Introduction
Claudins are tight junctional proteins which are
present at the epithelial and endothelial cell membranes (1,2), and
are the major integral membrane proteins forming the backbone of
tight junctions. Tight junctions form the primary barrier to the
paracellular transport of solutes across cells, and also play a
critical role in establishing and maintaining epithelial cell
polarity (3,4). The claudin family consists of 23
transmembrane proteins exhibiting distinct tissue- and
development-specific distribution patterns (5). The carboxylic terminal region of
claudin proteins contains a PDZ domain-binding motif that
potentially interacts with a number of PDZ domain-containing
proteins, such as ZO proteins (6,7).
These interactions also serve as adapters for other proteins
involved in cell signaling. A number of other cytosolic and nuclear
proteins, including regulatory proteins such as Rab3b, tumor
suppressors such as PTEN and transcription factors such as ZONAB,
also interact directly or indirectly with the tight junction
complex (8–10). These interactions suggest that
tight junctions, in addition to acting as barriers to the
paracellular flow of solutes, may play an important role in
regulating other cell functions, such as proliferation and tumor
suppression.
Modulations in tight junction structure and function
have been observed in epithelial tumorigenesis (11,12).
A tissue microarray study showed that claudin-1, -3 and -4 are
strongly expressed in most cases of intestinal-type gastric cancer,
but are less frequently expressed in diffuse-type gastric cancer
(13). Using cDNA microarray and
immunohistochemical analysis, our group previously showed that the
expression of claudin-4 was significantly higher in intestinal-type
than in diffuse-type gastric cancer (14,15).
Other studies have shown that claudin-2 expression gradually
increases during the multistage process of gastric carcinogenesis
(16,17). In addition, several studies have
found aberrant claudin expression in various types of cancer,
including increased expression of claudin-3 and -4 in prostate and
uterine cancers (18,19), high claudin-4 expression in
pancreatic cancer (20),
down-regulation of claudin-7 in head and neck cancer (21) and metastatic breast cancer
(22), and an increase in
claudin-3 and -4 in breast cancer (23). However, the exact role of claudin
overexpression and the functional importance of these proteins in
the development of gastric cancer remain unclear.
Gastric cancer is one of the most common malignant
tumors of the alimentary tract. At the time of diagnosis, it
usually shows extensive local tumor invasion and frequent spread to
metastatic sites, particularly the lymph nodes. It is thus
characterized by late clinical presentation, rapid progression and
a poor survival prognosis (24).
The spread of malignant tumors is a multistep process, and many of
the stages of tumor invasion require degradation or breakdown of
the extracellular matrix and connective tissue surrounding tumor
cells (25,26). The matrix metalloproteinases (MMPs)
are a family of zinc-containing enzymes involved in the degradation
of different components of the extracellular matrix. There is
considerable evidence indicating that individual MMPs play crucial
roles in tumor invasion and tumor spread (27–32).
Some studies have suggested a major role for MMP-2 and -9 in the
digestion of basement membrane type IV collagen as an important
mechanism for vessal invasion and metastasis in gastric cancer
(33,34).
Recent studies have indicated the modulatory effects
of claudins on MMP activation. Agarwal et al showed that
claudin-3 and -4 expression in ovarian epithelial cells enhanced
invasion and was associated with increased MMP-2 activity (35). Oku et al showed that
claudin-1 enhanced the invasive activity of oral squamous cell
carcinoma cells by promoting the cleavage of the laminin-5 γ2 chain
via MMP-2 and membrane-type MMP-1 (36). Takehara et al revealed that
the overexpression of claudin-4 specifically stimulated the
invasive activity of colonic cancer cells and increased MMP-2 and
-9 activity (37). In the present
study, we examined the expression levels of claudin-4, MMP-2 and -9
in gastric cancer in order to analyze their correlation with tumor
invasion, clinicopathologic parameters and clinical outcome of
gastric cancer patients. We also investigated the relationship
between claudin-4 expression and MMP-2 and -9 expression in gastric
cancer.
Materials and methods
Patients and specimens
A consecutive series of 189 tissue specimens was
collected from patients with gastric cancer who underwent subtotal
or total resection by gastrectomy at Chang Gung Memorial Hospital
(CGMH), Taiwan between January 2001 and December 2002. Written
informed consent was obtained before sample collection, and the
study was approved by the Institutional Review Board of CGMH. The
patients comprised 110 males and 79 females with a mean age of 62
years (range 24–90). The age and gender of the patients, tumor
location, tumor size, cell differentiation, depth of wall invasion,
status of lymph node metastasis, vascular invasion, lymphatic
invasion and desmoplastic reaction were obtained from
histopathology records. The stage of gastric cancer was described
according to the 1997 tumor-node-metastasis (TNM) classification of
malignant tumors of the American Joint Committee on Cancer.
Follow-up was conducted until December 2007, for a minimum
follow-up time of 5 years. The tissue specimens were formalin-fixed
and paraffin-embedded, then stained with H&E and classified by
a pathologist. The results were compared to histopathology records
from CGMH. Final pathology was determined by consensus, with review
if necessary.
Immunohistochemistry
Tissue blocks were constructed according to the
method of Schraml et al (38), and the most representative
morphological areas of the tumors were used in the study. The
specimen sections were deparaffinized, treated with 3% hydrogen
peroxide and microwaved after pre-treatment in 10 mM citric acid to
retrieve antigenicity. The sections were incubated with blocking
solution containing PBS and 1% bovine serum albumin for 20 min at
room temperature, and then incubated overnight at 4°C with an
anti-claudin-4 antibody (1:100; Zymed, San Francisco, CA, USA), an
anti-MMP-2 monoclonal antibody (1:50; Lab Vision Corporation,
Fremont, CA, USA) and an anti-MMP-9 monoclonal antibody (1:50; Lab
Vision Corporation), respectively. After washing 4 times with
Tris-buffered saline, the sections were incubated with biotinylated
secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
The immunocomplex was visualized by the immonoglobulin enzyme
bridge technique using the Dako LSAB 2 System, HRP kit (Dako Corp.,
Carpinteria, CA, USA) with 3,3'diaminobenzidine tetrachloride as a
substrate. The sections were counterstained with hematoxylin,
dehydrated with graded alcohol, cleared with xylene and mounted
with coverslips.
Scoring of immunostaining
The results of immunostaining were scored according
to a previous report (39) as
follows: the immunostaining reaction was evaluated by subjective
assessments of the median staining intensity (0, no stain; 1, weak;
2, moderate; 3, strong stain) and by the fraction of stained cells
in percentage categories (0, 0–9%; 1, 10–49%; 2, 50–89%; and 3,
≥90%). This scoring system was previously shown to be reproducible
(40). Scores of 0–3 were
determined as follows: percentage categories and staining were each
ranked as indicated above. The ranks for percentage and staining
intensity were multiplied by each other, divided by 3 and rounded
up to the nearest whole number (40). The results of immunostaining were
classified as negative (whole number 0) or positive (whole number
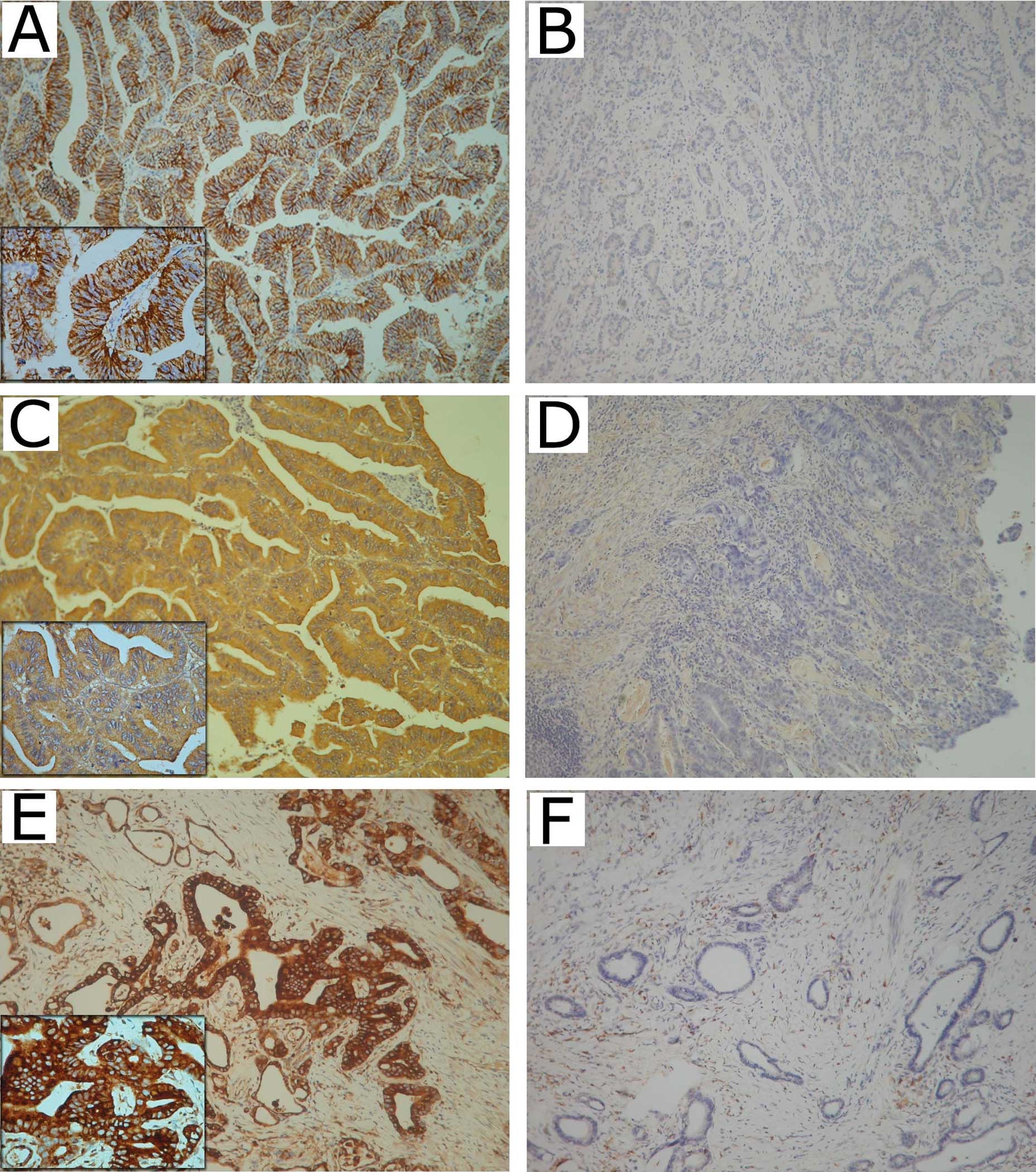
1–3), respectively (Fig. 1).
Statistical analysis
The χ2 test or Fisher's exact test were
used to test for an association between claudin-4, MMP-2 and -9
expression and the clinicopathologic parameters of the patients.
Disease-free survival was defined as the time from surgery to the
first relapse of cancer, occurence of a second primary tumor or
death of any cause. Univariate survival analysis was assessed by
the Kaplan-Meier method, and the significance of differences
between groups was analyzed using the log rank test or the log rank
test for trend. Stepwise multivariate survival analysis was
performed according to the Cox proportional hazards model. All
reported P-values were two-sided, and P-values <0.05 were
considered significant.
Results
Claudin-4, MMP-2 and MMP-9 expression in
gastric cancer
Claudin-4 was expressed in the membrane of gastric
adenocarcinoma cells in 84.7% (160/189) of cases. MMP-2 and -9 were
expressed in the cytoplasm of gastric adenocarcinoma cells in 81%
(153/189) and 89.4% (169/189) of the cases, respectively (Fig. 1).
Claudin-4, MMP-2 and MMP-9 expression in
relation to clinicopathologic parameters
The expression of claudin-4 was significantly higher
in males than in females (P=0.046), and was positively correlated
with tumor size (P=0.008) and desmoplastic reaction (P=0.027). The
expression of claudin-4 was significantly higher in gastric cancer
with advanced depth of wall invasion (P=0.008), lymph node
metastasis (P=0.005), lymphatic invasion (P=0.001) and high TNM
stage (P=0.004), but was not correlated with age, tumor location,
cell differentiation or vascular invasion (Table I).
 | Table I.Association of claudin-4, MMP-2 and
MMP-9 expression with clinicopathologic parameters. |
Table I.
Association of claudin-4, MMP-2 and
MMP-9 expression with clinicopathologic parameters.
| Factors | Cases | Claudin-4
expression
| MMP-2 expression
| MMP-9 expression
|
|---|
| Negative n (%) | Positive n (%) | P-value | Negative n (%) | Positive n (%) | P-value | Negative n (%) | Positive n (%) | P-value |
|---|
| | n=29 | n=160 | | n=36 | n=153 | | n=20 | n=169 | |
| Age (years) | | | | | | | | | | |
| ≤60 | 81 | 15 (51.7) | 66 (41.3) | 0.294 | 18 (50.0) | 63 (41.2) | 0.336 | 9 (45.0) | 72 (42.6) | 0.838 |
| >60 | 108 | 14 (48.3) | 94 (58.8) | | 18 (50.0) | 90 (58.8) | | 11 (55.0) | 97 (57.4) | |
| Gender | | | | | | | | | | |
| Male | 110 | 12 (41.4) | 98 (61.2) | 0.046 | 16 (44.4) | 94 (61.4) | 0.036 | 8 (40.0) | 102 (60.4) | 0.081 |
| Female | 79 | 17 (58.6) | 62 (38.8) | | 20 (55.6) | 59 (38.6) | | 12 (60.0) | 67 (39.6) | |
| Tumor location | | | | | | | | | | |
| Lesser curvature
lesion | 40 | 7 (24.1) | 33 (20.6) | 0.554 | 14 (38.9) | 26 (17.0) | 0.01 | 4 (20.0) | 36 (21.3) | 0.569 |
| Prepyloric | 121 | 20 (69.0) | 101 (63.1) | | 21 (58.3) | 100 (65.4) | | 15 (75.0) | 106 (62.7) | |
| Proximal
cardioesophageal | 21 | 2 (6.9) | 19 (11.9) | | 1 (2.8) | 20 (13.1) | | 1 (5.0) | 20 (11.8) | |
| Diffuse | 7 | 0 (0.0) | 7 (4.4) | | 0 (0.0) | 7 (4.6) | | 0 (0.0) | 7 (4.1) | |
| Tumor size
(cm) | | | | | | | | | | |
| ≤3 | 94 | 21 (72.4) | 73 (45.6) | 0.008 | 24 (66.7) | 70 (45.8) | 0.024 | 16 (80.0) | 78 (46.2) | 0.004 |
| >3 | 95 | 8 (27.6) | 87 (54.4) | | 12 (33.3) | 83 (54.2) | | 4 (20.0) | 91 (53.8) | |
|
Differentiation | | | | | | | | | | |
| Well | 18 | 1 (3.4) | 17 (10.6) | 0.226 | 0 (0.0) | 18 (11.8) | 0.04 | 0 (0.0) | 18 (10.7) | 0.153 |
| Moderate | 53 | 6 (20.7) | 47 (29.4) | | 8 (22.2) | 45 (29.4) | | 4 (20.0) | 49 (29.0) | |
| Poor | 118 | 22 (75.9) | 96 (60.0) | | 28 (77.8) | 90 (58.8) | | 16 (80.0) | 102 (60.3) | |
| Depth of wall
invasion | | | | | | | | | | |
| T1 | 47 | 14 (48.3) | 33 (20.6) | 0.008 | 14 (38.9) | 33 (21.6) | 0.151 | 12 (60.0) | 35 (20.7) | 0.002 |
| T2 | 35 | 5 (17.2) | 30 (18.8) | | 7 (9.4) | 28 (18.3) | | 3 (15.0) | 32 (18.9) | |
| T3 | 92 | 10 (34.5) | 82 (51.3) | | 13 (6.1) | 79 (51.6) | | 4 (20.0) | 88 (52.1) | |
| T4 | 15 | 0 (0.0) | 15 (9.4) | | 2 (5.6) | 13 (8.5) | | 1 (5.0) | 14 (8.3) | |
| Lymph node
metastasis | | | | | | | | | | |
| N0 | 86 | 22 (75.9) | 64 (40.0) | 0.005 | 22 (61.1) | 64 (41.8) | 0.186 | 15 (75.0) | 71 (42.0) | 0.038 |
| N1 | 44 | 3 (10.3) | 41 (25.6) | | 5 (13.9) | 39 (25.5) | | 2 (10.0) | 42 (24.9) | |
| N2 | 22 | 1 (3.4) | 21 (13.1) | | 4 (11.1) | 18 (11.8) | | 2 (10.0) | 20 (11.8) | |
| N3 | 37 | 3 (10.3) | 34 (21.3) | | 5 (13.9) | 32 (20.9) | | 1 (5.0) | 36 (21.3) | |
| Vascular
invasion | | | | | | | | | | |
| No | 165 | 28 (96.6) | 137 (85.6) | 0.134 | 34 (94.4) | 131 (85.6) | 0.263 | 0 (0.0) | 24 (14.2) | 0.082 |
| Yes | 24 | 1 (3.4) | 23 (14.4) | | 2 (5.6) | 22 (14.4) | | 20 (100.0) | 145 (85.8) | |
| Lymphatic
invasion | | | | | | | | | | |
| No | 102 | 24 (82.8) | 78 (48.8) | 0.001 | 22 (61.1) | 80 (52.3) | 0.339 | 5 (25.0) | 82 (48.5) | 0.046 |
| Yes | 87 | 5 (17.2) | 82 (51.2) | | 14 (38.9) | 73 (47.7) | | 15 (75.0) | 87 (51.5) | |
| Desmoplastic
reaction | | | | | | | | | | |
| None | 29 | 9 (31.0) | 20 (12.5) | 0.034 | 11 (30.6) | 18 (11.8) | 0.028 | 5 (25.0) | 24 (14.2) | 0.021 |
| Mild | 62 | 11 (37.9) | 51 (31.9) | | 11 (30.6) | 51 (33.3) | | 11 (55.0) | 51 (30.2) | |
| Moderate | 73 | 7 (24.1) | 66 (41.3) | | 12 (33.3) | 61 (39.7) | | 4 (20.0) | 69 (40.8) | |
| Marked | 25 | 2 (6.9) | 23 (14.4) | | 2 (5.6) | 23 (15.0) | | 0 (0.0) | 25 (14.8) | |
| TNM stage | | | | | | | | | | |
| I | 67 | 18 (62.1) | 49 (30.6) | 0.004 | 22 (61.1) | 64 (41.8) | 0.186 | 14 (70.0) | 53 (31.4) | 0.008 |
| II | 37 | 6 (20.7) | 31 (19.4) | | 5 (13.9) | 39 (25.5) | | 1 (5.0) | 36 (21.3) | |
| III | 37 | 1 (3.4) | 36 (22.5) | | 4 (11.1) | 18 (11.8) | | 2 (10.0) | 35 (20.7) | |
| IV | 48 | 4 (13.8) | 44 (27.5) | | 5 (13.9) | 32 (20.9) | | 3 (15.0) | 45 (26.6) | |
The expression of MMP-2 was significantly higher in
males than in females (P=0.036), and was significantly correlated
with tumor location (P=0.01), tumor size (P=0.024), cell
differentiation (P=0.04) and desmoplastic reaction (P=0.021), but
not with age, depth of wall invasion, lymph node metastasis,
vascular invasion, lymphatic invasion or TNM stage (Table I).
MMP-9 expression was positively correlated with
tumor size (P=0.004) and desmoplastic reaction (P=0.02). As with
claudin-4, the expression of MMP-9 was significantly higher in
gastric cancer with advanced depth of wall invasion (P=0.002),
lymph node metastasis (P=0.038), lymphatic invasion (P=0.046) and
higher TNM stage (P=0.008), but was not correlated with age,
gender, tumor location, cell differentiation or vascular invasion
(Table I).
Correlation of claudin-4 expression with
MMP-2 and MMP-9 expression
Further analysis of the relationship between
claudin-4 expression and MMP-2 and -9 expression revealed claudin-4
expression to be significantly correlated with the expression of
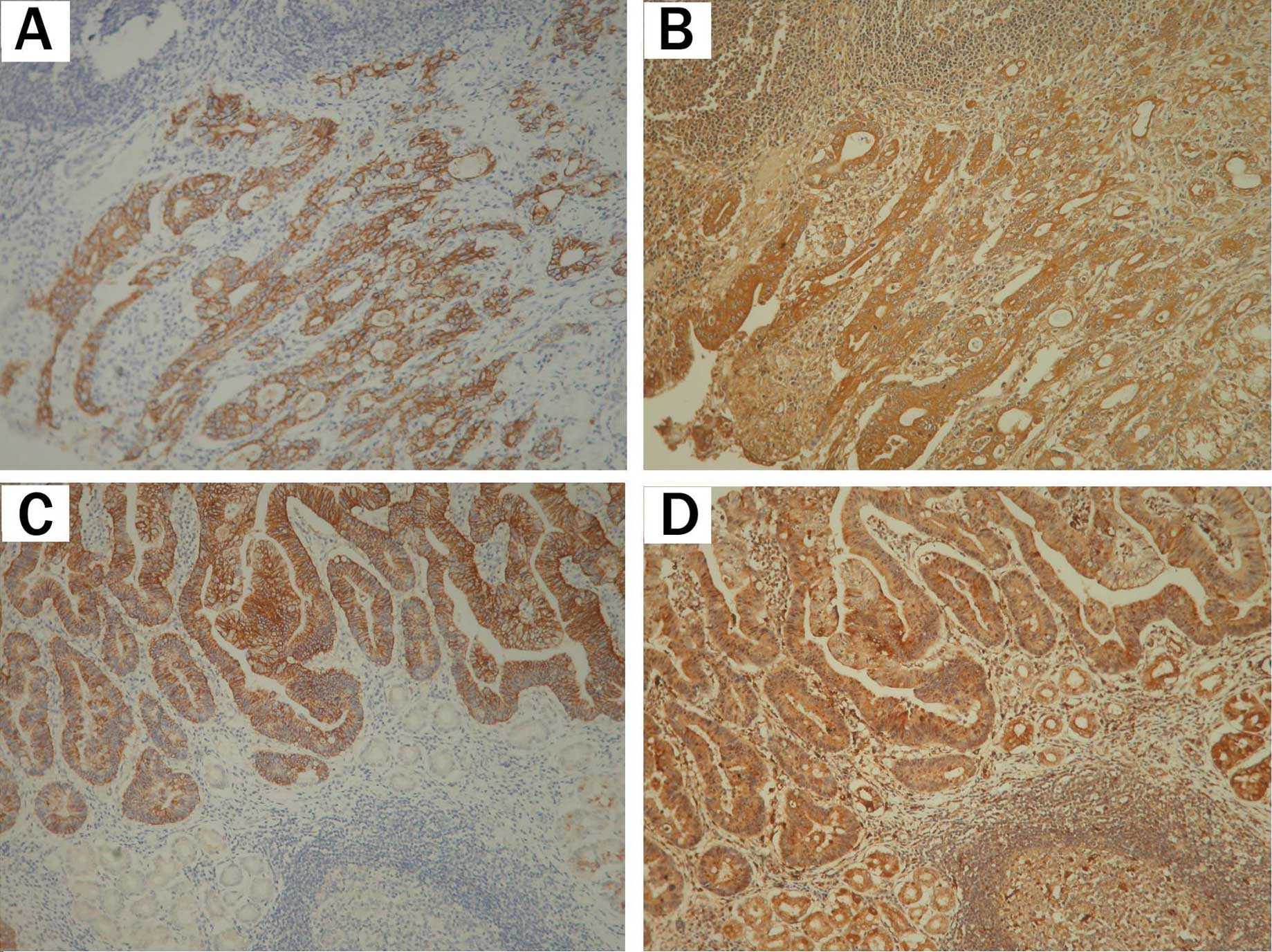
these two proteins (P=0.005 and 0.018, respectively; Table II). To better define the pattern of
co-expression between claudin-4 and these two proteins,
immunostaining was conducted in serial sections of gastric cancer.
Of 189 specimens, 135 (71.4%) were positive for claudin-4- and
MMP-2, and 147 (77.8%) were positive for claudin-4- and MMP-9
(Table II and Fig. 2).
 | Table II.Association of claudin-4 expression
with MMP-2 and MMP-9 expression. |
Table II.
Association of claudin-4 expression
with MMP-2 and MMP-9 expression.
| Claudin-4
expression
|
|---|
| Factors | Negative n (%) | Positive n (%) | P-value |
|---|
| n=29 | n=160 | |
| MMP-2
expression | | | |
| Negative (−) | 11 (37.9) | 25 (15.6) | 0.005 |
| Positive (+) | 18 (62.1) | 135 (84.4) | |
| MMP-9
expression | | | |
| Negative (−) | 7 (24.1) | 13 (8.1) | 0.018 |
| Positive (+) | 22 (75.9) | 147 (91.9) | |
Prognostic implications of claudin-4,
MMP-2 and MMP-9 expression in gastric cancer
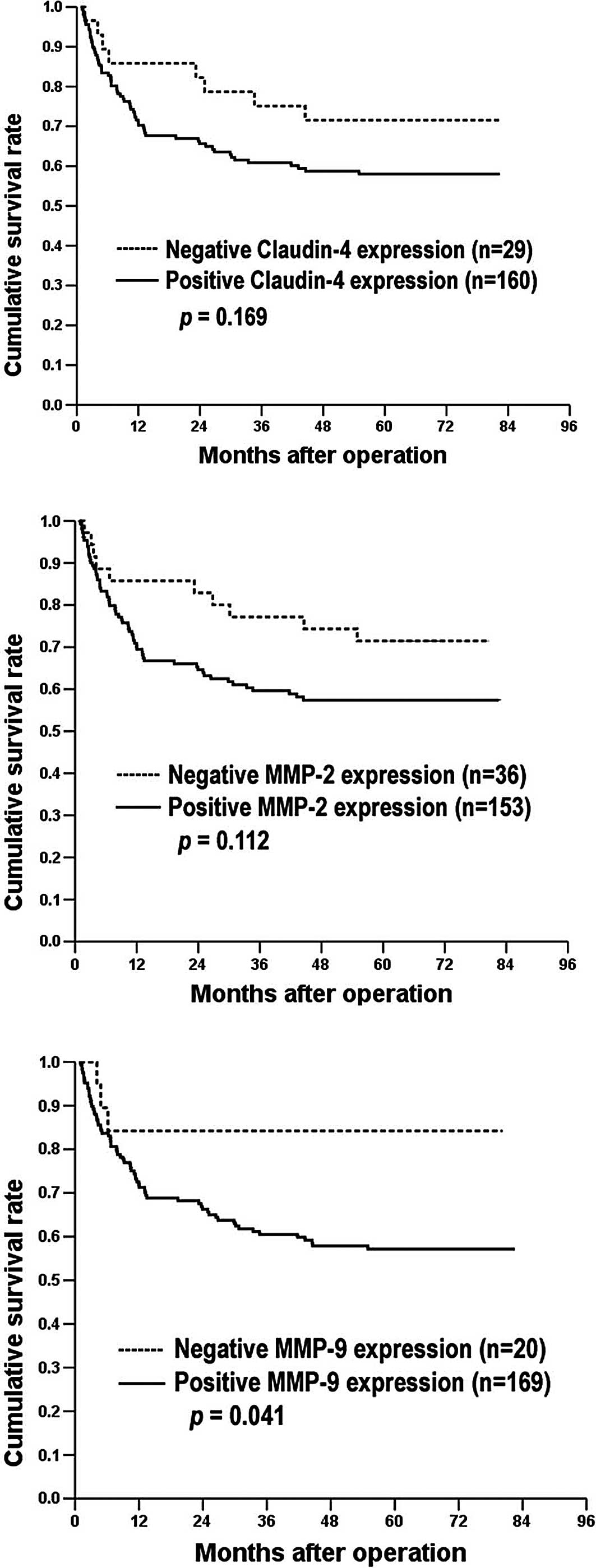
MMP-9 expression was correlated with a poor
prognosis (P=0.041; Table III and
Fig. 3C). Neither claudin-4 nor
MMP-2 expression was correlated with survival (Table III, Fig. 3A and B). Other prognostic factors
were type of gastrectomy, tumor location, large tumor size, poor
cell differentiation, advanced penetration depth, presence of nodal
metastases, presence of vascular or lymphatic invasion, marked
desmoplastic reaction and higher stage. In multivariate analysis,
depth of invasion, lymph node metastasis and lymphatic invasion
were independent prognostic factors (Table IV).
 | Table III.Univariate analysis of the
clinicopathologic parameters influencing disease-free survival in
189 gastric cancer patients undergoing gastrectomy. |
Table III.
Univariate analysis of the
clinicopathologic parameters influencing disease-free survival in
189 gastric cancer patients undergoing gastrectomy.
| Factors | No. cases | Mean survival
(months) | 95% CI of mean | 5-year survival
(%) | P-value |
|---|
| Age (years) | | | | | |
| ≤60 | 81 | 54.6 | 46.9–62.3 | 58.8 | 0.7750 |
| >60 | 108 | 54.9 | 48.1–61.7 | 61.2 | |
| Gender | | | | | |
| Male | 110 | 58.5 | 52.0–65.0 | 65.1 | 0.0820 |
| Female | 79 | 49.6 | 41.6–57.7 | 53.3 | |
| Type of
gastrectomy | | | | | |
| Total | 42 | 29.3 | 20.2–38.4 | 33.8 | <0.0001 |
| Subtotal | 147 | 60.8 | 55.4–66.1 | 67.7 | |
| Tumor location | | | | | |
| Lesser curvature
lesion | 40 | 65.9 | 56.7–75.1 | 74.5 | <0.0001 |
| Prepyloric | 121 | 56.7 | 50.4–62.9 | 62.3 | |
| Proximal
cardioesophageal | 21 | 24.8 | 13.2–36.4 | 30.2 | |
| Diffuse | 7 | 22.9 | 4.5–41.3 | 28.6 | |
| Tumor size
(cm) | | | | | |
| ≤3 | 94 | 72.2 | 67.2–77.1 | 83.4 | <0.0001 |
| >3 | 95 | 36.9 | 29.5–44.2 | 36.0 | |
|
Differentiation | | | | | |
| Well | 18 | 65.4 | 59.0–71.9 | 94.4 | 0.0190 |
| Moderate | 53 | 43.7 | 35.2–52.2 | 58.1 | |
| Poor | 118 | 52.6 | 46.1–59.0 | 51.5 | |
| Depth of
invasion | | | | | |
| T1 | 47 | 79.4 | 75.0–83.7 | 95.7 | <0.0001 |
| T2 | 35 | 74.2 | 67.2–81.3 | 85.4 | |
| T3 | 92 | 39.3 | 32.1–48.5 | 38.9 | |
| T4 | 15 | 8.2 | 4.6–11.7 | 0.0 | |
| Lymph node
metastasis | | | | | |
| N0 | 86 | 74.9 | 70.6–79.3 | 86.6 | <0.0001 |
| N1 | 44 | 60.2 | 50.0–70.4 | 69.6 | |
| N2 | 22 | 27.2 | 16.1–38.3 | 26.0 | |
| N3 | 37 | 11.2 | 7.0–15.4 | 0.0 | |
| Vascular
invasion | | | | | |
| No | 165 | 60.9 | 55.9–66.0 | 68.1 | <0.0001 |
| Yes | 24 | 11.1 | 5.4–16.9 | 4.5 | |
| Lymphatic
invasion | | | | | |
| No | 102 | 71.3 | 66.5–76.2 | 81.5 | <0.0001 |
| Yes | 87 | 34.7 | 27.1–42.4 | 34.3 | |
| Desmoplastic
reaction | | | | | |
| None | 29 | 67.4 | 56.5–78.2 | 79.3 | <0.0001 |
| Mild | 62 | 73.3 | 67.7–79.0 | 84.4 | |
| Moderate | 73 | 38.1 | 30.0–46.2 | 37.9 | |
| Marked | 25 | 34.5 | 21.3–47.8 | 39.5 | |
| TNM stage | | | | | |
| I | 67 | 79.4 | 76.3–82.6 | 93.9 | <0.0001 |
| II | 37 | 70.1 | 61.8–78.4 | 78.9 | |
| III | 37 | 44.8 | 33.5–56.0 | 47.6 | |
| IV | 48 | 10.4 | 6.9–13.9 | 0.0 | |
| Claudin-4 | | | | | |
| Negative | 29 | 64.2 | 53.0–75.3 | 71.5 | 0.1690 |
| Positive | 160 | 53.0 | 47.3–58.6 | 58.0 | |
| MMP-2 | | | | | |
| Negative | 36 | 63.0 | 53.5–72.6 | 71.5 | 0.1120 |
| Positive | 153 | 52.4 | 46.6–58.2 | 57.4 | |
| MMP-9 | | | | | |
| Negative | 20 | 68.3 | 56.0–80.7 | 84.2 | 0.0410 |
| Positive | 169 | 52.9 | 47.5–58.3 | 57.2 | |
 | Table IV.Multivariate Cox's proportional
hazards analysis for disease-free survival of 189 gastric cancer
patients undergoing gastrectomy. |
Table IV.
Multivariate Cox's proportional
hazards analysis for disease-free survival of 189 gastric cancer
patients undergoing gastrectomy.
| Factors | Relative risk (95%
CI) | P-value |
|---|
| Depth of
invasion | | <0.0001 |
| T2 vs. T1 | 5.543
(0.962–31.950) | 0.0550 |
| T3 vs.T1 | 20.420
(4.023–103.659) | 0.0003 |
| T4 vs. T1 | 35.392
(6.037–207.466) | <0.0001 |
| Lymph node
metastasis | | 0.0005 |
| N1 vs. N0 | 1.926
(0.831–4.465) | 0.1270 |
| N2 vs. N0 | 3.643
(1.516–8.756) | 0.0040 |
| N3 vs. N0 | 5.779
(2.387–13.990) | 0.0001 |
| Lymphatic
invasion | | |
| Yes vs. no | 2.115
(1.188–3.766) | 0.0110 |
Discussion
In this study, claudin-4, MMP-2 and MMP-9 expression
was examined in 189 cases of gastric cancer, and was associated
with patient clinicopathologic factors. Claudin-4 expression was
correlated with depth of wall invasion, lymph node metastasis and
lymphatic invasion, and was significantly correlated with MMP-2 and
-9 expression. These results are consistent with those obtained in
a cancer cell model (35,37). Agarwal et al showed that
claudin-4 expression in ovarian epithelial cells enhanced cell
invasion and was associated with increased MMP-2 activity (35). Takehara et al also showed
that the overexpression of claudin-4 in colonic cancer cells
stimulated invasive activity and MMP-2 and -9 activity (37). Although it is generally believed
that an alteration in claudin expression is involved in
tumorigenesis, the role of claudin-4 in the regulation of
cancer-related cell functions, such as invasion, remains unclear.
It is known that claudins affect cell physiology by recruiting
signal transduction-related molecules at tight junctions. Claudin-4
affects the expression and activity of MMP-2 and -9 either directly
or by modulating signal transduction; consequently, these two
proteins stimulate cell invasion.
Recent studies also indicate that the overexpression
of claudins is correlated with tumor invasion. Wu et al
demonstrated that the overexpression of claudin-1 was correlated
with the invasiveness and metastasis of gastric cancer (41). Kinugasa et al revealed that
the expression of claudin-1 and -2 was up-regulated in colorectal
cancer, and that this up-regulation was correlated with the depth
of tumor invasion (42). Dhawan
et al showed that claudin-1 expression increased with the
progression of colon carcinoma and metastasis (43). Nevertheless, some studies have
shown that the down-regulation of claudins is correlated with tumor
invasion. Ueda et al showed that decreased claudin-4
expression at the invasive front is correlated with cancer invasion
and metastasis in colorectal cancer (44). Oshima et al showed that
reduced expression of claudin-7 correlated with venous invasion and
liver metastasis in colorectal cancer (45). Usami et al reported that
reduced expression of claudin-7 at the invasive front of esophageal
squamous cell carcinoma may lead to tumor progression and
subsequent metastasis (46).
Morohashi et al found that decreased expression of claudin-1
correlated with lymphatic node metastasis in breast cancer
(47). These reports of decreased
claudin protein expression in cancer are consistent with the
generally accepted notion that tumorigenesis is accompanied by a
disruption of the tight junctions, a process that may play a key
role in the loss of cohesion and invasiveness observed in cancer
cells.
In gastric cancer, MMP-2 and -9 are linked to tumor
invasion and metastasis as well as to a poor prognosis (48–50).
Kabashima et al demonstrated a correlation between MMP-9
expression and lymphatic invasion and lymph node positivity in
gastric carcinoma (51). The
results of Kabashima et al are consistent with the results
of the present study, which indicate that MMP-9, but not MMP-2
expression, is positively correlated with lymph node metastasis and
lymphatic invasion as well as with a poor prognosis. By contrast,
certain reports have shown MMP-2 expression to be associated with
tumor invasion, lymph node metastasis and survival in gastric
cancer (52–54). Allgyer et al demonstrated an
association between the immunohistochemical detection of MMP-2 and
the prognosis of gastric cancer patients (52). Ji et al showed that the
expression of MMP-2 mRNA was significantly correlated with lymph
node metastasis and a poor prognosis in gastric cancer patients
(53). Monig et al also
found that the intensity of MMP-2 staining in tumor cells was
significantly correlated with the depth of tumor infiltration,
lymph node metastasis and distal metastasis in gastric cancer
patients (54). Further studies
are required to clearly distinguish the roles and involvement of
MMP-2 and -9 in the metastasis of gastric cancer.
In conclusion, claudin-4 expression was correlated
with the depth of wall invasion, lymph node metastasis and
lymphatic invasion in gastric cancer. Further analysis showed that
claudin-4 expression was significantly correlated with MMP-2 and -9
expression. We suggest that claudin-4 affects the expression and
activity of MMP-2 and -9 either directly or by modulating signal
transduction, and that these two proteins stimulate tumor cell
invasion.
Acknowledgements
This study was supported by a grant
from the National Science Council (NSC 98-2314-B-238-001) and a
grant from Vanung University, Taiwan (VIT-98-CM-01).
References
|
1.
|
Tsukita S and Furuse M: Pores in the wall:
claudins constitute tight junction strands containing aqueous
pores. J Cell Biol. 149:13–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tsukita S and Furuse M: Claudin-based
barrier in simple and stratified cellular sheets. Curr Opin Cell
Biol. 14:531–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Anderson JM: Molecular structure of tight
junctions and their role in epithelial transport. News Physiol Sci.
16:126–130. 2001.PubMed/NCBI
|
|
4.
|
Cereijido M, Valdes J, Shoshani L, et al:
Role of tight junctions in establishing and maintaining cell
polarity. Annu Rev Physiol. 60:161–177. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Morita K, Furuse M, Fujimoto K, et al:
Claudin multigene family encoding four-transmembrane domain protein
components of tight junction strands. Proc Natl Acad Sci USA.
96:511–516. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Itoh M, Furuse M, Morita K, et al: Direct
binding of three tight junction-associated MAGUKs, ZO-1, ZO-2 and
ZO-3, with the COOH termini of claudins. J Cell Biol.
147:1351–1363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yamamoto Y, Nishimura N, Morimoto S, et
al: Distinct roles of Rab3B and Rab13 in the polarized transport of
apical, basolateral, and tight junctional membrane proteins to the
plasma membrane. Biochem Biophys Res Commun. 308:270–275. 2003.
View Article : Google Scholar
|
|
9.
|
Balda MS, Garrett MD and Matter K: The
ZO-1-associated Y-box factor ZONAB regulates epithelial cell
proliferation and cell density. J Cell Biol. 160:423–432. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wu Y, Dowbenko D, Spencer S, et al:
Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of
MAGI3, a novel membrane-associated guanylate kinase. J Biol Chem.
275:21477–21485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mullin JM: Potential interplay between
luminal growth factors and increased tight junction permeability in
epithelial carcinogenesis. J Exp Zool. 279:484–489. 1997.
View Article : Google Scholar
|
|
12.
|
Soler AP, Miller RD, Laughlin KV, et al:
Increased tight junctional permeability is associated with the
development of colon cancer. Carcinogenesis. 20:1425–1431. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Resnick MB, Gavilanez M, Newton E, et al:
Claudin expression in gastric adenocarcinomas: a tissue microarray
study with prognostic correlation. Hum Pathol. 36:886–892. 2005.
View Article : Google Scholar
|
|
14.
|
Wu CM, Lee YS, Wang TH, et al:
Identification of differential gene expression between intestinal
and diffuse gastric cancer using cDNA microarray. Oncol Rep.
15:57–64. 2006.PubMed/NCBI
|
|
15.
|
Kuo WL, Lee LY, Wu CM, et al: Differential
expression of claudin-4 between intestinal and diffuse-type gastric
cancer. Oncol Rep. 16:729–734. 2006.PubMed/NCBI
|
|
16.
|
Song X, Chen H, Shen B, et al: Expression
of Cdx2 and claudin-2 in the multistage tissue of gastric
carcinogenesis. Oncology. 73:357–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Song X, Li X, Tang Y, et al: Expression of
claudin-2 in the multistage process of gastric carcinogenesis.
Histol Histopathol. 23:673–682. 2008.PubMed/NCBI
|
|
18.
|
Long H, Crean CD, Lee WH, et al:
Expression of Clostridium perfringens enterotoxin receptors
claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res.
61:7878–7881. 2001.PubMed/NCBI
|
|
19.
|
Santin AD, Zhan F, Cane S, et al: Gene
expression fingerprint of uterine serous papillary carcinoma:
identification of novel molecular markers for uterine serous cancer
diagnosis and therapy. Br J Cancer. 92:1561–1573. 2005. View Article : Google Scholar
|
|
20.
|
Nichols LS, Ashfaq R and Iacobuzio-Donahue
CA: Claudin 4 protein expression in primary and metastatic
pancreatic cancer: support for use as a therapeutic target. Am J
Clin Pathol. 121:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Al Moustafa AE, Alaoui-Jamali MA, Batist
G, et al: Identification of genes associated with head and neck
carcinogenesis by cDNA microarray comparison between matched
primary normal epithelial and squamous carcinoma cells. Oncogene.
21:2634–2640. 2002.
|
|
22.
|
Kominsky SL, Argani P, Korz D, et al: Loss
of the tight junction protein claudin-7 correlates with
histological grade in both ductal carcinoma in situ and invasive
ductal carcinoma of the breast. Oncogene. 22:2021–2033. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kominsky SL, Vali M, Korz D, et al:
Clostridium perfringens enterotoxin elicits rapid and specific
cytolysis of breast carcinoma cells mediated through tight junction
proteins claudin 3 and 4. Am J Pathol. 164:1627–1633. 2004.
View Article : Google Scholar
|
|
24.
|
Morson BC, Dawson IMP and Day DW: Morson
and Dawson's Gastrointestinal Pathology. 3rd edition. Blackwell
Science; Oxford: pp. 53–70. 1990
|
|
25.
|
Hart IR and Saini A: Biology of tumour
metastasis. Lancet. 339:1453–1457. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kohn EC and Liotta LA: Molecular insights
into cancer invasion: strategies for prevention and intervention.
Cancer Res. 55:1856–1862. 1995.PubMed/NCBI
|
|
27.
|
Murphy G and Docherty AJ: The matrix
metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol.
7:120–125. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Stetler-Stevenson WG, Liotta LA and
Kleiner DE: Extracellular matrix 6: role of matrix
metalloproteinases in tumor invasion and metastasis. FASEB J.
7:1434–1441. 1993.PubMed/NCBI
|
|
29.
|
Davies B, Waxman J, Wasan H, et al: Levels
of matrix metalloproteases in bladder cancer correlate with tumor
grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
30.
|
Boag AH and Young ID: Increased expression
of the 72-kd type IV collagenase in prostatic adenocarcinoma.
Demonstration by immunohistochemistry and in situ hybridization. Am
J Pathol. 144:585–591. 1994.PubMed/NCBI
|
|
31.
|
Muller D, Wolf C, Abecassis J, et al:
Increased stromelysin 3 gene expression is associated with
increased local invasiveness in head and neck squamous cell
carcinomas. Cancer Res. 53:165–169. 1993.PubMed/NCBI
|
|
32.
|
Urbanski SJ, Edwards DR, Hershfield N, et
al: Expression pattern of metalloproteinases and their inhibitors
changes with the progression of human sporadic colorectal
neoplasia. Diagn Mol Pathol. 2:81–89. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Sakurai Y, Otani Y, Kameyama K, et al:
Expression of interstitial collagenase (matrix metalloproteinase-1)
in gastric cancers. Jap J Cancer Res. 88:401–406. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Torii A, Kodera Y, Uesaka K, et al: Plasma
concentration of matrix metalloproteinase 9 in gastric cancer. Br J
Surg. 84:133–136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Agarwal R, D'Souza T and Morin PJ:
Claudin-3 and claudin-4 expression in ovarian epithelial cells
enhances invasion and is associated with increased matrix
metalloproteinase-2 activity. Cancer Res. 65:7378–7385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Oku N, Sasabe E, Ueta E, et al: Tight
junction protein claudin-1 enhances the invasive activity of oral
squamous cell carcinoma cells by promoting cleavage of laminin-5
gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type
MMP-1. Cancer Res. 66:5251–5257. 2006. View Article : Google Scholar
|
|
37.
|
Takehara M, Nishimura T, Mima S, et al:
Effect of claudin expression on paracellular permeability,
migration and invasion of colonic cancer cells. Biol Pharm Bull.
32:825–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Schraml P, Bucher C, Bissig H, et al:
Cyclin E overexpression and amplification in human tumours. J
Pathol. 200:375–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Ravn V, Havsteen H and Thorpe SM:
Immunohistochemical evaluation of estrogen and progesterone
receptors in paraffin-embedded, formalin-fixed endometrial tissues:
comparison with enzyme immunoassay and immunohistochemical analysis
of frozen tissue. Mod Pathol. 11:709–715. 1998.
|
|
40.
|
Ravn V, Rasmussen BB and Hojholt L:
Reproducibility of subjective immunohistochemical estrogen- and
progesterone-receptor determination in human endometrium. Pathol
Res Pract. 189:1015–1022. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Wu YL, Zhang S, Wang GR, et al: Expression
transformation of claudin-1 in the process of gastric
adenocarcinoma invasion. World J Gastroenterol. 14:4943–4948. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Kinugasa T, Huo Q, Higashi D, et al:
Selective up-regulation of claudin-1 and claudin-2 in colorectal
cancer. Anticancer Res. 27:3729–3734. 2007.PubMed/NCBI
|
|
43.
|
Dhawan P, Singh AB, Deane NG, et al:
Claudin-1 regulates cellular transformation and metastatic behavior
in colon cancer. J Clin Invest. 115:1765–1776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Ueda J, Semba S, Chiba H, et al:
Heterogeneous expression of claudin-4 in human colorectal cancer:
decreased claudin-4 expression at the invasive front correlates
cancer invasion and metastasis. Pathobiology. 74:32–41. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Oshima T, Kunisaki C, Yoshihara K, et al:
Reduced expression of the claudin-7 gene correlates with venous
invasion and liver metastasis in colorectal cancer. Oncol Rep.
19:953–959. 2008.PubMed/NCBI
|
|
46.
|
Usami Y, Chiba H, Nakayama F, et al:
Reduced expression of claudin-7 correlats with invasion and
metastasis in squamous cell carcinoma of the esophagus. Hum Pathol.
37:569–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Morohashi S, Kusumi T, Sato F, et al:
Decreased expression of claudin-1 correlates with recurrence status
in breast cancer. Int J Mol Med. 20:139–143. 2007.PubMed/NCBI
|
|
48.
|
Grigioni WF, D'Errico A, Fortunato C, et
al: Prognosis of gastric carcinoma revealed by interactions between
tumor cells and basement membrane. Mod Pathol. 7:220–225.
1994.PubMed/NCBI
|
|
49.
|
Tori A, Kodera Y, Ito M, et al: Matrix
metalloproteinase 9 in mucosally invasive gastric cancer. Gastric
Cancer. 1:142–145. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Schwartz GK: Invasion and metastasis in
gastric cancer: in vitro and in vivo models with clinical
correlations. Semin Oncol. 23:316–324. 1996.PubMed/NCBI
|
|
51.
|
Kabashima A, Maehare Y, Kakeji Y, et al:
Clinicopathological features and overexpression of matrix
metalloproteinases in intramucosal gastric carcinoma with lymph
node metastasis. Clin Cancer Res. 6:3581–3584. 2000.PubMed/NCBI
|
|
52.
|
Allgayer H, Babic R, Beyer BC, et al:
Prognostic relevance of MMP-2 (72-kDa collagenase IV) in gastric
cancer. Oncology. 55:152–160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Ji F, Chen YL, Jin EY, et al: Relationship
between matrix metalloproteinase-2 mRNA expression and
clinicopathological and urokinase-type plasminogen activator system
parameters and prognosis in human gastric cancer. World J
Gastroenterol. 11:3222–3226. 2001. View Article : Google Scholar
|
|
54.
|
Monig SP, Baldus SE, Hennecken JK, et al:
Expression of MMP-2 is associated with progression and lymph node
metastasis of gastric carcinoma. Histopathology. 39:597–602. 2001.
View Article : Google Scholar : PubMed/NCBI
|