protein expression levels of FAS were markedly decreased in cell
supernatant following treatment with PRF for 24 h, and
significantly elevated at 48 h. During early apoptosis, FAS
proteins may aggregate and bind to the plasma membrane, leading to
elevated intracellular FAS levels. In late apoptosis, loss of
plasma membrane integrity may facilitate the release of
membrane-bound FAS proteins into the culture medium in a soluble
form (57-59),
resulting in the observed increase in soluble FAS protein levels at
48 h.
DISC-associated caspase-8 can undergo
auto-proteolysis to activate caspase-8, which cleaves interacting
domain death agonist (Bid) into truncated (t)Bid (56,60).
tBid can translocate to mitochondria, thus perturbing the
equilibrium between pro-apoptotic and anti-apoptotic Bcl-2 family
members (60-62).
However, the lack of alterations in the mRNA expression levels of
BCL2 and BAX indicated that the PRF-induced cell apoptosis may
occur independently of the mitochondrial pathway. Nandana et
al (50) confirmed that Brucein
D (a bioactive quassinoid compound isolated from Brucea
javanica fruit) induces apoptosis in T24 cells by regulating the
Bcl-2/Bax pathway, exhibiting the classical DNA ladder feature.
Conversely, the typical DNA laddering would not be expected in
apoptotic mechanisms independent of the Bcl-2/Bax pathway. This was
further reinforced by the absence of characteristic DNA ladder
fragmentation, supporting extrinsic apoptotic pathway
involvement.
In parallel with the FAS pathway, TNFα binding to
TNFR1 disrupts its interaction with the inhibitory protein silencer
of death domains, enabling the recruitment of TNF
receptor-associated death domain (TRADD) through the intracellular
death domain (63-67).
TRADD interacts with FADD, activating procaspase-8 to caspase-8,
which cleaves caspase-3. In the present study, the concurrent
upregulation of TNFR1, TNFα and CASP3 further supported the
involvement of this TNFα-driven apoptotic mechanism. In addition,
the significant elevation of the TNF-α protein expression
demonstrated that TNF-α may be involved in PRF-induced apoptosis in
BC cells.
TRADD recruits TNFR-associated factor 2 and
receptor-interacting serine/threonine-protein kinase 1, leading to
the activation of the IKK complex, which degrades IκB (63,64).
This process can promote the release of NF-κB, allowing its nuclear
translocation and the subsequent transcription of pro-survival
genes (63,64). Paradoxically, the present NF-κB
downregulation indicated that PRF may suppress this survival
signaling axis, thus shifting the cellular balance toward
apoptosis. Although this interaction may be indirect, the inverse
association between NF-κB suppression and caspase activation may
provide evidence for the pro-apoptotic effects of PRF.
NF-κB exhibits biphasic regulation (68). NF-κB upregulation promotes cellular
survival via transcription of pro-survival genes (63,64).
However, its pathological hyperactivation promotes tumor
progression and chemoresistance through the upregulation of
anti-apoptotic factors and activation of survival signaling
(69,70). By contrast, crosstalk between TNF-α
and FAS could promote the establishment of a compensatory apoptotic
axis by amplifying the activation of caspase-8 and -3, thus
counteracting NF-κB-mediated cytoprotection (71). In the present study, PRF led to a
biphasic modulation of NF-κB, which was characterized by transient
upregulation at 24 h followed by downregulation at 48 h. The
aforementioned finding was consistent with context-dependent
biphasic NF-κB regulation, implicating NF-κB in PRF-induced
apoptosis. These results indicated a dual role for NF-κB in T24
cells, acting as both a pro-survival mediator and a
stress-responsive modulator of apoptosis.
The present study had limitations. Firstly, the
antitumor effects of PRF were not validated through in vivo
animal experiments. Secondly, the findings were verified solely in
the T24 cell line without replication in other BC cell lines,
thereby restricting the generalizability of the conclusions.
Investigation of the mechanistic pathways lacked protein-level
validation by western blot analysis. Fourthly, no positive control
was included in the DNA ladder assay.
In conclusion, the present study demonstrated that
PRF exhibited a significant inhibitory effect on the viability of
BC cells (T24 cell line). This inhibitory activity displayed a
time- and dose-dependent association, with more pronounced effects
observed at PRF concentrations of 100 and 200 µg/ml following
treatment for 24 and 48 h. Mechanistically, the results indicated
that the PRF-induced apoptosis was triggered by two mechanisms,
namely the death receptor-mediated pathways (FAS/TNFR1) in the
absence of mitochondrial pathways and the biphasic modulation of
NF-κB signaling, characterized by a switch from survival to
apoptosis. Overall, these findings highlighted the therapeutic
potential of PRF for the treatment of apoptosis-resistant BC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 82173568, 81703269,
81973097 and 82273687), the Natural Science Foundation of
Heilongjiang Province (grant no. LH2023H054), the Huoju Plan
Research Foundation of Mudanjiang Medical University (grant no.
2022-MYHJ-014), the Special Program for Supervisor Scientific
Research of Mudanjiang Medical University (grant nos. YJSZX2022137
and YJSZX2022141), the Administration of Science & Technology
Foundation of Mudanjiang (grant nos. HT2022NS112 and HT2022NS113),
the Scientific Research Project of the Health Commission of
Heilongjiang Province (grant no. 20221212060609) and the
Fundamental Research Funds for the Universities of Heilongjiang
Province (grant no. 2022-KYYWFMY-0712).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JD designed the study, analyzed and interpreted data
and wrote and revised the manuscript. YG and HG designed the study
and analyzed data. TJ and YM analyzed data and wrote the
manuscript. SR designed the study and wrote and revised the
manuscript. JD and SR confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tran L, Xiao JF, Agarwal N, Duex JE and
Theodorescu D: Advances in bladder cancer biology and therapy. Nat
Rev Cancer. 21:104–121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Katims AB, Tallman J, Vertosick E, Porwal
S, Dalbagni G, Cha EK, Smith R, Benfante N and Herr HW: Response to
2 induction courses of bacillus Calmette-Guèrin therapy among
patients with high-risk non-muscle-invasive bladder cancer: 5-Year
follow-up of a phase 2 clinical trial. JAMA Oncol. 10:522–525.
2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Teoh JY, Kamat AM, Black PC, Grivas P,
Shariat SF and Babjuk M: Recurrence mechanisms of
non-muscle-invasive bladder cancer-a clinical perspective. Nat Rev
Urol. 19:280–294. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nat Rev Cancer. 15:25–41. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Berdik C: Unlocking bladder cancer.
Nature. 551:S34–S35. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
James AC and Gore JL: The costs of
non-muscle invasive bladder cancer. Urol Clin North Am. 40:261–269.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abdollah F, Gandaglia G, Thuret R,
Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF,
Perrotte P, et al: Incidence, survival and mortality rates of
stage-specific bladder cancer in United States: A trend analysis.
Cancer Epidemiol. 37:219–225. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Witjes JA, Bruins HM, Cathomas R, Compérat
EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A,
Neuzillet Y, et al: European association of urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2020
guidelines. Eur Urol. 79:82–104. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Y, Chang Q and Li Y: Racial
differences in urinary bladder cancer in the United States. Sci
Rep. 8(12521)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schinkel JK, Shao S, Zahm SH, McGlynn KA,
Shriver CD and Zhu K: Overall and recurrence-free survival among
black and white bladder cancer patients in an equal-access health
system. Cancer Epidemiol. 42:154–158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kaye DR, Canner JK, Kates M, Schoenberg MP
and Bivalacqua TJ: Do African American patients treated with
radical cystectomy for bladder cancer have worse overall survival?
Accounting for pathologic staging and patient demographics beyond
race makes a difference. Bladder Cancer. 2:225–234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Casey MF, Gross T, Wisnivesky J, Stensland
KD, Oh WK and Galsky MD: The impact of regionalization of
cystectomy on racial disparities in bladder cancer care. J Urol.
194:36–41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dobruch J, Daneshmand S, Fisch M, Lotan Y,
Noon AP, Resnick MJ, Shariat SF, Zlotta AR and Boorjian SA: Gender
and bladder cancer: A collaborative review of etiology, biology,
and outcomes. Eur Urol. 69:300–310. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Radkiewicz C, Edgren G, Johansson ALV,
Jahnson S, Häggström C, Akre O, Lambe M and Dickman PW: Sex
Differences in urothelial bladder cancer survival. Clin Genitourin
Cancer. 18:26–34.e6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Babjuk M, Burger M, Compérat EM, Gontero
P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF,
Sylvester R, et al: European association of urology guidelines on
non-muscle-invasive bladder cancer (TaT1 and carcinoma in
situ)-2019 update. Eur Urol. 76:639–657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Messing EM, Tangen CM, Lerner SP,
Sahasrabudhe DM, Koppie TM, Wood DP Jr, Mack PC, Svatek RS, Evans
CP, Hafez KS, et al: Effect of intravesical instillation of
gemcitabine vs saline immediately following resection of suspected
low-grade non-muscle-invasive bladder cancer on tumor recurrence:
SWOG S0337 randomized clinical trial. JAMA. 319:1880–1888.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Monro S, Colón KL, Yin H, Roque J III,
Konda P, Gujar S, Thummel RP, Lilge L, Cameron CG and McFarland SA:
Transition metal complexes and photodynamic therapy from a
tumor-centered approach: Challenges, opportunities, and highlights
from the development of TLD1433. Chem Rev. 119:797–828.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sylvester RJ, van der Meijden APM and Lamm
DL: Intravesical bacillus Calmette-Guerin reduces the risk of
progression in patients with superficial bladder cancer: A
meta-analysis of the published results of randomized clinical
trials. J Urol. 168:1964–1970. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lamm DL, Blumenstein BA, Crissman JD,
Montie JE, Gottesman JE, Lowe BA, Sarosdy MF, Bohl RD, Grossman HB,
Beck TM, et al: Maintenance bacillus Calmette-Guerin immunotherapy
for recurrent TA, T1 and carcinoma in situ transitional cell
carcinoma of the bladder: A randomized southwest oncology group
study. J Urol. 163:1124–1129. 2000.PubMed/NCBI
|
|
22
|
Hermans TJN, Voskuilen CS, van der Heijden
MS, Schmitz-Dräger BJ, Kassouf W, Seiler R, Kamat AM, Grivas P,
Kiltie AE, Black PC and van Rhijn BWG: Neoadjuvant treatment for
muscle-invasive bladder cancer: The past, the present, and the
future. Urol Oncol. 36:413–422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi W, Yuan R, Chen X, Xin Q, Wang Y,
Shang X, Cong W and Chen K: Puerarin reduces blood pressure
in spontaneously hypertensive rats by targeting eNOS. Am J Chin
Med. 47:19–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shukla R, Pandey N, Banerjee S and
Tripathi Y: Effect of extract of Pueraria tuberosa on
expression of hypoxia inducible factor-1α and vascular endothelial
growth factor in kidney of diabetic rats. Biomed Pharmacother.
93:276–285. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Satpathy S, Patra A, Ahirwar B and Hussain
MD: Antioxidant and anticancer activities of green synthesized
silver nanoparticles using aqueous extract of tubers of
Pueraria tuberosa. Artif Cells Nanomed Biotechnol. 46 (Suppl
3):S71–S85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jearapong N, Chatuphonprasert W and
Jarukamjorn K: Miroestrol, a phytoestrogen from Pueraria
mirifica, improves the antioxidation state in the livers and uteri
of β-naphthoflavone-treated mice. J Nat Med. 68:173–180.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahmad B, Khan S, Liu Y, Xue M, Nabi G,
Kumar S, Alshwmi M and Qarluq AW: Molecular mechanisms of
anticancer activities of Puerarin. Cancer Manag Res.
12:79–90. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin YJ, Hou YC, Lin CH, Hsu YA, Sheu JJ,
Lai CH, Chen BH, Lee Chao PD, Wan L and Tsai FJ: Puerariae
radix isoflavones and their metabolites inhibit growth and
induce apoptosis in breast cancer cells. Biochem Biophys Res
Commun. 378:683–688. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang XL, Wang BB and Mo JS:
Puerarin 6''-O-xyloside possesses significant antitumor
activities on colon cancer through inducing apoptosis. Oncol Lett.
16:5557–5564. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bulugonda RK, Kumar KA, Gangappa D, Beeda
H, Philip GH, Muralidhara Rao D and Faisal SM: Mangiferin from
Pueraria tuberosa reduces inflammation via inactivation of
NLRP3 inflammasome. Sci Rep. 7(42683)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pandey N, Yadav D, Pandey V and Tripathi
VB: Anti-inflammatory effect of Pueraria tuberosa extracts
through improvement in activity of red blood cell anti-oxidant
enzymes. Ayu. 34:297–301. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Srivastava S, Pandey H, Singh SK and
Tripathi YB: Anti-oxidant, anti-apoptotic, anti-hypoxic and
anti-inflammatory conditions induced by PTY-2 against STZ-induced
stress in islets. Biosci Trends. 13:382–393. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shukla R, Banerjee S and Tripathi YB:
Pueraria tuberosa extract inhibits iNOS and IL-6 through
suppression of PKC-α and NF-kB pathway in diabetes-induced
nephropathy. J Pharm Pharmacol. 70:1102–1112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jin SE, Son YK, Min BS, Jung HA and Choi
JS: Anti-inflammatory and antioxidant activities of constituents
isolated from Pueraria lobata roots. Arch Pharm Res.
35:823–837. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim JM, Lee YM, Lee GY, Jang DS, Bae KH
and Kim JS: Constituents of the roots of Pueraria lobata
inhibit formation of advanced glycation end products (AGEs). Arch
Pharm Res. 29:821–825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sun Y, Zhang H, Cheng M, Cao S, Qiao M,
Zhang B, Ding L and Qiu F: New hepatoprotective isoflavone
glucosides from Pueraria lobata (Willd.) Ohwi. Nat Prod Res.
33:3485–3492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sook Kim Y, Soo Lee I and Sook Kim J:
Protective effects of Puerariae radix extract and its single
compounds on methylglyoxal-induced apoptosis in human retinal
pigment epithelial cells. J Ethnopharmacol. 152:594–598.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sucontphunt A, De-Eknamkul W, Nimmannit U,
Dan Dimitrijevich S and Gracy RW: Protection of HT22 neuronal cells
against glutamate toxicity mediated by the antioxidant activity of
Pueraria candollei var. mirifica extracts. J Nat Med.
65:1–8. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Koirala P, Seong SH, Jung HA and Choi JS:
Comparative evaluation of the antioxidant and anti-Alzheimer's
disease potential of coumestrol and puerarol isolated from
Pueraria lobata using molecular modeling studies. Molecules.
23(785)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Anukulthanakorn K, Parhar IS, Jaroenporn
S, Kitahashi T, Watanbe G and Malaivijitnond S: Neurotherapeutic
effects of Pueraria mirifica extract in early- and
late-stage cognitive impaired rats. Phytother Res. 30:929–939.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tiyasatkulkovit W, Malaivijitnond S,
Charoenphandhu N, Havill LM, Ford AL and VandeBerg JL:
Pueraria mirifica extract and Puerarin enhance
proliferation and expression of alkaline phosphatase and type I
collagen in primary baboon osteoblasts. Phytomedicine.
21:1498–1503. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Manonai J, Chittacharoen A, Udomsubpayakul
U, Theppisai H and Theppisai U: Effects and safety of
Pueraria mirifica on lipid profiles and biochemical markers
of bone turnover rates in healthy postmenopausal women. Menopause.
15:530–535. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Y, Wang WL, Xie WL, Li LZ, Sun J, Sun
WJ and Gong HY: Puerarin stimulates proliferation and
differentiation and protects against cell death in human
osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt
activation. Phytomedicine. 20:787–796. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang Y, Ma Y, Zheng Y, Song J, Yang X, Bi
C, Zhang D and Zhang Q: In vitro and in vivo anticancer activity of
a novel Puerarin nanosuspension against colon cancer, with
high efficacy and low toxicity. Int J Pharm. 441:728–735.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jia L, Hu Y, Yang G and Li P:
Puerarin suppresses cell growth and migration in
HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR
signaling pathway. Exp Ther Med. 18:543–549. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang WG, Liu XF, Meng KW and Hu SY:
Puerarin inhibits growth and induces apoptosis in SMMC-7721
hepatocellular carcinoma cells. Mol Med Rep. 10:2752–2758.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang WG, Yin XC, Liu XF, Meng KW, Tang K,
Huang FL, Xu G and Gao J: Puerarin induces hepatocellular
carcinoma cell apoptosis modulated by MAPK signaling pathways in a
dose-dependent manner. Anticancer Res. 37:4425–4431.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hu Q, Xiang H, Shan J, Jiao Q, Lv S, Li L,
Li F, Ren D and Lou H: Two pairs of diastereoisomeric isoflavone
glucosides from the roots of Pueraria lobata. Fitoterapia.
144(104594)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shi ZD, Hao L, Han XX, Wu ZX, Pang K, Dong
Y, Qin JX, Wang GY, Zhang XM, Xia T, et al: Targeting HNRNPU to
overcome cisplatin resistance in bladder cancer. Mol Cancer.
21(37)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nandana PI, Rasyid H, Prihantono P,
Yustisia I, Hakim L, Bukhari A and Prasedya ES: Cytotoxicity and
apoptosis studies of brucein D against T24 bladder cancer cells.
Asian Pac J Cancer Prev. 25:921–930. 2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sun Y, Liu X, Tong H, Yin H, Li T, Zhu J,
Chen J, Wu L, Zhang X, Gou X and He W: SIRT1 promotes cisplatin
resistance in bladder cancer via beclin1 deacetylation-mediated
autophagy. Cancers (Basel). 16(125)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mun JY, Baek SW, Jeong MS, Jang IH, Lee
SR, You JY, Kim JA, Yang GE, Choi YH, Kim TN, et al: Stepwise
molecular mechanisms responsible for chemoresistance in bladder
cancer cells. Cell Death Discov. 8(450)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dong J, Guo Y, Ji T, Gao Q, Guan H, Niu Y,
Ma Y and Rong S: Effect of Puerariae radix flavones on mRNA
expression of N-myc downstream regulatory gene 1 in bladder cancer
cell line T24. J Environ Health. 41:941–943. 2024.(In Chinese).
|
|
55
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lavrik IN and Krammer PH: Regulation of
CD95/Fas signaling at the DISC. Cell Death Differ. 19:36–41.
2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Peter ME and Krammer PH: The
CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35.
2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Rehm M, Huber HJ, Dussmann H and Prehn JH:
Systems analysis of effector caspase activation and its control by
X-linked inhibitor of apoptosis protein. EMBO J. 25:4338–4349.
2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cheng J, Zhou T, Liu C, Shapiro JP, Brauer
MJ, Kiefer MC, Barr PJ and Mountz JD: Protection from Fas-mediated
apoptosis by a soluble form of the Fas molecule. Science.
263:1759–1762. 1994.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sartorius U, Schmitz I and Krammer PH:
Molecular mechanisms of death-receptor-mediated apoptosis.
Chembiochem. 2:20–29. 2001.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li H and Lin X: Positive and negative
signaling components involved in TNFalpha-induced NF-kappaB
activation. Cytokine. 41:1–8. 2008.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wajant H and Scheurich P: TNFR1-induced
activation of the classical NF-κB pathway. FEBS J. 278:862–876.
2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Luo JL, Tan W, Ricono JM, Korchynskyi O,
Zhang M, Gonias SL, Cheresh DA and Karin M: Nuclear
cytokine-activated IKKalpha controls prostate cancer metastasis by
repressing Maspin. Nature. 446:690–694. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227.
2002.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ben-Neriah Y and Karin M: Inflammation
meets cancer, with NF-κB as the matchmaker. Nat Immunol.
12:715–723. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov.
8:33–40. 2009.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Vazquez-Santillan K, Melendez-Zajgla J,
Jimenez-Hernandez L, Martínez-Ruiz G and Maldonado V: NF-κB
signaling in cancer stem cells: A promising therapeutic target?
Cell Oncol (Dordr). 38:327–339. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cao X and Jin HM: Effects of Fas, NF-κB
and caspases on microvascular endothelial cell apoptosis induced by
TNFα. Chin J Pathophysiol. 8:15–18. 2001.(In Chinese).
|