Introduction
Breast conserving surgery has become the most
popular surgical procedure for primary breast cancer (1). The significance of extended resection
has become less important, since the long-term survival rate among
women who undergo breast-conserving surgery is the same as that
among women who undergo radical mastectomy (2). Thus, nowadays, local control is
expected to be minimally invasive on the basis that permanent
curability is estimated to be comparable. Various types of
non-surgical ablation have been introduced as a local control for
early breast cancer that also achieve cosmetic gains (3–7). A
new enzyme-targeting radiosensitization treatment containing
hydrogen peroxide and sodium hyaluronate for percutaneous
injection, Kochi Oxydol-Radiation Therapy for Unresectable
Carcinomas, type II (KORTUC II) (8), was recently developed. It markedly
enhances the radiotherapeutic effect of treatment for various types
of tumors that are not superficially exposed, such as breast cancer
and other types of soft tissue tumors (8). As precise assessment of therapeutic
efficacy by radiological imaging is essential for the success of
KORTUC II, contrast enhanced breast magnetic resonance imaging
(CE-breast MRI), ultrasonography (US) and
[18F]-fluorodeoxyglucose positron emission computed
tomography (FDG-PET-CT) were employed to assess therapeutic
outcomes. The aim of the present study was to report the
therapeutic outcome of KORTUC II for stage I breast cancer
precisely assessed by the aforementioned radiological imaging
modalities.
Materials and methods
KORTUC II radiosensitizer was used as a percutaneous
injection for breast cancer as approved by our local ethics
committee. Since hydrogen peroxide is an irritant and may cause
severe adverse effects, experimental studies were performed prior
to clinical applications in order to ascertain safety of the method
(9). In order to allow long-acting
radiosensitization of the local tumor tissue, sodium hyaluronate
was added to hydrogen peroxide in order to make the solution more
viscous and to slow the degradation of the hydrogen peroxide
(9).
Preparation of the radiosensitizing
agent
The radiosensitizing agent was composed of 0.83%
sodium hyaluronate and 0.5% hydrogen peroxide, and was prepared by
adding 0.5 ml of 3% hydrogen peroxide solution (Oxydol; Ken-ei
Pharmaceutical Co. Ltd., Osaka, Japan) to a commercially available
disposable syringe containing 2.5 ml of 1.0% sodium hyaluronate.
Hydrogen peroxide was added immediately before use in order to
avoid degradation of the sodium hyaluronate due to oxidation by
hydrogen peroxide.
Patient selection and radiotherapy
Fourteen female stage I (10) breast cancer patients (invasive
ductal carcinomas) were enrolled in the KORTUC II trial. Each
patient signed an informed consent form before participation in the
study. Patient data are summarized in Table I. Patients were eligible for the
study if they had stage I breast cancer and had either
contraindications to general anesthesia due to significant
comorbidity or had declined surgical and systemic chemotherapy
treatment.
 | Table I.Summary of the subject data. |
Table I.
Summary of the subject data.
| Case | Observation period
(months) | Age (years) | Assessed by
MRIa | Assessed by
USb | Flow signal on
color-Doppler USc | PS artifact on
USd |
|---|
| 1 | 29 | 67 | CR→CR | CR→CR | N→N | N→N |
| 2 | 48 | 77 | CR→CR | CR→CR | P→N | N→N |
| 3 | 38 | 79 | CR→CR | 27.3→45.5 | P→N | N→N |
| 4 | 38 | 76 | CR→CR | CR→CR | P→N | N→N |
| 5 | 32 | 71 | CR→CR | CR→CR | N→N | N→N |
| 6 | 20 | 74 | CR→CR | CR→CR | N→N | N→N |
| 7 | 19 | 79 | CR→CR | CR→CR | N→N | N→N |
| 8 | 16 | 89 | CR→CR | CR→CR | N→N | N→N |
| 9 | 16 | 64 | CR→CR | 53.8→CR | P→N | N→N |
| 10 | 13 | 43 | CR→CR | CR→CR | N→N | N→N |
| 11 | 11 | 62 | CR→CR | CR→CR | N→N | N→N |
| 12 | 11 | 61 | CR→CR | CR→CR | N→N | N→N |
| 13 | 7 | 78 | CR→NA | CR→CR | N→N | N→N |
| 14 | 4 | 51 | CR→NA | CR→NA | N→N | N→N |
For each patient, radiation therapy with high-energy
X-ray was delivered with an EXL-20TP linear accelerator equipped
with a multi-leaf collimator (Mitsubishi Electric Co. Ltd., Tokyo,
Japan) at an appropriate energy level (4 MV). Hypofraction
radiotherapy was administered using a tangential field approach;
the total dose was 44 Gy administered as a 2.75 Gy/fraction.
Radiation therapy was performed five times a week for each patient.
After the initiation of radiotherapy, an intratumoral injection of
KORTUC II was performed under ultrasonographic guidance twice a
week for 2 weeks, immediately prior to radiation therapy. A maximum
of 6 ml of the agent was injected at each session. Cone-down boost
irradiation was then delivered using an electron beam of
appropriate energy for each individual patient, and was
administered concurrently with a dose of 9 Gy in three fractions in
the last week of radiotherapy.
A risk category was assigned to each patient
according to the St. Gallen guidelines based on clinical tumor size
and the pathological results of a core needle biopsy taken before
therapy (11). Adjuvant systemic
chemotherapy was not administered to any patients: 12 of 14
patients were classified as low risk and, according to the St.
Gallen guidelines, chemotherapy is not recommended for low-risk
patients (11). However, 1 of the
2 subjects with intermediate risk (case 12 in Table I), for whom the St. Gallen
guidelines recommend the use of chemotherapy, declined systemic
chemotherapy with their fully informed consent (11). Another St. Gallen intermediate-risk
patient was too old to receive systemic chemotherapy (case 2 in
Table I).
Endocrine therapy
All patients with breast tumors positive for
hormonal receptor received endocrine therapy immediately after the
completion of radiotherapy. Tamoxifen (40 mg/day per os) or an
aromatase inhibitor (anastrozole 1 mg/day or exemestane 25 mg/day
per os) was used for pre-menopausal and post-menopausal patients,
respectively. Endocrine therapy was scheduled to continue for 5
years in all eligible patients.
Patient assessment (primary breast tumor
and toxicity of therapy)
Tumor response was assessed according to the RECIST
criteria (12) using CE-breast
MRI, FDG-PET-CT and US. Patients were assigned a toxicity grade
from a standard assessment scale (NIH common toxicity criteria).
Treatment-related complications were assessed in detail in order to
evaluate the feasibility of this approach. Posterior shadow
artifacts from each tumor on US and flow signal on color-Doppler US
were also assessed.
Each breast mass was scanned using a US unit
(LOGIQ700MR; GE Healthcare, Milwaukee, WI, USA) with a 7–11 MHz
linear-array transducer. CE-breast MRI was performed at 3.0 T
(Signa EXCITE HDx; GE Healthcare) with subjects in the prone
position. Dynamic MRI using a three-dimensional fast spoiled
gradient-echo sequence (VIBRANT, volume imaging for breast imaging;
TR 7.0 msec; TE 4.0 msec; flip angle 10°; FOV 36x36 cm; matrix
512×256; slice thickness 3 mm; increment 0 mm; NEX 0.7) was
obtained before and 8 times after (every 30 sec) a bolus injection
of 0.1 mmol/kg gadolinium-diethylenetriamine pentaacetic acid at a
rate of 3 ml/sec. Whole-body FDG-PET-CT scans were obtained on a
Discovery ST Elite PET-CT system (GE Healthcare) consisting of a
full ring dedicated PET and a 16-slice spiral CT. All patients were
instructed to fast for 6 h before receiving an intravenous
application of 3.5 MBq/kg FDG. Imaging was initiated ∼60 min after
the application of FDG. CT was acquired before PET with 50 mA/sec
at 130 kV without administration of a non-ionic contrast agent. All
images were reconstructed with a 5-mm slice thickness and a 3.7-mm
increment. After CT, a 3-D mode PET was performed. The PET emission
time per bed position was adapted to the patient body weight:
<65 kg, 2 min per bed position; 65–85 kg, 2.5 min; and >85
kg, 3 min. Any focally elevated PET signal above normal that could
be mapped to a tumor location was rated as positive for viable
breast cancer (13). The
interpreters of US (K.K.), CE-breast MRI (Y.M.) and FDG-PET-CT
(J.H.) were provided information regarding tumor location, but were
otherwise blinded to patient and therapy information.
Beginning and frequency of
observation
Assessment of the primary tumor started within 2–4
weeks of the completion of radiotherapy, regardless of the
endocrine therapy. CE-breast MRI and FDG-PET-CT were performed at
least once a year following the completion of radiotherapy. US and
a clinical examination were performed every 3 months. The mean
observation period was 21.6 months with a range of 4–48 months.
Results
Adverse events
All patients experienced mild local pain at the
injection site. For all 14 patients, radiation-induced dermatitis
was mild (grade I) and equivalent to dermatitis induced after
radiation therapy alone as described previously (8).
Assessment of primary breast tumors by
CE-breast MRI and FDG-PET-CT
Patient data are summarized in Table I. All patients were unable or
unwilling to undergo surgery, and therefore underwent non-surgical
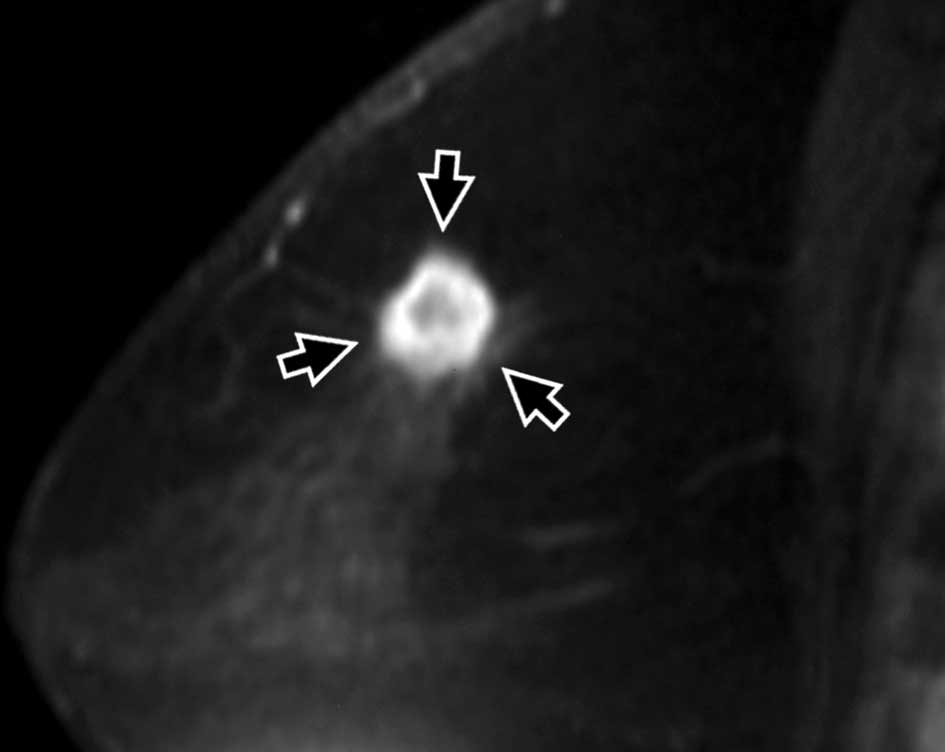
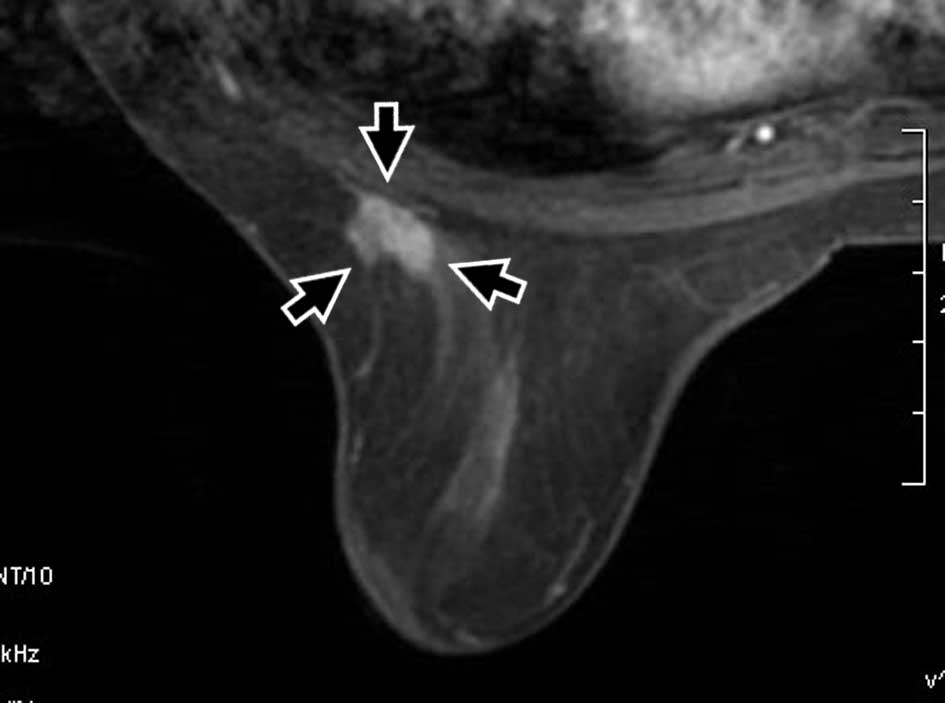
breast conservation therapy. All achieved a complete response (CR)
(Figs. 1 and 2). At the completion of the follow-up
period, none of the patients exhibited local recurrence. The
findings of CE-breast MRI did not differ from those of
FDG-PET-CT.
Assessment of primary breast tumors by
US
US depicted tumor-like findings in 2 of 14 patients
immediately after the completion of therapy (Fig. 1). CR was noted in 12 cases, partial
response (PR) in 1 case and stable disease (SD) in 1 case. One of
the tumor-like findings had disappeared by the end of follow-up
(case 9 in Table I). Another
tumor-like finding remained throughout the follow-up period (case 3
in Table I). No posterior shadow
artifacts appeared in any of the patients throughout the
observation period. Color Doppler-US depicted an intratumoral flow
signal in 4 of 14 tumors prior to therapy. This flow signal
disappeared from all patients after the completion of therapy
(Fig. 1). Absence of a flow signal
continued during the observation period.
Discussion
Breast cancer surgery has changed dramatically over
the past two decades. With the emergence of breast conserving
therapy, many breast cancer patients now have the option of
preserving a cosmetically acceptable breast without sacrificing
survival. In 1984, Dr William Halsted published a landmark paper
describing the outcome of the Halsted Radical Mastectomy (14). This procedure achieved improved
survival, and thus the Halsted Radical Mastectomy became the
standard care in breast cancer treatment. While survival from
breast cancer improved with the Halsted Radical Mastectomy, it was
clear that there was increased morbidity associated with this
technique.
In the mid-1970s, the National Study of the Adjuvant
Breast and Bowel Project (NSABP) published the results of the B-04
study, which demonstrated that there was no difference in survival
between a radical mastectomy vs. a modified radical mastectomy,
where the pectoralis muscles are preserved (15). Once the results of the NSABP B-04
landmark trial were reported, the surgical management of breast
cancer moved in a more conservative direction.
In the mid-1980s, the NSABP B-06 trial demonstrated
no difference in survival between mastectomy vs. lumpectomy
followed by radiation (16).
Recently, breast conserving surgery has become the most common
surgical procedure for breast cancer (1). However, breast conserving surgery
often degrades the cosmetic outcome to some degree. Therefore,
various types of minimally invasive options have been employed as
alternatives to surgical therapy, such as radiofrequency ablation
(RFA) (5,6), focused ultrasound ablation (FUS)
(3,4) and cryotherapy (7). These minimally invasive approaches
are currently being investigated. Although they obtain excellent
locoregional control (3–7), long-term control rates are unknown.
Moreover, RFA and cryotherapy demand insertion of a moderately
large needle into the breast (5–7).
General anesthesia is essential to carring out RFA (5,6), MRI
scanners to monitor the thermal distribution of FUS may be
prohibitively expensive (3,4), and
FUS takes too much time (3,4). It
is also important to note that these non-surgical approaches to
therapy require adjuvant radiation to non-ablated tissue in order
to exterminate residual cancerous tissue (3–7).
KORTUC II, radiation therapy intensified with radiosensitizer, is a
logical technique for the ablation of micro-cancerous nests in the
whole breast. KORTUC II has an advantage over other non-surgical
ablation therapies, as it treats the whole breast at once. General
anesthesia, insertion of a large needle and expensive equipment to
monitor thermal distribution are unnecessary with KORTUC II.
Currently, most radiation therapy for breast cancer
is performed using X-rays or high-energy electron beams from a
linear accelerator (17,18). However, these forms of low-linear
energy transfer (LET) radiation are not ideal for radiation therapy
when compared to high-LET radiation. To overcome the disadvantages
of these low-LET beams, KORTUC II, a new radiosensitizer containing
hydrogen peroxide and sodium hyaluronate for injection into the
tumor, was developed. Theoretically, KORTUC II inactivates
anti-oxidative enzymes, produces oxygen in tumor tissue and
converts a radioresistant tumor into a radiosensitive one. The
favorable efficacy of KORTUC II has been reported in vivo
and in preliminary clinical trials (8,9,19–22).
The favorable therapeutic efficacy for stage I breast cancer in the
present study suggests that KORTUC II is a powerful non-surgical
therapeutic option for the treatment of stage I breast cancer. In
the late 1960s and early 1970s, several studies investigated the
use of hydrogen peroxide in radiotherapy, but this line of
investigation appears to have been discontinued (23,24).
In the present study, sodium hyaluronate, ordinarily used for
intra-articular injection in chronic knee joint disorders, was
combined with hydrogen peroxide in order to preserve oxygen
concentration in tumor tissue for >24 h, and intratumoral
injections of hydrogen peroxide alone resulted in a rapid lowering
of oxygen concentration (unpublished data). The success of the
present study may provide a reason to renew investigations into the
use of hydrogen peroxide as a radiosensitizer.
Furthermore, worldwide advances in systemic therapy
for breast cancer are compatible with KORTUC II. Adjuvant endocrine
therapy, such as tamoxifen and aromatase inhibitors, increases the
survival rate and is an acceptable option when patients have
hormone receptor-positive breast cancer (25). The St. Gallen guidelines recommend
adjuvant endocrine therapy alone to low-risk patients (11), a group to which almost all of the
patients in our study population belonged. However, 2 patients in
this study were rated as intermediate risk, for which the St.
Gallen guidelines recommend administration of systemic adjuvant
chemotherapy (11). One of the
intermediate risk patients in the present study was too old for
systemic chemotherapy and the other patient, though suitable for
systemic chemotherapy, refused it. Although, systemic adjuvant
chemotherapy prevents cancer recurrence and improves survival
(17,18,26,27),
patient preference for adjuvant therapy could feasibly eliminate
the use of systemic chemotherapy (28). Patient preference may become the
determinant for whether or not systemic chemotherapy is appropriate
for intermediate risk stage I breast cancer patients, because of
the balance between significant toxicities and benefit (11).
Precise assessment of therapeutic efficacy is
important to gage the outcome of clinical trials. CE-breast MRI
obtains over 95% sensitivity in the detection of breast cancer
through enhancement of the lesion with gadolinium-based contrast
material (29,30), and accurately reveals the tumor
extent regardless of prior neoadjuvant chemotherapy (31–33).
US has been reported to be more reliable for the detection and
measurement of breast tumors than mammography, particularly in case
of dense breast tissue (34–36).
FDG-PET-CT is a reliable modality for the detection of primary
breast tumors (37,38). Therefore, this study employed MRI,
FDG-PET-CT and US as diagnostic tools for the precise assessment of
the therapeutic effects of KORTUC II for primary breast tumors. US
depicted tumor-like findings in 2 cases of CR as detected by
CE-breast MRI and FDG-PET-CT. To the best of our knowledge, the
diagnostic ability of FDG-PET-CT and US to detect primary breast
tumors has not been compared. However, CE-breast MRI obtains
equivalent to superior detection rates for bulky breast mass
compared to US (36,39,40).
Moreover, CE-breast MRI has been reported to have higher
sensitivity in the detection of small lesions (including
intraductal spread) compared to US (39,40).
Therefore, based on these US and MRI characteristics, the
tumor-like US findings after therapy were probably scar tissue.
Fibrous tissue in scar tissue develops after exposure to radiation
(41,42) and causes ultrasound attenuation and
a posterior shadow artifact (43).
However, none of the patients in the present study had posterior
shadow artifacts either before or after therapy, leading to the
conclusion that these stage I breast tumors and scars resulting
from KORTUC II therapy were too small to produce these types of
artifacts. In addition, the absence of a flow signal on the color
Doppler-US after therapy supports the possibility that the
tumor-like findings are scar tissue (44). The present results suggest that
tumor-like findings on US after therapy do not necessarily indicate
tumor recurrence. Consequently, a CR on CE-breast MRI and on
FDG-PET-CT was considered to be a reliable indicator of treatment
efficacy.
In conclusion, based on these successful therapeutic
outcomes, KORTUC II has a strong potential as a non-surgical
therapy approach for stage I breast cancer. Radiological imaging
modalities, including CE-breast MRI, US and FDG-PET-CT, can be used
to monitor therapeutic effects, and the combination of these
modalities is recommended to determine the success of this therapy.
However, further investigation is required to confirm the long-term
outcome of this new approach to stage I breast cancer therapy.
References
|
1.
|
Sonoo H and Noguchi S: Results of
questionnaire survey on breast cancer surgery in Japan 2004–2006.
Breast Cancer. 15:3–4. 2008.
|
|
2.
|
Veronesi U, Cascinelli N, Mariani L, Greco
M, Saccozzi R, Luini A, Aguilar M and Marubini E: Twenty-year
follow-up of a randomized study comparing breast-conserving surgery
with radical mastectomy for early breast cancer. N Engl J Med.
347:1227–1232. 2002.PubMed/NCBI
|
|
3.
|
Schmitz AC, Gianfelice D, Daniel BL, Mali
WP and van den Bosch MA: Image-guided focused ultrasound ablation
of breast cancer: current status, challenges, and future
directions. Eur Radiol. 18:1431–1441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Jolesz FA: MRI-guided focused ultrasound
surgery. Annu Rev Med. 60:417–430. 2009. View Article : Google Scholar
|
|
5.
|
Manenti G, Bolacchi F, Perretta T, Cossu
E, Pistolese CA, Buonomo OC, Bonanno E, Orlandi A and Simonetti G:
Small breast cancers: in vivo percutaneous US-guided radiofrequency
ablation with dedicated cool-tip radiofrequency system. Radiology.
251:339–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kinoshita T, Iwamoto E, Tsuda H and Seki
K: Radiofrequency ablation as local therapy for early breast
carcinomas. Breast Cancer. Jan 14–2010.(E-pub ahead of print).
|
|
7.
|
Littrup PJ, Jallad B, Chandiwala-Mody P,
D'Agostini M, Adam BA and Bouwman D: Cryotherapy for breast cancer:
a feasibility study without excision. J Vasc Interv Radiol.
20:1329–1341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ogawa Y, Kubota K, Ue H, Kataoka Y,
Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J,
Hamada N, Kariya S, Fukumoto M, Nishioka A and Inomata T: Phase I
study of a new radiosensitizer containing hydrogen peroxide and
sodium hyaluronate for topical tumor injection: a new
enzyme-targeting radiosensitization treatment, Kochi
Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II
(KORTUC II). Int J Oncol. 34:609–618. 2009. View Article : Google Scholar
|
|
9.
|
Ogawa Y, Kubota K, Ue H, et al:
Development and clinical application of a new radiosensitizer
containing hydrogen peroxide and hyaluronic acid sodium for topical
tumor injection – a new enzyme-targeting radiosensitization
treatment, KORTUC II (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas Type II). Strahlenther Onkol. 183:100–101.
2007.PubMed/NCBI
|
|
10.
|
UICC. TNM Classification of Malignant
Tumors. 6th edition. Wiley-Liss; New York: pp. 2002
|
|
11.
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: 10th St. Gallen conference. Progress
and promise: highlights of the international expert consensus on
the primary therapy of early breast cancer 2007. Ann Oncol.
18:1133–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
13.
|
Tatsumi M, Cohade C, Mourtzikos KA,
Fishman EK and Wahl RL: Initial experience with FDG-PET/CT in the
evaluation of breast cancer. Eur J Nucl Med Mol Imaging.
33:254–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Halsted WS: The results of operations for
the cure of cancer of the breast performed at the Johns Hopkins
Hospital from June, 1889, to January, 1894. Ann Surg. 20:497–555.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fisher B, Jeong JH, Anderson S, Bryant J,
Fisher ER and Wolmark N: Twenty-five-year follow-up of a randomized
trial comparing radical mastectomy, total mastectomy, and total
mastectomy followed by irradiation. N Engl J Med. 347:567–575.
2002.PubMed/NCBI
|
|
16.
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241.
2002.PubMed/NCBI
|
|
17.
|
Ogawa Y, Nishioka A, Inomata T, Ohnishi T,
Kariya S, Terashima M, Yoshida S, Tohchika N, Tanaka Y and Kumon M:
Conservation treatment intensified with an anti-estrogen agent and
CAF chemotherapy for stage I and II breast cancer. Oncol Rep.
7:479–484. 2000.PubMed/NCBI
|
|
18.
|
Ogawa Y, Nishioka A, Inomata T, Yokota N,
Sasaki T, Terashima M, Yoshida S, Tanaka Y and Tohchika N:
Conservation treatment intensified with tamoxifen and CAF
chemotherapy without axillary dissection for early breast cancer
patients with clinically-negative axillary nodes. Oncol Rep.
6:801–805. 1999.
|
|
19.
|
Ogawa Y, Takahashi T, Kobayashi T, Kariya
S, Nishioka A, Mizobuchi H, Noguchi M, Hamasato S, Tani T, Seguchi
H, Yoshida S and Sonobe H: Mechanism of apoptotic resistance of
human osteosarcoma cell line, HS-Os-1, against irradiation. Int J
Mol Med. 12:453–458. 2003.PubMed/NCBI
|
|
20.
|
Ogawa Y, Takahashi T, Kobayashi T, Kariya
S, Nishioka A, Ohnishi T, Saibara T, Hamasato S, Tani T, Seguchi H,
Yoshida S and Sonobe H: Apoptotic-resistance of the human
osteosarcoma cell line HS-Os-1 to irradiation is converted to
apoptotic-susceptibility by hydrogen peroxide: a potent role of
hydrogen peroxide as a new radiosensitizer. Int J Mol Med.
12:845–850. 2003.PubMed/NCBI
|
|
21.
|
Ogawa Y, Takahashi T, Kobayashi T, Kariya
S, Nishioka A, Hamasato S, Moriki T, Seguchi H, Yoshida S and
Sonobe H: Immunocytochemical characteristics of human osteosarcoma
cell line HS-Os-1: possible implication in apoptotic resistance
against irradiation. Int J Mol Med. 14:397–403. 2004.
|
|
22.
|
Ogawa Y, Ue H, Tsuzuki K, Tadokoro M,
Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J,
Nishioka A, Nakajima K, Ikeda M, Sano S and Inomata T: New
radiosensitization treatment (KORTUC I) using hydrogen peroxide
solution-soaked gauze bolus for unresectable and superficially
exposed neoplasms. Oncol Rep. 19:1389–1394. 2008.
|
|
23.
|
Chasin WD, Gross CC, Wang CC and Miller D:
Hydrogen peroxide and irradiation of tumors. Arch Otolaryngol.
85:151–155. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bianchini G, Salgarello G, Mennini T and
Lorini G: Intra-arterial infusion of hydrogen peroxide in
radiotherapy of malignant tumors. Radiol Med. 55:207–225.
1969.PubMed/NCBI
|
|
25.
|
Coates AS, Keshaviah A, Thurlimann B, et
al: Five years of letrozole compared with tamoxifen as initial
adjuvant therapy for postmenopausal women with endocrine-responsive
early breast cancer: update of study BIG 1–98. J Clin Oncol.
25:486–492. 2007.PubMed/NCBI
|
|
26.
|
Fisher B, Brown AM, Dimitrov NV, et al:
Two months of doxorubicin-cyclophosphamide with and without
interval reinduction therapy compared with 6 months of
cyclophosphamide, methotrexate, and fluorouracil in positive-node
breast cancer patients with tamoxifen-nonresponsive tumors: results
from the National Surgical Adjuvant Breast and Bowel Project B-15.
J Clin Oncol. 8:1483–1496. 1990.
|
|
27.
|
Fisher B, Anderson S, Tan-Chiu E, Wolmark
N, Wickerham DL, Fisher ER, Dimitrov NV, Atkins JN, Abramson N,
Merajver S, Romond EH, Kardinal CG, Shibata HR, Margolese RG and
Farrar WB: Tamoxifen and chemotherapy for axillary node-negative,
estrogen receptor-negative breast cancer: findings from National
Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol.
19:931–942. 2001.
|
|
28.
|
Jansen SJ, Otten W and Stiggelbout AM:
Review of determinants of patients' preferences for adjuvant
therapy in cancer. J Clin Oncol. 22:3181–3190. 2004.
|
|
29.
|
Esserman L, Hylton N, George T and Weidner
N: Contrast-enhanced magnetic resonance imaging to assess tumor
histopathology and angiogenesis in breast carcinoma. Breast J.
5:13–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Esserman L, Hylton N, Yassa L, Barclay J,
Frankel S and Sickles E: Utility of magnetic resonance imaging in
the management of breast cancer: evidence for improved preoperative
staging. J Clin Oncol. 171:110–119. 1999.PubMed/NCBI
|
|
31.
|
Tsuboi N, Ogawa Y, Inomata T, Yoshida D,
Yoshida S, Moriki T and Kumon M: Changes in the findings of dynamic
MRI by preoperative CAF chemotherapy for patients with breast
cancer of stage II and III: Pathologic correlation. Oncol Rep.
6:727–732. 1999.PubMed/NCBI
|
|
32.
|
Kubota K, Ogawa Y, Nishioka A, Kariya S,
Itoh S, Murata Y, Hamada N, Maeda H and Tanaka Y: Diagnostic
accuracy of mammography, ultrasonography and magnetic resonance
imaging in the detection of intraductal spread of breast cancer
following neoadjuvant chemotherapy. Oncol Rep. 17:915–918.
2007.
|
|
33.
|
Nakamura S, Kenjo H, Nishi T, Kazama T,
Doi O and Suzuki K: Efficacy of 3D-MR mammography for breast
conserving surgery after neoadjuvant chemotherapy. Breast Cancer.
9:15–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Leconte I, Feger C, Galant C, Berliere M,
Berg BV, D'Hoore W and Maldague B: Mammography and subsequent
whole-breast sonography of nonpalpable breast cancers: the
importance of radiologic breast density. AJR. 180:1675–1679. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Yang WT, Lam WW, Cheung H, Suen M, King WW
and Metreweli C: Sonographic, magnetic resonance imaging, and
mammographic assessments of preoperative size of breast cancer. J
Ultrasound Med. 16:791–797. 1997.PubMed/NCBI
|
|
36.
|
Berg WA, Gutierrez L, NessAiver MS, Carter
WB, Bhargavan M, Lewis RS and Ioffe OB: Diagnostic accuracy of
mammography, clinical examination, US, and MR imaging in
preoperative assessment of breast cancer. Radiology. 233:830–849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Adler LP, Crowe JP, Al-Kaisi NH and
Sunshine JL: Evaluation of breast masses and axillary lymph nodes
with [F-18] 2-deoxy-2-fluoro-D-glucose PET. Radiology. 187:743–750.
1993.
|
|
38.
|
Avril N, Dose J, Jänicke F, Bense S,
Ziegler S, Laubenbacher C, Römer W, Pache H, Herz M, Allgayer B,
Nathrath W, Graeff H and Schwaiger M: Metabolic characterization of
breast tumors with positron emission tomography using F-18
fluorodeoxyglucose. J Clin Oncol. 14:1848–1857. 1996.PubMed/NCBI
|
|
39.
|
Hata T, Takahashi H, Watanabe K, Takahashi
M, Taguchi K, Itoh T and Todo S: Magnetic resonance imaging for
preoperative evaluation of breast cancer: a comparative study with
mammography and ultrasonography. J Am Coll Surg. 198:190–197. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Van Goethem M, Schelfout K, Dijckmans L,
van Der Auwera C, Weyler J, Verslegers I, Biltjes I and De Schepper
A: MR mammography in the pre-operative staging of breast cancer in
patients with dense breast tissue: comparison with mammography and
ultrasound. Eur Radiol. 14:809–816. 2004.PubMed/NCBI
|
|
41.
|
Gottlober P, Kerscher MJ, Korting HC and
Peter RU: Sonographic determination of cutaneous and subcutaneous
fibrosis after accidental exposure to ionising radiation in the
course of the Chernobyl nuclear power plant accident. Ultrasound
Med Biol. 14:9–13. 1997. View Article : Google Scholar
|
|
42.
|
Adams MJ, Lipshultz SE, Schwartz C,
Fajardo LF, Coen V and Constine LS: Radiation-associated
cardiovascular disease: manifestations and management. Semin Radiat
Oncol. 13:346–356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Jones JP and Leeman S: Ultrasonic tissue
characterization. Acta Electronica. 26:3–31. 1984.
|
|
44.
|
Baz E, Madjar H, Reuss C, Vetter M,
Hackeloer B and Holz K: The role of enhanced Doppler ultrasound in
differentiation of benign vs. malignant scar lesion after breast
surgery for malignancy. Ultrasound Obstet Gynecol. 15:377–382.
2000. View Article : Google Scholar : PubMed/NCBI
|