Osteoporosis (OP) is a systemic metabolic bone
disease characterized by degradation of bone microstructure, bone
fragility, and increased fracture risk (1). OP affects 10.2% of adults >50 years
of age and is expected to increase to 13.6% by 2030(2). Annually, >150 million individuals
suffer from OP, and the disease poses a great threat to the quality
of life for >50% of postmenopausal women, with the most severe
risk being osteoporotic fractures (3). OP is influenced by the process of bone
loss, and its therapeutic goal is to halt bone mass reduction and
rectify the imbalance in bone remodeling. Currently, certain drugs
are effective in mitigating bone loss or increasing bone calcium
content. The commonly employed drugs for OP treatment include
bisphosphonates, estrogens, norepinephrine, and teriparatide
acetate. However, the prolonged and high-dose administration of
these agents may cause adverse effects such as hypercalcemia,
gastrointestinal reactions, and an increased risk of breast cancer
and heart disease (4,5). With the in-depth study into the
pathogenesis of OP, the relationship between intestinal
microecology and bone metabolism (BM) has become a worldwide
research hotspot. Studies indicate that the gut microbiota (GM) is
associated with the reduction of bone mass and the development of
OP in humans (6-8).
The intestinal microecosystem is the largest
ecosystem within the human body, harboring >1014
orders of magnitude of bacteria, with the total number of genes in
the gut microbiome genome being ~150-fold that of the human genome
(9). The GM is mainly dominated by
Firmicutes and Bacteroidetes, which together account
for over 90% of the relative abundance, followed by
Actinobacteria, Proteobacteria, and
Fusobacteria (10). The GM
exhibits variabilities between individuals and dynamic changes
throughout the lifetime of an individual and is influenced by a
variety of factors, including diet, age, lifestyle, medications,
and disease states. The GM plays a regulatory role in multiple
physiological functions, encompassing the enteric nervous system,
enteroendocrine system, immune system, and intestinal permeability
(11,12). Specific GM can modulate immunity,
improve defense function, promote skeletal health, and inhibit bone
calcium loss. Additionally, it can improve intestinal permeability,
reduce inflammatory responses, and facilitate nutrient absorption
(13). Imbalance of intestinal
flora affects calcium absorption, subsequently triggering
inflammatory and autoimmune changes, leading to bone loss and
reduced bone formation (14-18).
Therefore, GM is closely associated with the occurrence of OP, and
the restoration of GM imbalance has become a key approach to
treating OP, as revealed in Fig. 1.
In the present review, the latest advancements in the association
between GM and OP are examined, elucidating the role of GM in the
pathogenesis and treatment of OP. The aim of the review is to
explore novel therapeutic strategies and research directions for OP
and related diseases.
Articles on the treatment of OP with traditional
Chinese medicine (TCM) through regulation of GM were collected
using the keywords ‘osteoporosis’, ‘gut microbiota’, and
‘traditional Chinese medicine’ by searching in PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of
Science (https://www.clarivate.com.cn/). The search period was
from the inception of each database up to August, 2025. To ensure
the quality of literature and accuracy of data extraction, the
search was limited to articles published in English.
Intestinal microflora can affect the energy
homeostasis of the host organism and serve as a source of energy
for the differentiation and formation of osteoblasts (OBs) and
osteoclasts (OCs). In cases of aberrant energy metabolism, the OB
precursors will shift to adipose differentiation (19). The composition of bacterial
structures, characterized by varying quantities and ratios of
different bacterial genera, forms diverse GM structures, which in
turn have differential effects on BM (13,20).
Reduced bone mass in the lumbar vertebrae and femoral neck is
associated with excessive growth of gut bacteria, suggesting that
overgrowth of the GM may constitute a significant risk factor for
OP (21). Another study further
validated this finding (22). The
administration of antibiotics in the diet was shown to modulate the
GM, thereby promoting growth and skeletal development (11). The increase in bone mass in
germ-free mice (GFM) was associated with a decrease in the number
of OCs (12). Bone marrow cultures
revealed that the number of OCs was reduced in GFM, accompanied by
a significant reduction in the formation of
CD11b+/Gr1- and CD4+ T cells in
the precursor OC population (13).
Microbial transplantation of GFM led to an increase in the number
of bone marrow CD4+ T cells and OC precursors, along
with a decrease in bone mass, suggesting the key regulatory role of
the GM in the process of BM. Additionally, studies have identified
associations between GM and both postmenopausal (PM)OP and senile
OP (23,24). Elevated levels of trimethylamine
N-oxide (TMAO), a metabolite of GM, have been shown to have a
strong negative correlation with the degree of bone mineral density
(BMD) in OP. TMAO has been demonstrated to regulate the cell
function of BMSCs by activating the NF-κB signaling pathway, which
affects the balance of BM, leading to acceleration of bone loss and
further progression of OP (25).
Furthermore, estrogen-deficient GFM exhibited reduced bone mass,
while probiotic interventions effectively prevented loss of bone
mass (26). 5-Hydroxytryptamine
(5-HT) levels were increased after the introduction of specific
Escherichia coli in GFM (27). Lactobacillus reuteri not only
reduced the level of bone resorption markers and inhibited OC
formation, but also suppressed the expression of tumor necrosis
factor-α (TNF-α) and modulated the level of Wnt10b RNA in OBs
cultured in vitro (28,29).
In clinical research, changes have been observed in
the abundance and diversity of the GM, as well as in their
associated metabolites, in patients with OP (30). Distinct differences exist in the GM
between patients with OP and healthy populations (31). A previous meta-analysis encompassing
12 studies included fecal data from 2,033 subjects (604 patients
with OP and 1,429 healthy controls). It observed increased relative
abundances of Lactobacillus and Ruminococcus in the
OP group, along with a higher proportion of Bacteroides
(32). With an expanded sample
size, research has identified that an increase in
Bacteroidetes and a decrease in Firmicutes may be
important factors in the GM imbalance triggering OP (33). In a previous study,
Klebsiella, Escherichia-Shigella, and
Akkermansia were identified as biomarkers in patients with
OP. Among them, the abundance of Akkermansia was negatively
correlated with lumbar BMD, while Klebsiella and
Escherichia-Shigella were negatively correlated with femoral
neck and hip BMD (34). In another
study, an increase in the abundance of Actinomyces,
Eggerthella, Clostridium Cluster XlVa and
Lactobacillus was noted in the OP group, suggesting that
alterations in GM abundance may serve as an independent risk factor
for bone mass loss in the elderly (35). Research has indicated a positive
correlation between the relative abundance of
Actinobacillus, Blautia, Oscillospira,
Bacteroides, Phascolarctobacterium and OP, while a
negative correlation was identified with other Veillonellaceae,
Collinsella, and other Ruminococcaceae (36). Another study evaluated the causal
relationship between GM and bone development by determining
specific causal bacterial taxa using Mendelian randomization (MR).
The study revealed that Clostridiales and Lachnospiriaceae
regulate bone mass variation, indicating a causal relationship
between GM and bone development (37). Previous research revealed that
increased abundances of the family Pasteurellaceae, order
Pasteurellales, and genus Ruminococcaceae UCG004 were linked
to an increased risk of OP. Conversely, the family
Oxalobacteraceae, unknown family (ID.1000006161), the genus
Lachnospiraceae NK4A136 group, an unknown genus
(ID.1000006162), and order NB1n were associated with a
reduced risk of OP (38).
Additionally, other research found that changes in the GM,
including the Lactobacillus genus, are associated with
osteoporosis (39).
Other studies further demonstrated the association
between GM and PMOP, while also exploring the phenomenon of GM
regulating BM (30). Notably,
healthy postmenopausal women exhibited higher abundances of
Clostridia and Methanobacteraceae within their GM, whereas
women with osteopenic/osteoporotic conditions showed a greater
richness of Bacteroidetes in their fecal microbiota.
Therefore, alterations in GM composition are considered closely
associated with OP (40). The
abundance of Fusicatenibacter, Lachnoclostridium, and
Megamonas species was significantly higher in PMOP women
compared to women with osteopenia (41). Researchers have proposed GM as a
potential new target for the treatment of PMOP, highlighting that
GM can influence BM through various mechanisms, such as regulating
the host's immune system, particularly affecting inflammation and
autoimmune responses. For example, GM can promote the production of
regulatory T cells (Tregs) by producing short-chain fatty acids
(SCFAs) such as butyrate and propionate, thereby exerting
anti-inflammatory effects (42).
Reduced α-diversity in GM has been shown to be associated with PMOP
(43). Previous research has
revealed that GM has an essential role to perform as a target for
TCM intervention in bone disease treatment (44). Certain TCMs with natural prebiotic
properties may help combat OP by facilitating the development of
healthy probiotics. Hence, the gut-bone axis may provide an
explanation for the multi-target regulation of TCM in treating OP
(45).
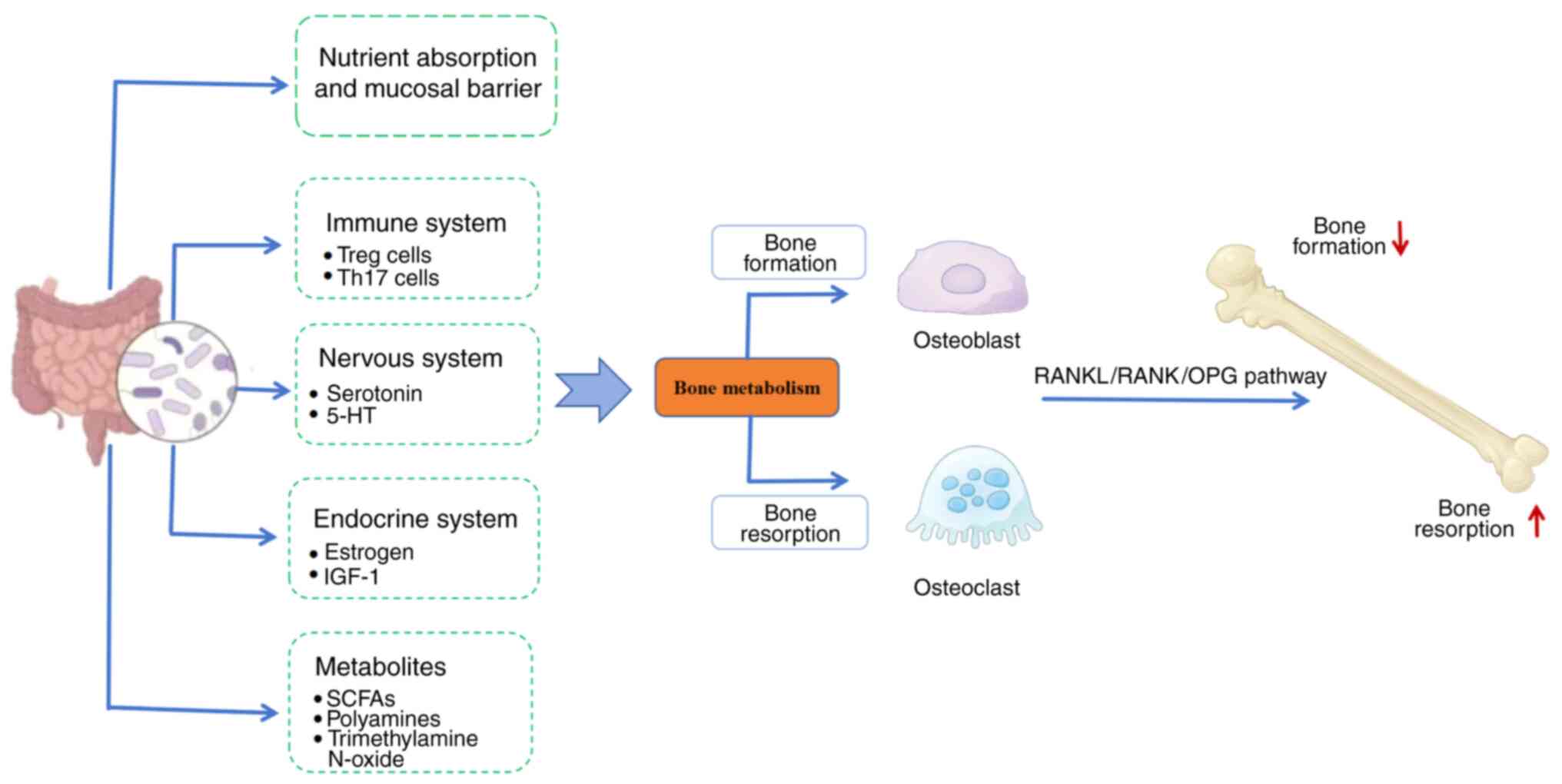
In recent years, the association between GM and OP
has increasingly become a research hotspot. The mechanisms by which
GM influences OP are highly complex. The pivotal role of GM in bone
regulation is elaborated in this section through a multifaceted
approach, including nutrient absorption, intestinal mucosal barrier
permeability, the immune, endocrine, and nervous systems, as well
as metabolites of GM, as illustrated in Fig. 2.
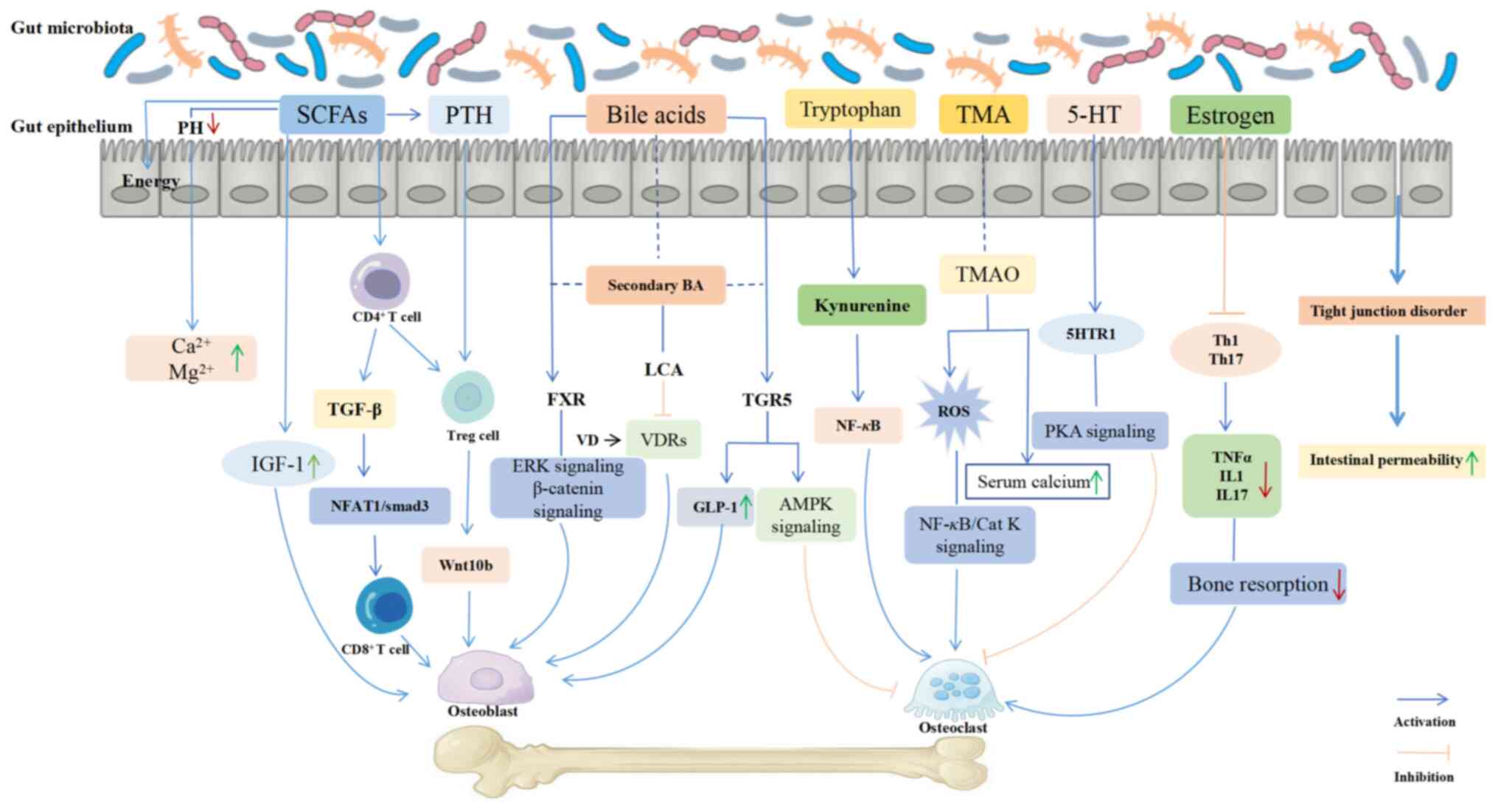
Calcium, the major mineral element in human skeletal
tissue, plays an important role in bone formation and is absorbed
in the form of calcium ions. Only 30% of dietary calcium intake is
absorbed by the bones in healthy individuals (46), underscoring the importance of
enhancing calcium absorption to promote bone production. The GM
facilitates the formation of bone calcium and reduces bone loss,
thereby promoting BM (13). The
impact of calcium intake on bone health is closely related to the
quantity and type of flora. Calcium supplements or a high-calcium
diet can increase the population of beneficial bacteria and
maintain the integrity of the GM ecosystem (47). Calcium absorption is associated with
a lower pH in the cecum (20), and
alterations in the microbiota can decrease intestinal pH, impede
the intestinal calcium-acid complexes, enhance calcium solubility,
and increase the amount of calcium available for absorption
(48). The expression of calcium
transporter proteins has been shown to be upregulated, enhancing
calcium absorption and mitigating the downstream effects of bone
resorption on bone (49). Vitamin D
is one of the important substances maintaining bone homeostasis
(50). When insufficient calcium
intake occurs, the GM can produce active vitamin D, promoting
calcium absorption in the gut (51). In addition, intestinal
microorganisms are considered one of the primary sources of
vitamins B and K, both of which play important roles in bone
homeostasis (52).
The intestinal mucosal barrier constitutes the
interface between the human body and the external environment, and
its integrity is critically important for preventing the invasion
of harmful substances, such as toxins and bacteria, into the body
(53).
The intestinal barrier is composed of a mucus layer,
GM, immune cells, and a monolayer of intestinal epithelial cells
(54). Lipopolysaccharides (LPS),
the main component of the bacterial cell wall of GM, induce chronic
inflammatory responses, leading to bone loss. Additionally, LPS
promotes the formation and activation of OCs, thereby further
contributing to bone loss (55).
Conversely, muramyl dipeptide functions to reduce bone resorption
and increase bone mass by downregulating the ratio of the receptor
activator of NF-κB ligand (RANKL)/osteoprotegerin (OPG), thereby
indirectly suppressing OC differentiation (56). Barrier damage is universally
observed in all types of OP models, mainly manifesting as
disruption of tight junction proteins, alteration in intestinal
villus morphology, and increased intestinal permeability (57,58).
This compromised intestinal barrier facilitates the translocation
of microbes or potential antigens to subepithelial mucosa, thereby
activating immune cells and triggering aberrant intestinal and
systemic immune responses, ultimately resulting in bone loss
(26). GM affects BM by influencing
mucosal barrier function, with increased membrane levels of
Toll-like receptor 4 (TLR4) on the membrane observed in primary
cells treated with RANKL or LPS. TNF-α is secreted via the
endotoxin/TLR4 signaling pathway and modulates RANKL-induced
osteoclastogenesis (59). The
integrity of the GM prevents bacterial LPS from contacting
macrophage TLR-4 in the lamina propria and prevents OP (60). The impact of GM on the intestinal
mucosal barrier is involved in glucocorticoid-induced OP (61).
OP is characterized as a systemic disease with
chronic low-grade inflammation. Changes in GM can elicit systemic
or localized immune responses, which are closely implicated in the
development of OP (62). The
immunological impact on bone conversion is often manifested by B-
and T-cell activation, along with an increase in osteoclastogenic
factors such as interleukin (IL)-17, IL-6, RANKL, and TNF-α. These
factors enhance the activity of OCs while simultaneously inhibiting
the formation and function of OBs, ultimately exacerbating the
imbalance in BM (63). GM maintains
contact with dendritic cells and immune cells at the vascular
endothelial boundary, stimulating the immune system to release
inflammatory factors. These factors subsequently influence the
immune cell population within bone tissue, thereby modulating the
bone remodeling process (64).
Hematopoietic stem cells in bone tissue have the potential to
differentiate into OCs and immune cells. GM regulates the BM
microenvironment by promoting the maturation of the host immune
system (65). The harmful flora in
GM can produce endotoxins. These endotoxins initiate inflammatory
responses by binding to the TLRs on the surface of host immune
cells, subsequently resulting in bone mass reduction (18). Type helper 17 (Th17) cells, an
integral subset of the CD4+ T-cell OC population, can be
generated and differentiated under GM dysregulation, and secrete
IL-17a, IL-1, IL-6, along with low levels of interferon-γ and TNF.
These cytokines contribute to the release of RANKL and the
formation of OCs (66). Regulatory
T cells are capable of stimulating bone marrow CD8+ T
cells to produce the osteogenic Wnt signaling pathway ligand
Wnt10b, thus promoting osteogenic differentiation (67). It has been found that the expression
of inflammatory factors such as TNF-α and IL-6 is reduced in mice
that maintain a balanced composition of GM. In addition, the number
of T cells in the body is reduced, accompanied by a decrease in the
number of OCs and an improvement in bone quality (12,68).
Endocrine hormones act on various organs within the
body, playing a role in the progression of a variety of
musculoskeletal disorders, including OP. Beneficial bacteria in the
GM can stimulate the secretion of incretins from intestinal cells
(17). Incretins, a group of
gastrointestinal hormones secreted by the intestine that exert
glucose concentration-dependent insulinotropic effects, include
glucose-dependent insulinotropic polypeptide (GIP) and
glucagon-like peptide-1 (GLP-1) (69). GIP can bind to surface receptors on
OBs, enhancing the expression of type I collagen genes, promoting
the maturation and mineralization of collagen matrix, increasing
alkaline phosphatase activity, and promoting TGF-β secretion, all
of which contribute to bone formation. GIP interacts with receptors
on pro-OCs to suppress the generation and activity of OCs, thereby
reducing bone resorption. Furthermore, GLP-1 facilitates insulin
secretion from pancreatic β-cells, which in turn promotes bone
formation; GLP-1 also promotes calcitonin secretion from thyroid
C-cells, suppressing bone resorption (70). Insulin-like growth factor-1 (IGF-1),
as a growth-promoting endocrine hormone, plays a key role in cell
proliferation, differentiation, and the cell cycle, exerting its
regulatory function through endocrine and paracrine/autocrine
mechanisms. IGF-1 was the first identified substance mediating the
association between GM and OP. Furthermore, it was the earliest
confirmed molecule involved in the interaction between intestinal
flora and OP (71). Research has
demonstrated that IGF-1 promotes the proliferation and
differentiation of OBs, as well as the mineralization of the bone
matrix. It has been shown to play an important role in the
maturation of the growth plate and the formation of the secondary
ossification center (72).
Estrogen, a steroid hormone secreted mainly by the
ovaries, promotes the generation of OCs, while also exerting
regulatory effects on the BM. Under normal physiological
conditions, estrogen, as a product of enterohepatic circulation,
needs to undergo enzymatic hydrolysis by the GM before re-entering
the internal circulation. When the GM is imbalanced, the
enterohepatic circulation is weakened, and the reabsorption
capacity of estrogen decreases, accelerating the loss of bone mass
and the course of OP (73,74). In a previous study it was found that
in GFM or mice subjected to prolonged antibiotic treatment, the
absence of estrogen did not lead to significant bone loss (26). By contrast, in normal animals, the
deficiency of estrogen led to increased intestinal permeability,
allowing a large number of pathogenic bacteria to invade the
intestine, which in turn triggered OP (75). Lactobacillus rhamnosus GG
ameliorated estrogen deficiency-induced osteoporosis by regulating
the gut microbiome and intestinal barrier and stimulating Th17/Treg
balance in the gut and bone (76).
Estrogen has been demonstrated to play a crucial role in
maintaining bone health by reducing bone resorption through the
maintenance of systemic and bone marrow T-cell homeostasis and also
to directly regulate the formation of OB and OC (76-78).
The central nervous system and GM mediate the
transmission of information between the brain and the gut by
chemical signals (such as acetylcholine, γ-aminobutyric acid, and
5-HT), a pathway known as the ‘brain-gut axis’ (79). GM can communicate directly with the
brain through various signaling molecules or indirectly through the
brain-gut axis. Similarly, the brain's regulation of GM can be
achieved either directly or indirectly by altering the intestinal
environment (80). The nervous
system regulates the secretion of gastric acid, mucus, bicarbonate,
intestinal peptides, and antimicrobial peptides through intestinal
cuprocytes, and influences the thickness and quality of the
intestinal mucus layer (81). In
addition, physiological changes in the gut can alter the microbial
habitat, thus regulating the composition and activity of the
microbial community (82). It has
also been found that Lactobacillus and
Bifidobacterium are positively correlated with serum leptin,
while Clostridium, Prevotella and Bacteroides
are negatively correlated with leptin levels (83). ObRb, as a receptor in the brainstem
nervous system, inhibits the release of 5-HT and the expression of
5-HT receptor 2C in the ventromedial hypothalamic nucleus upon
binding to leptin. Activation of the β2-adrenergic receptor on OBs
promotes bone resorption (84).
Conversely, when leptin concentrations decrease, the release of
5-HT is diminished, leading to a reduction in sympathetic activity,
which affects the homeostasis of BM. 5-HT, as a neurotransmitter,
has been confirmed to regulate BM function through the
gastrointestinal system (85).
Tryptophan hydroxylase 1 has been identified as the enzyme
catalyzing serotonin synthesis, and its inhibitors hold potential
therapeutic utility in the treatment of low bone mass (86). It has been revealed through
knockdown of the serotonin receptor gene in OBs that a variety of
serotonin receptors can be expressed in these cells (87,88). A
correlation between serotonin levels and BM in women has been
observed, in which increased serotonin levels were accompanied by
decreased bone formation and increased bone turnover (89).
During the metabolic process of an organism, the GM
can produce numerous active substances, such as SCFAs,
hypocholestatic acid, TMAO, indole derivatives, and polyamines.
These metabolites are relatively stable and can diffuse into the
body circulation through the intestinal tract, thereby indirectly
revealing the role of the GM on BM (90). SCFAs are saturated fatty acids with
a chain length of one to six carbon atoms, which are metabolized
from the fermentation of indigestible carbohydrates by GM,
including acetate, propionate, and butyrate (91). Additionally, the fermentation of
amino acids in GM also produces SCFAs, which were found to inhibit
the activity of histone deacetylase, induce the generation of
Tregs, and maintain the immune system. By binding to G
protein-coupled receptors (GPRs) on the cell surface, particularly
free fatty acid receptor 2, SCFAs have been shown to reduce
intracellular cyclic adenosine monophosphate levels, activate the
immune system, promote the proliferation and differentiation of
Tregs, inhibit intestinal inflammation and OC differentiation, and
regulate BM (92). SCFAs were shown
to induce the development of peripheral Tregs by acting through
GPR43 (acetate and propionate receptors). Additionally, the
precursor OCs derived from differentiated bone marrow cells express
GPR41 (propionate and butyrate receptors) and GPR109 (butyrate and
nicotinic receptors), confirming that SCFAs affect OB and OC
activity through GPR activation (93). SCFAs in tryptophan, such as
butyrate, acetate, propionate, and indole, were revealed to promote
muscle synthesis, inhibit catabolism, exhibit anti-inflammatory and
antioxidant effects, and regulate BM, thereby helping to prevent OP
(94). SCFAs can directly act on
bone cells, increasing bone density and bone strength (95). Experiments have demonstrated that
SCFAs can increase bone density and bone strength in mice. Under
pathological conditions (such as inflammation), a reduction in gut
probiotic populations in mice has been observed, leading to a
reduction in SCFAs levels, which contributes to the onset of OP
(96). SCFAs can also lower
intestinal pH, reducing the formation of inorganic salts such as
calcium phosphate that bind to calcium ions. In addition, SCFAs
have been shown to selectively increase the phosphorylation of
mitogen-activated protein kinase p38, which mediates the
phosphorylation of the downstream substrate heat shock protein 27
at Ser78 and Ser82, thereby affecting the absorption of minerals
and improving bone quality (97).
SCFAs have also been demonstrated to induce metabolic reprogramming
of OBs, resulting in enhanced glycolysis at the expense of
oxidative phosphorylation, thereby downregulating essential OC
genes to directly inhibit OC activity (98).
Bile acids, serving as metabolites of cholesterol,
are synthesized and secreted by hepatocytes. Within this process,
95% of bile acids enter the gut-liver axis. Under the influence of
GM, primary bile acids can be converted into secondary bile acids,
such as lithocholic acid, and deoxycholic acid (99). GM contributes to the processing and
metabolism of bile acids, and metabolism regulates BM mainly
through the sphincter farnesoid X receptor (FXR) and GPRs (100). LPS, a distinctive chemical
component of the outer membrane layer of Gram-negative bacteria,
can cause a chronic inflammatory response, promoting OC formation
and inducing bone loss (101).
In vitro experiments found that LPS inhibited RANKL activity
by reducing the expression of RANK and M-CSF receptor and
stimulated osteoclastogenesis in RANKL-pretreated cells via TNF-α
(102). Lactic acid significantly
reduced TNF-α and IL-6 production in LPS-stimulated macrophages,
thereby decreasing the release of RANKL from OBs and inhibiting the
differentiation of bone marrow-derived macrophages to OCs (103).
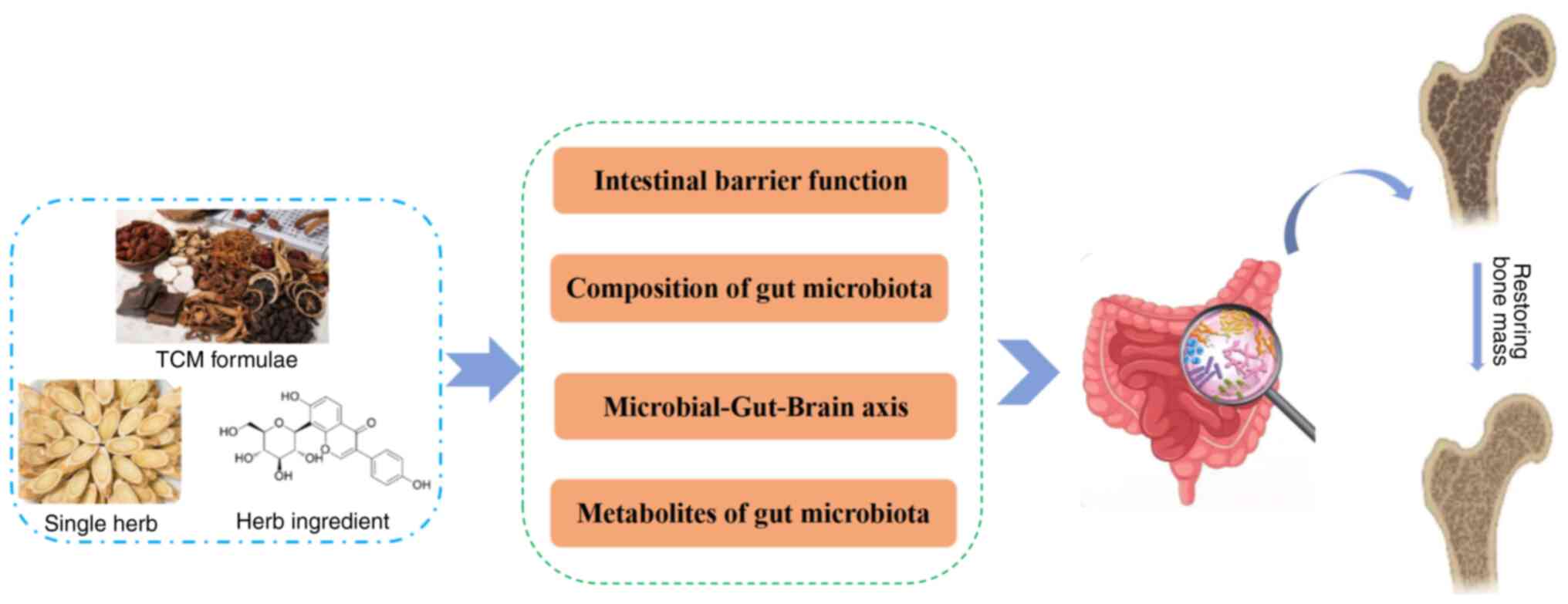
As an important stage in drug absorption, the
progression of GM is closely associated with OP and its variations
in metabolites. With deepening research, the efficacy of
traditional Chinese medicine in preventing and treating OP through
the modulation of GM, has been highlighted. Single-flavor Chinese
medicinal herbs and their active ingredients, as well as herbal
compounds, play an anti-inflammatory role, enhance the mucous
barrier, improve the immune system, and regulate metabolism
(104-134).
Specific mechanisms are detailed in Table SI.
Glycosylation in the structure of total flavonoids
of Epimedii folium affects the number and activity of GM, which in
turn increases the thickness of bone trabeculae, elevates bone
mineral density, enhances OB activity, and promotes calcium
deposition (104). Fructus
Ligustri Lucid has been shown to promote the generation of
SCFAs, and regulate calcium absorption and calcium homeostasis by
upregulating the serum levels of 1,25-dihydroxyvitamin D3 and
vitamin D-dependent calcium transporter gene expression (128,129). Fructus Ligustri Lucidi
aqueous extract may preserve bone quality through regulation of the
calcium balance and intestinal SCFA production in ovariectomized
rats (130). Traditional herbal
formula Gushukang (GSK) was clinically applied to treat primary
osteoporosis. GSK exerted beneficial effects on trabecular bone of
ovariectomized mice by improving calcium homeostasis through
regulation of paracellular calcium absorption in the duodenum and
transcellular calcium reabsorption in the kidney (131).
Puerarin has been demonstrated to repair intestinal
mucosal integrity by regulating the species and abundance of GM,
increasing the levels of tight junction proteins (ZO-1 and occludin
and their related mRNAs), and decreasing the release of
inflammatory factors (TNF-α, IL-6, IL-1β, LPS and their related
mRNAs). Concurrently, it was shown to increase the content of SCFAs
in the colon, influencing host metabolic pathways with
anti-inflammatory responses, such as amino acid metabolism, lipid
metabolism and butyrate. This action was revealed to maintain
intestinal homeostasis, alleviate inflammatory responses, and exert
anti-osteoporotic effects (112).
Berberine has been shown to enhance the intestinal barrier function
by upregulating butyrate production from GM, ameliorate both
systemic and local inflammation, and prevent alveolar bone loss
associated with estrogen deficiency (115).
The aqueous extract of Epimedii folium has been
shown to increase the abundance of Candidatus Saccharimonas.
It was demonstrated to be positively correlated with the production
of T-cell cytokines (IL-2, IL-4, IL-10 and IFN-γ) by mesenteric
lymphocytes (132). Lycium
barbarum polysaccharide has been shown to promote the
production of SCFAs by adjusting the structure of GM (110). Among them, butyric acid was
demonstrated to increase the number of Tregs in both intestine and
bone marrow, which in turn activated the Wnt signaling pathway in
OBs and stimulated osteogenesis (133). Jian Gu granule effectively
prevented bone loss and enhanced bone strength by restoring the
abundance of GM, increasing the levels of SCFAs, reducing the
permeability of colonic epithelium, increasing the proportion of
Tregs in the spleen, and altering cytokines associated with bone
immunomodulation (120).
The aqueous extract of Epimedii folium can improve
the ratio of dominant flora, such as Muribaculaceae and
Lactobacillus, and regulate the level of parathyroid hormone
in the body, which affects BM (104). Eucommia ulmoides extract
was shown to change the composition of intestinal microbiota and
enhance the production of SCFAs, thereby improving OP (106). Extracts of Sambucus
williamsii Hance, administered at a dose of 140 mg/kg,
significantly reduced the abundance of Ruminococcaceae
UGC-014, inhibited the synthesis of 5-HT, and increased bone
density (134).
In conclusion, herbal combinations, single-flavor
herbs, their extracts, and herbal monomers regulate the intestinal
microbiota and their metabolic homeostasis, thereby exerting an
anti-OP effect, as shown in Fig. 3.
Although numerous studies on this subject have been conducted, the
current understanding is limited to the association between the GM
and the herbal medicines or active ingredients. Specific strains of
microorganisms, genes, or metabolic enzymes involved remain
unidentifiable. Therefore, it is still necessary to employ
multi-omics technologies to elucidate the mechanism by which
Chinese medicines act on which intestinal microorganisms to exert
their therapeutic effects, and then to elucidate the metabolic
mechanism of Chinese medicines in the intestinal tract of patients
with OP in subsequent studies.
Research focusing on targeting the GM for the
treatment of OP has emerged as a hot topic. Studies have
demonstrated that interventions such as fecal microbiota
transplantation (FMT), probiotic and prebiotic supplementation, or
dietary modification can improve the composition of the GM. These
changes not only modulate local processes, but also systemic
responses, including BM, affecting host metabolism, the immune
system, and hormone secretion (135-175),
as shown in Table SII.
Probiotics are a group of active microorganisms that
colonize the intestinal tract and reproductive system. They confer
benefits on the host, producing precise health effects and
contributing to the microecological balance of the host (151). The regulation of OP by probiotics
represents an emerging field, where the mechanisms underlying their
enhancement to BM, prevention, and treatment of OP have become a
focal point in BM research, yielding certain advancements. Research
indicates that probiotics can maintain skeletal health by
inhibiting bone resorption, promoting bone formation and
mineralization, enhancing bone density and improving bone
microstructure (152). The
specific mechanisms include: i) Increasing calcium content in bone:
Feeding hens with Bacillus subtilis significantly increased
the calcium content of the tibia in hens (153) and prevented the reduction of bone
mass in chicks caused by Salmonella enteritidis infection
(154). ii) Inhibition of OC
activity: Under estrogen deficiency, Lactobacillus reuteri
ATCC PTA 6475 exerted a protective effect by reducing bone loss,
bone resorption markers and osteoclastogenesis (28). Lactobacillus reuteri ATCC PTA
6475 inhibited bone resorption and TNF-α production, and
significantly improved bone density in male mice, suggesting that
the effect of bone regulation may be related to sex (139). iii) Inhibition of inflammatory
response to reduce bone resorption: Mice were treated with
Lactobacillus paracasei DSM13434 and the amount of cortical
bone mineral content was increased, while the serum levels of bone
resorption marker C-terminal telopeptides and the urinary
fractional excretion of calcium were reduced (137). Lactobacillus rhamnosus GG
and probiotic supplement VSL #3 have been demonstrated to reduce
intestinal permeability and inhibit intestinal inflammation,
thereby preventing estrogen deficiency-induced bone loss (26). iv) Promoting OB activity:
Lactobacillus reuteri attenuated bone loss in type 1
diabetic mice by regulating GM, preventing the decline in
osteocalcin levels and mineralization deposition rates, suggesting
a positive effect of probiotics on osteoanabolism (30). v) Bifidobacterium longum
increased the bone mineral content in rats during experimental
trials (155). Lactobacillus
rhamnosus stimulated the secretion of insulin-like growth
factor, which in turn promoted bone mineralization (140). vi) Regulation of BM pathways:
Lactobacillus rhamnosus promoted the maturation and
differentiation of OB and osteocytes by upregulating the expression
of genes associated with the mitogen-activated protein kinase 1 and
3 pathway (156). In addition,
Bifidobacterium longum increased bone mineral density by
upregulating the expression of SPARC and Bmp-2 genes (157).
Prebiotics are a class of indigestible food
ingredients, mainly non-digestible oligosaccharides, including
isomaltooligosaccharides, inulin, oligofructose, soybean
oligosaccharides, lactuloses, and pyrodextrins. They can
selectively stimulate the growth of one or more types of intestinal
flora, which can be utilized by GM, and promote the health of the
host (158). It has been found
that prebiotics can promote the absorption of mineral elements,
increase bone mineralization and enhance bone density (152). i) Promoting calcium absorption:
Galactooligosaccharides increased the population of
Bifidobacteria in the gut, which was conducive to the
utilization of calcium and magnesium, thus improving bone mass
(145). A three-week oral
administration of galactooligosaccharides to adolescent females
significantly increased the proportion of intestinal
Bifidobacteria and the rate of calcium absorption (141). Agave fructans were shown to
promote the absorption of minerals in the intestinal tract, and
improve the content of bone minerals (144). In addition, oligofructose
effectively promoted calcium absorption in rats fed a high-calcium
diet (147). ii) Influence on bone
conversion: The combination of lactulose
galactooligosaccharides/oligofructose with calcium supplementation
has been shown to elevate bone mineralization, bone mineral
density, and increase the surface area of OBs in rats (159). Calcium supplementation combined
with short-chain fructooligosaccharides has been shown to mitigate
the rate of systemic and spinal bone loss in postmenopausal women
(160). iii) Improvement of bone
strength: Galactooligosaccharides, oligofructose, and inulin have
demonstrated bone-strengthening effects in both healthy and
ovariectomized rats (161,162). iv) Enhancement of other bone
health agents: The synergistic prebiotic combination of
fructo-oligosaccharide and soy isoflavones was shown to improve the
bone strength of ovariectomized rats. The prebiotic mixture had a
more pronounced enhancement in bone strength when soy isoflavone
content was relatively low, underscoring that this prebiotic blend
can yield synergistic effects. These effects enhanced the efficacy
of prebiotics, with superior bone-strengthening outcomes compared
with the use of prebiotics alone (152). Therefore, prebiotics play an
important role in regulating GM and maintaining bone
homeostasis.
FMT is the transfer of GM from a healthy donor to
an intestinal dysbiosis recipient, aiming to restore intestinal
microbial homeostasis and ameliorate intestinal dysbiosis (163). A significant increase in bone mass
was observed 4 weeks after GFM received FMT using cecal contents
from conventionally housed mice (12). Additionally, the bones of
conventional mice chronically infused with broad-spectrum
antibiotics reproduced the phenotype of GFM (164). The transplantation of fecal
bacteria from osteoporotic mice into normal mice resulted in a
significant decrease in the bone mass of the recipient (61). Transplantation of fecal bacteria
from young rats into aged rats improved intestinal homeostasis at
the portal and familial levels, while increasing the bone volume,
trabecular volume fraction, trabecular number, and trabecular
thickness in aged rats. This phenomenon suggests the direct
influence of that GM on BM within the organism (57). The transplantation of segmented
filamentous bacteria into GFM increased the number of Th17 cells,
leading to increased levels of IL-17, TNF-α, and IL-1, and induced
the expression of RANKL, thereby promoting osteoclastogenesis
(165). The transplantation of
Clostridium clusters IV and XIVa, which were isolated from
mice, into GFM resulted in an increase in the number of systemic
and lamina propria Tregs (166).
SCFAs also induced the differentiation of Tregs, which regulated
osteoclastogenesis through the secretion of IL-4, IL-10, and TGF-β
(167). Malnutrition in infants
and young children has been shown to lead to the dysbiosis of the
GM, subsequently disrupting the maturation of the immune system and
the normal growth of the skeletal system. It was demonstrated that
the transplantation of the GM from malnourished children into
healthy mice resulted in a significant reduction in bone mass over
time (168). Animal experiments
have shown that bone mass and immune factor levels are restored to
normal through metabolites and immunomodulation when GM is
transplanted into germ-free or depleted mice (12). Certainly, the adverse effects caused
by the allochthonous enterobacteria post-transplantation, as well
as the compliance issue arising from the differences in
administration routes between the upper and lower gastrointestinal
tract, remain areas for improvement and consideration. Long-term
and effective FMT therapy may radically improve the diversity of GM
in patients and become an effective method to treat OP (169).
Dietary structure can influence the type of GM and
the function of the gut. Low dietary fibre intake may disrupt the
integrity of the intestinal mucosal barrier, causing a relative
increase in the levels of Firmicutes and
Proteobacteria and a relative decrease in the levels of
Bacteroidetes in the intestines of dietary fibre-deficient
mice (170). A previous study
indicated that Tanzanian hunter-gatherers, and Malawian and
Venezuelan farmers consuming agri-foods had a greater diversity of
flora than populations from the United States (171). Higher dietary fibre consumption
may exert a positive influence on the progression of OP by altering
the structural composition of GM (172). High-fibre diets and oligofructose
may increase the number of Bifidobacterium species, optimize
the microbial composition of the GM, increase the content of SCFAs,
and lower the pH in the gut, thus promoting calcium absorption.
Dairy consumption encourages bone formation and inhibits bone
resorption (173). Studies have
also found that consumption of dairy products by adults aged 60
years and above can reduce the risk of OP. This beneficial effect
is partly attributed to the lactulose content of lactulose
derivatives. This interaction is capable of lowering serum
parathyroid hormone levels, thereby decreasing levels of bone
resorption markers (174,175).
GM is closely related to the occurrence and
development of OP and related metabolic diseases. Treating OP by
improving GM and its metabolites has been a hot and challenging
topic in medical research in recent years. On one hand, the GM can
directly or indirectly participate in bone mass regulation by
modulating host metabolism, calcium absorption, hormone levels, the
immune system, and the central nervous system. On the other hand,
GM-associated metabolites can also reliably and effectively reflect
the impact of GM on BM, potentially serving as novel targets for
the prevention and treatment of OP. The present review summarized
that TCM, probiotics, and prebiotics can ameliorate the composition
of GM and promote microbial-metabolite balance by regulating the
‘gut-bone axis’, thereby exerting therapeutic effects against OP.
These findings provide theoretical support and reference for
clinical treatment of OP and the development of innovative
therapeutic agents.
Currently, TCM has demonstrated potential in
treating OP by regulating GM. However, several challenges and
limitations remain in its practical applications: i) Research
primarily relies on laboratory animal studies, lacking high-quality
clinical trials to objectively reflect TCM clinical efficacy and
clarify its mechanisms of action; ii) TCM interventions in OP
studies often focus on single signaling pathways, failing to
systematically elucidate multi-pathway interactions; iii) Research
on GM profiles has primarily focused on common bacterial
communities, lacking studies on the mechanisms underlying complex
microbial interactions; and iv) The therapeutic effects of TCM on
OP currently remain confined to the levels of marker
microorganisms, microbial diversity, and differential metabolic
products, making it difficult to elucidate the biotransformation
processes of herbal active components through GM. Therefore,
further investigation is still required to elucidate how TCM
optimizes the structure and function of the host GM, how the host
GM converts the active components of TCM into metabolites that act
on target organs, and the mechanisms by which microbial metabolites
exert biological effects on the host. Moreover, research should
leverage modern molecular biology techniques, such as
macro-genomics, functional genomics and metabolomics, to elucidate
the mechanisms of TCM treatment for OP from a multi-component,
multi-target, and holistic regulatory perspective. Simultaneously,
techniques such as FMT, supplementation of deficient microbiota and
metabolites, and co-incubation of TCM with GM should be employed to
bridge the gap in understanding the ‘TCM active
components-GM-microbial metabolites’ interaction pathway.
Furthermore, it is imperative to enhance clinical trials to
substantiate the efficacy and safety of these treatments, while
methodologies such as molecular dynamic simulations should be
employed to identify critical therapeutic targets of TCM in the
treatment of OP.
As awareness of the impact of GM on health grows,
an increasing number of studies are focusing on the relationship
between gut microbial metabolism and BM. Regulating GM through
supplementation with probiotics and prebiotics to improve BM has
emerged as a new therapeutic target for OP. However, whether
moderate supplementation of prebiotics or probiotics is effective
in humans needs to be supported by large-scale multi-centre
clinical studies. In addition, it remains to be elucidated whether
the effects of different types of prebiotics or probiotics are
consistent, the differential effects of age, sex, and etiology on
the efficacy of OP as well as the optimal dosage, duration of
treatment, timing of initiation and cessation, and the specific
mechanisms underlying their actions. FMT as a treatment for
Clostridium infection has become more mature, but there are
still some unresolved problems in the treatment of OP. Therefore,
in clinical practice, the use of enterobacterial preparations
administered via capsule for transplantation can improve patient
compliance and reduce adverse reactions. Timely and long-term FMT
in patients with OP can fundamentally improve their GM composition
and intestinal barrier function, potentially emerging as an
effective therapeutic approach for OP in clinical treatment.
In summary, future research should focus on the
core area of the mechanisms by which GM and their metabolites treat
OP. By skillfully integrating cutting-edge technologies such as
artificial intelligence, genomics, and high-throughput screening,
and closely aligning with clinical practice needs, this approach
aims to lay a solid foundation for exploring and innovating anti-OP
treatment strategies.
Not applicable.
Funding: The present review was supported by the Yunnan
Provincial Science and Technology Department of the Chinese
Medicine Research Center [grant no. 2018FF001(-085)].
Not applicable.
MA and XL contributed equally to this article, and
they drafted the manuscript. CX, YL, YZ, RC reviewed the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Clynes MA, Harvey NC, Curtis EM, Fuggle
NR, Dennison EM and Cooper C: The epidemiology of osteoporosis. Br
Med Bull. 133:105–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harris K, Zagar CA and Lawrence KV:
Osteoporosis: Common questions and answers. Am Fam Physician.
107:238–246. 2023.PubMed/NCBI
|
|
3
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cosman F, de Beur SJ, LeBoff MS, Lewiecki
EM, Tanner B, Randall S and Lindsay R: National Osteoporosis
Foundation. Clinician's guide to prevention and treatment of
osteoporosis. Osteoporos Int. 25:2359–2381. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Health Quality Ontario. Vertebral
augmentation involving vertebroplasty or kyphoplasty for
cancer-related vertebral compression fractures: A systematic
review. Ont Health Technol Assess Ser. 16:1–202. 2016.PubMed/NCBI
|
|
6
|
Brzozowska MM, Sainsbury A, Eisman JA,
Baldock PA and Center JR: Bariatric surgery, bone loss, obesity and
possible mechanisms. Obes Rev. 14:52–67. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Goulet O: Potential role of the intestinal
microbiota in programming health and disease. Nutr Rev. 73 (Suppl
1):S32–S40. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maynard CL, Elson CO, Hatton RD and Weaver
CT: Reciprocal interactions of the intestinal microbiota and immune
system. Nature. 489:231–241. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qin J, Li R, Raes J, Arumugam M, Burgdorf
KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al: A
human gut microbial gene catalogue established by metagenomic
sequencing. Nature. 464:59–65. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Costea PI, Hildebrand F, Arumugam M,
Bäckhed F, Blaser MJ, Bushman FD, de Vos WM, Ehrlich SD, Fraser CM,
Hattori M, et al: Enterotypes in the landscape of gut microbial
community composition. Nat Microbiol. 3:8–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kau AL, Ahern PP, Griffin NW, Goodman AL
and Gordon JI: Human nutrition, the gut microbiome and the immune
system. Nature. 474:327–336. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sjögren K, Engdahl C, Henning P, Lerner
UH, Tremaroli V, Lagerquist MK, Bäckhed F and Ohlsson C: The gut
microbiota regulates bone mass in mice. J Bone Miner Res.
27:1357–1367. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ohlsson C and Sjögren K: Effects of the
gut microbiota on bone mass. Trends Endocrinol Metab. 26:69–74.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Espinoza JL, Elbadry MI and Nakao S: An
altered gut microbiota may trigger autoimmune-mediated acquired
bone marrow failure syndromes. Clin Immunol. 171:62–64.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fransen F, van Beek AA, Borghuis T, Aidy
SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge
MI, Boekschoten MV, Smidt H, et al: Aged gut microbiota contributes
to systemical inflammaging after transfer to germ-free mice. Front
Immunol. 8(1385)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lerner A, Neidhöfer S and Matthias T: The
gut microbiome feelings of the brain: A perspective for
non-microbiologists. Microorganisms. 5(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
McCabe L, Britton RA and Parameswaran N:
Prebiotic and probiotic regulation of bone health: Role of the
intestine and its microbiome. Curr Osteoporos Rep. 13:363–371.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Villa CR, Ward WE and Comelli EM: Gut
microbiota-bone axis. Crit Rev Food Sci Nutr. 57:1664–1672.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Donohoe DR, Garge N, Zhang X, Sun W,
O'Connell TM, Bunger MK and Bultman SJ: The microbiome and butyrate
regulate energy metabolism and autophagy in the mammalian colon.
Cell Metab. 13:517–526. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weaver CM: Diet, gut microbiome, and bone
health. Curr Osteoporos Rep. 13:125–130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Anantharaju A and Klamut M: Small
intestinal bacterial overgrowth: A possible risk factor for
metabolic bone disease. Nutr Rev. 61:132–135. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stotzer PO, Johansson C, Mellström D,
Lindstedt G and Kilander AF: Bone mineral density in patients with
small intestinal bacterial overgrowth. Hepatogastroenterology.
50:1415–1418. 2003.PubMed/NCBI
|
|
23
|
Ma S, Qin J, Hao Y and Fu L: Association
of gut microbiota composition and function with an aged rat model
of senile osteoporosis using 16s rrna and metagenomic sequencing
analysis. Aging (Albany NY). 12:10795–10808. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma S, Qin J, Hao Y, Shi Y and Fu L:
Structural and functional changes of gut microbiota in
ovariectomized rats and their correlations with altered bone mass.
Aging (Albany NY). 12:10736–10753. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lin H, Liu T, Li X, Gao X, Wu T and Li P:
The role of gut microbiota metabolite trimethylamine N-oxide in
functional impairment of bone marrow mesenchymal stem cells in
osteoporosis disease. Ann Transl Med. 8(1009)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li JY, Chassaing B, Tyagi AM, Vaccaro C,
Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, et
al: Sex steroid deficiency-associated bone loss is microbiota
dependent and prevented by probiotics. J Clin Invest.
126:2049–2063. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nzakizwanayo J, Dedi C, Standen G,
Macfarlane WM, Patel BA and Jones BV: Escherichia coli nissle 1917
enhances bioavailability of serotonin in gut tissues through
modulation of synthesis and clearance. Sci Rep.
5(17324)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Britton RA, Irwin R, Quach D, Schaefer L,
Zhang J, Lee T, Parameswaran N and McCabe LR: Probiotic l. Reuteri
treatment prevents bone loss in a menopausal ovariectomized mouse
model. J Cell Physiol. 229:1822–1830. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang J, Motyl KJ, Irwin R, MacDougald OA,
Britton RA and McCabe LR: Loss of bone and wnt10b expression in
male type 1 diabetic mice is blocked by the probiotic lactobacillus
reuteri. Endocrinology. 156:3169–3182. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He J, Xu S, Zhang B, Xiao C, Chen Z, Si F,
Fu J, Lin X, Zheng G, Yu G and Chen J: Gut microbiota and
metabolite alterations associated with reduced bone mineral density
or bone metabolic indexes in postmenopausal osteoporosis. Aging
(Albany NY). 12:8583–8604. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang J, Wang Y, Gao W, Wang B, Zhao H,
Zeng Y, Ji Y and Hao D: Diversity analysis of gut microbiota in
osteoporosis and osteopenia patients. PeerJ.
5(e3450)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang R, Liu P, Bai Y, Huang J, Pan R, Li
H, Su Y, Zhou Q, Ma R, Zong S and Zeng G: Changes in the gut
microbiota of osteoporosis patients based on 16SrRNA gene
sequencing:a systematic review and meta-analysis. J Zhejiang Univ
Sci B. 23:1002–1022. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li C, Huang Q, Yang R, Dai Y, Zeng Y, Tao
L, Li X, Zeng J and Wang Q: Gut microbiota composition and bone
mineral loss-epidemiologic evidence from individuals in Wuhan,
China. Osteoporos Int. 30:1003–1013. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun M, Liu Y, Tang S, Li Y, Zhang R and
Mao L: Characterization of intestinal flora in osteoporosis
patients based on 16S rDNA sequencing. Int J Gen Med. 17:4311–4324.
2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Das M, Cronin O, Keohane DM, Cormac EM,
Nugent H, Nugent M, Molloy C, O'Toole PW, Shanahan F, Molloy MG and
Jeffery IB: Gut microbiota alterations associated with reduced bone
mineral density in older adults. Rheumatology (Oxford).
58:2295–2304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ling CW, Miao ZL, Xiao ML, Zhou H, Jiang
Z, Fu Y, Xiong F, Zuo LS, Liu YP, Wu YY, et al: The association of
gut microbiota with osteoporosis is mediated by amino acid
metabolism: multiomics in a large cohort. J Clin Endocrinol Metab.
106:e3852–e3864. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ni JJ, Yang XL, Zhang H, Xu Q, Wei XT,
Feng GJ, Zhao M, Pei YF and Zhang L: Assessing causal relationship
from gut microbiota to heel bone mineral density. Bone.
143(115652)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zeng HQ, Li G, Zhou KX, Li AD, Liu W and
Zhang Y: Causal link between gut microbiota and osteoporosis
analyzed via Mendelian randomization. Eur Rev Med Pharmacol Sci.
28:542–555. 2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wei M, Li C, Dai Y, Zhou H, Cui Y, Zeng Y,
Huang Q and Wang Q: High-throughput absolute quantification
sequencing revealed osteoporosis-related gut microbiota alterations
in Han Chinese elderly. Front Cell infect Microbiol.
11(630372)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rettedal EA, Ilesanmi-Oyelere BL, Roy NC,
Coad J and Kruger MC: The gut microbiome is altered in
postmenopausal women with osteoporosis and osteopenia. JBMR Plus.
5(e10452)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang X, Chang T, Yuan Q, Wei W, Wang P,
Song X and Yuan H: Changes in the composition of gut and vaginal
microbiota in patients with postmenopausal osteoporosis. Front
Immunol. 13(930244)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q
and Zhou X: Intestinal microbiota: A potential target for the
treatment of postmenopausal osteoporosis. Bone Res.
5(17046)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kuo YJ, Chen CJ, Hussain B, Tsai HC, Hsu
GJ, Chen JS, Asif A, Fan CW and Hsu BM: Inferring associated with
osteopenia and osteoporosis in Taiwanese postmenopausal bacterial
community interactions and functionalities women. Microorganisms.
11(234)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang YW, Li YJ, Lu PP, Dai GC, Chen XX
and Rui YF: The modulatory effect and implication of gut microbiota
on osteoporosis: from the perspective of ‘brain-gut-bone’ axis.
Food Funct. 12:5703–5718. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li K, Jiang Y, Wang N, Lai L, Xu S, Xia T,
Yue X and Xin H: Traditional Chinese medicine in osteoporosis
intervention and the related regulatory mechanismof gut microbiome.
Am J Chin Med. 51:1957–1981. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Report of the dietary guidelines advisory
committee dietary guidelines for americans, 1995. Nutr Rev.
53:376–379. 1995.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hirata Y, Egea L, Dann SM, Eckmann L and
Kagnoff MF: Gm-csf-facilitated dendritic cell recruitment and
survival govern the intestinal mucosal response to a mouse enteric
bacterial pathogen. Cell Host Microbe. 7:151–163. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ohta A, Motohashi Y, Sakai K, Hirayama M,
Adachi T and Sakuma K: Dietary fructooligosaccharides increase
calcium absorption and levels of mucosal calbindin-d9k in the large
intestine of gastrectomized rats. Scand J Gastroenterol.
33:1062–1068. 1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Raveschot C, Coutte F, Frémont M,
Vaeremans M, Dugersuren J, Demberel S, Drider D, Dhulster P,
Flahaut C and Cudennec B: Probiotic lactobacillus strains from
mongolia improve calcium transport and uptake by intestinal cells
in vitro. Food Res Int. 133(109201)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rillaerts K, Verlinden L, Doms S,
Carmeliet G and Verstuyf A: A comprehensive perspective on the role
of vitamin D signaling in maintaining bone homeostasis: Lessons
from animal models. J Steroid Biochem Mol Biol.
250(106732)2025.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lin HR, Xu F, Chen D, Xie K, Yang Y, Hu W,
Li BY, Jiang Z, Liang Y, Tang XY, et al: The gut microbiota-bile
acid axis mediates the beneficial associations between plasma
vitamin d and metabolic syndrome in chinese adults: A prospective
study. Clin Nutr. 42:887–898. 2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Castaneda M, Strong JM, Alabi DA and
Hernandez CJ: The gut microbiome and bone strength. Curr Osteoporos
Rep. 18:677–683. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Schoultz I and Keita ÅV: The intestinal
barrier and current techniques for the assessment of gut
permeability. Cells. 9(1909)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cardoso-Silva D, Delbue D, Itzlinger A,
Moerkens R, Withoff S, Branchi F and Schumann M: Intestinal barrier
function in gluten-related disorders. Nutrients.
11(2325)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Smith BJ, Lerner MR, Bu SY, Lucas EA,
Hanas JS, Lightfoot SA, Postier RG, Bronze MS and Brackett DJ:
Systemic bone loss and induction of coronary vessel disease in a
rat model of chronic inflammation. Bone. 38:378–386.
2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Park OJ, Kim J, Yang J, Yun CH and Han SH:
Muramyl dipeptide, a shared structural motif of peptidoglycans, is
a novel inducer of bone formation through induction of Runx2. J
Bone Miner Res. 34(975)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ma S, Wang N, Zhang P, Wu W and Fu L:
Fecal microbiota transplantation mitigates bone loss by improving
gut microbiome composition and gut barrier function in aged rats.
PeerJ. 9(e12293)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xiao HH, Lu L, Poon CC, Chan CO, Wang LJ,
Zhu YX, Zhou LP, Cao S, Yu WX, Wong KY, et al: The lignan-rich
fraction from sambucus williamsii hance ameliorates dyslipidemia
and insulin resistance and modulates gut microbiota composition in
ovariectomized rats. Biomed Pharmacother.
137(111372)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
AlQranei MS, Senbanjo LT, Aljohani H,
Hamza T and Chellaiah MA: Lipopolysaccharide-tlr-4 axis regulates
osteoclastogenesis independent of rankl/rank signaling. BMC
Immunol. 22(23)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yuan S and Shen J: Bacteroides vulgatus
diminishes colonic microbiota dysbiosis ameliorating lumbar bone
loss in ovariectomized mice. Bone. 142(115710)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Schepper JD, Collins F, Rios-Arce ND, Kang
HJ, Schaefer L, Gardinier JD, Raghuvanshi R, Quinn RA, Britton R,
Parameswaran N and McCabe LR: Involvement of the gut microbiota and
barrier function in glucocorticoid-induced osteoporosis. J Bone
Miner Res. 35:801–820. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Locantore P, Del Gatto V, Gelli S,
Paragliola RM and Pontecorvi A: The interplay between immune system
and microbiota in osteoporosis. Mediators Inflamm.
2020(3686749)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Cline-Smith A, Axelbaum A, Shashkova E,
Chakraborty M, Sanford J, Panesar P, Peterson M, Cox L, Baldan A,
Veis D and Aurora R: Ovariectomy activates chronic low-grade
inflammation mediated by memory T cells, which promotes
osteoporosis in mice. J Bone Miner Res. 35:1174–1187.
2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Tsukasaki M and Takayanagi H:
Osteoimmunology: Evolving concepts in bone-immune interactions in
health and disease. Nat Rev Immunol. 19:626–642. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Charles JF and Nakamura MC: Bone and the
innate immune system. Curr Osteoporos Rep. 12:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hao ML, Wang GY, Zuo XQ, Qu CJ, Yao BC and
Wang DL: Gut microbiota: An overlooked factor that plays a
significant role in osteoporosis. J Int Med Res. 47:4095–4103.
2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Uluçkan Ö, Jimenez M, Karbach S, Jeschke
A, Graña O, Keller J, Busse B, Croxford AL, Finzel S, Koenders M,
et al: Chronic skin inflammation leads to bone loss by
il-17-mediated inhibition of wnt signaling in osteoblasts. Sci
Transl Med. 8(330ra37)2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Quach D and Britton RA: Gut microbiota and
bone health. Adv Exp Med Biol. 1033:47–58. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Campbell JE and Drucker DJ: Pharmacology,
physiology, and mechanisms of incretin hormone action. Cell Metab.
17:819–837. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Mabilleau G: Incretins and bone: Friend or
foe? Curr Opin Pharmacol. 22:72–78. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Baker JM, Al-Nakkash L and
Herbst-Kralovetz MM: Estrogen-gut microbiome axis: Physiological
and clinical implications. Maturitas. 103:45–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang Y, Cheng Z, Elalieh HZ, Nakamura E,
Nguyen MT, Mackem S, Clemens TL, Bikle DD and Chang W: Igf-1r
signaling in chondrocytes modulates growth plate development by
interacting with the pthrp/ihh pathway. J Bone Miner Res.
26:1437–1446. 2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Guo X, Zhong K, Zhang J, Hui L, Zou L, Xue
H, Guo J, Zheng S, Huang D and Tan M: Gut microbiota can affect
bone quality by regulating serum estrogen levels. Am J Transl Res.
14:6043–6055. 2022.PubMed/NCBI
|
|
74
|
Ren H, Sun R and Wang J: Relationship of
melatonin level, oxidative stress and inflammatory status with
osteoporosis in maintenance hemodialysis of chronic renal failure.
Exp Ther Med. 15:5183–5188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gilbert L, He X, Farmer P, Boden S,
Kozlowski M, Rubin J and Nanes MS: Inhibition of osteoblast
differentiation by tumor necrosis factor-alpha. Endocrinology.
141:3956–3964. 2000.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Guo M, Liu H, Yu Y, Zhu X, Xie H, Wei C,
Mei C, Shi Y, Zhou N, Qin K and Li W: Lactobacillus rhamnosus GG
ameliorates osteoporosis in ovariectomized rats by regulating the
Th17/Treg balance and gut microbiota structure. Gut Microbes.
15(2190304)2023.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yang X, Zhou F, Yuan P, Dou G, Liu X, Liu
S, Wang X, Jin R, Dong Y, Zhou J, et al: T cell-depleting
nanoparticles ameliorate bone loss by reducing activated T cells
and regulating the Treg/Th17 balance. Bioact Mater. 6:3150–3163.
2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Lorenzo J: From the gut to bone:
Connecting the gut microbiota with Th17 T lymphocytes and
postmenopausal osteoporosis. J Clin Invest.
131(e146619)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ohara TE and Hsiao EY:
Microbiota-neuroepithelial signalling across the gut-brain axis.
Nat Rev Microbiol. 23:371–384. 2025.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Mayer EA, Nance K and Chen S: The
gut-brain axis. Annu Rev Med. 73:439–453. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Hayes CL, Dong J, Galipeau HJ, Jury J,
McCarville J, Huang X, Wang XY, Naidoo A, Anbazhagan AN, Libertucci
J, et al: Commensal microbiota induces colonic barrier structure
and functions that contribute to homeostasis. Sci Rep.
8(14184)2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Treangen TJ, Wagner J, Burns MP and
Villapol S: Traumatic brain injury in mice induces acute bacterial
dysbiosis within the fecal microbiome. Front Immunol.
9(2757)2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Queipo-Ortuño MI, Seoane LM, Murri M,
Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F and Tinahones
FJ: Gut microbiota composition in male rat models under different
nutritional status and physical activity and its association with
serum leptin and ghrelin levels. PLoS One. 8(e65465)2013.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Yadav VK, Oury F, Suda N, Liu ZW, Gao XB,
Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et
al: A serotonin-dependent mechanism explains the leptin regulation
of bone mass, appetite, and energy expenditure. Cell. 138:976–989.
2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Bliziotes M, Eshleman A, Burt-Pichat B,
Zhang XW, Hashimoto J, Wiren K and Chenu C: Serotonin transporter
and receptor expression in osteocytic MLO-Y4 cells. Bone.
39:1313–1321. 2006.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Yadav VK, Balaji S, Suresh PS, Liu XS, Lu
X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, et al:
Pharmacological inhibition of gut-derived serotonin synthesis is a
potential bone anabolic treatment for osteoporosis. Nat Med.
16:308–312. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Chabbi-Achengli Y, Coudert AE, Callebert
J, Geoffroy V, Côté F, Collet C and de Vernejoul MC: Decreased
osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci
USA. 109:2567–2572. 2012.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Westbroek I, van der Plas A, de Rooij KE,
Klein-Nulend J and Nijweide PJ: Expression of serotonin receptors
in bone. J Biol Chem. 276:28961–28968. 2001.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Mödder UI, Achenbach SJ, Amin S, Riggs BL,
Melton LJ III and Khosla S: Relation of serum serotonin levels to
bone density and structural parameters in women. J Bone Miner Res.
25:415–422. 2010.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Cummings JH and Macfarlane GT: The control
and consequences of bacterial fermentation in the human colon. J
Appl Bacteriol. 70:443–459. 1991.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Nagpal R, Kumar M, Yadav AK, Hemalatha R,
Yadav H, Marotta F and Yamashiro Y: Gut microbiota in health and
disease: An overview focused on metabolic inflammation. Benef
Microbes. 7:181–194. 2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Montalvany-Antonucci CC, Duffles LF, de
Arruda JAA, Zicker MC, de Oliveira S, Macari S, Garlet GP, Madeira
MFM, Fukada SY, Andrade I Jr, et al: Short-chain fatty acids and
FFAR2 as suppressors of bone resorption. Bone. 125:112–121.
2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Smith PM, Howitt MR, Panikov N, Michaud M,
Gallini CA, Bohlooly-Y M, Glickman JN and Garrett WS: The microbial
metabolites, short-chain fatty acids, regulate colonic Treg cell
homeostasis. Science. 341:569–573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Feng B, Lu J, Han Y, Han Y, Qiu X and Zeng
Z: The role of short-chain fatty acids in the regulation of
osteoporosis: New perspectives from gut microbiota to bone health:
A review. Medicine (Baltimore). 103(e39471)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Charles JF, Ermann J and Aliprantis AO:
The intestinal microbiome and skeletal fitness: Connecting bugs and
bones. Clin Immunol. 159:163–169. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lucas S, Omata Y, Hofmann J, Böttcher M,
Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B,
Krönke G, et al: Short-chain fatty acids regulate systemic bone
mass and protect from pathological bone loss. Nat Commun.
9(55)2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Yonezawa T, Kobayashi Y and Obara Y:
Short-chain fatty acids induce acute phosphorylation of the p38
mitogen-activated protein kinase/heat shock protein 27 pathway via
gpr43 in the mcf-7 human breast cancer cell line. Cell Signal.
19:185–193. 2007.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Li P, Ji B, Luo H, Sundh D, Lorentzon M
and Nielsen J: One-year supplementation with lactobacillus reuteri
atcc pta 6475 counteracts a degradation of gut microbiota in older
women with low bone mineral density. NPJ Biofilms Microbiomes.
8(84)2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Winston JA and Theriot CM: Diversification
of host bile acids by members of the gut microbiota. Gut Microbes.
11:158–171. 2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Zheng XQ, Wang DB, Jiang YR and Song CL:
Gut microbiota and microbial metabolites for osteoporosis. Gut
Microbes. 17(2437247)2025.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Hernandez CJ, Guss JD, Luna M and Goldring
SR: Links between the microbiome and bone. J Bone Miner Res.
31:1638–1646. 2016.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Zou W and Bar-Shavit Z: Dual modulation of
osteoclast differentiation by lipopolysaccharide. J Bone Miner Res.
17:1211–1218. 2002.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Yang K, Xu J, Fan M, Tu F, Wang X, Ha T,
Williams DL and Li C: Lactate suppresses macrophage
pro-inflammatory response to LPS stimulation by inhibition of YAP
and NF-κB activation via GPR81-mediated signaling. Front Immunol.
11(587913)2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Huang J, Yuan L, Wang X, Zhang TL and Wang
K: Icaritin and its glycosides enhance osteoblastic, but suppress
osteoclastic, differentiation and activity in vitro. Life Sci.
81:832–840. 2007.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Li L, Chen B, Zhu R, Tian Y, Liu C, Jia Q,
Wang L, Tang J, Zhao D, Mo F, et al: Fructus ligustri lucidi
preserves bone quality through the regulation of gut microbiota
diversity, oxidative stress, TMAO and sirt6 levels in aging mice.
Aging (Albany NY). 11:9348–9368. 2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Zhao X, Wang Y, Nie Z, Han L, Zhong X, Yan
X and Gao X: Eucommia ulmoides leaf extract alters gut microbiota
composition, enhances short-chain fatty acids production, and
ameliorates osteoporosis in the senescence-accelerated mouse p6
(samp6) model. Food Sci Nutr. 8:4897–4906. 2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Zhao X, Ai J, Mao H and Gao X: Effects of
Eclipta prostrata on gut microbiota of SAMP6 mice with
osteoporosis. J Med Microbiol. 68:402–416. 2019.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Kerezoudi EN, Mitsou EK, Gioti K, Terzi E,
Avgousti I, Panagiotou A, Koutrotsios G, Zervakis GI, Mountzouris
KC, Tenta R and Kyriacou A: Fermentation of pleurotus ostreatus and
ganoderma lucidum mushrooms and their extracts by the gut
microbiota of healthy and osteopenic women: Potential prebiotic
effect and impact of mushroom fermentation products on human
osteoblasts. Food Funct. 12:1529–1546. 2021.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Liu J, Liu J, Liu L, Zhang G and Peng X:
Reprogrammed intestinal functions in astragalus
polysaccharide-alleviated osteoporosis: Combined analysis of
transcriptomics and DNA methylomics demonstrates the significance
of the gut-bone axis in treating osteoporosis. Food Funct.
12:4458–4470. 2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Li ZX, Zhuo JL, Yang N, Gao MB, Qu ZH and
Han T: Effect of Lycium barbarum polysaccharide on osteoblast
proliferation and differentiation in postmenopausal osteoporosis.
Int J Biol Macromol. 271(132415)2024.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Jin S, Liu X, Zheng Y, Zhu T, Tong D,
Zhang R and Liu Y: Genistein supplementation alleviates bone damage
by regulating gut microbiota composition and metabolism in obesity
and estrogen decline. Food Funct. 16:7900–7918. 2025.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Li B, Liu M, Wang Y, Gong S, Yao W, Li W,
Gao H and Wei M: Puerarin improves the bone micro-environment to
inhibit OVX-induced osteoporosis via modulating SCFAs released by
the gut microbiota and repairing intestinal mucosal integrity.
Biomed Pharmacother. 132(110923)2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Mei F, Meng K, Gu Z, Yun Y, Zhang W, Zhang
C, Zhong Q, Pan F, Shen X, Xia G and Chen H: Arecanut (areca
catechu l.) seed polyphenol-ameliorated osteoporosis by altering
gut microbiome via LYZ and the immune system in estrogen-deficient
rats. J Agric Food Chem. 69:246–258. 2021.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Zhang Z, Chen Y, Xiang L, Wang Z, Xiao GG
and Hu J: Effect of curcumin on the diversity of gut microbiota in
ovariectomized rats. Nutrients. 9(1146)2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Jia X, Jia L, Mo L, Yuan S, Zheng X, He J,
Chen V, Guo Q, Zheng L, Yuan Q, et al: Berberine ameliorates
periodontal bone loss by regulating gut microbiota. J Dent Res.
98:107–116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Wang N, Yang H, Tong X, Xu T, Zhao J and
Li YK: Ginkgolide B modulates the gut-bone axis to ameliorate bone
loss in ovariectomized mice. J Orthop Surg Res.
20(804)2025.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Dou J, Liang Z, Liu J, Liu N, Hu X, Tao S,
Zhen X, Yang L, Zhang J and Jiang G: Quinoa alleviates osteoporosis
in ovariectomized rats by regulating gut microbiota imbalance. J
Sci Food Agric. 104:5052–5063. 2024.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Hao F, Guo M, Zhao Y, Zhu X, Hu X, Zhu W,
Mei C, Zhou N, Qin K, Zhu H and Li W: Qing'e Pills ameliorates
osteoporosis by regulating gut microbiota and Th17/Treg balance in
ovariectomized rats. J Inflamm Res. 18:7611–7629. 2025.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Xie H, Hua Z, Guo M, Lin S, Zhou Y, Weng
Z, Wu L, Chen Z, Xu Z and Li W: Gut microbiota and metabonomics
used to explore the mechanism of Qing'e Pills in alleviating
osteoporosis. Pharm Biol. 60:785–800. 2022.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Sun P, Zhang C, Huang Y, Yang J, Zhou F,
Zeng J and Lin Y: Jiangu granule ameliorated OVX rats bone loss by
modulating gut microbiota-SCFAs-Treg/Th17 axis. Biomed
Pharmacother. 150(112975)2022.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Li J, HaomingYou Hu Y, Li R, Ouyang T, Ran
Q, Zhang G and Huang Y: Effects of traditional Chinese medicine
Zuo-Gui-Wan on gut microbiota in an osteoporotic mouse model. J
Orthop Surg Res. 20(128)2025.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Chen J, Ng S, Xu P, Chen S, Li S, Chen X,
Xie L and Ge J: Herbal formula xuling-jiangu improves bone
metabolic balance in rats with ovariectomy-induced osteoporosis via
the gut-bone axis. Front Pharmacol. 15(1505231)2024.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Li X, Li N, Pei H, Ren Y, Li L, Sun L, Wu
Y, Yuan J and Ma Y: Zhuanggu Shubi ointment mediated the
characteristic bacteria-intestinal mucosal barrier-bone metabolism
axis to intervene in postmenopausal osteoporosis. Front Cell Infect
Microbiol. 14(1500111)2024.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Gao MX, Tang XY, Zhang FX, Yao ZH, Yao XS
and Dai Y: Biotransformation and metabolic profile of
Xian-Ling-Gu-Bao capsule, a traditional chinese medicine
prescription, with rat intestinal microflora by ultra-performance
liquid chromatography coupled with quadrupole time-of-flight tandem
mass spectrometry analysis. Biomed Chromatogr. 32:2018.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Tang XY, Gao MX, Xiao HH, Dai ZQ, Yao ZH,
Dai Y and Yao XS: Effects of Xian-Ling-Gu-Bao capsule on the gut
microbiota in ovariectomized rats: Metabolism and modulation. J
Chromatogr B Analyt Technol Biomed Life Sci.
1176(122771)2021.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Liang Q, Du H, Wang Y, Lai Y, Ren M, Wei X
and Xiong Z: Integrated metabolomics and gut microbiota analysis to
explore the protective effects of Gushudan on postmenopausal
osteoporosis rats via gut-bone axis. J Pharm Biomed Anal.
263(116942)2025.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Chen XC, Li WJ, Zeng JY, Dong YP, Qiu JM,
Zhang B, Wang DY, Liu J and Lyu ZH: Shengu granules ameliorate
ovariectomy-induced osteoporosis by the gut-bone-immune axis. Front
Microbiol. 15(1320500)2024.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Ko CH, Siu WS, Lau CP, Lau CB, Fung KP and
Leung PC: Osteoprotective effects of fructus ligustri lucidi
aqueous extract in aged ovariectomized rats. Chin Med.
5(39)2010.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Zhang Y, Leung PC, Che CT, Chow HK, Wu CF
and Wong MS: Improvement of bone properties and enhancement of
mineralization by ethanol extract of fructus ligustri lucidi. Br J
Nutr. 99:494–502. 2008.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Chen B, Wei J, Zhu R, Zhang H, Xia B, Liu
Y, Dai X, Ye Z, Tian Y, Li R, et al: Fructus Ligustri Lucidi
aqueous extract promotes calcium balance and short-chain fatty
acids production in ovariectomized rats. J Ethnopharmacol.
279(114348)2021.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Li XL, Wang L, Bi XL, Chen BB and Zhang Y:
Gushukang exerts osteopreserve effects by regulating vitamin D and
calcium metabolism in ovariectomized mice. J Bone Miner Metab.
37:224–234. 2019.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Sang H, Xie Y, Su X, Zhang M, Zhang Y, Liu
K and Wang J: Mushroom bulgaria inquinans modulates host
immunological response and gut microbiota in mice. Front Nutr.
7(144)2020.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Tyagi AM, Yu M, Darby TM, Vaccaro C, Li
JY, Owens JA, Hsu E, Adams J, Weitzmann MN, Jones RM and Pacifici
R: The microbial metabolite butyrate stimulates bone formation via
t regulatory cell-mediated regulation of WNT10B expression.
Immunity. 49:1116–1131.e7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Xiao HH, Zhu YX, Lu L, Zhou LP, Poon CC,
Chan CO, Wang LJ, Cao S, Yu WX, Wong KY, et al: The lignan-rich
fraction from sambucus williamsii hance exerts bone protective
effects via altering circulating serotonin and gut microbiota in
rats. Nutrients. 14(4718)2022.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Lei M, Hua LM and Wang DW: The effect of
probiotic treatment on elderly patients with distal radius
fracture: A prospective double-blind, placebo-controlled randomised
clinical trial. Benef Microbes. 7:631–637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Takimoto T, Hatanaka M, Hoshino T, Takara
T, Tanaka K, Shimizu A, Morita H and Nakamura T: Effect of bacillus
subtilis c-3102 on bone mineral density in healthy postmenopausal
japanese women: A randomized, placebo-controlled, double-blind
clinical trial. Biosci Microbiota Food Health. 37:87–96.
2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Ohlsson C, Engdahl C, Fåk F, Andersson A,
Windahl SH, Farman HH, Movérare-Skrtic S, Islander U and Sjögren K:
Probiotics protect mice from ovariectomy-induced cortical bone
loss. PLoS One. 9(e92368)2014.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Schwarzer M, Makki K, Storelli G,
Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S,
Hudcovic T, Heddi A, et al: Lactobacillus plantarum strain
maintains growth of infant mice during chronic undernutrition.
Science. 351:854–857. 2016.PubMed/NCBI View Article : Google Scholar
|
|
139
|
McCabe LR, Irwin R, Schaefer L and Britton
RA: Probiotic use decreases intestinal inflammation and increases
bone density in healthy male but not female mice. J Cell Physiol.
228:1793–1798. 2013.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Avella MA, Place A, Du SJ, Williams E,
Silvi S, Zohar Y and Carnevali O: Lactobacillus rhamnosus
accelerates zebrafish backbone calcification and gonadal
differentiation through effects on the gnRH and IGF systems. PLoS
One. 7(e45572)2012.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Whisner CM, Martin BR, Schoterman MH,
Nakatsu CH, McCabe LD, McCabe GP, Wastney ME, van den Heuvel EG and
Weaver CM: Galacto-oligosaccharides increase calcium absorption and
gut bifidobacteria in young girls: A double-blind cross-over trial.
Br J Nutr. 110:1292–1303. 2013.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Abrams SA, Griffin IJ, Hawthorne KM, Liang
L, Gunn SK, Darlington G and Ellis KJ: A combination of prebiotic
short- and long-chain inulin-type fructans enhances calcium
absorption and bone mineralization in young adolescents. Am J Clin
Nutr. 82:471–476. 2005.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Whisner CM, Martin BR, Nakatsu CH, Story
JA, MacDonald-Clarke CJ, McCabe LD, McCabe GP and Weaver CM:
Soluble corn fiber increases calcium absorption associated with
shifts in the gut microbiome: A randomized dose-response trial in
free-living pubertal females. J Nutr. 146:1298–1306.
2016.PubMed/NCBI View Article : Google Scholar
|
|
144
|
García-Vieyra MI, Del Real A and López MG:
Agave fructans: Their effect on mineral absorption and bone mineral
content. J Med Food 2014. 17:1247–1255. 2014.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Weaver CM, Martin BR, Nakatsu CH,
Armstrong AP, Clavijo A, McCabe LD, McCabe GP, Duignan S,
Schoterman MH and van den Heuvel EG: Galactooligosaccharides
improve mineral absorption and bone properties in growing rats
through gut fermentation. J Agric Food Chem. 59:6501–6510.
2011.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Levi YLAS, Novais GS, Dias RB, Andraus
RAC, Messora MR, Neto HB, Ervolino E, Santinoni CS and Maia LP:
Effects of the prebiotic mannan oligosaccharide on the experimental
periodontitis in rats. J Clin Periodontol. 45:1078–1089.
2018.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Scholz-Ahrens KE, Açil Y and Schrezenmeir
J: Effect of oligofructose or dietary calcium on repeated calcium
and phosphorus balances, bone mineralization and trabecular
structure in ovariectomized rats. Br J Nutr. 88:365–377.
2002.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Bueno-Vargas P, Manzano M, Diaz-Castro J,
López-Aliaga I, Rueda R and López-Pedrosa JM: Maternal dietary
supplementation with oligofructose-enriched inulin in
gestating/lactating rats preserves maternal bone and improves bone
microarchitecture in their offspring. PLoS One.
11(e0154120)2016.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Zhang YW, Cao MM, Li YJ, Li YJ, Lu PP, Dai
GC, Zhang M, Wang H and Rui YF: Fecal microbiota transplantation
ameliorates bone loss in mice with ovariectomy-induced osteoporosis
via modulating gut microbiota and metabolic function. J Orthop
Translat. 37:46–60. 2022.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Ma P, Wang R, Chen H, Zheng J, Yang W,
Meng B, Liu Y, Lu Y, Zhao J and Gao H: Fecal microbiota
transplantation alleviates lipopolysaccharide-induced osteoporosis
by modulating gut microbiota and long non-coding RNA TUG1
expression. Front Cell Infect Microbiol. 15(1535666)2025.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Mazziotta C, Tognon M, Martini F,
Torreggiani E and Rotondo JC: Probiotics mechanism of action on
immune cells and beneficial effects on human health. Cells.
12(184)2023.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Lu L, Chen X, Liu Y and Yu X: Gut
microbiota and bone metabolism. FASEB J. 35(e21740)2021.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Abdelqader A, Irshaid R and Al-Fataftah
AR: Effects of dietary probiotic inclusion on performance, eggshell
quality, cecal microflora composition, and tibia traits of laying
hens in the late phase of production. Trop Anim Health Prod.
45:1017–1024. 2013.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Sadeghi AA: Bone mineralization of broiler
chicks challenged with salmonella enteritidis fed diet containing
probiotic (bacillus subtilis). Probiotics Antimicrob Proteins.
6:136–140. 2014.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Rodrigues FC, Castro AS, Rodrigues VC,
Fernandes SA, Fontes EA, de Oliveira TT, Martino HS and de Luces
Fortes Ferreira CL: Yacon flour and bifidobacterium longum modulate
bone health in rats. J Med Food. 15:664–670. 2012.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Maradonna F, Gioacchini G, Falcinelli S,
Bertotto D, Radaelli G, Olivotto I and Carnevali O: Probiotic
supplementation promotes calcification in danio rerio larvae: A
molecular study. PLoS One. 8(e83155)2013.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Parvaneh K, Ebrahimi M, Sabran MR, Karimi
G, Hwei AN, Abdul-Majeed S, Ahmad Z, Ibrahim Z and Jamaluddin R:
Probiotics (bifidobacterium longum) increase bone mass density and
upregulate sparc and bmp-2 genes in rats with bone loss resulting
from ovariectomy. Biomed Res Int. 2015(897639)2015.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Gibson GR and Roberfroid MB: Dietary
modulation of the human colonic microbiota: Introducing the concept
of prebiotics. J Nutr. 125:1401–1412. 1995.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Bryk G, Coronel MZ, Pellegrini G,
Mandalunis P, Rio ME, de Portela ML and Zeni SN: Effect of a
combination GOS/FOS® prebiotic mixture and interaction with calcium
intake on mineral absorption and bone parameters in growing rats.
Eur J Nutr. 54:913–923. 2015.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Slevin MM, Allsopp PJ, Magee PJ, Bonham
MP, Naughton VR, Strain JJ, Duffy ME, Wallace JM and Mc Sorley EM:
Supplementation with calcium and short-chain
fructo-oligosaccharides affects markers of bone turnover but not
bone mineral density in postmenopausal women. J Nutr. 144:297–304.
2014.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Bass EF, Baile CA, Lewis RD and Giraudo
SQ: Bone quality and strength are greater in growing male rats fed
fructose compared with glucose. Nutr Res. 33:1063–1071.
2013.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Yang LC, Wu JB, Lu TJ and Lin WC: The
prebiotic effect of anoectochilus formosanus and its consequences
on bone health. Br J Nutr. 109:1779–1788. 2013.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Vindigni SM and Surawicz CM: Fecal
microbiota transplantation. Gastroenterol Clin North Am.
46:171–185. 2017.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Rios-Arce ND, Schepper JD, Dagenais A,
Schaefer L, Daly-Seiler CS, Gardinier JD, Britton RA, McCabe LR and
Parameswaran N: Post-antibiotic gut dysbiosis-induced trabecular
bone loss is dependent on lymphocytes. Bone.
134(115269)2020.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Goto Y, Panea C, Nakato G, Cebula A, Lee
C, Diez MG, Laufer TM, Ignatowicz L and Ivanov II: Segmented
filamentous bacteria antigens presented by intestinal dendritic
cells drive mucosal th17 cell differentiation. Immunity.
40:594–607. 2014.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Atarashi K, Tanoue T, Shima T, Imaoka A,
Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al:
Induction of colonic regulatory T cells by indigenous clostridium
species. Science. 331:337–341. 2011.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Li L, Rao S, Cheng Y, Zhuo X, Deng C, Xu
N, Zhang H and Yang L: Microbial osteoporosis: The interplay
between the gut microbiota and bones via host metabolism and
immunity. Microbiologyopen. 8(e00810)2019.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Blanton LV, Charbonneau MR, Salih T,
Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ,
Trehan I, Jorgensen JM, et al: Gut bacteria that prevent growth
impairments transmitted by microbiota from malnourished children.
Science 351: 10.1126/science.aad3311 aad3311, 2016.
|
|
169
|
Zhang YW, Cao MM, Li YJ, Zhang RL, Wu MT,
Yu Q and Rui YF: Fecal microbiota transplantation as a promising
treatment option for osteoporosis. J Bone Miner Metab. 40:874–889.
2022.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Neumann M, Steimle A, Grant ET, Wolter M,
Parrish A, Willieme S, Brenner D, Martens EC and Desai MS:
Deprivation of dietary fiber in specific-pathogen-free mice
promotes susceptibility to the intestinal mucosal pathogen
citrobacter rodentium. Gut Microbes. 13(1966263)2021.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Sonnenburg ED, Smits SA, Tikhonov M,
Higginbottom SK, Wingreen NS and Sonnenburg JL: Diet-induced
extinctions in the gut microbiota compound over generations.
Nature. 529:212–215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Davis HC: Can the gastrointestinal
microbiota be modulated by dietary fibre to treat obesity? Ir J Med
Sci. 187:393–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Matkovic V, Landoll JD, Badenhop-Stevens
NE, Ha EY, Crncevic-Orlic Z, Li B and Goel P: Nutrition influences
skeletal development from childhood to adulthood: A study of hip,
spine, and forearm in adolescent females. J Nutr. 134:701S–705S.
2004.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Laird E, Molloy AM, McNulty H, Ward M,
McCarroll K, Hoey L, Hughes CF, Cunningham C, Strain JJ and Casey
MC: Greater yogurt consumption is associated with increased bone
mineral density and physical function in older adults. Osteoporos
Int. 28:2409–2419. 2017.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Rizzoli R: Dairy products and bone health.
Aging Clin Exp Res. 34:9–24. 2022.PubMed/NCBI View Article : Google Scholar
|