Introduction
Anaemia is defined as a low blood haemoglobin (Hb)
concentration and is a public health problem (1). It can occur in all stages of life,
particularly during pregnancy and childhood (2). Iron deficiency anaemia (IDA) is the
predominant cause of anaemia during pregnancy and is one of the
primary consequences of iron deficiency (ID) (3).
It is hypothesised that the occurrence of IDA in
pregnancy is >40%. IDA occurs due to an increased need for the
development of the fetoplacental unit, an increase in the mass of
erythrocytes (E), and an increase in the volume of plasma to
compensate for iron loss during childbirth. In developed countries,
the iron intake is often below nutritional needs (4).
Undiagnosed and untreated IDA can have a significant
impact on the health of both the mother and the child. In mothers,
IDA is associated with a generally poor condition, decreased
working capacity, fatigue, pallor, breathlessness, palpitations and
headaches. The risk of postpartum depression is significantly
increased compared with pregnant women without IDA. Fatigue and
depression often affect the relationship between mother and child.
During pregnancy, IDA is also associated with problems with the
placenta, intrauterine death, infections, retardation of
intrauterine growth, low birth weight, preterm delivery and
perinatal mortality. In infants, IDA is associated with poor
postnatal growth, reduced cognitive ability, and early ID (5). Iron is also necessary for the activity
of enzymes related to the development of the central nervous system
(4).
The World Health Organization (WHO) defines IDA as
anaemia accompanied by depleted iron stores and a compromised
supply of iron to the tissues (1).
IDA develops when the supply of iron is insufficient to sustain
erythropoiesis, leading to a decreased concentration of Hb
(6).
To prevent the development of IDA, the WHO has
developed a system for classifying IDA in pregnant women (1).
According to the WHO, IDA in pregnancy is present if
Hb is <110 g/l, serum ferritin <15 µg/l, and haematocrit
(HCT) <0.33 l/l (7). To
determine the presence of anaemia in pregnant women, the WHO
recommends the determination of Hb and ferritin concentrations
(8).
Laboratory determination plays a crucial role in
diagnosing IDA. For the differential diagnosis of IDA, it is
necessary to determine the complete blood count (CBC), which
includes Hb concentration and HCT, as diagnostic criteria for IDA.
In addition, ferritin concentration in the serum sample should be
determined. Ferritin is considered the gold standard for diagnosing
IDA, but it has its limitations. As an acute-phase reactant,
ferritin levels can be influenced by inflammation; therefore, it is
essential to determine C-reactive protein (CRP) levels for an
accurate diagnosis. It is recommended to determine ferritin levels
once CRP levels have returned to normal. Conversely, serum iron
(Fe) exhibits significant daily variation; therefore, determining
transferrin levels is also recommended (4). Hb concentration is not a reliable
parameter during pregnancy due to physiological changes in plasma
volume and E mass in pregnant women. Therefore, Hb is not a
sensitive or specific indicator of IDA (3). Regarding erythrocyte constants, the
mean corpuscular volume (MCV) initially rises slightly and then
falls, which can lead to drawing an incorrect conclusion. Mean
corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin
concentration (MCHC) are reduced only when anaemia is severe. Red
blood cell distribution width (RDW) represents a quantitative
measure of the variation in the size of E. This is a routine
parameter and part of a CBC, easily measurable, independent
indicator, and increases at least 4 weeks before MCV. RDW is an
early indicator of the change in E, which is essential for
diagnosing IDA (9). Hb and HCT
effectively provide the same data. They are roughly described by
the following formula: Hb x 3/1,000=HCT. Hb is measured directly
using spectrophotometry, while HCT is calculated as the product of
the E count and MCV (10,11).
During a normal pregnancy, Fe concentration, the
percentage of Fe saturation, and total iron binding capacity (TIBC)
have less diagnostic importance, as Fe and the percentage of Fe
saturation decrease as TIBC increases (9). It is also important to emphasise the
pre-analytical requirements for Fe determination. A pregnant woman
should not drink vitamin drinks up to 48 h before drawing blood.
Since Fe shows significant daily variation (up to 70%), blood
should be drawn before 10 AM. If a pregnant woman is taking Fe
preparations, the determination should be postponed for at least 10
days after the last oral Fe dose, 3 days after intravenous Fe
preparations, and 1 month after intramuscular Fe preparations. It
is also important not to carry out Fe determination during an acute
infection, as this can cause Fe to be trapped within the cells of
the reticuloendothelial system. Pregnant women often undergo Fe
therapy before pregnancy or begin taking Fe supplements early in
pregnancy, and this information should be considered when
interpreting laboratory results (12).
It should also be noted that reference intervals
differ between pregnant and non-pregnant women of the same age,
primarily due to the previously mentioned increase in plasma volume
during pregnancy (4).
It is essential to diagnose IDA as early as possible
to prevent complications for both the mother and the child.
Therefore, it is also important to use diagnostic parameters that
are simple, safe and cost-effective (13).
Inspired by the notion of calculated parameters with
strong diagnostic accuracy, in the present study, new parameters,
as ratios of routinely available parameters (E, Fe, HCT, MCV, and
RDW), are proposed for their diagnostic value.
The aim of this study was to evaluate the diagnostic
accuracy of various parameters and define the best possible
parameter for diagnosing IDA. Simple and widely applicable
calculated ratios were assessed, including Fe/E, MCV/RDW, RDW/E,
RDW/Fe and RDW/HCT, and a scoring model was proposed. In view of
the importance of early diagnosis and the limitations of current
diagnostic parameters, the scoring model may represent a meaningful
and easy-to-use diagnostic tool, allowing for diagnosis of IDA
earlier, while making it more accessible and cost-effective,
thereby facilitating timely therapeutic intervention (14).
Materials and methods
Study plan
The present study was performed at the Clinical
Hospital Center Rijeka (Rijeka, Croatia) between August 2019 and
January 2020. A total of 623 pregnant women who had low-risk,
full-term pregnancies, including women who had received Fe
supplements, were enrolled in the study. The median age of the
pregnant women was 32 years (age range, 16-47). Blood samples were
collected either on the day of delivery or the day before when they
were admitted to the hospital.
The study was performed in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
the Clinical Hospital Center Rijeka, Rijeka (approval no.
2170-29-02/1-19-2; class, 003-05/19-1/110; approval date, 15 July
2019; Rijeka, Croatia). Written informed consent was obtained from
all participants in the study.
The inclusion criteria were women who delivered
between 38 and 41 weeks of gestation, those with premature rupture
of membranes lasting no longer than 12 h, women giving birth for
the first time, and those who had multiple births (up to three
births).
The exclusion criteria were twin pregnancies, those
with gestational diabetes requiring medical treatment,
hypertension, preeclampsia, uterine anomalies, bleeding during
pregnancy, women who had had multiple births with ≥4 deliveries,
smokers and women with chronic bowel disease.
Sample collection and analysis
For blood collection, two blood tubes were used; one
tube (K3EDTA tube 3 ml, 13x75 mm; cat. no. 454217;
VACUETTE®, Greiner Bio-One Ltd.) to determine CBC and
the other tube (serum clot activator tubes with gel separator 8 ml,
16x100 mm; cat. no. 455071; VACUETTE®, Greiner Bio-One
Ltd.) for determining Fe concentration. The blood samples were sent
to the Medical Biochemical Laboratory of the Clinical Hospital
Center Rijeka (Rijeka, Croatia), and all test measurements were
performed within 3 h of collection.
The determination of CBC parameters (E, Hb, HCT, MCV
and RDW) were performed using the ADVIA 2120 Hematology Analyzer
(Siemens Healthineers) based on colourimetric, peroxidase-based
optical and laser optical methods, while the serum test (Fe) was
performed on an Olympus AU 640 Automatic Biochemical Analyzer
(Olympus Corporation), based on the spectrophotometric principle
with manufacturer-provided reagents. In addition to internal
quality control procedures, external quality control CROQALM HDMBLM
(croqalm.hdmblm.hr) and LABQUALITY EQAS
(my.labscala.fi) were also performed at the
Clinical Hospital Center Rijeka laboratory.
The clinical criterion for IDA diagnosis in the
present study was defined based on the WHO threshold of Hb <110
g/l, and pregnant women were classified as anaemic and non-anaemic
based on this threshold (6).
Statistical analysis
Statistical analysis was performed using MedCalc
software version 20.104 (MedCalc Software Ltd.). The Shapiro-Wilk
test was used to determine the normality of the distribution of
variables. E followed a normal distribution, thus data are
presented as the mean ± standard deviation (SD), while other
parameters did not follow a normal distribution, and are presented
as the median and interquartile range (IQR). Pearson's correlation
coefficient (r) was used for correlation analysis of normally
distributed data and Spearman's rank correlation coefficient (ρ)
was used for correlation analysis of non-normally distributed data
with a corresponding 95% confidence interval (95% CI).
The difference between anaemic and non-anaemic
groups was determined by the unpaired t-test for normal data
distribution and the Mann-Whitney U test for non-normal data
distribution. P<0.05 was considered to indicate a statistically
significant difference.
The diagnostic sensitivity and specificity, with the
respective 95% CI of the evaluated parameters in anaemic and
non-anaemic pregnant women in diagnosing IDA, were determined.
Receiver operating characteristics (ROC) analysis and the area
under the curve (AUC) were used for this purpose. The optimal
cut-off value was selected according to the Youden index.
Scoring model
For the purpose of earlier and more accurate IDA
recognition, a scoring model based on parameters routinely
determined in a medical biochemical laboratory was developed. The
model consisted of five components, each representing a ratio of
simple haematological and biochemical parameters: Fe/E, MCV/RDW,
RDW/E, RDW/Fe and RDW/HCT. Whether the measured values were higher
or lower than the limit values was considered. The limit values of
the ratios were obtained using ROC curve analysis based on data
from all 623 pregnant women (both anaemic and non-anaemic).
Concentrations higher than the limit values where the criterion was
met were scored 1, and those lower than the limit values where the
criterion was not met were scored 0. The final score was calculated
as the sum of all five components (ranging from 0-5) and reflects
the severity of anaemia, allowing for earlier diagnosis and timely
therapeutic intervention.
Results
Of the 623 pregnant women included, 51% were
primigravida, 32% had one previous birth and 17% had ≥2 previous
births (Table SI). Based on the Hb
concentration, 133 women (21%) were classified as anaemic, 209
(34%) were anaemic according to RDW/HCT and 149 (24%) according to
HCT (Table SII). These findings
suggest that RDW/HCT and HCT are more sensitive indicators of IDA
than Hb concentration.
All tested parameters in anaemic and non-anaemic
pregnant women are presented in Table
I. The values of all tested parameters Fe/E, MCV/RDW, RDW/E,
RDW/Fe, RDW/HCT, E, Fe, Hb, HCT, MCV and RDW were significantly
different between the anaemic and non-anaemic pregnant women
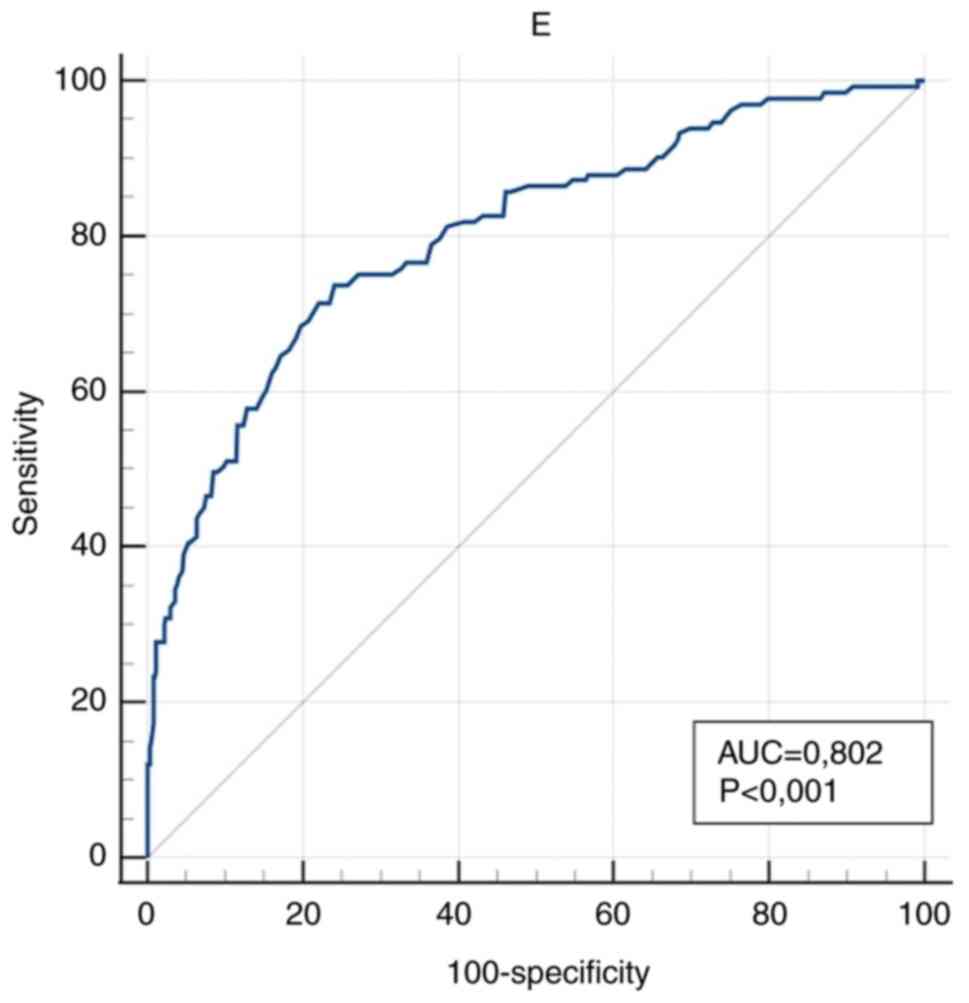
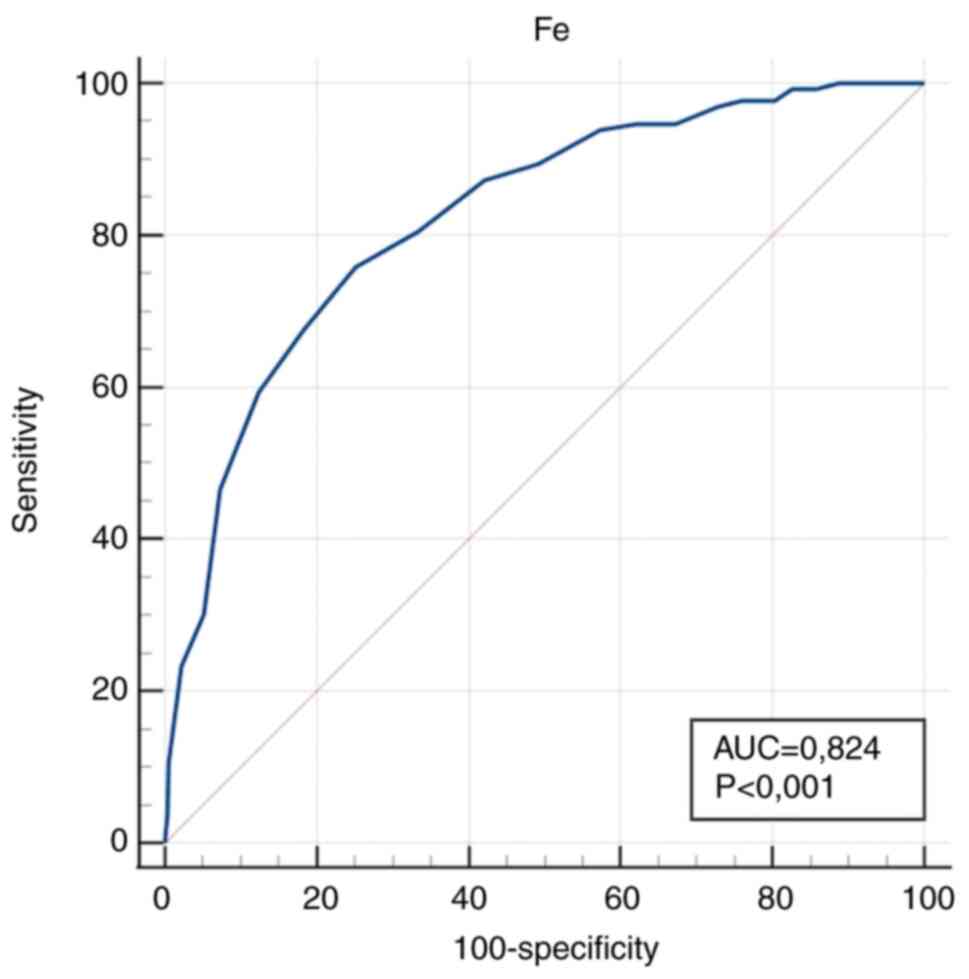
(P<0.001). In the anaemic group, E, Fe, HCT, MCV, Fe/E and
MCV/RDW values were statistically lower, and RDW, RDW/E, RDW/Fe and
RDW/HCT were statistically higher compared with non-anaemic
pregnant women.
 | Table IAssessed parameters in the anaemic and
non-anaemic pregnant women. |
Table I
Assessed parameters in the anaemic and
non-anaemic pregnant women.
| Parameter (unit) | Anaemic, n=133 | Non-anaemic,
n=490 | P-value |
|---|
| Hb (g/lb) | 102 (97-106) | 123 (117-130) |
<0.001a |
| E
(x1012/lc) | 3.77±0.38 | 4.20±0.33 |
<0.001a |
| Fe
(µmol/lb) | 8 (6-10) | 14 (10-18) |
<0.001a |
| HCT (l/lb) | 0.31 (0.29-0.32) | 0.36 (0.34-0.38) |
<0.001a |
| MCV (flb) | 82.5 (75.4-87.3) | 87.7 (84.6-90.8) |
<0.001a |
| RDW (%b) | 15.0 (14.1-15.9) | 14.3 (13.7-15.0) |
<0.001a |
| Fe/E (µmol
x10-12b) | 2.0 (1.5-2.8) | 3.2 (2.5-4.4) |
<0.001a |
| MCV/RDW (fl
x10-2b) | 5.5 (4.8-6.1) | 6.1 (5.7-6.6) |
<0.001a |
| RDW/E
(%lx10-12b) | 4.0 (3.8-4.3) | 3.4 (3.2-3.6) |
<0.001a |
| RDW/Fe
(lx10-2/µmolb) | 2.0 (1.4-2.7) | 1.1 (0.8-1.4) |
<0.001a |
| RDW/HCT
(l/lx10-2b) | 49.3
(46.3-54.5) | 39.4
(36.8-43.0) |
<0.001a |
The correlations between Hb and all parameters are
shown in Table II. A weak
correlation (correlation coefficients from 0.250-0.500) was found
for MCV, Fe/E and MCV/RDW, likely due to their previously mentioned
limitations in diagnosing IDA. A moderate correlation (correlation
coefficients from 0.500-0.750) was found for E, Fe, RDW/E and
RDW/Fe. A strong correlation (correlation coefficients >0.750)
was found for HCT and RDW/HCT. No significant correlation
(correlation coefficient <0.250) was found for RDW.
 | Table IICorrelations between Hb and all
tested parameters. |
Table II
Correlations between Hb and all
tested parameters.
| Parameter
(unit) | Correlation
coefficient (95% CI) | P-value |
|---|
| E
(x1012/l) | r=0.650 (0.602 to
0.693) |
<0.001a |
| Fe (µmol/l) | ρ=0.545 (0.487 to
0.598) |
<0.001a |
| HCT (l/l) | ρ=0.941 (0.932 to
0.950) |
<0.001a |
| MCV (fl) | ρ=0.423 (0.356 to
0.485) |
<0.001a |
| RDW (%) | ρ=-0.243 (-0.316 to
-0.167) |
<0.001a |
| Fe/E (µmol
x10-12) | ρ=0.426 (0.360 to
0.489) |
<0.001a |
| MCV/RDW (fl
x10-2) | ρ=0.360 (0.289 to
0.426) |
<0.001a |
| RDW/E
(%lx10-12) | ρ=-0.743 (-0.777 to
-0.706) |
<0.001a |
| RDW/Fe
(lx10-2/µmol) | ρ=-0.552 (-0.604 to
-0.495) |
<0.001a |
| RDW/HCT
(l/lx10-2) | ρ=-0.804 (-0.830 to
-0.774) |
<0.001a |
The diagnostic accuracy of the measured values
obtained from the ROC curves for the tested parameters at their
optimal diagnostic cut-off values is presented in Table III.
 | Table IIIDiagnostic accuracy of tested
parameters in distinguishing anaemic (n=133) and non-anaemic
(n=490) pregnant women. |
Table III
Diagnostic accuracy of tested
parameters in distinguishing anaemic (n=133) and non-anaemic
(n=490) pregnant women.
| Parameter
(unit) | Area under the
curve (95% CI) | P-value | Cut-off | Sensitivity (95%
CI) | Specificity (95%
CI) |
|---|
| E
(x1012/l) | 0.802
(0.768-0.833) |
<0.001a | ≤3.98 | 73.7
(65.3-80.9) | 75.9
(71.8-79.6) |
| Fe (µmol/l) | 0.824
(0.791-0.853) |
<0.001a | ≤10 | 75.9
(67.8-82.9) | 74.9
(70.8-78.6) |
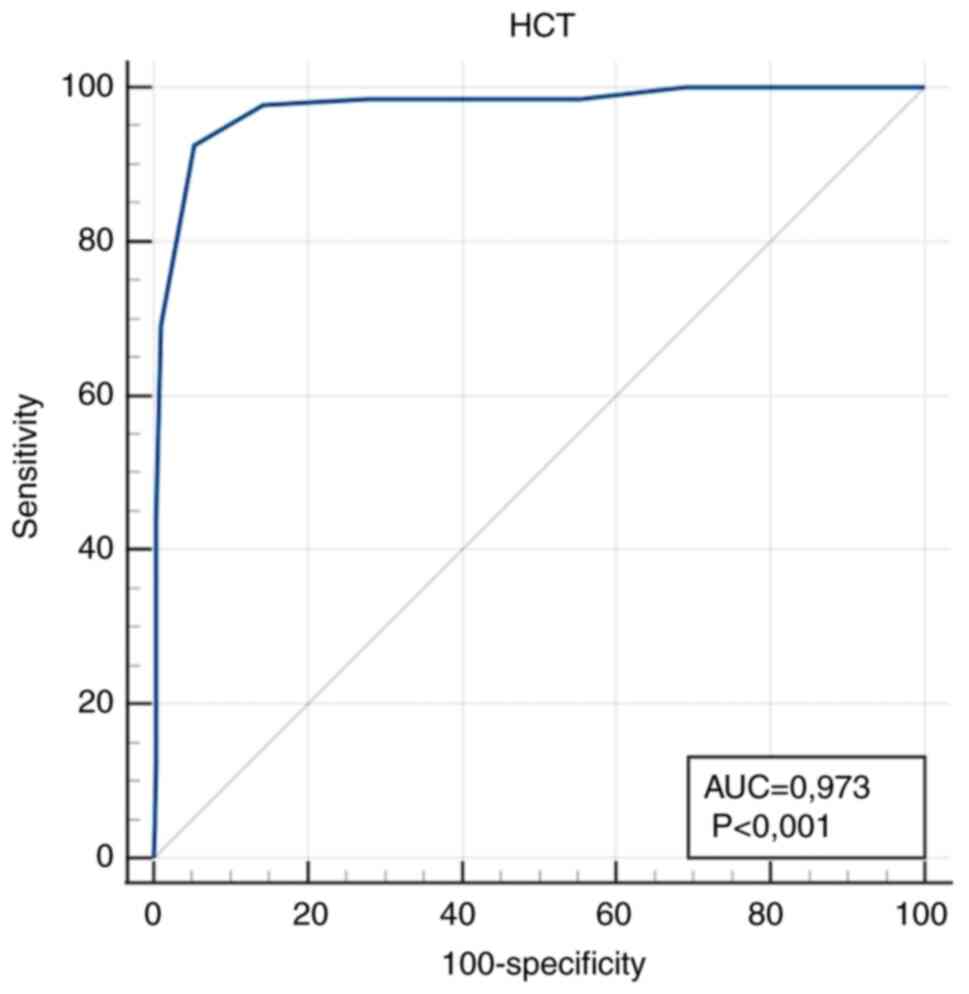
| HCT (l/l) | 0.973
(0.957-0.984) |
<0.001a | ≤0.32 | 92.5
(86.6-96.3) | 94.7
(92.3-96.5) |
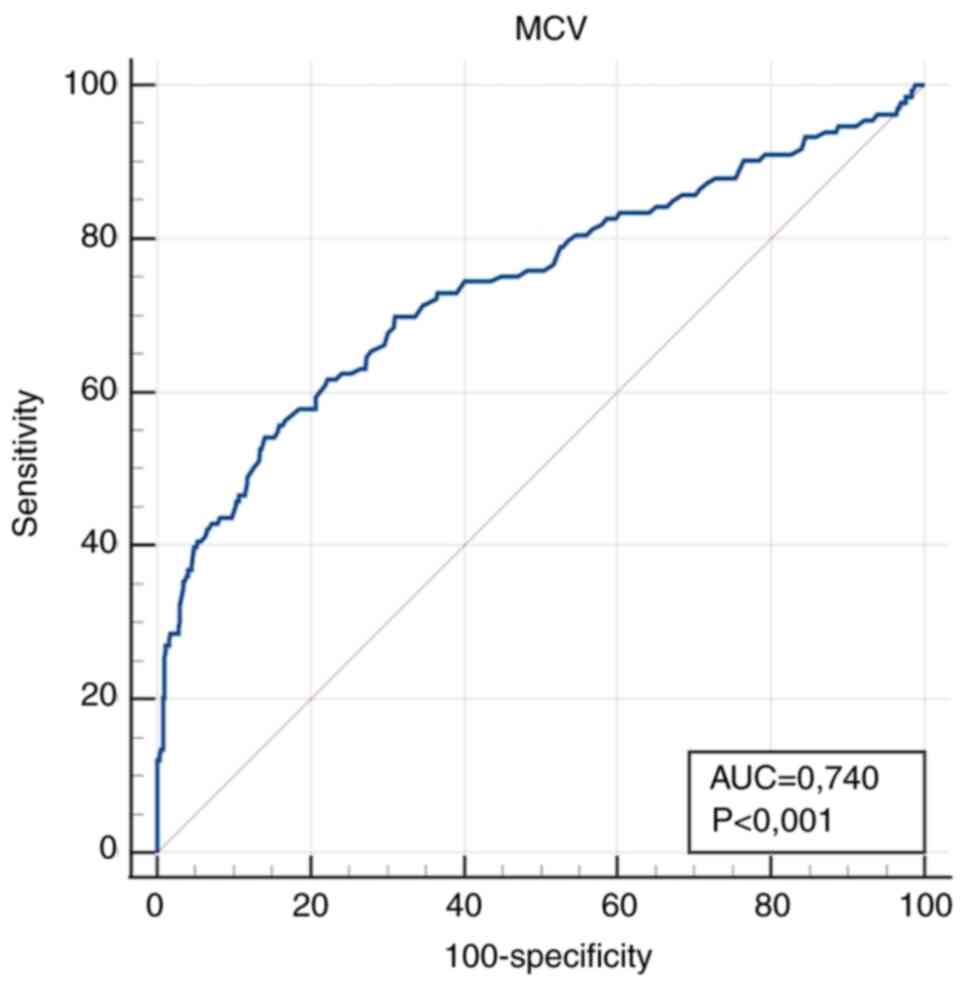
| MCV (fl) | 0.740
(0.704-0.774) |
<0.001a | ≤82.9 | 54.1
(45.3-62.8) | 85.9
(82.5-88.9) |
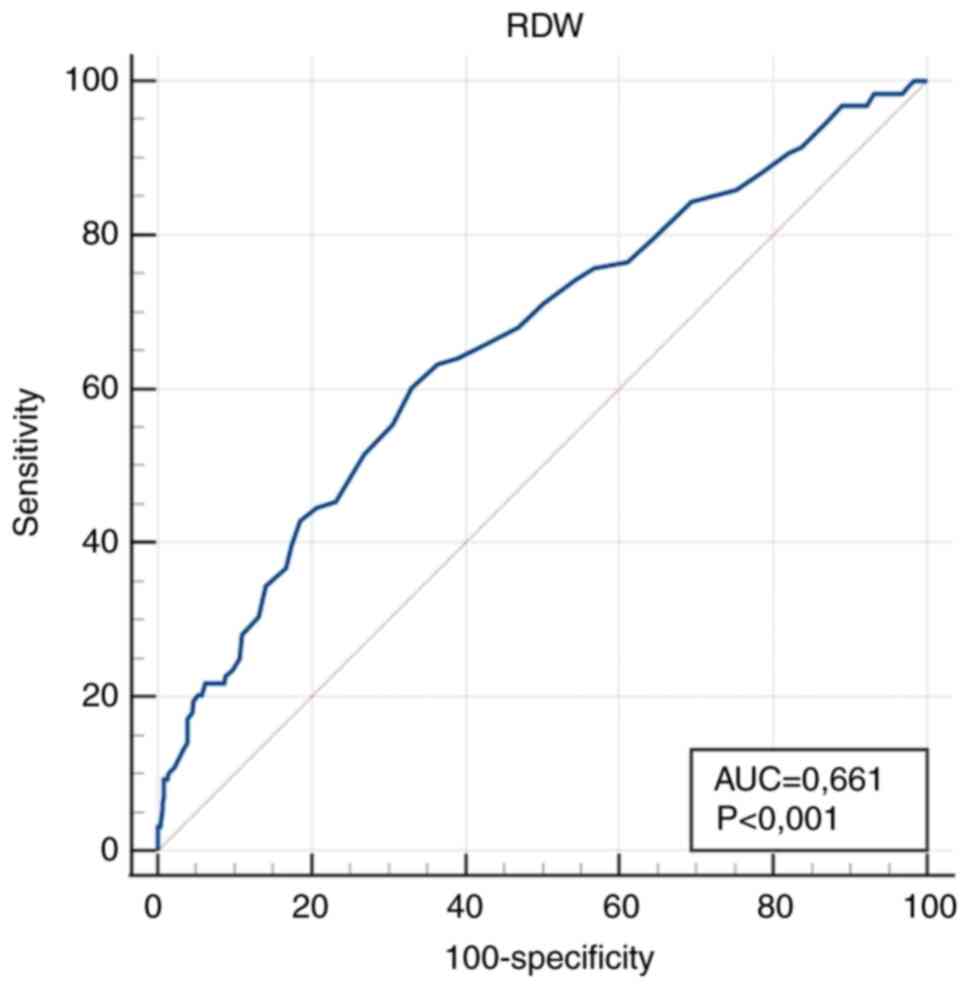
| RDW (%) | 0.661
(0.622-0.698) |
<0.001a | >14.7 | 60.2
(51.1-68.7) | 66.9
(62.6-71.1) |
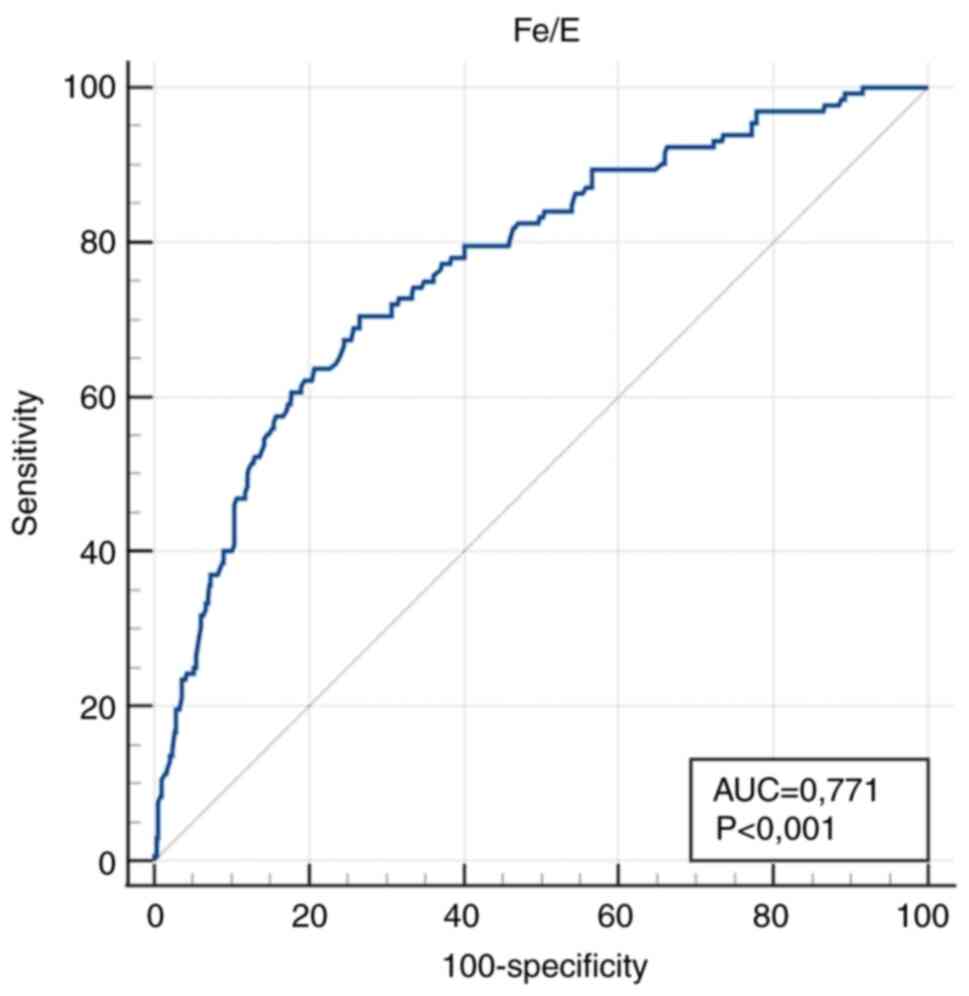
| Fe/E (µmol
x10-12) | 0.771
(0.735-0.803) |
<0.001a | ≤2.53 | 70.5
(61.9-78.1) | 73.4
(69.3-77.3) |
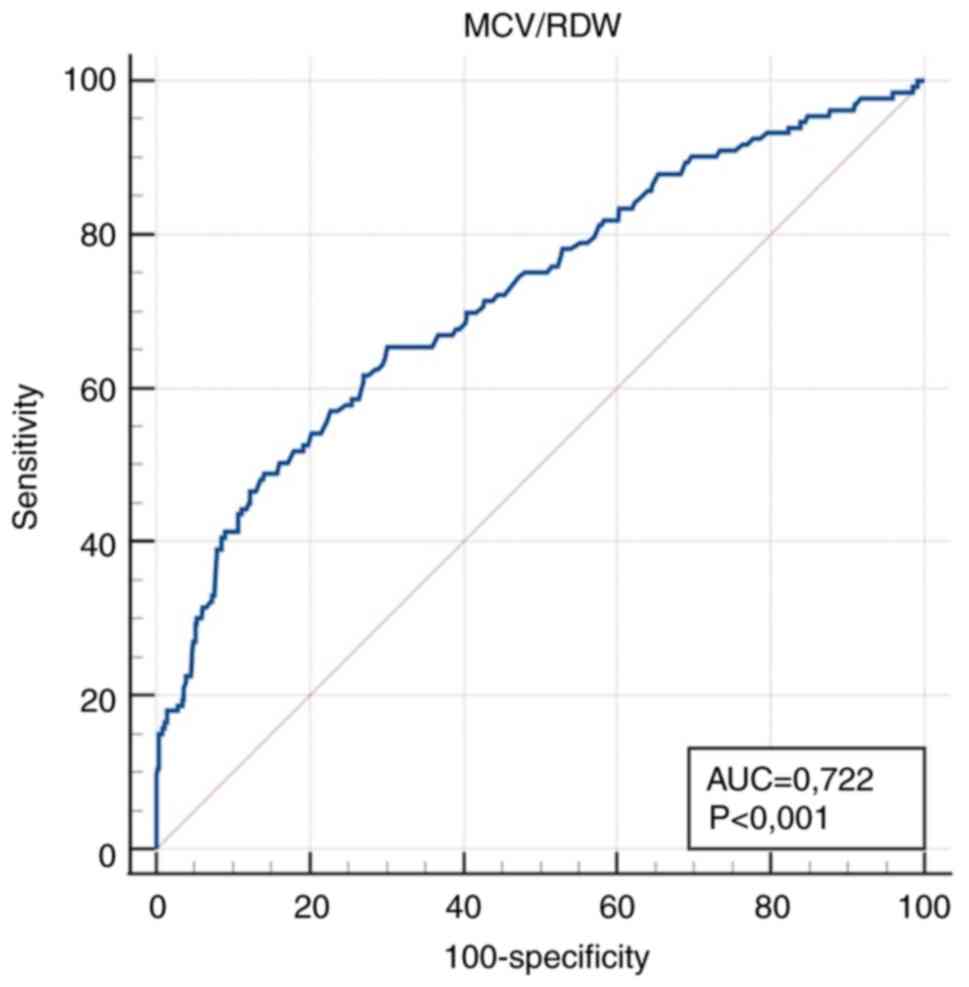
| MCV/RDW (fl
x10-2) | 0.722
(0.685-0.756) |
<0.001a | ≤5.8 | 65.4
(56.7-73.4) | 69.9
(65.7-74.0) |
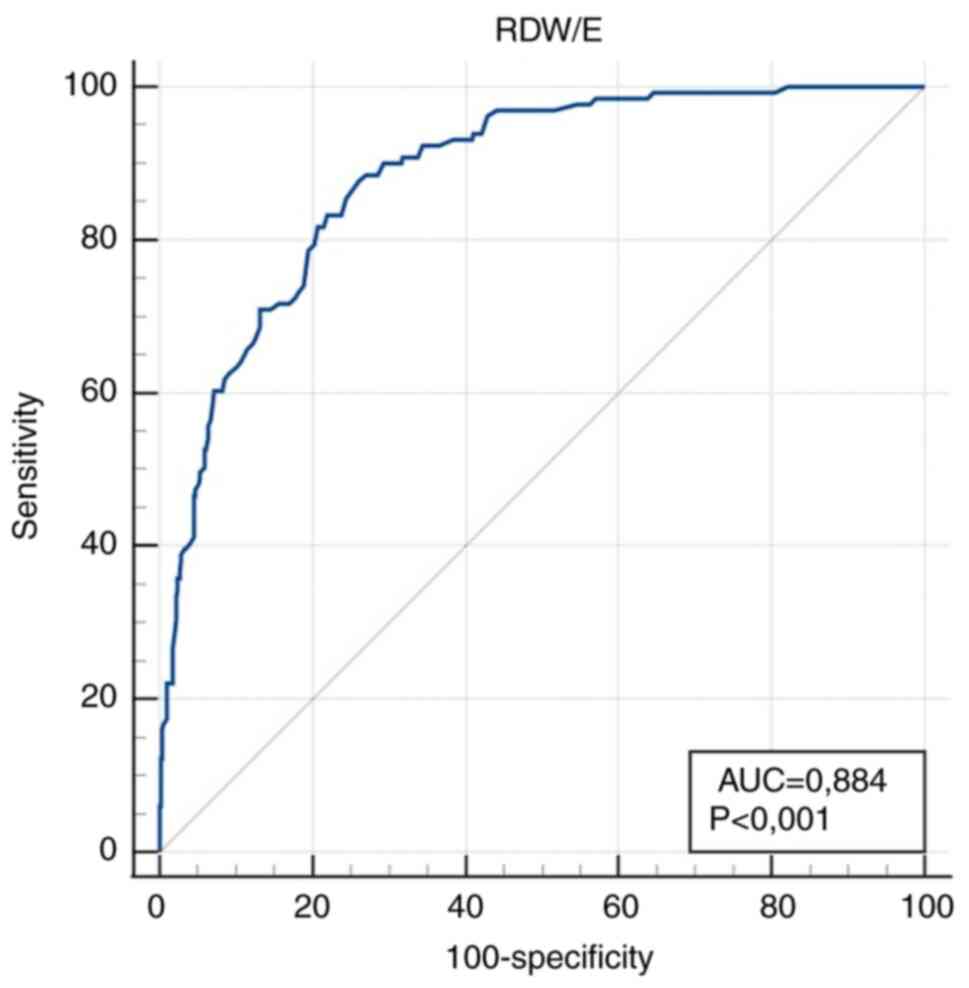
| RDW/E
(%lx10-12) | 0.884
(0.856-0.908) |
<0.001a | >3.63 | 87.8
(80.9-92.9) | 73.9
(69.8-77.8) |
| RDW/Fe
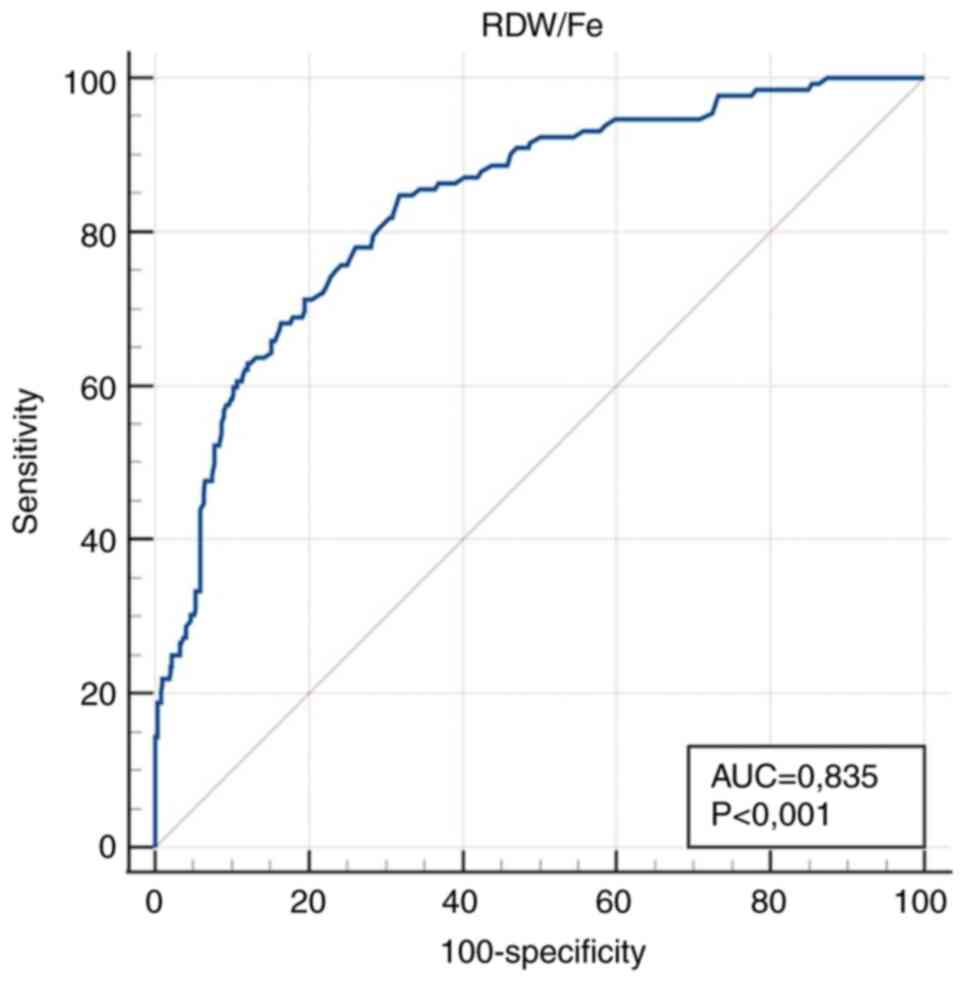
(lx10-2/µmol) | 0.835
(0.804-0.864) |
<0.001a | >1.27 | 84.9
(77.6-90.5) | 68.1
(63.8-72.2) |
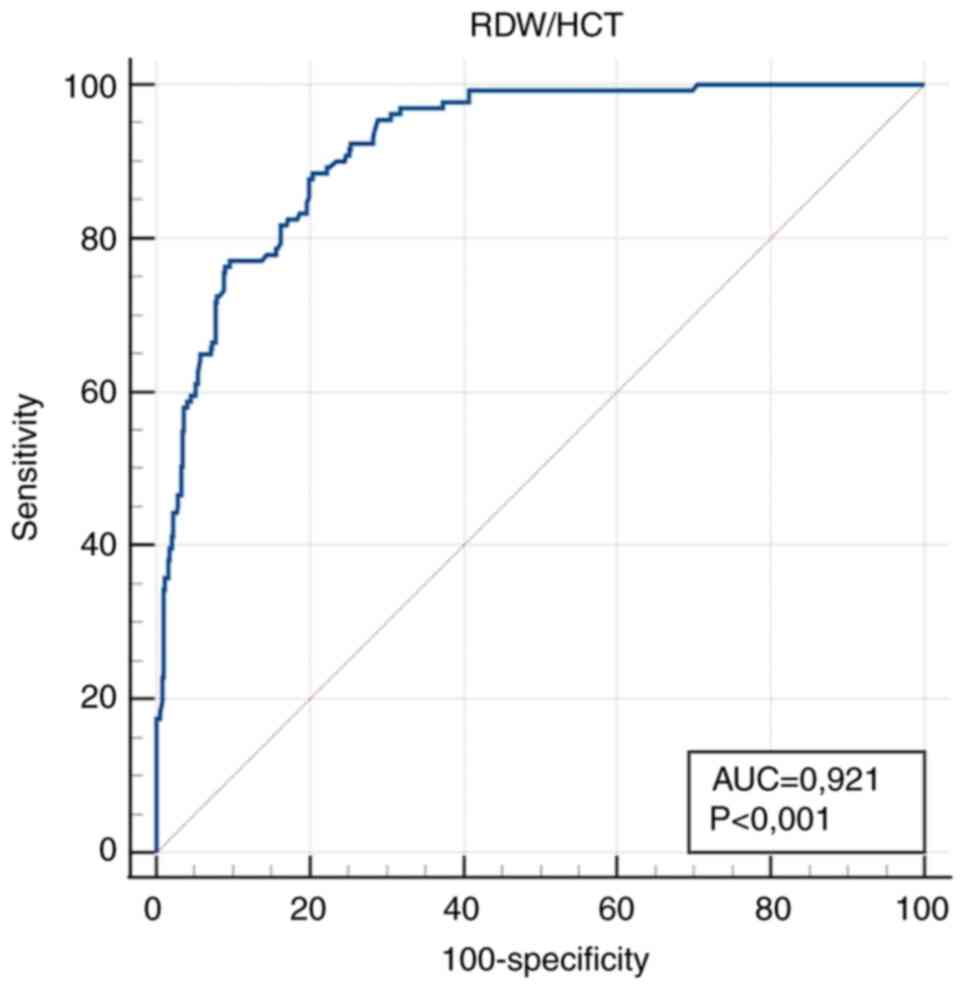
| RDW/HCT
(l/lx10-2) | 0.921
(0.897-0.941) |
<0.001a | >43.64 | 88.6
(81.8-93.4) | 79.5
(75.7-83.0) |
Markedly high diagnostic accuracy (AUC value
>0.9) for IDA diagnosis was obtained for RDW/HCT >43.64
l/lx10-2 as a calculated parameter, and HCT ≤0.32 l/l as
a simple parameter. High diagnostic accuracy (0.9> AUC >0.8)
was obtained for RDW/E, RDW/Fe, E and Fe.
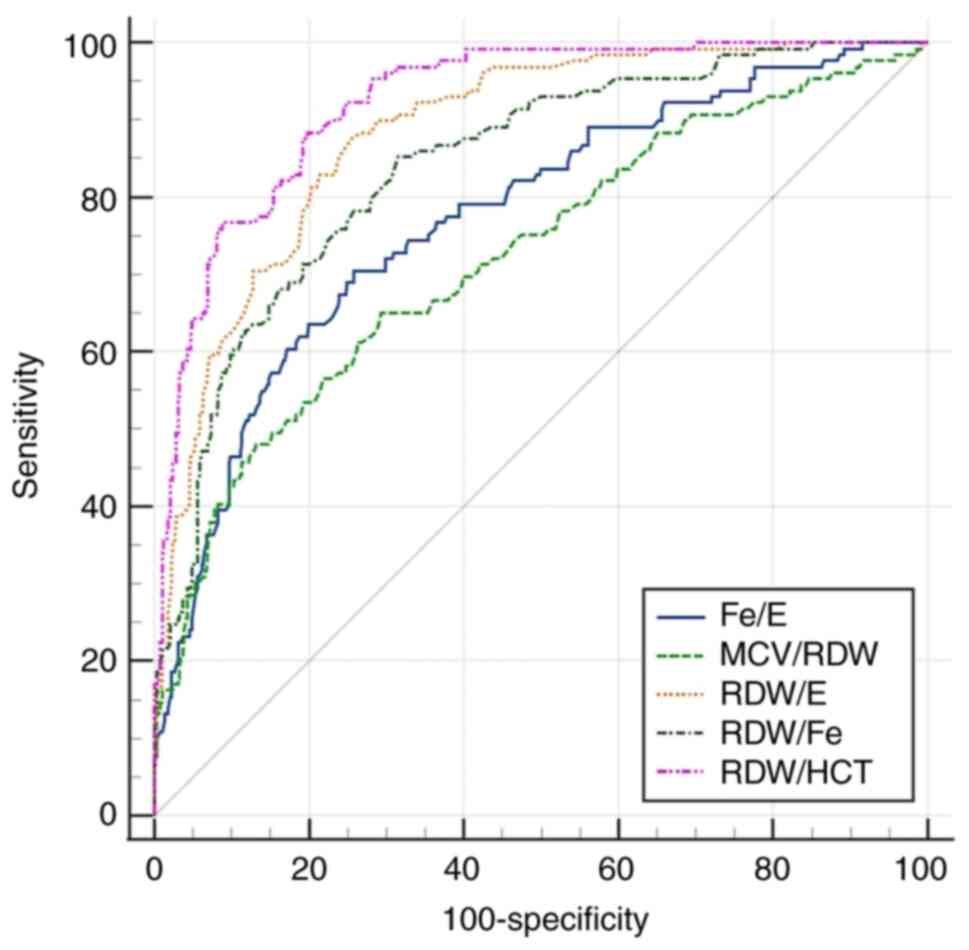
The ROC curves for all parameters, both simple and
calculated are shown in Fig. 1,
Fig. 2, Fig. 3, Fig.
4, Fig. 5, Fig. 6, Fig.
7, Fig. 8, Fig. 9 and Fig. 10, and the comparison of all the ROC
curves of all calculated parameters is presented in Fig. 11.
According to the cut-off values of other calculated
and simple parameters, the following numbers and percentages of
anaemic pregnant women were obtained: 223 (36%) for Fe/E, 234 (38%)
for MCV/RDW, 242 (39%) for RDW/E, 267 (43%) for RDW/Fe, 216 (35%)
for E, 224 (36%) for Fe, 141 (23%) for MCV, and 237 (38%) for RDW
(Table SIII).
The components and criteria of our scoring model are
shown in Table IV. According to
the of the scoring model, the following numbers and percentages of
133 anaemic pregnant women were obtained: 0, 0/133 (0%); 1, 3/133
(2%); 2, 14/133 (11%); 3, 20/133 (15%); 4, 34/133 (26%), and 5,
62/133 (47%). For the 490 non-anaemic pregnant women, the numbers
and percentages were as follows: 0, 217/490 (44%); 1, 80/490 (16%);
2, 76/490 (16%); 3, 60/490 (12%); 4, 33/490 (7%), and 5, 24/490
(5%) (Table SIV).
 | Table IVComponents and criteria of the
scoring model. |
Table IV
Components and criteria of the
scoring model.
| Component | Criterion | No/Yes |
|---|
| Fe/E (µmol
x10-12) | ≤2.53 | 0/1 |
| MCV/RDW (fl
x10-2) | ≤5.8 | 0/1 |
| RDW/E
(%lx10-12) | >3.63 | 0/1 |
| RDW/Fe
(lx10-2/µmol) | >1.27 | 0/1 |
| RDW/HCT
(l/lx10-2) | >43.64 | 0/1 |
| All | | 0-5 |
Discussion
During pregnancy, women are prone to developing IDA.
Routine laboratory parameters are only effective in detecting
already visible and manifested anaemia (3). Despite the availability of clinical
and laboratory indicators, adverse events may sometimes go
unrecognised in a timely manner, increasing the risk of
complications in both mother and child. The introduction of a
novel, simple and accessible laboratory indicator, either as a
standalone or as part of a summary model, allows timely recognition
and treatment of IDA (14). In the
present study, several calculated parameters were evaluated with
the aim of finding the one with the highest diagnostic accuracy. In
addition to introducing new combined parameters, a scoring model
based on the sum of these parameters was developed, and this model
may facilitate the timely recognition of IDA and help in making
clinical decisions.
The goal of the present study was to develop novel
combined parameters for IDA that demonstrate high diagnostic
accuracy, using simple, routine procedures performed in a standard
medical-biochemical laboratory. Additionally, another goal was to
establish a model that can be successfully applied at different
stages of pregnancy, thereby enabling timely diagnosis and
effective follow-up. The study subjects were pregnant women whose
blood samples were collected either the day before or on the day of
delivery, upon admission to the hospital. This is also a
limitation, as early recognition of IDA is crucial for reducing
complications and improving outcomes. The model that is proposed
requires validation, as the values obtained from the present study
would otherwise be applicable only to our specific sample of 623
pregnant women. When the original sample was divided into training
and validation subsets, the two groups produced statistical results
similar to those of the full sample. Although slight differences
were observed in the validation group during statistical analysis,
it is important to note that the same parameters demonstrated the
highest diagnostic accuracy. It is suggested that the model be
validated using a larger, entirely new cohort of pregnant women,
assessed at various stages of gestational age. The absence of such
validation is also acknowledged as a limitation of our study. The
scoring model presented in Table
IV should also be tested on an entirely independent cohort to
confirm its performance across various stages of the gestational
period, as this remains another limitation of the present
study.
It is hypothesised that the novel indicators are
superior for diagnostic purposes and may have potential for
prediction when applied in early pregnancy or at the initial
stages. The scoring model may be useful for predicting IDA in the
early stages of pregnancy. This will serve as a topic for further
research.
In the present study, RDW/HCT was revealed to be the
most effective parameter with high diagnostic sensitivity and
specificity. Among the simple parameters, HCT exhibited the best
performance. Both parameters showed the strongest correlation with
Hb. The RDW/HCT ratio was influenced by HCT, but compared with RDW
alone, RDW/HCT demonstrated superior diagnostic performance in
terms of greater sensitivity and specificity, as well as stronger
correlation with Hb. The ratio adjusts RDW in relation to the
overall red blood cell mass, highlighting subtle cases in which the
HCT may remain at the lower end of the normal range, while RDW is
disproportionately elevated. This increases sensitivity by
capturing both early anisocytosis and relative reductions in red
cell mass. In this way, a more independent indicator was obtained,
superior to HCT in terms of independence and to RDW in terms of
diagnostic accuracy. According to the available literature, there
were no similar studies with which to compare the findings obtained
in the present study.
While RDW/HCT and HCT were identified as the most
effective parameters, several other combined and simple parameters
also demonstrated good diagnostic performance. As aforementioned,
parameters that exhibited slightly lower AUC with good sensitivity
and specificity were Fe/E, RDW/E, RDW/Fe, E, and Fe. According to
the available literature, there were no studies with which to
compare these results. All combined and simple parameters exhibited
high AUC values, with the exception of RDW.
The values of all tested calculated parameters were
statistically significantly different between the anaemic and
non-anaemic pregnant women, indicating that these parameters were
good indicators of IDA. However, caution should be exercised when
interpreting the results, taking into account the pre-analytical
requirements of certain parameters. It should also be emphasised
that several factors must be considered when making a diagnosis;
laboratory findings are only one part of the process. The patient's
medical history and clinical manifestations, together with
laboratory results, contribute to the overall diagnostic picture.
In the present study, some patients with borderline anaemia were
asymptomatic, exhibiting no signs typically associated with
anaemia. By contrast, other patients presented with symptoms that
correlated with the severity of their anaemia. The patients
reported symptoms such as shortness of breath, rapid heartbeat,
increased fatigue, weakness, headache, dizziness, and poor
concentration (5). Although some of
these complaints may also be attributed to typical pregnancy
symptoms, particularly in the third trimester, laboratory analyses
confirmed anaemia in these patients. Conversely, pregnant women in
the control group who did not have anaemia did not report such
complaints, apart from fatigue and headaches (5), which were sporadic and only mildly
pronounced. It should be emphasised that exclusion criteria were
carefully designed, ensuring that pregnant women with conditions or
illnesses that could contribute to such symptoms were not included
in the study.
According to the reviewed literature, no studies
similar to the present one were identified. Most studies were
conducted during the first, second or third trimesters of pregnancy
(1,3,9,11,15,16,17).
As noted in the introduction, parameters from basic blood count
measurements were combined to determine IDA.
A low Hb concentration is an accepted and
recommended indicator of IDA. Unlike ferritin, Hb reflects
functional Fe but does not provide information regarding Fe stores.
This could explain its reduced ability to distinguish ID,
especially during the early stages. However, Hb, along with the
number of red blood cells and Fe, remains a good late indicator of
ID (18). Red blood cell counts and
Hb concentrations in pregnant women were useful but not entirely
reliable indicators of anaemia, due to physiological changes in
plasma volume and red blood cell mass as well as serum Fe
concentration, which is subject to diurnal variations (12).
The first laboratory indicator of ID is an increase
in RDW, which reflects the variation in the size of E and is a good
marker of anisocytosis. This occurs before anaemia becomes visible.
Often, RDW is elevated while MCV remains within the normal range,
and only with disease progression does MCV decrease (19). In the present study, RDW did not
prove to be a strong enough parameter, but calculated parameters
that included RDW showed notably improved diagnostic accuracy.
Earlier studies have shown that MCV can be used as
an indicator of ID and IDA (3,19).
This aligns with the position of the WHO, that MCV and MCH are the
most sensitive indicators of ID among standard CBC parameters
(19). However, in the present
study, MCV did not exhibit sufficient sensitivity.
As for HCT, it has been shown to have low
distinguishing power for ID but high distinguishing power for IDA.
A possible explanation is the similarity between Hb and HCT in
representing the oxygen-carrying capacity of E. Additionally, HCT
is often included as a parameter in point-of-care testing devices,
allowing for quick determination and reducing the time required for
sample transport to the laboratory (19). In the present study, HCT proved to
be an excellent indicator with high sensitivity and
specificity.
In the present study, a novel scoring model was
developed that was shown to offer an improved tool for identifying
IDA in the cohort, with each component contributing individually.
The criteria for a scoring model and the threshold values for each
component were established. The calculated parameters included in
the score demonstrated high sensitivity and specificity and are
considered superior indicators than the individual parameters
alone. These novel parameters make the score unique for detecting
IDA and have not been previously reported. Furthermore, as the
score increases, so does the probability of IDA. As aforementioned,
each component in the scoring model contributes individually. While
an individual calculated parameter may fall within a reference
range, the sum of the scores may still reflect the presence and
severity of IDA, which is the strength of the scoring model. The
prediction score proposed in the present study showed potential for
routine clinical use in the early detection of IDA.
In a published study by Sultana et al
(3), haematological parameters
(RDW, Hb, MCV, MCH and MCHC) were compared in pregnant women within
the first 20 weeks of pregnancy. The study concluded that RDW
(sensitivity 82.3%, specificity 97.4%) demonstrated the highest
diagnostic value for detecting IDA and was a reliable and useful
parameter. Conversely, in the present study, RDW showed a
sensitivity of 60.2% and a specificity of 66.9%, which may be
attributed to the different gestational ages of the pregnant women
included in the study.
In another study by the same authors (9), RDW, Hb and MCV were compared in all
pregnant women who visited their hospital during pregnancy. Once
again, RDW proved to be the most reliable parameter for determining
IDA. However, in the present study, RDW did not exhibit sufficient
sensitivity and specificity (Table
III).
Rabindrakumar et al (17) evaluated the role of red cell indices
as a screening tool for the early detection of ID in pregnant
women. They concluded that ID could be predicted in the early
stages using Hb and red cell indices, which are much less
expensive. The results of the present study support the conclusions
of the study by Rabindrakumar et al (17).
Bencaiova and Breymann (20) investigated the relationship between
Hb, Fe status and pregnancy outcome during the second trimester.
They concluded that mild anaemia and depleted Fe stores, when
detected early during pregnancy, were not associated with adverse
maternal and perinatal outcomes in women receiving Fe
supplementation. The present study also included pregnant women who
received Fe supplements, and similarly, there was no significant
association between Fe supplementation and adverse maternal
outcomes (21).
In conclusion, it was found that RDW/HCT and HCT had
the highest diagnostic accuracy and can be used in routine practice
for diagnosing IDA. These parameters, alongside the scoring model,
may be used in the clinic to predict maternal IDA based on
parameters obtained from routine blood tests. This would enable
clinicians to diagnose IDA more accurately in pregnant women and
initiate treatment promptly to ensure the best perinatal
outcomes.
Supplementary Material
Distribution of pregnant women by
gravidity.
Classification of anaemic pregnant
women by Hb, HCT and RDW/HCT: Number and percentage.
Classification of anaemic pregnant
women by calculated and simple parameters: Number and
percentage.
Classification of anaemic and
non-anaemic pregnant women by the scoring model: Number and
percentage.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DŽ, AV and TŠ conceived and designed the study. AV,
TŠ and DŽ collected the material and data. DŽ, AV, TB, GJ and LH
performed the data analysis. DŽ wrote the first draft of the
manuscript. All authors revised the manuscript. DŽ, AV and TŠ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was performed in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of the
Clinical Hospital Center Rijeka (Rijeka, Croatia; approval no.
2170-29-02/1-19-2; class, 003-05/19-1/110; approval date, 15 July
2019). Written informed consent was obtained from all participants
for participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Surekha MV, Srinivas M, Balakrishna N and
Kumar PU: Haemoglobin distribution width in predicting iron
deficiency anaemia among healthy pregnant women in third trimester
of pregnancy. IJIRAS. 4:390–395. 2017.
|
|
2
|
Navya KT, Prasad K and Singh BMK: Analysis
of red blood cells from peripheral blood smear images for anemia
detection: A methodological review. Med Biol Eng Comput.
60:2445–2462. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sultana GS, Haque SA, Sultana T, Rahman Q
and Ahmed ANN: Role of red cell distribution width (RDW) in the
detection of iron deficiency anaemia in pregnancy within the first
20 weeks of gestation. Bangladesh Med Res Counc Bull. 37:102–105.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Garzon S, Cacciato PM, Certelli C,
Salvaggio C, Magliarditi M and Rizzo G: Iron deficiency anemia in
pregnancy: Novel approaches for an old problem. Oman Med J.
35(e166)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ma M, Zhu M, Zhuo B, Li L, Chen H, Xu L,
Wu Z, Cheng F, Xu L and Yan J: Use of complete blood count for
predicting preterm birth in asymptomatic pregnant women: A
propensity score-matched analysis. J Clin Lab Anal. 34(e23313):
1–8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Obianeli C, Afifi K, Stanworth S and
Churchill D: . Iron deficiency anaemia in pregnancy: A narrative
review from a clinical perspective. Diagnostics.
14(2306)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Breymann C and Huch R: Anemia in pregnancy
and the puerperium. 3rd edition. UNI-MED: Bremen, p46, 2008.
|
|
8
|
Malczewska-Lenczowska J, Orysiak J,
Szczepańska B, Turowski D, Burkhard-Jagodzińska K and Gajewski J:
Reticulocyte and erythrocyte hypochromia markers in detection of
iron deficiency in adolescent female athletes. Biol Sport.
34:111–118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sultana GS, Haque SA, Mishu FA, Muttalib
MA and Rahman Q: Prediction of iron deficiency by red cell
distribution width, mean corpuscular volume and haemoglobin
concentration in pregnant women. BIRDEM Med J. 9:111–116. 2019.
|
|
10
|
Celkan TT: What does a hemogram say to us?
Turk Pediatri Ars. 55:103–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vora SM, Messina G and Pavord S: Utility
of erythrocyte indices in identifying iron depletion in pregnancy.
Obstetric Med. 14:23–25. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guder WG, Narayanan S, Wisser H and Zawta
B: Samples: From the Patient to the Laboratory: The impact of
preanalytical variables on the quality of laboratory results. 3rd
edition. Wiley-VCH GmbH & Co. KGaA, Weinheim, 2003.
|
|
13
|
Kumar U, Chandra H, Gupta AK, Singh N and
Chaturvedi J: Role of reticulocyte parameters in anemia of first
trimester pregnancy: A single center observational study. J Lab
Physicians. 12:15–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mayer L, Langer S, Gaće M, Hrabač P,
Šoštarić M, Fijan I, Špacir Prskalo Z, Cvetko A, Periša J,
Štefančić L, et al: Prediction score for complications after
colorectal cancer surgery based on neutrophils/lymphocytes ratio,
percentage of immature granulocytes, IG and IT ratios. Croat J of
Oncology. 47:1–5. 2019.
|
|
15
|
Resseguier AS, Guiguet-Auclair C,
Debost-Legrand A, Serre-Sapin AF, Gerbaud L, Vendittelli F and
Ruivard M: Prediction of iron deficiency anemia in third trimester
of pregnancy based on data in the first trimester: A prospective
cohort study in a high-income country. Nutrients.
14(4091)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tolunay HE and Elci E: Importance of
haemogram parameters for prediction of the time of birth in women
diagnosed with threatened preterm labour. J Int Med Res. 48:1–8.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rabindrakumar MSK, Wickramasinghe VP,
Gooneratne L, Arambepola C, Senanayake H and Thoradeniya T: The
role of haematological indices in predicting early iron deficiency
among pregnant women in an urban area of Sri Lanka. BMC Hematol.
18:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Örgül G, Hakh DA, Özten G, Fadiloğlu E,
Tanacan A and Beksaç MS: First trimester complete blood cell
indices in early and late onset preeclampsia. Turk J Obstet
Gynecol. 16:112–117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rivera AKB, Latorre AAE, Nakamura K and
Seino K: Using complete blood count parameters in the diagnosis of
iron deficiency and iron deficiency anemia in Filipino women. J
Rural Med. 18:79–86. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bencaiova G and Breymann C: Mild anemia
and pregnancy outcome in a Swiss collective. J Pregnancy 2014: Nov
13, 2014 (Epub ahead of print).
|
|
21
|
Shao J, Richards B, Kaciroti N, Zhu B,
Clark KM and Lozoff B: Contribution of iron status at birth to
infant iron status at 9 months: Data from a prospective
maternal-infant birth cohort in China. Eur J Clin Nutr. 75:364–372.
2021.PubMed/NCBI View Article : Google Scholar
|