Introduction
The population of US citizens aged 65 years and
older is projected to increase from 58 million in 2022 to 82
million by 2050(1). The increase in
aging-related comorbidities is closely linked to neurological
changes characterized by a reduction in the volume of the frontal
lobe and hippocampus (2,3), leading to progressive loss of myelin
integrity (4), calcium
dysregulation (5), mitochondrial
dysfunction and accumulation of reactive oxygen species (6). These changes likely contribute to
cognitive decline, sensorimotor impairments and increased
vulnerability to neurodegenerative disorders (7-9).
Alzheimer's disease (AD) and Parkinson's disease are among the most
prevalent aging-related neurodegenerative diseases that lack
effective preventative treatments (10). Given the multifaceted nature of
these diseases, holistic approaches that target multiple pathways
hold promise in addressing aging-related cognitive decline.
Garlic has been used as a seasoning or condiment in
cooking and folk medicine for thousands of years across various
cultures (11). Sulfur-containing
compounds, such as allicin and S-allyl cysteine (SAC), are known to
contribute to its pungent aroma. Besides its use in cooking, garlic
has been shown to exhibit antioxidant properties, and thus, is
considered to offer various health benefits. Among various garlic
supplements, aged garlic extract (AGE) has been noted for being an
odorless and stable product resulting from prolonged extraction of
fresh garlic at room temperature, and has essential bioavailable
properties (12-14).
Previous research has further indicated that AGE and its active
components, such as SAC and N-α-(1-deoxy-D-fructos-1-yl)-L-arginine
(FruArg), could offer various systemic health benefits (15). More specifically, studies have
highlighted the role of AGE in mitigating aging-related diseases
and cardiovascular health risks by lowering cholesterol levels,
reducing calcification in coronary arteries, reducing acute phase
reactants, and exerting striking protection against systolic and
diastolic hypertension (16-20).
Animal studies have also revealed that AGE and its active
components, such as diallyl trisulfide and SAC, could offer
cardioprotective effects, including reducing myocardial damage and
supporting glyco- and lipo-metabolism (21,22).
Human trials have corroborated these findings, indicating that AGE
can improve arterial elasticity, reduce inflammation, and stabilize
the ventricular mass in patients with diabetes and cerebrovascular
disease (CVD) (23-26).
Several single-center randomized clinical trials have also revealed
that AGE could attenuate the progression of subclinical
atherosclerosis, regenerate peripheral tissue perfusion measured
post occlusive reactive hyperemia by laser speckle contrast imaging
and increase microcirculation in patients with arteriolosclerosis
(27,28). There is strong evidence indicating
the ability of AGE to slow the progression of atherosclerosis, thus
serving as a nutraceutical in the primary prevention and mitigation
of CVD and stroke. Previous studies have suggested that AGE and its
bioactive components could enhance resiliency against inflammation
whilst mitigating learning and memory deficits by modulating
neuronal glucose transport and oxidative stress (15,29,30).
These multifaceted health benefits underscore the potential of AGE
to serve as a valuable nutraceutical to enhance neuroprotection and
improve systemic resilience for brain health.
Previous studies on short-term AGE supplementation
have demonstrated improvements in spatial learning and memory in
young rats with amyloid-β pathology (9 weeks-old) (30,31).
Another study has further demonstrated the ability of the AGE
supplement to improve learning and memory deficits in mice with
accelerated brain atrophy (12 months old) (32). The proposed molecular mechanisms of
short-term AGE supplementation include antiglycation and
antioxidation (14), activation of
antioxidant enzymes (31), and
decreasing oxidative stress and inflammation by modulating the
activities of NF-κB and activator protein-1 whilst inhibiting
matrix metalloproteinases (33).
There is also evidence showing the ability of AGE to inhibit lipid
peroxidation, reduce ischemic/reperfusion damage and inhibit
oxidative modification of low-density lipoprotein (34). Molecular studies using murine BV-2
microglial cells have further demonstrated the ability of AGE to
reduce nitric oxide production, and alter the expression levels of
20 proteins enriched in the cytosol, mitochondria, plasma membrane
and cytoskeleton (15,35,36).
Based on the results of previous studies using
cultured cells and short-term animal models, it was deemed valuable
to investigate the chronic effects of AGE supplementation on
different behavioral paradigms, including the assessment of
cognitive decline, sensorimotor deficits, memory impairments and
anxiety-like behaviors. In the present study, 43-week-old mice were
fed a diet supplemented with AGE for 40 weeks, and the chronic
effect on their neurological behavior, as well as brain proteomes
and molecular pathways, was examined. The results of the present
study revealed the long-term benefits of AGE supplementation during
the natural aging process.
Materials and methods
Animal husbandry
A total of 48 male C57BL/6J mice (RRID:
IMSR_JAX:000664) aged 42 weeks (±3 days) were obtained from The
Jackson Laboratory. In the present study, 42-week-old mice were
selected because this age is equivalent to middle-age in humans
(37,38). On the day of arrival, the mice were
randomly placed in a cage, with two mice per cage. Placing two mice
per cage has been shown previously to provide an essential
environment for social interaction and tracking food consumption
(39,40). Mice were housed in an AAALAC
accredited animal facility under standard conditions: The ambient
temperature 68-79˚F, relative humidity 30-70%, 10-15 air changes
per h, and a 12/12-h light/dark cycle.
After 1 week of habituation, the 43-week-old mice
(body weight 33.9±0.6 g) were administered either the AGE
supplement or the control diet for 40 weeks (~10 months). Control
mice were fed the nutritionally complete diet AIN93G (cat. no.
F3156-Rodent Diet; AIN-93G Bio-Serv®), and the AGE mice
were fed the AIN diet supplemented with AGE (cat. no. F10217 Rodent
Diet; AIN-93G-Modified; AGE-CSA-Missouri; Bio-Serv®).
AGE (40%; w/w aqueous solution) was provided by Wakunaga
Pharmaceutical Co., Ltd. The AGE diet was prepared using the AIN93G
base diet from Bio-Serv. AGE solution (2 kg) in 26 kg of the base
diet (25.2 kg base diet powder and 2 kg AGE solution) was used to
prepare 26 kg of the AGE diet. Because 2 kg of AGE solution
contains 0.8 kg dry weight and 1.2 kg of water, the diet was dried
at 120˚F in order to bring the moisture down to 5% after pelleting
for microbial stability. For the control diet, 8% water was added
to the dry mix as a pelleting aid. Mice were given the specific
diets along with ad libitum access to water. Food intake was
determined every week and body weights were measured every 2 weeks.
To avoid additional stress, body weight was measured after behavior
tests.
After feeding the mice the respective diets for 40
weeks, mice were subjected to behavior assessments. Prior to
behavior tests, mice were housed in a reverse 12/12-h light/dark
cycle environment for 2 weeks. All animal protocols were approved
by the University of Missouri Animal Care and Use Committee (ACUC)
(approval no. 25120; Columbia, USA).
The present study strictly adhered to the guidelines
set forth by the University of Missouri and the National Institutes
of Health for conducting animal studies and followed an approved
method of euthanasia (The American Veterinary Medical
Association-approved method). Mice should be euthanized before they
experience significant pain or distress. The general appearance of
the mice was checked by monitoring signs such as dehydration,
severe weight loss, persistent abnormal posture or hypothermia,
behavioral changes (such as inability to reach food or water, or
lethargy) and clinical signs (such as labored breathing). The mice
were euthanized with ~5% isoflurane and it was confirmed that there
was no response to a firm toe pinch, and no signs of breathing and
heartbeats, and the mice were continued to be exposed to ~5%
isoflurane for ≥1 min to ensure euthanasia. Subsequently, the mice
were perfused with saline through a cannula inserted into the left
ventricle of the heart to wash out the blood. Removing blood from
the brain results in cleaner tissue samples, which can enhance the
accuracy and reliability of results. The mice were decapitated, and
brain tissues were collected for future studies.
Assessment of multi-domain behavior
functions
The aim of the battery of neurobehavioral tests was
to assess the impact of dietary AGE supplementation on multiple
behavioral domains associated with natural aging in mice (Fig. S1). All behavior tests were carried
out in the dark phase (resting phase; with red lights in the
testing room). Prior to the behavior tests, mice were placed in the
testing room for ≥1 h for acclimation, and tests were conducted
from 9:00 am to 3:30 pm. A battery of comprehensive behavior tests
was established to evaluate different phenotypes under functional
and cognitive behavioral domains, including sensorimotor function,
exploratory behavior, anxiety/neophobia, memory and learning. To
test sensorimotor functions, a simple neuro-assessment of
asymmetric impairment (SNAP) test and an inverted screen test were
performed. For anxiety/neophobia responses, the light-dark
transition test, emergence test, open-field test, elevated-plus
maze and zero maze were used. To assess memory and learning, the
novel object recognition (NOR), three-chamber sociability and
Barnes maze tests were performed.
SNAP
The SNAP test was used to measure common
neurological function domains including sensorimotor responses. The
eight test phenotypes consist of interaction, cage grasp, visual
placing, pacing/circling, gait/posture, head tilt, visual field and
baton, as described previously (41). The SNAP test provides a relatively
sensitive, reliable and time-efficient means of assessing
neurological deficits in mice. Each individual test has a scoring
range of 0-5 based on the established guidelines for atypical
neurologic behavior (42).
Inverted screen test
The inverted screen test was used to measure motor
function, coordination and muscle strength under the functional
behavior domain. It is a quick but insensitive gross screening test
that provides a measure of muscular strength. This test was
performed as previously described by Deacon with modifications
(43). Untrained mice were
individually placed on top of a square (7.5x7.5 cm) wire screen
which is mounted horizontally on a metal rod. Within 2 sec, the
wire screen was rotated to an inverted position (180˚), displacing
the mouse to the bottom of the screens. The mouse was kept in this
position for 1 min (checking with a stopwatch). This position
causes the mouse to fall. Normally, the mouse can hold the screen
within a period of time and climb up the incline. The following
parameters were measured: i) latency to fall off the screen, ii)
could not climb up the screen, and iii) able to climb up the screen
within 1-min testing session.
Light-dark transition test
The light-dark box is a behavioral test that
measures novelty-seeking and anxiety-like behaviors in mice under
the cognitive domain. The test, performed according to the authors'
previous studies (44,45), has the same area as the open-field
test, except that half of the area is closed, and a small opening
(7.5 cm high and 5 cm wide) could be found in the middle. Briefly,
animals were placed in the light side of the box and were allowed
to explore the box for 5 min. Their movements were tracked and
analyzed using the ANY-maze software (RRID: SCR_014289; V7.16,
Stoelting Co., Wood Dale, IL; Windows XP OS). A total of 3
measurements were calculated for each mouse: i) latency to first
entry into the dark area, ii) number of entries to the dark area,
and iii) time spent in the light area.
Open field test
As described previously, the open-field test was
performed to measure locomotion, exploratory activity and
anxiety-like behaviors (44,46,47).
The open-field activity box is 40x40 cm and located on a white
platform. Animals were placed into the center of the square arena
for 10 min. After each trial, the box was cleaned with 10% ethanol
solution and air-dried. The animals' movements were tracked and
analyzed using the ANY-maze software. A series of 10x10-cm blocks
were identified to track mouse activity, with 4 blocks defined as
the center zone and the surrounding 12 blocks as the peripheral
zone. Phenotypic parameters such as total distance traveled and
time spent in different zones (that is, center vs. periphery) were
recorded as indicators of anxiety.
NOR test
The NOR test in the open-field platform evaluates
non-spatial learning and recognition memory under the cognition
behavioral domain, as previously described (48), with a few modifications. First, the
animal was allowed to become familiarized with an object for 5 min.
After a 20~30 min interval, the object was placed in a different
location in the same arena. Mice were allowed to explore the
familiar object in the different location for 5 min. After 24 h, a
new (novel) object was placed in the platform together with the
familiar object. Normally, the color or shape of the novel object
was changed, and the mouse was allowed to explore the object for 5
min. The entire test was recorded and measured using the ANY-maze
software. In the aforementioned test, the following parameters were
measured: i) Discrimination Index (DI) and ii) percentage of total
investigating time. The DI was calculated by taking the difference
between time investigating the novel object and the time
investigating the familiar object, divided by the total time
investigating both objects. The percentage of total investigating
time was calculated by dividing the time investigating the novel
object by the total time investigating both objects.
Emergence test
The emergence test is widely used to assess
neophobia and exploratory behavior in rodents (49,50).
The apparatus is an adaptation of the open-field test and intended
to evaluate novelty-based anxiety. Naturally, rodents have an
innate explorative drive as well as an aversion to brightly lit
open spaces. The task involves observation of the time taken for
the subject to exit the holding container (shelter) and explore the
arena. Mice with high levels of anxiety tend to spend more time in
shelter than in the open spaces.
Briefly, a large open field arena surrounded by high
walls was used for this test. A cylindrical shaped tube
(13.2x4.4x4.4 cm) that serves as the holding container or shelter,
was used to restrain the mouse before placing in the arena. The
shelter was equipped with lids and secured in place to prevent it
from rolling in the center of the arena. While carrying the mouse
in the tube, it was ensured not to close the lid tightly to
maintain proper ventilation. After placing the tube in the center,
the lid was removed. The number and time the subject spent in the
shelter (tube), number of pokes into the shelter (tube) door, and
time spent in the lit arena, were recorded and measured using the
ANY-maze software.
Elevated plus maze test
The elevated plus maze test was used to evaluate the
anxiety and fear domains. The test is based on the concept that
rodent's natural behavior is to stay in the enclosed areas and
display unconditional fear in open spaces and elevated heights.
Anxious animals tend to spend more time in the closed arms than
less anxious animals. The elevated plus maze was built according to
the description of Lister (51).
This maze was made of Plexiglas and consisted of a central square
(5x5 cm) from which radiated four arms (5x45 cm). Two of the arms
had walls (15 cm high) along the edge (closed arms), whereas the
other two arms did not have walls (open arms). A white line was
drawn halfway (22.5 cm) along each of the four arms to measure
motor activity. The maze was elevated 45 cm above the floor on a
plus-shaped plywood stand. The mouse was placed at the junction of
the four arms of the maze, facing a closed arm. They were allowed
to explore the apparatus for 5 min while an observer, a video
camera, and the automated tracking system recorded their actions.
Time spent in the open/closed arms and the duration of time in the
center were tracked by ANY-maze software and used those for scoring
various cognitive behavior parameters including: i) Number of
entries in the open or closed arms, ii) time spent in the open or
closed arms, iii) time spent in the center, and iv) percentage of
time spent in the open/closed arms.
Zero maze
The zero maze was used to test anxiety- and
exploratory-related behaviors in mice. It is an elevated,
ring-shaped runway, with the same amount of area devoted to the
adjacent open and closed quadrants. Mice were placed in one of the
closed quadrants at the start of each trial and were allowed to
explore the apparatus for 5 min. The ANY-maze software was used for
scoring cognitive behavior parameters including: i) Number of
entries in the open or closed segments, ii) time spent in the open
or closed segments, iii) time spent in the center, and iv)
percentage of time spent in the open/closed segments.
Three-chamber sociability test
A three-chamber social interaction test was
performed to assess sociability and social preference as described
previously (52). Mice were
habituated in the middle compartment for five min before testing.
The ‘strange’ animal (same age and strain) was placed in the wire
cage of either the left or right compartment. The strange mouse's
location represented stranger zone 1 while the other wire cage
remained empty (that is, empty zone). Mice were left to socialize
for 10 min. The time spent in the stranger zone 1 (interaction with
the new mouse) relative to the time spent in the empty zone was
measured.
The social preference test was conducted for another
10 min after termination of the sociability test. A second strange
mouse was introduced to the wire cage in place of the empty zone
and was considered stranger zone 2. The animal's preference for
interacting with a familiar animal (stranger zone 1) or an
unfamiliar animal (stranger zone 2) was determined by measuring the
same parameters. The indices of sociability and social preference
were computed, as previously described (53).
Barnes maze
Spatial learning and memory were tested using the
Barnes maze as described (44,54).
The maze consists of a circular platform (75 cm diameter) with 20
holes (5 cm diameter) evenly spaced around the platform's perimeter
and is elevated 56.5 cm above the floor by a stand. One hole was
designated as the escape hole for each mouse, and an escape box was
placed underneath the hole. For each mouse, the location of the
escape box remained the same throughout all test runs. To create an
aversive environment, three 72-watt lights were suspended above the
platform, encouraging the mice to flee from the highly lit
environment into the darkened environment of the box. Four shapes
(+, ∎, ∆, •) served as visual cues and each shape respectively was
placed on each side of the black background curtains to aid spatial
navigation. Behavioral testing consisted of 2 habituation trials on
the first day, and 8 evaluation trials (2 trials/day) over the next
4 days. Reversal training consisted of 2 trials per day for 3 days,
with an inter-trial interval of 20-30 min. This phase began 24 h
after the final acquisition trial. Prior to the start of reversal
training, each mouse was assigned a new escape hole, positioned
opposite the previously assigned location. Latency (that is, the
time mice found the escape box) and total errors (that is,
nose-pokes into non-escape holes) were recorded and analyzed by
ANY-maze software.
Data and statistical analysis for
behavior tests
The data are presented as the mean ± SEM. Results
were analyzed by one-way or two-way ANOVA using GraphPad Prism
Software (RRID:SCR_002798; V10.0; Dotmatics). Tukey's post hoc test
was applied for multiple comparisons. Differences were considered
statistically significant at P<0.05 for all analyses. An
unpaired t-test was applied for individual comparisons between two
groups.
Tissue collection for analysis of
global proteomes from brain cortex and hippocampus
A total of 10 mice, AGE (n=5) and control diet
(n=5), were euthanized at 88 weeks-old (following 11 months of AGE
supplementation) for label-free global proteomic quantitation by
tandem mass spectrometry (MS2) analysis. The sample
preparation for quantitative proteomic analysis has been described
previously (55). Briefly, the left
cortex and hippocampi from both hemispheres were dissected and
processed. Tissues were lysed with sample buffer containing 2%
sodium dodecyl sulphate (SDS), 0.5 M tetraethylammonium bicarbonate
(TEAB), pH 8.5, protease inhibitor cocktail (1:100), and
phosphatase inhibitor cocktail (1:100). Specimens were homogenized
by Glas-Col stringer 099C K43 (Glas-Col LLC, IN) and centrifuged at
17,000 x g for 20 min at 4˚C. The protein pellets (insoluble
fraction) were collected and further precipitated with 4X (w/v)
acetone and incubated at -20˚C overnight. Prior to MS2
analysis, protein pellets were washed two more times with
acetone.
Sample processing for MS2
analysis
Proteins in samples were denatured with 6 M urea, 2
M thiourea and 100 mM ammonium bicarbonate, and then reduced with
10 mM dithiothreitol (DTT) for 1 h at room temperature (RT) and
alkylated at RT in the dark with 30 mM iodoacetamide. Alkylation
was halted with 10 mM DTT for 15 min at RT. Protein concentration
was determined using Pierce 660 nm Protein Assay (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Proteins from each sample were digested with trypsin (Thermo Fisher
Scientific, Inc.) in a sequential digestion. Trypsin was added at a
1:100 enzyme to protein mass ratio. The first digest was incubated
at 37˚C for 5 h, and the second digest was incubated overnight. The
digested peptides were terminated with trifluoroacetic acid (TFA)
0.5%. For total protein quantification, 5-µg of peptide from each
sample was combined for spectral library using the method for
data-dependent acquisition with parallel accumulation-serial
fragmentation (DDA-PASEF). Subsequently, 500 ng peptides from each
sample were loaded to the liquid chromatography-MS and analyzed
using the method for data-independent acquisition (DIA-PASEF).
To create a comprehensive spectral library, digested
peptides were combined from each sample and fractionated using a
high pH reversed-phase peptide fractionation kit according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.). A total
of eight fractions were collected, lyophilized and resuspended with
0.1% formic acid in deionized water.
For proteome analyses, an EvoSep One liquid
chromatography system was used as described previously (56) and the peptides were analyzed by
eluting with a 44 min gradient or EvoSep One 30 samples per day
(SPD) program. The system used a 15 cm x 150 µm ID column with 1.5
µm C18 beads (Bruker PepSep) and a 20 µm ID zero dead volume
electrospray emitter (Bruker Daltonics). Mobile phases A and B were
0.1% formic acid in water and 0.1% formic acid in acetonitrile,
respectively. EvoSep One was coupled online to a modified trapped
ion mobility spectrometry quadrupole time-of-flight mass
spectrometer (timsTOF Pro 2, Bruker Daltonics) via a nano
electrospray ion source (Captivespray, Bruker Daltonics). To
generate a spectral library, eight high-pH reversed-phase
fractionated samples were analyzed by timsTOF Pro 2 in the
DDA-PASEF mode. PASEF and TIMS were set to ‘on’. One MS and ten
PASEF frames were acquired per cycle of 1.17 sec (~1 MS and 120
MS2). Target intensity for MS was set at 10,000
counts/sec with a minimum threshold of 2,500 counts/sec. Duty cycle
was locked to 100%. Ion mobility coefficient (1/K0) value was set
from 0.6 to1.6 V.s/cm2, collision energy was set from 20-59 eV. MS
data were collected over m/z range of 100 to 1,700. If the
precursor (within mass width error of 0.015 m/z) had >4X signal
intensity in subsequent scans, a second MS2 spectrum was
collected. Exclusion was active after 0.4 min. Isolation width was
set to 2 for m/z <700 m/z or 3 m/z for m/z >700.
The DIA-PASEF method was optimized with py_diAID, as
described previously (57), to
cover an m/z range from 300 to 1,350 Da and an ion mobility range
of 0.65 to 1.45 V.s/cm2. The method includes two IM windows per
DIA-PASEF scan with variable isolation window widths adjusted to
the precursor densities. A total of 25 DIA-PASEF scans were
deployed at a throughput of 30 SPDs (cycle time: 2.76 sec).
MS2 statistical
analysis
Data were analyzed with Spectronaut version 17
(Biognosys AG) with the default settings, except for the
prototypicity filter, which was set to ‘only protein group
specific’. Protein quantification and statistical analysis were
performed using MSstats. The MSstat set up included protein
quantification using the top 3 features, a q-value cutoff of 0.01,
the use of unique peptides, and the removal of protein with only
one feature. No imputation was used, and the data were
log2-transfered based on MS2 peak area.
Normalization was performed using the ‘equalize medians’
method.
Quantitative MS2 analysis
and bioinformatics
AGE supplementation-induced molecular phenome
changes in SDS-insoluble protein were evaluated by computing the
expression ratio and P-value for all MS2-identified
proteins from AGE mice relative to age-matched controls. The
expression ratio threshold was set to >1.3 and <0.77 for
respective increased and decreased proteins (P<0.05). For data
visualization, expression ratios and p values were log-transformed
to produce fold-change (log2 expression ratio) and
-log10 P-value.
The differentially changed proteins in AGE mice
cortex and hippocampus were imported into Ingenuity Pathway
Analysis (IPA; Redwood City, CA; RRID:SCR_008653) for canonical
pathway analysis, machine learning-driven disease pathway analysis,
upstream regulator analysis, and neurological behavior prediction.
Additionally, comparative analysis was performed by combining
expression profiles from AGE mice cortex and hippocampus to obtain
2x2 comparisons for top pathways. The data are licensed under the
Creative Commons Attribution-NonCommercial 4.0 International
License (CC BY-NC 4.0).
Results
Effects of AGE supplementation on the
general health of aging mice and sensorimotor function
No significant differences in average food intake or
body weight (Figs. S2 and S3, respectively) were observed when
comparing the AGE-supplemented diet-fed mice with the control
diet-fed mice. Additionally, no changes in mortality or apparent
abnormalities in general health were found in the mice during the
40-week feeding period. Neither group exhibited any significant
decrease in body weight throughout the study. The maximum observed
body weight loss occurred at the 34th feeding-week, with a weight
loss of ~8.1% in the AGE diet-fed group and 6.0% in the control
diet-fed group compared with the 1st feeding-week.
The SNAP test was used to assess the impact of AGE
supplementation on the sensorimotor function of the aging mice. The
inverted screen grip test was performed to assess the effects of
AGE on physical strength. No statistically significant differences
in coordination, motor strength, proprioception, gait and mental
status were observed following 40-week dietary AGE supplementation
(Fig. S4).
Effects of AGE supplementation on
anxiety and neophobia
Mice fed the AGE diet exhibited less anxiety in the
light-dark transition test and reduced neophobia-like behaviors in
the emergence test. These mice exhibited a decreased number of
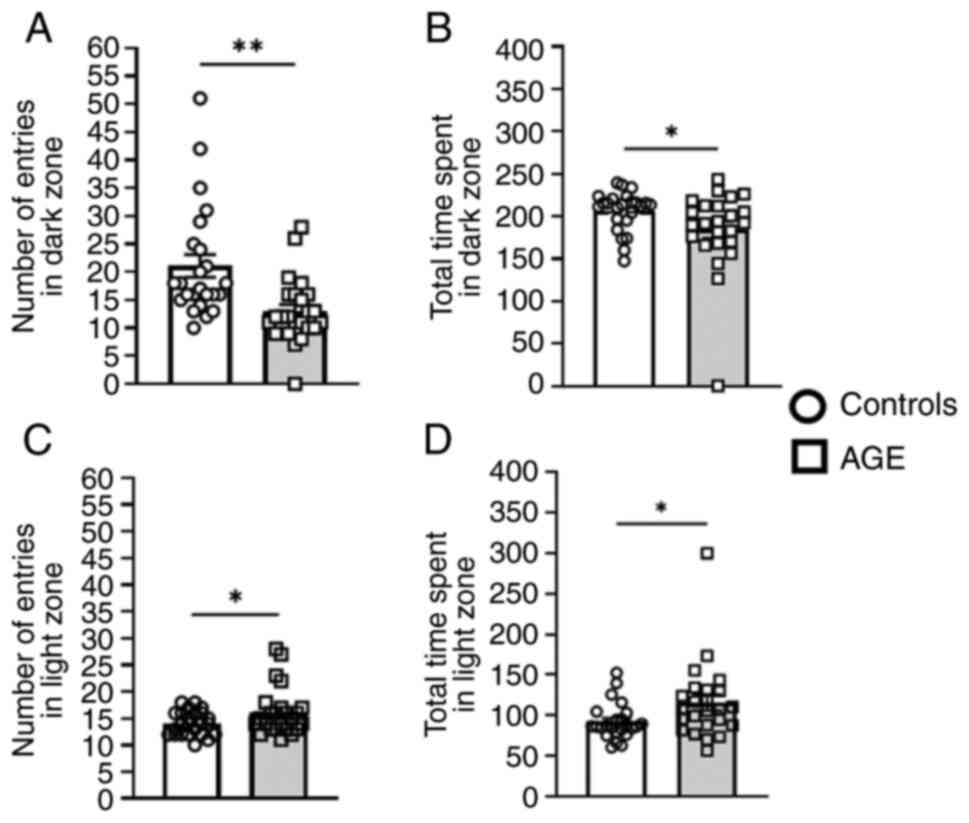
entries (Fig. 1A; P<0.01) and
time spent in the dark zone (Fig.
1B; P=0.046) relative to the aging controls. Furthermore, the
AGE group exhibited an increased number of entries (Fig. 1C; P<0.05) and time spent in the
light zone (Fig. 1D; P=0.034).
The AGE-supplemented diet-fed group also exhibited
less anxiety and neophobia and increased exploratory behavior in
the emergence test (Fig. 2A-G),
with statistically significant differences in the following
parameters: Less time spent in the shelter zone, more time spent in
the arena zone, higher number of line crossings in the arena zone,
longer distance travelled in the arena and less time immobile in
the arena zone. However, no differences were observed between the
AGE-supplemented and control diet groups in the open field test
(Fig. S5A-C), as well as the
elevated plus maze and zero maze tests (Fig. S6A-F).
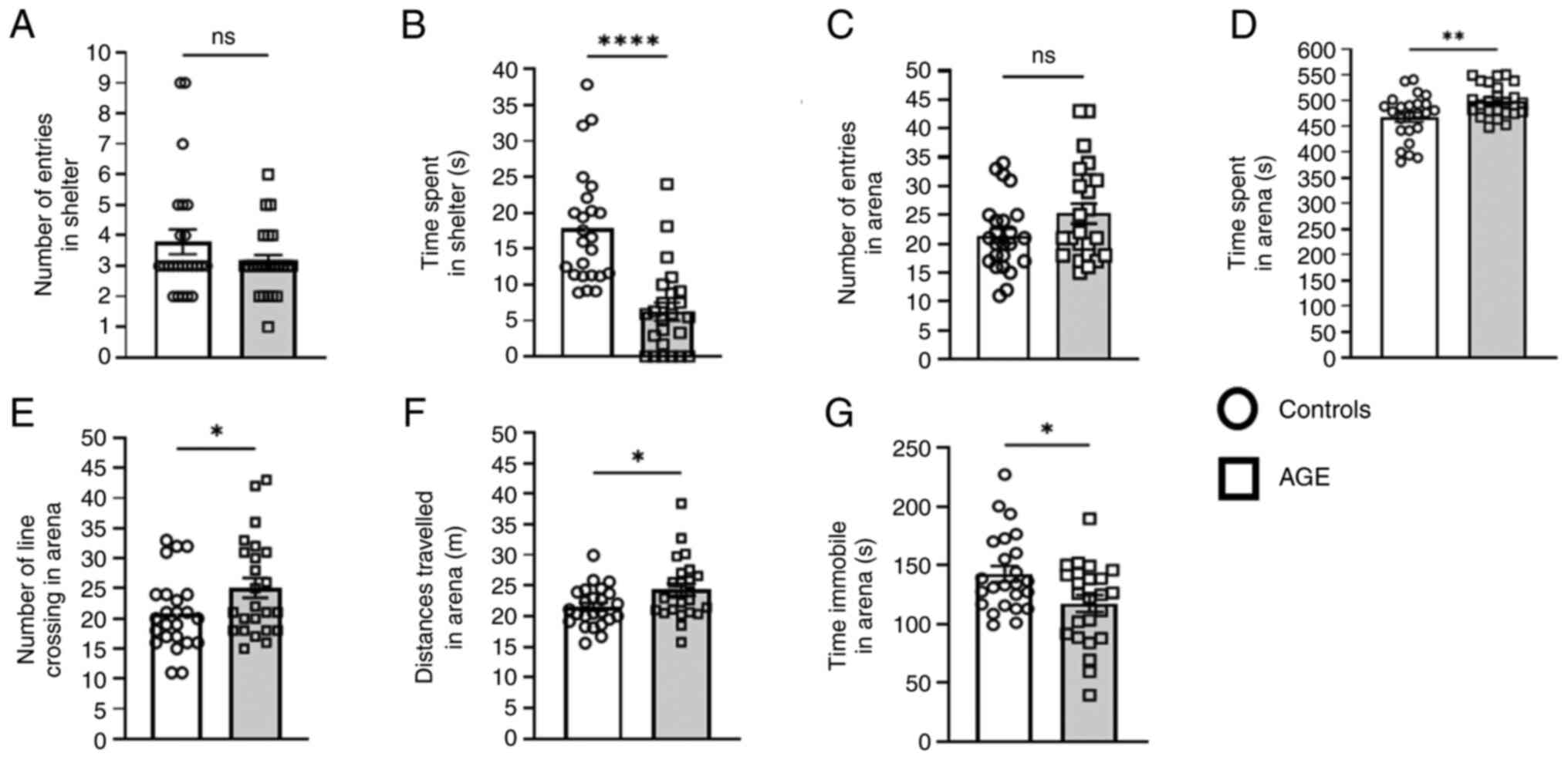 | Figure 2Effects of AGE supplementation on
neophobia behaviors. The emergence test was used to measure (A)
number of entries into shelter, (B) time spent in shelter, (C)
number of entries into arena, (D) time spent in arena, (E) number
of line crossings in arena, (F) distance travelled in arena and (G)
time immobile in the arena. Data are presented as the mean ± SEM.
Groups: Control, n=24; AGE diet, n=24. Significantly different from
age-matched control group, *P<0.05,
**P<0.01 and ****P<0.0001. AGE, aged
garlic extract; ns, not significant. |
Effects of AGE supplementation on
exploratory behavior, learning and memory
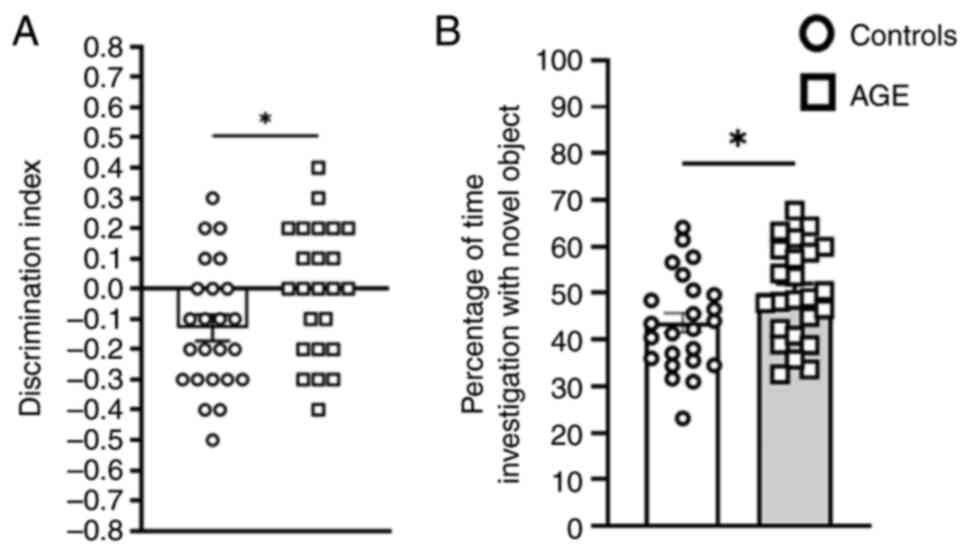
In the NOR test to evaluate ability of non-spatial
learning and recognition memory under the cognition behavioral
domain, AGE-supplemented mice displayed a greater DI (Fig. 3A; P=0.027), and increased percentage
of investigation time with the novel object location (Fig. 3B; P=0.039), indicating AGE
supplementation could improve learning levels, memory and
exploratory behaviors in aging mice.
Effects of AGE supplementation on
cognition and spatial reference learning in aging mice
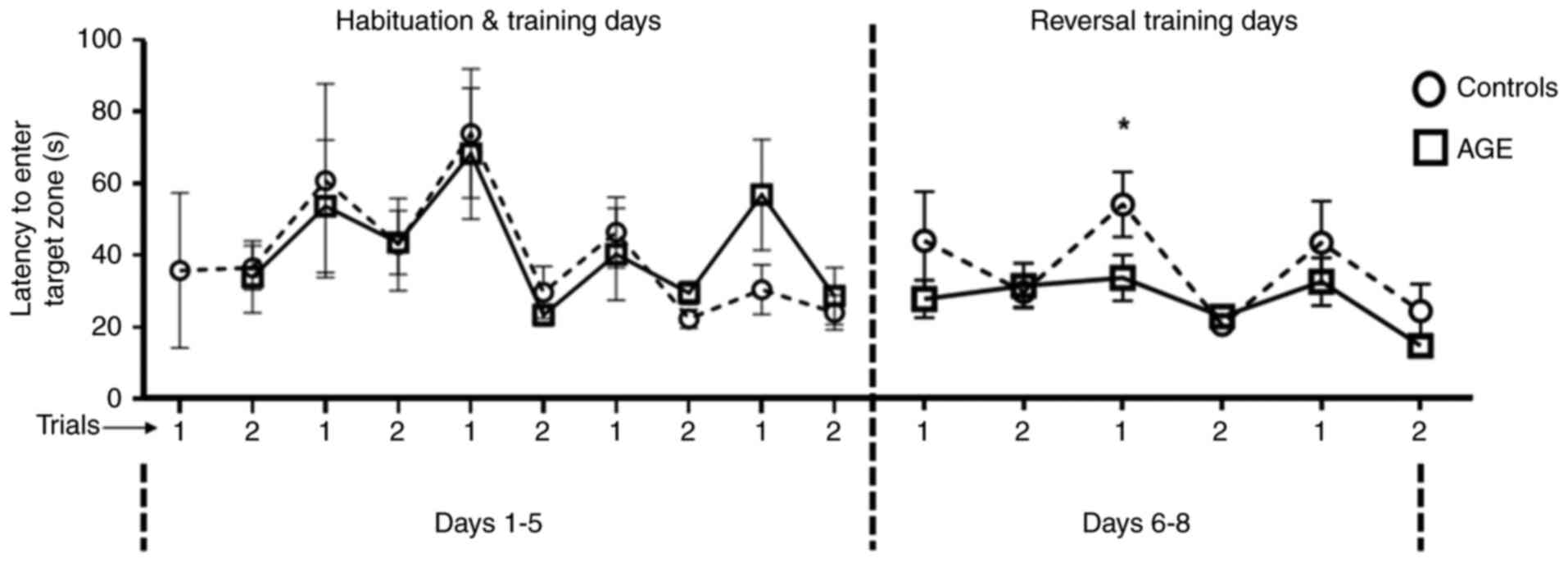
It was assessed if AGE supplementation could improve
spatial reference learning abilities in aging mice. During the
5-min Barnes maze test, a higher percentage of mice in the AGE
diet-fed group found the hiding box for acquired learning compared
with that in the control group. For those that found within the
allotted time, the latency to enter the hiding box was used to
estimate memory and learning abilities. In reversal training,
AGE-supplemented diet-fed mice displayed a shorter latency to enter
the target zone compared with mice in the control group. The
differences were most pronounced in trial 1 on day 2 of reversal
training (Fig. 4).
The present study evaluated whether AGE
supplementation could improve social recognition, sociability and
novelty preference behavior. The three-chamber social interaction
test was used for evaluating impact of AGE on the social index of
aging mice (time spent with stranger mouse 1 vs. empty cages) and
on the novelty preference index [time spent with familiar (stranger
mouse 1) vs. stranger mouse 2]. In the present study, AGE
supplementation did not improve cognitive domain parameters
(Fig. S7A and B) under the current conditions.
Effects of AGE supplementation on
proteomes are most pronounced in the hippocampus
The brain proteomic profiles were examined to
identify the molecular effects of AGE supplementation in aging
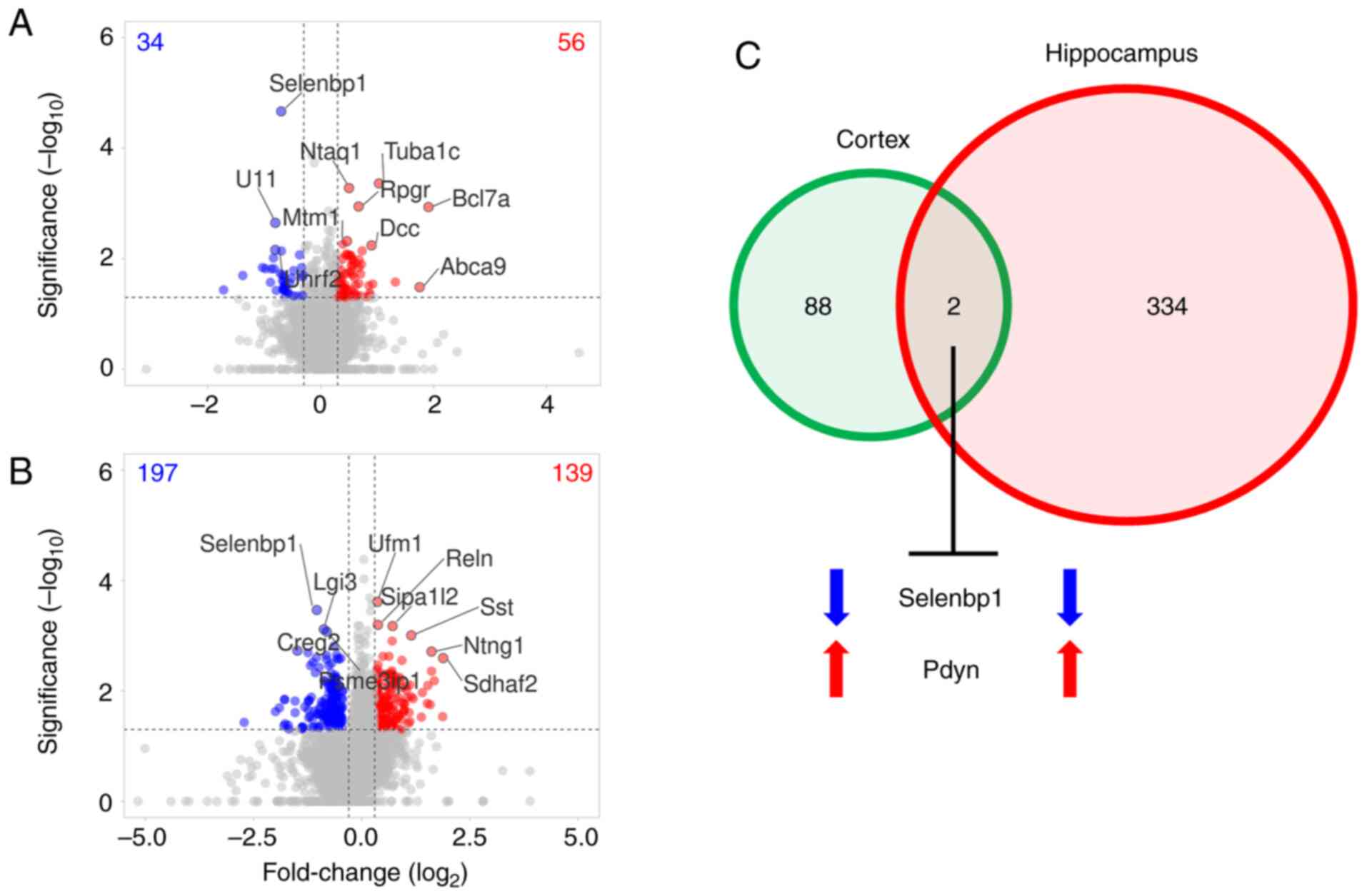
mice. Of 5,863 and 5,826 proteins identified using label-free
MS2, 90 and 336 proteins were significantly changed in
the AGE-supplemented mouse cortex and hippocampus, respectively
(Fig. 5A and B; Table
SI). A total of 34 and 197 proteins were decreased in the
cortex and hippocampus, respectively, whereas 139 and 56 proteins
were increased in the cortex and hippocampus, respectively.
Notably, dietary AGE supplementation decreased selenium-binding
protein 1 (Selenbp1; fold-change in the cortex, -0.700;
P=2.156x10-5; fold-change in the hippocampus, -1.039;
P=3.415x10-5) and increased prodynorphin (Pdyn;
fold-change in the cortex, 0.734; P=0.007; fold-change in the
hippocampus, 1.035; P=0.010) in both brain regions (Fig. 5C).
IPA predicts altered mouse phenomes in
AGE-supplemented diet-fed mice
With the bioinformatics tool to examine mouse
phenomes, which refers to a full set of observable traits (for
example, behavior, physiology and/or disease susceptibility), IPA
was able to use curated proteomic datasets to forecast changes in
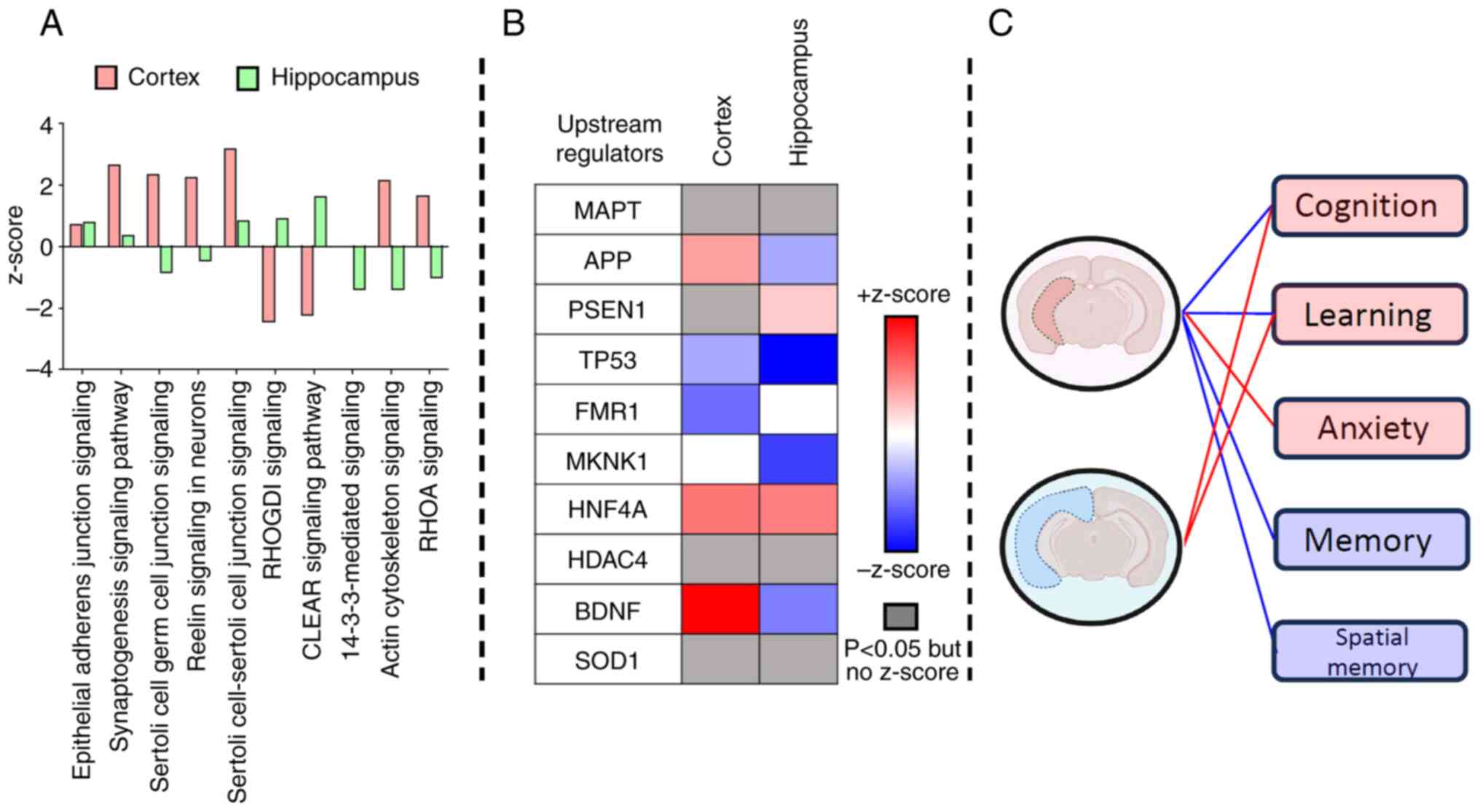
phenotypic outcomes. IPA comparative analysis showed that molecular
pathways in both the cortex and hippocampal regions were similarly
impacted but with some important distinctions. The commonly
affected pathways were ‘epithelial adherens junction signaling’
(increased; P<0.05), ‘synaptogenesis signaling pathway’
(increased; P<0.05) and ‘Sertoli cell-Sertoli cell junction
signaling’ (increased; P<0.05) (Fig.
6A; Table SII, sheet:
Canonical pathways). Among the top canonical pathways,
14-3-3-mediated signaling related to apoptosis was impacted
exclusively in the AGE-supplemented hippocampus (z-score, -1.387;
P=1.362x10-5) (Fig. 6A;
Table SII, sheet: Canonical
pathways). IPA upstream regulator analysis predicted
microtubule-associated protein tau (MAPT) to be most impacted by
dietary AGE supplementation based on P-value ranking, and this
occurred mostly in the hippocampus (cortex P=0.007; hippocampus
P=1.212x10-14) (Fig. 6B;
Table SII, sheet: Upstream
regulators). Amyloid precursor protein (APP) and brain-derived
neurotrophic factor (BDNF) were predicted to be increased in the
cortex and reduced in the hippocampus (Fig. 6B; Table
SII, sheet: Upstream regulators). Presenilin 1 (PSEN1) was
exclusively increased in the hippocampus (z-score, 0.658;
P=1.992x10-10) (Fig. 6B;
Table SII, sheet: Upstream
regulators). The p53 cell-cycle regulator was reduced in both the
cortex (z-score, -0.442; P=0.002) and hippocampus (z-score, -1.781;
P=1.917x10-8) (Fig. 6B;
Table SII, sheet: Upstream
regulators). IPA behavior predictions suggested that differential
expression changes in the cortex and hippocampus proteomes due to
AGE supplementation could impact cognition and learning, whereas
only changes to the hippocampus proteomes could impact anxiety,
memory and spatial memory (Fig. 6C;
Table SII, sheet: Behavior
prediction).
Discussion
In the natural aging process, there is a
well-documented decline in cognition and memory function. Based on
existing literature, a strong argument can be made that AGE, along
with its bioactive components, may delay cognitive impairment, and
thereby improve the behavioral phenotype associated with aging
(30,58-61).
As reported by Song et al (35), garlic (Allium sativum),
particularly in the form of AGE, contains nutraceutical
constituents such as SAC, S-allyl-mercapto-cysteine (SAMC) and
FruArg. SAC and SAMC, prominent organosulfur compounds, exhibit
potent antioxidant, anti-inflammatory and anti-apoptotic activities
that are consistent with the observed downregulation of
TP53-mediated apoptotic regulators and enhancement of
BDNF-associated neurotrophic signaling. FruArg, a carbohydrate
derived from garlic via the Maillard reaction, exhibits blood-brain
barrier permeability and transcriptional regulatory potential. In
lipopolysaccharide-stimulated microglial models, AGE and FruArg
reversed 67 and 55% of transcriptomic alterations, respectively,
implicating pathways such as Toll-like receptor signaling, IL-6 and
nuclear factor erythroid 2-related factor 2 pathways. These
findings strengthen the mechanistic framework linking AGE
bioactivities to both region-specific proteome shifts and the
behavioral improvements reported, highlighting the potential of
AGE-derived compounds in attenuating inflammatory activities and
enhancing brain resilience.
SAC has been shown to attenuate oxidative stress and
prevent neuronal cell death in models of neurodegeneration, while
SAMC has demonstrated the ability to modulate gene expression
related to mitochondrial function and cellular survival. Building
on these findings, the present experiments suggested that the
anxiolytic and cognitive benefits observed following AGE
supplementation may, at least in part, be mediated by the actions
of these sulfur-containing compounds and carbohydrate derivatives
on key signaling pathways involved in neuronal health and
plasticity.
Although evidence in humans is still being
developed, preclinical studies using different aging-related rodent
models have shown that AGE supplementation could improve cognition
through its neuroprotective molecular effects on antioxidant
enzymes (58-60),
resilience to amyloid-β toxicity (30,58,60,61),
and by promoting hippocampal neuroplasticity via modulation of
neurotransmitter receptor expression (30). These findings underscore the
potential for AGE to improve human health by altering the
aging-related brain pathology.
In the present study involving multi-faceted
behavioral experiments, aging mice given a diet supplemented with
AGE for >40 weeks showed improvements in age-related decline in
learning and spatial and working memory assessed in the Barnes maze
and NOR tests. These findings are in agreement with results from a
previous study using the passive avoidance test, which showed
improvements in scopolamine-induced amnesia in mice fed with crude
garlic extract (Lasuna) at 10 ml/kg body weight per os for
21 days (62). In another study
using the senescence-accelerated mouse model of aging, mice fed an
AGE diet for 8 months also showed amelioration of memory
acquisition deficits and memory retention impairments compared with
the age-matched controls without AGE supplementation (63). These data suggest that, in the
setting of natural and induced aging models, supplementation of AGE
can improve cognition and memory. AGE supplementation has also been
shown to attenuate cognitive impairment in the setting of
neurodegeneration (30,58,64,65).
To the best of our knowledge, the present study is among the first
to comprehensively assess multi-domain behavioral functions,
demonstrating that a 40-week AGE supplementation regimen could
enhance cognition and memory, and mitigate age-associated cognitive
decline.
Anxiety and related symptoms such as restlessness,
psychomotor agitation and excessive worrying are frequently
encountered in later life (66).
The current options for the treatment of aging-related anxiety
disorders encompass lifestyle modifications, behavioral and
cognitive therapies, mindfulness-based interventions, and/or
pharmacological treatment, including selective serotonin receptor
inhibitors, non-selective serotonin receptor inhibitors and
benzodiazepines (66). However,
these medications are often associated with severe side effects,
further complicating the management of age-related anxiety
(66). In the present study,
dietary supplementation of AGE could reduce anxiety-like behaviors
as manifested by reduced neophobia (fear of new things) and
increased exploratory behavior in the emergence test. These results
are consistent with a previous study demonstrating the potential of
AGE to alleviate anxiety associated with natural aging. A study by
Gilhotra and Dhingra (67)
investigated the neurochemical mechanisms underlying the anxiolytic
effects of garlic extract in mice. Their study focused on GABAergic
and nitrergic signaling pathways and employed behavioral paradigms
such as the elevated plus maze and the light-dark box test,
demonstrating that garlic extract reduced anxiety-like
behaviors.
Building on these behavior observations, the present
study leveraged machine learning-driven IPA to investigate the
molecular mechanisms underlying the anxiolytic effects of AGE.
Notably, the anti-anxiety potential of AGE may be linked to its
antioxidant properties, modulation of neurotransmitter systems and
anti-inflammatory effects. In particular, in Fig. 6 it is illustrated that several
upstream regulators, including TP53, MAPK interacting
serine/threonine kinase 1 (MKNK1) and BDNF, and their signaling
pathways relevant to neurobehavioral functions, were differentially
modulated in a region-specific manner following AGE
supplementation. In IPA, the z-score is a crucial statistic used to
infer the likely activation state (activation or inhibition) of
upstream regulators, which is based on the expression or
phosphorylation patterns of its downstream target molecules. As
shown in Fig. 6B, TP53 and MKNK1
exhibited strong negative z-scores in the hippocampus, suggesting
suppression of stress- or apoptosis-related signaling. Conversely,
BDNF was positively regulated in the cortex. These regulatory
changes may contribute to the anxiolytic effects of AGE by
modulating neuroplasticity, promoting neuroprotection or adjusting
stress-response pathways. These molecular changes with behavioral
domains are further contextualized in Fig. 6C, highlighting that the pathways
linked to anxiety were differentially impacted between the cortex
and hippocampus. More detailed mechanistic insights are presented
subsequently.
IPA analysis of AGE-induced differential protein
expression in the cortex and hippocampus predicted significant
changes in the key regulatory proteins MAPT, APP, PSEN1 and BDNF,
which likely mediate the distinct proteomic changes associated with
AGE supplementation through upstream regulation.
MAPT is normally increased in aging brains, and its
phosphorylation is associated with cognitive decline in AD
(68,69). MAPT was identified as a top upstream
regulator based on P-value ranking within the IPA framework,
reflecting significant enrichment of its downstream targets among
the differentially expressed proteins (P<0.01). While the
z-score is applied across regulators, it requires concordant
directionality between predicted effects and observed expression
changes. In the present dataset, MAPT-associated targets exhibited
mixed directional trends, resulting in a non-significant z-score
despite strong enrichment. MAPT expression itself did not differ
significantly between the cortex and hippocampus (Fig. 6B); rather, the analysis highlighted
its inferred regulatory influence. Notably, a greater number of
MAPT-related targets were differentially expressed in the
hippocampus, pointing to a region-specific sensitivity of
MAPT-regulated pathways to dietary AGE supplementation. Future
studies are needed to validate the directionality and potential
post-translational modifications of MAPT in response to AGE
treatment. Although directionality could not be assessed, MAPT
emerged as the most significant upstream regulator of molecular
changes in the hippocampus (-log10 BH-P-value=13.916),
compared with its regulatory impact in the cortex
(-log10 BH-P-value=2.143). Notably, >75% of proteins
were differentially altered due to AGE supplementation.
APP can be cleaved by β- and γ-secretases, resulting
in producing different amyloid-β variants in AD (70). Based on IPA, this protein was
predicted to be reduced in the hippocampus (z-score, -0.445;
-log10 BH-P-value=10.128) but not in the
AGE-supplemented mouse cortex (z-score, 2.714; -log10
BH-P-value=0.945). This prediction suggests a potential ability of
AGE to prevent the formation of AD plaques and possibly protect
against memory impairment.
PSEN1 or γ-secretase constitutes the catalytic
subunit of the γ-secretase complex, and its loss of function due to
mutations has been implicated in amyloid-β plaque formation and AD
pathogenesis (71). The increase in
PSEN1 in the hippocampus (z-score, 0.658; -log10
BH-P-value=9.701) due to AGE supplementation may be a protective
mechanism to counteract age-related memory loss.
BDNF is a key regulator for neuronal survival and
growth, neurotransmitter modulation and neuronal plasticity, which
are essential for learning and memory (72). Upon exogenous administration, BDNF
has been shown to participate in learning-related events, memory,
depression and cognition (73,74).
BDNF mRNA and protein levels are normally reduced in the cortex and
hippocampus of older adults relative to younger adults and infants
and are positively correlated with brain tissue volume (72,74).
In post-mortem AD brains, BDNF is globally reduced in the cortex
and hippocampus (72). In the
present study, AGE supplementation significantly increased cortical
BDNF (z-score, 2.030; -log10 BH-P-value=5.423),
suggesting that the AGE effects may preserve the cortical neurons,
thus attenuating the age-related decline in cognitive function and
short-term memory.
Comparative analysis of the commonly changed
pathways in the cortex and hippocampus revealed an increase in
synaptogenesis signaling in both brain regions, albeit mostly in
the cortex. In aged rodents and primates, loss of synaptic spines
in the hippocampus and cortex is known to reduce neural firing
rates during working memory tasks and to reduce non-synaptic bouton
density associated with learning deficits (75). A study showed that AGE
supplementation attenuates oxidative stress and amyloid pathology,
restores cortical and hippocampal synaptic proteins, such as
synaptophysin and synaptosome associated protein 25, as well as
enhanced cognition and memory (76).
In the hippocampus, AGE supplementation was found to
reduce 14-3-3 signaling pathways linked to apoptosis and
chaperone-mediated autophagy. Aberration of 14-3-3 signaling
pathways has been reported in aging, neurodegenerative diseases and
cancer (77). Aging has been shown
to disturb 14-3-3 chaperone and macroautophagic signaling activity,
which resulted in the accumulation of toxic molecular constituents,
including MAPT, amyloid-β and α-synuclein (77). Consequently, using AGE
supplementation to target 14-3-3 signaling may be a novel mechanism
to attenuate neuron cell death during the natural aging
process.
In conclusion, the present study revealed the
neuroprotective effects of dietary supplementation of AGE in
improving age-related cognitive decline and anxiety-like behaviors
in aging mice. Modulation of MAPT, APP, PSEN1 and BDNF further
suggested the ability of AGE to confer neuroprotective effects
against age-related neurodegeneration. Proteomic analysis
highlighted the increase in synaptogenesis and reduction in
apoptotic signaling, supporting the notion of AGE supplementation
as a nutraceutical to mitigate age-related cognitive decline. While
these findings provide promising insights into the molecular
changes induced by AGE, further validation is essential to
strengthen the present conclusions. To corroborate the proteomic
data, additional validation experiments using both western blotting
and immunohistochemical analyses, as well as other complementary
assays, will be performed in future studies. Understanding these
mechanisms can offer promising avenues for interventions in
age-related neurological disorders. Further research into the
translational potential of AGE-based interventions is warranted to
address the increasing disease burden of aging-associated cognitive
impairments and neuropsychiatric symptoms.
Supplementary Material
Study timeline including the AGE
feeding, behavior test duration, and tissue collection for global
proteomics are presented along with a list of the behavior tests
performed to assess AGE effects on motor function; anxiety,
neophobia and exploratory; and other cognition behavioral domains.
AGE, aged garlic extract; SNAP, simple neuro-assessment of
asymmetric impairment.
Effects of AGE supplementation on food
consumption. (A) Weekly food consumption and (B) bi-weekly food
consumption per body weight (grams/grams) for AGE mice (square with
solid lines) and controls (circle with dash lines). Data are
presented as the mean ± SEM. Groups: AGE, n=24; Controls, n=24.
AGE, aged garlic extract.
Effects of AGE supplementation on body
weight. Weekly gain in body weight data is presented as the mean ±
SEM. Groups: AGE, n=24; Controls, n=24. AGE, aged garlic
extract.
Effects of AGE supplementation on
gross motor function and strength. (A) Results from the simple
neuro-assessment of asymmetric impairment and (B) inverted screen
grip test. Data are presented as the mean ± SEM. Groups: AGE, n=24;
Controls, n=24. AGE, aged garlic extract; ns, not significant.
Effects of AGE supplementation on
locomotion-related anxiety-like behaviors. Results from the tested
open-field maze parameters showing AGE effects on (A) total time
spent in center zone, (B) total distance traveled in center zone,
and (C) distance traveled in center zone per minute. Data are
presented as the mean ± SEM. Groups: AGE, n=24; Controls, n=24.
AGE, aged garlic extract; ns, not significant.
Effects of AGE supplementation on
explorative-related anxiety-like behaviors. Results shown in (A-C)
represent the elevated plus maze and (D-F) represent the zero maze
to evaluate anxiety-like behaviors. (A and D) Time spent in open
arms/segments, (B and E) percentage of time spent in open
arms/segments, and (C and F) time spent in closed arms/segments
were measured. Data are presented as the mean ± SEM. Groups: AGE
(n=24) and Control (n=24). AGE, aged garlic extract; ns, not
significant.
Effects of AGE supplementation on
sociability. Results from the three-chamber social interaction test
including mice for (A) sociability and (B) novelty preferences.
Data are presented as the mean ± SEM. Groups: AGE, n=24; Controls,
n=24. AGE, aged garlic extract; ns, not significant.
Raw and processed global proteomics
data
Comparative analysis results (DE
groups: AGE vs Control; Comparison groups: Cor-tex vs Hippocampus)
from ingenuity pathway analysis
Acknowledgements
The authors would like to thank Dr Dennis K. Miller,
Associate Teaching Professor, Department of Psychology Sciences,
University of Missouri College of Arts & Science, for his
technical guidance in the setup of some neurobehavioral
assessments.
Funding
Funding: The present study was supported in part by the Wakunaga
Pharmaceutical Co., Ltd. (grant no. 00078665) and by the University
of Missouri School of Medicine (grant no. C2654010-C8904-0595).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The data generated in the
present study may be found in the Figshare under accession number
30066604 or at the following URL: https://doi.org/10.6084/m9.figshare.30066604.
Authors' contributions
MT and MJ developed methodology, validated and
curated data, conducted formal analysis and investigation, and
wrote the original draft. MJ visualized data. AZ conducted formal
analysis and investigation. WY validated and curated data. TTN
conducted formal analysis and investigation. AB conducted
investigation, validated and curated data. RL conducted
investigation and validated data. GYS contributed to data
interpretation, provided constructive criticism during the revision
process, and approved the final version. JC conceptualized the
study, developed methodology, validated data, performed formal
analysis, conducted investigation and project administration, and
curated data. ZG conceptualized and supervised the study, developed
methodology, performed software and formal analysis, visualized,
validated and curated data, conducted investigation and project
administration, provided resources, wrote the original draft, and
acquired funding. MT, MJ, AZ, WY, TTN, GYS, JC and ZG wrote,
reviewed and edited the manuscript. JC and ZG confirm the
authenticity of the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All experimental procedures involving mice were
conducted in accordance with the ethical standards of the
University of Missouri School of Medicine and the national
guidelines for the care and use of laboratory animals. The study
protocol was reviewed and approved (approval no. 25120) by the
Institutional Animal Care and Use Committee (IACUC) of the
University of Missouri School of Medicine (Columbia, USA).
Patient consent for publication
Not applicable.
Competing interests
Financial support was received from Wakunaga
Pharmaceutical Co., Ltd., however the funding agent had no
influence on the research, results, or interpretation of the study.
The authors declare that they have no competing interests.
References
|
1
|
Bureau USC: 2023 National Population
Projections Tables: Main Series, 2023.
|
|
2
|
No authors listed. 2023 Alzheimer's
disease facts and figures. Alzheimers Dement. 19:1598–1695.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hebert LE, Weuve J, Scherr PA and Evans
DA: Alzheimer disease in the United States (2010-2050) estimated
using the 2010 census. Neurology. 80:1778–1783. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bartzokis G, Cummings JL, Sultzer D,
Henderson VW, Nuechterlein KH and Mintz J: White matter structural
integrity in healthy aging adults and patients with Alzheimer
disease: A magnetic resonance imaging study. Arch Neurol.
60:393–398. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Toescu EC, Verkhratsky A and Landfield PW:
Ca2+ regulation and gene expression in normal brain aging. Trends
Neurosci. 27:614–620. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Melov S: Modeling mitochondrial function
in aging neurons. Trends Neurosci. 27:601–606. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murman DL: The impact of age on cognition.
Semin Hear. 36:111–121. 2015.
|
|
8
|
Xia X, Jiang Q, McDermott J and Han JJ:
Aging and Alzheimer's disease: Comparison and associations from
molecular to system level. Aging Cell. 17(e12802)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoshimura N, Tsuda H, Aquino D, Takagi A,
Ogata Y, Koike Y and Minati L: Age-related decline of sensorimotor
integration influences resting-state functional brain connectivity.
Brain Sci. 10(966)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heavener KS and Bradshaw EM: The aging
immune system in Alzheimer's and Parkinson's diseases. Semin
Immunopathol. 44:649–657. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yaniv Z and Bachrach U: Handbook of
Medicinal Plants. 1st Edition. Haworth Medical Press, Inc. New
York, 2005.
|
|
12
|
Amagase H: Clarifying the real bioactive
constituents of garlic. J Nutr. 136 (3 Suppl):716S–25S.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Amagase H, Petesch BL, Matsuura H, Kasuga
S and Itakura Y: Intake of garlic and its bioactive components. J
Nutr. 131 (3S):955S–962S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elosta A, Slevin M, Rahman K and Ahmed N:
Aged garlic has more potent antiglycation and antioxidant
properties compared to fresh garlic extract in vitro. Sci Rep.
7(39613)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song H, Cui J, Mossine VV, Greenlief CM,
Fritsche K, Sun GY and Gu Z: Bioactive components from garlic on
brain resiliency against neuroinflammation and neurodegeneration.
Exp Ther Med. 19:1554–1559. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Das D, M K, Mitra A, Zaky MY, Pathak S and
Banerjee A: A review on the efficacy of plant-derived bio-active
compounds curcumin and aged garlic extract in modulating cancer and
age-related diseases. Curr Rev Clin Exp Pharmacol. 19:146–162.
2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gadidala SK, Johny E, Thomas C, Nadella M,
Undela K and Adela R: Effect of garlic extract on markers of lipid
metabolism and inflammation in coronary artery disease (CAD)
patients: A systematic review and meta-analysis. Phytother Res.
37:2242–2254. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ried K: Garlic lowers blood pressure in
hypertensive subjects, improves arterial stiffness and gut
microbiota: A review and meta-analysis. Exp Ther Med. 19:1472–1478.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Varshney R and Budoff MJ: Garlic and heart
disease. J Nutr. 146:416S–421S. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wlosinska M, Nilsson AC, Hlebowicz J,
Hauggaard A, Kjellin M, Fakhro M and Lindstedt S: The effect of
aged garlic extract on the atherosclerotic process-a randomized
double-blind placebo-controlled trial. BMC Complement Med Ther.
20(132)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Asdaq SMB, Challa O, Alamri AS, Alsanie
WF, Alhomrani M, Almutiri AH and Alshammari MS: Cytoprotective
potential of aged garlic extract (AGE) and its active constituent,
S-allyl-l-cysteine, in presence of carvedilol during
isoproterenol-induced myocardial disturbance and metabolic
derangements in rats. Molecules. 26(3203)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin KH, Ng SC, Lu SY, Lin YM, Lin SH, Su
TC, Huang CY and Kuo WW: Diallyl trisulfide (DATS) protects cardiac
cells against advanced glycation end-product-induced apoptosis by
enhancing FoxO3A-dependent upregulation of miRNA-210. J Nutr
Biochem. 125(109567)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gruenwald J, Bongartz U, Bothe G and
Uebelhack R: Effects of aged garlic extract on arterial elasticity
in a placebo-controlled clinical trial using EndoPAT™
technology. Exp Ther Med. 19:1490–1499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hamal S, Cherukuri L, Birudaraju D,
Matsumoto S, Kinninger A, Chaganti BT, Flores F, Shaikh K, Roy SK
and Budoff MJ: Short-term impact of aged garlic extract on
endothelial function in diabetes: A randomized, double-blind,
placebo-controlled trial. Exp Ther Med. 19:1485–1489.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hutchins E, Shaikh K, Kinninger A,
Cherukuri L, Birudaraju D, Mao SS, Nakanishi R, Almeida S,
Jayawardena E, Shekar C, et al: Aged garlic extract reduces left
ventricular myocardial mass in patients with diabetes: A
prospective randomized controlled double-blind study. Exp Ther Med.
19:1468–1471. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wlosinska M, Nilsson AC, Hlebowicz J,
Fakhro M, Malmsjö M and Lindstedt S: Aged garlic extract reduces
IL-6: A double-blind placebo-controlled trial in females with a low
risk of cardiovascular disease. Evid Based Complement Alternat Med.
2021(6636875)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Budoff MJ, Ahmadi N, Gul KM, Liu ST,
Flores FR, Tiano J, Takasu J, Miller E and Tsimikas S: Aged garlic
extract supplemented with B vitamins, folic acid and L-arginine
retards the progression of subclinical atherosclerosis: A
randomized clinical trial. Prev Med. 49:101–107. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lindstedt S, Wlosinska M, Nilsson AC,
Hlebowicz J, Fakhro M and Sheikh R: Successful improved peripheral
tissue perfusion was seen in patients with atherosclerosis after 12
months of treatment with aged garlic extract. Int Wound J.
18:681–691. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gomez CD, Aguilera P, Ortiz-Plata A, López
FN, Chánez-Cárdenas ME, Flores-Alfaro E, Ruiz-Tachiquín ME and
Espinoza-Rojo M: Aged garlic extract and S-allylcysteine increase
the GLUT3 and GCLC expression levels in cerebral ischemia. Adv Clin
Exp Med. 28:1609–1614. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Thorajak P, Pannangrong W, Welbat JU,
Chaijaroonkhanarak W, Sripanidkulchai K and Sripanidkulchai B:
Effects of aged garlic extract on cholinergic, glutamatergic and
GABAergic systems with regard to cognitive impairment in Aβ-induced
rats. Nutrients. 9(686)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wichai T, Pannangrong W, Welbat J,
Chaichun A, Sripanidkulchai K and Sripanidkulchai B: Effects of
aged garlic extract on spatial memory and oxidative damage in the
brain of amyloid-β induced rats. Songklanakarin J Sci Technol.
41:311–318. 2019.
|
|
32
|
Moriguchi T, Saito H and Nishiyama N:
Anti-ageing effect of aged garlic extract in the inbred brain
atrophy mouse model. Clin Exp Pharmacol Physiol. 24:235–242.
1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim SR, Jung YR, An HJ, Kim DH, Jang EJ,
Choi YJ, Moon KM, Park MH, Park CH, Chung KW, et al: Anti-wrinkle
and anti-inflammatory effects of active garlic components and the
inhibition of MMPs via NF-κB signaling. PLoS One.
8(e73877)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Borek C: Antioxidant health effects of
aged garlic extract. J Nutr. 131 (3S):1010S–1015S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Song H, Lu Y, Qu Z, Mossine VV, Martin MB,
Hou J, Cui J, Peculis BA, Mawhinney TP, Cheng J, et al: Effects of
aged garlic extract and FruArg on gene expression and signaling
pathways in lipopolysaccharide-activated microglial cells. Sci Rep.
6(35323)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou H, Qu Z, Mossine VV, Nknolise DL, Li
J, Chen Z, Cheng J, Greenlief CM, Mawhinney TP, Brown PN, et al:
Proteomic analysis of the effects of aged garlic extract and its
FruArg component on lipopolysaccharide-induced neuroinflammatory
response in microglial cells. PLoS One. 9(e113531)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Flurkey K, Currer JM and Harrison DE:
Chapter 20-Mouse models in aging research. In: The Mouse in
Biomedical Research (Second Edition). Fox JG, Davisson MT, Quimby
FW, Barthold SW, Newcomer CE and Smith AL (eds). Academic Press,
Burlington, pp637-672, 2007.
|
|
38
|
Laboratory TJ: When are mice considered
‘old’? The Jackson Laboratory, Bar Harbor, ME, 2017. https://www.jax.org/news-and-insights/jax-blog/2017/november/when-are-mice-considered-old.
|
|
39
|
Hohlbaum K, Frahm S, Rex A, Palme R,
Thöne-Reineke C and Ullmann K: Social enrichment by separated pair
housing of male C57BL/6JRj mice. Sci Rep. 10(11165)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hennessy MB, Kaiser S and Sachser N:
Social buffering of the stress response: Diversity, mechanisms, and
functions. Front Neuroendocrinol. 30:470–482. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen M, Song H, Cui J, Johnson CE, Hubler
GK, DePalma RG, Gu Z and Xia W: Proteomic profiling of mouse brains
exposed to blast-induced mild traumatic brain injury reveals
changes in axonal proteins and phosphorylated tau. J Alzheimers
Dis. 66:751–773. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shelton SB, Pettigrew DB, Hermann AD, Zhou
W, Sullivan PM, Crutcher KA and Strauss KI: A simple, efficient
tool for assessment of mice after unilateral cortex injury. J
Neurosci Methods. 168:431–442. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Deacon RM: Measuring the strength of mice.
J Vis Exp: 2610, 2013.
|
|
44
|
Song H, Konan LM, Cui J, Johnson CE,
Langenderfer M, Grant D, Ndam T, Simonyi A, White T, Demirci U, et
al: Ultrastructural brain abnormalities and associated behavioral
changes in mice after low-intensity blast exposure. Behav Brain
Res. 347:148–157. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Walker JM, Klakotskaia D, Ajit D, Weisman
GA, Wood WG, Sun GY, Serfozo P, Simonyi A and Schachtman TR:
Beneficial effects of dietary EGCG and voluntary exercise on
behavior in an Alzheimer's disease mouse model. J Alzheimers Dis.
44:561–572. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Siedhoff HR, Chen S, Balderrama A, Sun GY,
Koopmans B, DePalma RG, Cui J and Gu Z: Long-term effects of
low-intensity blast non-inertial brain injury on anxiety-like
behaviors in mice: Home-cage monitoring assessments. Neurotrauma
Rep. 3:27–38. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Long JB, Bentley TL, Wessner KA, Cerone C,
Sweeney S and Bauman RA: Blast overpressure in rats: Recreating a
battlefield injury in the laboratory. J Neurotrauma. 26:827–840.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang TN and Hsueh YP: Novel object
recognition for studying memory in mice. Bio Protoc.
4(e1249)2014.
|
|
49
|
Leibrock C, Ackermann TF, Hierlmeier M,
Lang F, Borgwardt S and Lang UE: Akt2 deficiency is associated with
anxiety and depressive behavior in mice. Cell Physiol Biochem.
32:766–777. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lalonde R and Strazielle C: The relation
between open-field and emergence tests in a hyperactive mouse
model. Neuropharmacology. 57:722–724. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lister RG: The use of a plus-maze to
measure anxiety in the mouse. Psychopharmacology (Berl).
92:180–185. 1987.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kordás K, Kis-Varga Á, Varga A, Eldering
H, Bulthuis R, Lendvai B, Lévay G and Román V: Measuring
sociability of mice using a novel three-chamber apparatus and
algorithm of the LABORAS™ system. J Neurosci Methods.
343(108841)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kim JW, Seung H, Kwon KJ, Ko MJ, Lee EJ,
Oh HA, Choi CS, Kim KC, Gonzales EL, You JS, et al: Subchronic
treatment of donepezil rescues impaired social, hyperactive, and
stereotypic behavior in valproic acid-induced animal model of
autism. PLoS One. 9(e104927)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Walker JM, Fowler SW, Miller DK, Sun AY,
Weisman GA, Wood WG, Sun GY, Simonyi A and Schachtman TR: Spatial
learning and memory impairment and increased locomotion in a
transgenic amyloid precursor protein mouse model of Alzheimer's
disease. Behav Brain Res. 222:169–175. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jackson M, Chen S, Nguyen TT, Siedhoff HR,
Balderrama A, Zuckerman A, Li R, Greenlief CM, Cole G, Frautschy
SA, et al: The chronic effects of a single low-intensity blast
exposure on phosphoproteome networks and cognitive function
influenced by mutant tau overexpression. Int J Mol Sci.
25(3338)2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bache N, Geyer PE, Bekker-Jensen DB,
Hoerning O, Falkenby L, Treit PV, Doll S, Paron I, Müller JB, Meier
F, et al: A novel LC system embeds analytes in pre-formed gradients
for rapid, ultra-robust proteomics. Mol Cell Proteomics.
17:2284–2296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Skowronek P, Thielert M, Voytik E, Tanzer
MC, Hansen FM, Willems S, Karayel O, Brunner AD, Meier F and Mann
M: Rapid and in-depth coverage of the (phospho-)proteome with deep
libraries and optimal window design for dia-PASEF. Mol Cell
Proteomics. 21(100279)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nillert N, Pannangrong W, Welbat JU,
Chaijaroonkhanarak W, Sripanidkulchai K and Sripanidkulchai B:
Neuroprotective Effects of aged garlic extract on cognitive
dysfunction and neuroinflammation induced by β-amyloid in rats.
Nutrients. 9(24)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Li F and Kim MR: Effect of aged garlic
ethyl acetate extract on oxidative stress and cholinergic function
of scopolamine-induced cognitive impairment in mice. Prev Nutr Food
Sci. 24:165–170. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pannangrong W, Welbat JU, Chaichun A and
Sripanidkulchai B: Effect of combined extracts of aged garlic,
ginger, and chili peppers on cognitive performance and brain
antioxidant markers in Aβ-induced rats. Exp Anim. 69:269–278.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jeong JH, Jeong HR, Jo YN, Kim HJ, Shin JH
and Heo HJ: Ameliorating effects of aged garlic extracts against
Aβ-induced neurotoxicity and cognitive impairment. BMC Complement
Altern Med. 13(268)2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mukherjee D and Banerjee S: Learning and
memory promoting effects of crude garlic extract. Indian J Exp
Biol. 51:1094–1100. 2013.PubMed/NCBI
|
|
63
|
Nishiyama N, Moriguchi T and Saito H:
Beneficial effects of aged garlic extract on learning and memory
impairment in the senescence-accelerated mouse. Exp Gerontol.
32:149–160. 1997.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chauhan NB and Sandoval J: Amelioration of
early cognitive deficits by aged garlic extract in Alzheimer's
transgenic mice. Phytother Res. 21:629–640. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sripanidkulchai B: Benefits of aged garlic
extract on Alzheimer's disease: Possible mechanisms of action. Exp
Ther Med. 19:1560–1564. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Subramanyam AA, Kedare J, Singh OP and
Pinto C: Clinical practice guidelines for geriatric anxiety
disorders. Indian J Psychiatry. 60 (Suppl 3):S371–S382.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gilhotra N and Dhingra D: GABAergic and
nitriergic influence in antianxiety-like activity of garlic in
mice. J Appl Pharm Sci. 6:077–085. 2016.
|
|
68
|
Nelson PT, Alafuzoff I, Bigio EH, Bouras
C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del
Tredici K, et al: Correlation of Alzheimer disease neuropathologic
changes with cognitive status: A review of the literature. J
Neuropathol Exp Neurol. 71:362–381. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ziontz J, Bilgel M, Shafer AT, Moghekar A,
Elkins W, Helphrey J, Gomez G, June D, McDonald MA, Dannals RF, et
al: Tau pathology in cognitively normal older adults. Alzheimers
Dement (Amst). 11:637–645. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chow VW, Mattson MP, Wong PC and
Gleichmann M: An overview of APP processing enzymes and products.
Neuromolecular Med. 12:1–12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lanoiselée HM, Nicolas G, Wallon D,
Rovelet-Lecrux A, Lacour M, Rousseau S, Richard AC, Pasquier F,
Rollin-Sillaire A, Martinaud O, et al: APP, PSEN1, and PSEN2
mutations in early-onset Alzheimer disease: A genetic screening
study of familial and sporadic cases. PLoS Med.
14(e1002270)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tapia-Arancibia L, Aliaga E, Silhol M and
Arancibia S: New insights into brain BDNF function in normal aging
and Alzheimer disease. Brain Res Rev. 59:201–220. 2008.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Croll SD, Ip NY, Lindsay RM and Wiegand
SJ: Expression of BDNF and trkB as a function of age and cognitive
performance. Brain Res. 812:200–208. 1998.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Erickson KI, Miller DL and Roecklein KA:
The aging hippocampus: Interactions between exercise, depression,
and BDNF. Neuroscientist. 18:82–97. 2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Morrison JH and Baxter MG: The ageing
cortical synapse: hallmarks and implications for cognitive decline.
Nat Rev Neurosci. 13:240–250. 2012.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ray B, Chauhan NB and Lahiri DK: Oxidative
insults to neurons and synapse are prevented by aged garlic extract
and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg
mouse model. J Neurochem. 117:388–402. 2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Fan X, Cui L, Zeng Y, Song W, Gaur U and
Yang M: 14-3-3 proteins are on the crossroads of cancer, aging, and
age-related neurodegenerative disease. Int J Mol Sci.
20(3518)2019.PubMed/NCBI View Article : Google Scholar
|