1. Introduction
Lung cancer remains a leading cause of
cancer-related morbidity and mortality worldwide, with an estimated
1.8 million deaths annually (1,2).
Tobacco smoking is unequivocally recognized as the primary risk
factor, accounting for ~85% of lung cancer cases (3,4).
However, as cannabis use becomes increasingly widespread due to
legalization and social acceptance, concerns have arisen regarding
its potential role in respiratory pathology and carcinogenesis
(5).
Cannabis smoke shares numerous toxic and
carcinogenic compounds with tobacco smoke, including polycyclic
aromatic hydrocarbons (PAHs), volatile organic compounds (VOCs) and
reactive oxygen species (6). These
substances have been implicated in DNA damage, oxidative stress and
chronic inflammation, all of which are critical pathways in lung
carcinogenesis (7). Nevertheless,
epidemiological evidence linking cannabis use to lung cancer
remains inconclusive and is complicated by factors such as
concurrent tobacco use, variability in cannabis potency, and
differences in smoking patterns (8,9).
One of the unique aspects of cannabis smoke is its
bronchodilatory effect, mediated by
Δ9-tetrahydrocannabinol (THC) interacting with
cannabinoid receptors in the bronchial smooth muscle (10). This bronchodilation may transiently
counteract airflow obstruction, contrasting with the airway
constriction commonly induced by tobacco smoke (11). However, chronic cannabis use has
been associated with increased respiratory symptoms, including
cough, sputum production and wheezing, akin to chronic bronchitis
(12). It has been suggested that
while cannabis smoking may impair large airway function, it does
not typically cause emphysema or severe airflow limitation as
tobacco smoking does (13).
The paradoxical nature of cannabis and its health
impacts is further compounded by its pharmacological profile. In
addition to its harmful constituents, cannabis contains
cannabinoids, such as cannabidiol (CBD) and THC, which have
demonstrated anti-inflammatory, bronchodilatory, and even
antineoplastic properties in preclinical models (14,15).
These conflicting biological effects make it challenging to
ascertain the net impact of cannabis smoking on lung cancer
risk.
Given the increasing prevalence of cannabis use,
particularly among younger adults and populations with chronic
medical conditions, there is an urgent need to elucidate its
long-term effects on lung health and cancer development. The
present review aims to critically assess the current evidence
regarding cannabis smoking and its implications for lung cancer and
respiratory physiology. By examining both epidemiological data and
mechanistic insights, it is sought to provide a balanced
perspective on the potential respiratory risks associated with
cannabis use, while highlighting the gaps that warrant further
research.
2. Overview of cannabis
Cannabis, a plant with a rich history of use in both
medical and recreational contexts, has long been a subject of
scientific inquiry due to its complex effects on the human body.
The plant contains various chemical compounds known as
cannabinoids, the most studied being THC and CBD, which interact
with the body's endocannabinoid system (ECS). ECS plays a key role
in regulating various physiological processes, including mood,
appetite, pain sensation and immune responses (16).
General overview of cannabis. Cannabis has
been used for thousands of years, originating in Central Asia and
spreading across various cultures for medicinal, recreational and
industrial purposes. Its therapeutic potential was recognized early
on, with significant interest in its analgesic, anti-inflammatory
and antiemetic properties (17).
Despite historical stigma, cannabis has gradually
gained acceptance, particularly in modern times with the advent of
medical marijuana programs in various countries (18).
The psychoactive properties of cannabis are
primarily attributed to THC, which binds to cannabinoid receptors 1
(CB1) in the brain, producing a variety of effects, including
altered perceptions, euphoria, and, in some cases, anxiety or
paranoia. CBD, on the other hand, is non-psychoactive and has
gained attention for its potential to mitigate the effects of THC,
offering anxiolytic, anti-inflammatory and neuroprotective benefits
(19).
Modern use and legalization trends. In recent
years, the landscape of cannabis consumption has evolved markedly.
With the ongoing legalization of cannabis for medical and
recreational use, particularly in regions such as North America and
parts of Europe, cannabis has become more widely available. This
has led to changes in public attitudes and increased usage, with a
corresponding rise in demand for various cannabis products, ranging
from traditional dried flowers to oils, edibles and vaping products
(20).
Surveys show that cannabis use is rising across
various demographic groups, particularly in regions where
legalization has occurred. Legalization has also resulted in more
robust research on cannabis's effects, and as regulations evolve,
new cannabinoid-based therapies are emerging, leading to further
interest in its medical applications (21).
Health and safety considerations. The
increasing popularity of cannabis, particularly among younger
adults, older populations, and individuals with chronic conditions,
underscores the importance of studying its long-term health effects
(22). While cannabis is generally
considered to be safe when used in moderation, there are concerns
about its psychological and physical effects, particularly when
used heavily or at a young age. Long-term use has been linked to
cannabis use disorder (CUD), mental health disorders, and potential
risks associated with smoking, such as respiratory problems
(23).
It is crucial to understand the impact of cannabis
potency, as recent trends show an increase in the concentration of
THC, which may exacerbate these risks. While cannabis is often
observed as a safer alternative to other substances, such as
alcohol or opioids, further research is needed to fully understand
its health implications, especially as it becomes increasingly
integrated into both medical treatments and recreational use.
Potential medical benefits and risks.
Cannabis's therapeutic potential is being explored in a variety of
clinical settings. It is increasingly prescribed for a wide range
of conditions, including chronic pain, anxiety, epilepsy, nausea
and vomiting associated with chemotherapy, and neurological
disorders such as multiple sclerosis. However, the balance between
benefit and risk is complex, and much of the clinical evidence is
still emerging (24).
While cannabinoids show promise in treating various
conditions, there is a need for rigorous clinical trials to
establish clear dosing guidelines, safety profiles and long-term
effects. In particular, research is needed to evaluate the benefits
and risks associated with different consumption methods, such as
smoking, vaping and edibles (25).
Cannabis and its derivatives have been studied for a
wide range of medical indications. Robust evidence supports its
efficacy in the management of chronic pain, particularly
neuropathic pain, where cannabinoids can provide modest but
clinically meaningful benefit (25). Cannabinoid preparations also reduce
chemotherapy-induced nausea and vomiting and may improve appetite
in patients with human immunodeficiency virus/acquired
immunodeficiency syndrome or cancer-related cachexia (26,27).
Nabiximols, an oromucosal spray containing THC and CBD, has shown
effectiveness in reducing spasticity in multiple sclerosis
(28). There is emerging evidence
for potential roles in anxiety, post-traumatic stress disorder and
sleep disorders, though findings remain inconsistent and often
limited by small sample size (29,30).
Conversely, risks of cannabis use are substantial
and dose-dependent. Acute adverse effects include impaired
short-term memory, psychomotor performance deficits and increased
risk of accidents (5). Regular
heavy use has been associated with cognitive impairment,
development of CUD, and increased risk of psychosis in genetically
or clinically vulnerable individuals (31). Pulmonary risks, particularly from
smoked cannabis, include chronic bronchitis and airway inflammation
(32), while cardiovascular
concerns such as arrhythmia, myocardial infarction and stroke have
been reported, especially among young adults with underlying risk
factors (33,34). Additionally, prenatal cannabis
exposure has been linked to lower birth weight and potential
neurodevelopmental effects in offspring, though data remain mixed
(35).
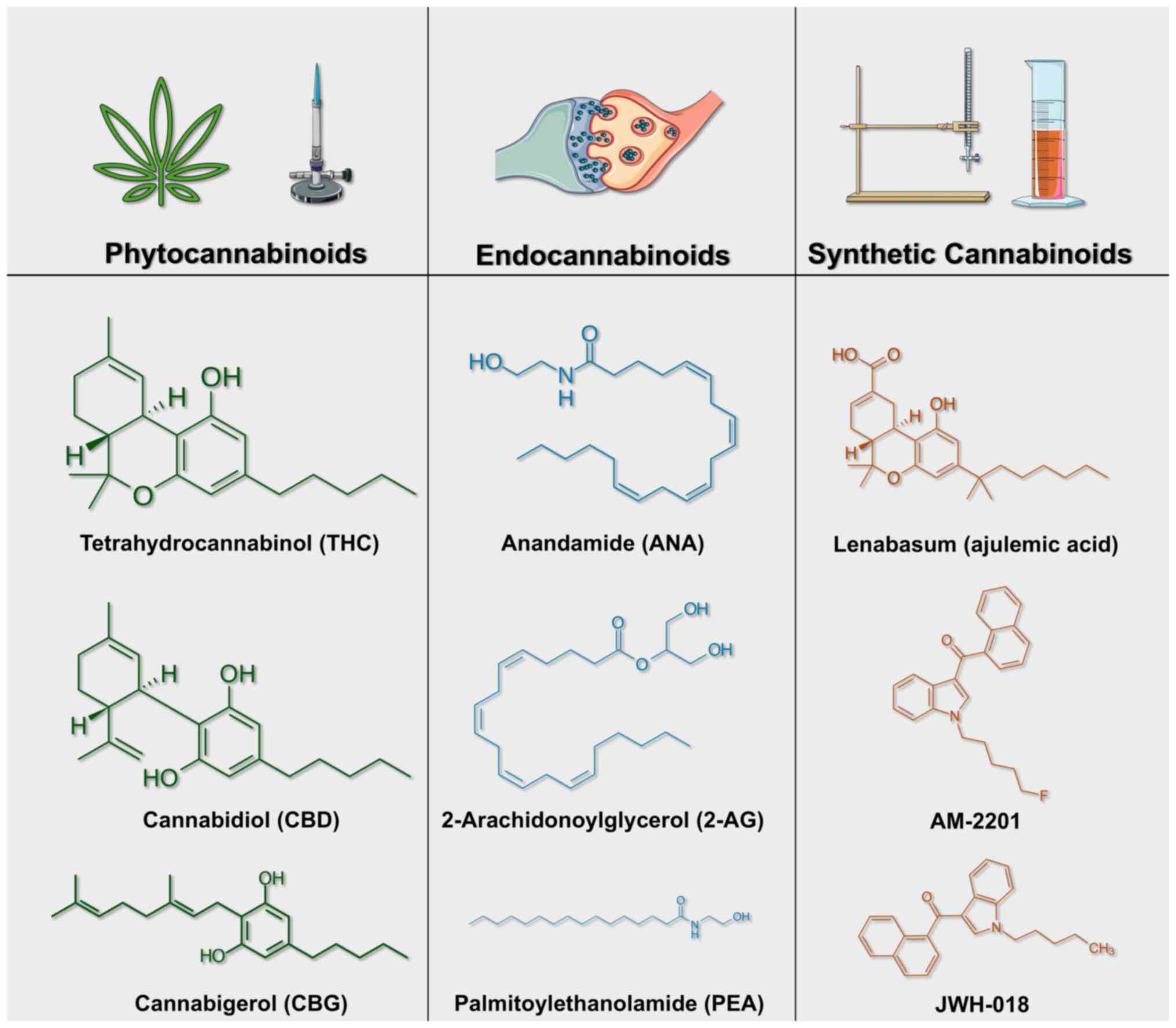
Chemical structures of common phytocannabinoids,
endocannabinoids and synthetic cannabinoids. Phytocannabinoids,
endocannabinoids and synthetic cannabinoids share structural
similarities while exhibiting distinct chemical compositions that
influence their pharmacological effects. Phytocannabinoids are
natural compounds produced by the Cannabis sativa plant,
with the most studied representatives being Δ9-THC, CBD,
cannabigerol (CBG), and cannabinol (CBN). Δ9-THC, the primary
psychoactive compound in cannabis, features a dibenzopyran ring
structure with a pentyl side chain. CBD, a non-psychoactive
counterpart, shares a similar ring structure but possesses a
hydroxyl group at the C1 position. CBG serves as a precursor to
both THC and CBD and is characterized by its linear structure and
pentyl side chain. CBN, an oxidative degradation product of THC,
retains a similar ring structure but lacks the double bond at
C9(36). These phytocannabinoids
differ in their affinities for cannabinoid receptors (CB1 and CB2)
and can interact with additional receptor systems such as transient
receptor potential (TRP) channels and peroxisome
proliferator-activated receptors (PPARs) (37).
Endocannabinoids are endogenous compounds produced
within the human body that bind to cannabinoid receptors and are
synthesized on demand rather than stored. The primary
endocannabinoids are anandamide (AEA) and 2-arachidonoylglycerol
(2-AG). Anandamide, an ethanolamide linked to arachidonic acid,
acts as a partial agonist at CB1 receptors and has limited efficacy
at CB2 receptors. By contrast, 2-AG, an ester of arachidonic acid
and glycerol, serves as a full agonist at both CB1 and CB2
receptors (27). Virodhamine,
another endocannabinoid, functions as a partial agonist at CB1 and
an antagonist at CB2, highlighting the complex signaling nature of
endogenous cannabinoids. Unlike classical neurotransmitters,
endocannabinoids are rapidly synthesized and released upon cellular
demand, followed by rapid degradation via enzymes such as fatty
acid amide hydrolase and monoacylglycerol lipase (37).
Synthetic cannabinoids (SCs) are laboratory-created
substances that mimic the effects of THC but are often
significantly more potent and toxic. These compounds are
structurally diverse, typically featuring an indole or indazole
core linked to a carbon tail, which enhances receptor binding
affinity and efficacy compared with natural cannabinoids (38). Examples include JWH-018 and JWH-073,
which are amino-alkyl-indole derivatives that act as full agonists
at CB1 receptors, and AB-FUBINACA, an indazole-based synthetic
cannabinoid with high CB1 affinity. Additionally, CP 47,497 and
HU-210 are synthetic cannabinoids known for their potent CB1
agonist activity (39). Synthetic
cannabinoids exhibit higher receptor affinity and are often
associated with severe toxicological effects due to their full
agonist behavior at cannabinoid receptors (38).
The structural differences among phytocannabinoids,
endocannabinoids and synthetic cannabinoids result in unique
pharmacological profiles, which influence their efficacy and
toxicity in both therapeutic and recreational settings (Fig. 1).
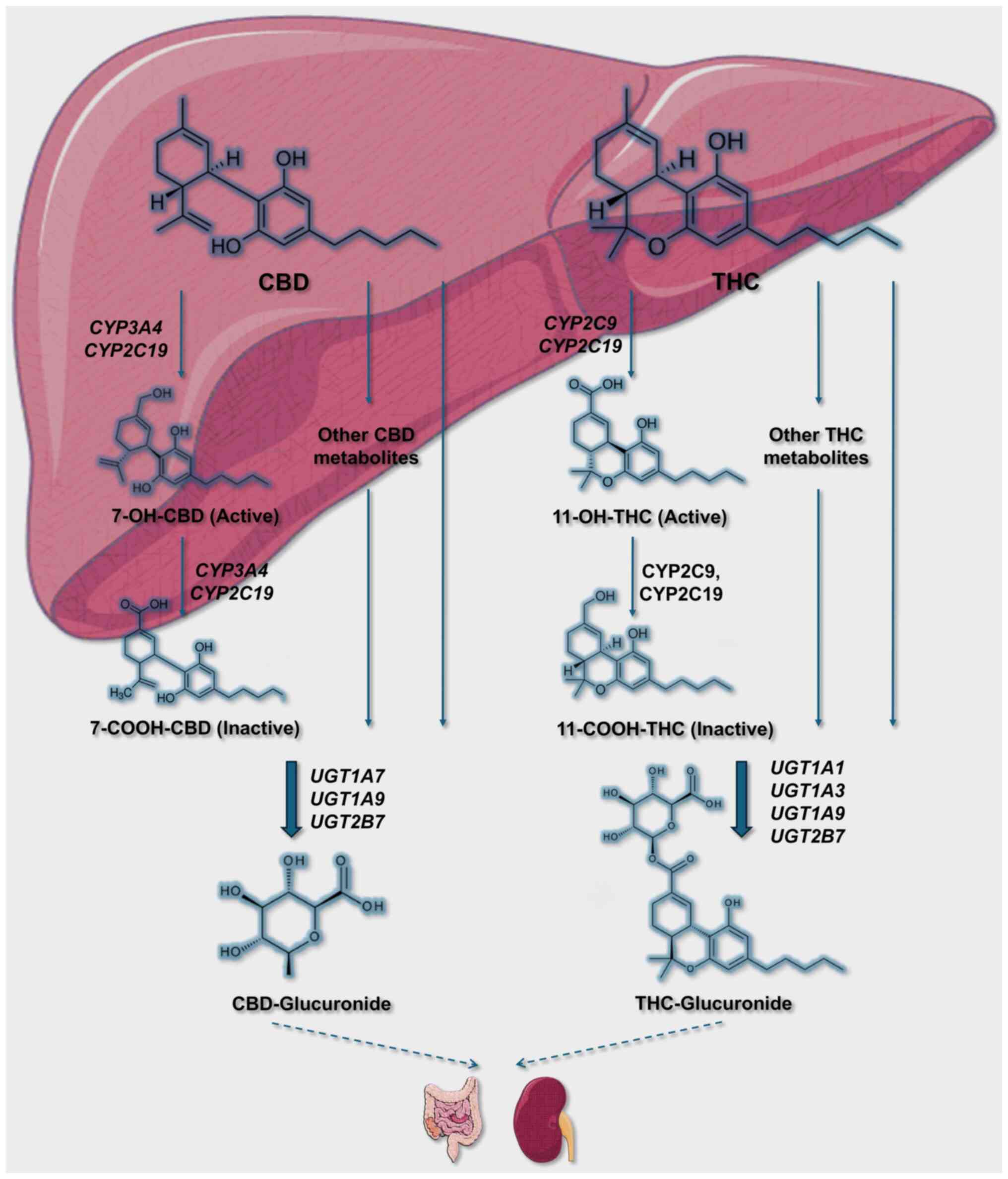
THC and CBD metabolism. THC and CBD are two
of the most abundant phytocannabinoids found in Cannabis
sativa, and they have distinct pharmacokinetic and metabolic
profiles. Both compounds are highly lipophilic and are rapidly
distributed in various tissues, including the brain and adipose
tissue, after absorption. Their metabolism primarily occurs in the
liver through the cytochrome P450 (CYP) enzyme system.
THC is predominantly metabolized in the liver by the
CYP450 system, specifically by the CYP2C9, CYP2C19 and CYP3A4
enzymes. Upon administration, THC undergoes extensive first-pass
metabolism, particularly when ingested orally, which significantly
reduces its bioavailability (~4-12%) compared with inhalation
(10-35%) (40). The major metabolic
pathways of THC include hydroxylation and oxidation, producing the
psychoactive metabolite 11-hydroxy-THC (11-OH-THC) and the inactive
metabolite 11-carboxy-THC (THC-COOH). 11-OH-THC is known to retain
psychoactive properties similar to THC itself and is rapidly formed
after ingestion, reaching peak plasma concentrations within 13 min
post-inhalation (41).
THC and its metabolites are primarily excreted
through feces (65 to 80%), while ~20-35% is excreted through urine,
mainly as conjugated metabolites (THC-COOH-glucuronide). Due to its
lipophilicity, THC is stored in adipose tissues, leading to a
prolonged elimination half-life of up to 13 days in chronic users
(41).
CBD is metabolized primarily by CYP2C19 and CYP3A4,
with subsequent involvement of CYP1A1, CYP1A2, CYP2C9 and CYP2D6
[Gonçalves et al (41)]. CBD
undergoes hydroxylation and oxidation, forming major metabolites
such as 7-hydroxy-CBD (7-OH-CBD) and its subsequent oxidized
product 7-carboxy-CBD (7-COOH-CBD). Like THC, CBD exhibits low oral
bioavailability (around 6%) due to extensive first-pass metabolism,
while its inhalation bioavailability ranges from 11-45% (40).
CBD is primarily excreted in feces (60%) and to a
lesser extent in urine (16%), mainly in the form of hydroxylated
and carboxylated metabolites. The plasma half-life of CBD varies
between 18-32 h (41).
Both THC and CBD are known to inhibit CYP enzymes,
potentially leading to interactions with other medications
metabolized by the same enzymes. CBD, in particular, is a potent
inhibitor of CYP2C19 and CYP3A4, increasing the risk of drug
interactions when co-administered with substrates of these enzymes
(42). This interaction profile is
clinically relevant, especially for patients on polypharmacy
regimens, such as those receiving anticonvulsants or antiepileptic
drugs (Fig. 2).
3. Patterns of cannabis consumption
Cannabis has been an integral component of human
society for millennia, with archaeological and historical evidence
suggesting that its use emerged independently in multiple regions.
This pattern of emergence indicates a complex process of cultural
exchange, particularly during the Bronze Age, when trans-Eurasian
trade played a significant role in the globalization of cannabis,
alongside the spread of other domesticated plants (43).
In contemporary society, cannabis use is pervasive,
with data from the United States indicating that ~7.5% of
individuals aged ≥12 years reported using marijuana in the
preceding month as of 2013(44).
The prevalence of cannabis use has continued to increase,
especially among adults, paralleling the legalization of medical
and recreational cannabis in several states (44,45).
The National Survey on Drug Use and Health has documented a
consistent rise in cannabis consumption, reporting an increase in
past-month cannabis use from 4.1% in 2001-2002 to 9.5% in
2012-2013(28). Demographic
analyses further reveal that cannabis use is more common among
males, younger adults, and individuals residing in higher-income
areas (44,45).
The shifting legal and social landscape surrounding
cannabis has also contributed to increased use among older adults.
For instance, there was a 57.8% relative increase in past-year
cannabis use among adults aged 50-64 and a remarkable 250% increase
among those aged 65 and older between 2006 and 2013(46). This trend underscores the need for a
comprehensive assessment of the long-term health implications of
cannabis use across diverse age groups.
CUD, characterized by problematic cannabis
consumption leading to significant impairment or distress, has
emerged as a pressing public health issue. In Australia, it was
reported that 6% of individuals experienced CUD within the past 12
months, with higher prevalence rates observed among males and
younger users. Moreover, CUD has been strongly associated with
other psychiatric conditions, including alcohol use disorders and
affective disorders (47).
The legalization of recreational cannabis in various
U.S. states, along with nationwide legalizations in countries such
as Uruguay and Canada, has transformed patterns of cannabis
consumption. Legalization has been associated with reduced prices,
increased potency and enhanced accessibility of cannabis products.
These changes have coincided with an increase in the frequency of
cannabis use among adults and a rise in emergency department visits
and hospitalizations related to cannabis-induced pathologies
(48).
4. Mechanisms of cannabinoid activity
The physiological effects of cannabis are primarily
mediated through the ECS, a complex network comprising cannabinoid
receptors (CB1 and CB2), endogenous cannabinoids
(anandamide and 2-arachidonoylglycerol) and associated enzymes
(39). The ECS plays a crucial role
in regulating various central nervous system (CNS) and peripheral
processes, including anxiety, depression, neurogenesis, cognition
and memory (49).
THC, the principal psychoactive component of
cannabis, exerts its effects primarily through CB1 receptors
located in the CNS. By contrast, CBD interacts with a range of
non-cannabinoid receptors and ion channels, modulating the
psychoactive and physiological effects of THC. The biphasic nature
of cannabinoid effects complicates the study of cannabis's impact
on human health, as low doses may elicit effects that are opposite
to those observed at higher doses (49).
Recent advances in ECS research have identified
numerous endocannabinoid-like compounds within the brain that may
influence a broad spectrum of brain functions, offering potential
therapeutic targets for various neurological and psychiatric
conditions (49). The intricate
dynamics of the ECS underscore the complexity of cannabis's
physiological effects, emphasizing the necessity for comprehensive
investigations to unravel the mechanisms through which cannabis
interacts with biological systems (15,49).
Cannabinoids exert their effects primarily through
activation of the ECS, which consists of the cannabinoid receptors
CB1 and CB2, endogenous ligands such as
anandamide and 2-arachidonoylglycerol (2-AG), and enzymes
responsible for ligand synthesis and degradation (50). CB1 receptors are widely
expressed in the CNS, where they regulate neurotransmitter release,
cognition and reward pathways, but they are also found in
peripheral tissues including the lungs, cardiovascular system and
gastrointestinal tract (51). By
contrast, CB2 receptors are predominantly expressed on
immune cells and modulate inflammation, immune surveillance and
cytokine release (52,53).
Beyond receptor-mediated pathways, cannabinoids can
influence a range of non-cannabinoid targets, including TRP
channels, PPARs and serotonin receptors, which contribute to their
complex physiological effects (19,54).
In the respiratory system, CB1 receptor activation can
promote bronchial smooth muscle relaxation but also increases
airway hyperreactivity with chronic exposure, while CB2
signaling is implicated in modulating pulmonary inflammation and
immune responses (32,54). At the cellular level, cannabinoids
can alter oxidative stress, apoptosis and angiogenesis, processes
relevant to both tissue injury and carcinogenesis (55,56).
Taken together, the mechanisms of cannabinoid
activity are multifaceted, involving both central and peripheral
CB1/CB2 receptor signaling as well as
non-canonical pathways. This complexity underlies the dual
potential of cannabinoids to provide therapeutic benefit in
conditions such as chronic pain and multiple sclerosis while
simultaneously contributing to adverse outcomes including cognitive
impairment, immune dysregulation, and possibly increased cancer
risk.
5. Cannabis composition and potency
trends
A major determinant of the health impact associated
with cannabis use is its potency, primarily dictated by the
concentrations of THC, the principal psychoactive component, and
CBD, a non-psychoactive constituent. Analysis of illicit cannabis
samples seized by the U.S. Drug Enforcement Administration from
1995 to 2014 revealed a substantial increase in THC potency, rising
from ~4% in 1995 to ~12% in 2014. Concurrently, CBD levels declined
from ~0.28% in 2001 to less than 0.15% in 2014, resulting in a
marked shift in the THC to CBD ratio from 14:1 to ~80:1(57). This trend toward higher THC
concentrations may be attributed to the cultivation of sinsemilla
(seedless cannabis), favored for its potent psychoactive effects
(57). Elevated THC levels are
associated with more pronounced psychoactive effects, potentially
increasing the risk of dependence, psychosis, and other adverse
health outcomes (57,58). Moreover, the reduction in CBD
content may diminish the mitigating effects of CBD on THC-induced
anxiety and psychosis, thereby exacerbating the health risks posed
by high-potency cannabis products (57). These compositional changes in
cannabis underscore the need to reassess previous evidence and
conduct contemporary studies that reflect current usage trends.
The increasing complexity of cannabis products,
including the development of high-potency strains and synthetic
cannabinoids, further complicates the evaluation of health risks.
Synthetic cannabinoids, which frequently exhibit higher affinity
for cannabinoid receptors than natural cannabis, can induce more
intense psychoactive effects and are associated with severe adverse
events (59). The interaction of
various cannabinoid compounds, including THC and CBD, within the
ECS adds another layer of complexity to understanding the nuanced
health outcomes of cannabis use (49,58).
The relationship between use of cannabis and broader
public health issues, such as the opioid epidemic, warrants
critical consideration. Research has explored the potential role of
cannabis as a substitute for opioids in pain management; however,
findings remain inconclusive (47,60).
Additionally, the co-use of cannabis with other substances,
including alcohol and prescription medications, highlights the need
to address polysubstance use within public health strategies
(46,61). Notably, the existing literature
often presents contradictory findings, partly due to methodological
limitations such as small sample sizes, insufficient control for
confounding variables, and variability in the potency and
composition of cannabis products (62,63).
Consequently, robust and methodologically sound research is
essential to accurately assess the health implications of
contemporary cannabis use.
Over the last two decades, the average THC content
in plant cannabis has increased substantially while CBD content has
declined, driving a markedly higher THC:CBD ratio (16,57,58).
This compositional shift has two broad implications. First, legacy
epidemiology that used ‘joint-years’ to quantify exposure largely
reflects low-potency eras; equal ‘joint-years’ today plausibly
deliver far greater psychoactive dose and, depending on smoking
topography, similar or greater particulate and PAH exposure per
session. Consequently, null or weak associations from older cohorts
(for example, pulmonary function plateaus at modest exposure) may
underestimate modern risk (6,13,64).
Potency may also interact with behavior. Although
users can partially titrate (for example, fewer puffs, shorter
sessions), titration is imperfect and varies by experience, product
type and setting, particularly with concentrates and
high-efficiency devices. Thus, higher THC can increase delivered
dose without proportionally reducing inhaled toxicants from
combustion, maintaining exposure to tar and PAHs that drive airway
inflammation and genotoxicity (6,24).
From a mechanistic perspective, rising THC with
declining CBD could tilt airway biology toward a more
pro-inflammatory milieu. CBD has documented anti-inflammatory and
antiproliferative properties in experimental systems, whereas THC
has complex, dose-dependent effects on immune function; a higher
THC:CBD ratio may therefore attenuate any CBD-mediated modulation
of THC's effects (14,16). Whether this compositional shift
translates to higher lung cancer risk remains unsettled:
Conventional observational studies are inconsistent and rarely
stratify by potency, but Mendelian randomization (MR) analyses
suggest a possible causal signal for lung cancer (and specifically
squamous histology) with genetic liability to cannabis use/use
disorder (65,66). Given that most case-control and
cohort data were accrued in lower-potency periods and seldom
capture THC concentration, extrapolating their null findings to
contemporary high-THC markets is precarious.
Potency is also salient for non-combustible routes.
High-THC concentrates delivered via vaporizers can markedly
increase systemic THC without combustion by-products, but the EVALI
outbreak-largely tied to adulterants in illicit THC
cartridges-highlights distinct acute pulmonary hazards unrelated to
THC potency per se; long-term oncologic effects of high-THC
vapor exposures remain unknown (24,67).
In light of these trends, future studies should
replace or complement ‘joint-years’ with dose-standardized metrics
(for example, mg-THC-years), capture product THC and CBD content,
device type, and co-use with tobacco, and analyze outcomes by
potency strata. Mechanistic studies should employ modern high-THC
smoke and aerosol models to test dose-response effects on airway
inflammation, DNA damage and macrophage function (6,14).
6. Respiratory effects of cannabis use
Molecular impact of cannabis on lung cells.
Understanding the molecular mechanisms underlying cannabis-induced
pulmonary effects is essential to elucidating the pathways through
which cannabis impacts lung pathophysiology. Cannabis smoke
contains numerous toxic and carcinogenic compounds analogous to
those present in tobacco smoke, raising significant concerns
regarding its potential to induce deleterious effects on
respiratory cellular architecture (68). Toxicogenomic analyses comparing the
effects of tobacco and marijuana smoke condensates on murine lung
epithelial cells have demonstrated that both types of smoke disrupt
similar toxicological pathways, including xenobiotic metabolism,
oxidative stress, inflammation and DNA damage response. However,
marijuana smoke condensates exerted a more pronounced impact on
steroid biosynthesis, apoptosis, and inflammation pathways,
indicating distinct and potentially more aggressive molecular
disruptions associated with cannabis smoke. Additionally, marijuana
smoke exposure resulted in increased oxidative stress, which may
contribute to the heightened cytotoxicity observed among cannabis
smokers (69). These toxicogenomic
findings underscore the unique and potentially more severe
pulmonary impact of cannabis smoke compared with tobacco smoke.
Supporting these observations, a comprehensive
analysis comparing the chemical composition of mainstream and
sidestream cannabis smoke with that of tobacco smoke identified the
presence of known carcinogens, including ammonia, hydrogen cyanide
and PAHs, in cannabis smoke at concentrations comparable to or even
exceeding those found in tobacco smoke. These findings raise
significant concerns regarding the carcinogenic potential of
cannabis, given the well-established association between tobacco
smoke exposure and lung cancer (6).
To further investigate the molecular association
between cannabis smoke and carcinogenesis, the DNA-damaging effects
of cannabis smoke were examined by quantifying acetaldehyde-derived
N2-ethyl-2'-deoxyguanosine (N2-ethyl-dG)
adducts in DNA. This analysis revealed that exposure to cannabis
smoke generated dose-dependent increases in DNA adducts, comparable
to those induced by tobacco smoke. These findings strongly suggest
the genotoxic potential of cannabis smoke, suggesting that chronic
cannabis use could initiate carcinogenic processes similar to those
observed with tobacco smoking (7).
In contrast to the evidence suggesting an oncogenic
potential, cannabinoids have also demonstrated dual roles in both
promoting and inhibiting tumorigenesis, depending on the specific
context and molecular pathways involved. Phytocannabinoids and
synthetic cannabinoids have exhibited antiproliferative effects on
tumor cells in vitro and in some animal models. However,
when considering the chronic inhalation of carcinogenic byproducts,
immunosuppression, and sustained inflammation associated with
cannabis smoke, any potential antitumor effects may be negated by
the net mutagenic and growth-promoting environment (14). Consequently, the complex interplay
between the pro- and anti-tumorigenic effects of cannabinoids
warrants further investigation to delineate the potential health
risks and therapeutic applications of cannabis use.
Immune modulation in cannabis smokers. In
addition to the direct cytotoxic and genotoxic effects on
epithelial cells, cannabis smoking alters lung immune homeostasis,
leading to an inflammatory response and functional impairment of
alveolar macrophages (AMs), which play a critical role in airway
surveillance and pathogen clearance. Chronic cannabis use has been
associated with an inflammatory infiltrate within the lungs,
reflecting chronic airway irritation and a potential predisposition
to airway remodeling.
Investigations into the cellular composition of
bronchoalveolar lavage (BAL) fluid in cannabis smokers revealed
significantly elevated total cell counts, particularly neutrophils,
in the BAL fluid of both marijuana-only and combined
marijuana-tobacco smokers compared with non-smokers (49). This inflammatory cellular response
may indicate chronic airway irritation and potential remodeling
processes.
Further examination of airway inflammation through
videobronchoscopy in young, healthy individuals who habitually
smoked cannabis demonstrated significant airway inflammation,
including vascular hyperplasia, submucosal edema and increased
neutrophil counts. These inflammatory changes were comparable in
frequency and magnitude to those observed in tobacco smokers,
suggesting that cannabis smoke induces substantial airway
inflammation similar to tobacco smoke (70).
In addition to airway inflammation, cannabis smoking
has been shown to impair the function of AMs, which are essential
for maintaining pulmonary immune defense. Functional assessments of
AMs obtained from marijuana smokers demonstrated impaired
phagocytosis and reduced fungicidal activity against Candida
albicans, despite normal rates of ingestion of the pathogen
(71). Furthermore, these
macrophages exhibited diminished production of superoxide anions
upon stimulation, a critical factor in microbial elimination
(5,71).
Further investigations have also highlighted the
diminished bactericidal activity of AMs from marijuana smokers,
specifically against Staphylococcus aureus. In addition to
impaired pathogen clearance, these macrophages exhibited reduced
production of key pro-inflammatory cytokines, including tumor
necrosis factor-alpha and interleukin-6, which are essential for
orchestrating an effective immune response (72).
Cannabis smoking has been shown to impair the
antimicrobial and immunomodulatory functions of AMs, thereby
potentially increasing susceptibility to opportunistic infections
and respiratory pathogens. One mechanistic basis for these
impairments involves the suppression of nitric oxide (NO)
production, a critical effector molecule in microbial defense.
Research has demonstrated that cannabis use significantly reduces
NO production in AMs, compromising their antimicrobial capabilities
(73).
Further investigation into the functional
consequences of reduced NO production revealed that AMs from
marijuana smokers exhibited impaired bactericidal activity. This
deficiency persisted unless the cells were primed with exogenous
cytokines, indicating that cannabis-induced suppression of
intrinsic cytokine priming mechanisms significantly hampers the
antimicrobial efficacy of AMs (74). These findings suggest that chronic
cannabis exposure may compromise the ability of AMs to eliminate
ingested bacteria effectively, weakening pulmonary immune
defense.
The immunologic impact of cannabis use extends
beyond impaired NO production, with studies also exploring the
epigenetic effects of Δ9-THC on immune cells. Notably,
research has demonstrated that THC induces histone modifications
that shift cytokine gene expression from a T-helper 1
(Th1) to a T-helper 2 (Th2) profile. This
epigenetic reprogramming may underlie the immunosuppressive effects
observed in cannabis smokers, as the transition from a
Th1 to a Th2 response is associated with
impaired cytokine production and diminished antimicrobial activity
of AMs (75). By altering the
balance of Th1 and Th2 responses, THC may
skew immune function toward a less effective state for combating
infections and maintaining pulmonary homeostasis.
Collectively, these studies indicate that cannabis
smoking could significantly impair the antimicrobial and
immunomodulatory functions of AMs. This compromised immune function
may increase vulnerability to respiratory infections and
opportunistic pathogens, raising concerns about the long-term
pulmonary health consequences of chronic cannabis use.
Structural and histopathologic impact. The
persistent inflammatory state and immunological dysfunction induced
by cannabis smoke are accompanied by distinctive structural and
histopathological changes in the bronchial mucosa.
Histopathological analyses have demonstrated that habitual
marijuana smokers exhibit significant bronchial mucosal
abnormalities, including goblet cell hyperplasia, vascular
hyperplasia and cellular disorganization. These changes are
indicative of chronic airway irritation and remodeling, which are
known precursors to more severe respiratory diseases (76).
Moreover, the combination of marijuana and tobacco
smoking appears to have additive detrimental effects on the
bronchial mucosa. It has been demonstrated that habitual users of
both substances experience more pronounced mucosal damage compared
with those smoking either substance alone. Specifically, combined
marijuana and tobacco smokers show higher frequencies of epithelial
and submucosal alterations, suggesting that concurrent use
exacerbates bronchial injury (76).
This synergistic effect is particularly concerning given the high
prevalence of dual use among cannabis and tobacco smokers,
potentially amplifying the risk of severe airway damage and chronic
respiratory conditions.
In addition to structural abnormalities, molecular
and cellular alterations have been observed in habitual marijuana
smokers. An investigation into bronchial biopsy specimens from a
cohort of 104 volunteers, comprising non-smokers and habitual
smokers of marijuana, tobacco, or both, revealed significant
histopathological and molecular changes in cannabis users. Among
individuals without lung cancer, habitual marijuana smokers
displayed epithelial changes characterized by increased cellular
proliferation markers, such as Ki-67, and elevated expression of
the epidermal growth factor receptor. Additionally, abnormalities
in DNA content were observed, suggesting early field cancerization
changes akin to those seen in tobacco-exposed epithelium (77).
While the clinical implications of these findings
remain uncertain, the presence of such molecular alterations raises
concerns that habitual marijuana use may establish a pro-oncogenic
microenvironment within the bronchial epithelium. This could
potentially increase the risk of malignant transformation,
especially when cannabis smoking is combined with tobacco use.
Consequently, the synergistic impact of combined smoking on airway
pathology highlights the importance of further research into the
long-term respiratory risks associated with dual substance use.
Structural lung changes associated with cannabis
smoking have been investigated using high-resolution computed
tomography (HRCT) scans. One study reported that cannabis smokers
exhibited decreased lung density, which may reflect structural
alterations such as airway wall thickening or early parenchymal
changes (9). However, the
prevalence of macroscopic emphysema among cannabis-only smokers was
relatively low (1.3%) compared with significantly higher rates
observed in combined cannabis and tobacco users (18.9%) and
tobacco-only smokers (16.3%) (9).
These findings suggest that while cannabis smoking may induce
airflow obstruction and structural modifications, the development
of emphysema likely requires higher exposure levels or the
concurrent use of tobacco.
In addition to the structural changes observed on
imaging, case reports have documented the occurrence of large
bullae and spontaneous pneumothorax among heavy cannabis smokers,
often at relatively young ages (78). Although large-scale epidemiological
studies are yet to explore this association comprehensively, the
presence of bullous disease in cannabis smokers raises concerns
about localized overdistension and alveolar rupture. The chronic
irritative and inflammatory environment associated with habitual
cannabis smoking could lead to structural weaknesses in the
alveolar walls, predisposing to bullous formations that are less
commonly observed in non-cannabis users.
This distinctive pathological signature of
cannabis-related lung damage warrants further investigation, as it
may represent a unique manifestation of lung injury that differs
from the well-documented effects of tobacco smoking. Understanding
the mechanisms that predispose cannabis smokers to such changes
will be crucial for identifying at-risk populations and
implementing appropriate public health interventions.
7. Cannabis use and pulmonary function
Cannabis impact on lung physiology.
Inhalation of cannabis smoke has been associated with numerous
alterations in pulmonary function and respiratory health. Although
both cannabis and tobacco combustion produce similar byproducts,
the unique constituents of cannabis, particularly THC, may elicit
distinct physiological responses within the pulmonary system,
influencing airway physiology, airflow obstruction and long-term
lung function.
A study analyzing data from the Tucson
epidemiological study of airways obstructive disease demonstrated
that among younger adults (under 40 years of age), smoking
non-tobacco cigarettes (presumed primarily marijuana) was
associated with increased respiratory symptoms and significant
reductions in expiratory flow rates at low lung volumes. After
adjusting for tobacco use, non-tobacco cigarette smokers,
particularly men, exhibited decreases in the forced expiratory
volume at 1 second (FEV1) to forced vital capacity (FVC)
ratio and flow rates that, in some instances, were even more
pronounced than those seen in tobacco smokers. However, a
limitation of that study was the characterization of non-tobacco
cigarettes, which may have encompassed substances other than
marijuana (11).
Further investigating this association, a study
involving habitual heavy smokers of marijuana alone, those who
smoked both marijuana and tobacco, tobacco-only smokers, and
non-smokers found that marijuana smokers, regardless of concurrent
tobacco use, reported significantly more respiratory symptoms,
including cough, sputum production and wheezing. Habitual marijuana
use was associated with decrements in specific airway conductance,
indicating large airway obstruction (11). However, unlike tobacco smokers,
these functional impairments did not consistently translate into a
characteristic obstructive defect on spirometry, suggesting that
cannabis may affect the airways differently from tobacco. This
disparity may indicate that cannabis predominantly alters large
airway caliber and reactivity rather than inducing small-airway
remodeling.
Aldington et al (9) conducted a cross-sectional study in the
Greater Wellington region of New Zealand to investigate the
differential effects of cannabis and tobacco smoking. The study
enrolled cannabis-only smokers, tobacco-only smokers, combined
users and non-smokers, and employed comprehensive pulmonary
evaluations, including HRCT scans, standard spirometry and a
detailed respiratory questionnaire (9). A dose-response relationship was
demonstrated between cannabis smoking and reductions in the
FEV1/FVC ratio, as well as decreased specific airway
conductance and increased total lung capacity (9). The study reported that a single
cannabis joint exerted a damaging effect on large airway function
comparable to 2.5 to 5 tobacco cigarettes, though emphysema
remained uncommon among cannabis-only users (9).
In addition, the Coronary Artery Risk Development in
Young Adults (CARDIA) study, which followed U.S. adults for 20
years, found a nonlinear association between marijuana exposure and
lung function. At low to moderate levels of use (for example, a few
joints per month), modest but significant increases in
FEV1 and FVC were observed. However, with cumulative
exposure beyond 10 joint-years, the slope became negative, and very
heavy use (>20 uses per month) led to a slight decline in
FEV1 and a more notable increase in FVC, ultimately
lowering the FEV1/FVC ratio (13). This pattern indicates that while
moderate cannabis use may not severely compromise lung function,
excessive consumption may eventually result in airflow
impairment.
Similarly, a cross-sectional study using data from
the U.S. National Health and Nutrition Examination Survey (NHANES)
found that lifetime marijuana use up to 20 joint-years did not
adversely affect spirometry measures, including the FEV1
and FEV1/FVC ratio. However, exceeding 20 joint-years
doubled the odds of an FEV1/FVC ratio below 70%,
suggesting that heavy, prolonged use might result in clinically
significant airway obstruction (64). The study noted that changes in the
FEV1/FVC ratio were primarily driven by increases in FVC
rather than pronounced declines in FEV1, suggesting that
cannabis might induce alterations in lung volume and elastic recoil
distinct from the classical obstructive patterns observed with
tobacco use (64).
Moreover, a study investigating the acute
bronchodilator effects of cannabinoids on human bronchi
demonstrated that cannabinoids inhibit cholinergic-induced
bronchial contractions through CB1 receptors, thereby
providing a mechanistic explanation for the bronchodilation
observed in cannabis smokers. This finding underscores the role of
cannabinoids in modulating airway tone by acutely relaxing
bronchial muscles (10).
Collectively, these findings highlight the complex
and multifaceted effects of cannabis on pulmonary function, ranging
from acute bronchodilation to chronic airflow obstruction and
structural changes, particularly with heavy and prolonged use.
Further research is necessary to delineate the dose-dependent
effects and long-term implications of cannabis inhalation on
respiratory health.
Cannabis use and respiratory disorders. A
study examining the association between cannabis use and chronic
bronchitis symptoms found that current marijuana use was
significantly linked to an elevated prevalence of chronic
bronchitis, coughing, phlegm production and wheezing. By
controlling for variables such as asthma, age, sex and tobacco use,
the study demonstrated that the association between cannabis use
and bronchitic symptoms remained independent and robust (12).
Further exploring the respiratory effects of
cannabis, a longitudinal study evaluated respiratory symptoms in a
population-based cohort of 1,037 young adults assessed at ages 18,
21, 26, 32 and 38(79). Frequent
cannabis use (defined as ≥52 times in the past year) was associated
with chronic bronchitic symptoms, including morning cough, sputum
production, and wheeze. Notably, these symptoms either resolved or
significantly diminished when individuals discontinued or
substantially reduced cannabis intake (79). This finding suggests that
cannabis-related bronchitic changes may primarily result from
inflammatory or irritative processes rather than fixed structural
damage, as evidenced by the reversibility of symptoms upon
cessation.
Together with findings from additional studies,
these results indicate that cannabis, similar to tobacco, exerts
irritant effects on the airways that clinically manifest as chronic
bronchitis-like symptoms. However, the mechanisms underlying these
effects may differ, given the potential for symptom resolution with
reduced cannabis exposure.
Furthermore, a study assessing the risk of chronic
obstructive pulmonary disease (COPD) in relation to cannabis use
found that while smoking tobacco alone was associated with an
increased risk of COPD and respiratory symptoms, cannabis use alone
did not significantly elevate COPD risk (74). However, individuals who smoked both
tobacco and cannabis exhibited a synergistic effect, with the risk
of COPD and symptom burden being greater than what would be
expected from tobacco smoking alone. This compounded risk was
particularly pronounced at higher cumulative cannabis exposure
levels (>50 joints lifetime) (80).
These findings suggest that although cannabis use
alone may not significantly increase the risk of developing COPD,
the concurrent use of tobacco and cannabis can lead to an augmented
risk. The synergy between the two substances warrants caution,
especially in populations with high rates of dual use. Moreover,
the apparent reversibility of bronchitis symptoms associated with
cannabis cessation highlights the potential for mitigating
respiratory harm through reduction or discontinuation of cannabis
use.
Comparative respiratory effects of cannabis and
tobacco. Numerous studies have compared the effects of cannabis
and tobacco on lung health outcomes, highlighting both similarities
and differences in their physiological impacts. Although both
substances share common inhalational byproducts, they differ
significantly in the mechanisms and outcomes of respiratory
impairment.
One study reported that cannabis smoking induces
significant airflow obstruction, albeit with distinct structural
consequences compared with tobacco (9,81).
While cannabis smoking was consistently associated with reductions
in the FEV1/FVC and specific airway conductance, the
prevalence of macroscopic emphysema remained relatively low unless
cannabis use was combined with tobacco smoking (9). By contrast, tobacco smoking is
strongly linked to both large and small airway dysfunction and
significantly higher rates of emphysema (9,81).
Notably, marijuana smoking impairs large airway function but does
not accelerate the decline in FEV1 over time as tobacco
smoking does (11). This
differential impact suggests that the pathophysiological mechanisms
underlying cannabis- and tobacco-induced lung damage may diverge,
with cannabis primarily affecting airway conductance and tobacco
contributing more broadly to both obstructive and restrictive lung
disease.
Further evidence highlights the synergistic
respiratory risks associated with concurrent cannabis and tobacco
use. Studies have demonstrated that dual use of these substances
markedly increases the risk of respiratory symptoms and COPD beyond
what would be expected from tobacco smoking alone (73,80).
This synergy may result from cumulative exposure to inhaled toxins,
compounded inflammatory responses, or overlapping detrimental
effects on airway architecture and immune function.
Overall, cannabis use consistently emerges as a risk
factor for chronic bronchitis symptoms, airflow obstruction and
immunological dysregulation. However, unlike tobacco, cannabis use
does not uniformly lead to emphysema or progressive declines in
FEV1, suggesting differing underlying mechanistic
pathways. Nonetheless, the established synergy between cannabis and
tobacco underscores the importance of integrated public health
strategies that address dual substance use.
To comprehensively elucidate the causal pathways and
individual susceptibilities associated with cannabis-related lung
damage, large prospective cohort studies are needed. These studies
should employ standardized cannabis exposure metrics and rigorously
control for confounding factors, including tobacco use and
pre-existing respiratory conditions. Additionally, molecular and
mechanistic studies are warranted to explore how cannabis smoke
specifically affects airway structure and function compared with
tobacco, which could inform targeted interventions and harm
reduction strategies.
Several longitudinal and case-control studies have
directly compared the respiratory effects of cannabis and tobacco.
In the Dunedin Multidisciplinary Health and Development Study,
cumulative cannabis use was associated with increased FVC but no
consistent decline in FEV1, contrasting with the clear
dose-dependent FEV1 decline observed in tobacco users
(79). The CARDIA cohort similarly
reported that low-to-moderate cannabis exposure was associated with
modest increases in FEV1 and FVC, though heavy
cumulative use attenuated these benefits, whereas tobacco
demonstrated progressive declines in both parameters (13). In a New Zealand case-control study
incorporating HRCT, emphysema and reduced specific airway
conductance were more strongly linked to tobacco than to cannabis,
although dual users experienced the greatest impairment, suggesting
possible additive or synergistic effects (9).
Cannabis and tobacco also differ in their effects on
bronchitic symptoms and emphysema. Regular cannabis use has been
consistently associated with chronic bronchitis symptoms, including
cough, phlegm and wheeze (12,82).
These symptoms often remit after cessation of cannabis use, whereas
tobacco-related bronchitic changes typically persist (82). Imaging studies have found cannabis
smoking to be associated with airway-centered emphysema, though
with lower frequency than tobacco-associated emphysema (83). Tobacco smoking remains the dominant
risk factor for emphysema and COPD, but the combined use of
cannabis and tobacco appears to exacerbate respiratory symptoms and
structural damage (9,83).
Cancer risk comparisons also reveal divergent
patterns. While tobacco smoking is an established carcinogen with a
clear dose-response relationship to lung cancer, the evidence for
cannabis is more mixed. A large case-control study in Los Angeles
reported no significant association between cannabis use and lung
or upper aerodigestive tract cancers after adjustment for tobacco
(8). By contrast, a New Zealand
case-control study found an increased risk of lung cancer
associated with heavy cannabis use, with an exposure-response trend
(84). More recently, MR analyses
have provided genetic evidence for a possible causal relationship
between cannabis use and lung cancer, particularly squamous cell
carcinoma (65,66). These findings suggest that, while
tobacco remains the far stronger driver of malignancy, heavy
cannabis exposure may not be benign with respect to cancer
risk.
8. Cannabis and lung cancer risk
The potential association between cannabis
inhalation and lung cancer risk has become increasingly relevant in
the context of evolving legalization and rising global usage.
Although cannabis smoke shares carcinogens with tobacco smoke,
epidemiological findings regarding lung cancer risk remain
inconclusive and complex. Confounding factors such as tobacco
co-use and challenges in accurately quantifying lifetime cannabis
exposure, often due to its legal status, further complicate the
investigation.
A large retrospective cohort study within the
Kaiser Permanente health system in California, involving 64,855
individuals, reported no significant association between overall
cannabis use and cancer incidence, including lung cancer, after
adjusting for sociodemographic factors and tobacco use (85). However, the study noted
site-specific associations, particularly an elevated risk of
prostate cancer among non-tobacco smokers who used cannabis and a
near-significant increase in cervical cancer risk. These findings
suggest that while overall cancer risk may not be markedly
elevated, cannabis use could predispose to specific cancer types
(85).
Similarly, a population-based case-control study in
Los Angeles, encompassing 1,212 incident cancer cases and 1,040
controls, initially indicated a positive association between heavy
cannabis use (>30 joint-years) and various cancer types,
including lung cancer. However, after adjusting for confounders
such as cigarette smoking, these associations were no longer
significant, with an adjusted odds ratio (OR) for lung cancer of
0.62 [95% confidence interval, (CI): 0.32-1.2] among individuals
with ≥60 joint-years of cannabis use, indicating no significant
association (8).
Conversely, a case-control study conducted in New
Zealand demonstrated a dose-response relationship between cannabis
use and lung cancer risk, reporting an 8% increase in lung cancer
risk per joint-year of cannabis smoking (95% CI: 2-15%) after
adjusting for tobacco smoking. Notably, individuals in the highest
tertile of cannabis use exhibited a significantly elevated risk
[relative risk (RR)=5.7; 95% CI: 1.5-21.6] (84). Another hospital-based case-control
study in Tunisia, involving 149 incident lung cancer cases and 188
controls, found a significant association between past cannabis use
and lung cancer risk, with an OR of 4.1 (95% CI: 1.9-9.0) after
accounting for age, tobacco use and occupational exposures
(66). However, the study did not
identify a clear dose-response relationship regarding the intensity
or duration of cannabis use (87).
Further supporting this association, a pooled
analysis of three hospital-based case-control studies conducted in
Tunisia, Morocco and Algeria, involving 430 lung cancer cases and
778 controls, demonstrated an adjusted OR of 2.4 (95% CI: 1.6-3.8)
for cannabis smoking after adjusting for country, age, tobacco
smoking and occupational exposure (87). Although the study observed an
increasing risk with joint-years of use, it did not identify a
clear dose or duration relationship (65). Importantly, all cannabis smokers in
this cohort were also tobacco users, raising concerns about
residual confounding by tobacco or other factors (87).
In a longitudinal cohort study conducted over 40
years, involving 49,321 young men in Sweden, heavy cannabis use
(defined as more than 50 lifetime uses) was associated with a more
than two-fold increase in lung cancer risk [hazard ratio (HR)=2.12;
95% CI: 1.08-4.14] after controlling for confounders such as
tobacco use, alcohol consumption, respiratory conditions and
socioeconomic status (88). The
large sample size, extended follow-up period, and robust adjustment
for confounders strengthen the validity of these findings.
MR studies have also provided insights into the
potential causal relationship between cannabis use and lung cancer.
One MR study assessing the relationship between genetic liability
to cannabis use and lung cancer susceptibility reported a
significant association with squamous cell carcinoma (OR=1.22; 95%
CI: 1.07-1.39; P=0.003) (65).
Another MR analysis found that CUDs were linked to an increased
risk of both breast cancer (OR=1.007; P=0.007) and lung cancer
(OR=1.122; P=0.014) (66). MR
studies have the advantage of reducing confounding and reverse
causation biases inherent in observational designs, though they
rely on the validity of genetic instruments.
The convergence of evidence from these studies
suggests a potential link between heavy cannabis use and increased
lung cancer risk, particularly when usage exceeds critical
thresholds or is combined with tobacco smoking. The potential
dose-response relationship observed in several studies underscores
the importance of moderating heavy and chronic cannabis use to
mitigate cancer risk.
However, methodological challenges persist,
including confounding by tobacco use, variability in cannabis
potency and consumption patterns, and limitations in accurately
measuring exposure. Future research should prioritize prospective
cohort studies with comprehensive exposure metrics and meticulous
control for confounding variables, including occupational and
environmental factors. Additionally, mechanistic studies exploring
the biological underpinnings of cannabis-induced carcinogenesis are
warranted to elucidate the pathways through which cannabis
inhalation may contribute to cancer development.
A major limitation across the literature is
residual confounding from tobacco, given the high prevalence of
dual use and the collinearity of exposure metrics (pack-years,
depth of inhalation, and mixing practices such as ‘blunts’ and
tobacco-mixed joints). Even with statistical adjustment,
under-reporting of tobacco, imprecise pack-year quantification, and
differing smoking topography can bias risk estimates toward the
null for cannabis or inflate apparent cannabis risks (62). Evidence for interaction is most
apparent in structural and functional lung outcomes: In a
comprehensive HRCT/physiology study from New Zealand, emphysema was
uncommon among cannabis-only users but more frequent in
tobacco-only smokers and highest in dual users, consistent with at
least additive-if not synergistic-effects on airway and parenchymal
injury (9). Symptom-based studies
likewise show greater bronchitic burden when cannabis and tobacco
are combined (12).
Whether any studies adequately isolate
cannabis-specific risks depend on outcome and design. Several
pulmonary studies enrolled cannabis-only groups and demonstrate
large-airway dysfunction and symptomatology independent of tobacco
(9,11), while population cohorts show
non-linear lung-function patterns with cannabis exposure after
tobacco adjustment (13) and
increased odds of airflow obstruction at very high lifetime use
(64). For lung cancer, findings
remain mixed: Large case-control work from Los Angeles reported no
association after adjusting for tobacco (8), whereas a New Zealand study suggested
an exposure-response increase in risk per joint-year despite
adjustment (84). Studies from
North Africa are difficult to interpret because nearly all cannabis
users also smoked tobacco, making cannabis-specific effects
inseparable (87). A long-term
Swedish cohort observed higher lung-cancer risk in very heavy
cannabis users after controlling for tobacco and other confounders,
though residual bias cannot be excluded (88). Notably, MR analyses-less sensitive
to confounding-support a possible causal signal for lung cancer
(including squamous histology) with genetic liability to cannabis
use/use disorder, but these approaches have their own assumptions
and do not capture route, dose, or combustion exposures (65,66).
Methodologically, future studies should i) recruit
never-tobacco smokers to derive cannabis-only estimates; ii) verify
tobacco exposure with biomarkers (for example, cotinine) alongside
detailed cannabis metrics (product potency, device, mg-THC-years);
iii) model additive and multiplicative interaction between cannabis
and tobacco with formal measures of synergy; and iv) triangulate
observational findings with modern experimental smoke/aerosol
models that reflect contemporary high-THC products (6,62).
Findings on dose-response relationships between
cannabis use and respiratory outcomes are inconsistent. Some
studies suggest a threshold effect, with minimal impairment at
lower exposures but measurable declines beyond ~20 joint-years. In
the CARDIA cohort, low-to-moderate cannabis use was associated with
transient increases in FVC, while heavier cumulative use predicted
declines in FEV1/FVC (13). Similarly, an analysis of NHANES data
found increased odds of airflow obstruction when exposure exceeded
20 joint-years (64). By contrast,
other studies have not identified a clear threshold, reporting
either preserved or increased FVC despite high cumulative exposure
(89) or mixed associations with
spirometry and imaging markers (9).
A systematic review and meta-analysis also highlighted the
variability across studies, finding consistent links with
bronchitic symptoms but less uniform associations with lung
function decline (62).
These inconsistencies may partly reflect
methodological heterogeneity. The ‘joint-years’ metric fails to
capture differences in potency, device efficiency, or smoking
topography, while secular increases in THC and shifts in the
THC:CBD ratio complicate comparisons with earlier cohorts (57). Residual confounding from tobacco
use, differences in modeling exposure categories vs. continuous
measures, and survivorship bias in heavy users further cloud
interpretation (6,9). Nonetheless, true biological
non-linearity is also plausible: Acute bronchodilation and
hyperinflation effects at low exposure may transiently mask airway
obstruction, with chronic inflammation and remodeling emerging at
higher doses (10). For lung
cancer, some case-control studies found no dose-response gradient
after tobacco adjustment (8),
whereas others reported elevated risks with heavy cannabis use
(84), and MR studies suggest a
potential causal link, particularly with squamous histology
(65,66).
Beyond epidemiological associations, several
mechanistic studies provide biological plausibility for a link
between cannabis use and lung cancer. Cannabis smoke contains
numerous of the same carcinogens and mutagens as tobacco smoke,
including PAHs and nitrosamines, often at equal or higher
concentrations due to the combustion process and inhalation
technique (6). Regular cannabis
users typically inhale more deeply and hold smoke longer in the
lungs, which may increase exposure of airway epithelium to
carcinogens (90).
At the cellular level, cannabis smoke has been
shown to induce DNA damage, chromosomal aberrations, and impaired
DNA repair in human lung epithelial cells (91). In vivo studies demonstrate
that marijuana smoke exposure disrupts mitochondrial function and
promotes oxidative stress, which can accelerate carcinogenic
pathways (91). Histopathological
investigations have revealed squamous metaplasia, atypia and
dysplasia in bronchial biopsies from habitual cannabis smokers,
mirroring precancerous changes observed in tobacco smokers
(77).
Interestingly, cannabinoids themselves may have
dual roles. THC and CBD have demonstrated anti-proliferative and
pro-apoptotic effects against certain tumor cell lines, suggesting
potential anti-cancer properties (56). However, chronic exposure through
smoking may overwhelm these effects due to the high burden of
combustion-derived carcinogens. Thus, the balance between
cannabinoid-mediated tumor suppression and smoke-induced
carcinogenesis remains unresolved.
These mechanistic observations underscore that
cannabis smoking is not biologically inert and can contribute to
molecular changes associated with lung carcinogenesis.
9. Clinical implications
Clinical implications of cannabis use on lung
health and cancer risk are multifaceted and warrant careful
consideration, particularly in light of its increasing prevalence
due to legalization and social acceptance. Chronic cannabis smoking
has been associated with respiratory symptoms similar to those
observed in chronic bronchitis, including cough, sputum production
and wheezing. Notably, these symptoms appear to be reversible upon
cessation of cannabis use, suggesting that the primary mechanism
may be related to airway irritation and inflammation rather than
irreversible lung damage (92).
Pulmonary function may not be significantly
impaired with moderate cannabis use, but heavy and prolonged
consumption has been linked to airflow obstruction and altered lung
volumes. Unlike tobacco smoking, cannabis appears to primarily
affect large airway caliber without consistently inducing
small-airway remodeling or emphysema. However, the concurrent use
of tobacco and cannabis has synergistic effects, amplifying the
risk of chronic bronchitis, COPD and airflow obstruction beyond
what would be expected from tobacco use alone (9,89).
In terms of oncological concerns, the risk of lung
cancer associated with cannabis smoking remains inconclusive.
Despite the presence of carcinogens similar to those found in
tobacco smoke, epidemiological studies have not consistently
demonstrated a strong association between cannabis use and lung
cancer. Emerging evidence suggests that heavy, long-term cannabis
use may increase lung cancer risk, especially when combined with
tobacco smoking. Potential carcinogenic mechanisms involve exposure
to harmful compounds such as PAHs and VOCs, which can induce DNA
damage, oxidative stress and chronic inflammation-key processes
involved in carcinogenesis. Furthermore, cannabinoids like THC and
CBD have shown both tumor-promoting and antineoplastic properties
in experimental models, yet the chronic inhalation of smoke may
counteract any potential protective effects by fostering a
mutagenic environment (9,88).
Immunological and infectious risks are also
noteworthy, as chronic cannabis smoking has been shown to impair
the antimicrobial functions of AMs, reducing phagocytic activity
and NO production. This suppression may increase susceptibility to
respiratory infections and opportunistic pathogens, with altered
cytokine profiles and impaired antimicrobial activity weakening
pulmonary immune defense. These effects may be particularly
concerning among heavy users or those who concurrently smoke
tobacco (72,93).
From a public health perspective, it is crucial to
educate patients about the potential respiratory risks associated
with chronic cannabis use, especially those with pre-existing
respiratory conditions or who practice dual substance use.
Implementing harm reduction strategies, such as recommending
alternatives to smoking such as vaporization or oral formulations,
could mitigate the inhalation of harmful byproducts. Additionally,
regular monitoring for respiratory symptoms and lung function
assessment should be considered for heavy cannabis users,
particularly those with a history of tobacco use.
Beyond smoking, alternative routes of cannabis
administration such as vaping and oral ingestion present distinct
implications for lung health. Smoking remains the most strongly
associated with respiratory symptoms including cough, sputum
production, wheeze and bronchitic changes, as well as structural
airway alterations and airflow obstruction (9,11).
Vaping reduces exposure to combustion-related toxins such as tar
and PAHs, which may lower certain toxicological risks (6,62).
However, emerging data highlight important safety concerns. Vaping
has been associated with airway irritation, bronchitic symptoms and
impaired pulmonary function in young adults (94), and the outbreak of e-cigarette or
EVALI underscored the risk of acute, severe pulmonary damage,
largely linked to additives such as vitamin E acetate in illicit
cartridges. By contrast, oral formulations-including edibles and
oils-bypass the respiratory tract and therefore avoid combustion-
and inhalation-related injury. Nevertheless, oral use is associated
with delayed onset, variable absorption, and risks of
overconsumption due to unpredictable pharmacokinetics (25). Taken together, smoking carries the
greatest burden of chronic bronchitic and structural respiratory
effects, vaping may reduce some combustion-related harms but
introduces risks of acute lung injury and uncertain long-term
consequences, while oral routes minimize pulmonary risks but raise
challenges related to dosing and psychoactive effects.
Further research is essential to address the
existing gaps, particularly through robust longitudinal cohort
studies that assess the dose-response relationship between cannabis
use and lung cancer risk. Modern high-potency cannabis products and
changing patterns of consumption require contemporary
investigations to improve understanding of the long-term
consequences. Moreover, mechanistic studies exploring the molecular
pathways through which cannabis smoke induces lung damage and
carcinogenesis are needed to provide more comprehensive insights.
Finally, public health policies should prioritize strategies to
reduce dual substance use, given the compounded respiratory risks
associated with the concurrent consumption of cannabis and
tobacco.
10. Strengths and limitations
Several reviews have previously summarized the
respiratory and oncological consequences of cannabis use,
underscoring its association with bronchitic symptoms, impaired
lung function and potential links to carcinogenesis (95-105).
While these reviews have contributed significantly to understanding
cannabis-related pulmonary risks, most either focused primarily on
epidemiological associations or on narrower clinical outcomes,
without integrating the increasing body of mechanistic,
immunological and histopathological evidence. Furthermore, the
majority of earlier reviews were based on data from periods when
cannabis potency was considerably lower than in contemporary
markets, with fewer analyses accounting for the impact of the
rising THC to CBD ratio.
By contrast, the present review seeks to provide an
updated and integrative perspective by combining epidemiological
evidence with mechanistic insights into airway inflammation,
alveolar macrophage dysfunction, and carcinogen-mediated DNA
damage. Particular emphasis was also placed on dual
cannabis-tobacco use and its synergistic effects on chronic
bronchitis, airflow obstruction and cancer risk, a dimension that
remains underexplored in prior syntheses. Importantly, our review
incorporates the most recent literature, including large-scale
longitudinal studies, systematic reviews, and MR analyses recently
published, thereby offering a timely reassessment of the pulmonary
and oncological consequences of cannabis in the context of
increasing legalization, higher product potency, and evolving
consumption patterns. This comprehensive approach distinguishes the
current review from earlier publications and provides clinicians
and policymakers with a more contemporary framework for evaluating
the health impacts of cannabis use.
11. Conclusions
While cannabis smoking is associated with
respiratory symptoms resembling chronic bronchitis and impaired
alveolar macrophage function, the link between cannabis use and
lung cancer remains inconclusive, with conflicting epidemiological
evidence. Moderate cannabis use does not appear to significantly
impair pulmonary function, but heavy and prolonged consumption may
lead to airflow obstruction and increased lung volume without
causing emphysema as observed with tobacco smoking. Additionally,
the combination of cannabis and tobacco use poses synergistic
risks, amplifying respiratory and possibly oncogenic outcomes.
While non-combustible methods such as vaping have been proposed as
harm reduction strategies, they are not without risks. Compared
with smoking, vaping cannabis eliminates some combustion-related
toxins, such as PAHs and tar and may therefore reduce direct
exposure to certain carcinogens. However, vaping introduces its own
safety concerns, particularly with the emergence of e-cigarette or
EVALI, largely linked to vitamin E acetate and other additives in
illicit cartridges. Studies have described acute lung injury,
hypoxemia, and even fatalities associated with vaping-related
toxicity. Moreover, the high bioavailability of THC through vaping
can encourage more intense use, potentially exacerbating dependence
and neurocognitive risks. Thus, although vaping may mitigate some
harms associated with smoke inhalation, it does not represent a
risk-free alternative. A critical comparison indicates that while
smoking carries stronger associations with chronic bronchitis,
airflow obstruction and potential oncogenesis, vaping raises
concerns of acute pulmonary toxicity and long-term safety
uncertainties. Therefore, both methods warrant careful public
health evaluation rather than unqualified endorsement as safer
consumption routes.
As cannabis legalization expands and consumption
patterns evolve, further longitudinal studies are essential to
clarify the long-term health impacts and guide public health
strategies aimed at harm reduction and safer use practices.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DAA and VEG conceptualized the study. VEG, DAA, WZ
and DAS made a substantial contribution to data interpretation and
analysis and wrote and prepared the draft of the manuscript. WZ and
VEG analysed the data and provided critical revisions. All authors
contributed to manuscript revision, read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
References
|
1
|
World Health Organization (WHO): Global
cancer burden growing, amidst mounting need for services.
https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services#:~:text=Lung%20cancer%20was%20the%20leading,660%20000%20deaths%2C%206.8%25.
Accessed March 22, 2025.
|
|
2
|
Georgakopoulou VE, Lempesis IG, Trakas N,
Sklapani P, He Y and Spandidos DA: Lung cancer and obesity: A
contentious relationship (Review). Oncol Rep.
52(158)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Warren GW and Cummings KM: Tobacco and
lung cancer: Risks, trends, and outcomes in patients with cancer.
Am Soc Clin Oncol Educ Book. 33:359–364. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Trakas N, Georgakopoulou VE, Melemeni D,
Damaskos C, Mantzouranis K, Garmpis N, Gkoufa A, Papalexis P,
Chlapoutakis S, Sklapani P, et al: Association between smoking
cessation and alterations in forced expiratory volume in one second
(FEV1). A Follow-up study from a Greek Tobacco Cessation Clinic.
Addict Health. 14:87–95. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Volkow ND, Baler RD, Compton WM and Weiss
SR: Adverse health effects of marijuana use. N Engl J Med.
370:2219–2227. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moir D, Rickert WS, Levasseur G, Larose Y,
Maertens R, White P and Desjardins S: A comparison of mainstream
and sidestream marijuana and tobacco cigarette smoke produced under
two machine smoking conditions. Chem Res Toxicol. 21:494–502.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Singh R, Sandhu J, Kaur B, Juren T,
Steward WP, Segerbäck D and Farmer PB: Evaluation of the DNA
damaging potential of cannabis cigarette smoke by the determination
of acetaldehyde derived N2-ethyl-2'-deoxyguanosine adducts. Chem
Res Toxicol. 22:1181–1188. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hashibe M, Morgenstern H, Cui Y, Tashkin
DP, Zhang ZF, Cozen W, Mack TM and Greenland S: Marijuana use and
the risk of lung and upper aerodigestive tract cancers: Results of
a population-based case-control study. Cancer Epidemiol Biomarkers
Prev. 15:1829–1834. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aldington S, Williams M, Nowitz M,
Weatherall M, Pritchard A, McNaughton A, Robinson G and Beasley R:
Effects of cannabis on pulmonary structure, function and symptoms.
Thorax. 62:1058–1063. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grassin-Delyle S, Naline E, Buenestado A,
Faisy C, Alvarez JC, Salvator H, Abrial C, Advenier C, Zemoura L
and Devillier P: Cannabinoids inhibit cholinergic contraction in
human airways through prejunctional CB1 receptors. Br J Pharmacol.
171:2767–2777. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tashkin DP, Coulson AH, Clark VA, Simmons
M, Bourque LB, Duann S, Spivey GH and Gong H: Respiratory symptoms
and lung function in habitual heavy smokers of marijuana alone,
smokers of marijuana and tobacco, smokers of tobacco alone, and
nonsmokers. Am Rev Respir Dis. 135:209–216. 1987.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moore BA, Augustson EM, Moser RP and
Budney AJ: Respiratory effects of marijuana and tobacco use in a
U.S. sample. J Gen Intern Med. 20:33–37. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pletcher MJ, Vittinghoff E, Kalhan R,
Richman J, Safford M, Sidney S, Lin F and Kertesz S: Association
between marijuana exposure and pulmonary function over 20 years.
JAMA. 307:173–181. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bifulco M, Laezza C, Pisanti S and
Gazzerro P: Cannabinoids and cancer: Pros and cons of an antitumour
strategy. Br J Pharmacol. 148:123–135. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ladin DA, Soliman E, Griffin L and Van
Dross R: Preclinical and clinical assessment of cannabinoids as
Anti-cancer agents. Front Pharmacol. 7(361)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pacher P, Kogan NM and Mechoulam R: Beyond
THC and endocannabinoids. Annu Rev Pharmacol Toxicol. 60:637–659.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Andre CM, Hausman JF and Guerriero G:
Cannabis sativa: The plant of the thousand and one molecules. Front
Plant Sci. 7(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hill KP: Medical marijuana for treatment
of chronic pain and other medical and psychiatric problems: A
clinical review. JAMA. 313:2474–2483. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pertwee RG: The diverse CB1 and CB2
receptor pharmacology of three plant cannabinoids:
Delta9-tetrahydrocannabinol, cannabidiol and
delta9-tetrahydrocannabivarin. Br J Pharmacol. 153:199–215.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Carliner H, Brown QL, Sarvet AL and Hasin
DS: Cannabis use, attitudes, and legal status in the U.S.: A
review. Prev Med. 104:13–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dilley JA, Richardson SM, Kilmer B, Pacula
RL, Segawa MB and Cerdá M: Prevalence of cannabis use in youths
after legalization in washington state. JAMA Pediatr. 173:192–193.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hill KP, Gold MS, Nemeroff CB, McDonald W,
Grzenda A, Widge AS, Rodriguez C, Kraguljac NV, Krystal JH and
Carpenter LL: Risks and benefits of cannabis and cannabinoids in
psychiatry. Am J Psychiatry. 179:98–109. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hoch E, Volkow ND, Friemel CM, Lorenzetti
V, Freeman TP and Hall W: Cannabis, cannabinoids and health: A
review of evidence on risks and medical benefits. Eur Arch
Psychiatry Clin Neurosci. 275:281–292. 2025.PubMed/NCBI View Article : Google Scholar
|
|
24
|
National Academies of Sciences,
Engineering and Medicine; Health and Medicine Division; Board on
Population Health and Public Health Practice; Committee on the
Health Effects of Marijuana: An Evidence Review and Research
Agenda. The Health Effects of Cannabis and Cannabinoids: The
Current State of Evidence and Recommendations for Research.
Washington (DC), National Academies Press (US), Jan 12. 4, 2017.
Therapeutic Effects of Cannabis and Cannabinoids. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK425767/.
|
|
25
|
Whiting PF, Wolff RF, Deshpande S, Di
Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K,
Ryder S, et al: Cannabinoids for medical use: A systematic review
and Meta-analysis. JAMA. 313:2456–2473. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Machado Rocha FC, Stéfano SC, De Cássia
Haiek R, Rosa Oliveira LM and Da Silveira DX: Therapeutic use of
Cannabis sativa on chemotherapy-induced nausea and vomiting among
cancer patients: Systematic review and Meta-analysis. Eur J Cancer
Care (Engl). 17:431–443. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Badowski ME: A review of oral cannabinoids
and medical marijuana for the treatment of chemotherapy-induced
nausea and vomiting: A focus on pharmacokinetic variability and
pharmacodynamics. Cancer Chemother Pharmacol. 80:441–449.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Koppel BS, Brust JC, Fife T, Bronstein J,
Youssof S, Gronseth G and Gloss D: Systematic review: Efficacy and
safety of medical marijuana in selected neurologic disorders
[RETIRED]: Report of the Guideline development subcommittee of the
american academy of neurology. Neurology. 82:1556–1563.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Walsh Z, Gonzalez R, Crosby K, S Thiessen
M, Carroll C and Bonn-Miller MO: Medical cannabis and mental
health: A guided systematic review. Clin Psychol Rev. 51:15–29.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bonn-Miller MO, Boden MT, Bucossi MM and
Babson KA: Self-reported cannabis use characteristics, patterns and
helpfulness among medical cannabis users. Am J Drug Alcohol Abuse.
40:23–30. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Di Forti M, Marconi A, Carra E, Fraietta
S, Trotta A, Bonomo M, Bianconi F, Gardner-Sood P, O'Connor J,
Russo M, et al: Proportion of patients in south London with
first-episode psychosis attributable to use of high potency
cannabis: A case-control study. Lancet Psychiatry. 2:233–238.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tashkin DP: Effects of marijuana smoking
on the lung. Ann Am Thorac Soc. 10:239–247. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Thomas G, Kloner RA and Rezkalla S:
Adverse cardiovascular, cerebrovascular, and peripheral vascular
effects of marijuana inhalation: What cardiologists need to know.
Am J Cardiol. 113:187–190. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jouanjus E, Lapeyre-Mestre M and Micallef
J: French Association of the Regional Abuse and Dependence
Monitoring Centres (CEIP-A) Working Group on Cannabis
Complications*. Cannabis use: Signal of increasing risk of serious
cardiovascular disorders. J Am Heart Assoc.
3(e000638)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gunn JK, Rosales CB, Center KE, Nuñez A,
Gibson SJ, Christ C and Ehiri JE: Prenatal exposure to cannabis and
maternal and child health outcomes: A systematic review and
Meta-analysis. BMJ Open. 6(e009986)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Calapai F, Cardia L, Esposito E,
Ammendolia I, Mondello C, Lo Giudice R, Gangemi S, Calapai G and
Mannucci C: Pharmacological aspects and biological effects of
cannabigerol and its synthetic derivatives. Evid Based Complement
Alternat Med. 2022(3336516)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lu HC and Mackie K: An Introduction to the
endogenous cannabinoid system. Biol Psychiatry. 79:516–525.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Roque-Bravo R, Silva RS, Malheiro RF,
Carmo H, Carvalho F, da Silva DD and Silva JP: Synthetic
cannabinoids: A pharmacological and toxicological overview. Annu
Rev Pharmacol Toxicol. 63:187–209. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mills B, Yepes A and Nugent K: Synthetic
cannabinoids. Am J Med Sci. 350:59–62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chayasirisobhon S: Mechanisms of action
and pharmacokinetics of cannabis. Perm J. 25:1–3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gonçalves J, Rosado T, Soares S, Simão AY,
Caramelo D, Luís Â, Fernández N, Barroso M, Gallardo E and Duarte
AP: Cannabis and Its secondary metabolites: Their use as
therapeutic drugs, toxicological aspects, and analytical
determination. Medicines (Basel). 6(31)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zendulka O, Dovrtělová G, Nosková K,
Turjap M, Šulcová A, Hanuš L and Juřica J: Cannabinoids and
cytochrome P450 interactions. Curr Drug Metab. 17:206–226.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Long T, Wagner M, Demske D, Leipe C and
Tarasov PE: Cannabis in eurasia: Origin of human use and Bronze Age
trans-continental connections. Vegetation History and
Archaeobotany. 26:245–258. 2017.
|
|
44
|
Azofeifa A, Mattson ME, Schauer G, McAfee
T, Grant A and Lyerla R: National estimates of marijuana use and
related indicators-National survey on drug use and health, united
states, 2002-2014. MMWR Surveill Summ. 65:1–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Han BH, Sherman S, Mauro PM, Martins SS,
Rotenberg J and Palamar JJ: Demographic trends among older cannabis
users in the United States, 2006-13. Addiction. 112:516–525.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hasin DS, Saha TD, Kerridge BT, Goldstein
RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, et
al: Prevalence of marijuana use disorders in the united states
between 2001-2002 and 2012-2013. JAMA Psychiatry. 72:1235–1242.
2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Teesson M, Slade T, Swift W, Mills K,
Memedovic S, Mewton L, Grove R, Newton N and Hall W: Prevalence,
correlates and comorbidity of DSM-IV cannabis use and cannabis use
disorders in australia. Aust N Z J Psychiatry. 46:1182–1192.
2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hall W and Lynskey M: Assessing the public
health impacts of legalizing recreational cannabis use: The US
experience. World Psychiatr. 19:179–186. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mechoulam R and Parker LA: The
endocannabinoid system and the brain. Annu Rev Psychol. 64:21–47.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Di Marzo V and Piscitelli F: The
endocannabinoid system and its modulation by phytocannabinoids.
Neurotherapeutics. 12:692–698. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Howlett AC, Barth F, Bonner TI, Cabral G,
Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR,
et al: International union of pharmacology. XXVII. Classification
of cannabinoid receptors. Pharmacol Rev. 54:161–202.
2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Munro S, Thomas KL and Abu-Shaar M:
Molecular characterization of a peripheral receptor for
cannabinoids. Nature. 365:61–65. 1993.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Klein TW: Cannabinoid-based drugs as
anti-inflammatory therapeutics. Nat Rev Immunol. 5:400–411.
2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Morales P and Reggio PH: An update on
non-CB1, non-CB2 cannabinoid related G-Protein-coupled receptors.
Cannabis Cannabinoid Res. 2:265–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Garmpis N, Damaskos C, Dimitroulis D,
Garmpi A, Diamantis E, Sarantis P, Georgakopoulou VE, Patsouras A,
Prevezanos D, Syllaios A, et al: Targeting the endocannabinoid
system: From the need for new therapies to the development of a
promising strategy. What About Pancreatic Cancer? In Vivo.
36:543–555. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Velasco G, Sánchez C and Guzmán M:
Anticancer mechanisms of cannabinoids. Curr Oncol. 2
(Suppl):S23–S32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
ElSohly MA, Mehmedic Z, Foster S, Gon C,
Chandra S and Church JC: Changes in cannabis potency over the last
2 decades (1995-2014): Analysis of current data in the united
states. Biological Psychiatry. 79:613–619. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Atakan Z: Cannabis, a complex plant:
Different compounds and different effects on individuals. Ther Adv
Psychopharmacol. 2:241–254. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Le Boisselier R, Alexandre J,
Lelong-Boulouard V and Debruyne D: Focus on cannabinoids and
synthetic cannabinoids. Clin Pharmacol Ther. 101:220–229.
2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wilkie G, Sakr B and Rizack T: Medical
marijuana use in oncology: A review. JAMA Oncol. 2:670–675.
2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hancock-Allen JB, Barker L, VanDyke M and
Holmes DB: Notes from the field: Death following ingestion of an
edible marijuana product-Colorado, March 2014. MMWR Morb Mortal
Wkly Rep. 64:771–772. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ghasemiesfe M, Ravi D, Vali M, Korenstein
D, Arjomandi M, Frank J, Austin PC and Keyhani S: Marijuana use,
respiratory symptoms, and pulmonary function: A systematic review
and Meta-analysis. Ann Intern Med. 169:106–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Biehl JR and Burnham EL: Cannabis smoking
in 2015: A concern for lung health? Chest. 148:596–606.
2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kempker JA, Honig EG and Martin GS: The
effects of marijuana exposure on expiratory airflow. A study of
adults who participated in the U.S. National Health and Nutrition
Examination Study. Ann Am Thorac Soc. 12:135–141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Baumeister SE, Baurecht H, Nolde M,
Alayash Z, Gläser S, Johansson M and Amos CI: International Lung
Cancer Consortium. Johnson EC and Hung RJ: Cannabis use, pulmonary
function, and lung cancer susceptibility: A mendelian randomization
study. J Thorac Oncol. 16:1127–1135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Niu D, Li C, Qu H and Zheng Y: Does
cannabis elevate cancer risk?: Evidence from Mendelian
randomization. Wien Klin Wochenschr. 136:311–318. 2024.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Layden JE, Ghinai I, Pray I, Kimball A,
Layer M, Tenforde MW, Navon L, Hoots B, Salvatore PP, Elderbrook M,
et al: Pulmonary Illness Related to E-cigarette use in illinois and
wisconsin-final report. N Engl J Med. 382:903–916. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Maertens RM, White PA, Williams A and Yauk
CL: A global toxicogenomic analysis investigating the mechanistic
differences between tobacco and marijuana smoke condensates in
vitro. Toxicology. 308:60–73. 2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Barbers RG, Gong H Jr, Tashkin DP, Oishi J
and Wallace JM: Differential examination of bronchoalveolar lavage
cells in tobacco cigarette and marijuana smokers. Am Rev Respir
Dis. 135:1271–1275. 1987.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Roth MD and Baldwin GC: Mechanisms for
impaired effector function in alveolar macrophages from marijuana
and cocaine smokers. J Neuroimmunol. 147:82–86. 2004.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sherman MP, Campbell LA, Gong H Jr, Roth
MD and Tashkin DP: Antimicrobial and respiratory burst
characteristics of pulmonary alveolar macrophages recovered from
smokers of marijuana alone, smokers of tobacco alone, smokers of
marijuana and tobacco and nonsmokers. Am Rev Respir Dis.
144:1351–1356. 1991.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Baldwin GC, Tashkin DP, Buckley DM, Park
AN, Dubinett SM and Roth MD: Marijuana and cocaine impair alveolar
macrophage function and cytokine production. Am J Respir Crit Care
Med. 156:1606–1613. 1997.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Roth MD, Baldwin GC and Tashkin DP:
Effects of delta-9-tetrahydrocannabinol on human immune function
and host defense. Chem Phys Lipids. 121:229–239. 2002.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Shay AH, Choi R, Whittaker K, Salehi K,
Kitchen CM, Tashkin DP, Roth MD and Baldwin GC: Impairment of
antimicrobial activity and nitric oxide production in alveolar
macrophages from smokers of marijuana and cocaine. J Infect Dis.
187:700–704. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
75
|
Yang X, Hegde VL, Rao R, Zhang J,
Nagarkatti PS and Nagarkatti M: Histone modifications are
associated with Δ9-tetrahydrocannabinol-mediated alterations in
antigen-specific T cell responses. J Biol Chem. 289:18707–18718.
2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Fligiel SE, Roth MD, Kleerup EC, Barsky
SH, Simmons MS and Tashkin DP: Tracheobronchial histopathology in
habitual smokers of cocaine, marijuana, and/or tobacco. Chest.
112:319–326. 1997.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Barsky SH, Roth MD, Kleerup EC, Simmons M
and Tashkin DP: Histopathologic and molecular alterations in
bronchial epithelium in habitual smokers of marijuana, cocaine
and/or tobacco. J Natl Cancer Inst. 90:1198–1200. 1998.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Gracie K and Hancox RJ: Cannabis use
disorder and the lungs. Addiction. 116:182–190. 2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Hancox RJ, Shin HH, Gray AR, Poulton R and
Sears MR: Effects of quitting cannabis on respiratory symptoms. Eur
Respir J. 46:80–87. 2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tan C, Hatam N and Treasure T: Bullous
disease of the lung and cannabis smoking: Insufficient evidence for
a causative link. J R Soc Med. 99:77–80. 2006.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Bloom JW, Kaltenborn WT, Paoletti P,
Camilli A and Lebowitz MD: Respiratory effects of non-tobacco
cigarettes. Br Med J (Clin Res Ed). 295:1516–1518. 1987.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Tashkin DP, Simmons MS and Tseng CH:
Impact of changes in regular use of marijuana and/or tobacco on
chronic bronchitis. COPD. 9:367–374. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Murtha L, Sathiadoss P, Salameh JP,
Mcinnes MDF and Revah G: Chest CT Findings in Marijuana Smokers.
Radiology. 307(e212611)2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Aldington S, Harwood M, Cox B, Weatherall
M, Beckert L, Hansell A, Pritchard A, Robinson G and Beasley R:
Cannabis and respiratory disease research group. Cannabis use and
risk of lung cancer: A case-control study. Eur Respir J.
31:280–286. 2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sidney S, Quesenberry CP, Friedman GD and
Tekawa IS: Marijuana use and cancer incidence (California, United
States). Cancer Causes Control. 8:722–728. 1997.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Voirin N, Berthiller J, Benhaïm-Luzon V,
Boniol M, Straif K, Ayoub WB, Ayed FB and Sasco AJ: Risk of lung
cancer and past use of cannabis in Tunisia. J Thorac Oncol.
1:577–579. 2006.PubMed/NCBI
|
|
87
|
Berthiller J, Straif K, Boniol M, Voirin
N, Benhaïm-Luzon V, Ayoub WB, Dari I, Laouamri S, Hamdi-Cherif M,
Bartal M, et al: Cannabis smoking and risk of lung cancer in men: A
pooled analysis of three studies in Maghreb. J Thorac Oncol.
3:1398–1403. 2008.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Callaghan RC, Allebeck P and Sidorchuk A:
Marijuana use and risk of lung cancer: A 40-year cohort study.
Cancer Causes Control. 24:1811–1820. 2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Hancox RJ, Poulton R, Ely M, Welch D,
Taylor DR, McLachlan CR, Greene JM, Moffitt TE, Caspi A and Sears
MR: Effects of cannabis on lung function: A population-based cohort
study. Eur Respir J. 35:42–47. 2010.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Wu TC, Tashkin DP, Djahed B and Rose JE:
Pulmonary hazards of smoking marijuana as compared with tobacco. N
Engl J Med. 318:347–351. 1988.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Sarafian TA, Habib N, Oldham M, Seeram N,
Lee RP, Lin L, Tashkin DP and Roth MD: Inhaled marijuana smoke
disrupts mitochondrial energetics in pulmonary epithelial cells in
vivo. Am J Physiol Lung Cell Mol Physiol. 290:L1202–L129.
2006.PubMed/NCBI View Article : Google Scholar
|
|
92
|
National Academies of Sciences,
Engineering and Medicine; Health and Medicine Division; Board on
Population Health and Public Health Practice; Committee on the
Health Effects of Marijuana: An Evidence Review and Research
Agenda. The Health Effects of Cannabis and Cannabinoids: The
Current State of Evidence and Recommendations for Research.
Washington (DC), National Academies Press (US), Jan 12. 7, 2017.
Respiratory Disease. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425753/.
|
|
93
|
Hazzard AA, McCrorey M, Salman T, Johnson
DE, Luo Z, Fu X, Keegan AP, Benitez A, Fitting S and Jiang W:
Cannabis use, oral dysbiosis, and neurological disorders.
NeuroImmune Pharm Ther. 3:183–193. 2024.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Braymiller JL, Barrington-Trimis JL,
Leventhal AM, Islam T, Kechter A, Krueger EA, Cho J, Lanza I, Unger
JB and McConnell R: Assessment of nicotine and cannabis vaping and
respiratory symptoms in young adults. JAMA Netw Open.
3(e2030189)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Joshi M, Joshi A and Bartter T: Marijuana
and lung diseases. Curr Opin Pulm Med. 20:173–179. 2014.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Gates P, Jaffe A and Copeland J: Cannabis
smoking and respiratory health: Consideration of the literature.
Respirology. 19:655–662. 2014.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Martinasek MP, McGrogan JB and Maysonet A:
A systematic review of the respiratory effects of inhalational
marijuana. Respir Care. 61:1543–1551. 2016.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Jett J, Stone E, Warren G and Cummings KM:
Cannabis use, lung cancer, and related issues. J Thorac Oncol.
13:480–407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Ghasemiesfe M, Barrow B, Leonard S,
Keyhani S and Korenstein D: Association between marijuana use and
risk of cancer: A systematic review and meta-analysis. JAMA Netw
Open. 2(e1916318)2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Clark TM: Scoping review and meta-analysis
suggests that cannabis use may reduce cancer risk in the United
States. Cannabis Cannabinoid Res. 6:413–34. 2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Nigro E, Formato M, Crescente G and
Daniele A: Cancer initiation, progression and resistance: Are
phytocannabinoids from Cannabis sativa L. promising compounds?
Molecules. 26(2668)2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Kaplan AG: Cannabis and lung health: Does
the bad outweigh the good? Pulm Ther. 7:395–408. 2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Joshi M, Joshi A and Bartter T: Marijuana
and the lung: Evolving understandings. Med Clin North Am.
106:1093–1107. 2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Khoj L, Zagà V, Amram DL, Hosein K,
Pistone G, Bisconti M, Serafini A, Cammarata LM, Cattaruzza MS and
Mura M: Effects of cannabis smoking on the respiratory system: A
state-of-the-art review. Respir Med. 221(107494)2024.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Nargis N, Westmaas JL, Orr E, Alqahtani
MM, Choudhury PP, Islami F and Jemal A: Cancer risk and
legalisation of access to cannabis in the USA: overview of the
evidence. Lancet Public Health. 10:e160–e164. 2025.PubMed/NCBI View Article : Google Scholar
|