Introduction
Seminoma, comprising 55% of all testicular
malignancies, is the most common subtype of testicular cancer
(1). The widely accepted hypothesis
for the origin of testicular germ cell tumors (TGCT) is that they
derive from primordial germ cell or gonocytes whose maturation is
disturbed. Seminoma usually occurs in patients ranging from 15 to
44 years of age (2). It is reported
that epidemiological risk factors of TGCT include previous TGCT in
the contralateral testis, cryptorchidism, hypospadias, male
infertility and exposure to environment factors, such as
organochlorines, polychlorinated biphenyls, polyvinyl chloride,
phthalates, cannabis and tobacco (3). A primary abdominal seminoma harboring
KIT mutation was reported, which was misdiagnosed as
gastrointestinal stromal tumor via biopsy pathology, revealing the
diagnostic pitfalls caused by atypical pathological features,
insufficient immunohistochemical data and inadequate understanding
of the KIT mutation-associated disease spectrum.
Case presentation
A 53-year-old male patient was admitted to a local
hospital due to fatigue in March 2024, with no previous medical
history. The 53-year-old male patient was found to have a huge
abdominal mass with a maximum diameter of 16 cm, via computed
tomography (CT). Ultrasound-guided needle biopsy was performed to
establish a definitive histopathological diagnosis prior to
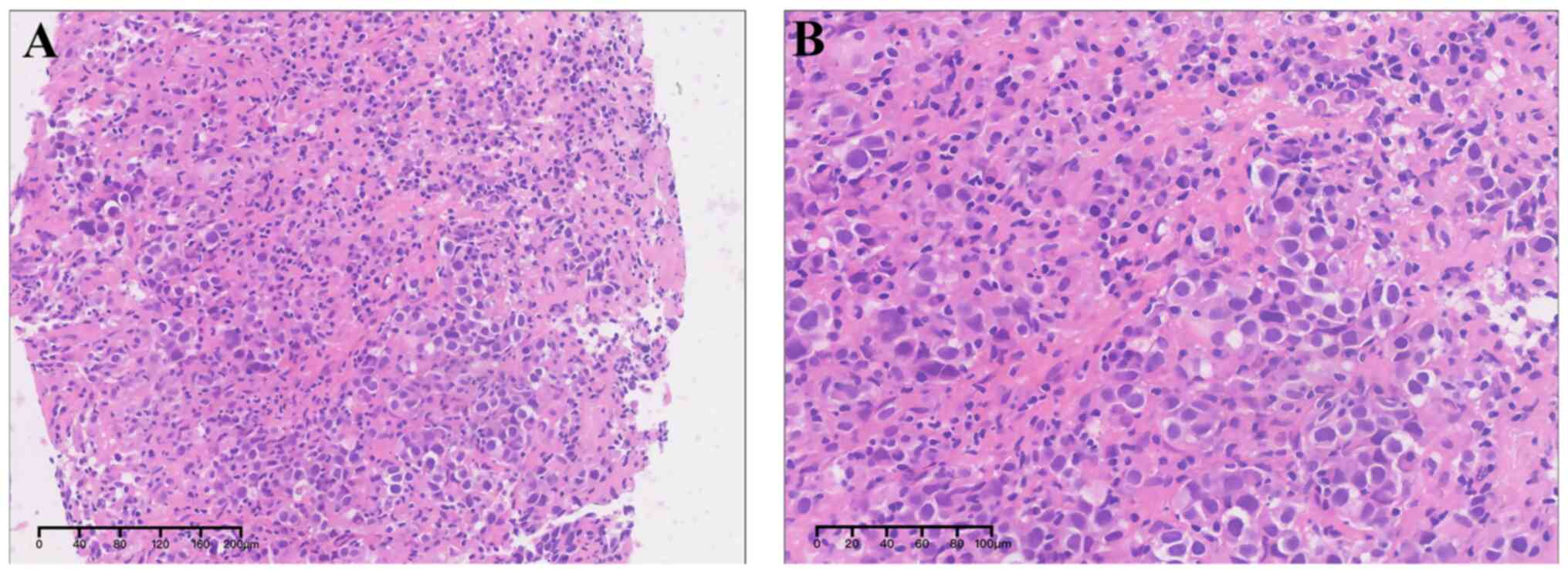
multidisciplinary therapeutic planning. Histologically,
significantly atypical cells, characterized by nuclei pleomorphism,
increased nucleoplasmic ratio, prominent nucleoli, granular
chromatin and visible mitotic figures, were observed to be
diffusely distributed, with scattered infiltration of inflammatory
cells, small vessel formation, and fibrous tissue hyperplasia
(Fig. 1). Immunohistochemical
staining for CD117, DOG-1, CD34, Des, and S100 was performed using
the DAKO immunohistochemical automatic staining machine (DAKO
Omnis/GI100) following Envision 2-step protocol. The primary
antibodies used were CD117 rabbit anti-human (clone 104D2; 1:100
dilution), Des rabbit anti-human (clone D33; 1:100 dilution), and
S100 rabbit anti-human (clone A5109; 1:500 dilution) purchased from
Dako; Agilent Technologies, Inc., DOG-1 rabbit anti-human (clone
SP31, 1:100 dilution) purchased from Abcam, and CD34 mouse
anti-human (clone QBEnd/10, 1:100 dilution) purchased from Shanghai
Long Island Antibody Diagnostic Inc. In brief, the tissue sections
were subjected to dewaxing and heat-induced antigen retrieval at
95˚C. Endogenous peroxidase activity was blocked with 0.3% hydrogen
peroxide at 37˚C for 30 min. Antigen retrieval was performed in
citrate buffer (10 mmol/l, pH 6.0) at 100°C for 30 min, followed by
three 5-minute washes in PBS. The sections were then blocked with
10% normal goat serum [Biorigin (Beijing) Inc.] for 30 min.
Subsequently, diluted primary antibodies were applied and incubated
overnight at 4˚C. After incubation, Dako Envision kit HRP (cat. no.
K4006; Dako; Agilent Technologies, Inc.) was added as a secondary
antibody and incubated at 37˚C for 20 min, followed by three PBS
washes. Color development was carried out using DAB, and
counterstaining was performed with hematoxylin for 30 sec to 1 min.
Finally, the sections underwent gradient dehydration, clearing, and
mounting with neutral balsam. An Olympus BX43F microscope was used
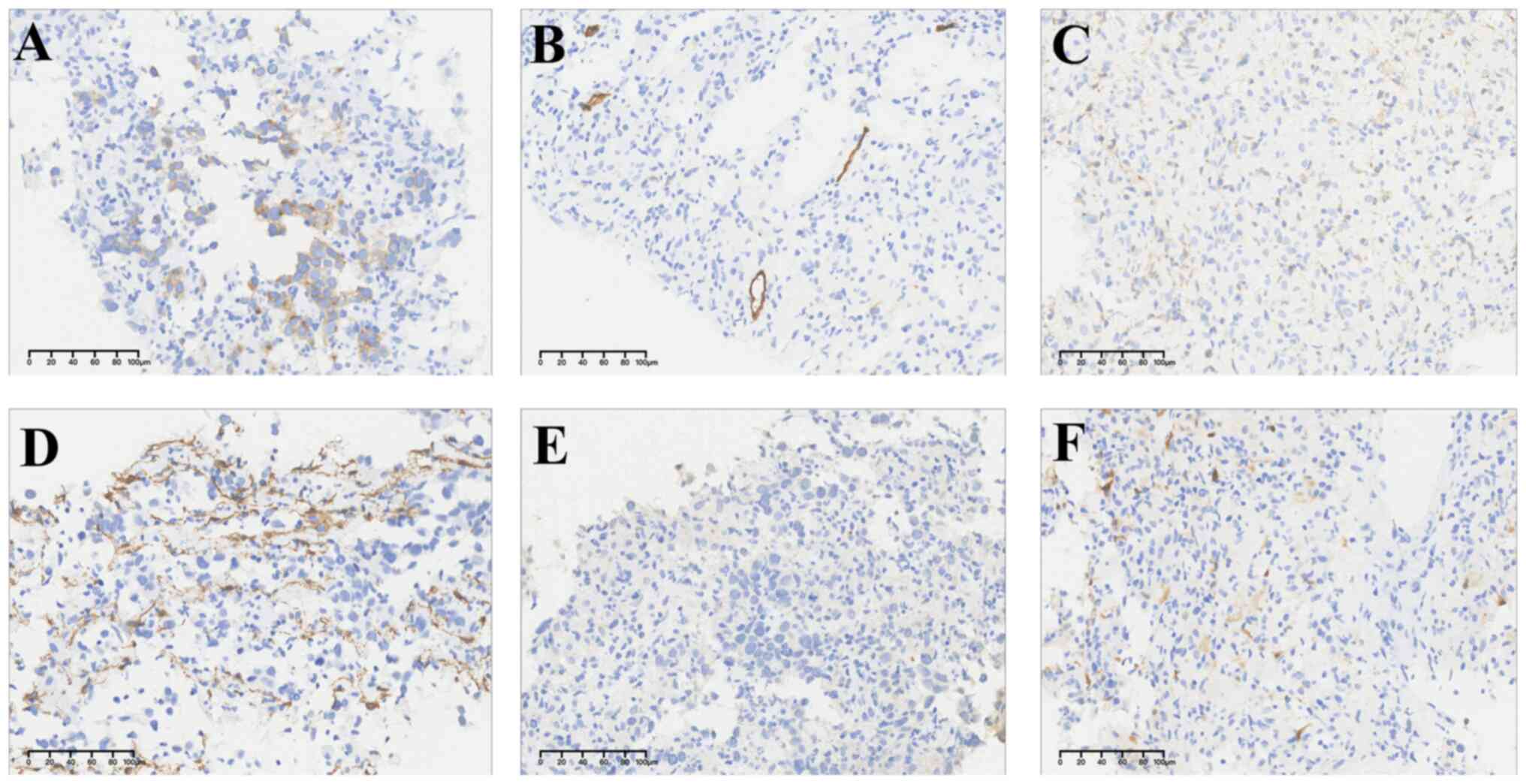
to interpret results. Immunohistochemically, the tumor cells were
positive for CD117, but negative for DOG-1, CD34, SMA, Des and S100
(Fig. 2). Amplicon-based targeted
next-generation sequencing (NGS) in the affiliated hospital of
Jiangnan University detected molecular alternations, revealing the
mutation in exon 17 of KIT gene (p.Y823D). A diagnosis of malignant
epithelioid GIST was made. Given the massive lesion exceeding 16 cm
in maximal diameter, surgical evaluation deemed the tumor
unresectable for radical resection at the external institution,
prompting attempted targeted therapy to assess potential tumor
downsizing for further surgical intervention. Then the patient
received daily oral imatinib 400 mg. However, 3 months after
targeted therapy in another hospital, CT revealed tumor
enlargement. Although sunitinib, regorafenib and remipatinib were
applied in turn, tumor progression appeared. Given the poor
efficacy, the patient was admitted to the multidisciplinary team
clinic of Zhongshan Hospital for pathology consultation in July
2024. Questioning the original diagnostic results due to the
morphology and the clinical response to treatment, a series of
immunohistochemical markers were applied to establish the
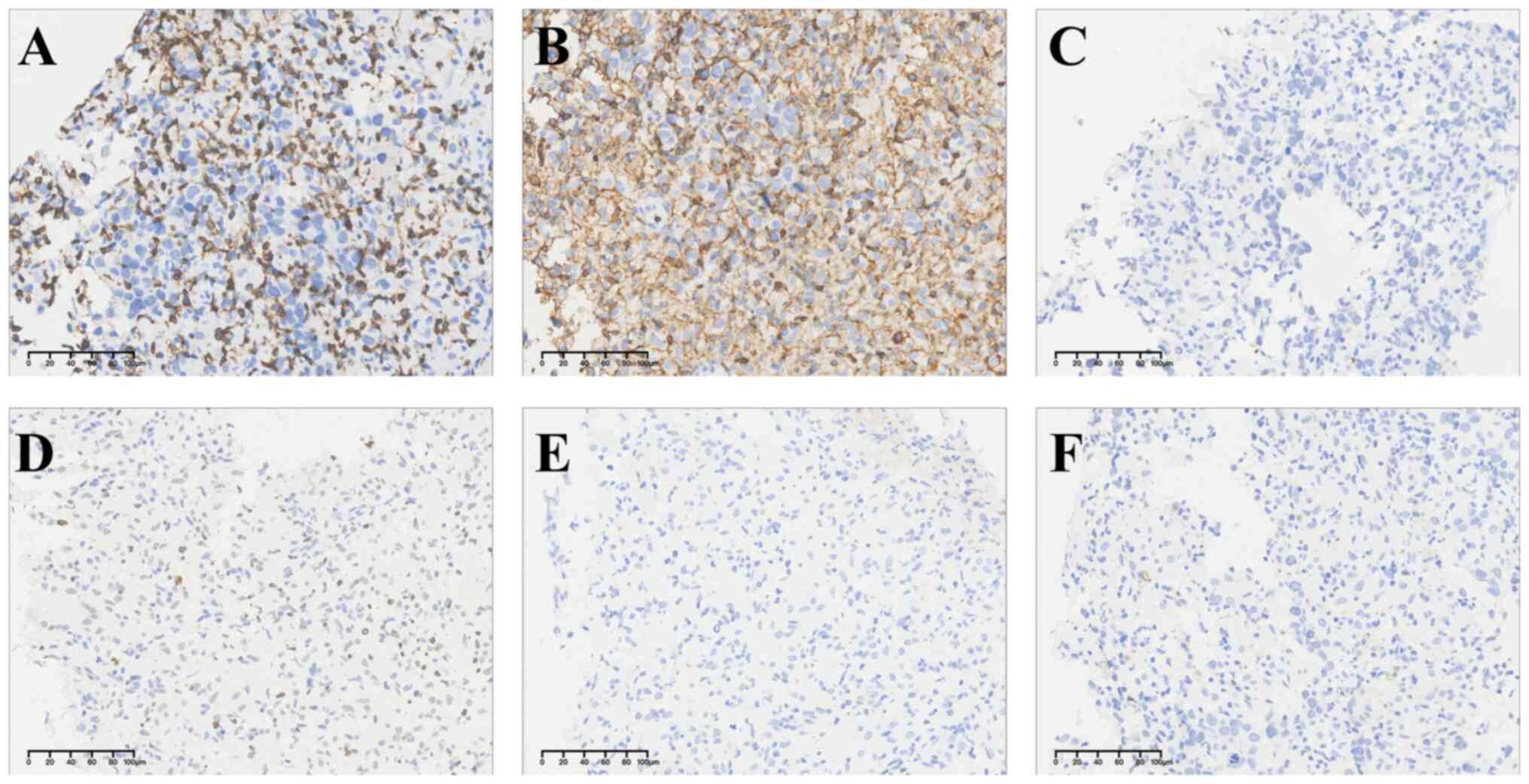
diagnosis. Immunohistochemistry exhibited that LCA and CD3 merely
stained lymphocyte, excluding lymphohematopoietic tumors. No CK7,
CK8, EMA, CgA, Syn, CD56, WT-1, or Calretinin immunoreactivity was
seen in tumor cells, which largely ruled out the possibility of
epithelial tumor, neuroendocrine neoplasm and mesothelioma
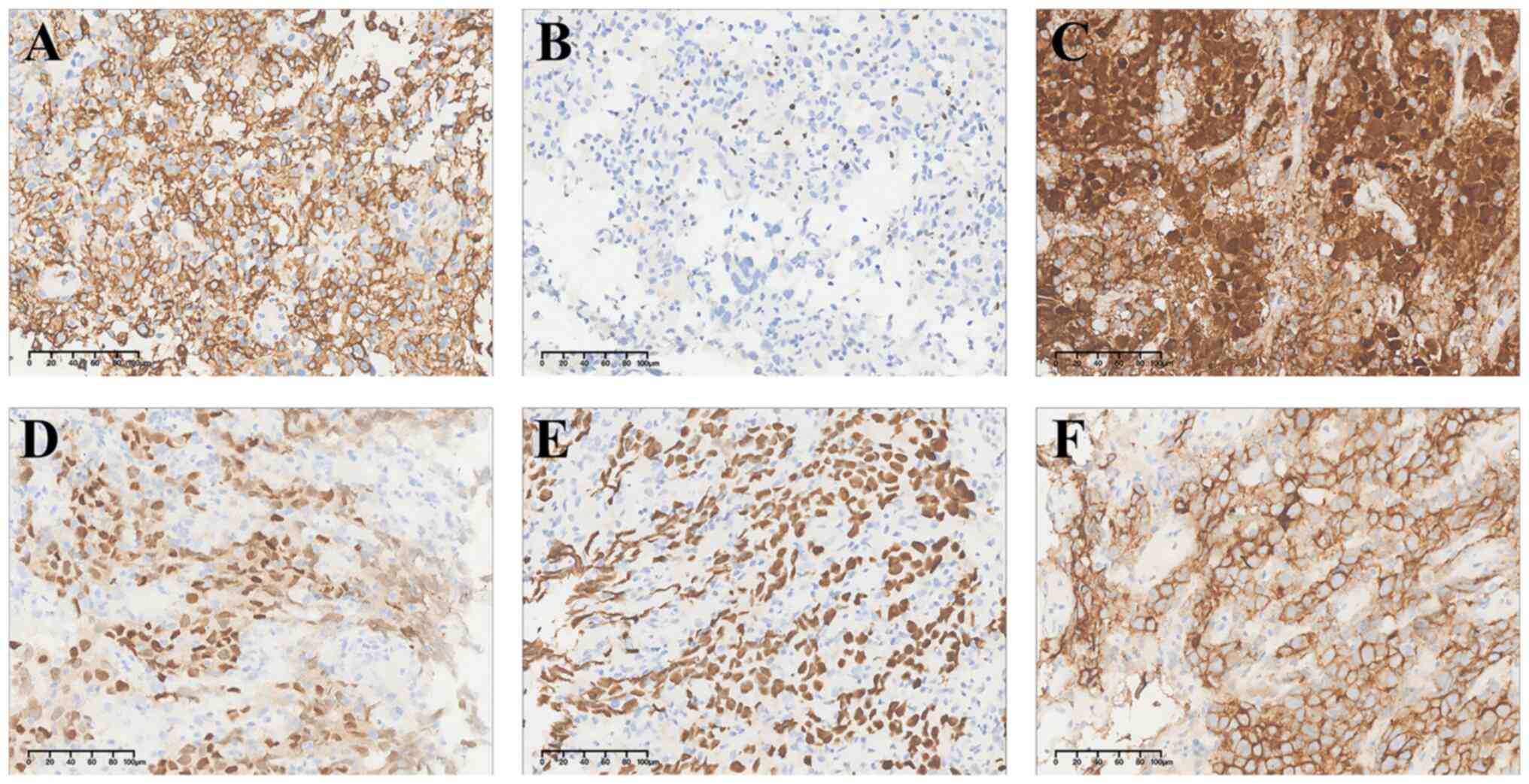
(Fig. 3). OCT4, SALL4, PLAP, SOX17
and D2-40 were strongly expressed in tumor cells, suggesting a
possible origin of tumor in the reproductive system (Fig. 4). Different from original unit
testing results, sanger sequencing and NGS analysis in Zhongshan
Hospital (Shanghai, China) simultaneously detected the point
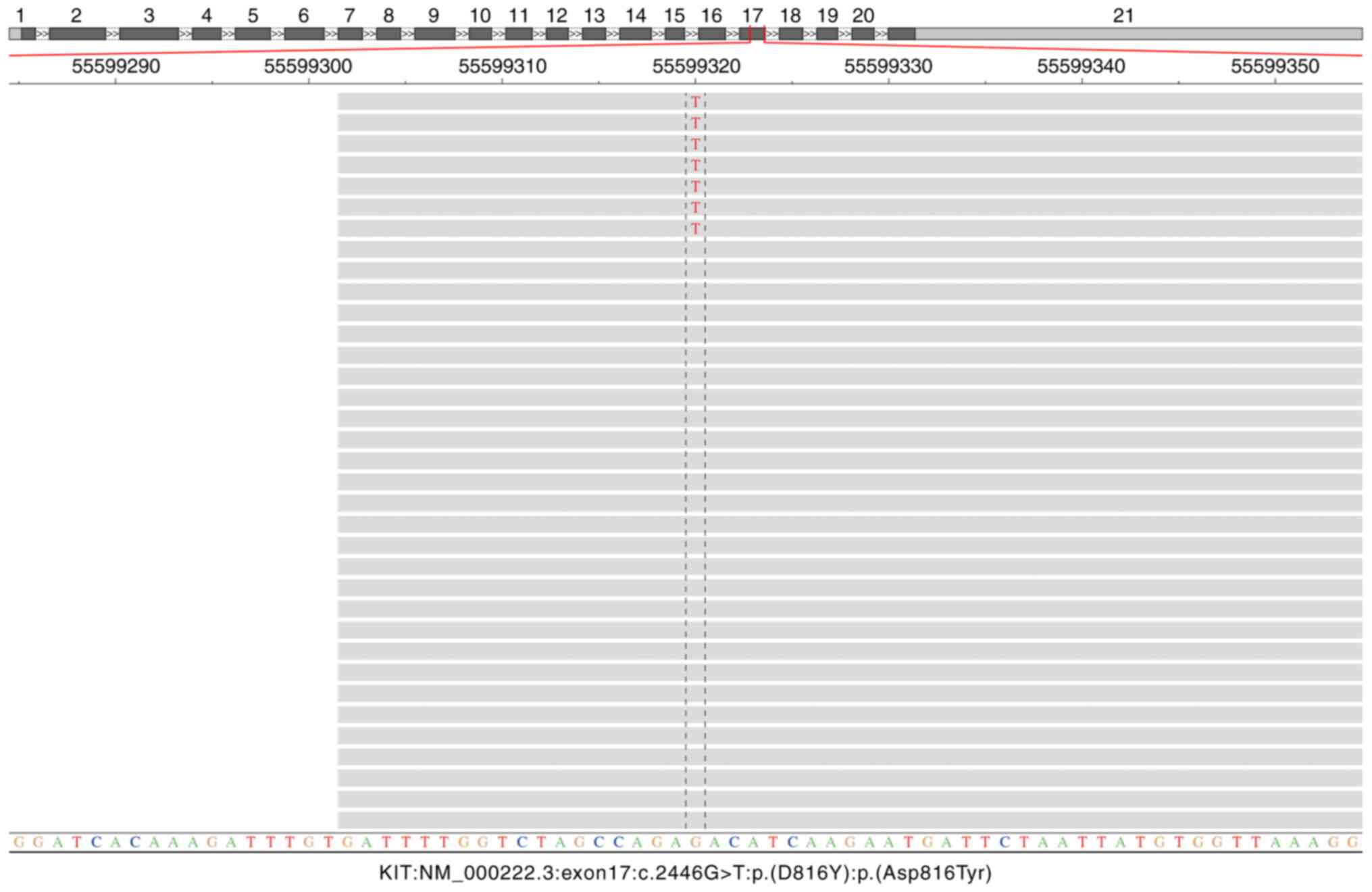
mutation at codon 816 of exon 17 of KIT (p.D816Y) (Fig. 5). NGS workflow was as follows:
Nucleic acids were extracted from formalin-fixed paraffin-embedded
(FFPE) tissue sections using the FFPE DNA Automated Nucleic Acid
Extraction Kit (cat. no. 8.02.0071), according to the
manufacturer's instructions. DNA concentration was measured by
Qubit fluorometer (Thermo Fisher Scientific, Inc.), and the total
amount of DNA exacted from tissue samples should exceed 60 ng. The
extracted DNA was sheared into fragments of 200-250 bp using a
Covaris LE220 system. (Index NGS libraries were constructed through
sequential steps of end repair, A-tailing, adaptor ligation, and
PCR amplification using the NEBNext® Ultra™ II DNA Prep
Kit (cat. no. E7645; NEB). Targeted capture was performed using the
AmoyDx® Master Panel covering 571 genes associated with
DNA mutations. The resulting libraries were quantified by the
Quantus™ Fluorometer, showing a concentration of at least 4.5 pM,
and library fragment size distribution was assessed with the
Agilent 2100 Bioanalyzer. Pooled libraries were sequenced on an
Illumina NovaSeq 6000 platform with 2x150 bp pair-end reads,
employing the Illumina Novaseq 6000 S1 Reagent kit v1.5 (300
cycles; cat. no. 20028317; Illumina, Inc.). Demultiplexing and
FASTQ file generation were carried out using bcl2fastq v2.17
software (Illumina, Inc.), which also initiated the automated
downstream analysis pipeline. Ultimately, a diagnosis of seminoma
was confirmed. The present study was performed in accordance with
the research policies approved by Zhongshan Hospital, Fudan
University [approval no. B2020-101(2)R]. Informed consent was waived from the
Ethics Committee of Zhongshan Hospital, Fudan University.
Discussion
Patients with seminoma generally present with a
palpable mass and may experience pain. Serum levels of
alpha-fetoprotein (AFP) are elevated, but this is not a specific
marker. Patients with syncytiotrophoblasts have increased serum
human chorionic gonadotrophin (HCG), usually not exceeding 1,000
mIU/ml. Imaging shows that the mass has well-defined borders and
exhibits homogeneous hypoechogenicity.
Gross examination reveals a solid tumor, which is
often lobulated, with gray-white to yellow cut surfaces,
well-defined margins and homogeneous texture. Hemorrhage or
necrosis is barely observed in seminoma; otherwise, seminoma mixed
with components from other germ cell tumors may be suggested.
Histologically, classical seminoma is characterized by uniformly
shaped germ cells arranged in a diffuse sheet-like pattern, which
is separated into small lobules, strands, or nests by fibrous
vascular septa. Tumor cells have clear boundaries and transparent
cytoplasm rich in glycogen. The nuclei are large and centrally
located, with regular shapes and clumped chromatin. One or more
prominent nucleoli are often visible, along with varying numbers of
mitotic figures. Also, the infiltration of different lymphocytes
and plasma cells can be observed in the tumor stroma. A total of
~25% of seminomas may show a granulomatous reaction and
multinucleated giant cells, which can obscure their original
characteristics. Anaplastic seminoma is noted for significant
cellular atypia, accompanying with an increased number of mitotic
figures (>3/HPF) and few interstitial lymphocytes.
Immunohistochemistry can aid in diagnosis when the morphology is
atypical. Tumor cells are positive for germ cell markers such as
SALL4, OCT3/4, D2-40, PLAP, CD117, and SOX17, while negative for
CK, CD30 and AFP (4). HCG,
cytokeratin and pregnancy associated proteins are expressed in
syncytiotrophoblasts.
The pathological diagnosis in this case contained
two critical errors. First, a seminoma was misdiagnosed as a GIST.
Second, the genetic analysis revealed a point mutation at codon 816
(p.D816Y) in exon 17 of the KIT gene, which was discordant with the
external laboratory report documenting a mutation at codon 823
(p.Y823D) within the same exon. The misdiagnosis of GIST by the
external institution may be attributed to a combination of atypical
clinical and histopathological features. The patient's presentation
with a large abdominal mass instead of a testicular lesion deviated
from the classic seminoma phenotype, while limited tissue sampling
via biopsy hindered definitive morphological assessment, with tumor
cells lacking hallmark characteristics such as clear cytoplasm and
prominent nucleoli. Furthermore, the uncritical acceptance of CD117
immunopositivity and KIT mutation detection as pathognomonic for
GIST likely contributed to diagnostic inaccuracy, reflecting
insufficient integration of morphological, immunohistochemical and
clinical data.
A critical reappraisal reveals diagnostic clues that
could have alerted pathologists to the misclassification.
Morphologically, GISTs predominantly exhibit spindle cell
morphology, with mixed or pure epithelioid subtypes accounting for
<50% of cases. Notably, such epithelioid GISTs predominantly
arise in the stomach and are typically associated with PDGFRA
mutations or SDH deficiency, rendering the coexistence of KIT
mutations with epithelioid morphology biologically incongruous
(5). Furthermore, the
immunohistochemical profile in this case, solitary CD117 expression
without DOG1 or CD34 co-expression, deviates from diagnostic
standards. As emphasized in the Clinical Practice Guidelines for
GIST Diagnosis, definitive GIST classification mandates rigorous
exclusion of other CD117-positive neoplasms. The differential
diagnosis should comprehensively encompass both epithelial
malignancies (for example, adenoid cystic carcinoma, secretory
carcinoma, basal cell adenoma, eccrine spiradenoma, renal
oncocytoma, chromophobe renal carcinoma, basal cell adenoma,
eccrine spiradenoma, renal oncocytoma, chromophobe renal cell
carcinoma, thymic carcinoma, pulmonary small cell carcinoma) and
non-epithelial tumors (for example, angiosarcoma, granulocytic
sarcoma, mast cell neoplasms, malignant melanoma and seminoma),
particularly when confronted with atypical morphological and
immunophenotypic features (6).
Besides, the molecular findings in this case exhibited biological
incongruity with typical GIST profiles. Although KIT mutations
constitute the most common genetic alterations in GIST, primary
tumors predominantly harbor exon 9 (10~15%) or exon 11 (60~70%)
mutations (7). Exon 13 and 17
mutations are exceptionally rare in primary GISTs (1~2%), with exon
17 variants historically containing p.A795P (1 case), p.D816F (1
case), p.D816Y (1 case), pD820A (1 case), p.D820Y (1 case), p.D820V
(1 case), p.N822K (15 cases), p.N822Y (1 case) and p.Y823D (1 case)
(8-10).
It is also demonstrated that exon 17 mutation of KIT is mainly
found as a secondary gene mutation in drug-resistance GIST after
the failure of targeted therapy, with specific codons including
N822K (43.94%), Y823D (22.73%), D816H (15.15%), D820Y (6.06%),
C809G (4.55%), N822Y (4.55%) and A829P (3.03%) (11,12).
Crucially, the mere presence of KIT mutations cannot confirm GIST
diagnosis, as such alternations are documented in 8~15% of other
CD117-positive malignancies including angiosarcoma, granulocytic
sarcoma, mast cell neoplasms, malignant melanoma and seminoma
(13). KIT gene mutations occur in
8.2~25.8% of seminomas, predominantly localized to exon 17
(14,15).
The most frequent hotspot involves codon 816
(p.D816V/H), accounting for approximately 35% of cases, followed by
codon 882 (p.N822K) with 22% frequency. Additional exon 17 variants
(e.g., p. K642E, p.N655K, p.D820Y, p.Y823C, p.Y823D, p.A829T) have
been reported at lower frequencies. Exon 11 mutations (3~6%)
predominantly affect codons 557 (p.W557S), 576 (p.L576P), and 578
(p.Y578C) (16). Critically, the
p.D816V mutation in this case strongly supports seminoma over GIST,
as exon 17 mutations are exceptionally rare (<1%) in primary
GISTs while prevalent in seminoma.
Different genotypes of KIT mutations are associated
with diversified response to specific TKIs. Imatinib, initially
developed as a BCR-ABL tyrosine kinase inhibitor, was subsequently
identified to potently inhibit KIT receptor tyrosine kinase
activity and to be the first-line therapy for GISTs, particularly
in patients harboring KIT exon 11 mutation or specific exon 17
variants (for example, p.N22K and p. Y823D) (7). However, several exon 17 mutants, such
as p.D816V/H, are resistant to imatinib treatment (13). In the present study, this further
explained the disease progression in the short term after imatinib
treatment in this patient.
Patients with stage I or localized disease are
treated with radical orchiectomy followed by risk-adapted
management: Active surveillance (preferred for low-risk patients)
or adjuvant therapy based on established risk factors (tumor size
>4 cm, rete testis invasion). Stage IIA/B requires
post-orchiectomy treatment with either radiotherapy (20~30 Gy to
involved fields) or chemotherapy (BEP x 3 cycles).
Advanced/unresectable disease mandates first-line cisplatin-based
chemotherapy (BEP x 3-4 cycles), achieving 83% disease-specific
survival at 5 years (17). Notably,
targeted therapies (imatinib/sunitinib/pazopanib) demonstrate
limited efficacy with objective response rate <15%, reflecting
intrinsic resistance mechanism in germ cell malignancies (18).
There are several limitations in the present study.
First, a critical limitation of this report was the unavailability
of primary CT imaging findings essential for diagnosing
extragonadal seminoma. During pathological consultation at our
institution for diagnostic verification, only textual radiological
descriptions from an external facility were provided and original
DICOM images were unobtainable. Furthermore, no supplemental CT
imaging or therapeutic interventions were pursued at our center,
constraining comprehensive clinicopathological correlation. In
addition, another limitation was the omission of serum tumor
markers (AFP, β-HCG and LDH) during initial diagnosis of the case.
Although these biomarkers provide substantial diagnostic utility in
seminoma detection, particularly in extragonadal presentations,
their measurement was overlooked due to the original misdiagnosis
of GIST based solely on CD117 immunopositivity and reported KIT
mutation. This precluded timely diagnostic rectification through
serological correlation.
In conclusion, this diagnostic pitfall underscores
the imperative for morphology-driven, multidisciplinary evaluation
when interpreting CD117-positive tumors. Solitary CD117 expression
requires systematic exclusion of other CD117-positive malignancies
to diagnose GIST. Reliance solely on molecular findings risks
misclassification. Although KIT mutations support GIST diagnosis in
appropriate contexts, rare mutation loci more strongly align with
non-GIST neoplasms. Furthermore, interlaboratory variability in
molecular alternation necessitates validation by multiple detection
methods including sanger sequencing and NGS, and correlation with
histomorphology. Notably, rapid disease progression on multi-line
targeted therapy, despite reported KIT mutation, should prompt
immediate reassessment of diagnostic validity, including expert
histopathology review and expanded molecular profiling.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Nature
Science Foundation of China (grant no. 32571067).
Availability of data and materials
The data generated in the present study may be found
in the SRA database under accession number PRJNA1304866 or at the
following URL: https://www.ncbi.nlm.nih.gov/sra/PRJNA1304866.
Authors' contributions
XZ, WY and YH contributed to the conception and the
design of the study. LR and CX contributed to the acquisition,
analysis, and interpretation of the data. XZ and YH contributed to
drafting the manuscript and revising the manuscript. All authors
read and approved the final version of the manuscript. XZ and WY
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed in accordance with
the research policies approved by Zhongshan Hospital, Fudan
University [approval no. B2020-101(2)R]. Informed consent was waived from the
Ethics Committee of Zhongshan Hospital, Fudan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marko J, Wolfman DJ, Aubin AL and
Sesterhenn IA: Testicular seminoma and its mimics: From the
radiologic pathology archives. Radiographics. 37:1085–1098.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ghazarian AA, Trabert B, Devesa SS and
McGlynn KA: Recent trends in the incidence of testicular germ cell
tumors in the United States. Andrology. 3:13–18. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Fukawa T and Kanayama HO: Current
knowledge of risk factors for testicular germ cell tumors. Int J
Urol. 25:337–344. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lau SK, Weiss LM and Chu PG: D2-40
immunohistochemistry in the differential diagnosis of seminoma and
embryonal carcinoma: A comparative immunohistochemical study with
KIT (CD117) and CD30. Mod Pathol. 20:320–325. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Medeiros F, Corless CL, Duensing A,
Hornick JL, Oliveira AM, Heinrich MC, Fletcher JA and Fletcher CD:
KIT-negative gastrointestinal stromal tumors: Proof of concept and
therapeutic implications. Am J Surg Pathol. 28:889–894.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Casali PG, Blay JY, Abecassis N, Bajpai J,
Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee
JVMG, et al: Gastrointestinal stromal tumours:
ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 33:20–33. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ibrahim A and Montgomery EA:
Gastrointestinal stromal tumors: Variants and some pitfalls that
they create. Adv Anat Pathol. 31:354–363. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pai T, Bal M, Shetty O, Gurav M, Ostwal V,
Ramaswamy A, Ramadwar M and Desai S: Unraveling the spectrum of KIT
mutations in gastrointestinal stromal tumors: An Indian tertiary
cancer center experience. South Asian J Cancer. 6:113–117.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Calibasi G, Baskin Y, Alyuruk H, Cavas L,
Oztop I, Sagol O, Atila K, Ellidokuz H and Yilmaz U: Molecular
analysis of the KIT gene in gastrointestinal stromal tumors with
novel mutations. Appl Immunohistochem Mol Morphol. 22:37–45.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lasota J, Corless CL, Heinrich MC,
Debiec-Rychter M, Sciot R, Wardelmann E, Merkelbach-Bruse S,
Schildhaus HU, Steigen SE, Stachura J, et al: Clinicopathologic
profile of gastrointestinal stromal tumors (GISTs) with primary KIT
exon 13 or exon 17 mutations: A multicenter study on 54 cases. Mod
Pathol. 21:476–484. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Serrano C, Bauer S, Gómez-Peregrina D,
Kang YK, Jones RL, Rutkowski P, Mir O, Heinrich MC, Tap WD,
Newberry K, et al: Circulating tumor DNA analysis of the phase III
VOYAGER trial: KIT mutational landscape and outcomes in patients
with advanced gastrointestinal stromal tumor treated with
avapritinib or regorafenib. Ann Oncol. 34:615–625. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Du J, Wang S, Wang R, Wang SY, Han Q, Xu
HT, Yang P and Liu Y: Identifying secondary mutations in Chinese
patients with Imatinib-resistant gastrointestinal stromal tumors
(GISTs) by next generation sequencing (NGS). Pathol Oncol Res.
26:91–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lennartsson J and Rönnstrand L: Stem cell
factor receptor/c-Kit: From basic science to clinical implications.
Physiol Rev. 92:1619–1649. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McIntyre A, Summersgill B, Grygalewicz B,
Gillis AJ, Stoop J, van Gurp RJ, Dennis N, Fisher C, Huddart R,
Cooper C, et al: Amplification and overexpression of the KIT gene
is associated with progression in the seminoma subtype of
testicular germ cell tumors of adolescents and adults. Cancer Res.
65:8085–8089. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kemmer K, Corless CL, Fletcher JA,
McGreevey L, Haley A, Griffith D, Cummings OW, Wait C, Town A and
Heinrich MC: KIT mutations are common in testicular seminomas. Am J
Pathol. 164:305–313. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakhaei-Rad S, Soleimani Z, Vahedi S and
Gorjinia Z: Testicular germ cell tumors: Genomic alternations and
RAS-dependent signaling. Crit Rev Oncol Hematol.
183(103928)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Vries G, Rosas-Plaza X, van Vugt MATM,
Gietema JA and de Jong S: Testicular cancer: Determinants of
cisplatin sensitivity and novel therapeutic opportunities. Cancer
Treat Rev. 88(102054)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Galvez-Carvajal L, Sanchez-Muñoz A,
Ribelles N, Saez M, Baena J, Ruiz S, Ithurbisquy C and Alba E:
Targeted treatment approaches in refractory germ cell tumors. Crit
Rev Oncol Hematol. 143:130–138. 2019.PubMed/NCBI View Article : Google Scholar
|