Introduction
Cervical cancer remains a significant global health
concern, ranking as the fourth most common cancer among women
worldwide. In 2020, ~604,000 new cases and 342,000 associated
deaths were reported, with a disproportionate burden in low- and
middle-income countries (1). The
management of locally advanced cervical cancer (LACC), typically
defined as International Federation of Gynecology and Obstetrics
(FIGO) stages IB2 to IIB (2),
presents a particular therapeutic challenge.
Concurrent chemoradiotherapy (CCRT) is the
internationally accepted standard for FIGO IB2-IIB, resulting in
superior overall survival (OS) and progression-free survival (PFS)
compared with surgery alone or radiotherapy alone (e.g., higher
response and survival rates; CCRT outperforms neoadjuvant therapy
plus surgery in studies such as EORTC-55994) (3). However, the role of neoadjuvant
chemotherapy (NACT) followed by radical surgery (RS) as an
alternative treatment strategy has been a subject of ongoing
debate. NACT involves administering chemotherapy before the primary
treatment modality, such as RS in this case. The rationale behind
this approach includes reducing tumor size to facilitate surgical
resection, eradicating micrometastatic disease early and
potentially improving surgical outcomes by decreasing the extent of
disease (4).
Several studies have explored the efficacy and
safety of NACT followed by RS compared with RS alone in patients
with LACC (5-8).
A systematic review and meta-analysis conducted by Zhao et
al (9) evaluated the outcomes
of NACT combined with RS vs. RS alone in patients with cervical
cancer at all stages. The analysis included 13 studies with a total
of 2,158 participants. The findings indicated that, with regard to
OS, disease-free survival (DFS), PFS, local and distant recurrence,
and parametrial infiltration, neoadjuvant chemotherapy plus radical
surgery was similar to radical surgery alone (9). Nevertheless, a review by Gadducci and
Cosio (4) assessed the impact of
NACT followed by radical hysterectomy in patients with LACC. The
study reported that this combined approach resulted in higher PFS
and OS times compared with primary radical hysterectomy. These
benefits were observed regardless of the total cisplatin dose,
chemotherapy cycle length or tumor stage (4).
Despite these promising results, the integration of
NACT into standard treatment protocols for LACC is not universally
accepted. Concerns have been raised regarding potential delays in
definitive treatment, the risk of disease progression during
chemotherapy, and the added toxicity associated with additional
treatment modalities. Moreover, the heterogeneity of chemotherapy
regimens and variations in surgical expertise across different
centers contribute to the ongoing debate (1). Recent guidelines from the European
Society of Gynecological Oncology (ESGO) highlight that while NACT
followed by RS is a treatment option, it should not replace the
standard CCRT approach. The guidelines emphasize the need for
individualized treatment planning, considering factors such as
tumor characteristics, patient comorbidities and resource
availability (1,10).
Given the ongoing debate and the potential
implications for patient care, a comprehensive systematic review
and meta-analysis are warranted to critically evaluate the efficacy
and safety of NACT followed by RS vs. RS alone in LACC. The present
analysis aimed to provide clarity on the optimal treatment
approach, thereby informing clinical practice and guiding future
research endeavors.
Materials and methods
Study design
The present systematic review and meta-analysis were
conducted to evaluate the efficacy and safety of NACT followed by
RS vs. RS alone in patients with LACC. The methodology adhered to
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines (11).
Search strategy
A comprehensive literature search was performed
across multiple databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/), the Cochrane Central
Register of Controlled Trials (CENTRAL; https://www.cochranelibrary.com/central/about-central)
and the Cochrane Database of Systematic Reviews (https://www.cochranelibrary.com/cdsr/about-cdsr). The
search encompassed studies published from database inception up to
March 2025, without language restrictions. The following medical
subject headings (MeSH) terms and keywords were used: ‘Cervical
cancer’, ‘neoadjuvant chemotherapy’, ‘radical surgery’, ‘radical
hysterectomy’ and ‘locally advanced’. The search had the following
format in PubMed: [‘Cervical Cancer’(MeSH) OR ‘Cervical
Neoplasms’(Title/Abstract) OR ‘cervical carcinoma’
(Title/Abstract)] AND [‘Neoadjuvant Chemotherapy’(MeSH) OR
‘neoadjuvant chemotherapy’(Title/Abstract) OR ‘preoperative
chemotherapy’(Title/Abstract)] AND [‘Radical Surgery’ (MeSH) OR
‘radical hysterectomy’(Title/Abstract) OR ‘surgical
resection’(Title/Abstract)] AND [‘Locally Advanced’(Title/Abstract)
OR ‘FIGO stage IB2’(Title/Abstract) OR ‘FIGO stage
IIB’(Title/Abstract)]. Similar strategies were applied to EMBASE,
CENTRAL and the Cochrane database. Additionally, the reference
lists of relevant articles (9) were
manually screened to identify further pertinent studies. Although
no language restrictions were applied during the database search,
only articles published in English were ultimately included.
Non-English studies were excluded if full translations were not
available or if data extraction was not feasible due to language
barriers.
Inclusion and exclusion criteria
Studies were considered eligible if they met the
following criteria: i) Population: Women diagnosed with LACC,
specifically FIGO stages IB2 to IIB. ii) Intervention: NACT
followed by RS. iii) Comparison: RS alone. iv) Outcomes: Reported
at least one of the following, namely, OS, PFS and DFS times,
recurrence rate, lymph node metastasis or parametrial infiltration.
v) Study design: Randomized controlled trials (RCTs), prospective
non-RCTs or retrospective cohort studies.
Exclusion criteria included studies focusing on
early-stage (FIGO stage IA to IB1) or metastatic cervical cancer,
those lacking a comparison between the specified interventions and
studies with insufficient data for extraction (1,9,12).
Data extraction and quality
assessment
Two independent reviewers screened titles and
abstracts for relevance, followed by full-text assessments to
confirm eligibility. Discrepancies were resolved through discussion
or consultation with a third reviewer. Data extracted included
study characteristics (author, year, study design and sample size),
patient demographics, treatment protocols and reported outcomes.
Risk of bias was assessed using different tools depending on study
design. For observational studies, the Newcastle-Ottawa Scale (NOS)
(13) was applied. For randomized
controlled trials, the Cochrane Risk of Bias (RoB) 2.0 tool
(https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials)
was used to assess the randomization process, allocation
concealment, blinding, outcome measurement and completeness of
data. The NOS scale assesses three aspects: i) Selection of the
study groups (maximum of 4 stars); ii) comparability of groups
(maximum of 2 stars), indicating appropriate matching or analysis
to control for confounders; and iii) outcome/exposure (maximum of 3
stars), reflecting the adequacy of outcome measurement,
length/adequacy of follow-up and attrition. A total of up to 9
stars can be awarded. Higher scores suggest a lower risk of
bias.
Statistical analysis
All statistical analyses were conducted using
comprehensive meta-analysis software, version 2 (Biostat
International, Inc.). A random-effects model was employed to
account for potential heterogeneity among studies, and odds ratios
(ORs) with corresponding 95% confidence intervals (CIs) were
calculated for dichotomous outcomes. Survival outcomes (OS and DFS)
were summarized using ORs, as hazard ratios (HRs) were not
consistently reported or extractable across the included studies.
To distinguish between outcome-specific treatment effects, separate
meta-analyses were performed for OS and DFS as distinct subgroups,
rather than stratifying by study design or patient characteristics.
This approach allowed the assessment of whether the treatment
benefit varied according to the type of survival outcome. A number
of studies provided dichotomized survival data (e.g., 5-year
survival rates or recurrence status at follow-up) without
time-to-event estimates or Kaplan-Meier curves suitable for HR
reconstruction. Therefore, ORs were used to enable a consistent
pooled analysis, while acknowledging that ORs are less informative
than HRs for time-to-event outcomes and that they may overestimate
effect sizes when event rates are high. To assess the robustness of
the results, sensitivity analyses were performed by systematically
excluding each study to evaluate its impact on the overall
findings.
Heterogeneity was assessed using precision interval
analysis, which offers an alternative to the traditional I²
statistic by focusing on the accuracy of the combined effect size
estimate. Publication bias was evaluated through visual inspection
of funnel plots, along with Begg and Mazumdar's rank correlation
test (Kendall's τ values closer to zero indicate less serious
publication bias) and Egger's regression test (if the intercept
term is equal to or close to zero this indicates less serious
publication bias). Furthermore, the fail-safe N test was used to
determine how many missing studies would be needed to nullify the
observed effect, and the Duval and Tweedie's trim-and-fill method
was applied to adjust for potential publication bias by estimating
and adding hypothetical missing studies. P<0.05 was considered
to indicate a statistically significant difference for all
analyses.
Results
Study selection
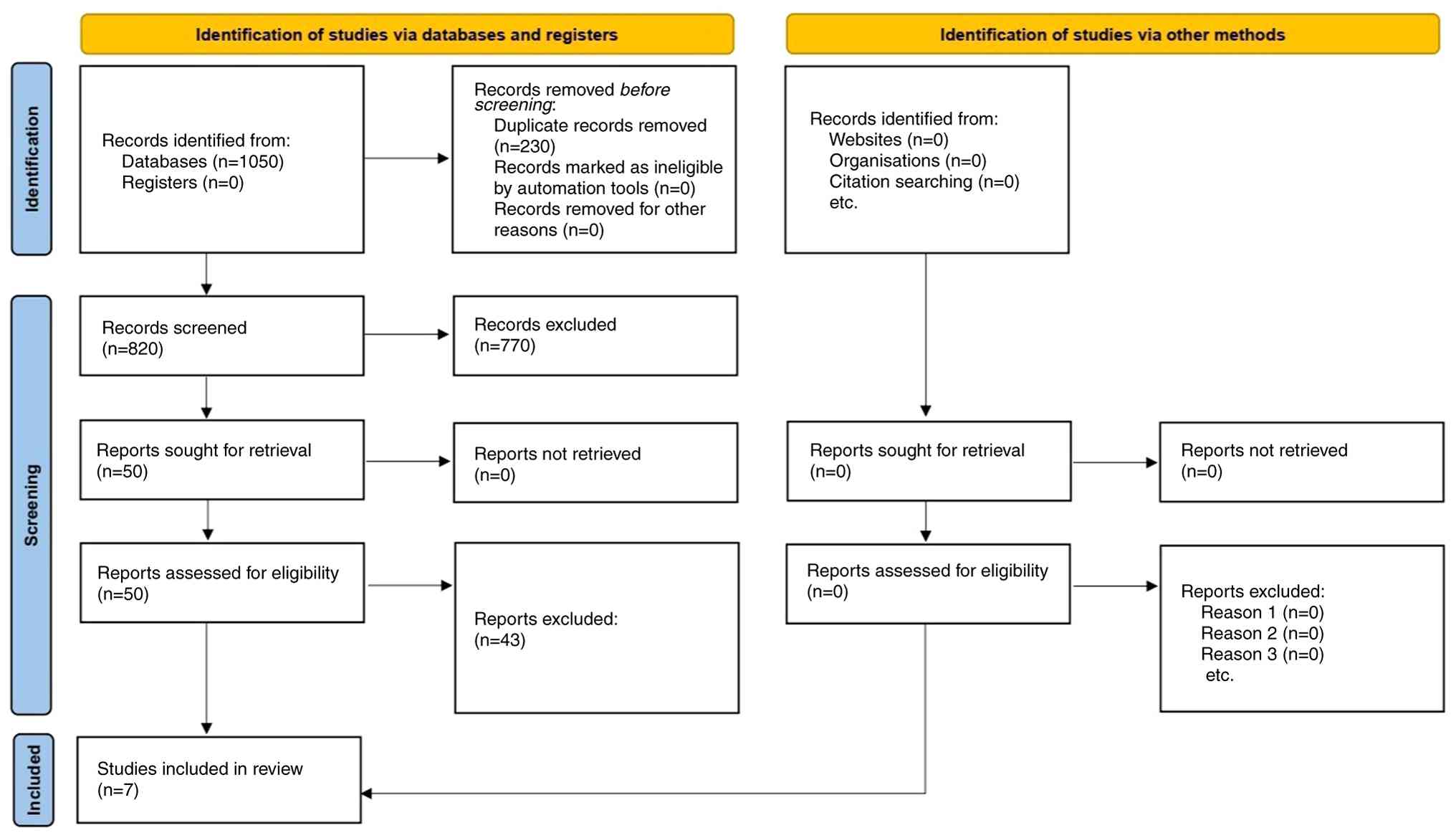
The initial database search yielded 1,050 records.
After removing duplicates, 820 unique records remained. Following
title and abstract screening, 50 articles were selected for
full-text review. Of these, 43 were excluded for the following
reasons: Early-stage or metastatic cervical cancer populations
(n=13), absence of a comparator group matching the inclusion
criteria (n=10), insufficient outcome data (n=9), duplicate or
overlapping cohorts (n=6) and non-English publications without
accessible translations (n=5), resulting in 7 studies being
included in the final analysis (5-8,14-16).
A PRISMA flow diagram illustrating the study selection process is
provided in Fig. 1. Among the 7
included studies, 3 were RCTs (6,8,15), and
4 were observational cohort studies (5,7,14,16).
Table I provides a comprehensive
summary of the general characteristics and demographics of the
included studies comparing NACT followed by RS vs. RS alone in
LACC. The table presents key data on study sample sizes, patient
demographics, FIGO staging, histological subtypes, treatment
groups, lymph node metastasis rates, survival outcomes, and NACT
chemotherapy regimens and doses. The total sample size of the
included patients in this meta-analysis is 2,231.
 | Table IGeneral characteristics and
demographics of included studies. |
Table I
General characteristics and
demographics of included studies.
| First author,
year | Sample size, n | Mean age (range),
years | FIGO stages | Histological
types | Treatment groups | Lymph node
metastasis, % | Survival outcome | NAC regimen and
doses | (Refs.) |
|---|
| Behtash et al,
2006 | 182 | 48 (26-75) | IB2-IIA | Squamous (majority),
adenocarcinoma | NACT + RS (n=22) vs.
RS alone (n=160) | 36 RS; 47.1 NACT | OS not significantly
different (P=0.06) | 50 mg/m² cisplatin +
1 mg/m² vincristine (every 10 days for 3 cycles) | (5) |
| Chen et al,
2008 | 142 | 44 (25-74) | IB2-IIB | Squamous,
adenocarcinoma | NACT + RS (n=72) vs.
RS alone (n=70) | 16.0 NACT responders;
45.5 non-responders | NACT responders ha
better DFS and lower recurrence (P<0.001) | 100 mg/m² cisplatin
(day 1) + 4 mg/ m² mitomycin C (IM, days 1-5) + 24 mg/kg/day 5-FU
(IV, days 1-5) | (6) |
| Gong et al,
2016 | 800 | Not specified | IB2-IIB | Squamous,
adenocarcinoma | NACT + RS (n=411) vs.
RS alone (n=389) | Not specified | 5-year PFS 80.3%
(NACT) vs. 81.0% (RS) | Carboplatin +
cisplatin, Carboplatin + cisplatin, xpingyangmycin, ifosfamide,
5-FU, paclitaxel, vincristine (1-3 cycles) | (7) |
| Katsumata et
al, 2013 | 134 | Not specified | IB2-IIB | Squamous cell
carcinoma | NACT + RS (n=67) vs.
RS alone (n=67) | Not specified | 5-year OS: 70.0%
(NACT) vs. 74.4% (RS) (P=0.85) | 7 mg Bleomycin
(days 1-5) + 0.7 mg/m² vincristine (day 5) + 7 mg/m² mitomycin (day
5) + 14 mg/m² cisplatin (days 1-5) | (8) |
| Kim et al,
2011 | 524 | 50.6±11.5 | IB1-IIA | Squamous
(majority), non-squamous | NACT + RS (n=73)
vs. RS alone (n=451) | 23.9 RS; 23.3
NACT | Not specified | 5-FU/cisplatin or
paclitaxel/carboplatin (median, 3 cycles) | (16) |
| Yang et al,
2016 | 219 | 47 (23-66) | IB2-IIB | Squamous,
adenocarcinoma, adenosquamous | NACT + RS (n=109)
vs. RS alone (n=110) | Not specified | No significant
difference in DFS/OS between groups | 60 mg/m² irinotecan
(IV, days 1, 8 and 15) + 70 mg/m² cisplatin (IV, day 1) or 175
mg/m² paclitaxel (IV, day 1) + 70 mg/m² cisplatin (IV, day 1) (1-2
cycles) | (15) |
| Namkoong et
al, 1995 | 230 | 48 (30-58) | IB-IIB | Squamous
(majority), adenocarcinoma | NACT + RS (n=92)
vs. RS alone (n=138) | 18.5 NACT; 34.0
RS | 4-Year tumor-free
survival: 81.5% (NACT) vs. 66.7% (RS) (P=0.0067) | 4 mg/m² vinblastine
(days 1-2), 15 mg/m² bleomycin (days 1, 7 and 14), 60 mg/m²
cisplatin (day 1); every 3 weeks | (14) |
Outcomes and synthesis of results
A subgroup-specific meta-analyses was performed
based on survival outcome type (OS vs. DFS). Fig. 2 presents the survival
outcome-specific meta-analyses for DFS and OS. For DFS, the pooled
OR was 0.98 (95% CI, 0.62-1.56; P=0.941), indicating no significant
benefit of NACT followed by RS compared with surgery alone.
Although several individual studies reported improvements in DFS,
these effects did not translate into a significant pooled estimate.
By contrast, the pooled analysis of OS data showed a more favorable
effect. When restricted to OS outcomes only, the pooled OR was 0.53
(95% CI: 0.32-0.87; P=0.012), suggesting a statistically
significant survival benefit associated with NACT. However, when
all studies contributing OS and DFS data are combined, the overall
effect estimate becomes more modest and borderline significant (OR,
0.74; 95% CI, 0.53-1.04; P=0.078). These findings highlight a
potential outcome-specific benefit of NACT for OS but not for
DFS.
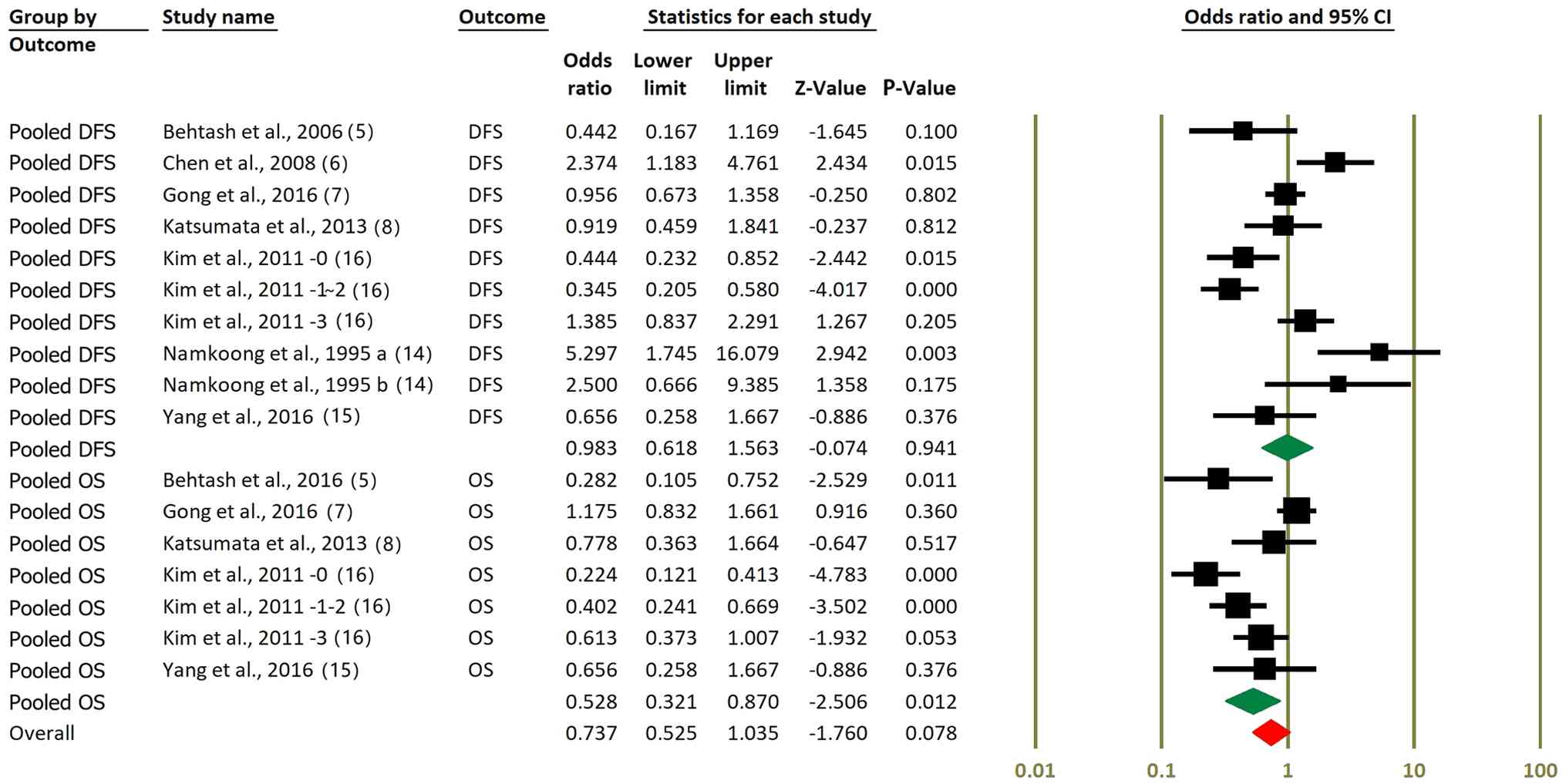 | Figure 2Forest plots comparing DFS and OS in
NACT + RS vs. RS alone. Pooled ORs and 95% CIs for DFS are
presented, showing no significant difference between NACT + RS and
RS alone (OR, 0.98; P=0.941). Pooled OS data are presented, with a
significant benefit shown in subgroup analysis (OR, 0.53; P=0.012);
however, when all OS and DFS data were combined, the advantage
approached but did not reach statistical significance (OR, 0.74;
P=0.078). Kim et al (16)
-0, -1~2 and -3 represent the number of metastatic lymph nodes;
this study statistically analyzed OS and DFS after grouping based
on the number of lymph node metastases. Namkoong et al
(14) 1995 a and b represent
squamous cell carcinoma and adenocarcinoma, respectively. DFS,
disease-free survival; OS, overall survival; NACT, neoadjuvant
chemotherapy; RS, radical surgery; OR, odds ratio; CI, confidence
interval. |
Sensitivity analysis
In the leave-one-out sensitivity analysis for OS
(Fig. 3A), removing each study one
at a time resulted in ORs ranging from 0.45 to 0.62, all with
statistically significant P-values of <0.05. The combined
estimate remains significant (OR, 0.53; 95% CI, 0.32-0.87,
P=0.012), indicating that no single trial primarily drives the
benefit in OS. By contrast, for DFS (Fig. 3B), excluding individual studies
yields summary ORs consistently near 1.0, with P-values from 0.467
to 0.990, highlighting the lack of a significant overall effect on
DFS and confirming that the null finding is robust against the
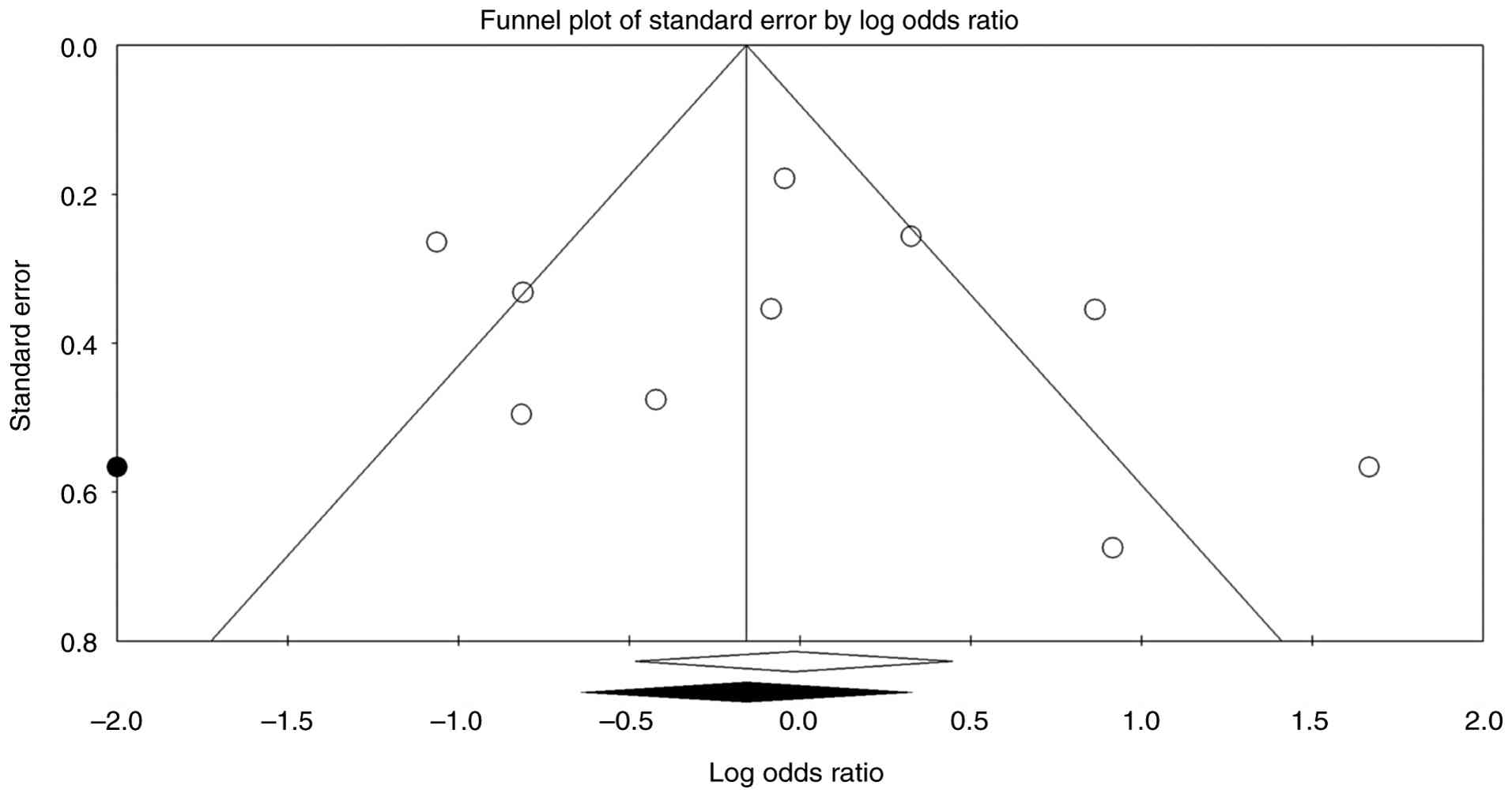
omission of any single study. The funnel plot of log ORs against
standard error appears fairly symmetrical, suggesting no major
signs of publication bias (Fig. 4).
Begg and Mazumdar's rank correlation produces Kendall's τ values
near 0.2 with non-significant P-values (two-tailed P>0.4), while
Egger's regression test also shows no evidence of small-study
effects (P>0.5). Duval and Tweedie's trim-and-fill analysis
detects no missing studies on either side of the mean, leaving the
pooled estimate unchanged. The fail-safe N similarly indicates that
no additional studies are needed to bring the meta-analysis to a
non-significant level. Overall, these findings suggest that the
observed effect estimates are not heavily influenced by publication
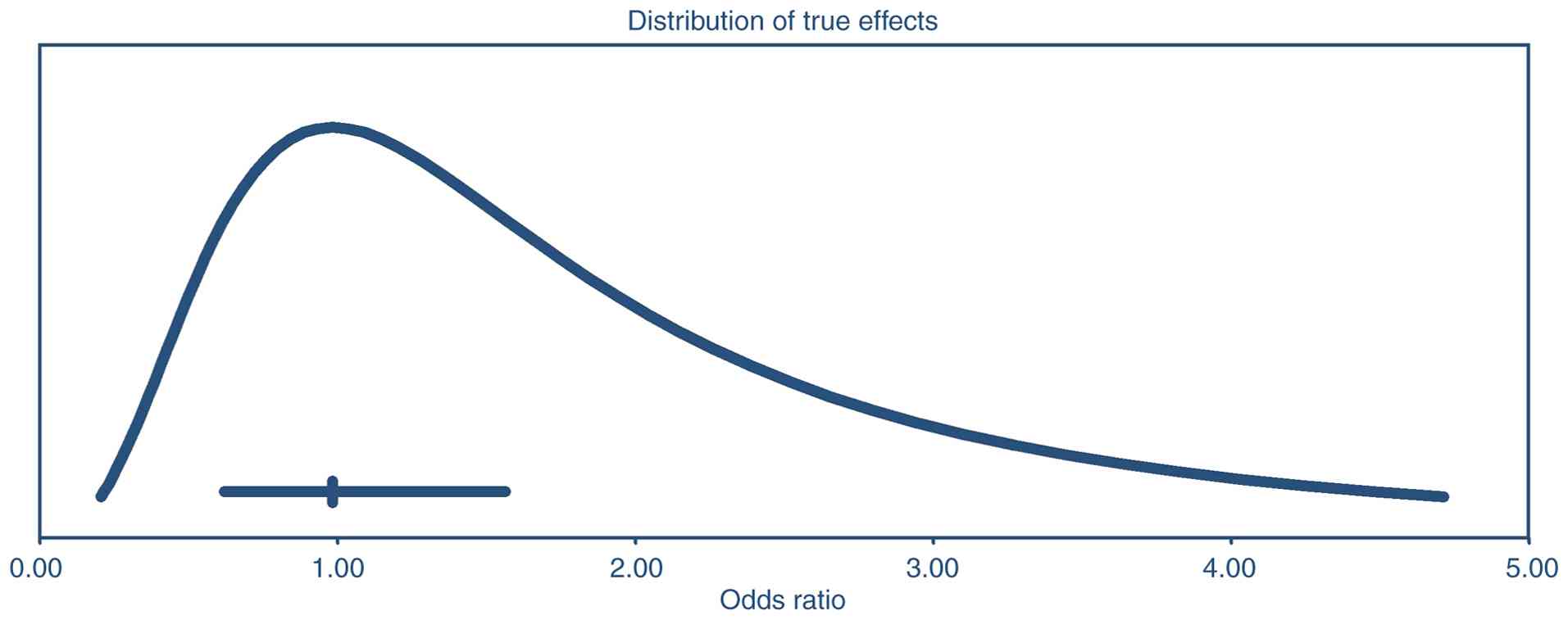
bias. The prediction-interval analysis (Fig. 5) indicates that, although the
average effect size (OR, 0.98) has a 95% CI of 0.62-1.56 implying
no clear departure from unity; the true effect across similar
populations could range widely, from about 0.21 to 4.71. Therefore,
even though the pooled estimate does not significantly differ from
no effect, there is substantial heterogeneity; some settings might
observe smaller effects (possibly null or worse), while others
could see much larger effects.
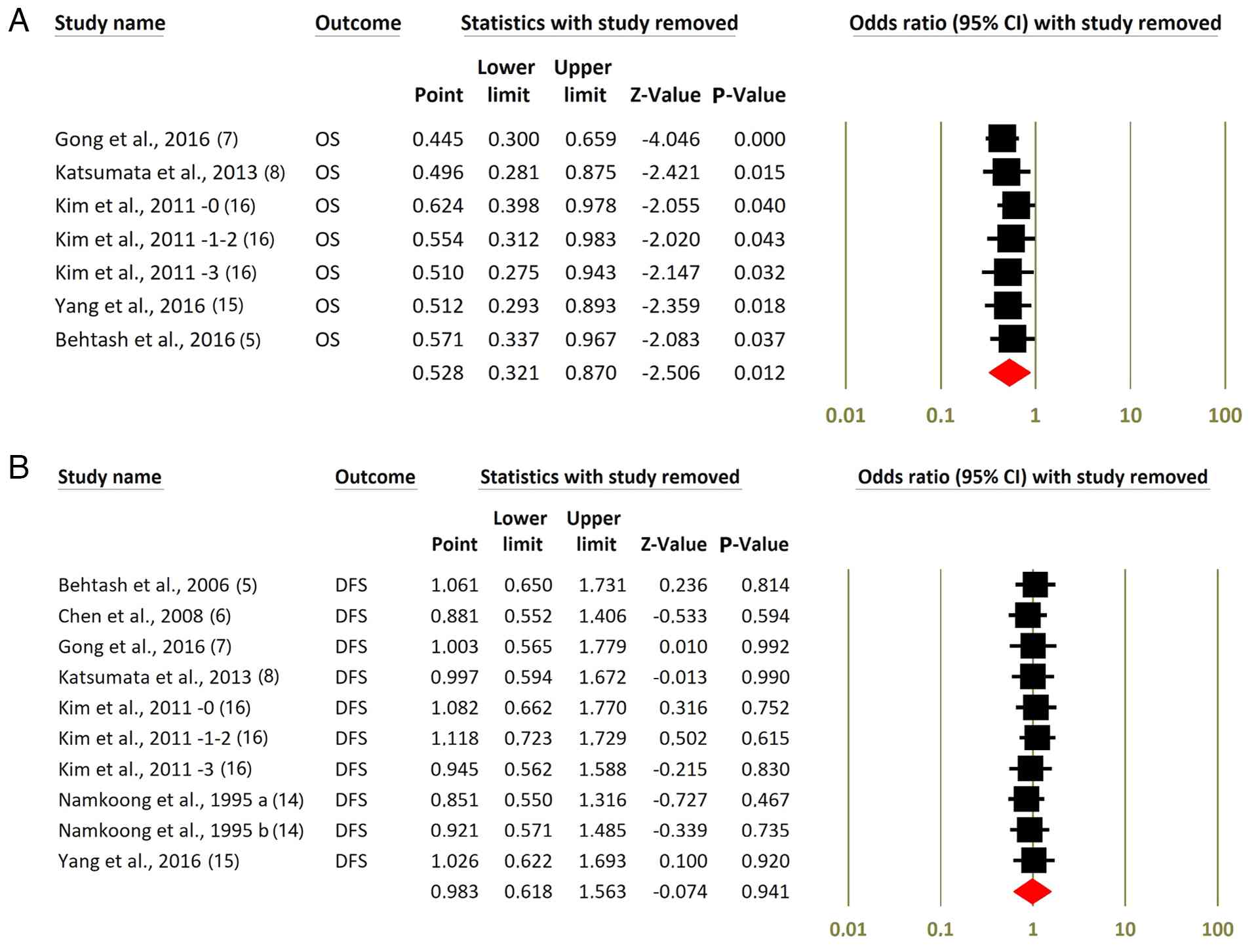 | Figure 3Leave-one-out sensitivity analysis for
OS and DFS. Leave-one-out sensitivity analysis presented for (A) OS
and (B) DFS in patients treated with NACT followed by RS vs. RS
alone. (A) The exclusion of individual studies yields ORs
consistently <1.0, with statistically significant P-values,
indicating a stable OS benefit for NACT + RS. (B) By contrast,
excluding individual studies does not meaningfully alter the DFS
estimate, with ORs consistently near 1.0 and non-significant
P-values, confirming the robustness of the null effect on DFS. Kim
et al (16) -0, -1~2 and -3
represent the number of metastatic lymph nodes; this study
statistically analyzed OS and DFS after grouping based on the
number of lymph node metastases. Namkoong et al (14) 1995 a and b represent squamous cell
carcinoma and adenocarcinoma, respectively. OS, overall survival;
DFS, disease-free survival; NACT, neoadjuvant chemotherapy; RS,
radical surgery; OR, odds ratio; CI, confidence interval. |
Quality assessment
The quality of observational studies was evaluated
using the NOS scale (Table II),
and the quality of RCTs was evaluated using the Cochrane RoB 2.0
tool (Table III). Among the
studies assessed, the study by Gong et al (7) received the highest total score (9
points), reflecting strong methodological rigor in terms of
participant selection, group comparability and outcome assessment.
Namkoong et al (14)
achieved a score of 8, demonstrating a well-balanced design with
appropriate controls for confounders and thorough outcome
evaluation. Behtash et al (5) and Kim et al (16) received scores of 6, suggesting
moderate quality due to potential limitations in comparability or
outcome assessment. These studies may have had fewer controls for
confounding factors or shorter follow-up periods compared with
others in the analysis. The three RCTs (6,8,15)
demonstrated generally low risk of bias in randomization, outcome
assessment and data completeness, although blinding was not
feasible in most surgical trials. No major threats to internal
validity were identified. Overall, the included studies were of
acceptable quality for meta-analysis, with most receiving high
scores, indicating a low risk of bias. While some studies exhibited
minor limitations in comparability or outcome assessment, the
overall methodological rigor supports the reliability of the pooled
findings.
 | Table IIQuality assessment of included
studies using the Newcastle-Ottawa Scale. |
Table II
Quality assessment of included
studies using the Newcastle-Ottawa Scale.
| First author,
year | Selection (score
0-4) | Comparability
(score 0-2) | Outcome/exposure
(score 0-3) | Total score (score
0-9) | (Refs.) |
|---|
| Behtash et
al, 2006 | 3 | 1 | 2 | 6 | (5) |
| Gong et al,
2016 | 4 | 2 | 3 | 9 | (7) |
| Kim et al,
2011 | 3 | 1 | 2 | 6 | (16) |
| Namkoong et
al, 1995 | 3 | 2 | 3 | 8 | (14) |
 | Table IIIRisk of bias assessment for included
randomized controlled trials using Cochrane RoB 2.0
toola. |
Table III
Risk of bias assessment for included
randomized controlled trials using Cochrane RoB 2.0
toola.
| First author,
year | Bias arising from
the randomization process | Bias due to
deviations from intended interventions | Bias due to missing
outcome data | Bias in the
measurement of the outcome | Overall risk of
bias | (Refs.) |
|---|
| Chen et al,
2008 | Low risk | Some concerns | Low risk | Low risk | Some concerns | (6) |
| Katsumata et
al, 2013 | Low risk | Low risk | Low risk | Low risk | Low risk | (8) |
| Yang et al,
2016 | Some concerns | Low risk | Low risk | Low risk | Some concerns | (15) |
Heterogeneity analysis
Meta-regression was conducted to assess whether
study-level variables explained heterogeneity in OS estimates. The
covariates included chemotherapy regimen, proportion of FIGO stage
IIB patients, publication year, study design and sample size. None
of the included covariates showed a statistically significant
association with the treatment effect. Specifically, the proportion
of FIGO IIB patients (coefficient, -0.305; P=0.642), chemotherapy
regimen (coefficient, 2.470; P=0.551), publication year
(coefficient, 0.008; P=0.666), study design (coefficient, -5.735;
P=0.559) and sample size (coefficient, 0.016; P=0.631) were not
significant moderators. These findings suggest that the variability
in OS effect sizes could not be adequately explained by these
study-level characteristics, indicating residual unexplained
heterogeneity (Table IV). Also,
meta-regression analysis was performed to explore sources of
heterogeneity in DFS outcomes using five covariates: Chemotherapy
regimen, proportion of FIGO stage IIB patients, publication year,
study design (randomized controlled trial vs. observational) and
sample size. The proportion of FIGO IIB patients was significantly
associated with effect size (coefficient, -0.049; P=0.027),
suggesting that higher proportions of advanced-stage patients were
linked to less favorable treatment effects. None of the other
covariates, including chemotherapy regimen (coefficient, 0.098;
P=0.289), publication year (coefficient, 0.003; P=0.936), study
design (coefficient, -0.240; P=0.496) or sample size (coefficient,
0.0003; P=0.932), reached statistical significance. These results
indicate that stage distribution may partially explain the observed
heterogeneity (I²=58%), whereas other study-level characteristics
were not significant moderators (Table
V).
 | Table IVMeta-regression results for OS
outcomesa. |
Table IV
Meta-regression results for OS
outcomesa.
| Covariate | Coefficient | Standard error | Z-value | P-value | 95% CI (lower) | 95% CI (upper) |
|---|
| Constant | -0.1140 | 0.1636 | -0.70 | 0.5578 | -0.8178 | 0.5897 |
| Chemotherapy
regimen | 2.4702 | 3.4722 | 0.71 | 0.5506 | -12.4694 | 17.4097 |
| FIGO IIB patients
(%) | -0.3046 | 0.5615 | -0.54 | 0.6418 | -2.7205 | 2.1112 |
| Publication
year | 0.0080 | 0.0160 | 0.50 | 0.6664 | -0.0607 | 0.0767 |
| Study design
(0=observational; 1=RCT) | -5.7345 | 8.2507 | -0.70 | 0.5589 | -41.2343 | 29.7652 |
| Sample size | -0.0156 | 0.0278 | -0.56 | 0.6307 | -0.1352 | 0.1040 |
 | Table VMeta-regression results for DFS
outcomesa. |
Table V
Meta-regression results for DFS
outcomesa.
| Covariate | Coefficient | Standard error | Z-value | P-value | 95% CI (lower) | 95% CI (upper) |
|---|
| Constant | -1.8372 | 2.0150 | -0.91 | 0.3916 | -6.1210 | 2.4460 |
| Chemotherapy
regimen | 0.0975 | 0.0856 | 1.14 | 0.2888 | -0.0854 | 0.2805 |
| FIGO IIB patients
(%) | -0.0486 | 0.0211 | -2.30 | 0.0269 | -0.0917 | -0.0055 |
| Publication
year | 0.0034 | 0.0411 | 0.08 | 0.9357 | -0.0886 | 0.0954 |
| Study design
(0=observational; 1=RCT) | -0.2396 | 0.3393 | -0.71 | 0.4958 | -1.0358 | 0.5566 |
| Sample size | 0.0003 | 0.0031 | 0.09 | 0.9320 | -0.0064 | 0.0071 |
Incidence of complications, adverse
reactions, recurrence rate and distant metastasis rate
Several of the included studies evaluated the safety
profile of NACT followed by RS compared with RS alone in LACC. Gong
et al (7) found that
patients in the NACT + RS group had a lower incidence of
postoperative complications (7.30 vs. 13.62%; P=0.002) and
postoperative radiotherapy/radiochemotherapy-related adverse
effects (3.16 vs. 4.63%; P<0.001) compared with the RS-alone
group. On the other hand, Behtash et al (5) reported similar recurrence rates in
both groups, but the NACT group exhibited a slightly higher rate of
distant metastases, although this was not statistically
significant.
Operation time and intraoperative
blood loss
Patients undergoing NACT followed by surgery in the
study by Gong et al (7) had
a longer operative time (233.66 vs. 224.37 min; P=0.008) and higher
intraoperative blood loss (750.34 vs. 684.41 ml; P=0.011) compared
with the RS alone group, indicating increased complexity in
surgery. By contrast, Katsumata et al observed that surgical
morbidity rates were comparable between both groups, suggesting
that pre-surgical chemotherapy did not necessarily complicate
surgical outcomes (8).
Hematological toxicity
In the trial reported by Katsumata et al
(8), grade 3 or 4 hematological
toxicity was more frequent in the NACT group, particularly
leukopenia (41%), neutropenia (56%) and thrombocytopenia (27%).
Similarly, Yang et al (15)
reported that neutropenia and diarrhea were significantly higher in
the irinotecan-cisplatin (IP) chemotherapy group compared with that
in the paclitaxel-cisplatin (TP) group, although both regimens had
tolerable toxicities.
Postoperative radiation
requirements
Katsumata et al (8) noted lower rates of postoperative
radiation requirements in the NACT group (58 vs. 80%; P=0.015),
which translated to fewer late radiation-related adverse events.
Late adverse events such as urinary retention, lymphedema,
vesicovaginal fistula and bowel obstruction were more frequent in
the RS-alone group.
Discussion
The management of LACC has historically revolved
around two main strategies: Primary CCRT or the combination of NACT
followed by RS (1,4,17,18).
In recent years, the potential role of NACT before surgery has
garnered increasing interest, as it may offer advantages such as
downstaging the tumor, facilitating more complete resections and
potentially reducing distant metastases (5,6).
However, the evidence supporting a definitive survival benefit,
particularly when compared with the standard of care, remains
inconsistent (7,8,19). The
meta-analysis of the present study evaluated the efficacy and
safety of NACT followed by RS relative to RS alone in women with
LACC (FIGO stages IB2-IIB). These results confirm previous
observations for OS while casting doubt on earlier claims regarding
DFS improvement. In the present pooled analysis of 7 studies,
encompassing a total of 2,231 patients, a complex picture
concerning survival outcomes was observed. For DFS, the combined OR
hovered near 0.98, with a 95% CI of 0.62-1.56 and a P-value of
0.941. This result indicates that there was no statistically
significant difference in DFS between patients receiving NACT
followed by RS and those undergoing surgery alone. Particularly,
several individual studies within the meta-analysis did report
improvements in DFS, but these positive effects did not translate
into a significant advantage when pooled across all studies. By
contrast, the meta-analysis of OS demonstrated a somewhat more
favorable outcome, particularly in a subset of included data. In
that subgroup, the OR for OS was 0.53 (95% CI, 0.32-0.87; P=0.012),
indicating a statistically significant benefit of NACT plus RS over
RS alone. However, when all OS data points were combined in the
larger overall analysis, the OR shifted to 0.74 (95% CI, 0.53-1.04;
P=0.078), crossing the threshold of statistical significance and
thus suggesting only a borderline benefit. These findings suggest
that NACT could be beneficial in selected cases; however, the
evidence does not support a consistent survival advantage across
all settings. Given that CCRT is the standard of care and
consistently yields better outcomes than surgery-based approaches
alone, the modest OS advantage of NACT + RS observed against RS
should not be misinterpreted as an alternative to CCRT. Without
direct comparisons to CCRT, the clinical implications of NACT + RS
are most relevant in resource-limited settings or for patients
unable to undergo radiotherapy.
In the present study, the leave-one-out sensitivity
analyses for OS demonstrated that, upon excluding any individual
trial, the pooled estimate remained comparatively stable (OR range,
0.45-0.62), consistently showing a significant or
borderline-significant advantage for NACT plus RS. IBy contrast,
the exclusion of individual studies in the DFS analysis
consistently yielded ORs near 1.0 (P-values ranging from 0.467 to
0.990), emphasizing the lack of evidence for DFS benefit and the
robustness of this null finding. Furthermore, no strong indications
of publication bias were found based on Begg and Mazumdar's rank
correlation test, Egger's regression test, the trim-and-fill method
or the fail-safe N, all of which suggest that the conclusions about
the overall effect estimates are not likely to be confounded by
missing or selectively reported studies.
The results of the present study align in part with
the those of the meta-analysis conducted by Zhao et al
(9), which included 13 studies and
found that the combination of NACT and RS significantly improved OS
and reduced local and distant recurrence, lymph node metastasis and
parametrial infiltration in LACC. In that analysis, however, more
consistent improvements in DFS were noted, whereas the pooled data
of the present study did not show a statistically significant
benefit in disease-free intervals. One possible explanation for
this discrepancy is that Zhao et al (9) included a slightly different set of
studies and varying DFS definitions, follow-up durations and timing
of assessments, which may have influenced reported outcomes.
Gadducci and Cosio (4) similarly
identified improvements in both PFS, a surrogate closely related to
DFS, and OS with NACT followed by RS in LACC. The meta-analysis
emphasized that these benefits seemed to persist regardless of the
total cisplatin dose, cycle length or tumor stage. The results of
this study indicate that although neoadjuvant chemotherapy may
improve OS rates, when all the data are taken into account, this
advantage is not particularly clear. Such mixed results are not
unusual in the setting of relatively heterogeneous treatment
protocols; the variability in chemotherapy regimens, dosing and
timing could attenuate any potential benefit in pooled
analyses.
It is also instructive to situate the findings of
the present study against the prevailing standard of CCRT. Although
the analysis of the present study did not compare NACT plus RS
against CCRT per se, several large RCTs and meta-analyses have
established that CCRT is highly effective for LACC, leading to
robust local control and improved survival rates (10,17).
The 2023 ESGO/ESTRO/ESP guidelines (10) recognize that NACT followed by
surgery can be an option for selected patients, but do not broadly
recommend it as a replacement for CCRT. This stance echoes the
ambivalence in the present study's findings, which do not show a
consistent or statistically robust advantage of NACT in terms of
DFS across the board, and only a borderline or context-dependent
improvement in OS.
The lack of a significant improvement in DFS,
compared with a borderline benefit in OS, raises several
hypotheses. One possibility is that, while NACT may help debulk
tumors and eradicate micrometastatic disease early, the subsequent
RS and any adjuvant treatment might be sufficient to minimize local
recurrences in both groups, thus balancing DFS outcomes over time
(12,20). Another factor could be that patients
who receive NACT but do not respond well might go on to have
surgery delayed or complicated by chemotherapy-induced side
effects, ultimately erasing some of the theoretical benefits on
DFS. Additionally, DFS in LACC can be influenced by multiple
confounders, such as tumor biology, patient comorbidities and
post-surgical treatment decisions (e.g., radiotherapy after surgery
in cases with high-risk features) (20). By contrast, the modest improvement
in OS might suggest that some patients, perhaps those with highly
chemosensitive tumors, experience eradication or substantial
downstaging of micrometastatic disease, thus improving their
overall life expectancy. It is conceivable that such a ‘response
subgroup’ benefits disproportionately from early systemic therapy,
tipping OS statistics in favor of NACT plus RS. Indeed, if a subset
of patients is particularly responsive to neoadjuvant regimens,
they might experience longer survival even if local recurrences
(the primary driver of DFS) are not markedly affected at the
population level. Additional research is needed to refine the
identification of these potential responders based on tumor
biology, molecular markers and precise imaging.
Moderate heterogeneity was noted across the included
studies in the present analysis. The prediction-interval analysis
for DFS indicated that while the summary effect size showed no
significant difference from unity (OR, 0.98), the distribution of
true effects across settings could range widely, from 0.21 to 4.71.
This wide range suggests that while some centers or specific
patient subgroups might observe a substantial benefit of NACT,
others could see minimal or no advantage. Also, in some contexts,
NACT + RS may be harmful. These findings highlight the uncertainty
of generalizing the results of the present study and suggest that
treatment decisions should be individualized and guided by
context-specific factors, such as chemotherapy regimen, surgical
expertise and patient characteristics. Factors influencing this
variability likely include differences in chemotherapy regimens
(e.g., platinum-based doublets vs. single-agent cisplatin),
treatment duration (number of cycles), dosing intensity, and
subsequent surgical technique and expertise. Further heterogeneity
stems from differences in the baseline characteristics of patient
populations: Tumor size, histological subtype (squamous vs.
adenocarcinoma) and patient performance status can all modulate
outcomes (1,4). To explore the sources of heterogeneity
in OS outcomes, a meta-regression analysis was conducted
incorporating key study-level variables, including chemotherapy
regimen, study design, publication year, proportion of FIGO stage
IIB patients and total sample size. None of these covariates
demonstrated a statistically significant moderating effect on the
observed treatment outcomes. For instance, the proportion of stage
IIB patients (coefficient, -0.305; P=0.642) and chemotherapy
regimen (coefficient, 2.470; P=0.551) showed no association with
effect size. Similarly, neither study design nor sample size
significantly explained between-study variability. These findings
indicate that the moderate heterogeneity (I²=58%) observed in the
pooled OS estimate could not be accounted for by the tested
moderators, suggesting the presence of residual clinical or
methodological variability not captured in the model. Future trials
should collect patient-level data and apply consistent reporting
standards to resolve these uncertainties.
Despite these variations, the overall methodological
quality of the included studies (as evaluated by the NOS and
Cochrane RoB tool) was acceptable to high, with most of the studies
having a score of ≥6. This finding suggests that the risk of bias
at the study design level, especially selection bias and outcome
assessment bias, was generally low. Even the studies with moderate
scores (6 points) offered reasonably robust data. Hence, the
observed inconsistencies are more likely attributable to genuine
clinical heterogeneity than to methodological flaws.
From a clinical standpoint, the findings of the
present study support the principle of individualized patient
selection for NACT. Even if the benefit in DFS is not established,
a borderline improvement in OS for certain populations indicates
that NACT plus RS may be advantageous for carefully chosen
patients. For instance, younger patients with good performance
status and bulky but resectable tumors might derive greater
benefit, particularly if their tumors are chemosensitive (5,6).
Conversely, older patients or those with significant comorbidities
may be at higher risk of toxicity from chemotherapy, without a
sufficient downstaging advantage to justify the addition of
NACT.
The safety profile of NACT followed by RS is
generally acceptable, with a lower incidence of postoperative
complications and a reduction in the occurrence of adverse
reactions related to radiotherapy (8). However, NACT is associated with
increased hematological toxicity and chemotherapy-related side
effects. Surgical complexity (operative time and blood loss) may be
slightly increased in the NACT group, though this does not appear
to impact long-term surgical morbidity. These findings suggest that
while NACT offers some advantages in reducing post-surgical
radiation complications, patient selection remains critical to
balance the risks of chemotherapy toxicity and surgical challenges
(8).
One strength of the present study is the consistent
absence of publication bias across four complementary tests. The
inclusion of both prospective and retrospective studies, while
adding heterogeneity, broadens the generalizability of the results
of the present study. A leave-one-out sensitivity analysis was also
conducted, to ensure that no single study unduly influenced the
conclusions of the present study.
The findings of the present should be interpreted in
the context of certain limitations. First, the relatively small
number of included studies (n=7) imposes constraints on the
statistical power, particularly for subgroup analyses. Second, the
analysis relied on available data for DFS and OS, but there was
variation in how outcomes were defined and measured across studies.
Some studies may have reported PFS or event-free survival as
opposed to DFS, and they used different follow-up intervals. Third,
heterogeneity in chemotherapy regimens, the number of treatment
cycles and surgical expertise can all cause variations in effect
sizes. This heterogeneity is partly captured by the wide prediction
intervals, indicating that the true effect of NACT in a given
clinical setting could deviate substantially from the pooled
estimate. Additionally, one important methodological limitation of
the present meta-analysis is the use ORs instead of HRs to analyze
survival outcomes. While HRs are the preferred and statistically
appropriate metric for time-to-event data such as OS and DFS, a
number of the included studies reported survival outcomes in
dichotomous form (e.g., 3- or 5-year survival status) without
providing HRs, log-rank P-values or Kaplan-Meier curves suitable
for reconstruction. Due to this constraint, ORs were used to ensure
analytic consistency across all studies. However, it is important
to recognize that ORs may overestimate the strength of association,
particularly when event rates are high and they do not capture the
time component of survival. This issue has been acknowledged in the
statistical analysis section and it is recommended that future
meta-analyses prioritize time-to-event metrics where available to
provide more accurate estimates of treatment effects. Finally, NACT
plus RS directly was not compared with the current standard
treatment, CCRT. While such a comparison was beyond the scope of
the present analysis, future systematic reviews might aim to
include all three arms (NACT plus RS, RS alone and CCRT) to provide
a more direct elucidation of optimal management strategies. Some
studies have attempted such three-arm comparisons, albeit with
incomplete data for direct head-to-head comparisons (4,17).
In conclusion, the present meta-analysis highlights
the nuanced and, in some respects, inconclusive role of NACT
followed by RS for the treatment of LACC. While there appears to be
a borderline improvement in OS, the data do not demonstrate a
clear, statistically significant advantage for DFS when all
available studies are pooled. These findings are consistent with
broader uncertainties in the literature, where some individual
trials and meta-analyses reveal positive effects of NACT and others
show limited or no benefits compared with upfront surgery or
standard CCRT. Clinicians should interpret these results within the
broader clinical context, weighing patient factors such as tumor
bulk, performance status, comorbidities and treatment preferences.
NACT plus RS may offer a meaningful therapeutic alternative in
select populations or settings where radiotherapy resources are
limited, but it is not currently poised to replace CCRT as the
gold-standard treatment. Future well-designed randomized trials,
possibly incorporating biological or molecular markers of
chemosensitivity, may help refine patient selection and confirm or
refute the borderline OS advantage observed in the present
study.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Lianyungang City Health
Technology Project (Lianyungang Municipal Health Commission; grant
no. 202407) and the Lianyungang City Cancer Prevention and
Treatment Science and Technology Development Project (Lianyungang
Anti-Cancer Association; grant no. MS202404).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FW designed the present study, performed data
analysis and drafted the manuscript. FW collected data and
contributed to manuscript writing. RP assisted with data collection
and statistical analysis. FW and RP confirm the authenticity of all
the raw data. YZ contributed to the data interpretation. YZ
provided technical support and revised the manuscript. RZ
supervised the study and reviewed the manuscript. YZ conceived the
study, provided overall supervision and finalized the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Mereu L, Pecorino B, Ferrara M, Tomaselli
V, Scibilia G and Scollo P: Neoadjuvant chemotherapy plus radical
surgery in locally advanced cervical cancer: Retrospective
single-center study. Cancers (Basel). 15(5207)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Foucher T, Bendifallah S, Ouldamer L,
Bricou A, Lavoue V, Varinot J, Canlorbe G, Carcopino X, Raimond E,
Monnier L, et al: Patterns of recurrence and prognosis in locally
advanced FIGO stage IB2 to IIB cervical cancer: Retrospective
multicentre study from the FRANCOGYN group. Eur J Surg Oncol.
45:659–665. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Qiu J, Sun S, Liu Q, Fu J, Huang Y and Hua
K: A comparison of concurrent chemoradiotherapy and radical surgery
in patients with specific locally advanced cervical cancer (stage
IB3, IIA2, IIICr): Trial protocol for a randomized controlled study
(C-CRAL trial). J Gynecol Oncol. 34(e64)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gadducci A and Cosio S: Neoadjuvant
chemotherapy in locally advanced cervical cancer: Review of the
literature and perspectives of clinical research. Anticancer Res.
40:4819–4828. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Behtash N, Nazari Z, Ayatollahi H,
Modarres M, Ghaemmaghami F and Mousavi A: Neoadjuvant chemotherapy
and radical surgery compared to radical surgery alone in bulky
stage IB-IIA cervical cancer. Eur J Surg Oncol. 32:1226–1230.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen H, Liang C, Zhang L, Huang S and Wu
X: Clinical efficacy of modified preoperative neoadjuvant
chemotherapy in the treatment of locally advanced (stage IB2 to
IIB) cervical cancer: Randomized study. Gynecol Oncol. 110:308–315.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gong L, Zhang JW, Yin RT, Wang P, Liu H,
Zheng Y, Lou JY and Peng ZL: Safety and efficacy of neoadjuvant
chemotherapy followed by radical surgery versus radical surgery
alone in locally advanced cervical cancer patients. Int J Gynecol
Cancer. 26:722–728. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Katsumata N, Yoshikawa H, Kobayashi H,
Saito T, Kuzuya K, Nakanishi T, Yasugi T, Yaegashi N, Yokota H,
Kodama S, et al: Phase III randomised controlled trial of
neoadjuvant chemotherapy plus radical surgery vs radical surgery
alone for stages IB2, IIA2, and IIB cervical cancer: A Japan
clinical oncology group trial (JCOG 0102). Br J Cancer.
108:1957–1963. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao H, He Y, Yang SL, Zhao Q and Wu YM:
Neoadjuvant chemotherapy with radical surgery vs radical surgery
alone for cervical cancer: A systematic review and meta-analysis.
Onco Targets Ther. 12:1881–1891. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cibula D, Raspollini MR, Planchamp F,
Centeno C, Chargari C, Felix A, Fischerová D, Jahnn-Kuch D, Joly F,
Kohler C, et al: ESGO/ESTRO/ESP guidelines for the management of
patients with cervical cancer-update 2023. Int J Gynecol Cancer.
33:649–666. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Vizza E, Corrado G, Zanagnolo V, Tomaselli
T, Cutillo G, Mancini E and Maggioni A: Neoadjuvant chemotherapy
followed by robotic radical hysterectomy in locally advanced
cervical cancer: A multi-institution study. Gynecol Oncol.
133:180–185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang Y, Huang L, Wang D, Ren P, Hong Q
and Kang D: The ROBINS-I and the NOS had similar reliability but
differed in applicability: A random sampling observational studies
of systematic reviews/meta-analysis. J Evid Based Med. 14:112–122.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Namkoong SE, Park JS, Kim JW, Bae SN, Han
GT, Lee JM, Jung JK and Kim SJ: Comparative study of the patients
with locally advanced stages I and II cervical cancer treated by
radical surgery with and without preoperative adjuvant
chemotherapy. Gynecol Oncol. 59:136–142. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang Z, Chen D, Zhang J, Yao D, Gao K,
Wang H, Liu C, Yu J and Li L: The efficacy and safety of
neoadjuvant chemotherapy in the treatment of locally advanced
cervical cancer: A randomized multicenter study. Gynecol Oncol.
141:231–239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim HS, Kim JH, Chung HH, Kim HJ, Kim YB,
Kim JW, Park NH, Song YS and Kang SB: Significance of numbers of
metastatic and removed lymph nodes in FIGO stage IB1 to IIA
cervical cancer: Primary surgical treatment versus neoadjuvant
chemotherapy before surgery. Gynecol Oncol. 121:551–557.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ronsini C, Solazzo MC, Braca E, Andreoli
G, Vastarella MG, Cianci S, Capozzi VA, Torella M, Cobellis L and
De Franciscis P: Locally advanced cervical cancer: Neoadjuvant
treatment versus standard radio-chemotherapy-an updated
meta-analysis. Cancers (Basel). 16(2542)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Coutinho F, Gokhale M, Doran C, Monberg M,
Yamada KS and Chen L: Treatment patterns and outcomes among locally
advanced cervical cancer patients receiving concurrent
chemoradiotherapy. J Clin Oncol. 41(e17511)2023.
|
|
19
|
Kenter GG, Greggi S, Vergote I, Katsaros
D, Kobierski J, van Doorn H, Landoni F, van der Velden J, Reed N,
Coens C, et al: Randomized phase III study comparing neoadjuvant
chemotherapy followed by surgery versus chemoradiation in stage
IB2-IIB cervical cancer: EORTC-55994. J Clin Oncol. 41:5035–5043.
2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fu Y, Zhu T and Li H: Effectiveness of
cervical cancer therapy using neoadjuvant chemotherapy in
combination with radical surgery: A meta-analysis. Transl Cancer
Res. 6:228–237. 2017.
|