Introduction
Platinum and taxane are used as two key drugs for
the gold standard regimen in ovarian cancer (OC) treatment,
although recent innovative treatment strategies have achieved more
successful results using an intraperitoneal approach (1) or the dose-dense principle (2). However, platinum resistance remains a
major obstacle in cancer therapy. Clinical trials are aimed at
circumventing patient relapse within 6 or 12 months after
first-line chemotherapy, and new trials are underway to deal with
drug resistant OC, including clear-cell and mucinous carcinomas.
Recently, we reported that mitochondrial (MT) ultrastructural
morphology closely correlates with platinum response in OC
(3). We also reported that
mitochondria play an important role in platinum sensitivity, as
MT-DNA is involved in platinum cell cytotoxicity (4). Furthermore, it has been shown that
the stimulation of MT cytochrome C release results in the
enhancement of platinum sensitivity in ovarian carcinoma cells
(5). Finally, several studies have
shown a direct drug action on mitochondria, inducing the loss of
membrane potential and the release of apoptogenic proteins from
isolated mitochondria (6,7).
Taxanes are important drugs commonly used to treat
OC. Taxane resistance is hard to evaluate in the clinical setting,
unlike platinum resistance as determined by GOG criteria, which
defines clinical resistance as patient relapse within 6 months of
previous platinum chemotherapy. However, basic or translational
research studies have addressed the MT-associated mechanisms of
paclitaxel resistance, including the association of the Bcl-2
molecule to drug-induced apoptosis (8,9),
paclitaxel-induced MT functional abnormality (10), microtubule stabilization associated
with MT aggregation (11) and
caspase activation of mitrochondria upstream (12). Other investigators have shown
MT-dependent apoptosis induced by paclitaxel, etoposide and
UV-irradiation (13). Cell
cytotoxicity of irinotecan and 5-fluorouracil has also been
reported to be related to MT membrane potential (14). These findings indicate that most
chemotherapeutic drugs used for OC treatment affect the
MT-associated apoptotic cascade; therefore, mitochondria may play a
central role in sensitivity to these drugs.
Among all OCs, serous adenocarcinoma is considered
to be the most sensitive to standard chemotherapeutic regimens,
while other types are less sensitive. Silverberg reported the
prognostic significance of a histopathologic grading system of
epithelial OC, emphasizing that histopathologic typing is less
valuable than grading in predicting survival, but better at
predicting tumor responsiveness to chemotherapy agents (15). Malpica et al reported that a
two-tier system for grading ovarian serous carcinoma based on
nuclear atypia (uniformity vs. pleomorphism) correlated well with
the prognosis of survival (16,17).
The pathological features of ovarian cancer following chemotherapy
have been detailed previously (18).
We recently developed a scoring system of MT
morphological findings to determine its potential correlation with
responsiveness to chemotherapy, and the application of this system
to a series of cases treated in a uniform manner in our institution
was analyzed (3). In this study,
this scoring system was extended to investigate whether it can be
applied to sensitivity to various drugs, histologic type and
patient prognosis.
Materials and methods
Patients
The study group comprised 41 women with advanced
stage or recurrent OC who had been treated with primary surgery
followed by taxane plus platinum-based chemotherapy at the
Department of Obstetrics and Gynecology of Jikei University School
of Medicine, Japan, between January 2001 and December 2006.
Patients were enrolled in the study after providing informed
consent. The study protocol and all accompanying forms and surveys
were reviewed and approved by the institutional review board.
Histologic diagnosis
Tumor stage and histologic subtype were determined
according to FIGO and WHO guidelines, respectively. All available
histological sections were reviewed by two expert pathologists
involved in the study. The Gynecologic Oncology Group (GOG) grading
system (19) was used in this
study.
Clinical evaluation
Clinical examination, serum CA125 assay, chest
X-ray, abdominal-pelvic ultrasound and computed tomography scan
(CT) were routinely used to evaluate clinical response. Additional
investigations were performed when appropriate. Response was
characterized according to the Response Evaluation Criteria in
Solid Tumors (RECIST) (20). When
no measurable tumor was observed, CA125 response criteria
(Gynecologic Cancer Intergroup-Modified Rusting definition)
(21) were used. Serum CA125
values were measured immediately before and after chemotherapy.
In vitro drug sensitivity assay
When the appropriate number of viable cells was
available from the tumor mass, continuous oxygen consumption (slope
of dissolved oxygen; SDO) was monitored with a dissolved oxygen
meter. When cells are sensitive to anticancer agents, the number of
viable cells decreases in association with the decreased amount of
total cellular oxygen consumption. The amount of oxygen consumption
correlates with cellular drug sensitivity, and it is a rapid method
for assessing chemosensitivity (22,23).
The ovarian carcinoma cell line 2008 and its platinum-resistant
variant C13 were used as controls. The human cell line 2008 was
established from a patient with a serous cystadenocarcinoma of the
ovary (24), and a resistant
subline C13 was obtained by 13 monthly selections with cisplatin
followed by chronic exposure to cisplatin (25).
Electron microscopy
The method of electron microscopy is described in
detail in our previous report (3).
Briefly, for electron microscopic analysis, cells or samples were
fixed in situ with 2.0% glutalaldehyde. After washing, cells
were fixed for 1 h at 4°C in 1% OsO4. Samples were
dehydrated in graded concentrations of ethanol and embedded in Epon
812 epoxy resin. After polymerization, ultra-thin sections were cut
parallel to the block surface using a Reichert OUM4 ultramicrotome,
stained with uranyl acetate and lead citrate and then examined
using a JEOL-1200EX electron microscope at 60 kV acceleration
voltage at a magnification of x1,000, x1,200 and x2,500.
By careful examination and comparison of
mitochondria in typical platinum-sensitive and -resistant OC cells,
we evaluated ∼50 mitochondria and focused on seven independent
parameters depicting the most prominent differences between these
two representative types of cells: i) MT size (longest diameter:
<0.7 μm, 0.7–0.8, >0.8 μm); ii) Cresta structure (clear,
intermediate, destroyed); iii) electron density (low, intermediate,
high); iv) MT distribution (no./100 μm2: <40, 40–80,
>80); v) pattern of MT distribution (perinuclear, intermediate,
dispersed); vi) ovoid ratio (shortest diameter/longest diameter:
≥0.7, intermediate, ≤0.3); vii) MT architecture (three types:
tubular, adrenal or hepatocyte) (Table
I). Each of these evaluated parameters was assigned a point
score of 0–2, with the exception of MT architecture, which was
scored as 0 or 2. The different features were then summed up for a
possible total score of 14. The 2008 and C13 cell lines served as
examples of typical electron microscopy of drug-sensitive and
-resistant cell mitochondria, respectively. In our previous report,
the electron density and the MT distribution pattern were selected
for the final scoring system based on an epithelial OC (3). However, in this study, non-epithelial
ovarian tumors comprised ∼25% of the study population, and the MT
scoring was performed using the full set of seven parameters.
 | Table I.Scoring system of the mitochondrial
ultrastructure. |
Table I.
Scoring system of the mitochondrial
ultrastructure.
| Feature | 0 | 1 | 2 |
|---|
| MT size (longest
diameter) | Small (≤0.7 μm) | Intermediate | Large (≥0.8 μm) |
| Cresta structure | Clear | Intermediate | Destroyed |
| Electron density | Low | Intermediate | High |
| MT distribution (/100
μm2) | High (≥100/100
μm2) | Intermediate | Low (≤50/100
μm2) |
| Distribution
pattern | Perinuclear | Intermediate | Dispersed |
| Oval ratio
(short/long) | Short ovoid
(≥0.7) | Intermediate | Long ovoid
(≤0.3) |
| MT type | Tubular | | Adrenal
hepatocyte |
Statistical analysis
Differences between samples or groups of samples
were determined by the Student's t-test using two-sided P-values.
Multiple logistic regression was carried out to investigate the
relationship between drug response and the above seven features.
Clinical response and in vitro sensitivity analysis were
compared to the results of the Fisher's exact test. Receiver
operator characteristic (ROC) analysis was performed to establish
whether an optimal ‘cut-off’ point could be determined for the MT
scoring system and subsequent drug sensitivity.
Progression-free survival (PFS) was calculated from
the date of surgery to the date of recurrence as determined by CT
or MRI scan. One case with an independent increase of CA125 without
detectable tumor was excluded. Kaplan-Meier estimates were used to
construct the survival curves (26).
Results
Patient and tumor characteristics
Patient demographics are summarized in Table II. The median age of the patients
was 51 years (range 24–71). According to the FIGO classification, 4
patients had stage I tumors, 2 had stage II, 24 had stage III and 5
had stage IV tumors. In 6 patients, only a recurrent tumor was
available for review.
 | Table II.Clinical features of the patients. |
Table II.
Clinical features of the patients.
| Characteristic | Total
| Low score
| High score
|
|---|
| No. | % | No. | % | No. | % |
|---|
| No. | 41 | | 16 | | 25 | |
| Age (median) | 51.5 | | 54 | | 47 | |
| Stage | | | | | | |
| Ia | 4 | 9.8 | 3 | 18.8 | 1 | 4.0 |
| II | 2 | 4.9 | 0 | 0.0 | 2 | 8.0 |
| III | 24 | 58.5 | 10 | 62.5 | 14 | 56.0 |
| IV | 5 | 12.2 | 1 | 6.3 | 4 | 16.0 |
| Recurrence | 6 | 14.6 | 2 | 100.0 | 4 | 16.0 |
| Histology | | | | | | |
| Serous | 16 | 43.1 | 10 | 62.5 | 6 | 24.0 |
| Mucinous | 1 | 2.3 | 1 | 6.3 | 0 | 0.0 |
| Endometrioid | 6 | 13.6 | 1 | 6.3 | 5 | 20.0 |
| Clear cell | 6 | 13.6 | 1 | 6.3 | 5 | 20.0 |
|
Undifferentiated | 2 | 4.5 | 0 | 0.0 | 2 | 8.0 |
| Unclassified | 1 | 11.4 | 1 | 6.3 | 0 | 0.0 |
| Othersb | 9 | 11.4 | 2 | 12.5 | 7 | 28.0 |
| Tumor gradec | | | | | | |
| 1 | 5 | 31.3 | 1 | 10.0 | 4 | 66.7 |
| 2 | 6 | 37.5 | 4 | 40.0 | 2 | 33.3 |
| 3 | 5 | 31.3 | 5 | 50.0 | 0 | 0.0 |
| Max. tumor
sized | | | | | | |
| ≤1 cm | 28 | 68.3 | 14 | 82.4 | 14 | 58.3 |
| >1 cm | 13 | 31.7 | 3 | 17.6 | 10 | 41.7 |
| No. of residual
lesions | | | | | | |
| ≤5 | 29 | 70.7 | 14 | 82.4 | 15 | 62.5 |
| >5 | 12 | 29.3 | 3 | 17.6 | 9 | 37.5 |
Patients with stage I did not receive chemotherapy,
and the remaining 37 cases were analyzed for response to
chemotherapy. For the recurrent cases, platinum-resistant disease
was defined as progression either during therapy or within 6 months
of completing therapy with platinum or platinum-containing
chemotherapy. In this study, all recurrent cases had a PFS time of
>6 months, indicating that they were all platinum-sensitive.
Histologically, 19 carcinomas were serous and 6 were endometrioid
and clear-cell, respectively. Two were undifferentiated, 1 was
mucinous and 1 was unclassified adenocarcinoma. The remaining 9
were other ovarian tumors, including sarcoma, small-cell carcinoma
and other rare tumors. Since grading is important for serous and
endometrioid carcinoma, these tumors were graded, resulting in a
grade of G1 in 5 patients, G2 in 6 and G3 in 5 cases. After
surgery, 28 patients had residual disease ≤1 cm and 13 patients had
larger residual tumors. Chemotherapy response was not related to
the stage, histology, grade, tumor size or residual lesion.
Scoring system for mitochondrial
ultrastructure
Seven features were analyzed and scored
independently in our grading system. Of the 37 patients receiving
post-operative chemotherapy, 15 achieved a response, while 22 were
resistant to treatment. The total MT score was 5.13±1.13 (mean ±
SE) in the 15 responsive cases and 11.41±0.43 in the 22 resistant
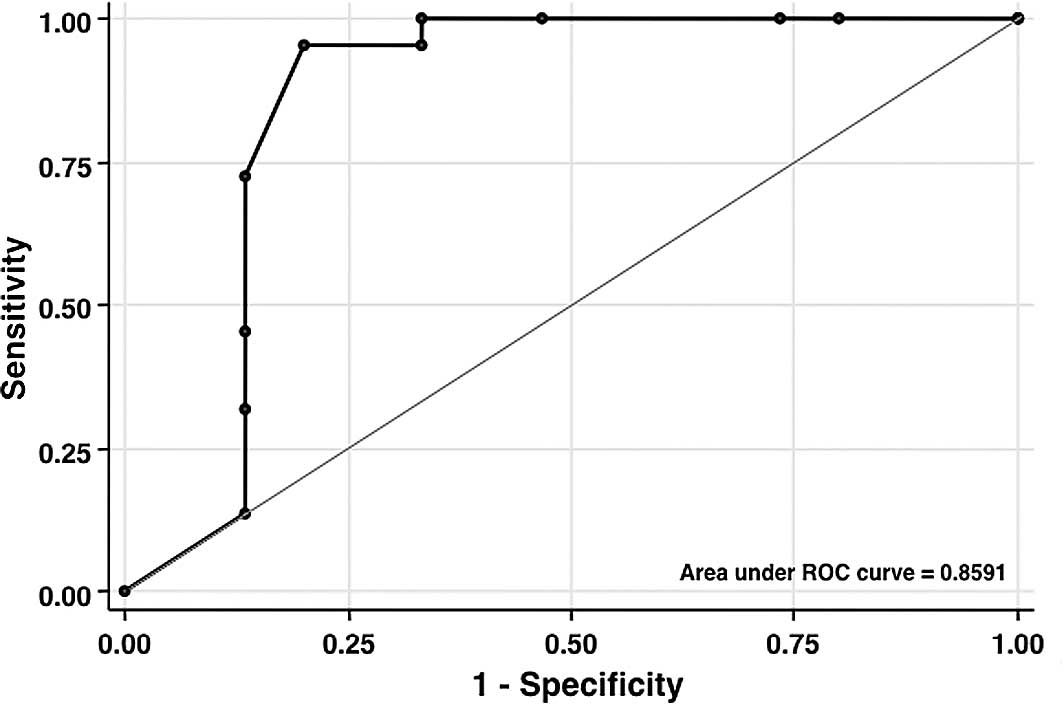
cases (P<0.001). As shown in Fig.
1, ROC analysis revealed that the resistant total ‘cut-off’
score was ≥10 points (P<0.05; AUC=0.86) with 95.5% sensitivity,
80.0% specificity and 89.2% positive predictive value. Table III lists MT scores for different
drugs related to sensitivity. This was highly associated with
cellular drug sensitivity, with the exception of doxorubicin. The
total scores were in the range of 4-6 in the groups sensitive to
platinum, taxane and irinotecan, while they were 10–12 in the
resistant groups. ROC analysis revealed that the resistant
‘cut-off’ scores for platinum, taxane and irinotecan were ≥11, 5
and 10 points, respectively.
 | Table III.Results of the scoring system. |
Table III.
Results of the scoring system.
| Drug(s) | Sensitive mean ± SE
(no.) | Resistant mean ± SE
(no.) | P-value | 95% CI | Cut-off |
|---|
| Clinical TCa | 5.13±1.13 (15) | 11.41±0.42
(22) | <0.0001 | 3.73–8.82 | 10 |
| Platinum | 5.75±1.13 (16) | 11.60±0.68 (5) | 0.0002 | 3.07–8.63 | 11 |
| Taxane | 4.97±1.21 (12) | 10.40±1.27
(10) | 0.0030 | 1.75–9.05 | 5 |
| Irrinotecan | 4.27±1.13 (11) | 10.15±1.53 (6) | 0.0062 | 1.52–9.94 | 10 |
| Doxorubicin | 6.31±1.35 (13) | 9.00±1.75 (7) | 0.1221 | −2.08–7.46 | - |
Based on the above ROC analysis, patients were
classified into two groups: patients with low scores (<10
points; n=16) or patients with high scores (≥10 points; n=25)
(Table II). Most patients in the
low-score group corresponded to chemotherapy responders, while
patients in the high-score group were from the non-responder group.
The distribution, stage and histology were mostly well-balanced
between these two groups. The most prognostically relevant clinical
features are shown in Table II,
including maximum tumor size and the presence of residual disease
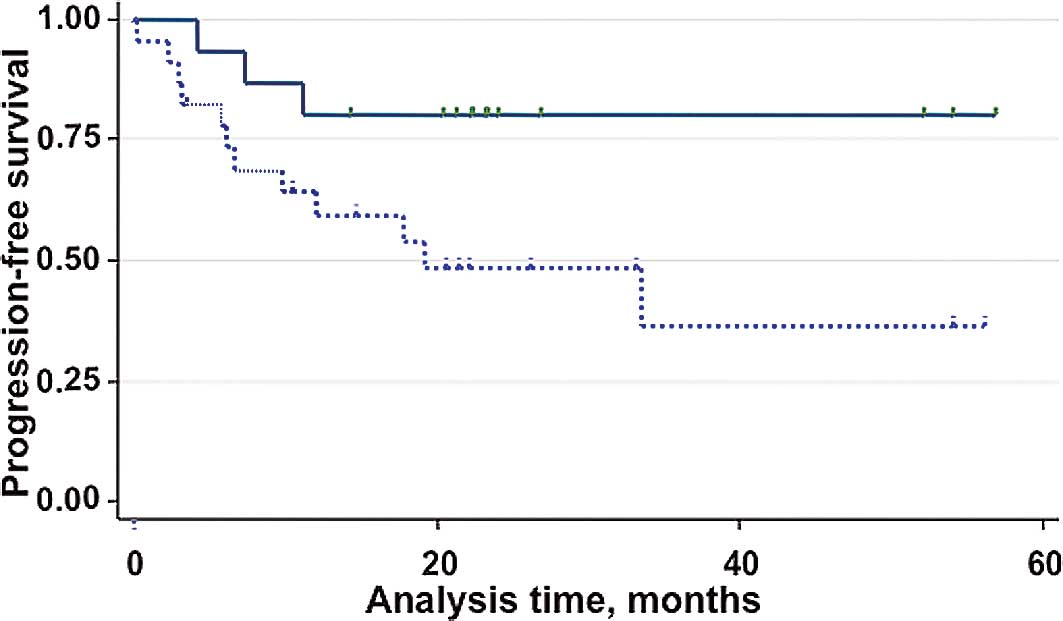
prior to chemotherapy. Fig. 3
shows the Kaplan-Meier PFS estimates by MT Score. During the median
follow-up of 23 months, 11 patients relapsed. The PFS curves show a
difference in favor of the low-score group of patients compared to
the high-score group (risk ratio 3.99, P=0.045), corresponding to
an absolute difference in the 6-month PFS of 10% (83 vs. 73%) and
in the 12-month PFS of 21% (80 vs. 59%).
Discussion
Two recent randomized Phase III trials demonstrated
that intraperitoneal (1) or weekly
dose-dense (2) chemotherapy with
carboplatin and paclitaxel resulted in a more favorable survival
than standard chemotherapy for OC patients. However, it is not
clear whether these two strategies circumvent drug resistance. For
the rare type of platinum-resistant OC, such as clear-cell
carcinoma or mucinous adenocarcinoma, worldwide clinical trials
using combinations other than the platinum/taxane combination are
currently underway. Drug sensitivity (27) or resistance (28) screening prior to chemotherapy may
be useful tools when the system relates to the clinical response or
prognosis of the patients. An extreme drug-resistant (EDR) test
could avoid drug toxicity and the subsequent cost of treatment of
patients with highly drug-resistant tumors. EDR tests involve the
limited outcome of the elimination of chemotherapy in a resistant
tumor (28,29). These tests may correlate strongly
to our MT scoring system as this assay is not associated with cell
survival, but rather measures cellular metabolic activity monitored
by MT status. In our previous report, the electron density and MT
distribution pattern were selected for the final scoring system
based on the platinum sensitivity of epithelial OC (3). However, the present study consisted
of 9 non-epithelial ovarian tumors comprising 25% of the study
population, and the scoring system was also applied to non-platinum
agents. MT scoring was performed using the full set of seven
parameters.
Here, we report that our MT scoring system is
strongly associated with the clinical response to platinum/taxane
and is a good predictor of patient response to chemotherapy.
However, several issues require discussion.
First of all, the pathologic grading system has been
shown to correlate with the survival prognosis in ovarian serous
adenocarcinoma (15–17), while histologic type is a good
predictor of cellular sensitivity to chemotherapeutic drugs. Our
study patients had various types of tumors, including clear-cell
carcinoma, mucinous adenocarcinoma, sarcoma and other rare tumors.
Mitochondria are cellular ubiquitous organelles and are
indispensable organs of the cell involved in controlling
respiratory or metabolic functions in tumors of any histologic type
or grading. Notably, MT change correlates well with drug
sensitivity and with patient survival regardless of the histologic
type or grading system. This clearly indicates that the MT scoring
system has a clinical advantage over the latter two systems.
Second, the MT scoring system was originally
developed on the basis of MT changes in platinum-resistant cells
(4). Notably, the system can be
applied to taxane- and irinotecan-resistant cells, suggesting that
MT-impaired cells are resistant to most of the drugs among the
standard battery of agents usually selected for the treatment of
OC.
This finding is supported by the fact that, in the
clinic, platinum-resistant patients are usually resistant to most
other chemotherapeutic regimens, including topotecan (30), gemcitabine (31) and weekly taxol (32), as the series of GOG126 trials
demonstrated. Our data suggest that a high MT score is a common
morphological feature for drug resistance, at least to platinum,
taxane and irinotecan. Mitochondria are involved in the mechanism
of resistance for the above three drugs. Notably, the resistant
‘cut-off’ scores for platinum, taxane and irinotecan vary for each
drug, suggesting that mitochondria are differentially involved in
the mechanism of resistance, although it is not clear how the MT
morphological changes relate to its function.
Third, 4 out of 6 recurrent cases showed high MT
scores, although more than 6 months had elapsed since the last dose
of chemotherapy, suggesting that these cases may have exhibited
intrinsic resistance rather than acquired resistance.
Unfortunately, the cases did not have any measurable disease at the
time of previous chemotherapy, and the response was not
evaluated.
Finally, the relevant outcome was based on whether
chemotherapy actually resulted in improved survival for the
patients in the low-MT score cohort. The data showed significant
difference in the 6-month and 1-year PFS. In particular, regarding
the 1-year PFS, more than half the patients in the high-score
cohort relapsed compared to 20% in the low-score cohort. This
result was consistent with the GOG resistance criteria, defining
patient relapse within 6–12 months after the last chemotherapy as a
platinum-resistant case. However, there are several other issues to
be considered. The MT score highly depends on the condition of the
sampled tumor cells. Uncontrollable factors, such as the
vascularity of a tumor or the amount of necrosis, limit the
extrapolation of the MT scoring system to the clinical results.
Data incorporating these factors are not available, while these
applications must be considered as speculative at this time.
Although the MT scoring system has significant predictive value,
any potential role for MT scoring would also vary according to
tumor type, the goal of the chemotherapy (adjuvant vs. neoadjuvant
or salvage) and the roster of available chemotherapies to choose
from.
The number of cases in our study was small.
Carefully designed prospective studies with a larger sample and
with clinical follow-up are required to further investigate the
clinical relevance of this MT scoring system. However, the data
presented here provide evidence that the system is of considerable
value as a biomarker for chemosensitivity in OC.
In conclusion, this study showed that the MT scoring
system is closely correlated with clinical response as well as
cellular sensitivity to platinum, taxane and irinotecan, resulting
in the association with PFS.
Acknowledgements
The authors wish to thank Dr Esther
Oliva of Massachusetts General Hospital, Boston, MA, USA, for the
critical review of this manuscript.
References
|
1.
|
Armstrong D, Bundy B, Wenzel L, Huang H,
Baergen R, Lele S, Copeland L, Walker J and Burger R; for the
Gynecologic Oncology Group: Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Katsumata N, Yasuda M, Takahashi F,
Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura
E, Ochiai K and Noda K; for the Japanese Gynecologic Oncology
Group: Dose-dense paclitaxel once a week in combination with
carboplatin every 3 weeks for advanced ovarian cancer; a phase III,
open-label, randomized controlled trial. Lancet. 374:1331–1338.
2009. View Article : Google Scholar
|
|
3.
|
Saitou M, Isonishi S, Hamada T, Kiyokawa
T, Tachibana T, Ishikawa H and Yasuda M: Mitochondrial
ultrastructure associated chemotherapy response in ovarian cancer.
Oncol Rep. 21:199–204. 2008.PubMed/NCBI
|
|
4.
|
Hirama M, Isonishi S, Yasuda M and
Ishikawa H: Characterization of mitochondria in cisplatin-resistant
human ovarian carcinoma cells. Oncol Rep. 16:997–1002.
2006.PubMed/NCBI
|
|
5.
|
Isonishi S, Saitou M, Ochiai K, Yasuda M
and Tanaka T: Enhancement of sensitivity to cisplatin by orobol is
associated with increased mitochondrial cytochrome c release in
human ovarian carcinoma cells. Gynecol Oncol. 90:413–420. 2003.
View Article : Google Scholar
|
|
6.
|
Kim TS, Yun BY and Kim IY: Induction of
the mitochondrial permeability transition by selenium compounds
mediated by oxidation of the protein thiol groups and generation of
the superoxide. Biochem Pharmacol. 66:2301–2311. 2003. View Article : Google Scholar
|
|
7.
|
Chilin A, Dodoni G, Frezza C, Guiotto A,
Barbieri V, Di Lisa F and Canton M:
4-Hydroxymethyl-1,6,8-trimethylfuro [2,3-h] quinolin-2(1H)-one
induces mitochondrial dysfunction and apoptosis upon its
intracellular oxidation. J Med Chem. 48:192–199. 2005.
|
|
8.
|
Ferlini C, Raspaglio G, Mozzetti S,
Distefano M, Filippetti F, Martinelli E, Ferrandina G, Gallo D,
Ranelletti FO and Scambia G: Bcl-2 down-regulation is a novel
mechanism of paclitaxel resistance. Mol Pharmacol. 64:51–58. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shajahan AN, Wang A, Decker M, Minshall
RD, Liu MC and Clarke R: Caveolin-1 tyrosine phosphorylation
enhances paclitaxel-mediated cytotoxicity. J Biol Chem.
282:5934–5943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Flatters SJ and Bennett GJ: Studies of
peripheral sensory nerves in paclitaxel-induced painful peripheral
neuropathy: evidence for mitochondrial dysfunction. Pain.
122:245–257. 2006. View Article : Google Scholar
|
|
11.
|
Liu L, Vo A, Liu G and McKeehan WL:
Distinct structural domains within C19ORF5 support association with
stabilized microtubules and mitochondrial aggregation and genome
destruction. Cancer Res. 65:4191–4201. 2005. View Article : Google Scholar
|
|
12.
|
André N, Carré M, Brasseur G, Pourroy B,
Kovacic H, Briand C and Braguer D: Paclitaxel targets mitochondria
upstream of caspase activation in intact human neuroblastoma cells.
FEBS Lett. 532:256–260. 2002.PubMed/NCBI
|
|
13.
|
Matassa AA, Carpenter L, Biden TJ,
Humphries MJ and Reyland ME: PKC delta is required for
mitochondrial-dependent apoptosis in salivary epithelial cells. J
Biol Chem. 276:29719–29728. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Grivicich I, Regner A, da Rocha AB, Grass
LB, Alves PA, Kayser GB, Schwartsmann G and Henriques JA:
Irinotecan/5-fluorouracil combination induces alterations in
mitochondrial membrane potential and caspases on colon cancer cell
lines. Oncol Res. 5:385–392. 2005.
|
|
15.
|
Silverberg SG: Histopathologic grading of
ovarian carcinoma: a review and proposal. Intl J Gynecol Pathol.
19:7–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Malpica A, Deavers MT, Tornos C, Kurman
RJ, Soslow R, Seidman JD, Munsell MF, Gaertner E, Frishberg D and
Silva EG: Interobserver and intraobserver variability of a two-tier
system for grading ovarian serous carcinoma. Am J Surg Pathol.
31:1168–1174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Malpica A, Deavers MT, Lu K, Bodurka DC,
Atkinson EN, Gershenson DM and Silva EG: Grading ovarian serous
carcinoma using a two-tier system. Am J Surg Pathol. 28:496–504.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mc Cluggage WG, Lyness RW, Atkinson RJ,
Dobbs SP, Harley I, Mc Clelland HR and Price JH: Morphological
effects of chemotherapy on ovarian carcinoma. J Clin Pathol.
55:27–31. 2002.
|
|
19.
|
Benda JA and Zaino R: GOG Pathology
Manual. Gynecologic Oncology Group; Buffalo, NY: 1994
|
|
20.
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
21.
|
Rustin GJ: Use of CA-125 to assess
response to new agents in ovarian cancer trials. J Clin Oncol.
21:S187–S193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ishikawa T, Zhu BL and Maeda H: Effects of
therapeutic agents on cellular respiration as an indication of
metabolic activity. Hum Exp Toxicol. 25:135–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Amano Y, Okumura C, Yoshida M, Katayama H,
Unten S, Arai J, Tagawa T, Hoshina S and Ishikawa H: Measuring
respiration of cultured cell with oxygen electrode as a metabolic
indicator for drug screening. Human Cell. 12:3–10. 1999.PubMed/NCBI
|
|
24.
|
Disaida PJ, Sinkovics JG, Rutledge FN and
Smith JP: Cell-mediated immunity to human malignant cells. Am J
Obstet Gynecol. 114:979–989. 1972.PubMed/NCBI
|
|
25.
|
Andrews PA, Murphy MP and Howell SB:
Differential potentiation of alkylating agent cytotoxicity in human
ovarian carcinoma cells by glutathione depletion. Cancer Res.
45:6250–6253. 1985.
|
|
26.
|
Parmar MKB and Machin D: Survival
Analysis: A Practical Approach. John Wiley & Sons Inc.; New
York: pp. 160–177. 1995
|
|
27.
|
Andreotti PE, Cree IA, Kurbacher CM,
Hartmann DM, Linder D, Harel G, Gleiberman I, Caruso PA, Ricks SH,
Untch M, Sartori C and Bruckner HW: Chemosensitivity testing of
human tumors using a microplate adenosine triphosphate luminescence
assay. Clinical correlation for cisplatin resistance of ovarian
carcinoma. Cancer Res. 55:5276–5282. 1995.
|
|
28.
|
Brown E and Markman M: Tumor
chemosensitivity and chemoresistance assays. Cancer. 72:1020–1025.
1996. View Article : Google Scholar
|
|
29.
|
Tate Thigpen J: Debate; therapy for a
patient with platinum-resistant/refractory recurrent ovarian cancer
should be selected based on results of an in vitro drug
sensitivity/resistance assay. Clin Ovarian Cancer. 1:96–110.
2008.
|
|
30.
|
Markman M, Blessing JA, DeGeest K, Morgan
M, Look KY, Herzog TJ and Rose PG: Lack of efficacy of 24-hour
infusional topotecan in platinum-refractory ovarian cancer; a
Gynecologic Oncology Group trial. Gynecol Oncol. 75:444–446. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Brewer C, Blessing J, Nagourney R, Morgan
M and Hanjani P: Cisplatin plus gemcitabine in platinum-refractory
ovarian or primary peritoneal cancer; a phase II study of the
Gynecologic Oncology Group. Gynecol Oncol. 103:446–450. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Markman M, Blessing J, Rubin SC, Connor J,
Hanjani P and Waggoner S: Phase II trial of weekly paclitaxel (80
mg/m2) in platinum and paclitaxel-resistant ovarian and
primary peritoneal cancers; a Gynecologic Oncology Group study.
Gynecol Oncol. 101:436–440. 2006.PubMed/NCBI
|