Introduction
Orchids belong to the family Orchidaceae, one of
largest families of flowering plant and the most diverse family
with >25,000 species in ~880 genera (1). Several orchids have been used for a
variety of purposes, including ornamental plants, sources of
natural antioxidants and bioactive compounds and for cosmeceutical
use (2). Dendrobium spp. is
a large genus with >1,000 species that can grow in a variety of
environments and as epiphytes in tropical and subtropical Asia and
eastern Australia (3). Several
Dendrobium species have been used in traditional medicine
due to the high number of bioactive compounds, such as polyphenols,
flavonoids, alkaloids and polysaccharides (3,4). These
compounds have key biological activities. For example, phenolics
and flavonoids exhibit antioxidant capacity, which could promote
human health and prevent chronic and degenerative diseases,
including cancer, caused by free radicals (5). D. crepidatum contains bibenzyl
derivatives, polyphenols and flavonoids that have antioxidant and
cytotoxic effects on cancer cells (6). D. denneanum and D.
nobile contain bioactive polysaccharides and exhibit
antioxidant properties (7,8).
Skin cancer is one of the most frequent malignancies
worldwide and can be classified into melanoma skin cancer (MSC) and
non-MSC (9). According to global
data from 2000 to 2024, MSC ranks 17th in prevalence and 23rd as a
leading cause of cancer-related death, with the highest incidence
and mortality rate reported in Europe. While Asia has the lowest
incidence rate of MSC, it records the second-highest mortality rate
(9). In humans, malignant melanoma
cells consist of activating mutations of B-RAF oncogene, resulting
in abnormal cell proliferation (10). The SK-MEL-28 cell line is a type of
human malignant melanoma cell and the most aggressive form of skin
cancer. The migration and invasion of melanoma cells are involved
with proteolytic enzymes such as serine and cysteine proteases and
matrix metalloproteases (MMPs) in remodeling dermal extracellular
matrix (ECM) (11). The ECM
comprises a large variety of macromolecules, including
fibrous-forming proteins (collagen and elastin) and carbohydrate
strings (proteoglycans, glycosaminoglycans and glycoproteins), and
enzymes (proteinases and MMPs). Collagens are predominant and the
main targets for MMPs, especially collagenases, in ECM remodeling
(11,12). Also, the accumulation of hyaluronan
(HA), a polysaccharide subfamily of glycosaminoglycans, is
attributed to the progression and metastasis of cancer cells
(13). D. draconis, D.
falconeri, D. findlayanum and D. loddigesii have
been reported for their ability to inhibit the proliferation of
numerous types of cancer cell (14), including colon cancer (15) and leukemia (16). Most cancer cells are inhibited by
many pathways, including autophagy and apoptosis (both intrinsic
and extrinsic). Secondary metabolites, such as bibenzyl compounds
from D. loddigesii, induce apoptosis in melanoma cells
(17). In addition, the crude
extract and certain compounds isolated from Dendrobium have
been found to regulate the changes of expression of genes and
proteins involved in apoptosis, especially in the intrinsic pathway
(18-23).
The bcl-2 gene family is a key factor in the regulation of
cell apoptosis, including anti-apoptotic genes (bcl-2 and
bcl-XL) and pro-apoptotic genes (bax and bid)
(18). Some traditional Chinese
medicine anticancer drugs have been found to induce cell apoptosis
through targeting the proteins of the bcl-2 family and increasing
the ratio of bax to bcl-2 (19). D. officinale extracts
increase the protein levels of Bax and caspase-3 and decreased
Bcl-2 in extract-fed rats with gastric cancer (20). D. officinale extract
primarily contains polysaccharides, including mannose, which
exhibit anti-tumor activity and induce apoptosis in osteosarcoma
cells via caspase upregulation (21). Moreover, Nudol, a phenanthrene
derivative from D. nobile, notably enhances the mRNA
expression levels of cytc and caspases in
osteosarcoma cells (22).
Quercetin, a flavonoid compound commonly found in plant extracts,
exerts anti-cancer activity by inducing nuclear fragmentation and
intrinsic apoptosis via an increase in p53 expression
(23).
Our previous study investigated orchid hybrids that
were primarily created for horticultural purposes and found that
the propanolic extract from Dendrobium Pearl Vera [a
Dendrobium hybrid; DH-P] exhibited antioxidant activity and
could reduce melanin production and viability of melanoma SK-MEL-28
cells treated with phenolic contents at 15 µg/ml (24). To the best of our knowledge,
however, there are no published reports on the bioactive compounds
in such Dendrobium hybrids. Swainson et al (24) identified certain annotated bioactive
compounds using high-resolution mass spectrometry (HR-MS) coupled
with the Orbitrap (ion trap-based mass analyzer) using an
untargeted approach. Quadrupole time-of-flight (QTOF) is one of the
most popular mass analyzers used in analytical screening of
targeted and non-targeted substances due to its robustness, high
sensitivity and specificity. The present study aimed to identify
other bioactive compounds in the DH plant using QTOF and determine
the effects of DH-P on free radicals, enzyme activity involved in
ECM, cell migration and the mechanism of SK-MEL-28 cell death at
the gene level.
Materials and methods
Chemicals and reagents
The standards used for the HR-MS experiment were
quercetin (Sigma-Aldrich; Merck KGaA; cat. no. Q4951), dendrobine
(Chengdu Biopurify Phytochemicals, Ltd.; cat. no. BP0479) and
gigantol (Sigma-Aldrich; Merck KGaA; cat. no. Sml2036). The human
melanoma cell line was SK-MEL-28 (American Type Culture Collection;
cat. no. HTB-72). Other chemicals, reagents and media used were
HPLC, analytical or molecular biology grade, as appropriate.
Preparation of DH-P
DH plants (a hybrid between Dendrobium Topaz
Dream and D. bigibbum; CordyBiotech Co., Ltd.) were cultured
in vitro for 3-4 months in Murashige and Skoog medium
(PhytoTech Labs; cat. no. M524) containing 6.8 g/l agar and 17 g/l
sucrose, at 25±2˚C under 300 lux light for 16 h/day. Whole plants
of DH were harvested and ground using liquid nitrogen. The ground
sample was extracted using 2-propanol with a ratio of 1:5 (w/v).
Samples were macerated at room temperature for 24 h. The filtrate
was collected through filter papers (Whatman plc; Cytiva; cat. no.
1001-090) and samples from three extractions were pooled. The
filtrate was concentrated using a rotary evaporator and
lyophilized. The extraction was performed twice independently. DH-P
was redissolved in 95% ethanol for further use.
Screening of secondary metabolites
using thin-layer chromatography (TLC)
DH-P was prepared to a final concentration of 10
mg/ml in 95% ethanol. Then, extract samples were subjected to TLC
analysis for the presence of phenolics and flavonoids as previously
described (25). Quercetin was used
as a reference for detection. A total of ~5 drops/sample were
applied on a 10x5 cm silica gel plate (Merck KGaA) and separated in
a mobile phase consisting of a ratio of ethyl acetate-to-acetic
acid-to-water of 8:1:1 (v/v/v). The gel plate was sprayed with 0.1%
2-aminoethyl diphenylborinate solution to visualize phenolics and
flavonoids using ultraviolet viewing cabinets (Spectronics
Corporation) at 366 nm.
Measurement of total phenolic
content
The total phenolic content in DH-P was determined in
a microwell plate as previously described with modification
(26). In total, 20 µl standard or
DH-P were added, followed by 100 µl 10% v/v Folin-Ciocalteu
solution in 96-well plates at room temperature for 3 min; then, 80
µl 1 M sodium carbonate was added at room temperature for 20 min.
The absorbance was measured at 765 nm using a microplate reader
(Tecan Group, Ltd.; cat. no. M200). Gallic acid (GA) was used as a
standard. Each sample was performed in triplicate. The total
phenolic content was reported as µg GA equivalent (E)/g tissue.
Measurement of total flavonoid
content
Total flavonoid content in DH-P was determined in a
microwell plate as previously described with a slight modification
(27). Distilled water (104 µl) was
mixed with 60 µl methanol in each well. Then, 20 µl standard or
DH-P and 8 µl 0.5 M potassium acetate solution were added, followed
by 8 µl 5% (w/v) aluminum chloride solution. The mixture was shaken
in an orbital shaker (Edmund Bühler GmbH) for 5 min and incubated
in the dark at room temperature for 30 min. The absorbance was
measured at 415 nm. Quercetin was used as a standard. Each sample
was performed in triplicate. The total flavonoid content was
reported as µg quercetin equivalent (QE)/g tissue.
Measurement of total alkaloid
content
The total alkaloid content in DH-P was determined as
previously reported (28). A sample
(1 ml) of standard or DH-P was transferred to a separating funnel
and mixed with 5 ml each bromocresol green solution and phosphate
buffer (pH 4.7). The complex was extracted with 10 ml chloroform
using sequential addition of 1, 2, 3 and 4 ml. The extracted
complex was collected from each addition, pooled and measured for
absorbance at 470 nm. Atropine was used as a standard. Each sample
was performed in triplicate. The total alkaloid content was
reported as µg atropine equivalent (AE)/g tissue.
Identification of compounds using
ultra-high-performance liquid chromatography (UHPLC)-electrospray
ionization-QTOF-tandem mass spectrometry (ESI-QTOF-MS/MS)
DH-P at a concentration of 1 mg/ml in 95% ethanol
was filtered through a 0.2 µm nylon filter. The composition was
separated using UHPLC-ESI-QTOF-MS. A pure compound (dendrobine,
gigantol and quercetin) was used as a reference. These compounds
have been reported to accumulate in Dendrobium species and
exhibit antioxidant and anticancer properties (4,23). The
standard was prepared at a final concentration of 20 µg/ml in
methanol. All samples were analyzed by the Scientific Equipment
Center (Kasetsart University, Thailand). The chromatographic
separation and parameters were set up for the detection of
dendrobine, gigantol and quercetin as previously described
(29-31).
An ExionLC™ AD system (AB Sciex LLC) equipped with a C18
column (100.0x4.6 mm; internal diameter, 2.6 µm; Phenomenex, Inc.)
was used for separation. Metabolite analysis of the matrix was
performed on a SCIEX X500R QTOF system with Turbo V™
source (AB Sciex LLC) using the ESI probe operated in either
positive or negative ion mode. Information-dependent acquisition
was also performed on the X500R QTOF system. The TOF MS (scan
range, 100-500 Da) parameters were as follows: declustering
potential (DP), 50 V; collision energy (CE), 10 V and accumulation
time, 0.25 sec for positive mode and DP, -60 V; CE, -10 V and
accumulation time, 0.25 sec for negative mode. The TOF MS/MS (scan
range 50-500 Da) parameters were DP, 50 V; CE, 35 V; CE spread
(CES), 0 V and accumulation time, 0.1 sec for the positive mode,
but DP, -60 V; CE, -35 V; CES, 0 V; accumulation time, 0.1 sec for
the negative mode. MS/MS fragmentation patterns of compounds
detected in the crude extract were compared with those in the
National Institute of Standards and Technology research library
(version 2017; https://chemdata.nist.gov/). Compounds with a mass
error within ±5 ppm were selected as putative metabolites present
in DH-P.
2,2-Diphenyl-1-picrylhydrazyl (DPPH)
radical scavenging capacity
DPPH assay was performed in a microplate as
previously described, with minor modification (32). A sample (25 µl) of the control or
DH-P was added in each well with 160 µl 150 µM DPPH radical
solution (Sigma Aldrich; cat. no. D9132). The mixture was incubated
in the dark at room temperature for 30 min. The scavenging activity
of the samples against DPPH radicals was detected at 517 nm. Each
sample was run in triplicate. Ethanol was used as a background
control. Ascorbic acid was used as a positive control and standard.
Scavenging activity was calculated based on the absorbance (A) as
follows: % Radical scavenging=(1-Asample/A
control) x100. Antioxidant activity was expressed as
vitamin C equivalent antioxidant capacity (VCEAC) in µg/ml.
2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)
radical scavenging capacity
ABTS assay was performed in a microplate as
previously described, with minor modification (33). The working solution was prepared by
mixing 0.5 ml ABTS+ (Applichem; cat. no. A1088) with 30
ml methanol at a ratio of 1:60 v/v. The absorbance at 734 nm was
determined to be 1.1±0.02 AU before use in the experiment. The
reaction was performed by mixing 10 µl control or DH-P with 190 µl
ABTS+ working solution at room temperature for 2 h. The
scavenging activity was detected at 734 nm. Each sample was run in
triplicate. Ethanol was used as a background control. Ascorbic acid
was used as a positive control and standard. ABTS scavenging
activity was calculated as for DPPH.
Ferric reducing antioxidant power
(FRAP)
FRAP assay was performed in a microplate as
previously described with a minor modification (34). A total of 10 µl control or DH-P was
added with 140 µl FRAP solution in the dark at 37˚C for 30 min. The
absorbance of the mixture was read at 593 nm. Each sample was run
in triplicate. Ethanol was used as a background control. A standard
curve was generated using ascorbic acid (0-1,000 µM) and used for
calculation of the antioxidant activity. Reducing power was
reported as the FRAP value/mg extract.
Inhibition of collagenase
activity
Collagenase inhibition assay was performed using
Collagenase Activity Colorimetric Assay kit (Sigma-Aldrich; Merck
KGaA; cat. no. MAK293) according to the manufacturer's
instructions. The mixture was prepared by adding collagenase assay
buffer and DH-P sample or inhibitor control (1 M
1,10-phenanthroline). Ethanol was used as a background control. The
mixture was added with 5 µl 0.35 U/ml collagenase enzyme at room
temperature for 10 min. A total of 20 µl 1 mM
N-[3-(2-furyl)acryloyl]-L-leucyl-glycyl-L-prolyl-L-alanine
substrate and 30 µl collagenase buffer were added. The reaction was
immediately measured in the kinetic mode at absorbance of 345 nm
and 37˚C for 5-15 min. Each sample was performed in triplicate.
Collagenase inhibition was calculated according to the
manufacturer's instructions.
Inhibition of hyaluronidase
activity
Hyaluronidase inhibition assay was performed as
previously described (35). A
reaction was performed by mixing 35 µl 1% dimethyl sulfoxide
(DMSO), 5 µl DH-P or inhibitor control (1 mg/ml oleanolic acid) and
5 µl 1.5 U/ml hyaluronidase. Ethanol was used as a control. After
the mixture had been incubated at 37˚C for 10 min, 5 µl 0.03% (w/v)
hyaluronic acid in 300 mM sodium phosphate (pH 5.35) was added and
further incubated at 37˚C for 45 min. Then, 5 µl acid albumin
substrate [0.1% BSA (Sigma-Aldrich; cat. no. 12659) in 24 mM sodium
acetate and 79 mM acetic acid (pH 3.75)] was added. The absorbance
was determined at 600 nm and measured immediately following the
addition of the substrate and continuously for 10 min at room
temperature. Each sample was run in triplicate. The hyaluronidase
inhibition was calculated as follows: % Hyaluronidase
inhibition=(1-Asample/Acontrol) x100.
Cell culture and cytotoxicity
Human melanoma SK-MEL-28 cells (passage 14) were
cultured at a seeding density of ~20,000 cells/cm2 in 75
cm2 flask in Eagle's Minimum Essential Medium (EMEM;
ATCC; cat. no. 30-2003) supplemented with 10% fetal bovine serum
(FBS) (Gibco; cat. no. 10270-106) and 1% penicillin/streptomycin.
SK-MEL-28 cells (passage 15-22) were seeded in 96-well plates at a
density of 5,000 cells/well and cultured for 24 h in EMEM. DH-P at
final concentrations of 0, 10, 20, 30, 40, 50, 60, 80 or 100 µg/ml
in 3.17% ethanol was added to each well. DMSO at a final
concentration of 5% was used as an inhibitor control. Wells
containing 3.17% ethanol at a final concentration served as
controls. Cell cultures were maintained at 37˚C with 5%
CO2 throughout the treatment period. The experiments
were performed twice independently with three replicates for each
treatment. Following treatment for 96 h, 40 µl MTT solution was
added and incubated at 37˚C for 3 h. After removing the
supernatant, 50 µl 100% DMSO was added. The absorbance was measured
at 570 nm. The cell viability was calculated as follows: % Cell
viability=Asample/Acontrol x100. The
half-maximal inhibitory concentration (IC50) was
determined from a dose response curve (GraphPad Software Version
10.3.1, Inc.; Dotmatics).
Wound healing assay
SK-MEL-28 cells were cultured in serum-free EMEM in
24-well plates at a density of 1x105 cells/well (~80%
confluency) at 37 ˚C for 24 h. After removing media, a gap in the
middle of each well was created by scratching with a sterile 100-µl
pipette tip. Loosely attached cells were removed using PBS (pH
7.4). Cells were treated with the DH-P at 20 µg/ml for 0, 24, 48,
and 96 h. DMSO (5%) was used as an inhibitor control. A drug-free
plate with 3.17% ethanol served as a control. Cell cultures were
maintained at 37˚C with 5% CO2 throughout the treatment
period. The cell migration was photographed under a light
microscope at 0, 24, 48 and 96 h after treatment. Gap distances
were measured using ImageJ software Version 1.53v (National
Institutes of Health). The experiment was performed twice
independently with three replicates for each treatment.
Cell migration assay
Cell migration was assessed using the EZCell Cell
Invasion Assay kit (Abcam; cat. no. ab287890) according to the
manufacturer's instructions. Briefly, diluted human fibronectin
solution at a 1:5 ratio was coated on the top chamber at 25˚C for 3
h. A total of ~5x104 cells in FBS-free EMEM were seeded
in the top chamber with DH-P at 0.5 (10 µg/ml), 2/3 IC50
(13.3 µg/ml), and IC50 (20 µg/ml). Cells exposed to
3.17% ethanol served as controls. Then, 200 µl FBS-containing EMEM
was added to the bottom chamber. The chamber was incubated at 37˚C
in a humidified CO2 incubator for 72 h according to the
manufacturer's instructions. Cells that had migrated to the bottom
chamber were stained with dissociation/dye solution at 37˚C with 5%
CO2 for 1 h. Each treatment was run in four replicates.
The absorbance of the invaded cells was read at excitation/emission
wavelengths of 535/587 nm using a fluorescence microplate reader
(Tecan Group, Ltd.). The percentage of invasive cell numbers was
calculated from a standard curve.
Analysis of nuclear morphology by
Hoechst 33342 staining
SK-MEL-28 cells were seeded at a density of
3x105 cells/well in a 35x10 mm dish chamber (SPL Life
Sciences) for 24 h. Cells were treated with DH-P at 20 µg/ml for 0,
48 and 96 h. Dishes containing 3.17% ethanol and 5% DMSO were used
as controls and inhibitor controls, respectively. Cells were
incubated at 37˚C with 5% CO2 during treatment. Cells
were observed for DNA damage at 0, 48 and 96 h after treatment.
After removing media, nuclei were stained with 10 µg/ml Hoechst
33342 (Sigma-Aldrich; Merck KGaA) in PBS (pH 7.4) at room
temperature for 15 min in the dark, as previously described
(36). The morphology of nuclei was
examined using a fluorescence microscope at 40X magnification
(Olympus Corporation). Each treatment was run in triplicate.
Gene expression analysis using reverse
transcription-quantitative (RT-q)PCR
SK-MEL-28 cells were treated with DH-P at 20 µg/ml
for 48 h. Cells exposed to 3.17% ethanol served as controls. Cells
were incubated at 37˚C with 5% CO2 during the treatment
period. A total of ~1x106 cells/group was pooled and
used to isolate total RNA with TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The cDNA was synthesized using a
FIREScript RT cDNA Synthesis kit (Solis BioDyne). The cDNA samples
were used as templates in the qPCR, while total RNA was used to
screen for gDNA contamination. The qPCR reaction mixture was at a
final volume of 20 µl containing 1X HOT FIREPol EvaGreen qPCR Mix
Plus (NO-ROX; Solis BioDyne), 0.4 µM primer pair (Table I) and cDNA template (1 ng).
Thermocycling conditions were as follows: Initial activation at
95˚C for 12 min, followed by 35 cycles of denaturation at 95˚C for
15 sec, annealing at primer-specific temperatures (Table I) for 20 sec and extension at 72˚C
for 20 sec and final extension at 72˚C for 10 min. β-actin was used
as the internal reference, and deionized water as the negative
control. The experiments were performed independently twice, each
with three biological replicates/treatment. Each gene from every
sample was assessed in duplicate. The relative gene expression was
determined using the comparative 2-∆∆Cq method (37).
 | Table IPrimer pairs used to amplify genes
involved in apoptosis via intrinsic pathway. |
Table I
Primer pairs used to amplify genes
involved in apoptosis via intrinsic pathway.
| Gene | NCBI accession
no. | Primer | Sequence,
5'à3' | Annealing
temperature, ˚C | Amplicon size,
bp |
|---|
| β-actin | HQ154074.1 | Forward |
CATCCTGCGTCTGGACCTGG | 58 | 107 |
| | | Reverse |
TAATGTCACGCACGATTTCC | | |
| bax | JX524562.1 | Forward |
TCTGACGGCAACTTCAACTG | 50.5 | 201 |
| | | Reverse |
CGTCCCAAAGTAGGAGAGG | | |
| bcl-2 | KY098799.1 | Forward |
GAGGATTGTGGCCTTCTTTG | 50.8 | 118 |
| | | Reverse |
GTGCCGGTTCAGGTACTCAG | | |
|
caspase-3 | NM_004346.4 | Forward |
TGGAATTGATGCGTGATGTT | 48.2 | 161 |
| | | Reverse |
ACTTCTACAACGATCCCCTC | | |
|
caspase-9 | AF093130.1 | Forward |
GAGGGAGTCAGGCTCTTCCT | 51.6 | 228 |
| | | Reverse |
GCTCGACATCACCAAATCCT | | |
| p53 | AB082923.1 | Forward |
GTTCCGAGAGCTGAATGAGG | 51.6 | 157 |
| | | Reverse |
TGAGTCAGGCCCTTCTGTCT | | |
Statistical analysis
Data were analyzed using GraphPad Prism Version
10.3.1 (GraphPad Software, Inc.; Dotmatics). All data are presented
as the mean ± SEM of ≥2 independent experimental repeats. One-way
ANOVA followed by Dunnett's or Tukey's test was used to analyze
inhibition of ECM activity and cell viability and migration,
respectively. An unpaired t-test was performed in gene expression
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection and quantification of
secondary metabolites in DH-P
Dendrobium accumulates large amounts of
compounds beneficial to human health, especially polyphenols,
flavonoids and alkaloids. Based on the TLC analysis, the two
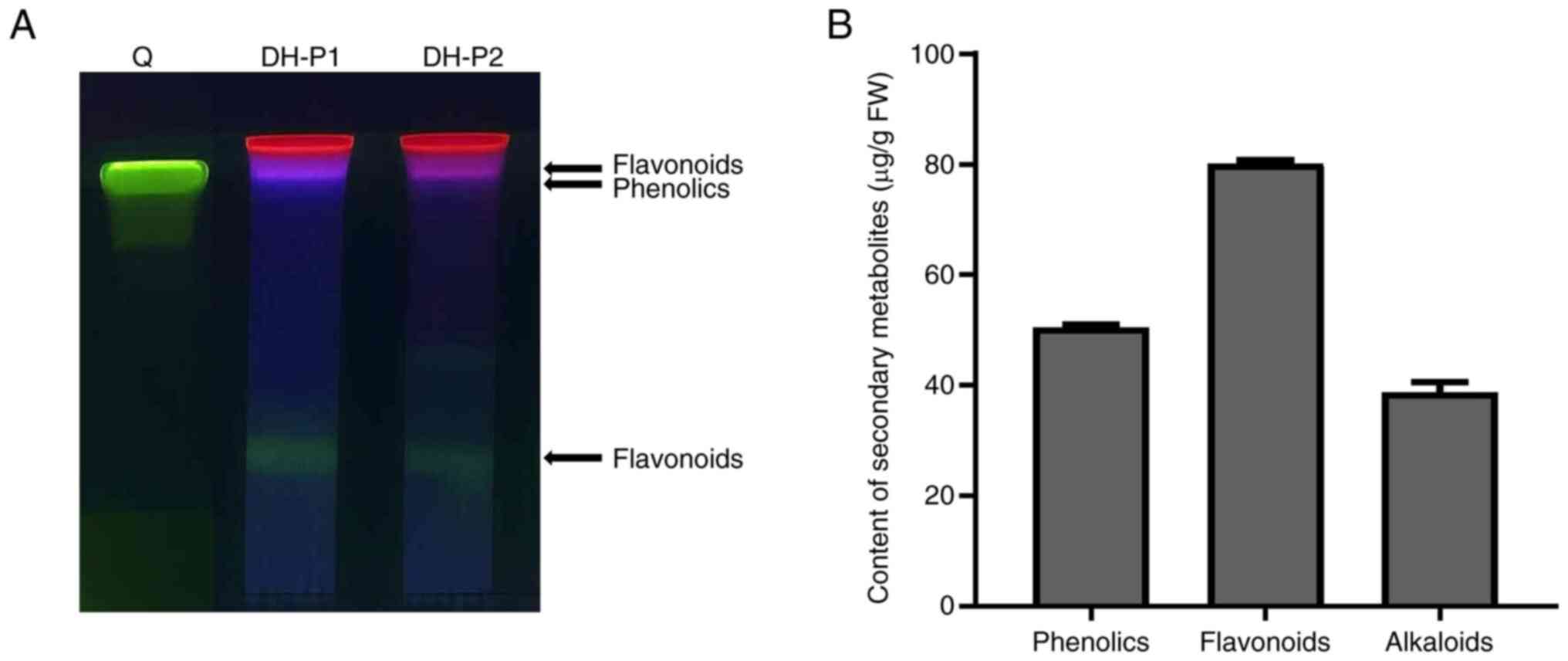
independent replications of DH-P had similar amounts and types of
compound (Fig. 1A). Violet and
green fluorescence were observed in DH-P, indicating the presence
of flavonoids and phenolics. However, quercetin (a common flavonol
produced in plants) was not observed. Furthermore, total phenolics,
flavonoids and alkaloids in DH-P were quantified and comprised
50.51±0.44, 80.11±0.69 and 38.75±1.77 µg/g fresh weight (FW),
respectively (Fig. 1B).
Identification of tentative compounds
in DH-P using UHPLC-ESI-QTOF-MS/MS
The present study used a targeted approach based on
HR-MS (QTOF system) to investigate the chemical components in DH-P.
The chromatographic separation and parameters were set according to
separate detection methods for dendrobine (29), gigantol (30) and quercetin (31). These three targeted compounds were
not detected in DH-P. In total, 119 compounds were matched using
the MS2 spectra with the NIST Research Library v.2017. Each
compound was then manually re-evaluated for mass error and searched
for its functions. The focus was on compounds with biological
activities such as anti-oxidant, anti-cancer, anti-inflammatory,
anti-aging, depigmentation and anti-microbial activity. A total of
eight tentative compounds of interest were identified using the
detection method for quercetin (analyzed in a negative mode, with
mass error <±5 ppm). The chemical formula, retention time,
observed mass and calculated mass of the precursor ions, mass error
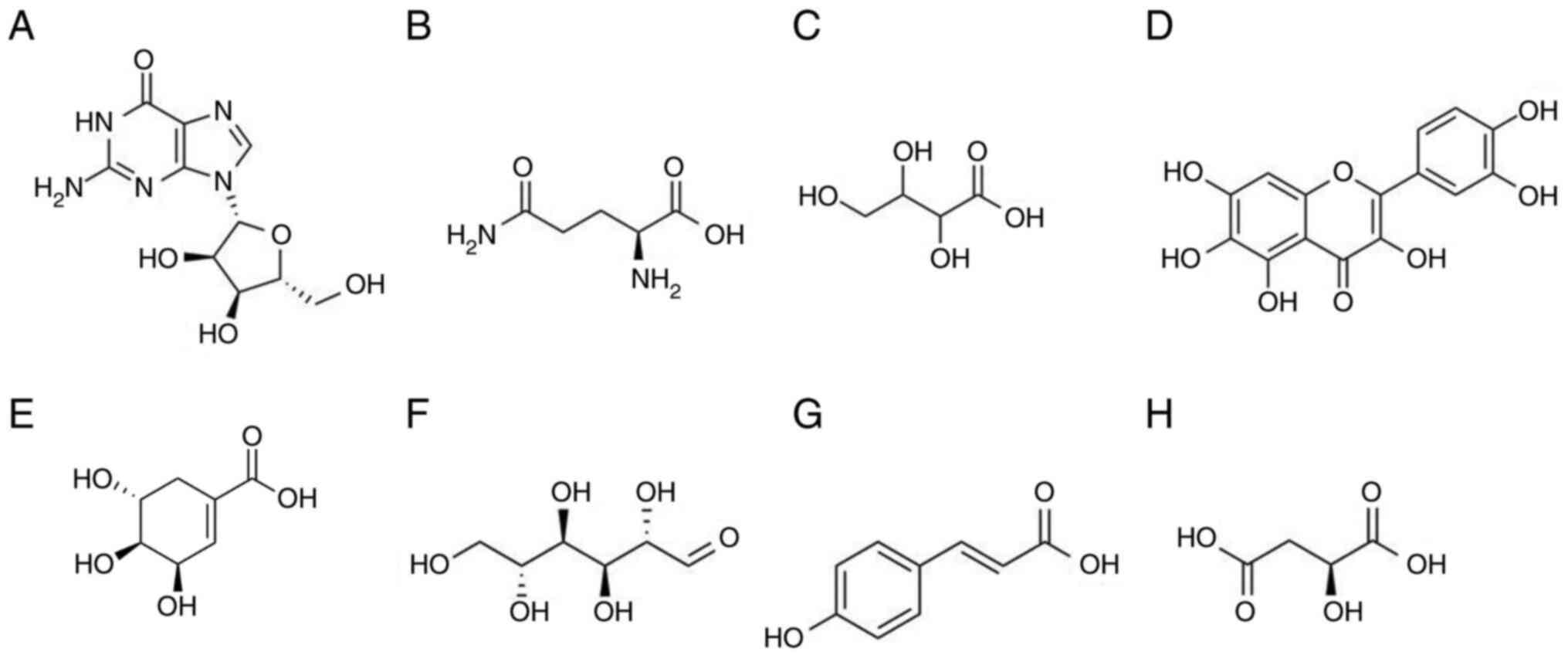
and biological activity of compounds are summarized in Table II (38-45).
The tentative compounds were guanosine, L-glutamine, threonic acid,
quercetagetin, shikimic acid, D-(+)-mannose, p-coumaric acid
and L-malic acid (Table II;
Figs. 2 and S1).
 | Table IITentative compounds in DH-P
identified using ultra-high-performance liquid
chromatography-electrospray ionization-quadrupole time-of-flight
tandem mass spectrometry and National Institute of Standards and
Technology (NIST) research library v.2017. |
Table II
Tentative compounds in DH-P
identified using ultra-high-performance liquid
chromatography-electrospray ionization-quadrupole time-of-flight
tandem mass spectrometry and National Institute of Standards and
Technology (NIST) research library v.2017.
| Tentative
Compound | Chemical
formula | Retention time,
min | Observed
[M-H]-, m/z | Calculated
[M-H]-, m/z | Mass error,
ppm | Main MS2 fragments,
m/z | Biological
activity | (Refs.) |
|---|
| L-glutamine |
C5H10N2O3 | 2.370 | 145.0622 | 145.0619 | 2.0681 | 127.0514;
84.0456 | Anti-oxidant | (38) |
| D-(+)-mannose |
C6H12O6 | 2.462 | 179.0563 | 179.0561 | 1.1170 | 71.0139;
59.0140 | Anti-cancer;
anti-bacterial | (39) |
| Threonic acid |
C4H8O5 | 2.480 | 135.0302 | 135.0299 | 2.2217 | 75.0086;
59.0142 | Stimulatory action
on vitamin C uptake | (40) |
| Guanosine |
C10H13N5O5 | 2.490 | 282.0847 | 282.0844 | 1.0635 | 150.0419; 133.0157;
108.0201 | Anti-oxidant;
anti-inflammatory | (41) |
| Shikimic acid |
C7H10O5 | 2.487 | 173.0450 | 173.0455 | -2.8894 | 93.0343; 71.0140;
65.0398 | Depigmentation | (42) |
| L-malic acid |
C4H6O5 | 2.527 | 133.0139 | 133.0142 | -2.2554 | 115.0046; 72.9932;
71.0139 | Anti-oxidant;
anti-microbial | (43) |
| Quercetagetin |
C15H10O8 | 9.437 | 317.0306 | 317.0303 | 0.9463 | 151.0037;
109.0299 | Anti-cancer | (44) |
| p-coumaric
acid |
C9H8O3 | 9.560 | 163.0406 | 163.0401 | 3.0667 | 119.0507;
91.0179 | Anti-oxidant;
anti-microbial; anti-tumor; anti-inflammatory | (42,45) |
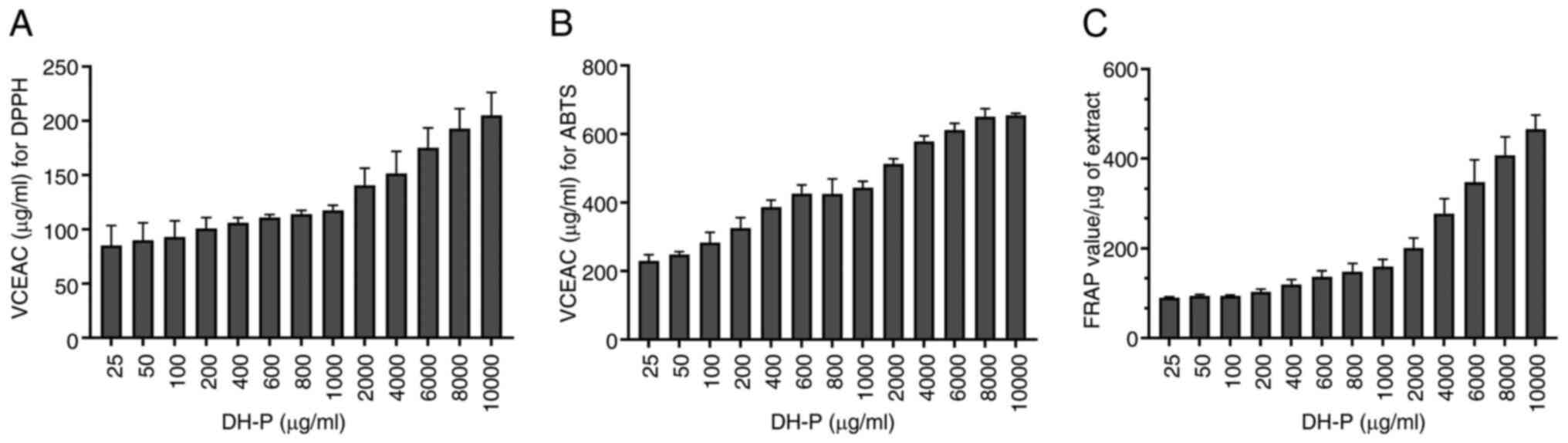
Inhibition of free radicals
The effect of DH-P (25-10,000 µg/ml) on scavenging
DPPH and ABTS radicals and reducing power for the ferric ion
(Fe3+) were investigated. Increased concentrations of
DH-P had a greater inhibitory effect on DPPH and ABTS radicals,
suggesting that the radical scavenging was concentration-dependent
(Fig. 3A and B). However, DH-P scavenged ABTS radicals
more effectively than DPPH. At the highest concentration (10,000
µg/ml), DH-P had VCEAC of 205.09±20.99 and 644.73±18.47 µg/ml for
DPPH and ABTS radicals, respectively. A similar trend of scavenging
performance was observed in the FRAP assay, suggesting that the
inhibition was concentration-dependent (Fig. 3C). The highest concentration (10,000
µg/ml) of DH-P had a reducing power for Fe3+ equivalent
to 465.83±31.37 FRAP value/µg extract. Consistent results across
three different antioxidant assays validated the strong radical
scavenging and reducing properties of DH-P.
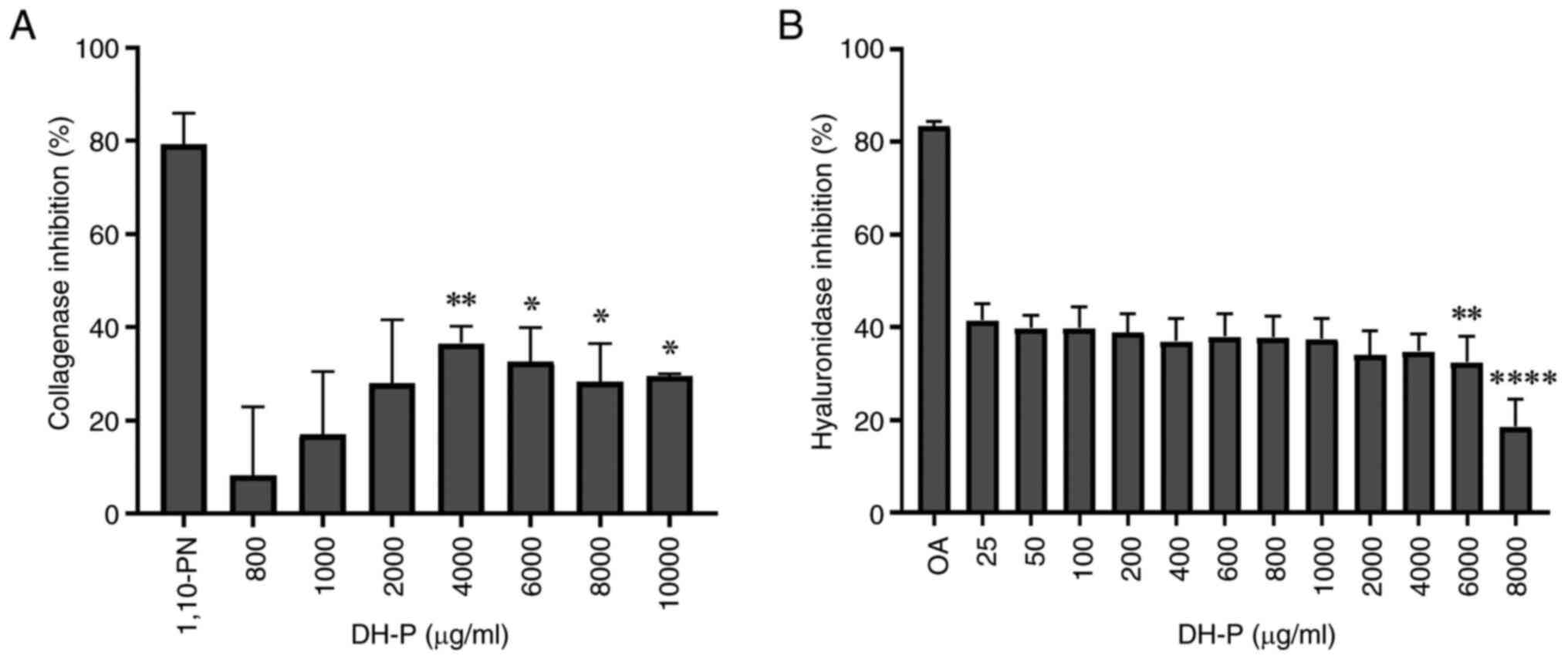
Inhibition of collagenase and
hyaluronidase activity
The present study investigated the effect of DH-P on
the inhibition of enzymes responsible for degrading ECM components,
such as collagenase and hyaluronidase (Fig. 4). DH-P inhibited the activity of
collagenase and hyaluronidase, however, its inhibitory effects were
less potent than those of the respective inhibitor controls,
1,10-PN and OA. DH-P significantly inhibited collagenase activity
at 4,000-10,000 µg/ml, with a slight decrease in inhibitory effects
as the concentration increased. DH-P at a concentration of 4,000
µg/ml exerted the maximum inhibitory activity for the collagenase
enzyme (36.57±3.62%; Fig. 4A). The
inhibitory activity of hyaluronidase was observed across DH-P
concentrations 25-4,000 µg/ml with no significant difference, with
the maximum inhibition being 41.52±3.49%; inhibition was
significantly decreased by DH-P ≥6,000 µg/ml (Fig. 4B).
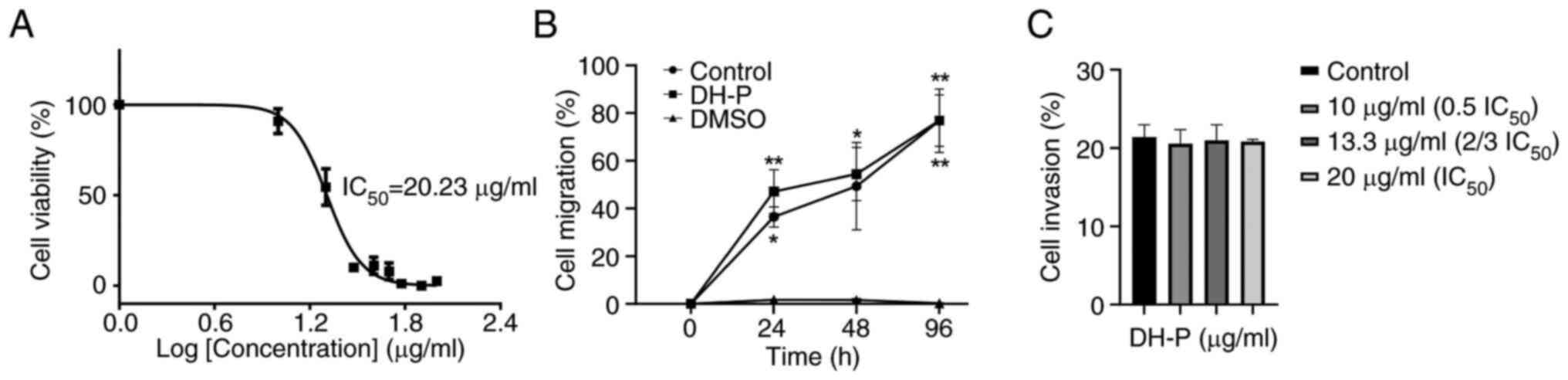
Inhibition of melanoma cell viability
and migration
The cytotoxicity was evaluated on melanoma SK-MEL-28
cells treated with DH-P (0-100 µg/ml) for 96 h. The viability of
SK-MEL-28 gradually decreased as the concentration of DH-P
increased (Figs. 5A and S2). DH-P at 20 µg/ml significantly
decreased viability of SK-MEL-28 cells. In addition, SK-MEL-28 cell
viability was significantly inhibited when cells were exposed to
DH-P ≥30 µg/ml (Fig. S2). DH-P had
an IC50 value of 20.23±1.04 µg/ml (Fig. 5A). Therefore, DH-P at 20 µg/ml was
applied for subsequent experiments.
Cells treated with 20 µg/ml DH-P migrated at a
similar rate as the control group at all time points, while 5% DMSO
(inhibitor control) inhibited cell migration (Figs. 5B and S3). In addition, there was no significant
difference in the invaded cell number between DH-P at all
concentrations and the control group, suggesting that DH-P had no
inhibitory effect on migration of SK-MEL-28 cells (Fig. 5C).
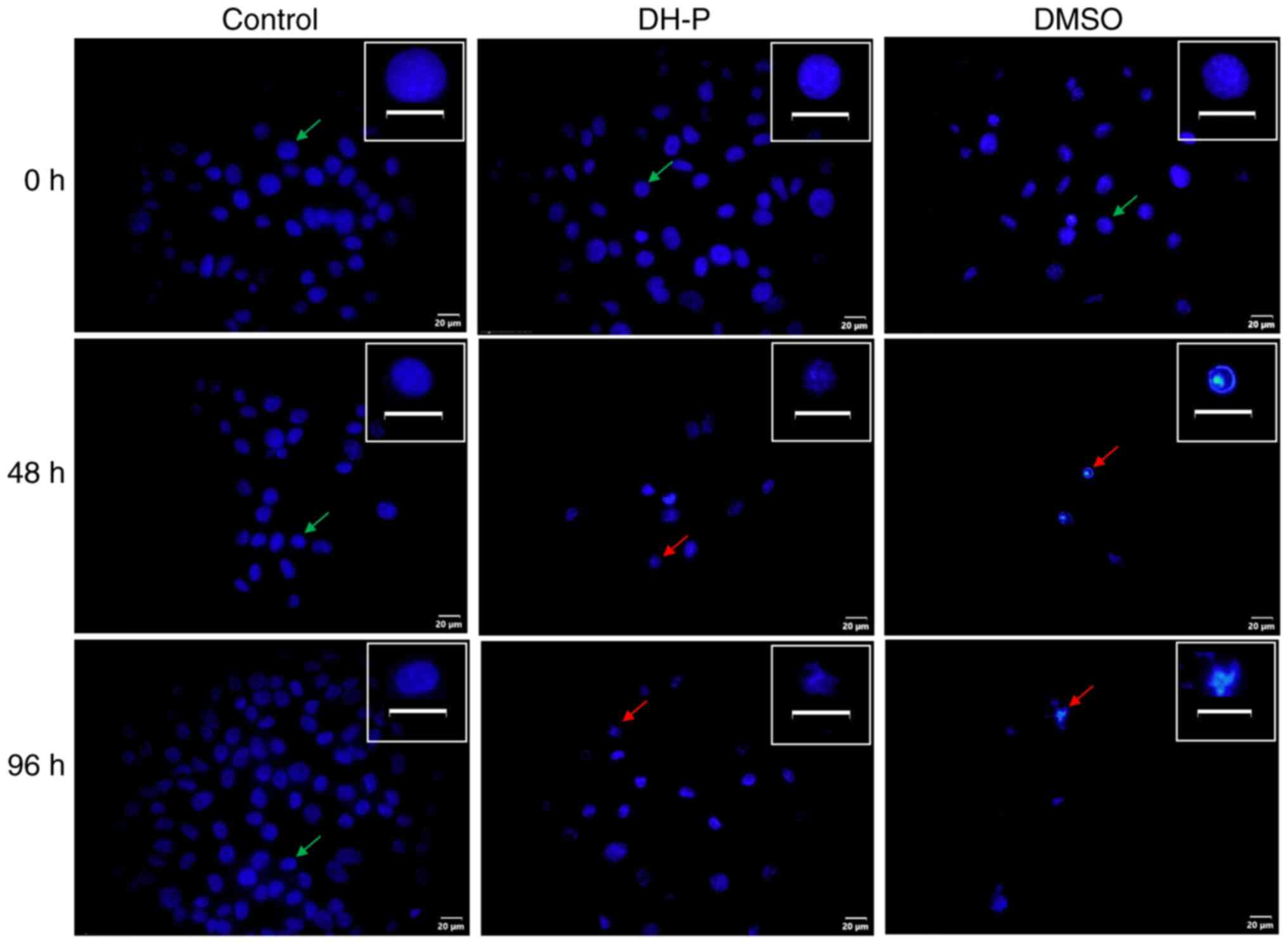
Analysis of cell apoptosis
As DH-P could inhibit viability of SK-MEL-28 cells,
cell death induced through apoptosis was investigated. Hoechst
staining was performed to observe cell and nuclear morphological
changes (Fig. 6). Following
exposure to DH-P at 20 µg/ml for 48 and 96 h, nuclei were condensed
or fragmented. In addition, the cell number decreased notably
compared with the control group. Furthermore, cells treated with 5%
DMSO (inhibitor control) were decreased in number, and the nuclei
were fragmented.
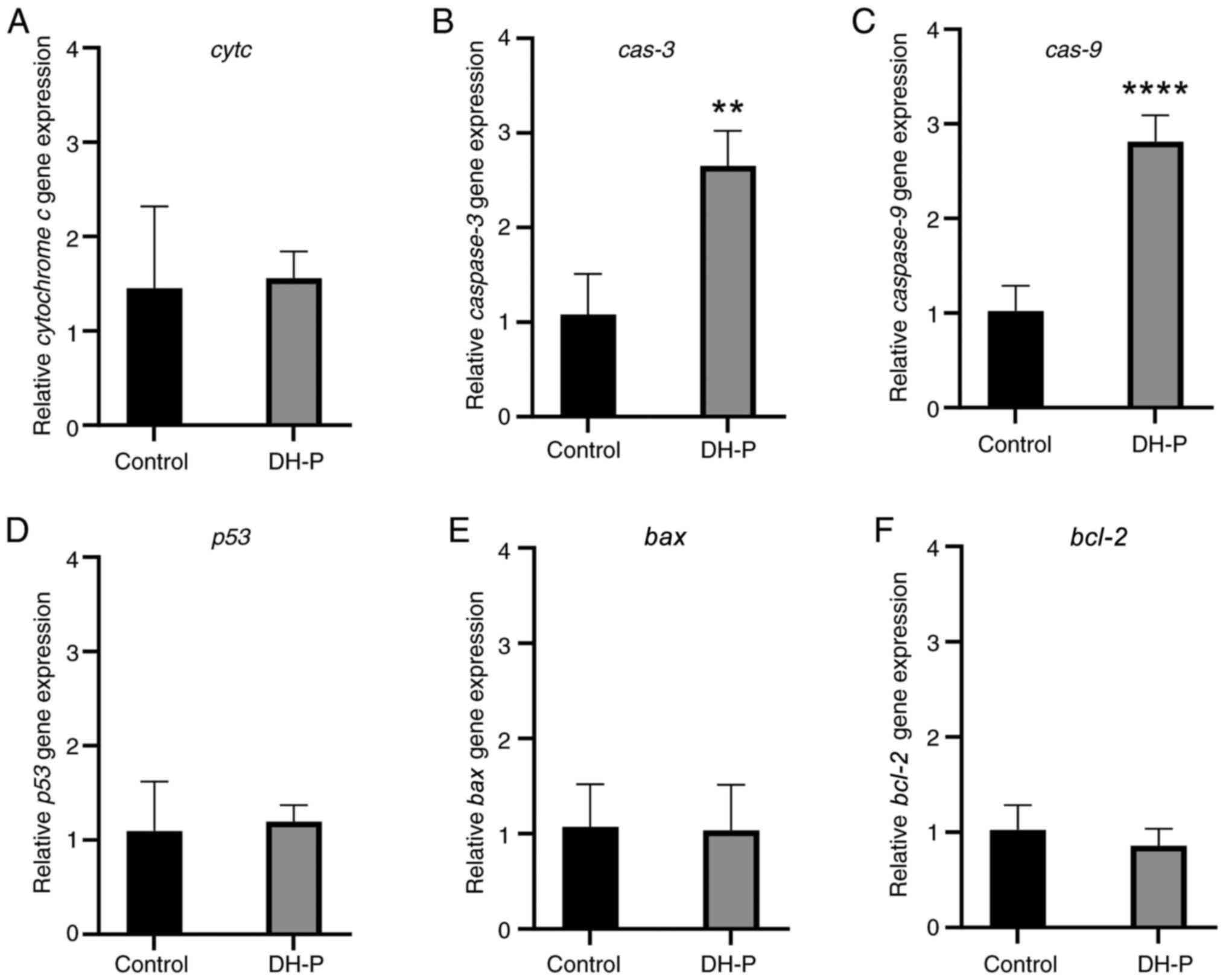
The expression of genes involved in intrinsic
apoptosis was evaluated to confirm whether cell death was induced
through apoptosis (Fig. 7). The
relative fold-changes in the expression of the apoptotic genes
bax, bcl-2, cytc, cas-3, cas-9
and p53 were 1.035, 0.858, 1.558, 2.650, 2.813 and 1.195,
respectively, compared with the control group. Only cas-3
and cas-9 were significantly upregulated by DH-P. There was
no significant change in the expression of bax,
bcl-2, cytc and p53.
Discussion
Dendrobium spp. is a large genus with
>1,000 species (3).
Dendrobium contains high amounts of bioactive compounds,
such as polyphenols, flavonoids, alkaloids and polysaccharides,
which confer biological activities beneficial for human health and
wellness (4,17,46).
Dendrobium Pearl Vera is a hybrid whose genetic material is
derived from D. bigibbum (93.75%) and D.
canaliculatum (6.25%), which are used as cosmetic ingredients
and a treatment for sores, respectively (24,46).
The present findings agreed with several reports on
Dendrobium benefits (3-8,30),
especially for skin treatment and health improvement, revealing
that Dendrobium Pearl Vera accumulates phenolics, flavonoids
and alkaloids. These bioactive compounds exert key biological
effects, such as anti-oxidant, anti-aging, and anti-cancer
properties (2,4-6).
Crude extracts from DH plant at 1 mg/ml exhibited scavenging
activity and human tyrosinase inhibition (24). In addition, DH-P at various
concentrations demonstrated inhibition of free radicals and enzyme
activity involved in ECMs degradation, as well as induction of
melanoma cell apoptosis, which may be attributed to its bioactive
compounds.
Reactive oxygen species (ROS) serve a key role in
cancer growth and development. Elevated ROS levels promote
proliferation and metastasis, thereby contributing to cancer
progression (5,47). DH-P contains secondary metabolites
that may serve as free radical scavengers or metal ion chelators
(24), suggesting its bioactive
components may inhibit SK-MEL28 cell proliferation by reducing ROS
levels. In addition, dysregulation of metal homeostasis, such as
iron, may mediate the production of ROS and alter MMP activity,
causing destruction of the cell membrane leading to skin aging and
malignant cancer (47-49).
DH-P also affected the activity of collagenase and hyaluronidase.
Skin aging and cancer progression occur via decreased skin strength
and resiliency, mostly due to alteration of the ECM biomolecules
such as collagen, elastin and HA (11,13,49).
Among these, collagen fibrils, the most abundant structural protein
in the skin, are particularly degraded by collagenases and other
MMPs (12,49). Hydrophilic ECM biomolecules,
including the non-sulfated glycosaminoglycan and HA, may be present
in lower quantities under physiological conditions, but the
increase of HA significantly affects the progression and metastasis
of cancer cells (13). Phenolic and
flavonoids protect against skin destruction and hyperpigmentation
by inhibiting the enzymes involved in the degradation of EMC
biomolecules as well as inhibiting the melanin production by
melanoma cells (24,50). Dendrobium Pearl Vera
accumulates bioactive secondary metabolites; therefore, the extract
may also protect skin damage from pollutants and sunlight exposure,
which are common stimuli for the generation of free radicals and an
increase in dermal EMC-degrading enzyme activity. DH-P at 4,000
µg/ml exerted maximal collagenase activity inhibition and
maintained hyaluronidase activity, suggesting this dose may prolong
the structural integrity of ECM. It has been reported that D.
crepidatum contains bibenzyl derivatives, polyphenols and
flavonoids that exert antioxidant and cytotoxic effects on HeLa
(human cervical carcinoma) and U251 (human glioblastoma) cancer
cell lines (6). Alkaloids have
medicinal properties, especially for cancer prevention, and are
commonly found in orchids, including Dendrobium Pearl Vera,
with most alkaloids in orchids being classified either as
pyrrolizidines or dendrobines (51).
To the best of our knowledge, few bioactive
compounds have been reported with antioxidant and anticancer
activity in DH-P, such as 3,5-di-tert-butyl-4-hydroxybenzaldehyde,
5-hydroxymethyl-2-furaldehyde, 4-guanidinobutyric acid,
L-norleucine and acetophenone (24). Notably, the present study expanded
the chemical components in DH-P to identify other chemicals, using
QTOF. A total of eight tentative compounds [guanosine, L-glutamine,
threonic acid, quercetagetin, shikimic acid, D-(+)-mannose,
p-coumaric acid, and L-malic acid] were identified with
beneficial activities for skin and medicinal use, such as
anti-oxidant, anti-aging, anti-microbial anti-cancer,
anti-inflammatory and whitening effects. The therapeutic potential
of plant-derived derivatives may be attributed to combined action
of diverse active phytochemicals with varying chemical structures
that act synergistically to enhance biological activities (44,52).
Based on the present TLC analysis, flavonoids and phenolics were
detected in varying amounts and types. Notably, different plant
species may exhibit variations in fluorescent spot coloration
(25,53). The strong inhibitory effect of DH-P
on SK-MEL-28 cell viability may result from the interactions among
its constituent compounds.
Several reports suggested that extracts from
Dendrobium spp. inhibit proliferation and metastasis on
various types of cancer cell (4,6,17); to
the best of our knowledge, however, research is limited on the
inhibitory effects of Dendrobium extract on skin cancer
cells (10,24,36).
Cytotoxic effects against cancer cells are classified as ‘very
active’, ‘active’, ‘moderately active’ or ‘no cytotoxic activity’
when the IC50 value is <10, 10-100, 101-500 or
>500 µg/ml, respectively (54).
Based on the IC50 value (20.23 µg/ml) of DH-P for
SK-MEL-28 cells, it was classified as an active inhibitor toward
human melanoma cells. By contrast, SK-MEL-28 cells that survived
treatment with 20 µg/ml DH-P could migrate and invade at the same
rate as the control group at all time points, implying the
progression and aggressiveness of escaping melanoma cells.
SK-MEL-28 is a malignant melanoma cell line and represents the most
aggressive form of skin cancer (9,10).
Malignant cell migration and invasion are the primary
manifestations of tumor biology and key components of metastasis,
which is a notable cause of death in oncology patients (55).
Apoptosis is a form of programmed cell death
occurring naturally and is induced in cancer cells by natural
compounds (17-22).
Bioactive compounds, such as quercetin, prevent the cell cycle
progression, inhibit cell proliferation and promote cell apoptosis
in numerous types of cancer, such as lung, colorectal, pancreatic,
breast and prostate cancer (56).
The extracts from D. draconis, D. falconeri, D.
findlayanum, D. loddigesii, as well as Dendrobium
Pearl Vera, exhibit anti-cancer activity (14). Cell apoptosis is characterized by
cell shrinkage and chromatin condensation and fragmentation that
produces compact nuclei and/or the formation of apoptotic bodies
(44). Decreased cell number and
fragmentation of nuclei were observed in SK-MEL-28 cells treated
with DH-P, suggesting cell apoptosis might have occurred (36). In addition, the mRNA expression of
apoptotic executors cas-9 and cas-3 significantly
increased in SK-MEL-28 cells treated with DH-P, demonstrating that
cell death was caused by apoptosis. The caspases are a family of
cysteine proteases and key mediators of apoptosis. Cas-8 and -9 are
determinants in the extrinsic and intrinsic pathway, respectively.
Cas-3 is key for apoptotic chromatin condensation and DNA
fragmentation (44). Although the
expression levels of bax, bcl-2, cytc and
p53 were unchanged, the significant increase of cas-9
and cas-3 expression demonstrated a hallmark of apoptosis
via the intrinsic pathway. Polysaccharides from D.
officinale induce apoptosis in Saos-2 osteosarcoma cells via
the intrinsic pathway, with no change in p53, Bax and Cas-9 protein
levels, but an increase in Cas-3 expression (21). Certain compounds in
Dendrobium, such as moscatilin and quercetin, exert
anti-cancer effects on melanoma cells and induce cell apoptosis via
the intrinsic pathway (17,23). Quercetagetin was identified in DH-P
and has been reported to induce the apoptotic process through the
intrinsic apoptotic pathway in cervical (CaSki), breast
(MDA-MB-231) and lung (SK-Lu-1) cancer cells (44).
In conclusion, based on the inhibitory effects of
DH-P on free radicals, ECM enzymes and SK-MEL-28 cell
proliferation, Dendrobium Pearl Vera is a promising natural
candidate as an anti-oxidant and for skin cancer prevention as an
anti-cancer agent.
Supplementary Material
Tandem mass spectrometry spectra
matched with the NIST research library v.2017. The MS2 spectra from
precursor ions in DH-P (blue) were matched with NIST research
library v.2017 (gray) and identified for the tentative compounds.
(A) Guanosine. (B) L-glutamine. (C) Threonic acid. (D)
Quercetagetin. (E) Shikimic acid. (F) D-(+)-mannose. (G)
p-coumaric acid. (H) L-malic acid.
Viability of SK-MEL-28 cells treated
with DH-P for 96 h. N=6. ****P<0.0001 vs. control.
ns, not significant; DH-P, propanolic extract from
Dendrobium Pearl Vera hybrid.
Migration of SK-MEL28 cells treated
with DH-P at 20 μg/ml and 5% DMSO (inhibitor control).
Magnification, x10. DH-P, propanolic extract from Dendrobium
Pearl Vera hybrid.
Acknowledgements
The authors would like to thank CordyBiotech Co.,
Ltd. for providing plant material.
Funding
Funding: The present study was supported by Basic Research Fund
(grant no. BRF5/2567) and International SciKU Branding (ISB),
Faculty of Science, Kasetsart University, Bangkok, Thailand.
Availability of data and materials
The data generated in the present study may be
found in Figshare under accession number
10.6084/m9.figshare.30204490 at the following URL: doi.org/10.6084/m9.figshare.30204490).
Authors' contributions
RK and PM performed the experiments. RK, PM, and AA
designed the experiments, analyzed and interpreted the data, and
wrote the manuscript. PK, NPT, NMS, WP and PW conceived the study
and analyzed data. AA and PW confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Givnish TJ, Spalink D, Ames M, Lyon SP,
Hunter SJ, Zuluaga A, Iles WJD, Clements MA, Arroyo MTK,
Leebens-Mack J, et al: Orchid phylogenomics and multiple drivers of
their extraordinary diversification. Proc R Soc B.
282(20151553)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Minh TN, Khang do T, Tuyen PT, Minh LT,
Anh LH, Quan NV, Ha PT, Quan NT, Toan NP, Elzaawely AA and Xuan TD:
Phenolic compounds and antioxidant activity of phalaenopsis orchid
hybrids. Antioxidants (Basel). 5(31)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moretti M, Cossignani L, Messina F,
Dominici L, Villarini M, Curini M and Marcotullio MC: Antigenotoxic
effect, composition and antioxidant activity of Dendrobium
speciosum. Food Chem. 140:660–665. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cakova V, Bonte F and Lobstein A:
Dendrobium: Sources of active ingredients to treat
age-related pathologies. Aging Dis. 8:827–849. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tungmunnithum D, Thongboonyou A, Pholboon
A and Yangsabai A: Flavonoids and other phenolic compounds from
medicinal plants for pharmaceutical and medical aspects: An
overview. Medicines (Basel). 5(93)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paudel MR, Chand MB and Pant B and Pant B:
Assessment of antioxidant and cytotoxic activities of extracts of
Dendrobium crepidatum. Biomolecules. 9(478)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Luo A, He X, Zhou S, Fan Y, He T and Chun
Z: In vitro antioxidant activities of a water-soluble
polysaccharide derived from Dendrobium nobile Lindl.
extracts. Int J Biol Macromol. 45:359–363. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Luo A, Ge Z, Fan Y, Luo A, Chun Z and He
X: In vitro and in vivo antioxidant activity of a water-soluble
polysaccharide from Dendrobium denneanum. Molecules.
16:1579–1592. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Roky AH, Islam MM, Ahasan AMF, Mostaq MS,
Mahmud MZ, Amin MN and Mahmud MA: Overview of skin cancer types and
prevalence rates across continents. Cancer Pathog Ther. 3:89–100.
2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Daveri E, Valacchi G, Romagnoli R,
Maellaro E and Maioli E: Antiproliferative effect of rottlerin on
Sk-Mel-28 melanoma cells. Evid Based Complement Alternat Med.
2015(545838)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moro N, Mauch C and Zigrino P:
Metalloproteinases in melanoma. Eur J Cell Biol. 93:23–29.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karousou E, Parnigoni A, Moretto P, Passi
A, Viola M and Vigetti D: Hyaluronan in the cancer cells
microenvironment. Cancers (Basel). 15(798)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ghai D, Verma J, Kaur A, Thakur K, Pawar S
and Sembi J: Bioprospection of orchids and appraisal of their
therapeutic indications. In: Bioprospecting of plant biodiversity
for industrial molecules. Santosh KU and Sudhir PS (eds.) John
Wiley & Sons, West Sussex, pp464, 2021.
|
|
15
|
Zhao X, Sun P, Qian Y and Suo H: D.
candidum has in vitro anticancer effect in HCT-116 cancer cells
and exerts in vivo anti-metastratic effects in mice. Nutr Res
Pract. 8:487–493. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Prasad R and Koch B: Antitumor activity of
ethanolic extract of Dendrobium formosum in T-cell lymphoma:
An in vitro and in vivo study. Biomed Res Int.
2014(753451)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cardile V, Avola R, Graziano ACE and Russo
A: Moscatilin, a bibenzyl derivative from the orchid Dendrobium
loddigesii, induces apoptosis in melanoma cells. Chem Biol
Interact. 323(109075)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Robertson JD and Orrenius S: Molecular
mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev
Toxicol. 30:609–627. 2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y, Zhang S, Geng JX and Hu XY: Curcumin
inhibits human non-small cell lung cancer A549 cell proliferation
through regulation of Bcl-2/Bax and cytochrome C. Asian Pac J
Cancer Prev. 14:4599–4602. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao Y, Liu Y, Lan XM, Xu GL, Sun YZ, Li F
and Liu HN: Effect of Dendrobium officinale extraction on
gastric carcinogenesis in rats. Evid Based Complement Alternat Med.
2016(1213090)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang X, Duan S, Tao S, Huang J, Liu C,
Xing S, Ren Z, Lei Z, Li Y and Wei G: Polysaccharides from
Dendrobium officinale inhibit proliferation of osteosarcoma
cells and enhance cisplatin-induced apoptosis. J Funct Foods.
73(104143)2020.
|
|
22
|
Zhang Y, Zhang Q, Xin W, Liu N and Zhang
H: Nudol, a phenanthrene derivative from Dendrobium nobile,
induces cell cycle arrest and apoptosis and inhibits migration in
osteosarcoma cells. Drug Des Devel Ther. 13:2591–2601.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reyes-Farias M and Carrasco-Pozo C: The
anti-cancer effect of quercetin: Molecular implications in cancer
metabolism. Int J Mol Sci. 20(3177)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Swainson NM, Pengoan T, Khonsap R,
Meksangsee P, Hagn G, Gerner C and Aramrak A: In vitro inhibitory
effects on free radicals, pigmentation, and skin cancer cell
proliferation from Dendrobium hybrid extract: A new plant
source of active compounds. Heliyon. 9(e20197)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maliński MP, Kikowska MA, Soluch A,
Kowalczyk M, Stochmal A and Thiem B: Phytochemical screening,
phenolic compounds and antioxidant activity of biomass from
Lychnis flos-cuculi L. in vitro cultures and intact plants.
Plants (Basel). 10(206)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Attard E: A rapid microtitre plate
Folin-Ciocalteu method for the assessment of polyphenols. Cent Eur
J Biol. 8:48–53. 2013.
|
|
27
|
Bhattacharyya P, Kumaria S, Diengdoh R and
Tandon P: Genetic stability and phytochemical analysis of the in
vitro regenerated plants of Dendrobium nobile Lindl., an
endangered medicinal orchid. Meta Gene. 2:489–504. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shamsa F, Monsef H, Ghamooshi R and
Verdian-rizi M: Spectrophotometric determination of total alkaloids
in some Iranian medicinal plants. Thai J Pharm Sci. 32:17–20.
2008.
|
|
29
|
Sarsaiya S, Jain A, Fan X, Jia Q, Xu Q,
Shu F, Zhou Q, Shi J and Chen J: New insights into detection of a
dendrobine compound from a novel endophytic Trichoderma
longibrachiatum strain and its toxicity against phytopathogenic
bacteria. Front Microbiol. 11(337)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chan CF, Wu CT, Huang WY, Lin WS, Wu HW,
Huang TK, Chang MY and Lin YS: Antioxidation and melanogenesis
inhibition of various Dendrobium tosaense extracts.
Molecules. 23(1810)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kızıltaş H, Bingöl Z, Gören A, Alwasel SH
and Gülçin I: Anticholinergic, antidiabetic and antioxidant
activities of Ferula orientalis L. determination of its
polyphenol contents by LC-HRMS. Rec Nat Prod. 15:513–528. 2021.
|
|
32
|
Herald TJ, Gadgil P and Tilley M:
High-throughput micro plate assays for screening flavonoid content
and DPPH-scavenging activity in sorghum bran and flour. J Sci Food
Agric. 92:2326–2331. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Thaipong K, Boonprakob U, Crosby K,
Cisneros-Zevallos L and Byrne DH: Comparison of ABTS, DPPH, FRAP,
and ORAC assays for estimating antioxidant activity from guava
fruit extracts. J Food Compos Anal. 19:669–675. 2006.
|
|
34
|
Bolanos de la Torre AA, Henderson T, Nigam
PS and Owusu-Apenten RK: A universally calibrated microplate ferric
reducing antioxidant power (FRAP) assay for foods and applications
to Manuka honey. Food Chem. 174:119–123. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nema NK, Maity N, Sarkar BK and Mukherjee
PK: Matrix metalloproteinase, hyaluronidase and elastase inhibitory
potential of standardized extract of Centella asiatica.
Pharm Biol. 51:1182–1187. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu Y, Zhang P, Yang H, Ge Y and Xin Y:
Effects of demethoxycurcumin on the viability and apoptosis of skin
cancer cells. Mol Med Rep. 16:539–546. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Takaoka M, Okumura S, Seki T and Ohtani M:
Effect of amino-acid intake on physical conditions and skin state:
A randomized, double-blind, placebo-controlled, crossover trial. J
Clin Biochem Nutr. 65:52–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen S, Wang K and Wang Q: Mannose: A
promising player in clinical and biomedical applications. Curr Drug
Deliv. 15:1435–1444. 2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang HY, Hu P and Jiang J:
Pharmacokinetics and safety of calcium L-threonate in healthy
volunteers after single and multiple oral administrations. Acta
Pharmacol Sin. 32:1555–1560. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bettio LE, Gil-Mohapel J and Rodrigues AL:
Guanosine and its role in neuropathologies. Purinergic Signal.
12:411–426. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen YH, Huang L, Wen ZH, Zhang C, Liang
CH, Lai ST, Luo LZ, Wang YY and Wang GH: Skin whitening capability
of shikimic acid pathway compound. Eur Rev Med Pharmacol Sci.
20:1214–1220. 2016.PubMed/NCBI
|
|
43
|
Borah HJ, Borah A, Yadav A and Hazarika S:
Extraction of malic acid from Dillenia indica in organic
solvents and its antimicrobial activity. Sep Sci Technol.
58:314–325. 2023.
|
|
44
|
Alvarado-Sansininea JJ, Sánchez-Sánchez L,
López-Muñoz H, Escobar ML, Flores-Guzmán F, Tavera-Hernández R and
Jiménez-Estrada M: Quercetagetin and patuletin: Antiproliferative,
necrotic and apoptotic activity in tumor cell lines. Molecules.
23(2579)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pei K, Ou J, Huang J and Ou S:
p-Coumaric acid and its conjugates: Dietary sources,
pharmacokinetic properties and biological activities. J Sci Food
Agric. 96:2952–2962. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang YH: Traditional uses and
pharmacologically active constituents of Dendrobium plants
for dermatological disorders: A review. Nat Prod Bioprospect.
11:465–487. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shen T, Wang Y, Cheng L, Bode AM, Gao Y,
Zhang S, Chen X and Luo X: Oxidative complexity: The role of ROS in
the tumor environment and therapeutic implications. Bioorg Med
Chem. 127(118241)2025.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ying JF, Lu ZB, Fu LQ, Tong Y, Wang Z, Li
WF and Mou XZ: The role of iron homeostasis and iron-mediated ROS
in cancer. Am J Cancer Res. 11:1895–1912. 2021.PubMed/NCBI
|
|
49
|
Cole MA, Quan T, Voorhees JJ and Fisher
GJ: Extracellular matrix regulation of fibroblast function:
Redefining our perspective on skin aging. J Cell Commun Signal.
12:35–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kanlayavattanakul M, Lourith N and Chaikul
P: Biological activity and phytochemical profiles of
Dendrobium: A new source for specialty cosmetic materials.
Ind Crops Prod. 120:61–70. 2018.
|
|
51
|
Śliwiński T, Kowalczyk T, Sitarek P and
Kolanowska M: Orchidaceae-derived anticancer agents: A review.
Cancers (Basel). 14(754)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pezzani R, Salehi B, Vitalini S, Iriti M,
Zuñiga FA, Sharifi-Rad J, Martorell M and Martins N: Synergistic
effects of plant derivatives and conventional chemotherapeutic
agents: An update on the cancer perspective. Medicina (Kaunas).
55(110)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bernardi T, Bortolini O, Massi A,
Sacchetti G, Tacchini M and De Risi C: Exploring the synergy
between HPTLC and HPLC-DAD for the investigation of wine-making
by-products. Molecules. 24(3416)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yuliet S, Nurfajar R, Sindia S, Khaerati K
and Saraswaty V: Immunomodulatory activity of Pepolo (Bischofia
javanica Blume) stem bark ethanolic extract in
Staphylococcus aureus-stimulated macrophages and anticancer
activity against MCF-7 cancer cells. J Appl Pharm Sci. 12:109–116.
2022.
|
|
55
|
Wu JS, Jiang J, Chen BJ, Wang K, Tang YL
and Liang XH: Plasticity of cancer cell invasion: Patterns and
mechanisms. Transl Oncol. 14(100899)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tang SM, Deng XT, Zhou J, Li QP, Ge XX and
Miao L: Pharmacological basis and new insights of quercetin action
in respect to its anti-cancer effects. Biomed Pharmacother.
121(109604)2020.PubMed/NCBI View Article : Google Scholar
|