Introduction
Follicular lymphoma (FL) is the most common indolent
B-cell lymphoma. It is generally associated with translocation t
(14; 18) (q32;q21), which participates in tumour cell survival
through overexpression of the anti-apoptotic Bcl2 protein (1,2).
Numerous treatments have been proposed, from delayed therapy after
a watch-and-wait period to autologous transplantation or immune
therapy, from allogenic transplantation to vaccination or the use
of anti-CD20 monoclonal antibodies, with or without chemotherapy.
To date, only clinical prognostic classification, such as the
Follicular Lymphoma International Index (FLIPI), is used to guide
treatment (3).
Accumulation of genomic alterations and clonal
selection account for subsequent FL progression and transformation
(4). However, a role of the FL
cell microenvironment in determining clinical behaviour and disease
prognosis has also recently been substantiated (5–8). In
this cancer, tumour cells reside and proliferate in follicular
structures in close association with helper T cells and follicular
dendritic cells (7,9). Biological analyses have been
conducted to determine the exact role of these different cellular
subpopulations. Staudt and Dave (10) and Dave et al (11) initially used gene expression
profiling and observed a major role of T-lymphocytes and
macrophages, while defining two prognostic subgroups.
Tumours induce immunologic tolerance by several
mechanisms involving tolerogenic antigen-presenting cells,
Foxp3+CD4+CD25+ regulatory T cells
(Tregs). An increased frequency of Tregs has been noted in the
peripheral blood of patients with bronchial carcinoma as compared
to healthy individuals (12), and
similar findings were also reported in patients with a variety of
cancer types. Tregs that are found within the tumour
microenvironment are highly suppressive and abrogate the effector
function of cytotoxic T cells as well as NK cell-mediated
cytotoxicity (13).
In follicular lymphoma, FOXP3+ T cells
were first suggested to be associated with poor survival (14). Then conflicting results were
published indicating the positive impacts of the
CD4+CD25+FOXP3+ phenotype
(15–17). In addition, CD8 and CD4 T cells
were mentioned as being key cells in the progression and/or
transformation process (18,19),
and mast cells were found to be associated with a poor outcome
(20). Some studies have outlined
the unfavourable outcome of FL associated with high numbers of
tumour-associated macrophages, with or without rituximab treatment
(21–23)
We thus conducted immunohistochemical analyses to
investigate the presence of CD8+ T cells,
FOXP3+ regulatory T cells and macrophages in follicular
lymphoma, while focusing particularly on their intra- vs.
interfollicular localisation. To obtain a more dynamic picture of
the immune response, we correlated the CD8+/Treg ratio
in these two compartments with clinical parameters and outcome.
Materials and methods
Patients and samples (Table I)
Fifty-eight patients consecutively diagnosed at a
single institution between 1990 and 2005 were included in the
present study. All cases were reviewed according to criteria of the
WHO Classification (24). We
observed 39 grade 1 (67%), 10 grade 2 (17%) and 9 grade 3 (16%)
tumours. The median age of the patients was 58 years (interquartile
range, 48–69 years), and the male/female ratio was 32/26. Advanced
stage (Ann Arbor IV) lymphomas were noted in 14 cases (24%). The
population distribution according to FLIPI was as follows: 21 low
risk cases (36%), 26 intermediate risk cases (45%) and 11 high risk
cases (19%).
All patients were treated with combination
chemotherapy without rituximab. Thirty-two patients were in
complete remission (CR) and two in partial remission (PR).
Twenty-four patients died after a median follow-up of 2 years
(range 1–9). The median survival was 8.5 years. Fifteen samples
were identified from patients whose survival was less than 5 years,
and 14 samples were identified from patients whose survival was
more than 10 years after diagnosis. In addition to the diagnostic
tissue samples, specimens at relapse were available for 26
patients. Ten reactive lymph nodes were used as controls.
Immunohistochemistry
Tissue samples were formalin-fixed and
paraffin-embedded. Sections (4-μm) were dewaxed and blocked in a
hydrogen peroxide/methanol solution. Antigen retrieval was
performed by heating the slides in EDTA (Sigma) (diluted 1/10, pH
7.0) at 100°C. All immunohistochemical staining was carried out
manually using anti-CD8 (clone C8/144B, Dako; dilution 1/25),
anti-CD68 (KP1, Dako; dilution 1/500) and anti-FoxP3 (236A/E7,
CliniSciences; dilution 1/150). Detection was performed with the
Dako Real™ detection system, using peroxidase/DAB+ (ref. 5001).
CD8, CD68 and FOXP3-positive cells were counted in
20 HPF and expressed as the number of cells/mm2.
Positive infiltrating cells were counted independently in
perifollicular and follicular (germinal centre and mantle zone)
compartments. A minimum of six different follicular and
interfollicular areas were selected in tumours to minimize the
heterogeneous distribution of positive cells.
Double-labelling immunohistochemistry for CD8 and
Foxp3 was performed in selected cases using the Benchmark XT
automated stainer. After pretreatment as described above, the
sections were exposed to CD8 (clone C8/144B, Dako, at a 1:25
dilution). The ultraView Universal DAB detection system was used
with DAB chromogen. The sections were then processed for the Foxp3
antibody at a 1:100 dilution with the alkaline phosphatase-based
ultraView Red detection system and Fast Red/Naphthol chromogen.
Statistical analysis
The population characteristics were described with
proportions for categorical variables and median and interquartile
values for continuous unusually distributed variables (Shapiro-Wilk
statistics). The immunochemical results were compared according to
the clinical features using the Mann-Whitney U test. The
Kaplan-Meier method was used to provide estimates of probability of
survival. The following period was defined as time from diagnosis
to time of last follow-up or death. The survival and potential
prognostic factors were compared with the log rank test after
conversion of the immunochemical variables into dichotomous
variables using the median value. In all of the statistical
analyses, p<0.05 was considered significant. All statistical
analyses were performed using SAS software (SAS Institute, Cary,
NC, USA).
Results
Immunohisochemistry (IHC) results
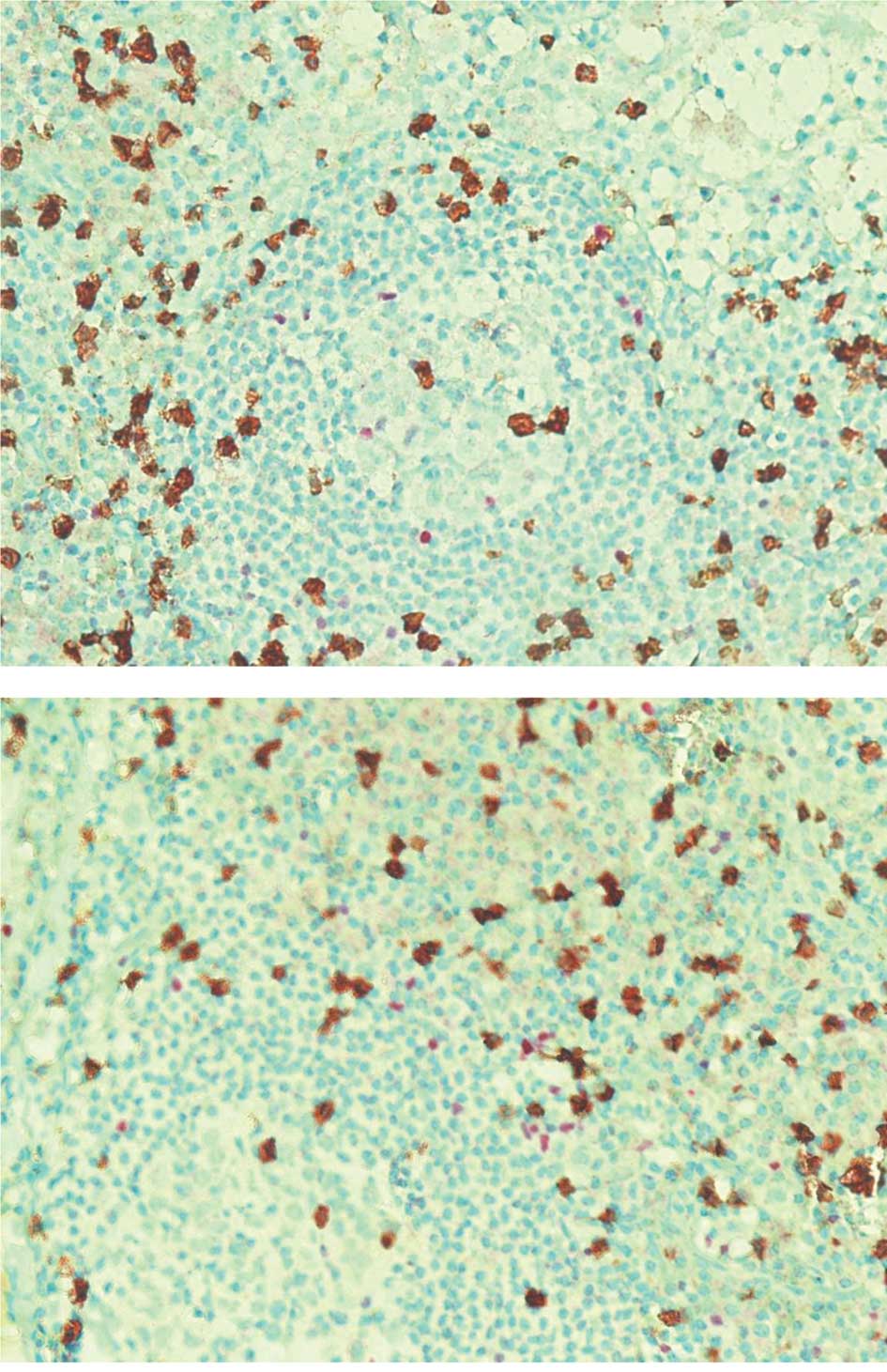
In FL at diagnosis, CD8, POXP3 and CD68-positive
cells were more numerous in interfollicular than in follicular
locations: 155.35, 86 and 96.2/mm2 compared with 33.5,
39.35 and 39.25/mm2, respectively (Table II). An example is illustrated in
Fig. 1. The CD8/FOXP3 median ratio
was 1.66 in interfollicular locations compared with 1.04 in
follicular locations.
 | Table II.CD8, FOXP3 and CD68 immunostaining in
58 FL patients and 10 reactive lymph nodes. |
Table II.
CD8, FOXP3 and CD68 immunostaining in
58 FL patients and 10 reactive lymph nodes.
| Location | CD8+
cells/mm2 median (IQ 25–75) | FOXP3+
cells/mm2 median (IQ 25–75) | CD68+
cells/mm2 median (IQ 25–75) | Ratio
CD8/FOXP3 |
|---|
| FL patients | Follicular | 33.50
(20.4–61.0) | 39.35
(23.6–55.8) | 39.25
(29.4–54.3) | 1.04 |
|
Interfollicular | 155.35
(118.8–359.2) | 86.00
(64.0–164.0) | 96.20
(78.2–130.8) | 1.66 |
| Reactive lymph
nodes | Follicular | 25 | 8 | 14 | 2.49 |
|
Interfollicular | 411 | 150 | 235 | 4.07 |
In reactive lymph nodes, the CD8/FOXP3 median ratio
was higher than that in FL, both in interfollicular (4.07 vs. 1.66)
and follicular locations (2.49 vs. 1.04). (Table II).
Correlations between IHC results and
clinical features
The interfollicular and follicular CD8/Treg ratios
were compared with the main clinical features of the patients at
diagnosis. The interfollicular CD8/FOXP3+ cell ratio was
significantly higher in patients with grade 3 tumours (2.04 vs.
1.63) and with a high risk FLIPI index (2.99 vs. 1.53) compared
with those with grade 1–2 tumours or a low-intermediate FLIPI
index. The same results were obtained with the follicular
CD8+/FOXP3+ cell ratio (Table III). We did not note any
significant relationship with stage or patient age.
 | Table III.Relationship between
CD8/FOXP3+ cell ratio and clinical features. |
Table III.
Relationship between
CD8/FOXP3+ cell ratio and clinical features.
| Expressed in median
(IQ 25–75) | Grade 1 and 2
(n=49) | Grade 3 (n=9) | P-value |
|
| Follicular
CD8/FOXP3+ cell ratio | 0.886
(0.47–1.54) | 3.463
(1.66–4.01) | 0.002 |
| Interfollicular
CD8/FOXP3+ cell ratio | 1.634
(1.02–2.29) | 2.048
(1.65–3.66) | 0.050 |
|
| FLIPI low and
intermediate risk (n= 47) | FLIPI high risk
(n=11) | |
|
| Follicular
CD8/FOXP3+ cell ratio | 0.916
(0.48–1.57) | 2.091
(1.08–8.04) | 0.020 |
| Interfollicular
CD8/FOXP3+ cell ratio | 1.536
(1.02–2.01) | 2.993
(1.762–8.676) | 0.001 |
Correlations between IHC results and
outcome
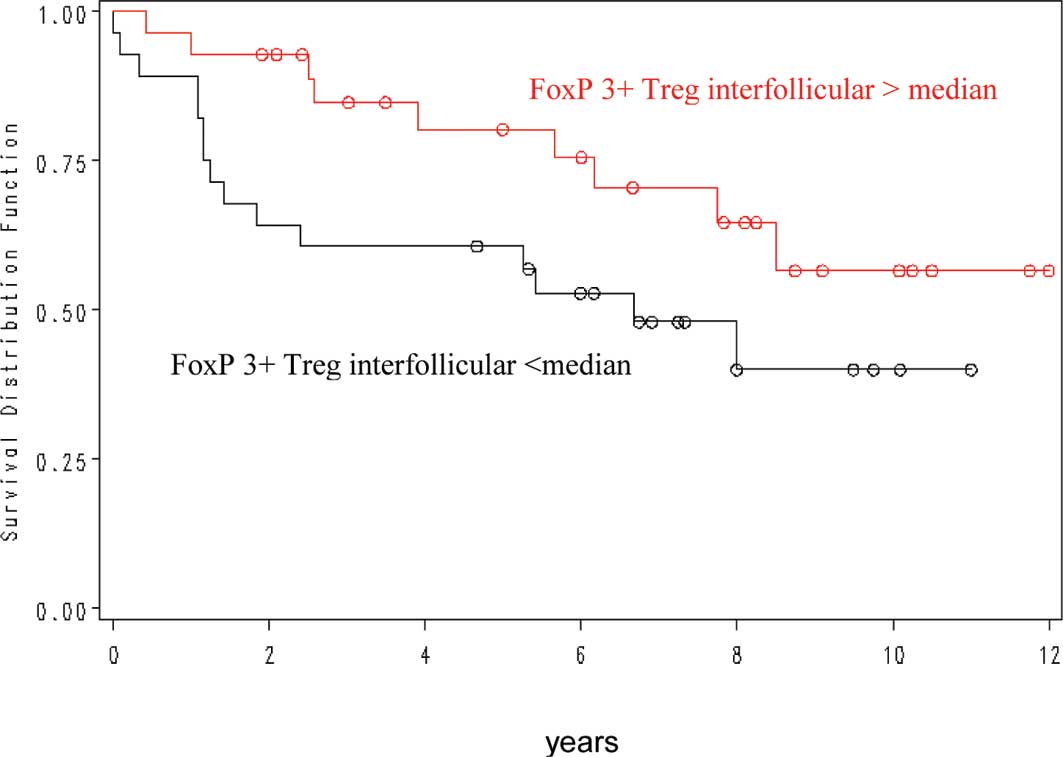
Concerning the interfollicular FOXP3+
cell number, more than 86 cells/mm2 were correlated with
a more favourable outcome (p=0.03) (Fig. 2). In contrast, intrafollicular
FOXP3, intrafollicular and interfollicular CD8 and CD68 cell
numbers were not predictive of patient outcome.
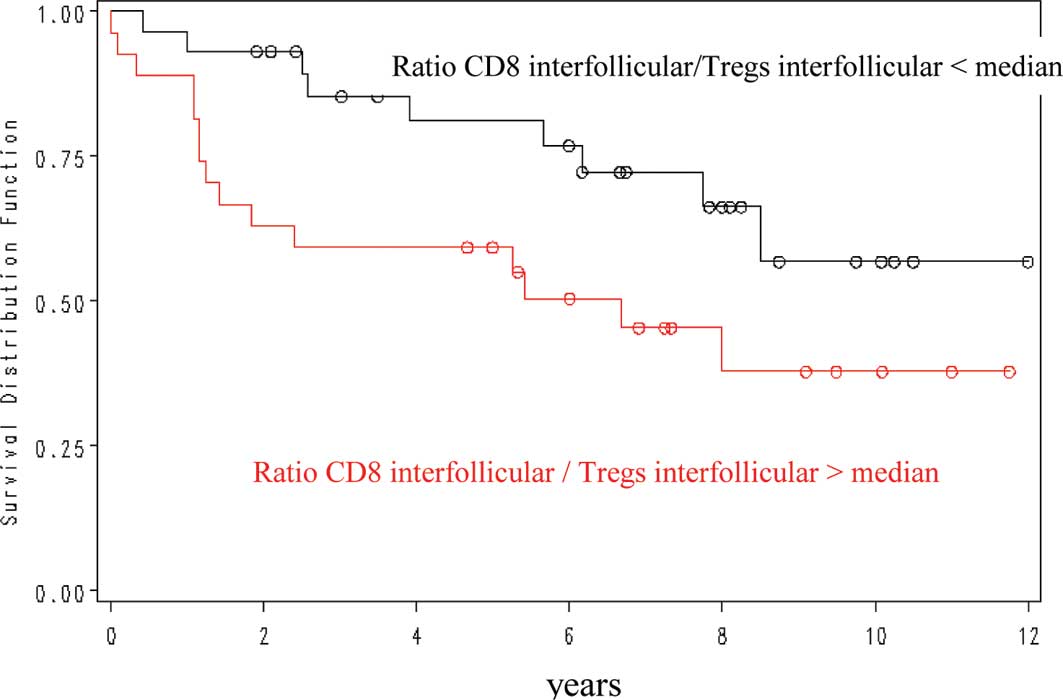
The interfollicular CD8/FOXP3 ratio showed a
positive prognostic value for overall survival, with a 5-year OS of
82 vs. 59% for a ratio of less or more than 1.68 (Fig. 3). Other ratios were not
significantly correlated with outcome.
CD8, CD68 and FOXP3-positive cells in FL
at first relapse compared with at diagnosis
When comparing these two groups, we observed a
significant difference between the number of CD68 cells in
intrafollicular (39.23 at diagnosis vs. 32 at relapse, p=0.07) and
interfollicular locations (96 at diagnosis vs. 62 at relapse,
p=0.06). The CD8/FOXP3 ratio in interfollicular locations was
significantly different (1.66 at diagnosis vs. 2.2 at relapse,
p=0.05) (Table IV).
 | Table IV.CD68, CD8 and POXP3-positive cells at
first relapse compared with at diagnosis. |
Table IV.
CD68, CD8 and POXP3-positive cells at
first relapse compared with at diagnosis.
| Expressed in median
(IQ 25–75) | Diagnosis
(n=58) | Relapse (n=26) | P-value |
|---|
| Follicular
CD68+ cells/mm2 | 39 (29.4–54.3) | 32 (20.5–46.8) | 0.007 |
| Interfollicular
CD68+ cells/mm2 | 96
(86.2–112.8) | 62 (54.1–73.2) | 0.005 |
| Interfollicular
CD8/FOXP3+ cell ratio | 1.66
(1.07–2.26) | 2.2
(1.32–3.87) | 0.050 |
Discussion
The present study of 58 diagnostic lymph node
samples from FL patients showed that the interfollicular
CD8/FOXP3+ cell ratio was significantly higher in
patients with grade 3 tumours (2.04 vs. 1.63) and with a high risk
FLIPI index (2.99 vs. 1.53) compared with those with grade 1–2
tumours or a low-intermediate FLIPI index. We obtained the same
results with the follicular CD8+/FOXP3+ cell
ratio. Moreover, the inter-follicular CD8/FOXP3 ratio was found to
have a prognostic value for overall survival, with a 5-year OS of
82 vs. 59% for a ratio of less or more than 1.68. An
interfollicular FOXP3+ cell number of more than 86
cells/mm2 was correlated with a more favourable outcome
(p=0.03).
These results are in accordance with the findings of
two recent studies in which a perifollicular pattern of
FOXP3-positive cells was more commonly found in the diagnosis of FL
lymph nodes from patients whose subsequent outcome was favourable
(15,17). Tzankov et al reported a
favourable prognostic influence of an increased amount of
FOXP3+ cells in FL, cHL and in GC-like diffuse large
B-cell lymphomas, and defined a cutoff value of 88.6
FOXP3+ cells/mm2 in a ROC analysis (16). These authors did not take the
interfollicular vs. follicular location into account. In our study,
an interfollicular but not follicular FOXP3+ cell number
of more than 86 cells/mm2 was correlated with a more
favourable outcome (p=0.03). Recent studies have shown that tumour
B cells alone induce conventional T cells to express FOXP3 and
acquire a regulatory function (25). The presence of FOXP3-positive cells
is also a protective variable in classical Hodgkin’s disease in
which an inverse relationship between TIA-1 and FOXP3 expression
and survival has been demonstrated. Moreover, higher levels of
FOXP3 twinned with low TIA-1 were beneficial (16,26,27).
In a recent study, De Jong et al showed that the presence of
CD4/FOXP3+ cells is beneficial to the host, irrespective
of the treatment used (28).
When comparing the two groups at diagnosis and
relapse, we observed a significant difference between the CD8/FOXP3
ratio in interfollicular locations (1.66 at diagnosis vs. 2.2 at
relapse, p=0.05). In the Tzankov study, a 2.7-fold (from 94 to
33/mm2) proportional decrease in FOXP3+ cells
in transformed DLBCL evolving from FL was observed after
immunochemical screening, and this was similar to the 4.4-fold
decrease observed by Carreras et al (9.6-2.2%) (17).
Concerning the presence of CD68+ cells,
we did not obtain a significant correlation with poor survival.
However, a recent study outlined the influence of specific
therapeutic regimens on the prognostic impact of CD68-postive
macrophages in follicular lymphoma (28). Surprisingly, the numbers of CD68
cells both in intrafollicular and interfollicular locations were
significantly lower at relapse as compared to the diagnostic
samples (respectively 32 vs. 39.23, p=0.07 and 62 vs. 96,
p=0.05).
In inflammatory lymph nodes, we found an
interfollicular CD8/FOXP3 ratio (2.74) close to that of the 15 FL
patients with less than a 5-year survival (2.02). These findings
are in agreement with those of Glas et al, who showed that
FL with a poor prognosis and a poor response to anti-CD20 therapy
had gene expression signatures that were similar to that of normal
activated lymphoid tissue (29).
As proposed by De Jong, the clinical behaviour and
prognosis of FL seem to be inherited rather than acquired
properties according to a dual pathway model (30,31).
The poor prognosis pathway may be characterised by genetic
alterations inducing highly activated specific T cells. In the
‘good prognosis’ pathway, different alterations (der18q and +7/+8)
and additional anti-apoptotic signals are involved, so protection
from apoptosis may therefore be more important than immunologic
support. The findings of a recent study support this hypothesis,
showing that host genetic variability in immune genes, particularly
IL-8, IL-2, IL-12B and IL-1RN, appear to be associated with overall
survival in FL (32).
In conclusion, the presence of a high CD8/FOXP3
ratio is the hallmark of an active immune response during tumour
development, with lymphoma cells acting as either target or
bystander and reflecting a more aggressive disease.
References
|
1.
|
Tsujimoto Y, Cossman J, Jaffe E and Croce
CM: Involvement of the bcl-2 gene in human follicular lymphoma.
Sciences. 228:1440–1443. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Clearly ML and Sklar J: Nucleotide
sequence of t(14,18) chromosomal breakpoint in follicular lymphoma
and demonstration of a breakpoint-cluster region near a
transcriptionally active locus on chromosome 18. Proc Natl Acad Sci
USA. 82:7439–7443. 1995. View Article : Google Scholar
|
|
3.
|
Solal-Céligny P, Roy P, Colombat P, White
J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero
D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI,
Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haïoun C, LeBlanc M,
Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P,
Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H,
Vitolo U, Zinzani PL, Zucca E and Montserrat E: Follicular Lymphoma
International Prognostic Index. Blood. 104:1258–1265. 2004.
|
|
4.
|
Lossos IS and Levy R: Higher-grade
transformation of follicle center lymphoma is associated with
somatic mutation of 5′ noncoding regulatory region of Bcl-6 gene.
Blood. 96:635–639. 2000.
|
|
5.
|
Küppers R: Prognosis in follicular
lymphoma – it’s in the microenvironment. N Engl J Med.
351:2152–2153. 2004.
|
|
6.
|
Natkunam Y: The biology of germinal
center. Hemat Am Soc Hematol Educ Program. 210–215. 2007.
View Article : Google Scholar
|
|
7.
|
Amé-Thomas P, Maby-El Hajjami H, Monvoisin
C, Jean R, Monnier D, Caulet-Maugendre S, Guillaudeeux T, Lamy T,
Fest T and Tarte K: Human mesenchymal stem cells isolated from bone
marrow and lymphoid organs support tumor B-cell growth: role of
stromal cells in follicular lymphoma pathogenesis. Blood.
109:693–702. 2007.PubMed/NCBI
|
|
8.
|
Herreros B, Sanchez-Aguilera A and Piris
MA: Lymphoma microenvironment: culprit or innocent. Leukemia.
22:49–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Carbone A, Gloghini A, Cabras A and Elia
G: Differentiating germinal center-derived lymphomas through their
cellular microenvironment. Am J Haematol. 84:435–438. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Staudt LM and Dave S: The biology of human
lymphoid malignancies revealed by gene expression profiling. Adv
Immunol. 87:163–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Dave SS, Wright G, Tan B, Rosenwald A,
Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM,
Miller TP, LeBlanc M, Greiner TC, Weisenburger DD, Lynch JC, Vose
J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Connors
JM, Lansdorp PM, Ouyang Q, Lister TA, Davies AJ, Norton AJ,
Muller-Hermelink HK, Ott G, Campo E, Montserrat E, Wilson WH, Jaffe
ES, Simon R, Yang L, Powell J, Zhao H, Goldschmidt N, Chiorazzi M
and Staudt LM: Prediction of survival in follicular lymphoma based
on molecular features of tumor-infiltrating immune cells. N Engl J
Med. 351:2159–2169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Woo EY, Chu CS, Goletz TJ, et al:
Regulatory CD4+ CD25+ T cells in tumors from
patients with early-stage non-small cell lung cancer and late-stage
ovarian cancer. Cancer Res. 61:4766–4772. 2001.PubMed/NCBI
|
|
13.
|
Shevach EM: Mechanisms of
foxp3+ T regulatory cell-mediated suppression. Immunity.
30:636–645. 2009.
|
|
14.
|
Farinha P, Al-Tourah A, Gill K, Klasa R,
Connors JM and Gascoyne RD: The architectural pattern of
FOXP3-positive T cells in follicular lymphoma is an independent
predictor of survival and histologic transformation. Blood.
115:289–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lee AM, Clear AJ, Calaminici M, Davies AJ,
Jordan S, MacDougall F, Matthews J, Norton AJ, Gribben JG, Lister
TA and Goff LK: Numbers of CD4+ cells and location of
forkhead box protein-P3-positive cells in diagnostic follicular
lymphoma tissue microarrays correlates with outcome. J Clin Oncol.
24:5052–5059. 2006.
|
|
16.
|
Tzankov A, Meier C, Hirschmann P, Went P,
Pileri SA and Dirnhofer S: Correlation of high numbers of
intratumoral FoxP3 regulatory T cells with improved survival in
germinal center-like diffuse large B-cell lymphoma, follicular
lymphoma and classical Hodgkin’s lymphoma. Haematologica.
93:193–200. 2008.PubMed/NCBI
|
|
17.
|
Carreras J, Lopez-Guillermo A, Fox BC,
Colomo L, Martinez A, Roncador A, Montserrat E, Campo E and Banham
AH: High numbers of tumors-infiltrating FoxP3-positive regulatory T
cells with improved overall survival in follicular lymphoma. Blood.
108:2957–2964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Glas AM, Kersten MJ, Delahaye L, Witteveen
AT, Kibbelaar RE, Velds A, Wessels LA, Joosten P, Kerkhoven RM,
Bernards R, Van Krieken JH, Kluin PM, van’t Veer LJ and de Jong D:
Gene expression profiling in follicular lymphoma to assess clinical
aggressiveness and to guide the choice of treatment. Blood.
105:301–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Engelbrekt W, Sander B, Christensson B and
Kimby E: CD8+ T-cell content in diagnostic lymph nodes
measured by flow cytometry is a predictor of survival in follicular
lymphoma. Clin Cancer Res. 13:388–397. 2007.
|
|
20.
|
Taskinen M, Karjalainen-Lindsberg ML and
Leppä S: Prognostic influence of tumor-infiltrating mast cells in
patients with follicular lymphoma treated with rituximab and CHOP.
Blood. 111:4664–4667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Alvaro T, Lejeûne M, Salvado MT, Lopez C,
Jaén J and Bosch R: Immunohistochemical patterns of reactive
microenvironment are associated with clinicobiologic behavior in
follicular lymphoma patients. J Clin Oncol. 24:5350–5357. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Taskinen M, Karjalainen-Lindsberg ML,
Nyman H, Eerola LM and Leppä S: A high tumor-associated macrophage
content predicts favorable outcome in follicular lymphoma patients
treated with rituximab and
cyclophosphamidedoxorubicin-vincristine-prednisone. Clin Cancer
Res. 13:5784–5789. 2007. View Article : Google Scholar
|
|
23.
|
Canioni D, Salles G, Mounier N, Brousse N,
Keuppens M, Morchhausser F, Lamy T, Sonnet A, Rousselet MC,
Foussard C and Xerri L: High numbers of tumor-associated
macrophages have an adverse prognostic value that can be
circumvented by rituximab in patients with follicular lymphoma
enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol.
26:440–446. 2008. View Article : Google Scholar
|
|
24.
|
Swerrdlow SH, Campo E, Lee Harris N, Jaffe
E, Pileir A, Stein H, Thiel J and Vardiman J: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. (4th edition).
International Agency for Research on Cancer. 2008.
|
|
25.
|
Ai W, Hou JZ, Zeiser R, Czerwinski D,
Negrin RS and Levy R: Follicular lymphoma B cells induce the
conversion of conventional CD4+ cells to T-regulatory
cells. Int J Cancer. 124:239–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Alvaro T, Lejeune M, Salvado MT, Bosch R,
Garcia JF, Jaén J, Banham AH, Roncador G, Montalban C and Piris M:
Outcome in Hodgkin’s lymphoma can be predicted from the presence of
accompanying cytotoxic and regulatory T cells. Clin Cancer Res.
11:1467–1473. 2005.
|
|
27.
|
Chetaille B, Bertucci F, Finetti P,
Esterni B, Stamatoullas A, Picquenot JM, Copin MC, Morschhauser F,
Casasnovas O, Petrella T, Molina T, Vekhoff A, Feugier P,
Bouabdallah R, Birnbaum D, Olive D and Xerri L: Molecular profiling
of classical Hodgkin lymphoma tissues uncovers variations in the
tumor microenvironment and correlations with EBV infection and
outcome. Blood. 113:2765–3775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
De Jong D, Koster A, Hagenbeek A,
Reameakers J, Veldhuizen D, Heisterkamps S, De Boer JP and
Vanglabeeke M: Impact of tumor microenvironment on prognosis in
follicular lymphoma is dependent on specific treatment protocols.
Haematologica. 94:70–77. 2009.PubMed/NCBI
|
|
29.
|
Glas AM, Kersten MJ, Delahaye LJ,
Witteveen AT, Kibbelaar RE, Velds A, Wessels LF, Joosten P,
Kerkhoven RM, Bernards R, van Krieken JH, Kluin PM, van ‘t Veer LJ
and De Jong D: Gene-expression and immunohistochemical study of
specific T-cell subsets and accessory cell types in the
transformation and prognosis of follicular lymphoma. J Clin Oncol.
25:390–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
De Jong D: Molecular pathogenesis of
follicular lymphoma: A cross talk of genetic and immunologic
factors. J Clin Oncol. 23:6358–6363. 2005.PubMed/NCBI
|
|
31.
|
Kelley T, Beck R, Absi A, Jin T, Pohlman B
and Hsi E: Biologic predictors in follicular lymphoma: importance
of markers of immune response. Leuk Lymphoma. 48:2403–2411. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Glas AM, Kersten MJ, Delahaye LJ,
Witteveen AT, Kibbelaar RE, Velds A, Wessels LF, Joosten P,
Kerkhoven RM, Bernards R, van Krieken JH, Kluin PM, van ‘t Veer LJ
and de Jong D: Prognostic significance of host immune gene
polymorphisms in follicular lymphoma survival. Blood.
109:5439–5446. 2007. View Article : Google Scholar : PubMed/NCBI
|