Introduction
Iron deficiency anemia (IDA) is a prevalent
nutritional deficiency, globally affecting >1.6 billion people,
particularly women of reproductive age, pregnant women, and
individuals with chronic diseases such as chronic kidney disease
(CKD) and inflammatory bowel disease (IBD) (1). IDA arises when iron levels are
inadequate to support hemoglobin synthesis. This condition
manifests as fatigue, diminished cognitive performance, and
decreased quality of life (2).
Timely management of IDA is essential to reduce the risk of
complications such as heart failure and mortality, especially among
pregnant women and individuals with chronic illnesses.
Oral iron supplementation, usually given as ferrous
salts, has been the first-choice treatment for IDA because it is
convenient and affordable. However, oral iron often causes
gastrointestinal side effects like constipation, nausea and stomach
discomfort, which can reduce patient compliance (3). Additionally, oral iron absorption can
be greatly affected in patients with gastrointestinal disorders,
chronic inflammation, or those using medications that impact
gastrointestinal function (4).
The low absorption rate and prolonged treatment
duration associated with oral iron have prompted the exploration of
intravenous (IV) iron as an alternative. IV iron administration
bypasses the gastrointestinal tract, resulting in rapid restoration
of iron stores and accelerated improvement of anemia-related
symptoms. Evidence indicates that IV iron increases hemoglobin
concentrations more efficiently than oral iron, especially in
patients with IBD and CKD (5). A
meta-analysis comparing IV and oral iron for perioperative anemia
management demonstrated that IV iron significantly increased
hemoglobin, ferritin, and mean corpuscular volume without a higher
risk of adverse effects (5). For
pregnant women with IDA, IV iron administration results in higher
hemoglobin and ferritin levels at delivery compared with oral iron.
This approach also causes fewer gastrointestinal side effects. IV
iron is therefore preferable for patients who are unable to
tolerate oral iron therapy (6).
IV iron has also shown superiority in treating
postpartum anemia. A systematic review reported that women
receiving IV iron achieved higher hemoglobin concentrations at six
weeks postpartum than those receiving oral iron, with a
significantly lower incidence of gastrointestinal side effects such
as constipation and dyspepsia (3).
In patients with CKD not undergoing dialysis, IV iron has been
shown to improve hemoglobin and ferritin levels more effectively
than oral iron, with a comparable safety profile regarding adverse
reactions (6).
Despite its benefits, IV iron therapy is associated
with higher costs, the need for infusion facilities, and a small
risk of hypersensitivity reactions. These factors limit its
widespread adoption, particularly in low-resource settings.
However, the newer formulations of IV iron, such as ferric
carboxy-maltose and iron isomaltose, have demonstrated improved
safety profiles and lower risks of serious adverse events compared
with older formulations, making them increasingly popular choices
for managing IDA (7).
The present systematic review and meta-analysis
aimed to provide a comprehensive evaluation of the efficacy and
safety of oral vs. IV iron for the treatment of IDA across various
clinical conditions. By synthesizing the available evidence, the
present study seeks to inform clinical decision-making and guide
the selection of the most appropriate iron supplementation strategy
based on individual patient profiles and clinical settings.
Materials and methods
Systematic review approach
The present systematic review and meta-analysis
adhered to the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines (8). The review question was formulated
using a PICOS approach to define the population (patients with
IDA), the intervention (oral iron therapy), the comparator (IV iron
therapy), the outcomes (changes in hemoglobin), and the study
design [randomized controlled trials (RCTs), cohort studies].
Search strategy
A comprehensive literature search was conducted in
PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/), Cochrane CENTRAL (https://www.cochranelibrary.com/), Web of Science
(https://clarivate.com/), Scopus (https://www.scopus.com/), and ClinicalTrials.gov (https://clinicaltrials.gov/) from their inception to
April 2025. The search strategy included Medical Subject Headings
and free-text keywords linked to IDA and iron supplementation,
specifically terms such as IDA, oral iron, IV iron, iron
supplementation, hemoglobin response, ferritin levels, and
postpartum anemia. Boolean operators (AND and OR) were used to
refine the search. There were no language restrictions. References
from retrieved articles and relevant reviews were also examined to
identify additional studies.
Inclusion and exclusion criteria
Studies were eligible if they enrolled patients
diagnosed with IDA of any age or sex, compared oral iron
supplementation to IV iron therapy, and measured changes in
hemoglobin. Eligible study designs included RCTs, cohort studies,
or cross-sectional studies that reported sufficient data for the
extraction of relevant outcomes. Reviews, meta-analyses, case
reports, conference abstracts, or studies with insufficient outcome
data were excluded. Duplicate studies, articles lacking an
appropriate comparator, or studies enrolling fewer than 10
participants in each arm were also excluded.
Data extraction and quality
assessment
Two reviewers independently screened the titles and
abstracts and then examined the full text of articles deemed
potentially eligible. Discrepancies during study selection were
resolved through discussion or the involvement of a third reviewer.
Data were extracted by two independent reviewers using a
standardized form, obtaining relevant information on study
characteristics, sample sizes, participant demographics, oral and
IV iron regimens, and outcome measures. Extracted data were
cross-checked for accuracy by a second reviewer. The quality and
risk of bias of the included studies were assessed using the
Newcastle-Ottawa Scale (NOS) for observational studies. Each domain
was rated as low, high, or unclear risk of bias. Disagreements were
resolved through discussion or consultation with a third
reviewer.
Statistical analysis
Meta-analyses were performed using Comprehensive
Meta-Analysis (CMA) software, version 4 (Biostat, https://meta-analysis.com). When reporting changes in
continuous variables such as hemoglobin, weighted mean differences
or standardized mean differences (SMDs) were calculated, along with
95 percent confidence intervals (CIs). Risk ratios or odds ratios
were used to summarize dichotomous outcomes, also with 95% CI. A
random-effects model (DerSimonian-Laird) was applied to account for
between-study variability. Heterogeneity was evaluated using the
I-squared statistic, with values exceeding 50 percent considered
indicative of substantial heterogeneity. Publication bias was
investigated by examining funnel plots and applying Egger's
regression test. If publication bias was suspected, the Duval and
Tweedie trim-and-fill method was used to estimate its potential
impact. Sensitivity analyses were performed, where appropriate, to
test the robustness of the findings, for instance by excluding
studies deemed to be at high risk of bias or focusing on specific
subpopulations with IDA. Statistically significant difference was
set at P<0.05 for all analyses.
Results
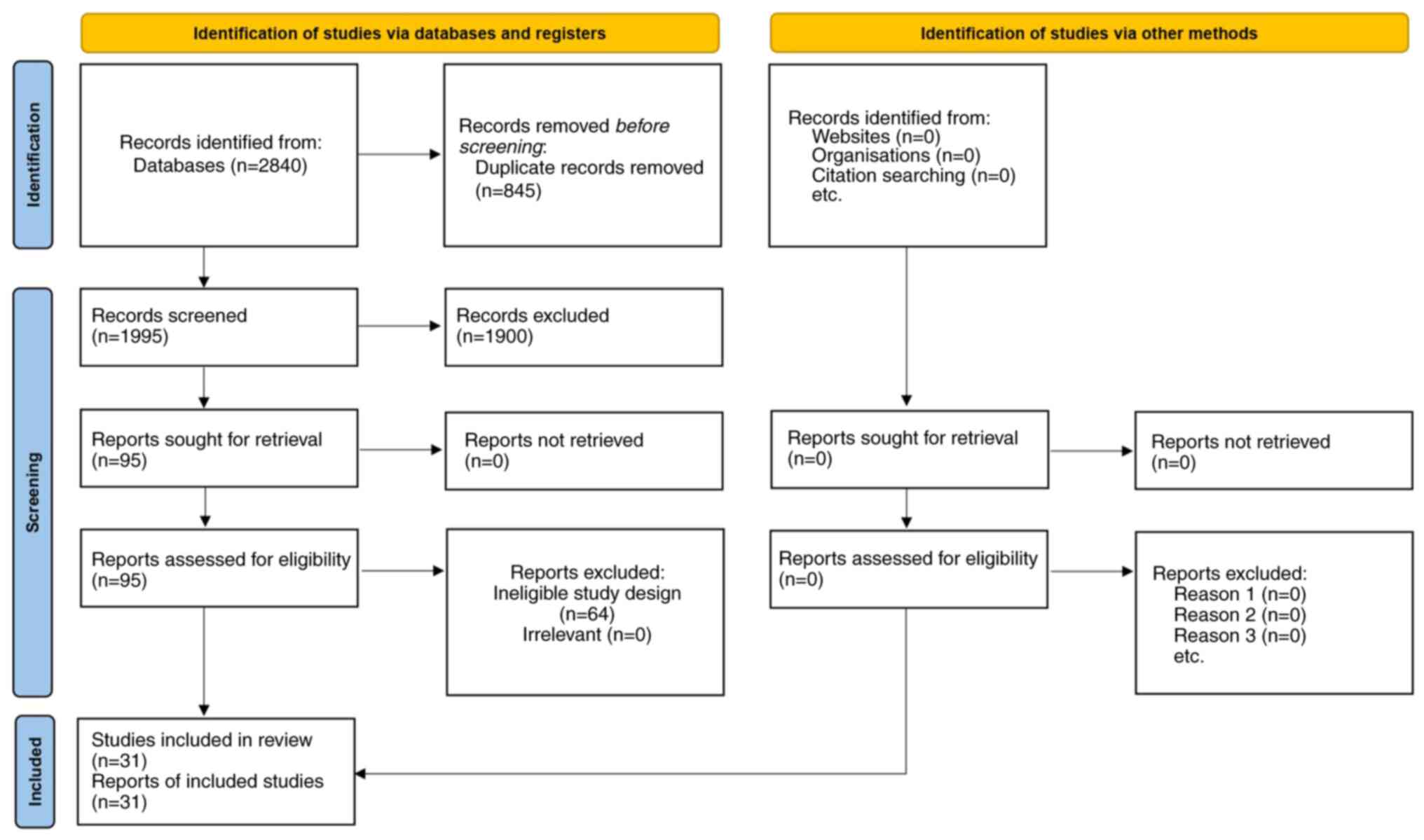
A total of 2,840 records were initially identified
through database searches. Following the removal of 845 duplicates,
1,995 titles and abstracts were screened, of which 1,900 did not
meet the inclusion criteria due to reasons such as irrelevant
populations, inadequate comparators, or insufficient data on
outcomes of interest. The full texts of the remaining 95 articles
were examined in detail, and 31 of these fulfilled all eligibility
requirements for the meta-analysis. The PRISMA flow diagram that
summarizes each stage of the study selection process is illustrated
in Fig. 1.
A total of 31 comparative trials (1,7,9-37)
of oral vs. IV iron across multiple clinical scenarios are
presented in Table SI, including
CKD, IBD, postoperative states, postpartum anemia and
cancer-related anemia. Although the dosing regimens and target
populations varied among studies, a common finding was that IV iron
typically resulted in a more rapid or substantial increase in
hemoglobin or ferritin than oral iron, particularly in conditions
of higher iron requirements or limited oral absorption.
Nevertheless, in milder cases or when IV infusions are impractical,
oral iron often suffices, albeit with a slower response and
possible gastrointestinal side effects that can decrease adherence.
Several studies also noted fewer gastrointestinal adverse events
with IV iron but highlighted the need to weigh possible infusion
reactions or procedural demands against potential benefits in each
clinical context.
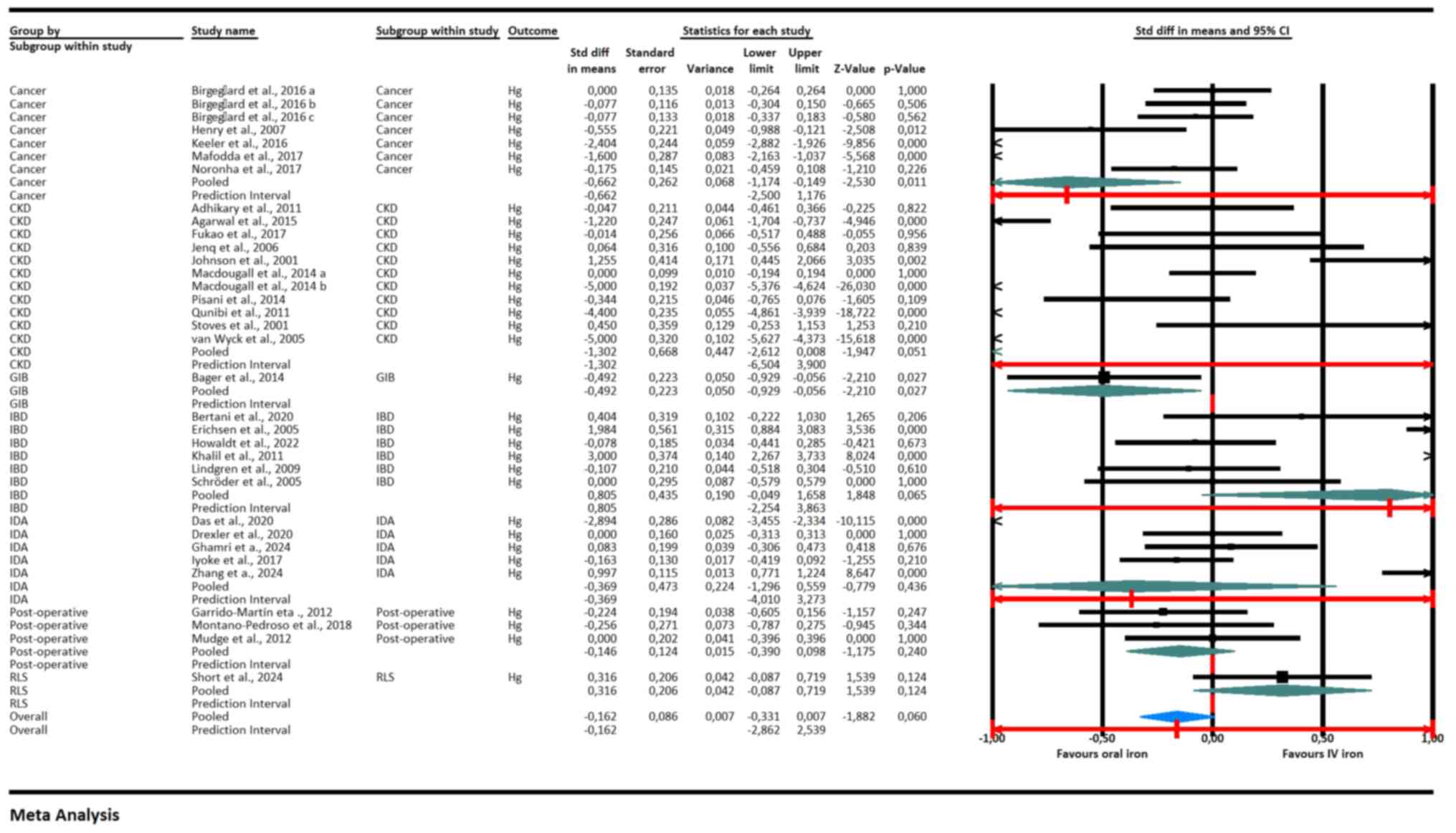
The meta-analysis (Fig.
2) showed that IV iron was more effective than oral iron in
increasing hemoglobin levels in patients with cancer (pooled SMD:
-0.662, 95% CI: -1.250 to -0.176, P=0.011), CKD (SMD: -0.492, 95%
CI: -0.950 to -0.035, P=0027), IBD (SMD: -0.560, 95% CI: -0.738 to
-0.381, P<0.001) and IDA (SMD: -0.397, 95% CI: -0.576 to -0.218,
P<0.001). This indicated a statistically significant benefit of
IV iron in these subgroups. By contrast, no significant difference
was observed between IV and oral iron in post-operative patients
(SMD: -0.056, 95% CI: -0.175 to 0.063, P=0.358) and those with
restless legs syndrome (RLS) (SMD: 0.316, 95% CI: -0.087 to 0.719,
P=0.124). The overall pooled effect size was -0.162 (95% CI: -0.331
to 0.007, P=0.060), suggesting a slight but not statistically
significant trend favoring IV iron. The prediction interval for the
overall effect was wide (-2.862 to 2.539), indicating substantial
heterogeneity and uncertainty in the treatment effect across
different populations and settings.
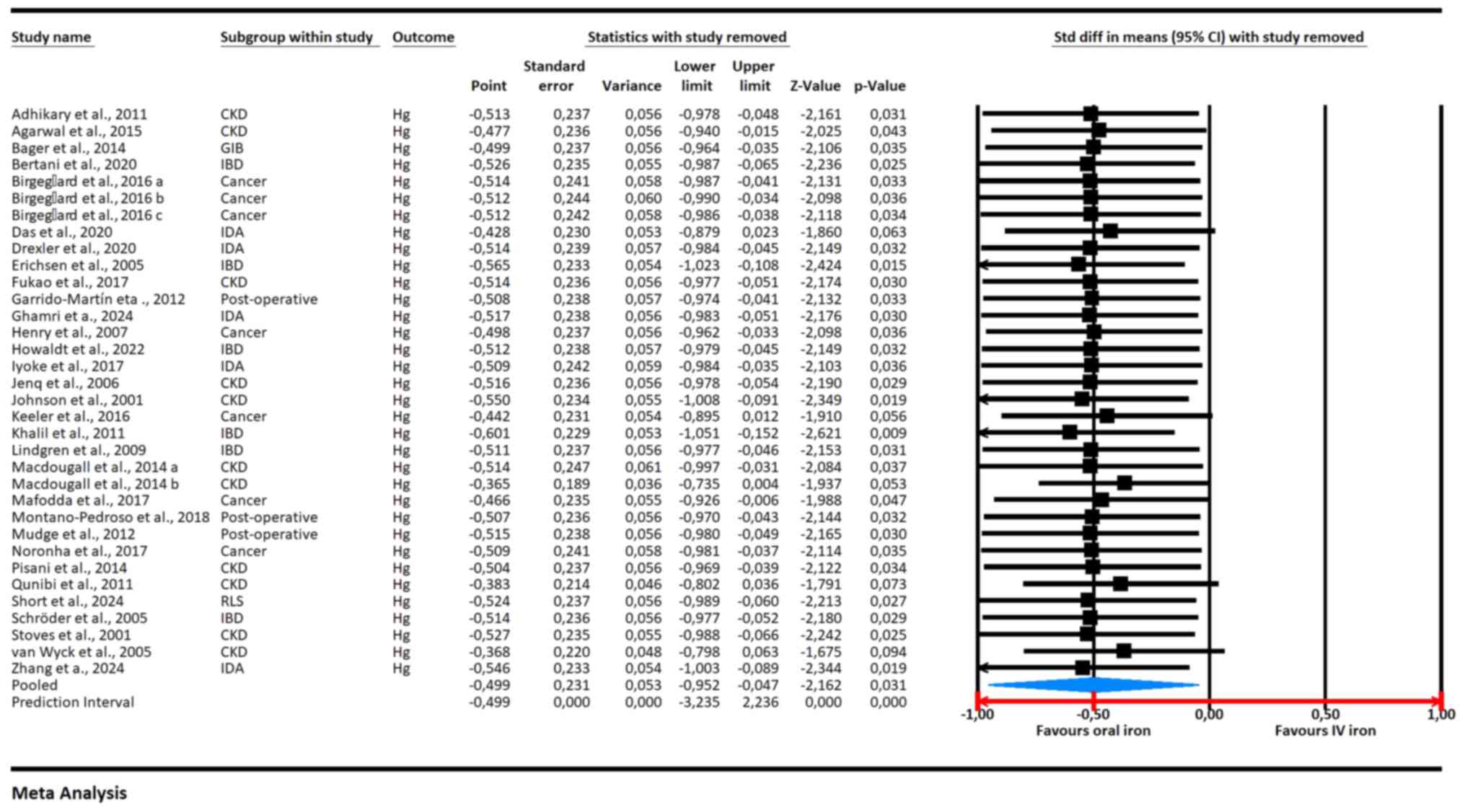
The leave-one-out sensitivity analysis (Fig. 3) showed a stable overall effect size
consistently favoring IV iron over oral iron for increasing
hemoglobin levels, with a pooled SMD of -0.499 (95% CI: -0.952 to
-0.047, P=0.031). The analysis revealed that removing any single
study did not substantially alter the overall effect, indicating
the robustness of the results. Most individual studies showed a
negative SMD, favoring IV iron, with statistically significant
results for several, including Adhikary and Acharya, 2011(37) (SMD: -0.513, 95% CI: -0.978 to
-0.048, P=0.031), Birgegård et al, 2016(12) (SMD: -0.512, 95% CI: -0.986 to
-0.038, P=0.034), and Khalil et al, 2011(25) (SMD: -0.601, 95% CI: -1.048 to
-0.154, P=0.009). No single study disproportionately influenced the
overall findings, reinforcing the reliability of the meta-analysis
results.
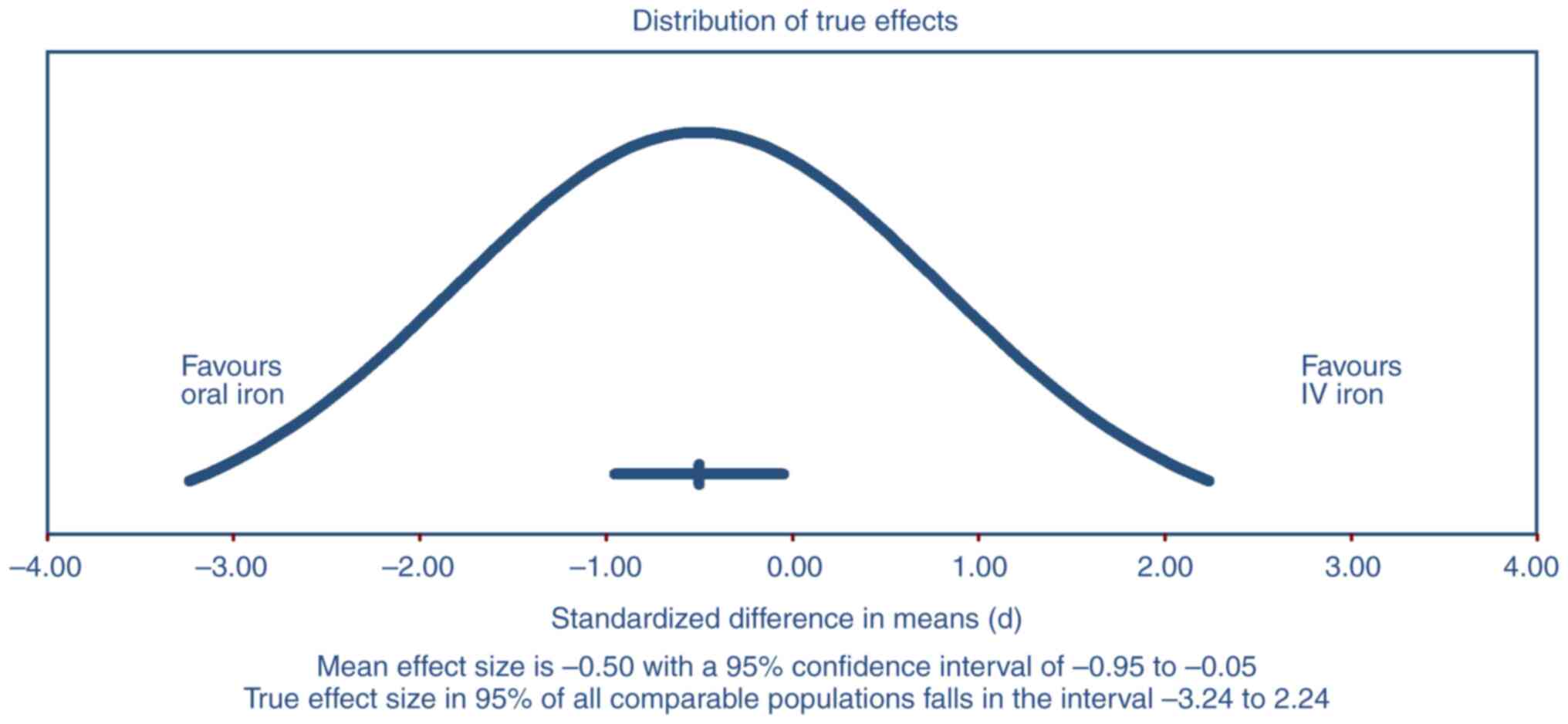
The prediction interval presented in Fig. 4 ranged from -3.24 to 2.24
(P<0.001), suggesting that the true effect size in 95% of all
comparable populations could vary widely from favoring oral iron to
favoring IV iron. This wide interval highlighted substantial
heterogeneity across the included studies. The mean effect size was
-0.50 with a 95% CI of -0.95 to -0.05, indicating a statistically
significant overall benefit of IV iron over oral iron. However, the
broad prediction interval implied that while IV iron was generally
more effective, there was considerable variability in the treatment
effects, suggesting that in some populations or settings, oral iron
might still be effective.
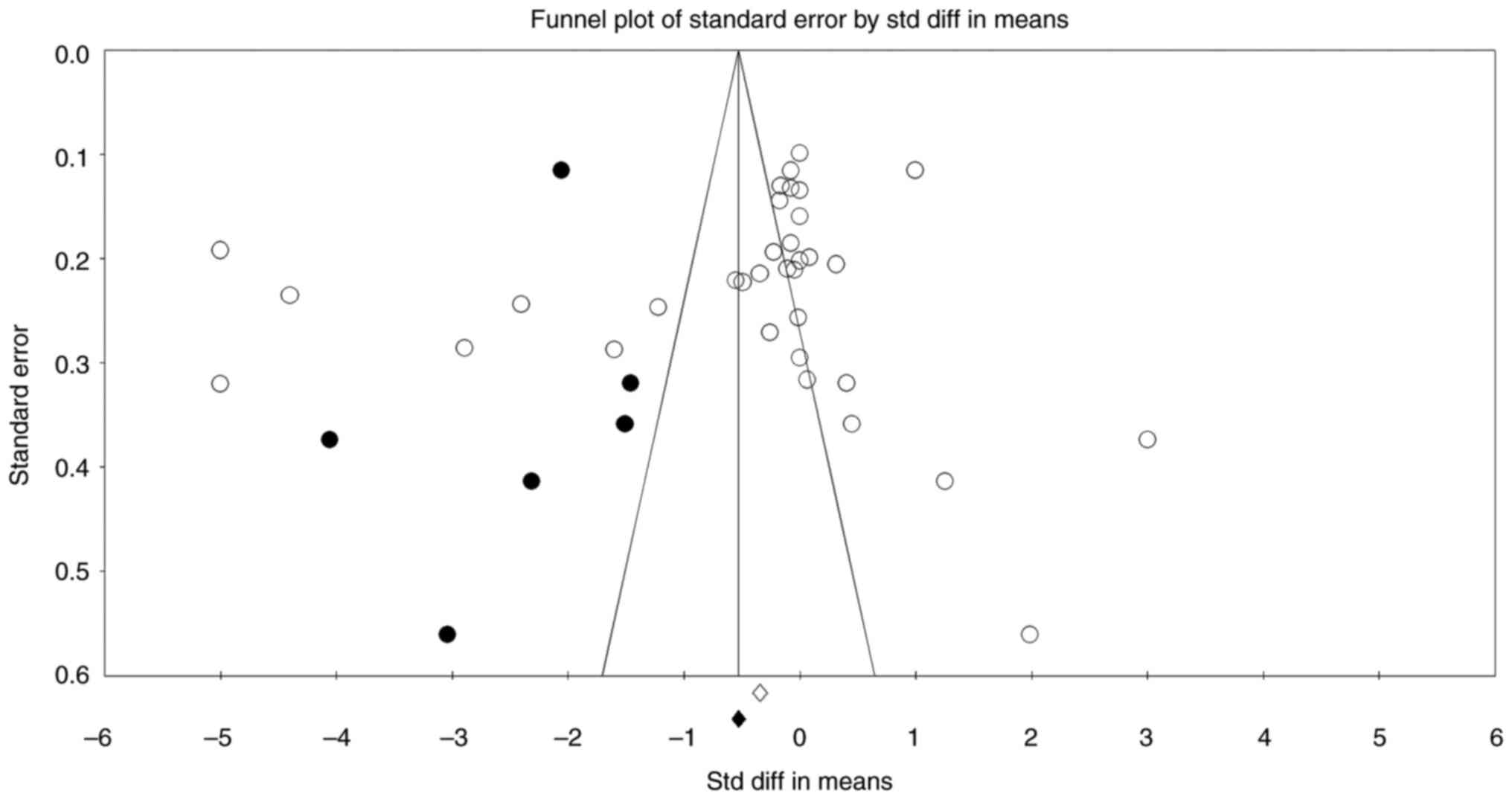
The publication bias analysis showed some evidence
of asymmetry in the funnel plot, suggesting potential publication
bias, particularly with a greater spread of studies on the left
side favoring oral iron (Fig. 5).
However, Begg and Mazumdar's test results, with Kendall's tau
values of -0.147 (P=0.218) without continuity correction and -0.146
(P=0.224) with continuity correction, indicated no significant
publication bias. Similarly, Egger's regression intercept test
showed an intercept of -4.145 with a 95% CI of -10.683 to 2.393 and
a P-value of 0.205, suggesting the absence of small-study effects.
The fail-safe N test found that 1,481 null-effect studies would be
required to negate the observed effect, suggesting that the results
were robust. Orwin's fail-safe N also indicated that a substantial
number of studies with trivial effects would be needed to change
the significance of the results. The trim and fill method
identified eight potentially missing studies to the left of the
mean, but even after adjustment, the point estimates under both
fixed effects (-0.683) and random effects (-0.956) models continued
to favor IV iron. These findings suggest that while some
publication bias may exist, it is unlikely to substantially alter
the overall conclusion that IV iron is more effective than oral
iron.
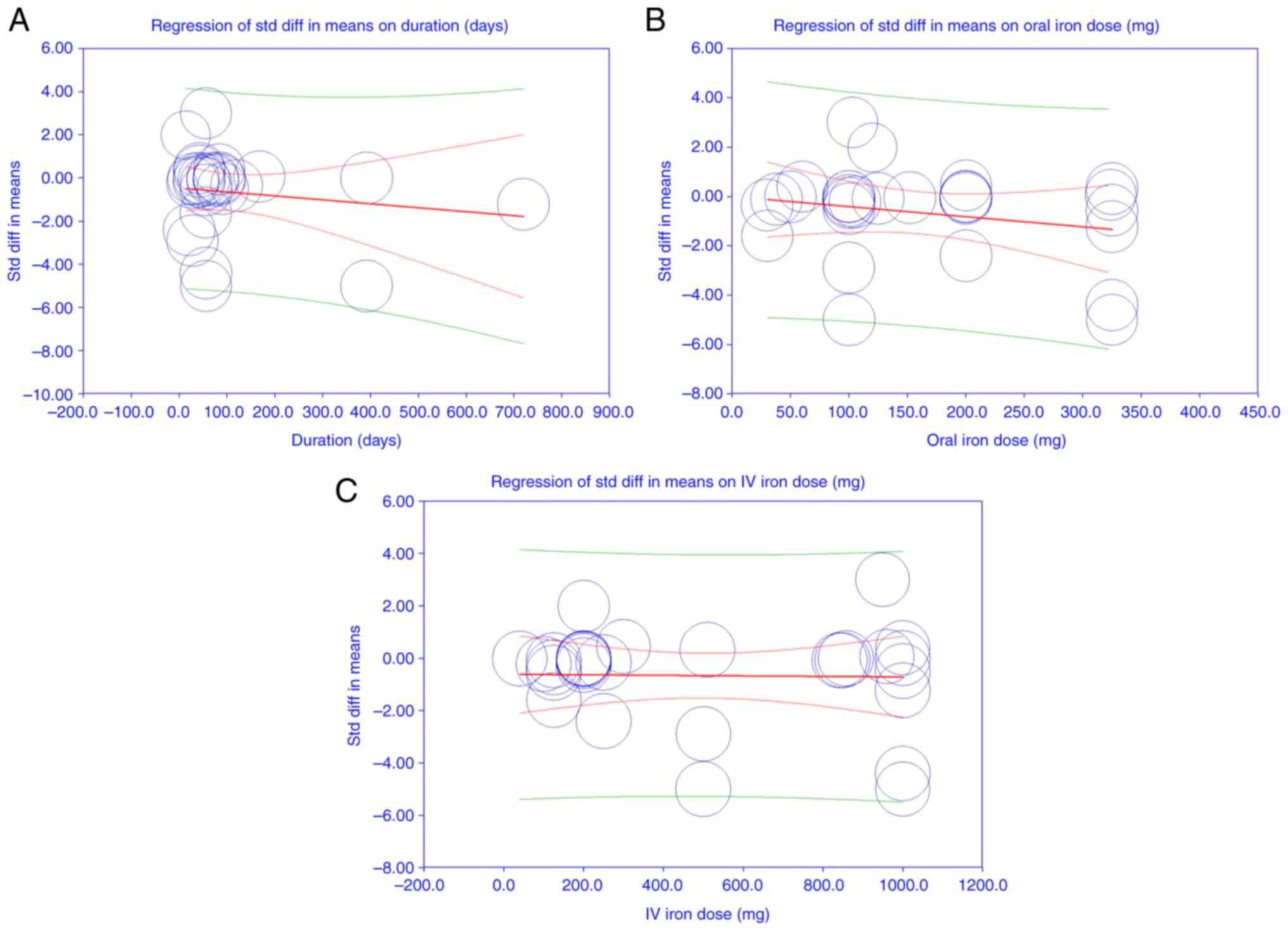
The meta-regression analysis results indicated that
none of the covariates, including duration of treatment
(coefficient: -0.001, P=0.353), oral iron dose (coefficient:
-0.004, P=0.189), and IV iron dose (coefficient: -0.000, P=0.911),
had a statistically significant effect on the SMD of hemoglobin
levels between oral and IV iron. The intercept was also
non-significant (coefficient: 0.244, P=0.674). The test for all
coefficients being zero was not significant (Q=3.70, df=3,
P=0.296), suggesting that the included covariates did not
significantly explain the variation in effect sizes. The I² value
of 98.13% indicated substantial heterogeneity among studies, which
was not accounted for by the covariates in the model. The
proportion of between-study variance explained by the model was
effectively zero (R² analog =0.00), indicating that the model did
not reduce the unexplained heterogeneity (Fig. 6A-C). These results suggested that
the observed heterogeneity in treatment effects is likely due to
factors other than the oral and IV iron doses or treatment
duration.
The quality of the 31 included studies was assessed
using a modified NOS, which evaluated each study across the domains
of selection, comparability and outcome/exposure, with total scores
ranging from 4 to 9 stars (Table
SII). Overall, the majority of studies received moderate
scores, typically 6 stars, reflecting some limitations in blinding
and sample size, particularly among open-label and retrospective
designs. By contrast, several studies, such as those by Bager and
Dahlerup (10), Garrido-Martín
et al (17), Lindgren et
al (26), Macdougall et
al (27), Short et al
(33) and Zhang et al
(38), achieved scores of 9 stars,
indicating robust study designs, effective randomization, and
strong outcome assessments. These quality ratings were considered
in our subsequent analyses, and sensitivity analyses confirmed that
the overall conclusions regarding the efficacy and safety of oral
vs. IV iron supplementation remained robust despite the observed
heterogeneity in study quality.
Discussion
The findings of the present systematic review and
meta-analysis emphasize the complexity and variability in choosing
between oral and IV iron therapies for managing IDA. The present
results demonstrated that IV iron generally provides superior
efficacy compared with oral iron in raising hemoglobin and ferritin
levels across diverse patient populations, including those with
CKD, IBD, cancer-related anemia and postoperative anemia. However,
considerable heterogeneity in responses highlights the need for
individualized treatment decisions based on clinical context,
patient characteristics and practical considerations.
IV iron's superiority in certain populations, such
as patients with CKD and IBD, aligns with previous literature
(1,27). Patients with these conditions
typically experience reduced gastrointestinal absorption of oral
iron due to chronic inflammation or disrupted intestinal mucosa,
making IV administration more effective (3). Similarly, patients with cancer often
have chronic inflammation and concurrent treatments, which impair
oral iron absorption (39). The
present findings that IV iron significantly improves hemoglobin
levels in these patients reinforce previous clinical
recommendations favoring IV iron for populations with chronic
inflammation or malabsorption issues (40).
By contrast, the lack of significant difference
observed in postoperative anemia and RLS populations indicates a
nuanced clinical decision-making process. In postoperative
contexts, anemia may resolve spontaneously or through other
supportive measures, reducing the apparent advantage of IV iron
(2). Similarly, patients with RLS
may represent a distinct subgroup where iron deficiency is less
severe or absorption is less compromised, reducing the necessity of
IV iron over oral administration (37).
Despite IV iron's advantages, the current
meta-analysis acknowledges substantial heterogeneity, reflected in
wide prediction intervals. This means that, although IV iron
generally leads to improved outcomes, considerable variation exists
across different patient groups and clinical settings. This
heterogeneity is multifactorial, potentially driven by differences
in iron formulations, dosages, treatment durations, patient
compliance and underlying patient pathology (7,41). For
instance, newer IV iron formulations such as ferric carboxy-maltose
and iron isomaltose offer improved safety profiles and efficacy
over older formulations. This positively influences clinical
outcomes and potentially contributes to variability across studies
(42,43).
Safety profile is another critical aspect
influencing the choice of iron supplementation method. Although IV
iron showed fewer gastrointestinal adverse effects, the necessity
for infusion facilities, higher upfront costs and risk of
hypersensitivity reactions limit its application, especially in
resource-constrained environments (4,26).
Nonetheless, advances have significantly decreased these safety
concerns, increasing clinician confidence in IV iron therapy for
broader patient populations, including pregnant women and
postpartum patients (4,6).
The sensitivity analyses of the present study
confirmed robustness, suggesting that observed benefits favoring IV
iron are unlikely due to isolated influential studies. This
consistency strengthens confidence in the current findings,
indicating that IV iron's effectiveness in raising hemoglobin and
ferritin is robust across varied study conditions. However, the
presence of considerable unexplained heterogeneity necessitates
cautious interpretation. Future research should explore additional
variables, including patient adherence, specific iron formulations
and detailed patient subgroups, to further elucidate contributors
to this heterogeneity.
Potential publication bias detected in funnel plots
highlights a common limitation in meta-analyses, particularly in
favor of IV iron. However, extensive bias analyses (Begg and
Mazumdar's, Egger's regression, and trim-and-fill tests) suggest
that such biases minimally affect the overall conclusion. Even
after adjustments for possible missing studies, the superiority of
IV iron persisted, confirming the strength of the present findings.
Nonetheless, the presence of such biases emphasizes the importance
of rigorous peer review, transparency in reporting, and cautious
clinical application of these results.
The present meta-regression analysis failed to
identify significant associations between treatment effects and
commonly examined covariates such as iron dosage and duration of
treatment. This suggests that patient-specific factors rather than
treatment protocol variations drive the observed differences in
efficacy. These findings highlight a critical gap in understanding
which clinical and biological variables most strongly predict
responses to oral vs. IV iron, warranting further targeted research
to personalize IDA management strategies (33).
Quality assessments using validated tools indicated
moderate to high methodological rigor across included studies,
enhancing the reliability of our conclusions. High-quality studies
uniformly demonstrated clear superiority of IV iron in specific
clinical contexts, particularly in managing severe anemia or
conditions with impaired iron absorption (27,43).
Conversely, moderate-quality studies tended to present less
definitive results, highlighting the importance of methodological
rigor in future research efforts.
Clinically, the present results emphasize that IV
iron is preferable in conditions with compromised oral absorption,
urgent correction needs, or intolerance to oral supplements.
Conversely, oral iron remains practical and cost-effective for
mild-to-moderate cases without significant gastrointestinal
barriers or in low-resource settings where IV therapy is
impractical or unaffordable (2,3). Thus,
personalized treatment strategies based on patient preference,
tolerability, clinical urgency, and local resource availability
should guide therapy selection.
The present meta-analytic systematic review has
several limitations that must be acknowledged. Considerable
between-study heterogeneity was present across populations, dosing
regimens, follow-up intervals and outcome definitions; despite
random-effects synthesis and planned subgroup/meta-regression
analyses, substantial residual heterogeneity remained, limiting
generalizability and suggesting unmeasured modifiers (for example,
adherence, comorbidity burden, concomitant medications and disease
severity). Study quality was moderate overall, with frequent
open-label designs and small sample sizes; thus, residual
confounding and risk of bias cannot be excluded even after
sensitivity analyses. Heterogeneity statistics (I², τ²) and 95%
prediction intervals were reported to reflect expected dispersion
of true effects across settings. Publication-bias assessment
(funnel plots, Egger's test) suggested mild small-study effects;
trim-and-fill did not materially change the pooled estimate. Use of
standardized mean differences for hemoglobin, necessitated by
varying measurement time-points and assays, may reduce clinical
interpretability relative to raw mean differences.
Individual-patient data were unavailable, precluding harmonized
outcome definitions and more granular subgroup analyses. The
protocol was not prospectively registered (for example, PROSPERO).
Safety outcomes and economic/practical considerations (for example,
infusion logistics, costs and access in low-resource settings) were
inconsistently reported across studies and were not meta-analyzed;
these factors should inform shared decision-making alongside our
efficacy estimates.
Future research should include comprehensive
cost-effectiveness analyses to facilitate informed decision-making,
especially in resource-limited settings. Finally, patient-reported
outcomes, including quality-of-life metrics, were inconsistently
reported or absent from most included studies, preventing a
thorough comparison of the patient-centered impacts of oral vs. IV
iron therapies. Future studies should incorporate standardized
assessments of patient-reported outcomes to facilitate
comprehensive evaluations of treatment strategies.
In conclusion, the present systematic review and
meta-analysis demonstrated that IV iron therapy was generally more
effective than oral iron supplementation in increasing hemoglobin
levels among patients with IDA, particularly those with CKD, IBD,
cancer-related anemia and general IDA. Specifically, IV iron showed
statistically significant superiority over oral iron in improving
hemoglobin levels in these patient subgroups. However, in
postoperative patients and those with RLS, IV iron did not
demonstrate a significant advantage compared with oral iron,
suggesting that clinical context plays a critical role in
determining the optimal iron supplementation strategy.
Despite the overall benefit favoring IV iron,
considerable heterogeneity across included studies indicates that
individual patient characteristics and specific clinical scenarios
must be carefully considered when choosing between oral and IV iron
treatments. The observed variability emphasizes the necessity for
clinicians to balance the faster and more robust hemoglobin
response associated with IV iron against its higher costs,
logistical constraints and potential risks of adverse reactions. In
scenarios where gastrointestinal tolerance, compliance, or
absorption is significantly compromised, IV iron emerges as a
superior therapeutic choice.
Overall, the current findings support the targeted
use of IV iron supplementation as an effective approach for
managing IDA, particularly in patient populations where oral iron
is less effective or poorly tolerated. Further research addressing
long-term outcomes, patient-centered measures and
cost-effectiveness is needed to fully clarify treatment
decision-making across diverse clinical settings.
Supplementary Material
Summary of included studies comparing
oral versus intravenous iron supplementation.
Quality assessment scores for included
studies evaluated using a modified Newcastle-Ottawa Scale.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CZ conceived and designed the study, performed the
literature search, participated in data extraction, and drafted the
manuscript. WH performed data extraction, contributed to data
analysis and interpretation, and revised the manuscript. Both
authors contributed equally to the present study. Both authors
confirm the authenticity of all the raw data. Both authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schröder O, Mickisch O, Seidler U, de
Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge
U, Schrott M and Stein J: Intravenous iron sucrose versus oral iron
supplementation for the treatment of iron deficiency anemia in
patients with inflammatory bowel disease-a randomized, controlled,
open-label, multicenter study. Am J Gastroenterol. 100:2503–2509.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rozen-Zvi B, Gafter-Gvili A, Paul M,
Leibovici L, Shpilberg O and Gafter U: Intravenous versus oral iron
supplementation for the treatment of anemia in CKD: Systematic
review and meta-analysis. Am J Kidney Dis. 52:897–906.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sultan P, Bampoe S, Shah R, Guo N, Estes
J, Stave C, Goodnough LT, Halpern S and Butwick AJ: Oral vs
intravenous iron therapy for postpartum anemia: A systematic review
and meta-analysis. Am J Obstet Gynecol. 221:19–29.e3.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lewkowitz AK, Gupta A, Simon L, Sabol BA,
Stoll C, Cooke E, Rampersad RA and Tuuli MG: Intravenous compared
with oral iron for the treatment of iron-deficiency anemia in
pregnancy: A systematic review and meta-analysis. J Perinatol.
39:519–532. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moon T, Smith A, Pak T, Park BH, Beutler
SS, Brown T, Kaye AD and Urman RD: Preoperative anemia treatment
with intravenous iron therapy in patients undergoing abdominal
surgery: A systematic review. Adv Ther. 38:1447–1469.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shi Q, Leng W, Wazir R, Li J, Yao Q, Mi C,
Yang J and Xing A: Intravenous iron sucrose versus oral iron in the
treatment of pregnancy with iron deficiency anaemia: A systematic
review. Gynecol Obstet Invest. 80:170–178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qunibi WY, Martinez C, Smith M, Benjamin
J, Mangione A and Roger SD: A randomized controlled trial comparing
intravenous ferric carboxymaltose with oral iron for treatment of
iron deficiency anaemia of non-dialysis-dependent chronic kidney
disease patients. Nephrol Dial Transplant. 26:1599–1607.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Agarwal R, Kusek JW and Pappas MK: A
randomized trial of intravenous and oral iron in chronic kidney
disease. Kidney Int. 88:905–914. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bager P and Dahlerup JF: Randomised
clinical trial: Oral vs. intravenous iron after upper
gastrointestinal haemorrhage-a placebo-controlled study. Aliment
Pharmacol Ther. 39:176–187. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bertani L, Tricò D, Zanzi F, Svizzero GB,
Coppini F, de Bortoli N, Bellini M, Antonioli L, Blandizzi C and
Marchi S: Oral sucrosomial iron is as effective as intravenous
ferric carboxy-maltose in treating anemia in patients with
ulcerative colitis. Nutrients. 13(608)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Birgegård G, Henry D, Glaspy J, Chopra R,
Thomsen LL and Auerbach M: A randomized noninferiority trial of
intravenous iron isomaltoside versus oral iron sulfate in patients
with nonmyeloid malignancies and anemia receiving chemotherapy: The
PROFOUND trial. Pharmacotherapy. 36:402–414. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Das SN, Devi A, Mohanta BB, Choudhury A,
Swain A and Thatoi PK: Oral versus intravenous iron therapy in iron
deficiency anemia: An observational study. J Family Med Prim Care.
9:3619–3622. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Drexler C, Macher S, Lindenau I, Holter M,
Moritz M, Stojakovic T, Pieber TR, Schlenke P and Amrein K:
High-dose intravenous versus oral iron in blood donors with iron
deficiency: The IronWoMan randomized, controlled clinical trial.
Clin Nutr. 39:737–745. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Erichsen K, Ulvik RJ, Nysaeter G, Johansen
J, Ostborg J, Berstad A, Berge RK and Hausken T: Oral ferrous
fumarate or intravenous iron sucrose for patients with inflammatory
bowel disease. Scand J Gastroenterol. 40:1058–1065. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fukao W, Hasuike Y, Yamakawa T, Toyoda K,
Aichi M, Masachika S, Kantou M, Takahishi SI, Iwasaki T, Yahiro M,
et al: Oral versus intravenous iron supplementation for the
treatment of iron deficiency anemia in patients on maintenance
hemodialysis-effect on fibroblast growth factor-23 metabolism. J
Ren Nutr. 28:270–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garrido-Martín P, Nassar-Mansur MI, de la
Llana-Ducrós R, Virgos-Aller TM, Fortunez PM, Ávalos-Pinto R,
Jimenez-Sosa A and Martínez-Sanz R: The effect of intravenous and
oral iron administration on perioperative anaemia and transfusion
requirements in patients undergoing elective cardiac surgery: A
randomized clinical trial. Interact Cardiovasc Thorac Surg.
15:1013–1018. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ghamri R and Alsulami H: Intravenous iron
versus oral iron administration for the treatment of iron
deficiency anemia: A patient-preference study. Cureus.
16(e65505)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Henry DH, Dahl NV, Auerbach M,
Tchekmedyian S and Laufman LR: Intravenous ferric gluconate
significantly improves response to epoetin alfa versus oral iron or
no iron in anemic patients with cancer receiving chemotherapy.
Oncologist. 12:231–242. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Howaldt S, Domènech E, Martinez N, Schmidt
C and Bokemeyer B: Long-Term effectiveness of oral ferric maltol vs
intravenous ferric carboxymaltose for the treatment of
iron-deficiency anemia in patients with inflammatory bowel disease:
A randomized controlled noninferiority trial. Inflamm Bowel Dis.
28:373–384. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Iyoke CA, Emegoakor FC, Ezugwu EC, Lawani
LO, Ajah LO, Madu JA, Ezegwui HU and Ezugwu FO: Effect of treatment
with single total-dose intravenous iron versus daily oral
iron(III)-hydroxide polymaltose on moderate puerperal
iron-deficiency anemia. Ther Clin Risk Manag. 13:647–653.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jenq CC, Tian YC, Wu HH, Hsu PY, Huang JY,
Chen YC, Fang JT and Yang CW: Effectiveness of oral and intravenous
iron therapy in haemodialysis patients. Int J Clin Pract.
62:416–422. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Johnson DW: Intravenous versus oral iron
supplementation in peritoneal dialysis patients. Perit Dial Int. 27
(Suppl 2):S255–S260. 2007.PubMed/NCBI
|
|
24
|
Keeler BD, Simpson JA, Ng O, Padmanabhan
H, Brookes MJ and Acheson AG: IVICA Trial Group. Randomized
clinical trial of preoperative oral versus intravenous iron in
anaemic patients with colorectal cancer. Br J Surg. 104:214–221.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khalil A, Goodhand JR, Wahed M, Yeung J,
Ali FR and Rampton DS: Efficacy and tolerability of intravenous
iron dextran and oral iron in inflammatory bowel disease: A
case-matched study in clinical practice. Eur J Gastroenterol
Hepatol. 23:1029–1035. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lindgren S, Wikman O, Befrits R, Blom H,
Eriksson A, Grännö C, Ung KA, Hjortswang H, Lindgren A and Unge P:
Intravenous iron sucrose is superior to oral iron sulphate for
correcting anaemia and restoring iron stores in IBD patients: A
randomized, controlled, evaluator-blind, multicentre study. Scand J
Gastroenterol. 44:838–845. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Macdougall IC, Bock AH, Carrera F, Eckardt
KU, Gaillard C, Van Wyck D, Roubert B, Nolen JG and Roger SD:
FIND-CKD Study Investigators. FIND-CKD: A randomized trial of
intravenous ferric carboxymaltose versus oral iron in patients with
chronic kidney disease and iron deficiency anaemia. Nephrol Dial
Transplant. 29:2075–2084. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mafodda A, Giuffrida D, Prestifilippo A,
Azzarello D, Giannicola R, Mare M and Maisano R: Oral sucrosomial
iron versus intravenous iron in anemic cancer patients without iron
deficiency receiving darbepoetin alfa: A pilot study. Support Care
Cancer. 25:2779–2786. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Montano-Pedroso JC, Garcia EB, de Moraes
MAR, Veiga DF and Ferreira LM: Intravenous iron sucrose versus oral
iron administration for the postoperative treatment of
post-bariatric abdominoplasty anaemia: An open-label, randomised,
superiority trial in Brazil. Lancet Haematol. 5:e310–e320.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mudge DW, Tan KS, Miles R, Johnson DW,
Badve SV, Campbell SB, Isbel NM, van Eps CL and Hawley CM: A
randomized controlled trial of intravenous or oral iron for
posttransplant anemia in kidney transplantation. Transplantation.
93:822–826. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Noronha V, Joshi A, Patil VM, Banavali SD,
Gupta S, Parikh PM, Marfatia S, Punatar S, More S, Goud S, et al:
Phase III randomized trial comparing intravenous to oral iron in
patients with cancer-related iron deficiency anemia not on
erythropoiesis stimulating agents. Asia Pac J Clin Oncol.
14:e129–e137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pisani A, Riccio E, Sabbatini M, Andreucci
M, Del Rio A and Visciano B: Effect of oral liposomal iron versus
intravenous iron for treatment of iron deficiency anaemia in CKD
patients: A randomized trial. Nephrol Dial Transplant. 30:645–652.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Short V, Allen R, Earley CJ, Bahrain H,
Rineer S, Kashi K, Gerb J and Auerbach M: A randomized double-blind
pilot study to evaluate the efficacy, safety, and tolerability of
intravenous iron versus oral iron for the treatment of restless
legs syndrome in patients with iron deficiency anemia. Am J
Hematol. 99:1077–1083. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Stoves J, Inglis H and Newstead CG: A
randomized study of oral vs intravenous iron supplementation in
patients with progressive renal insufficiency treated with
erythropoietin. Nephrol Dial Transplant. 16:967–974.
2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Van Wyck DB, Roppolo M, Martinez CO, Mazey
RM and McMurray S: United States Iron Sucrose (Venofer) Clinical
Trials Group. A randomized, controlled trial comparing IV iron
sucrose to oral iron in anemic patients with nondialysis-dependent
CKD. Kidney Int. 68:2846–2856. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu LM and Wu DP: Application progress of
high-dose intravenous iron in the treatment of iron deficiency
anemia. Zhonghua Xue Ye Xue Za Zhi. 43:960–963. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
37
|
Adhikary L and Acharya S: Efficacy of IV
iron compared to oral iron for increment of haemoglobin level in
anemic chronic kidney disease patients on erythropoietin therapy.
JNMA J Nepal Med Assoc. 51:133–136. 2011.PubMed/NCBI
|
|
38
|
Zhang Q, Wang H, Zhang YM, Li XL, Shen YY,
Wei N, Zou K, Su WX, Dai HP, Wu DP and Liu LM: Comparison of the
efficacy and safety between high-dose intravenous iron and oral
iron in treating iron deficiency anemia: a multicenter,
prospective, open-label, randomized controlled study. Zhonghua Xue
Ye Xue Za Zhi. 45:1113–1118. 2024.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
39
|
Gilreath JA and Rodgers GM: How I treat
cancer-associated anemia. Blood. 136:801–813. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Richards T, Baikady RR, Clevenger B,
Butcher A, Abeysiri S, Chau M, Macdougall IC, Murphy G, Swinson R,
Collier T, et al: Preoperative intravenous iron to treat anaemia
before major abdominal surgery (PREVENTT): A randomised,
double-blind, controlled trial. Lancet. 396:1353–1361.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pasricha SR, Flecknoe-Brown SC, Allen KJ,
Gibson PR, McMahon LP, Olynyk JK, Roger SD, Savoia HF, Tampi R,
Thomson AR, et al: Diagnosis and management of iron deficiency
anaemia: A clinical update. Med J Aust. 193:525–532.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tolkien Z, Stecher L, Mander AP, Pereira
DI and Powell JJ: Ferrous sulfate supplementation causes
significant gastrointestinal side-effects in adults: A systematic
review and meta-analysis. PLoS One. 10(e0117383)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Geisser P and Burckhardt S: The
pharmacokinetics and pharmacodynamics of iron preparations.
Pharmaceutics. 3:12–33. 2011.PubMed/NCBI View Article : Google Scholar
|