Introduction
Psoriasis is a chronic immune-mediated inflammatory
skin disease characterized by keratinocyte hyperproliferation and
infiltration of inflammatory cells, including CD4+ T and
mast cells, into the skin (1).
Although the precise molecular mechanisms underlying psoriasis
pathogenesis are not completely understood, accumulating evidence
highlights a key role for T helper 17 (Th17) cells as the primary
source of IL-17, a key cytokine in psoriasis development (2,3). Th17
cells differentiate from naïve CD4+ T cells primarily in
response to IL-23 through activation of the JAK2/STAT3 signaling
pathway (4,5). Thus, the IL-23/IL-17 axis is key to
the immunopathogenesis of psoriasis (4). During disease onset, proinflammatory
cytokines such as tumor necrosis factor (TNF)-α and IL-1β promote
abnormal keratinocyte proliferation and differentiation (6). These cytokines suppress keratin 10
expression while upregulating involucrin and keratins 6, 16 and 17,
which serve as molecular markers of psoriatic lesions (7). This dysregulation reflects
keratinocyte activation and contributes to hallmark
histopathological features of psoriasis, including epidermal
hyperplasia, parakeratosis and acanthosis (8).
Although various therapeutic agents for psoriasis
such as methotrexate, cyclosporine, retinoic acid, vitamin D3
analogs and biological agents such as alefacept are widely used, a
definitive cure remains elusive (2,3).
Moreover, long-term use of certain agents, including cyclosporine
and retinoic acid, is associated with significant adverse effects
(9). As a result, alternative
strategies, including combination therapies involving conventional
and biological agents, have been explored. However, these
approaches are limited by cumulative toxicity and safety concerns
(10). Therefore, the development
of novel, safe and effective therapeutic options for psoriasis
remains a clinical need.
Marine algae are regarded as the ‘plant-based
therapy of the future’ due to their abundance of health-promoting
compounds with activity against various diseases and disorders
(11). One of the most notable
components of seaweeds is their high content of sulfated
polysaccharides (SPs). SPs derived from seaweeds exhibit a broad
spectrum of biological activities, including anti-inflammatory,
immunomodulatory, antioxidant and antiviral effects, making them
promising candidates for pharmaceutical and nutraceutical
applications (12). Gracilaria
fisheri, a species commonly found in Thailand, is rich in
sulfated galactan (SG), a type of SP known for its diverse
biological properties, especially its anti-inflammatory and
immunomodulatory effects (13).
Determining molecular characteristics such as molecular weight is
key for enhancing the biological activity of SPs (14). Our previous study prepared low
molecular weight SG (LSG) from G. fisheri and demonstrated
its superior antioxidant activity compared with SG (15). Oligosaccharides derived from G.
fisheri modulate immune responses by inhibiting proinflammatory
cytokines such as TNF-α and IL-1β, which are key mediators in
psoriasis pathogenesis (13).
Moreover, SG has been shown to interfere with signaling pathways
such as NF-κB and JAK/STAT, which serve central roles in the
regulation of inflammatory gene expression (16,17).
Given these properties, LSG derived from G.
fisheri presents a promising candidate for the development of
novel anti-psoriatic agents. The present study employed an
imiquimod (IMQ)-induced psoriasis-like mouse model to evaluate the
therapeutic potential of LSG from G. fisheri.
Materials and methods
LSG
LSG was prepared using a previously established
method (18). SG was isolated from
the red seaweed G. fisheri using a cold-water extraction
method as described by Wongprasert et al (19). SG was stirred in 0.1 M HCl (RCI
Labscan Ltd.; ratio 10:1) for 6 h at room temperature. The mixture
was neutralized to pH 8 and precipitated with 95% ethanol
(RCILabscan). The resulting pellet was collected, re-suspended in
distilled water and dialyzed against distilled water in a dialysis
bag for 24 h. LSG was obtained by freeze-drying overnight at -60˚C.
The chemical structure and molecular weight of LSG were confirmed
by fourier transform infrared (FTIR), nuclear magnetic resonance
(NMR) and gel permeation chromatography (GPC) analyses. LSG (2 mg)
was analyzed using FTIR spectroscopy with an IRAffinity-1S (IR) and
MIRacle 10 (ATR; Shimadzu). The compound was transferred to a film
and analyzed. Spectral baselines were corrected to the 400-4,000
cm-¹ range. For NMR analysis, LSG (10 mg) was dissolved
in deuterium oxide (D2O, 99.9%; 0.5 ml) in 5 mm NMR
tubes and analyzed using a Varian Bruker 400 MHz spectrometer. For
GPC, LSG was dissolved in deionized water (1:1 w/v), and 20 µl of
the solution was analyzed using a Shimadzu LC-20AD system with an
LC-20A oven column and a RID-10A detector, equipped with a TSKgel
Guard PWH Size Exclusion Guard column. The column temperature was
maintained at 60.0±0.1˚C.
Animal model
Male BALB/c mice (n=48; age, 6-8 weeks; average
weight, 23.96±1.34 grams) were purchased from Nomura International
Siam (Bangkok, Thailand). All animals were housed under SPF
conditions with controlled humidity (50-70%), temperature (25±2˚C)
and a 12/12-h light/dark cycle. Mice had free access to a standard
pellet diet and water ad libitum throughout the experiment.
All experimental procedures were approved by the Ethical Committee
of the University of Phayao, Phayao, Thailand (approval no.
1-024-65).
IMQ-induced psoriasis and experimental
design
The dorsal hair of BALB/c mice was removed 1 day
before starting the experiment. IMQ cream (5% IMQ; Aldara™, Ensign
Laboratories Pty Ltd.) was applied daily at a topical dose of 62.5
mg for 7 consecutive days to establish an IMQ-induced psoriasis
mouse model characterized by moderate-to-severe psoriasis-like skin
lesions, including pronounced erythema, scale formation, epidermal
thickening and inflammatory cell infiltration, corresponding to
Psoriasis Area and Severity Index (PASI) scores of 2-3 for each
parameter (20,21). MTX was used as a positive control
because it is commonly used as a first-line treatment for
moderate-to-severe plaque psoriasis (22). The concentration of 5.0 mg/kg LSG
was selected based on previous studies (23,24).
The mice were randomly divided into six groups (n=8/group) as
follows: i) Normal control (NC), mice were intraperitoneally
injected with 200 µl normal saline, followed by the topical
application of petroleum cream (Chemipan Corporation Co., Ltd.);
ii) methotrexate (MTX) control, mice were intraperitoneally
injected with 200 µl 1.0 mg/kg MTX, followed by the topical
application of petroleum cream; iii) LSG control, mice were
intraperitoneally injected with 200 µl 5.0 mg/kg LSG, followed by
the topical application of petroleum cream; iv) IMQ, mice were
intraperitoneally injected with 200 µl normal saline, followed by
the topical application of IMQ cream; v) MTX + IMQ, mice were
intraperitoneally injected with 200 µl 1.0 mg/kg MTX, followed by
the topical application of IMQ cream; and vi) LSG + IMQ, mice were
intraperitoneally injected with 200 µl 5.0 mg/kg LSG, followed by
the topical application of IMQ cream (Fig. S1). After 7 days of treatment, all
mice were anesthetized with 5% isoflurane in oxygen and maintained
at 1.5-3%, followed by cervical dislocation. The thoracic cavity
was opened, and blood was collected via cardiac puncture. Skin
lesions, lymph nodes, spleen, kidney and liver were harvested for
further analysis.
Assessment of PASI
The severity of skin lesions was assessed using a
modified human scoring system based on PASI (21,25).
Mice were evaluated daily for erythema, scaling and thickening.
Each parameter was scored independently on a scale from 0 to 4 (0,
none; 1, mild; 2, moderate; 3, marked; 4, obvious; Table I) (21). The cumulative score was used to
evaluate the overall severity of skin inflammation.
 | Table IDescriptive Psoriasis Area and
Severity Index scores in a mouse model following imiquimod
induction. |
Table I
Descriptive Psoriasis Area and
Severity Index scores in a mouse model following imiquimod
induction.
| | Score |
|---|
| Sign | 0 | 1 | 2 | 3 | 4 |
|---|
| Erythema
(redness) | Normal flesh tone
(faint cuts may be visible but not prominent) | Slight redness over
the skin surface | Noticeable uniform
redness | Pronounced redness
with scattered dark red areas | Intense, dark red
coloration |
| Thickness | No thickening, skin
remains soft and flexible | Mild puckering with
no measurable increase in thickness (~1 mm) | Thickness of 1-2 mm
upon pinching, with ridges visible in some regions | Thickness >2 mm
upon pinching, with ridges easily noticeable | Thickness >2 mm
without pinching; ridges appear tight and distinct |
| Scaling
(desquamation) | No evidence of
flaking | Small, localized
dry spots without peeling | Dry patches
covering most of the surface, with flaking in creases | Moderate flaking
affecting a large portion of the surface | Extensive and
severe flaking over a wide surface area, particularly pronounced
involvement in certain regions |
Body and lymphatic organ weight
Body weight was recorded on days 1, 3, 5 and 7 of
administration. On day 8, the mice were euthanized, and right
axillary lymph nodes and spleens were removed, cleaned and weighed.
The weights of the right axillary lymph nodes and spleens were
normalized to body weight to calculate the organ index.
Histopathological analysis
Skin lesions were collected, fixed in 4%
paraformaldehyde for 48 h at 4˚C and embedded in paraffin. Paraffin
blocks were sectioned at 4 µm thickness using a rotary microtome.
Tissue sections were deparaffinized with xylene and rehydrated
through a descending graded ethanol series. Sections were stained
with Mayer's hematoxylin (cat. no. 05-06002/L) and Eosin Y
alcoholic solution (cat. no. 05-11007/L; both Bio Optica Milano
SpA). Sections were dehydrated, cleared, mounted and cover slipped
for histopathological observation under a light microscope.
Epidermal and dermal thickness were measured in four randomly
selected areas of view/section at 10X magnification using ImageJ
software version 1.32j (National Institutes of Health). To
visualize blood vessels in the skin lesions, Periodic Acid-Schiff
(PAS) staining was performed using a PAS staining kit (cat. no.
1.01646.0001, Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol (26).
Additionally, Giemsa staining was performed using Giemsa solution
(cat. no. RA-002-05, Biotechnical Co., Ltd.), following the method
described by Pudgerd et al (27). Stained sections were observed and
photographed under a light microscope. Mast cells were counted
manually in four randomly selected fields of view/section at 10X
magnification.
Immunohistochemistry analysis
Skin lesions were fixed in 4% paraformaldehyde for
48 h at 4˚C and embedded in paraffin. Paraffin blocks were
sectioned at 4 µm thickness. Tissue sections were deparaffinized
with xylene and rehydrated through a descending graded ethanol
series. Tissue sections were incubated in 3%
H2O2 for 15 min to block endogenous
peroxidase activity, followed by antigen retrieval using Tris-EDTA
buffer (pH 9.0) at 100˚C for 20 min, and washed with 1X PBS-T
(0.05% Tween 20). Tissue sections were incubated with protein
blocking solution (0.5% BSA, 0.5% casein in PBS; cat. no. AB64226,
Abcam) for 1 h at room temperature. Sections were incubated with
primary antibodies against CD4+ (rabbit monoclonal
anti-CD4+, cat. no. ab183685, Abcam; 1:50) and Ki67
(rabbit polyclonal anti-Ki67, cat. no. ab15580, Abcam; 1:100) for 3
h at room temperature. Following washing with 1X PBS-T, sections
were incubated with peroxidase-conjugated secondary antibody
(AffiniPure goat anti-rabbit IgG H&L, cat. no. 111-035-003,
Jackson ImmunoResearch Laboratories, Inc.; 1:500) for 1 h at room
temperature. Positive signals were developed using NOVA Red
substrate (cat. no. SK-4800, Vector Laboratories, Inc.; Maravai
LifeSciences) and counterstained with Mayer's hematoxylin for 20
sec at room temperature. For quantitative analysis, slides were
imaged at 10X magnification under a light microscope.
CD4+ and Ki67-positive cells were counted manually in
four randomly selected areas of view/section.
Cytokine ELISA
Skin tissue and blood serum samples were collected
to assess cytokine levels using ELISA kits. Dorsal skin was excised
and any attached connective tissue was removed. A 0.1
cm2 section of tissue was homogenized in 1 ml normal
saline using a tissue homogenizer. The homogenate was centrifuged
at 2,500 x g for 15 min at 4˚C. The supernatant was collected and
stored at -20˚C until analysis. For blood serum, samples were
allowed to clot at room temperature for 30 min, followed by
centrifugation at 2,500 x g for 15 min at 4˚C. The serum was
separated and stored at -20˚C until use (27). Cytokine concentrations including
IL-1β (ELISA MAX™ Deluxe Set Mouse IL-1β kit, cat. no. 432604),
TNF-α (cat. no. 430904), IL-17A (cat. no. 432504), and IL-23 (cat.
no. 433704), were measured using commercial ELISA kits, according
to the manufacturer's protocols (all BioLegend, Inc.). Absorbance
was measured at 450 nm using a VersaMax™ microplate reader and data
were analyzed with SoftMax Pro software version 6 (Molecular
Devices, LLC).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from skin tissue using TRI
Reagent® (Molecular Research Center, Inc., cat. no.
TR118) according to the manufacturer's instructions. A total of 1
µg total RNA was reverse-transcribed into cDNA using the iScript™
Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories,
Inc.; cat. no. 1708840). All RT-qPCR assays were conducted using
the QIAquant Real-Time PCR Thermal Cycler (Qiagen, Inc.) with the
SensiFAST™ SYBR® No-ROX Kit (Bioline, cat. no.
BIO-98050), according to the manufacturer's protocols.
Thermocycling conditions were as follows: Initial denaturation at
95˚C for 2 min, followed by 40 cycles of 95˚C for 5 sec, 60˚C for
10 sec of annealing, and 72˚C for 20 sec of extension. Relative
gene expression of IL-1β, TNF-α, keratin 6, 16 and 17, involucrin,
JAK1, 2 and 3, STAT1, 2 and 3, BCL2 and CCND1 was normalized to
β-actin and calculated using the 2-ΔΔCq method (28). Primer sequences for all target genes
are listed in Table II.
 | Table IIPrimer sequences. |
Table II
Primer sequences.
| Gene | Primer | Sequence,
5'→3' | Accession no. | (Refs.) |
|---|
| Keratin 6 | Forward |
GTGGCCTCAGCTCTTCTACC | BC080820.1 | (27) |
| | Reverse |
TCTGAGCACGGGATTCTGC | | |
| Keratin 16 | Forward |
TGGATGGCGAGAATATCCACAG | AF064006.1 | (27) |
| | Reverse |
GCTCCTTGAGGATGGACCG | | |
| Keratin 17 | Forward |
GCCCACCTGACTCAGTACAA | BC032161.2 | (27) |
| | Reverse |
GGAGCTGAGTCCTTAACGGG | | |
| Involucrin | Forward |
ATGTCCCATCAACACACACTG | NM_008412.3 | (27) |
| | Reverse |
TGGAGTTGGTTGCTTTGCTTG | | |
| JAK1 | Forward |
CTCCGAACCGAATCATCACT | NM_146145.2 | (55) |
| | Reverse |
GCCGTTTTTCTGCTTCTTTG | | |
| JAK2 | Forward |
TTGTGGTATTACGCCTGTGTATC | L16956.1 | (56) |
| | Reverse |
ATGCCTGGTTGACTCGTCTAT | | |
| JAK3 | Forward |
CCATCACGTTAGACTTTGCCA | L40172.1 | (56) |
| | Reverse |
GGCGGAGAATATAGGTGCCTG | | |
| STAT1 | Forward |
TCACAGTGGTTCGAGCTTCAG | NM_001205313.1 | (56) |
| | Reverse |
GCAAACGAGACATCATAGGCA | | |
| STAT2 | Forward |
TCCTGCCAATGGACGTTCG | NM_019963.2 | (56) |
| | Reverse |
GTCCCACTGGTTCAGTTGGT | | |
| STAT3 | Forward |
AGCAGAATCTCAACTTCAGACC | NM_213660.4 | (57) |
| | Reverse |
TTCGTGGTAAACTGGACACC | | |
| BCL2 | Forward |
AGGAGCAGGTGCCTACAAGA | AH001858.2 | (55) |
| | Reverse |
GCATTTTCCCACCACTGTCT | | |
| CCND1 | Forward |
GCGTACCCTGACACCAATCT | NM_007631.3 | (55) |
| | Reverse |
ATCTCCTTCTGCACGCACTT | | |
| β-actin | Forward |
GGCTGTATTCCCCTCCATCG | NM_007393.5 | (27) |
| | Reverse |
CCAGTTGGTAACAATGCCATGT | | |
Western blot analysis
Total protein from skin tissue was extracted using
lysis buffer containing 20 mM Tris-HCL, 100 mM NaCl, 5 mM
phenylmethylsulfonyl fluoride and 100X protease inhibitor solution.
Protein concentration was determined with a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). A total of 50
µg protein/lane was separated on a 12.5% SDS-polyacrylamide gel and
transferred to a nitrocellulose membrane. The membrane was blocked
with 2% BSA (cat. no. 112018, Merck KGaA) in TBS-T (100 mM
Tris-base, 150 mM NaCl, 0.1% Tween 20) for 2 h at room temperature
and incubated overnight at 4˚C with primary antibodies (1:1,000)
against mouse anti-Ki67 (cat. no. 14569982, Invitrogen, Thermo
Fisher Scientific, Inc.), rabbit anti-TNF-α (cat. no. 3707S), mouse
anti-IL-6 (cat. no. 12019S), rabbit anti-IL-10 (cat. no. 12163S;
all Cell Signaling Technology Inc.) and rabbit anti-β-actin (cat.
no. 4970S, Cell Signaling Technology, Inc.). After three washes
with TBS-T for 10 min each, the membranes were incubated with
HRP-conjugated secondary antibody (1:2,000; cat. nos. 31460 and
626520; Invitrogen, Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. Membranes were washed with TBS-T and immunoreactivity
was visualized using the Clarity™ Western ECL substrate kit
(Bio-Rad Laboratories, Inc.). Protein bands were detected using the
Amersham™ ImageQuant™ 800 biomolecular imager (Cytiva). Relative
protein expression was quantified using ImageJ software version
1.32j (National Institutes of Health), with band intensities
normalized to β-actin.
Statistical analysis
All data are presented as mean ± SD of triplicate
experiments. Statistical analysis was performed using one-way ANOVA
followed by Tukey's post hoc multiple comparison test. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using GraphPad Prism
software, version 10.3.1.509 (Dotmatics).
Results
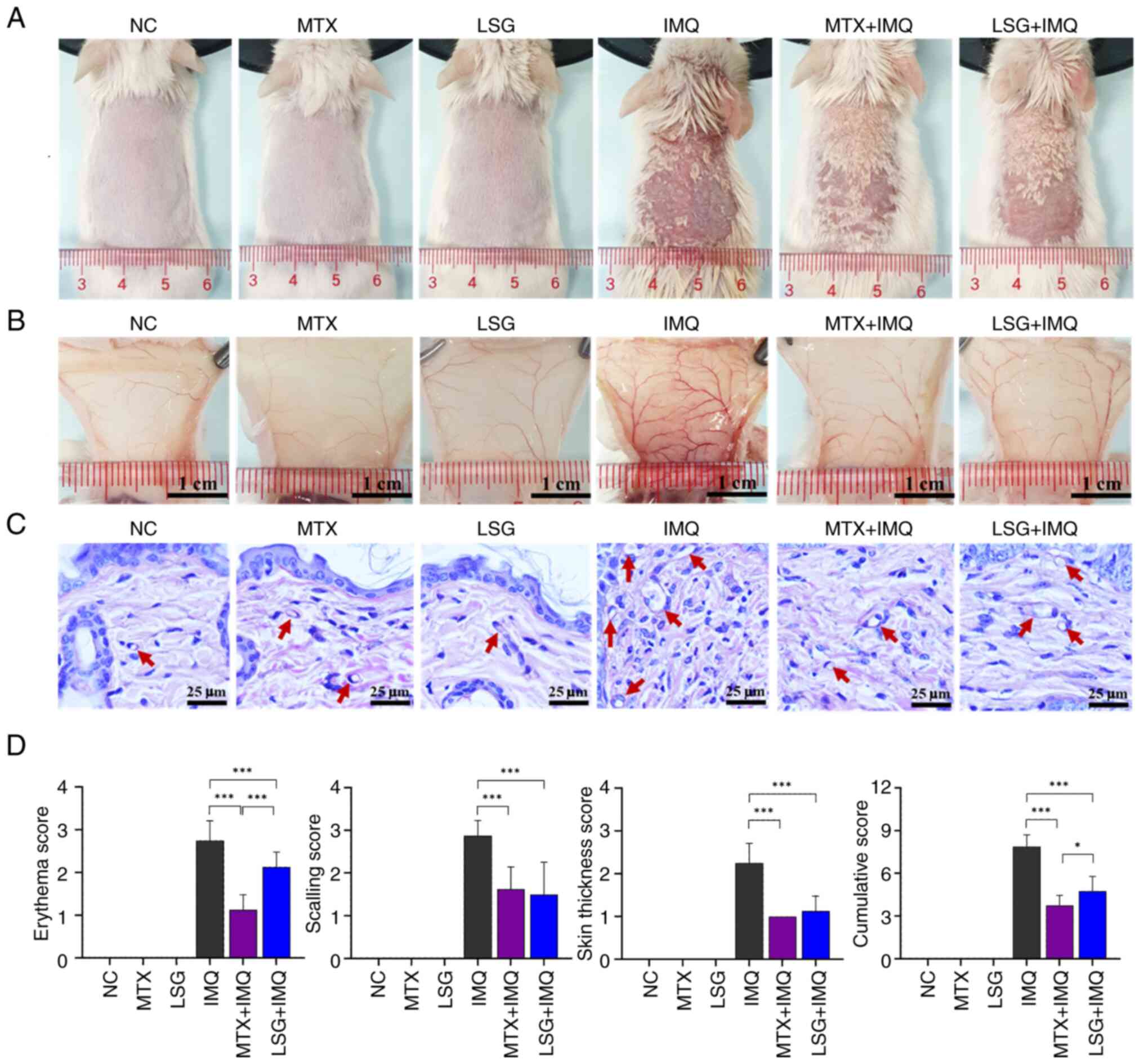
Effect of LSG on psoriatic skin
lesions in IMQ-induced mouse model of psoriasis
LSG had a molecular weight of 7.87 kDa and consisted
of complex structures with alternating 3-linked β-D-galactopyranose
and 4-linked 3,6-anhydro-α-L-galactopyranose or
α-L-galactopyranose-6- sulfate units (Fig. S2) (18).
To investigate whether LSG ameliorated skin lesions
in psoriasis, IMQ was applied to induce a psoriasis-like mouse
model, with and without LSG treatment, and compared with MTX
treatment. Mice treated with IMQ exhibited rapid proliferation of
immune cells and keratinocytes, leading to increased cytokine
production. This mimics the features of human plaque psoriasis,
which is characterized by erythema, skin thickening, scaling, and
epidermal changes associated with increased inflammation and
vascular alterations (29).
Symptoms of the psoriatic condition were observed over 7 days of
continuous IMQ application in the IMQ group. However, LSG + IMQ
group showed a decrease in IMQ-induced psoriatic traits, similar to
MTX + IMQ group (Figs. 1A and
S3).
Subcutaneous vasculature in the lesional area was
visualized post-mortem to evaluate inflammatory angiogenesis. Mice
in the IMQ group displayed dense and complex vascular networks,
characterized by prominent branching and bifurcation, indicative of
increased neovascularization and vascular remodeling commonly
associated with psoriatic inflammation (8,29). By
contrast, both the MTX + IMQ and LSG + IMQ groups exhibited
decreased vascular complexity, suggesting that these treatments
effectively suppressed IMQ-induced angiogenic responses. NC, MTX
and LSG groups exhibited sparse and minimally branched vasculature,
consistent with normal skin lacking inflammatory stimulation
(Fig. 1B and C).
PASI scores were calculated to provide a qualitative
assessment of disease progression and therapeutic efficacy
(Figs. 1D and S4). Mice in the LSG + IMQ group showed
significantly lower PASI scores in all parameters compared with the
IMQ group, indicating a marked decrease in psoriatic severity.
There were significant differences between the LSG + IMQ and MTX +
IMQ groups. Collectively, the PASI data supported the protective
effect of LSG against IMQ-induced psoriatic skin inflammation.
Psoriatic mice treated with LSG
exhibit decreased keratinocyte proliferation and
differentiation
Histopathological analysis of dorsal skin was
performed to evaluate the effect of LSG in IMQ-induced psoriasis.
Mice in the IMQ group developed typical psoriasis-like changes,
including hyperkeratosis, parakeratosis and thickening of the
epidermal stratum spinosum, whereas the LSG + IMQ group showed
decreased psoriasis-like alterations (Fig. 2A). Quantitative analysis confirmed
that epidermal thickness was significantly decreased in the LSG +
IMQ group compared with the IMQ group (Fig. 2B). No significant difference in
epidermal thickness was observed between the LSG + IMQ and MTX +
IMQ groups. Although dermal thickness did not differ significantly
between the IMQ-treated groups, both the MTX + IMQ and LSG + IMQ
groups showed decreased dermal thickness compared with the IMQ
group (Fig. 2C).
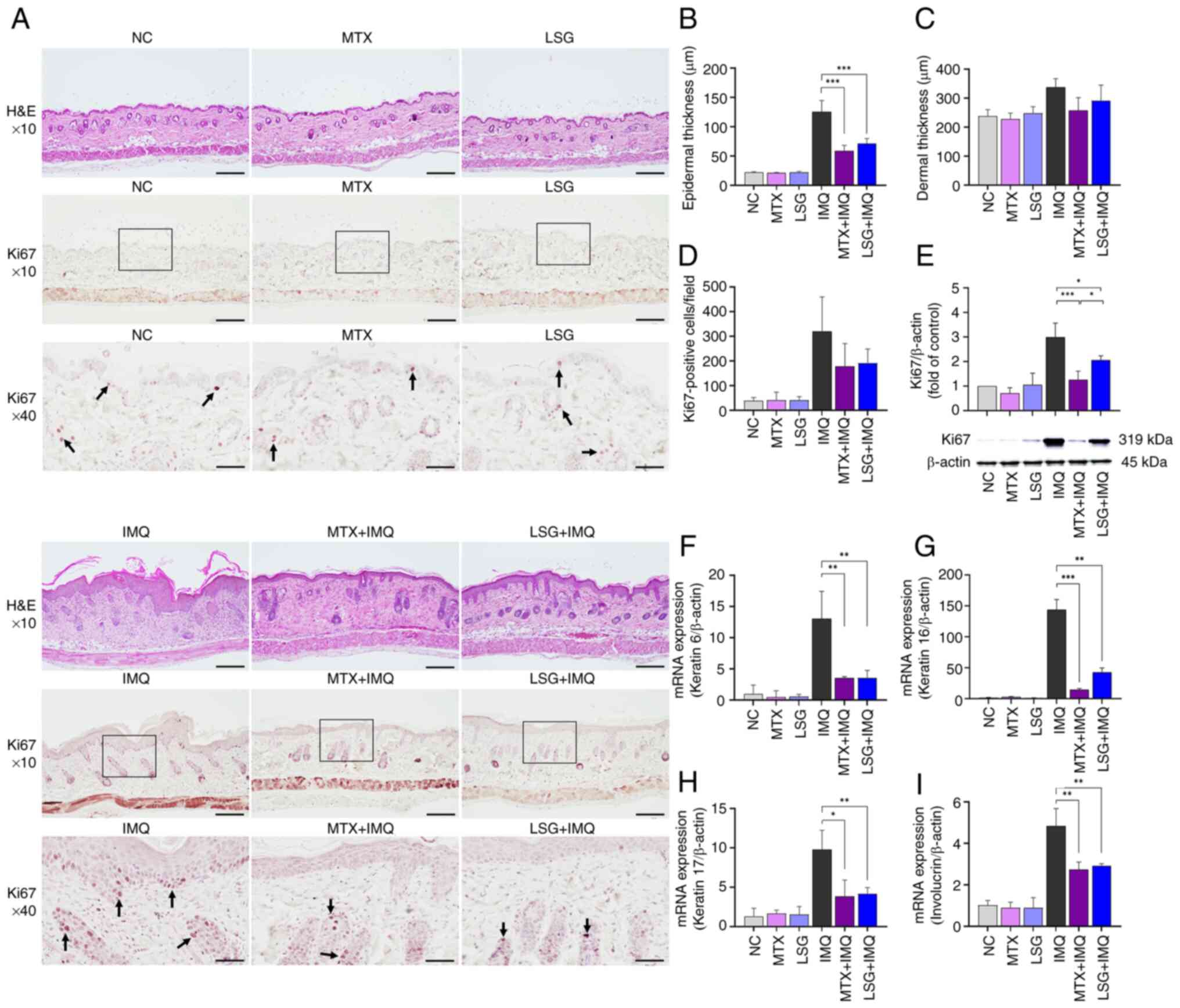 | Figure 2Effect of LSG on keratinocyte
proliferation and differentiation. (A) Representative
H&E-stained sections and Ki67 immunostaining of dorsal skin.
Arrows indicate Ki67-positive cells. Magnification, x10 (scale bar,
200 µm) and x40 (scale bar, 50 µm). Quantification of (B) epidermal
and (C) dermal thickness and (D) Ki67-positive cells (n=8). (E)
Ki67 protein expression assessed by western blot and quantified
relative to β-actin (n=3). Reverse transcription-quantitative PCR
analysis of keratinocyte differentiation markers keratin (F) 6, (G)
16 and (H) 17 and (I) involucrin (n=4). *P<0.05,
**P<0.01 and ***P<0.001. LSG, low
molecular weight sulfated galactan; H&E, Hematoxylin and Eosin;
NC, Normal control; MTX, Methotrexate; IMQ, Imiquimod. |
Epidermal thickening in psoriasis results from
increased keratinocyte proliferation, with Ki67 serving as a
proliferation marker (30).
Immunohistochemistry showed fewer Ki67-positive cells in the
epidermis of the MTX + IMQ and LSG + IMQ groups compared with the
IMQ group (Fig. 2A and D). Ki67 protein expression was
significantly decreased in the LSG + IMQ compared with the IMQ
group (Fig. 2E). To assess
keratinocyte differentiation, the expression of keratin 6, 16 and
17 and involucrin (established biomarkers of keratinocyte
differentiation) (7) was evaluated
by RT-qPCR. Expression levels of keratin 6, 16 and 17 were
significantly decreased in the LSG + IMQ compared with the IMQ
group. No significant differences were observed between the LSG +
IMQ and MTX + IMQ groups (Fig.
2F-H). Expression of involucrin, a structural protein involved
in psoriatic plaque formation, was also decreased in the LSG + IMQ
group compared with the IMQ group (Fig.
2I). Taken together, these findings indicated that LSG
treatment reduces keratinocyte proliferation and
differentiation.
LSG decreases cutaneous inflammation
in IMQ-induced psoriatic mice
Psoriasis is a T cell-mediated autoimmune skin
disease characterized by overexpression of proinflammatory
cytokines (31). TNF-α and IL-1β
serve key roles in regulating inflammation and promoting Th17 cell
development, which is central to the pathogenesis of psoriasis
(32). In addition, IL-23 activates
CD4+ T cells and promotes the release of IL-17A, which
drives keratinocyte proliferation in psoriasis (4). ELISA and western blot analyses were
performed to determine whether LSG modulates inflammatory cytokine
expression during psoriasis development. ELISA showed that levels
of TNF-α, IL-1β, IL-17A, and IL-23 were significantly decreased in
the skin lesions of the LSG + IMQ group compared with the IMQ
group. These effects were consistent with those observed in the MTX
+ IMQ group (Fig. 3A-H). Western
blotting further demonstrated that protein expression of TNF-α and
IL-6 proinflammatory cytokines was significantly decreased in the
LSG + IMQ compared with the IMQ group. By contrast, IL-10, an
anti-inflammatory cytokine, showed increased expression in the LSG
+ IMQ group (Fig. 3I-L). These
findings highlight the efficacy of LSG in attenuating cutaneous
inflammation in psoriatic mice.
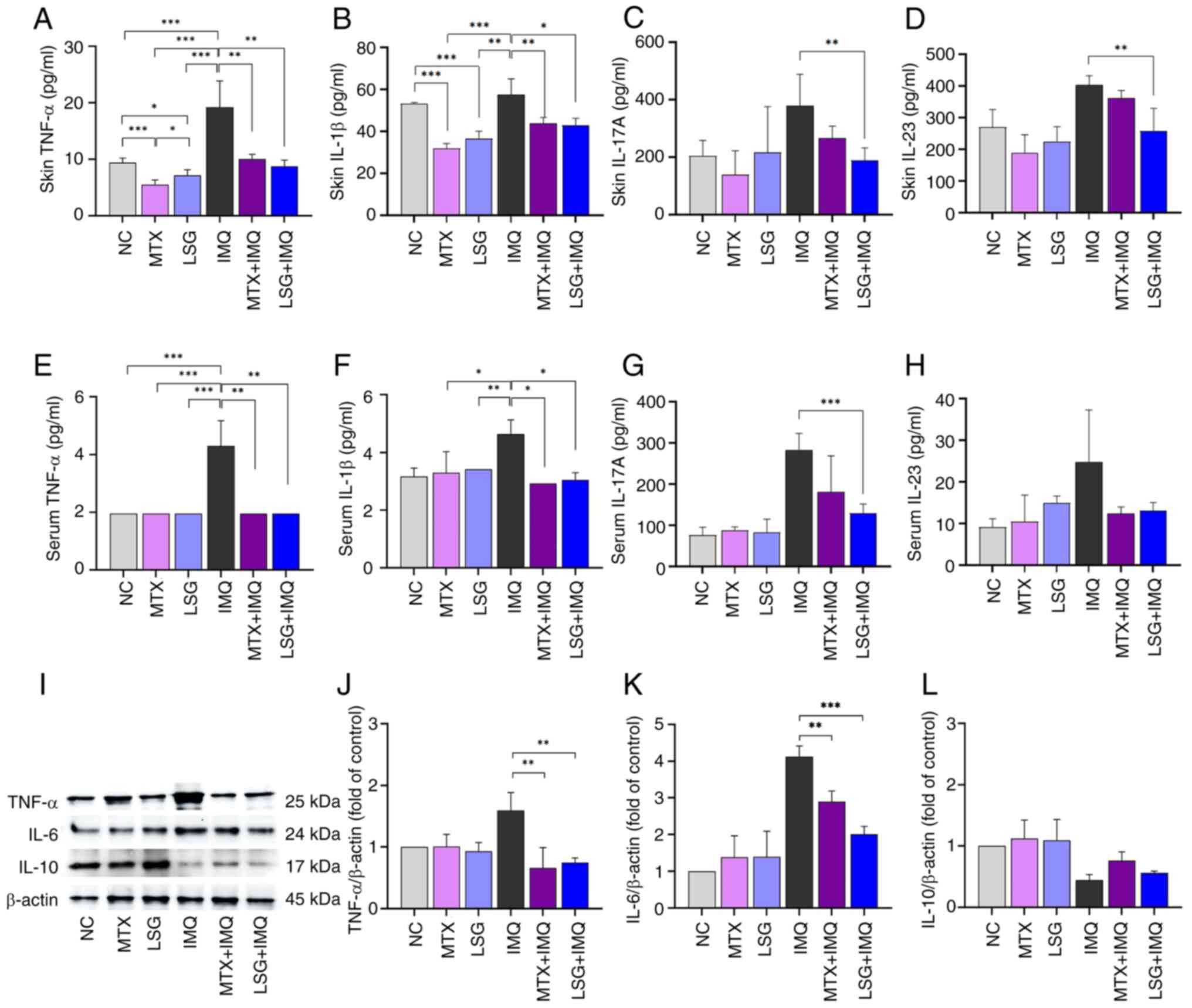 | Figure 3Effect of LSG on cutaneous
inflammation in IMQ-induced psoriatic mice. Cytokine levels in skin
tissue and blood serum were evaluated by ELISA and western blotting
(n=5). ELISA quantification of (A) TNF-α, (B) IL-1β, (C) IL-17A and
(D) IL-23 in skin tissue. Serum levels of (E) TNF-α, (F) IL-1β, (G)
IL-17A and (H) IL-23. (I) Protein expression of (J) TNF-α, (K) IL-6
and (L) IL-10 in skin tissue, normalized to β-actin.
*P<0.05, **P<0.01 and
***P<0.001. LSG, Low molecular weight sulfated
galactan; NC, Normal control; MTX, methotrexate; IMQ,
Imiquimod. |
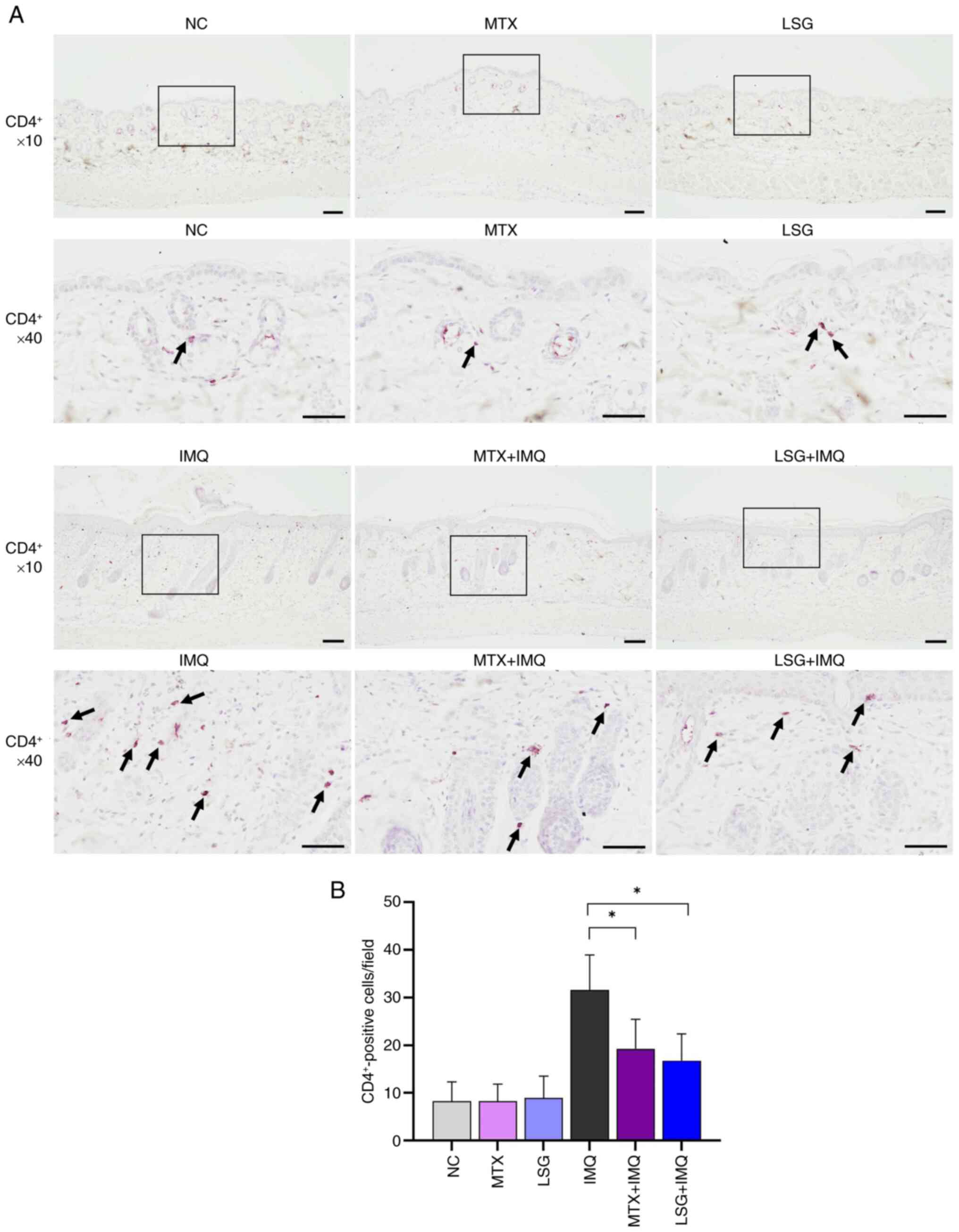
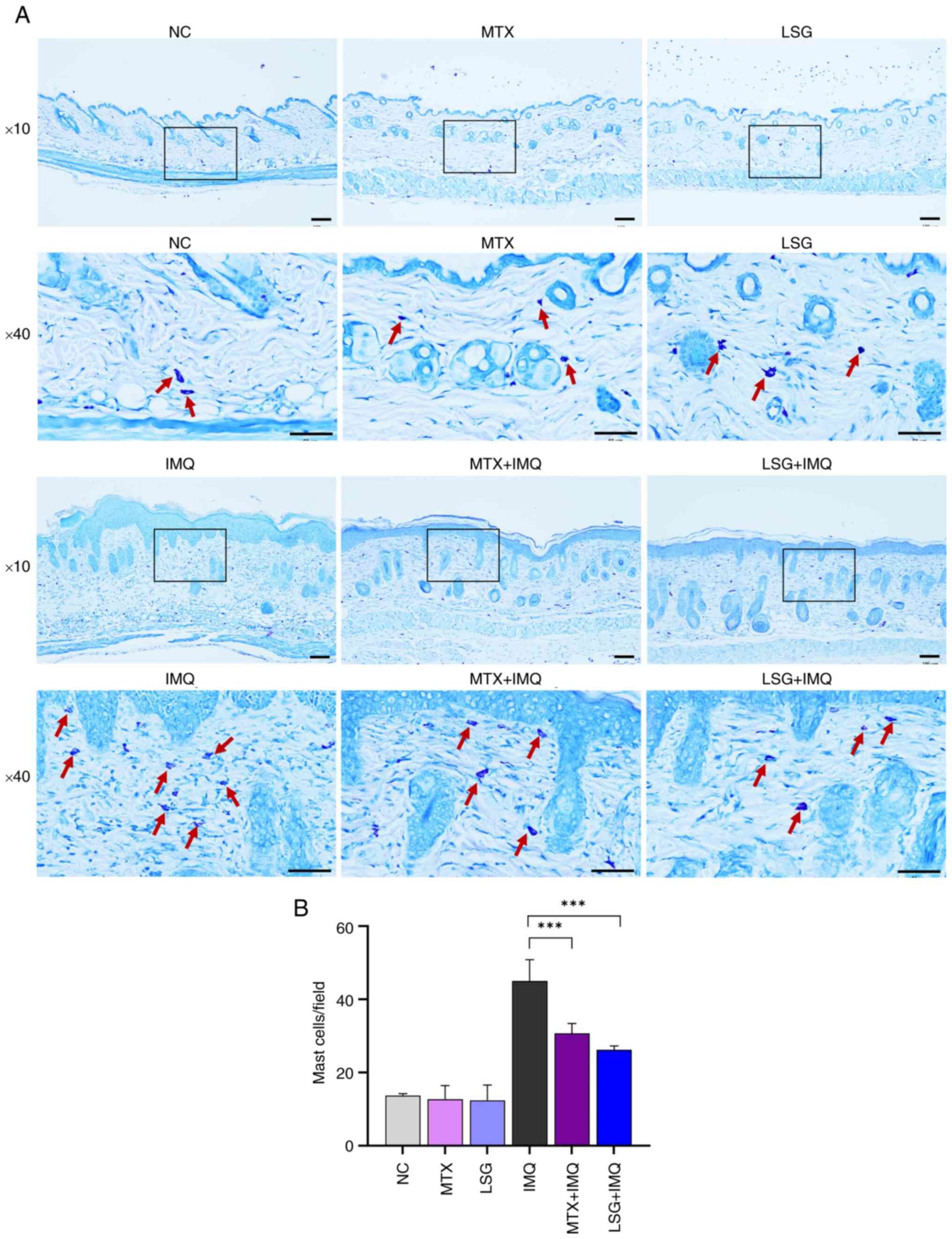
LSG decreases CD4+ and mast
cell recruitment in IMQ-induced psoriatic mice
By reducing levels of TNF-α, IL-1β, and IL-17A, LSG
decreased the recruitment of inflammatory Th17 cells. To assess the
effect of LSG on inflammatory cell infiltration in psoriatic skin
lesions, immunohistochemical staining for CD4+ cells was
performed. IMQ group demonstrated accumulation of CD4+
cells in psoriatic skin lesions. By contrast, CD4+ cell
infiltration was significantly decreased in both the MTX + IMQ and
LSG + IMQ groups compared with the IMQ group (Fig. 4A and B). Giemsa staining of skin sections
revealed that mast cell infiltration was significantly lower in the
MTX + IMQ and LSG + IMQ groups compared with the IMQ group
(Fig. 5A and B). These findings suggested that LSG
reduces inflammatory cell infiltration in psoriatic skin.
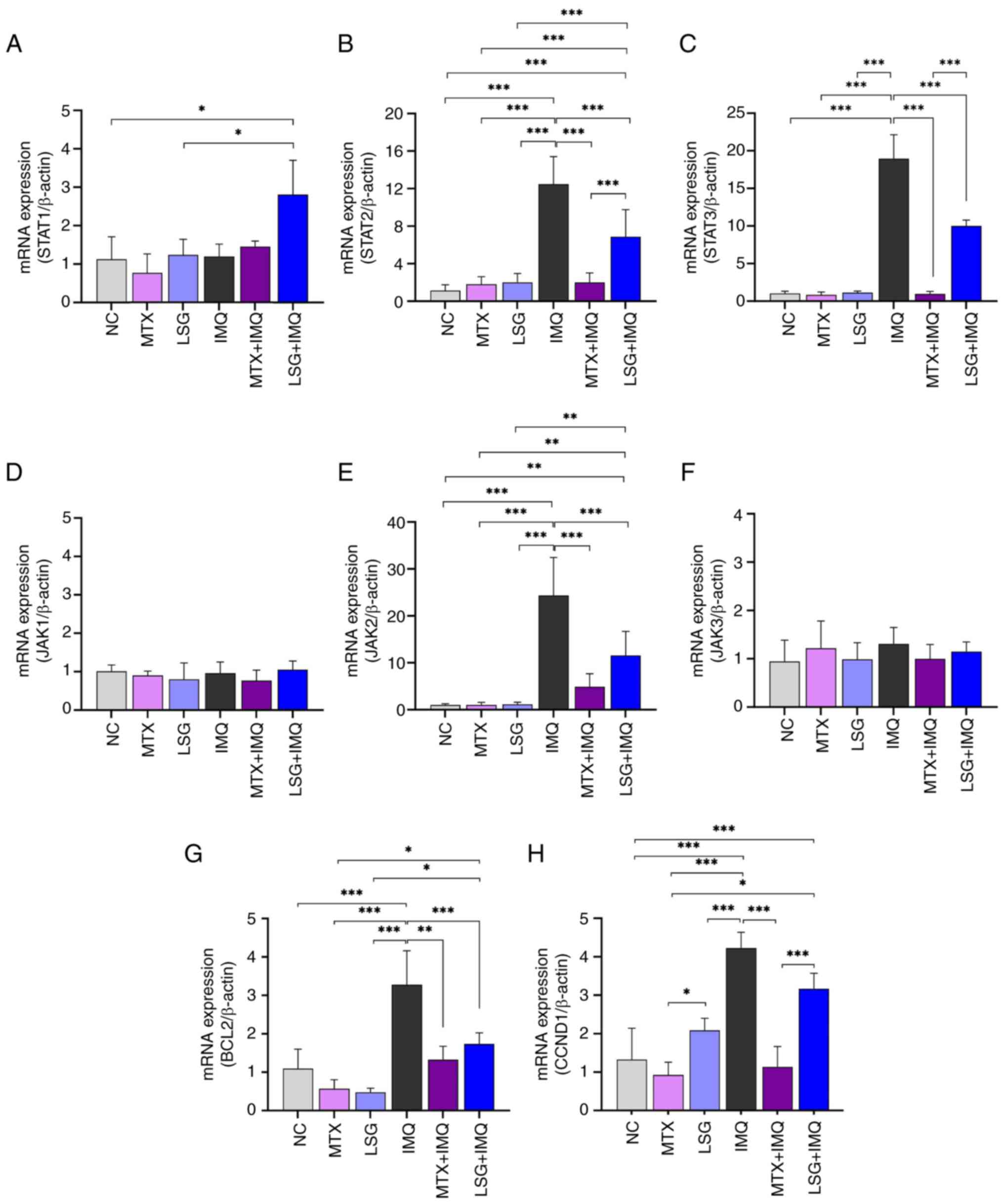
LSG alleviates inflammatory cytokine
production by inhibiting the JAK/STAT pathway in IMQ-induced
psoriatic mice
Proinflammatory cytokines are mediated by IL-17A and
IL-23 via activation of the JAK/STAT signaling pathway (5). The decreased expression of IL-17A and
IL-23 in the LSG + IMQ group may affect JAK/STAT pathway
activation. To investigate this, RT-qPCR was used to analyze
mRNA expression of JAK/STAT pathway components, demonstrating
downregulation of JAK2, STAT2 and STAT3 (Fig. 6A-F). Consistent with these findings,
expression of the JAK/STAT target genes BCL2 and CCND1 was also
decreased in the LSG + IMQ group to similar levels observed in the
MTX + IMQ group, compared with the IMQ group (Fig. 6G and H).
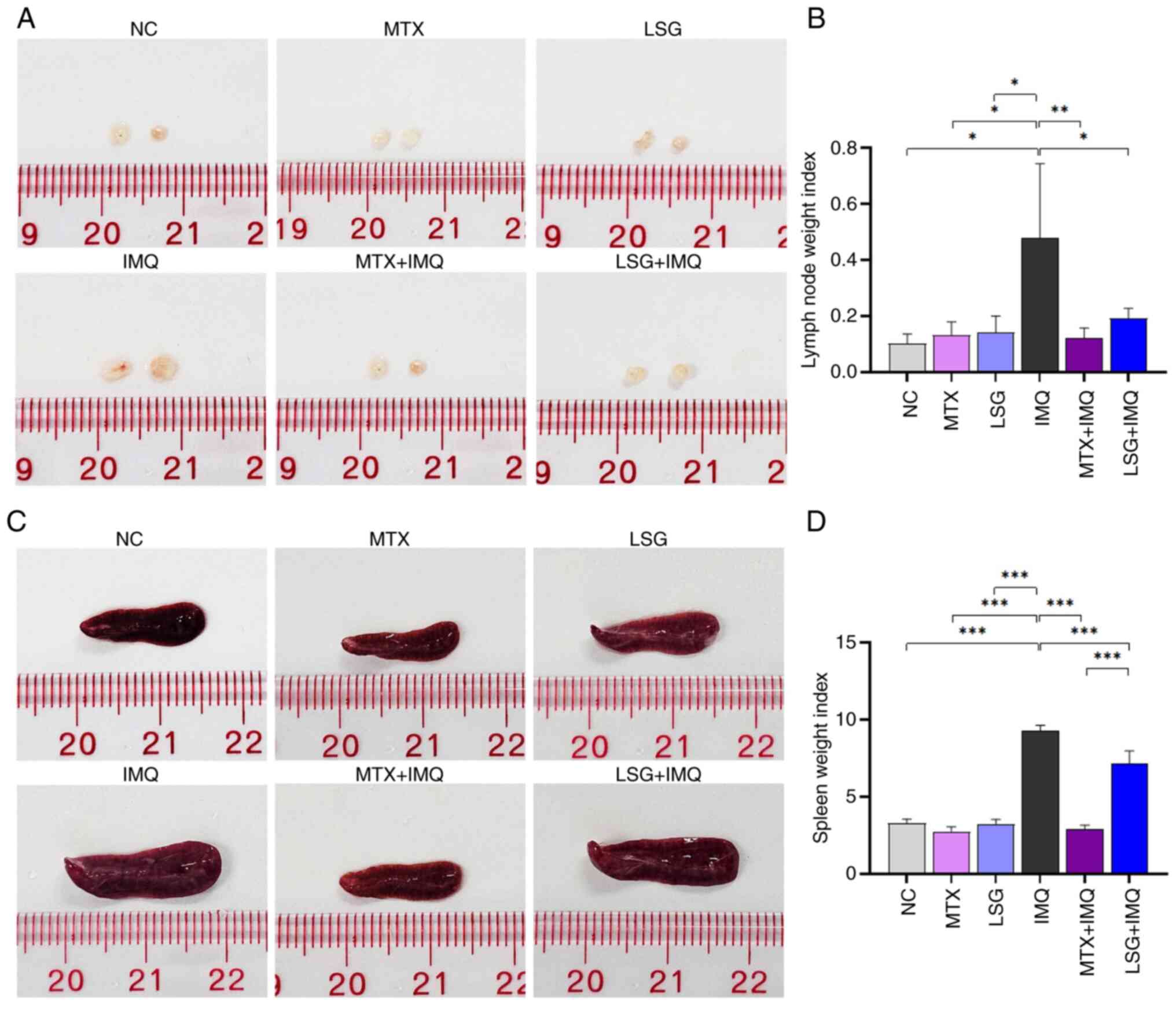
LSG suppresses IMQ-induced enlargement
of lymph node and spleen without causing toxicity to other vital
organs
Psoriasis, as an inflammatory disease, leads to
immune system activation and is associated with increased lymph
node and spleen weight (33). To
assess systemic inflammation, lymph nodes and spleens were
collected and weighed. IMQ treatment resulted in increased size and
weight of lymph nodes and spleens in the IMQ group compared with
controls (NC, MTX, and LSG; Fig.
7). By contrast, the LSG + IMQ group showed a significant
decrease in both lymph node (Fig.
7A and B) and spleen (Fig. 7C and D) size and weight compared with the IMQ
group. No significant differences were observed between the MTX +
IMQ and LSG + IMQ groups. These findings suggested LSG decreased
systemic inflammation by suppressing IMQ-induced organomegaly.
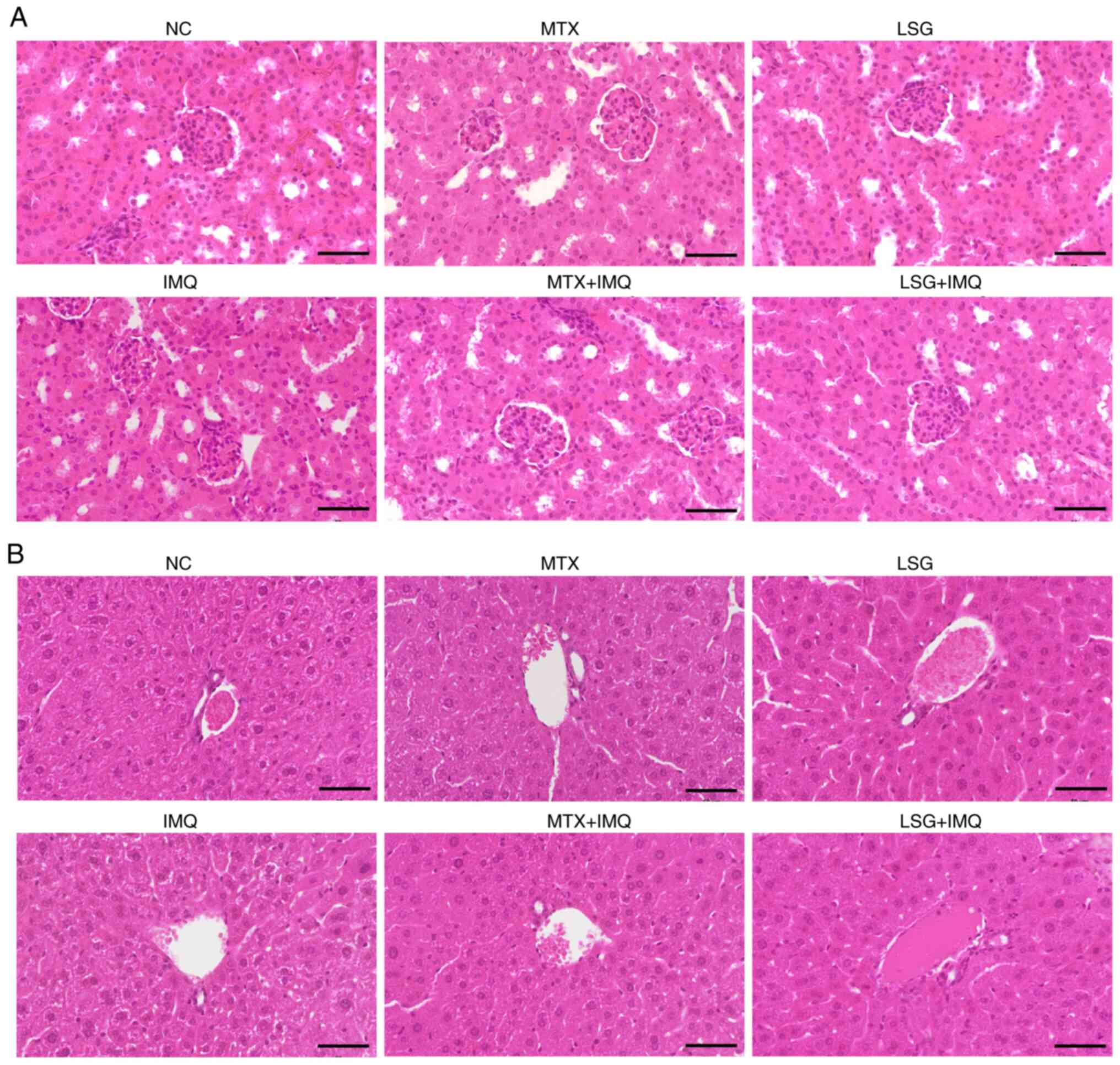
To evaluate the potential toxicity of LSG on major
internal organs, histopathological examination of the kidney and
liver was performed using H&E staining (Fig. 8). Representative micrographs of
renal (Fig. 8A) and hepatic tissue
(Fig. 8B) showed preserved normal
architecture without evidence of degeneration, necrosis,
inflammatory infiltration or vascular congestion. Renal histology
revealed intact glomeruli and renal tubules with no morphological
abnormalities across all groups, including those receiving MTX or
LSG with or without IMQ. Similarly, hepatic tissue exhibited normal
lobular architecture with well-preserved hepatocyte morphology and
no signs of hepatocellular damage. These findings suggested that
LSG at the administered dose did not induce detectable hepatic or
renal toxicity in IMQ-induced psoriatic mice, supporting its safety
for systemic use.
Discussion
There is increasing research on seaweed-derived SPs
for the treatment of numerous ailments (11,12),
particularly inflammatory conditions, due to their efficacy and
minimal side effects. Studies have shown that oligosaccharides
derived from G. fisheri modulate immune responses by
inhibiting proinflammatory cytokines such as TNF-α and IL-1β, key
mediators in psoriasis pathogenesis (13,34).
Furthermore, modification of SG to obtain LSG from G.
fisheri has been found to significantly enhance its biological
activity (15,18). To the best of our knowledge, the
present study is the first to provide in vivo evidence that
intraperitoneal administration of LSG exerts potent
anti-inflammatory and anti-psoriatic effects in an IMQ-induced
psoriasis-like mouse model. Administration methods include oral,
injectable and topical routes. Intraperitoneal administration
allows rapid absorption of large volumes of substances and is the
preferred injection route for non-irritant, isotonic solutions
(35). Numerous studies have
investigated the intraperitoneal injection of polysaccharides for
their anti-inflammatory and anti-psoriatic effects (23,36,37).
For example, psoriatic mice that received β-glucan
intraperitoneally show decreased psoriatic arthritis-like symptoms
(23). In another study, mice
treated with intraperitoneal acitretin-dextran nanoparticles for
six consecutive days exhibited amelioration of psoriasis-like skin
disease (36). Additionally, the
intraperitoneal injection of fucoidans at doses of 10, 50 and 250
mg/kg significantly reduced inflammation induced by sodium
carboxymethyl cellulose in mice (37).
The IMQ-induced psoriasis animal model is used as it
replicates key features of human psoriatic lesions, including
epidermal thickening, erythema, scaling, vascular proliferation and
infiltration of T and other immune cells (38). The PASI score is a standard tool for
evaluating disease severity and treatment response, with a
reduction >50% generally considered a significant improvement
(39). Administration of LSG
significantly decreased erythema, scaling and skin thickening, as
reflected by decreased PASI scores throughout the treatment period,
indicating its anti-psoriatic effect. An additional factor that may
have influenced lesion severity was hair regrowth during IMQ
application. New hair growth appeared earlier in certain treatment
groups, particularly in IMQ group, which decreased the effective
skin surface area directly exposed to IMQ. Nevertheless, the
overall amount of IMQ applied and the treated area remained
unchanged; some of the compound may have initially adhered to the
hair shafts. However, as the hair was relatively short, the drug
was still able to permeate the skin over time. This was supported
by the observation that the mice exhibited itching and scratching
in these regions, similar to the areas without hair. However, to
maintain a fully exposed dorsal skin surface for photographic
observation, mice in control groups were re-shaved mid-protocol,
whereas IMQ-treated groups were not re-shaved to avoid additional
irritation. Hair regrowth in treated groups may also indicate a
biological effect of the intervention. IMQ is associated with the
activation of Th17 cells, leading to skin inflammation (40), while at the same time Th17-mediated
mechanisms have been implicated in alopecia areata (41). The earlier reappearance of hair in
treatment groups compared with untreated controls suggests that the
treatment may have alleviated local inflammation and restored a
skin microenvironment permissive for hair growth. Although hair
regrowth was not quantitatively assessed, this supports the
hypothesis that the therapeutic regimens not only attenuate
psoriasiform inflammation but may also promote recovery of skin
homeostasis. Future studies incorporating both surface area control
and systematic monitoring of hair regrowth may clarify the dual
role of this phenomenon. Histopathological and immunohistochemical
staining (Ki67 and CD4+) supported these findings,
showing attenuated epidermal hyperplasia, normalized epidermal
architecture and decreased vascular development and infiltration of
inflammatory cells, including CD4+ T and mast cells in
the dermis (27). In psoriatic
lesions, CD4+ T cells are typically concentrated in the
upper dermis (31). Mast cells also
serve a key role in the development and progression of psoriasis,
with increased infiltration contributing to heightened inflammation
(42). The decrease in dermal
CD4+ T and mast cells following LSG treatment suggested
LSG may help inhibit disease progression.
Ki67 is a protein used as a marker of cell
proliferation, particularly to assess keratinocyte activity in skin
conditions (43). In healthy skin,
Ki67 expression is typically low but increases in conditions
involving active keratinocyte division, such as wound healing or
inflammatory skin disorders such as psoriasis (44). Previous studies have shown that Ki67
upregulation in psoriatic epidermis is decreased following
treatment with acitretin-conjugated dextran, MTX and oxymatrine
(36,45). Consistent with these findings, the
present results demonstrated that LSG significantly reduced both
the number of Ki67-positive cells and overall Ki67 expression in
IMQ-induced psoriatic mice. These effects were comparable with
those observed with MTX, a standard anti-psoriatic agent.
Additional biomarkers associated with keratinocyte proliferation
and differentiation in psoriasis include keratin 6, 16 and 17 and
involucrin (7). In the present
study, IMQ-induced upregulation of keratin 6, 16 and 17 and
involucrin was significantly decreased following LSG treatment.
This aligns with previous work demonstrating that
tryptophol-containing emulgel, a topical drug delivery system that
combines the properties of an emulsion and a gel, ameliorates
IMQ-induced psoriasis in mice by reducing the expression of these
markers (27).
The pathogenesis and progression of psoriasis
involve the activation of immune cells, including T and
antigen-presenting cells. Among these, Th cells, particularly Th1
and Th17 subsets, serve a key role in driving the immunological
alterations characteristic of the disease (46). Activation of Th1 and Th17 cells
leads to the aberrant production of proinflammatory cytokines such
as TNF-α, IL-1β, IL-17A, IL-23 and IL-6, all of which are key
mediators in psoriasis (6). In
IMQ-induced psoriatic mice, LSG exhibited immunomodulatory effects
by decreasing both systemic and cutaneous levels of TNF-α, IL-1β,
IL-17A, IL-23 and IL-6 (a key cytokine in psoriasis pathogenesis)
(3,8). These findings suggest that LSG
suppresses inflammatory cytokine expression under psoriatic
conditions. Mechanistically, the present findings indicated that
LSG administration interferes with key signaling pathways involved
in psoriasis. RT-qPCR revealed that LSG significantly decreased
mRNA expression of JAK2, STAT2 and STAT3, along with downregulation
of downstream targets BCL2 and CCND1 in psoriatic skin. The
decreased dermal expression of BCL2 suggests inhibition of the
JAK2/STAT3 pathway (27). The
downregulation of JAK2, STAT2 and STAT3 mRNA following LSG
administration may be a consequence of upstream cytokine
suppression, particularly IL-23 and IL-17A, which drive JAK/STAT
activation. While direct inhibition of JAK/STAT signaling by LSG
cannot be excluded, evidence suggests an indirect mechanism
mediated by decreased proinflammatory cytokine levels (47). Future mechanistic studies, including
kinase activity assays, are warranted to determine whether LSG
directly interacts with JAK/STAT components. This pathway serves a
critical role in IL-23-mediated STAT3 activation, which promotes
Th17 cell differentiation and subsequent IL-17A production,
contributing to psoriatic inflammation and keratinocyte dysfunction
(4). Although NF-κB activity was
not directly assessed in the present study, its role as a master
regulator of psoriatic inflammation is well established (4,48).
Notably, SPs from other marine sources inhibit NF-κB activation
(49), suggesting that LSG may
exert similar effects.
Psoriasis, as an inflammatory disease, leads to
immune system activation and is commonly associated with
organomegaly, manifested by increased lymph node and spleen weight,
which serves as an indicator of disease severity (33). LSG in IMQ-induced psoriatic mice
significantly decreased lymph node and spleen size and weight
compared with untreated psoriatic mice, suggesting LSG alleviated
disease progression driven by immune activation (50). Additionally, no signs of hepatic or
renal toxicity were observed in LSG-treated mice during the 7-day
treatment period, as assessed by histopathological analysis. Serum
biochemical markers for renal and hepatic function (alanine
aminotransferase, aspartate transaminase; AST, creatinine) were not
measured due to limited serum availability, as the samples were
primarily used for inflammatory cytokine analysis. Therefore,
potential subclinical organ toxicity cannot be fully excluded,
although histological analysis did not reveal any renal or hepatic
damage. However, in a previous study, intraperitoneal
administration of SP from Caulerpa cupressoides var.
lycopodium at a concentration of 9 mg/kg for 14 days
produced no signs of systemic toxicity in mice (51). Thus, short-term LSG is unlikely to
induce systemic toxicity, but longer-term studies and comprehensive
toxicological evaluations are required to confirm the absence of
cumulative or delayed adverse effects. This contrasts with
conventional treatments such as MTX and cyclosporine, which are
associated with cumulative toxicity and systemic side effects
(9).
LSG promotes wound healing by promoting fibroblast
proliferation, collagen synthesis and re-epithelialization
(52). These regenerative effects,
combined with its anti-inflammatory properties, suggest LSG may be
effective in managing inflammatory skin conditions such as
psoriasis. Additionally, LSG derived from G. fisheri
exhibits strong free radical scavenging activity and enhances
antioxidant capacity via activation of the nuclear factor erythroid
2-related factor 2/antioxidant response element signaling pathway,
indicating broader therapeutic potential beyond dermatological
applications (15). Previous
studies on G. fisheri derived SG have primarily focused on
wound-healing models (18,52). An octanoyl-esterified SG accelerates
wound closure in rats, improves collagen deposition, increases
α-smooth muscle actin (SMA) and vimentin expression and decreases
expression of TNF-α at the wound site. Likewise, our recent study
reported that a SG derivative improves histopathology and modulates
wound healing proteins such as Ki67, α-SMA, E-cadherin and vimentin
in a rat excision wound model (52). These findings highlight the
regenerative and anti-fibrotic potential of G.
fisheri-derived polysaccharides in tissue repair. To the best
of our knowledge, the present study is the first to demonstrate
that LSG from G. fisheri attenuates IMQ-induced psoriasis,
an immune-mediated dermatosis characterized by keratinocyte
hyperproliferation and Th17-driven inflammation. This extends the
therapeutic scope of G. fisheri derived SG beyond wound
repair to the modulation of immune-driven skin inflammation.
Bioactivity of Gracilaria-derived polysaccharides is
influenced by molecular weight and degree of sulfation, with lower
molecular weight fractions exhibiting anti-inflammatory effects
(53); consistent with this, the
LSG preparation used in the present study displayed potent
immunomodulatory effects in vivo. In comparison with
standard therapies such as cyclosporine and acitretin, MTX exerts
anti-proliferative and immunosuppressive effects primarily via the
inhibition of dihydrofolate reductase (54), whereas LSG acts via
immunomodulation, downregulation of inflammatory cytokines and
suppression of JAK/STAT signaling. Unlike MTX, which can cause
cumulative hepatotoxicity and bone marrow suppression with
long-term use, LSG demonstrated no detectable hepatic or renal
toxicity over the 7-day treatment period. From a pharmacological
perspective, LSG represents a class of marine-derived
polysaccharides with potent bioactivity and a favorable safety
profile, making it a promising candidate for further drug
development (12). Collectively,
these findings suggest that LSG from G. fisheri exerts
strong anti-psoriatic effects by inhibiting key inflammatory
pathways and decreasing keratinocyte hyperproliferation and
abnormal differentiation. However, the optimal delivery route,
long-term efficacy and large-scale production feasibility remain to
be further investigated.
In conclusion, the present study demonstrated the
efficacy of LSG derived from G. fisheri in alleviating
IMQ-induced psoriasis by decreasing psoriatic skin lesions and
attenuating inflammatory responses. These effects were evidenced by
decreased production of proinflammatory cytokines and infiltration
of inflammatory cells in both skin lesions and systemic tissue. In
addition, LSG treatment downregulated mRNA expression of key
components of the JAK/STAT signaling pathway. Although the precise
molecular mechanisms underlying its therapeutic effects remain to
be elucidated, the immunomodulatory and anti-inflammatory
properties suggest that LSG has potential as a marine-derived
therapeutic agent for psoriasis management. Further studies are
warranted to clarify the specific signaling pathways involved and
validate its clinical applicability in human models.
Supplementary Material
Schematic diagram showing the
experimental timeline. PC, Petroleum cream; NS, Normal saline; NC,
Normal control; MTX, Methotrexate; LSG, Low molecular weight
sulfated galactan; IP, Intraperitoneal; IMQ, Imiquimod.
GPC, FTIR and NMR profiles of LSG from
Gracilaria fisheri. GPC, gel permeation chromatography;
FTIR, Fourier-transform infrared; NMR, nuclear magnetic resonance;
LSG, Low molecular weight sulfated galactan.
Effect of LSG on psoriasis-like skin
in IMQ-induced psoriatic mice. Representative dorsal skin on days
1, 3, 5, and 7. LSG treatment visibly decreased the severity of
skin lesions from day 5 to 7. LSG, Low molecular weight sulfated
galactan; IMQ, Imiquimod; NC, Normal control; MTX,
Methotrexate.
Effect of LSG on IMQ-induced psoriasis
severity and body weight in mice. Daily evaluation of (A) erythema,
(B) scaling and (C) skin thickening using a modified human PASI
scoring system. (D) Cumulative PASI score. (E) Body weight. LSG,
Low molecular weight sulfated galactan; IMQ, Imiquimod; NC, Normal
control; PASI, Psoriasis area and severity index; MTX,
Methotrexate.
Acknowledgements
The authors would like to thank the Laboratory
Animal Research Center, University of Phayao, for providing the
animal facility, and the School of Medical Sciences, University of
Phayao, Phayao, Thailand for access to the laboratory facility. The
authors would also to thank Dr Dylan Southard for language editing
of the manuscript via the Khon Kaen University Publication Clinic
(Khon Kaen, Thailand).
Funding
Funding: The present study was supported by the University of
Phayao and the Thailand Science Research and Innovation Fund
(Fundamental Fund 2024; grant no. 256/2567) and the Faculty of
Medicine, Khon Kaen University, Thailand (grant no. IN67066).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJ, AP and TR conceived and designed the study. KJ,
AP, LP, SJ, TC, PP and TR performed the experiments. KJ, AP, KW and
TR analyzed data. KJ, AP and TR wrote the manuscript. KJ, AP, KW,
and TR edited the manuscript. KW and TR supervised the study. KJ,
AP and TR confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the University of Phayao, Phayao, Thailand in
accordance with the Ethics of Animal Experimentation guidelines
established by the National Research Council (approval no.
1-024-65).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moon CI, Lee J, Baek YS and Lee O:
Psoriasis severity classification based on adaptive multi-scale
features for multi-severity disease. Sci Rep.
13(17331)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rendon A and Schäkel K: Psoriasis
pathogenesis and treatment. Int J Mol Sci. 20(1475)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Griffiths CEM, Armstrong AW, Gudjonsson JE
and Barker JNWN: Psoriasis. Lancet. 397:1301–1315. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bugaut H and Aractingi S: Major role of
the IL17/23 axis in psoriasis supports the development of new
targeted therapies. Front Immunol. 12(621956)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xue Y, Zhang L, Chu L, Song Z, Zhang B, Su
X, Liu W and Li X: JAK2/STAT3 pathway inhibition by AG490
ameliorates experimental autoimmune encephalomyelitis via
regulation of Th17 cells and autophagy. Neuroscience. 552:65–75.
2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dutta S, Chawla S and Kumar S: Psoriasis:
A review of existing therapies and recent advances in treatment. J
Rational Pharmacother Res. 4:12–23. 2018.
|
|
7
|
Qiao P, Zhi D, Yu C, Zhang C, Wu K, Fang
H, Shao S, Yin W, Dang E, Li K and Wang G: Activation of the C3a
anaphylatoxin receptor inhibits keratinocyte proliferation by
regulating keratin 6, keratin 16, and keratin 17 in psoriasis.
FASEB J. 36(e22322)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lowes MA, Bowcock AM and Krueger JG:
Pathogenesis and therapy of psoriasis. Nature. 445:866–873.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Balak DMW, Gerdes S, Parodi A and
Salgado-Boquete L: Long-term safety of oral systemic therapies for
psoriasis: A comprehensive review of the literature. Dermatol Ther
(Heidelb). 10:589–613. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Woo YR, Cho DH and Park HJ: Molecular
mechanisms and management of a cutaneous inflammatory disorder:
Psoriasis. Int J Mol Sci. 18(2684)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar A, Soratur A, Kumar S and Maran BAV:
A review of marine algae as a sustainable source of antiviral and
anticancer compounds. Macromol. 5(11)2025.
|
|
12
|
B J and R R: A critical review on
pharmacological properties of sulfated polysaccharides from marine
macroalgae. Carbohydr Polym. 344(122488)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Siringoringo B, Huipao N, Tipbunjong C,
Nopparat J, Wichienchot S, Hutapea AM and Khuituan P: Gracilaria
fisheri oligosaccharides ameliorate inflammation and colonic
epithelial barrier dysfunction in mice with acetic acid-induced
colitis. Asian Pac J Trop Biomed. 11:440–449. 2021.
|
|
14
|
Kang J, Jia X, Wang N, Xiao M, Song S, Wu
S, Li Z, Wang S, Cui SW and Guo Q: Insights into the
structure-bioactivity relationships of marine sulfated
polysaccharides: A review. Food Hydrocoll. 123(107049)2021.
|
|
15
|
Rudtanatip T, Pariwatthanakun C, Somintara
S, Sakaew W and Wongprasert K: Structural characterization,
antioxidant activity, and protective effect against hydrogen
peroxide-induced oxidative stress of chemically degraded
Gracilaria fisheri sulfated galactans. Int J Biol Macromol.
206:51–63. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen YF, Zheng JJ, Qu C, Xiao Y, Li FF,
Jin QX, Li HH, Meng FP, Jin GH and Jin D: Inonotus obliquus
polysaccharide ameliorates dextran sulphate sodium induced colitis
involving modulation of Th1/Th2 and Th17/Treg balance. Artif Cells
Nanomed Biotechnol. 47:757–766. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pai SK, Chakraborty K, Pai AA and Dhara S:
Therapeutic promise of a sulfated (1→4) galactan from edible sea
grape Caulerpa racemosa: Modulation of cytokine expression in
lipopolysaccharide-induced CALU-1 cells. Int J Biol Macromol.
313(144117)2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rudtanatip T, Somintara S, Sakaew W,
El-Abid J, Cano ME, Jongsomchai K, Wongprasert K and Kovensky J:
Sulfated galactans from Gracilaria fisheri with
supplementation of octanoyl promote wound healing activity in vitro
and in vivo. Macromol Biosci. 22(2200172)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wongprasert K, Rudtanatip T and Praiboon
J: Immunostimulatory activity of sulfated galactans isolated from
the red seaweed Gracilaria fisheri and development of
resistance against white spot syndrome virus (WSSV) in shrimp. Fish
Shellfish Immunol. 36:52–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Karamani C, Antoniadou IT, Dimou A,
Andreou E, Kostakis G, Sideri A, Vitsos A, Gkavanozi A, Sfiniadakis
I, Skaltsa H, et al: Optimization of psoriasis mouse models. J
Pharmacol Toxicol Methods. 108(107054)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Neu SD, Strzepa A, Martin D, Sorci-Thomas
MG, Pritchard KA Jr and Dittel BN: Myeloperoxidase inhibition
ameliorates plaque psoriasis in mice. Antioxidants (Basel).
10(1338)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang A, Ma X, Wei F, Li Y, Liu Q and Zhang
H: Evidence on the therapeutic role of thiolutin in
imiquimod-induced psoriasis-like skin inflammation in mice. Immun
Inflamm Dis. 11(e877)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fahlquist-Hagert C, Sareila O, Rosendahl S
and Holmdahl R: Variants of beta-glucan polysaccharides
downregulate autoimmune inflammation. Commun Biol.
5(449)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
dos Santos Ferreira J, da Silva Prudêncio
R, de Sousa AK, Sousa SG, de Sousa de Lima FM, dos Santos Carvalho
A, da Costa ACC, Silva DMM, da Graça Sales Furtado M, Rocha DML, et
al: Sulfated polysaccharide extracted from the alga Gracilaria
domingensis modified with propionic anhydride negatively
modulates acute inflammation and experimental hypernociception.
Bioact Carbohydr Diet Fibre. 32(100459)2024.
|
|
25
|
van der Fits L, Mourits S, Voerman JSA,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lindford A, Juteau S, Jaks V, Klaas M,
Lagus H, Vuola J and Kankuri E: Case report: Unravelling the
mysterious Lichtenberg figure skin response in a patient with a
high-voltage electrical injury. Front Med (Lausanne).
8(663807)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pudgerd A, Jongsomchai K, Muangkaew W,
Phuapittayalert L, Jamsuwan S, Chanmanee T, Vanichviriyakit R and
Sukphopetch P: A tryptophol-containing emulgel ameliorates
imiquimod-induced mice psoriasis. Sci Rep. 15(19398)2025.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aratwar A, Maji I, Chilvery S, Mahajan S,
Aalhate M, Gupta U, Godugu C and Singh PK: Contemplating novel W/O
emulsion-based gel for anti-psoriatic activity of Tofacitinib in
imiquimod-induced Balb/C mice model. AAPS PharmSciTech.
26(12)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sezer E, Böer-Auer A, Cetin E, Tokat F,
Durmaz E, Sahin S and Ince U: Diagnostic utility of Ki-67 and
Cyclin D1 immunostaining in differentiation of psoriasis vs other
psoriasiform dermatitis. Dermatol Pract Concept. 5:7–13.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang P, Su Y, Li S, Chen H, Wu R and Wu
H: The roles of T cells in psoriasis. Front Immunol.
14(1081256)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li L, Lu J, Liu J, Wu J, Zhang X, Meng Y,
Wu X, Tai Z, Zhu Q and Chen Z: Immune cells in the epithelial
immune microenvironment of psoriasis: Emerging therapeutic targets.
Front Immunol. 14(1340677)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hatem S and El-Kayal M: Novel
anti-psoriatic nanostructured lipid carriers for the cutaneous
delivery of luteolin: A comprehensive in-vitro and in-vivo
evaluation. Eur J Pharm Sci. 191(106612)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Khongthong S, Theapparat Y, Roekngam N,
Tantisuwanno C, Otto M and Piewngam P: Characterization and
immunomodulatory activity of sulfated galactan from the red seaweed
Gracilaria fisheri. Int J Biol Macromol. 189:705–714.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lambert LJ, Muzumdar MD, Rideout WM III
and Jacks T: Chapter 15-Basic mouse methods for clinician
researchers: Harnessing the mouse for biomedical research. In:
Basic Science Methods for Clinical Researchers. Academic Press,
pp291-312, 2017.
|
|
36
|
Lan J, Li Y, Wen J, Chen Y, Yang J, Zhao
L, Xia Y, Du H, Tao J, Li Y and Zhu J: Acitretin-conjugated dextran
nanoparticles ameliorate psoriasis-like skin disease at low
dosages. Front Bioeng Biotechnol. 9(816757)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Manggau M, Kasim S, Fitri N, Aulia S,
Agustiani AN, Raihan M and Nurdin WB: Antioxidant,
anti-inflammatory and anticoagulant activities of sulfate
polysaccharide isolate from brown alga Sargassum polycystum. IOP
Conf Ser Earth Environ Sci. 967(012029)2022.
|
|
38
|
Tripathi D, Srivastava M, Rathour K, Rai
AK, Wal P, Sahoo J, Tiwari KR and Pandey P: A promising approach of
dermal targeting of antipsoriatic drugs via engineered nanocarriers
drug delivery systems for tackling psoriasis. Drug Metab Bioanal
Lett. 16:89–104. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dawoud ADH and Abdalbagi M: Evaluation of
anti-psoriatic activity of Aloe sinkatana extract on imiquimod
induced psoriatic-like dermatitis in mice. Pharm Biomed Res.
11:41–48. 2025.
|
|
40
|
Cho Y, Kwon J and Kim TS: Imiquimod
promotes Th1 and Th17 responses via NF-κB-driven IL-12 and IL-6
production in an in vitro co-culture model. Exp Ther Med.
30(175)2025.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Minokawa Y, Sawada Y and Nakamura M:
Lifestyle factors involved in the pathogenesis of alopecia areata.
Int J Mol Sci. 23(1038)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhou XY, Chen K and Zhang JA: Mast cells
as important regulators in the development of psoriasis. Front
Immunol. 13(1022986)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Assawawongkasem N, Techangamsuwan S,
Piyaviriyakul P, Puchadapirom P and Sailasuta A: Involucrin,
cytokeratin 10 and Ki67 expression in a threedimensional cultured
canine keratinocyte cell line in comparison to canine skin and
cutaneous squamous cell carcinoma and a pilot study on
custom-designed siRNA-INV transfection. Thai J Vet Med. 50:43–55.
2020.
|
|
44
|
Ramezani M, Shamshiri A, Zavattaro E,
Khazaei S, Rezaei M, Mahmoodi R and Sadeghi M: Immunohistochemical
expression of P53, Ki-67, and CD34 in psoriasis and psoriasiform
dermatitis. Biomedicine (Taipei). 9(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shi HJ, Zhou H, Ma AL, Wang L, Gao Q,
Zhang N, Song HB, Bo KP and Ma W: Oxymatrine therapy inhibited
epidermal cell proliferation and apoptosis in severe plaque
psoriasis. Br J Dermatol. 181:1028–1037. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Armstrong AW and Read C: Pathophysiology,
clinical presentation, and treatment of psoriasis: A review. JAMA.
323:1945–1960. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fu S, Bao X, Mao Z, Lv Y, Zhu B, Chen Y,
Zhou M, Tain S, Zhou F and Ding Z: Tetrastigma hemsleyanum
polysaccharide ameliorates cytokine storm syndrome via the
IFN-γ-JAK2/STAT pathway. Int J Biol Macromol.
275(133427)2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Blauvelt A and Chiricozzi A: The
immunologic role of IL-17 in psoriasis and psoriatic arthritis
pathogenesis. Clin Rev Allergy Immunol. 55:379–390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jayawardhana HHACK, Lee HG, Liyanage NM,
Nagahawatta DP, Ryu B and Jeon YJ: Structural characterization and
anti-inflammatory potential of sulfated polysaccharides from
Scytosiphon lomentaria; attenuate inflammatory signaling pathways.
J Funct Foods. 102(105446)2023.
|
|
50
|
Takuathung MN, Wongnoppavich A, Panthong
A, Khonsung P, Chiranthanut N, Soonthornchareonnon N and
Sireeratawong S: Antipsoriatic effects of Wannachawee recipe on
imiquimod-induced psoriasis-like dermatitis in BALB/c mice. Evid
Based Complement Alternat Med. 2018(7931031)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rodrigues JAG, Vanderlei ESO, de Araújo
IWF, Quinderé ALG, Coura CO and Benevides NMB: In vivo
toxicological evaluation of crude sulfated polysaccharide from the
green seaweed Caulerpa cupressoides var. lycopodium
in Swiss mice. Acta Sci Technol. 35:603–610. 2013.
|
|
52
|
Jongsomchai K, Pudgerd A, Sakaew W,
Wongprasert K, Kovensky J and Rudtanatip T: Sulfated galactan
derivative from Gracilaria fisheri improves histopathology
and alters wound healing-related proteins in the skin of excision
rats. Front Biosci (Landmark Ed). 29(388)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Khandwal D, Patel S, Pandey AK and Mishra
A: A comprehensive, analytical narrative review of polysaccharides
from the red seaweed Gracilaria: Pharmaceutical applications
and mechanistic insights for human health. Nutrients.
17(744)2025.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Johnston A, Gudjonsson JE, Sigmundsdottir
H, Ludviksson BR and Valdimarsson H: The anti-inflammatory action
of methotrexate is not mediated by lymphocyte apoptosis, but by the
suppression of activation and adhesion molecules. Clin Immunol.
114:154–163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen Y, Song S, Wang Y, Zhang X, Zhang J,
Wu L, Wu J and Li X: Topical application of berberine ameliorates
imiquimod-induced psoriasis-like dermatitis in BALB/c mice via
suppressing JAK1/STAT1 signaling pathway. Arab J Chem.
17(105612)2024.
|
|
56
|
Maglakelidze N, Gao T, Feehan RP and Hobbs
RP: AIRE deficiency leads to the development of alopecia
areata-like lesions in mice. J Invest Dermatol. 143:578–587.e3.
2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Plaza R, Vidal S, Rodriguez-Sanchez JL and
Juarez C: Implication of STAT1 and STAT3 transcription factors in
the response to superantigens. Cytokine. 25:1–10. 2004.PubMed/NCBI View Article : Google Scholar
|